Abstract
The Hyperpolarization-activated, Cyclic Nucleotide-gated (HCN) channels, or If/Ih channels, are conventionally considered as monovalent-selective channels. Recently we discovered that Ca2+ ions can permeate through HCN4 and Ih channels in neurons. This raises the possibility of Ca2+ permeation in If, the Ih counterpart in cardiac myocytes, due to their structural homology. We performed simultaneous measurement of fura-2 Ca2+ signals and whole-cell currents produced by HCN2 and HCN4 channels (the two cardiac isoforms present in ventricles) expressed in HEK293 cells and by If in rat ventricular myocytes. We observed Ca2+ influx when HCN/If channels are activated. Ca2+ influx was increased with stronger hyperpolarization or longer pulse duration. Cesium, an If channel blocker, inhibited If and Ca2+ influx at the same time. Quantitative analysis revealed that Ca2+ flux contributed to about 0.5% of IHCN2 or If. The associated increase in Ca influx was also observed in spontaneously hypertensive rat (SHR) myocytes in which If current density is higher than that of normotensive rat ventricle. In the absence of EGTA (a Ca2+ chelator), pre-activation of If channels significantly reduced the action potential duration and the effect was blocked by another selective If channel blocker, ZD7288. In the presence of EGTA, however, pre-activation of If channels has no effects on action potential duration. Our data extend our previous discovery of Ca2+ influx in ih channels in neurons to If channels in cardiac myocytes.
Keywords: Ca2+ flux, HCN/If channels
INTRODUCTION
Ca2+ entry triggers a variety of essential cellular activities including cardiac muscle contraction (2). The fractional Ca2+ current, Pf, is defined as the percentage of current carried by Ca2+ in the total current through cation channels (32). Pf has been identified in many ion-conducting channels such as nicotinic acetylcholine receptors (nAChRs) (32), glutamate receptors [N-methyl-D-aspartate receptors] (NMDA-Rs) (17, 24), α-amino-3- hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPA-Rs) (4), cyclic nucleotide-gated (CNG) channels (8), and voltage-dependent Ca2+ channels (30). Ca2+ influx through these channels contributes to transmitter release, axon guidance, or muscle contraction (2,4,30,32).
In neurons the time- and voltage-dependent inward cation current, Ih, is generated by HCN channels (10,14,23). It has been shown that activation of Ih channels in crayfish neurons facilitates secretion (3). However, only monovalent cations was expected to permeat through the Ih channels. Recently, we demonstrated the presence of a fractional Ca2+ current through Ih channels in dorsal root ganglion (DRG) neurons (28). We found that Ca2+ influx through Ih channels at negative potentials contributes to activity-evoked secretion in DRG neurons (28).
The cardiac counterpart of Ih, If, shares same molecular components. Among four HCN channel isoforms that have been cloned, three of them, HCN1, HCN2, and HCN4, are present in heart (25). Two isoforms, HCN4 and HCN2, are present in the ventricles (25). Our previous finding of Ca2+ influx through Ih channel raised the possibility of Ca2+ entry through If channels in cardiac myocytes and subsequent contribution to cardiac function at negative membrane potentials.
In this paper, we demonstrated that a fractional Ca2+ current is present in currents induced by HCN2 and HCN4 channels, which were ectopically expressed in HEK293 cells, and in If of rat ventricular myocytes, designated as Pf (If). Preliminary results for understanding its potential in cardiac function are shown and the future investigation for establishing its physiological role in cardiac pacemaker cells is discussed.
MATERIALS AND METHODS
Heterologous expression of HCN channels
The full-length cDNA of mouse HCN2 was subcloned into EcoR I/Xba I sites in pCMS-EGFP vector (Clontech), human HCN4 was a gift from Forshungszentrum Julich. HEK293 cells were grown in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin. When cells approached confluence, they were seeded into 35 mm dishes, and subsequently transfected with the HCN plasmids using a Ca2+ phosphate method (28).
Cell dissociation
Adrenal chromaffin cells were isolated from Wistar rats and cultured as previously described (7,31). Cells were used in the experiments after 2-6 days in culture. Single ventricular cardiac myocytes were isolated from adult Sprague-Dawley rats (2-3 months old; weight, 225-300g, from SLACCAS Inc., Shanghai) using a previously described Langendorff method (21). Briefly, the heart was removed, placed in Tyrode's solution containing (in mM): NaCl, 137; MgCl2, 0.5; glucose, 10; KCl, 5.4; CaCl2, 1.8; and HEPES, 11.8 (pH adjusted to 7.4 with NaOH), and squeezed gently to expel the blood. Ventricular myocytes were prepared using a Langendorff perfusion apparatus. Briefly, the hearts were removed and perfused with calcium-free Tyrode's containing (in mM): NaCl, 130; MgSO4, 1.2; KCl, 5.4; KH2PO4, 1.2; HEPES-NaOH, 6 (pH adjusted to 7.2 with NaOH) with collagenase (Liberase Blendzyme 4, 0.1 mg/ml, Roche Molecular Biochemicals) for approximately 9 min. After washing out the collagenase with calcium-free Tyrode's, single cells were dissociated by mincing the ventricle and shaking the tissue in “Kraftbrühe” (KB) solution containing (in mM): KCl, 83; K2HPO4, 30; MgSO4, 5; Na-pyruvate, 5; Na-β-hydroxybutyrate, 5; creatine, 5; taurine, 20; glucose, 10; EGTA, 0.5; HEPES-KOH, 5; ATP-Na2, 5 (pH adjusted to 7.2 with KOH). Cells were washed and resuspended in KB.
The use and care of animals in this study complied with the guidelines of the Animal Research Advisory Committee in the Shanghai Institutes of Biological Sciences.
Whole-cell patch clamp recordings
Ionic currents were studied in the whole-cell patch-clamp configuration using an EPC-9 amplifier (HEKA Elektronik, Germany). The membrane was held at −40 mV unless otherwise stated. A RCP-2B perfusion system was used for switching external solutions. The system has a fast exchange time (100ms) controlled electronically among 7 channels (Inbio, Wuhan, China (29)).
Experiments on chromaffin and HEK cells were conducted at room temperature (22-24°C). Ventricular myocytes were studied at 32-35°C.
Solutions used in experiments are defined in Table 1. Pipettes with resistances of 2-5 MΩ were used for all three types of cells.
Table 1.
Composition of solutions: internal solutions * (mM)
| HEK cells | rat myocytes | chromaffin cells | |
|---|---|---|---|
| NaCl | 10 | – | – |
| KCl | 145 | 140 | – |
| CsCl | – | – | 153 |
| MgCl2 | 1 | 1 | 1 |
| Hepes | 5 | 10 | 5 |
| ATP | 2 | 2 | 2 |
| EGTA | – | 0.1 | - |
| pH | 7.2 | 7.2 | 7.2 |
| External solutions (mM) | |||
|---|---|---|---|
| HEK cells | rat myocytes | chromaffin cells | |
| NaCl | 138 | 137 | – |
| NMG | – | – | 138 |
| KCl | 5.6 | 5.4 | 5.6 |
| CaCl2 | 2.6 | 1.8 | 2.6 |
| MgCl2 | 1.2 | 1 | 1.2 |
| #BaCl2 | – | 5 | – |
| #4-AP | – | 2 | – |
| #CdCl2 | – | 0.2 | – |
| HEPES | 10 | 5 | 10 |
| Glucose | 10 | 10 | 10 |
| pH | 7.4 | 7.4 | 7.4 |
For fluorescence calibration experiments, 1 mM fura-2 potassium salt was added to internal solutions. For other Ca2+ imaging experiments, 0.1mM fura-2 salt was added to internal solutions.
CdCl2 was used to prevent Ca2+ entry from VDCCs that can be activated by steps back from hyperpolarization to the holding potential in ventricular myocytes. BaCl2 was used to block the background potassium current, iK1, which masks the activation of If. 4-AP was used to inhibit the activation of the transient outward potassium current, ito, which can overlap the deactivation of If.
Like our previous work, fluorescence calibration for Pf were performed on chromaffin cells (4,24,28,32). An intracellular solution containing high CsCl (see Table 1) was used to measure voltage-gated Ca2+ currents.
DMEM and fetal bovine serum were purchased from Gibco/Invitrogen. Fura-2 salt was from Molecular Probes. All other chemicals were from Sigma.
Theory and measurement of fractional Ca2+ currents
Intracellular Ca2+ concentration, [Ca2+]i was measured by dual-wavelength ratiometric fluorometry. The Fura-2 was excited with light alternating between 340 and 380 nm using a monochromator-based system (TILL Photonics), and the resulting fluorescence signals were measured using a cooled CCD. Relative changes in [Ca2+]i were calculated from the ratio of F340 to F380, which were sampled at 1 Hz. The image data were transferred and analyzed by Igor software (WaveMetrix) (28).
Fractional Ca2+ current, Pf, is defined as the percentage of Ca2+ current in the total current passing through the cation channels (IHCN in this case). According to the original definition (28,32),
| (Eq. 1) |
where IHCN is the HCN current, and IHCN,Ca is the proposed fractional IHCN current carried by Ca2+.
The change of Fd, ΔFd, is the “modified Ca2+-sensitive fura-2 signal” immediately before (Fd’) and after (Fd”) a voltage-pulse induced Ca2+ influx (30). Under the condition that all entering Ca2+ ions are bound by fura-2, Fd is a measure of Ca2+ influx (30). Fd is determined by the difference of fluorescence signals at 340nm and 380nm.
| (Eq. 2) |
| (Eq. 3) |
fmax is determined by measuring Ca2+ influx through voltage-gated Ca2+ channels in chromaffin cells under the condition that intracellular fura-2 is sufficiently high (> 0.4 mM, ref. 32).
Under physiological conditions, only Ca2+ ions contribute to the Ca2+ channels (30), or Pf = 100%. From Eq.1 we have
| (Eq. 4) |
where ICa is the current through voltage-gated Ca2+ channels. Although the calibration of fmax is measured in chromaffin cells, the accuracy of Pf(If) determined in myocytes should be safe because fmax is insensitive to cell types (4).
To record the time course of fura-2 dialysis, we used the Ca2+-independent fluorescence signal F360 (32), which can be calculated from F340 and F380.
| (Eq. 5) |
whereα is the “isocoefficient”. According to Eq. 5, α can be determined by any experimental recording that shows rapid changes in Ca2+ concentration. In our setup, α = 0.35. Since F360 is Ca2+-independent, it can be used as an indicator of the intracellular fura-2 concentration, [fura]i. After establishing the whole-cell recording configuration, fura-2 was dialyzed into the cell. Dialysis was accompanied by a proportional F360 increase. Once F360 reached a steady-state level, we assumed that [fura]i was equal to the fura-2 concentration in the pipette (Fig.2, also see ref 32).
Figure 2.
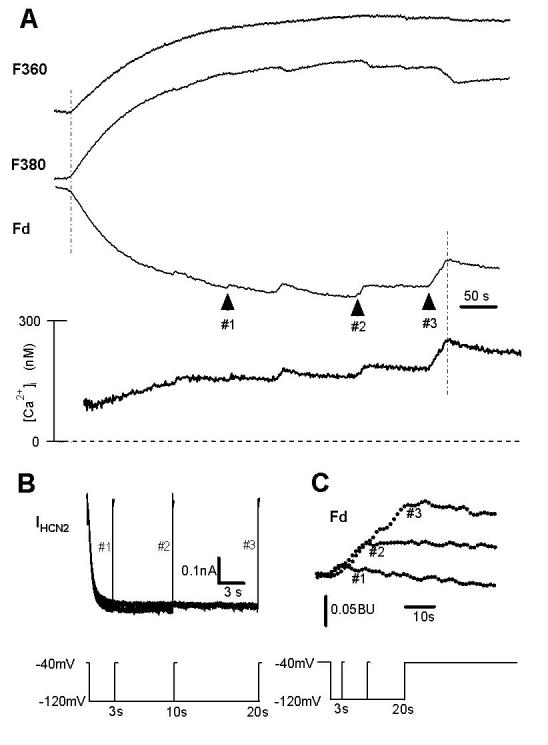
Ca2+ influx through HCN2 channels in HEK293 cells.
( A ). Ca2+ signals in response to a 3 s (arrow #1), 10 s (arrow #2), and 20 s (arrow #3) hyperpolarizing pulse (see protocol in panel B). Fluorescence signals during fura-2 loading (0.1 mM in the pipette) at 360 nm (upper trace F360, Ca2+-insensitive fluorescence, indicating the process of fura-2 entry into the cell); at 380 nm (second trace F380, Ca2+-sensitive fluorescence, indicating Ca2+ influx), Fd = F340 - F380 (third trace Fd, modified signal indicating Ca2+ influx) are shown together with intracellular free Ca2+ concentration (lower trace [Ca2+]i). The cell was hyperpolarized to −120 mV for 3 s twice, for 10 s twice, and for 20 s once. Arrow #1 indicates the rise of Ca2+ influx corresponding to the second 3s pulse, arrow #2 the second 10s pulse, and arrow #3 the 20s pulse. Similar results were observed in 14 cells.
( B ). HCN2 currents at −120 mV for 3 s, 10 s, and 20 s. The voltage protocol is shown in the lower panel.
( C ). Enlarged Fd signals corresponding to arrows #1, #2 and #3 in panel A.
We applied equations 1-4 to determine the Pf of HCN2 and If channels by measuring the fura-2 signals.
Data were analysed with IGOR Pro3.12 software (Wavemetrics, Inc., Lake Oswego, OR). Unless otherwise stated, the data were presented as mean ± SD. Statistical significance was tested with Student's t-test. P < 0.05 was considered statistically significant.
RESULTS
Hyperpolarization-induced Ca2+ influx was present in HCN-expressing HEK cells
In a non-transfected HEK cell held at −70mV, a hyperpolarizing pulse to −120mV induced neither a time-dependent inward IHCN (lower) nor Ca2+ flux (upper in the left panel of Fig.1B). On the other hand, in response to the same hyperpolarizing pulse a HCN2-transfected cell exhibits a typical IHCN2 (lower right panel in Fig. 1B) and Ca2+ flux (upper part of the right panel in Fig.1B) at the same time, suggesting that the Ca2+ signals induced by hyperpolarization are due to the activation of the HCN channels. In both experiments, the cells were killed at the end (see arrows) so that Ca2+ flux could be measured by the fura-2 signals (ΔFd, see ref. 30). Similar results were observed in 5 non-transfected cells and 5 HCN2-transfected cells. In addition, we discovered the requirement of extracellular Ca2+ for hyperpolarization- induced Ca2+ flux. In response to the same pulse shown in Fig.1B, no Ca2+ flux could be detected in the absence of extracellular Ca2+ (arrow 2). However, the Ca2+ flux appeared in the presence of 2mM Ca2+ (arrows 1 and 3). These data support the hypothesis that the extracellular Ca2+ and open HCN channels are required to induce Ca2+ flux.
Figure 1.
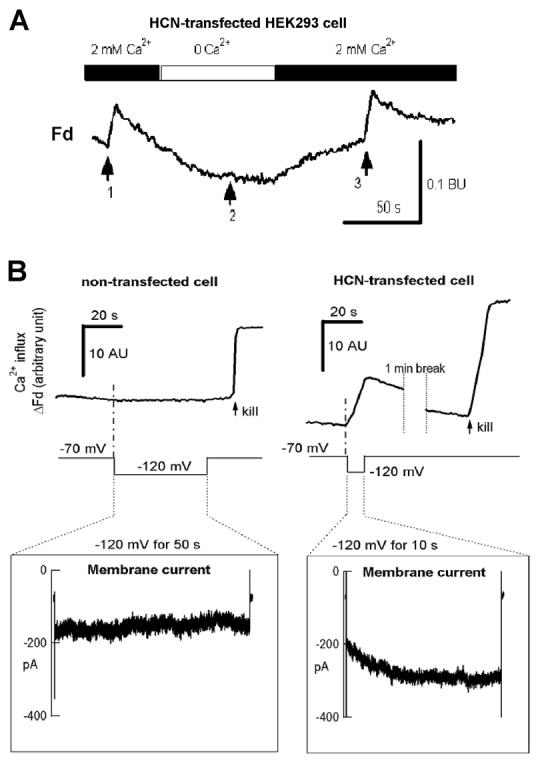
HCN channels induced Ca influx in HEK293 cells
( A ). Extracellular Ca2+ is required for hyperpolarization-induced Ca2+ influx in HEK 293 cells expressing HCN4. In response to a hyperpolarizing pulse to −120 mV for 5 s (arrows 1 and 3) from the holding potential of −70mV, calcium influx (arrow 1) was abolished when Ca2+ was removed from the bath (arrow 2), and re-appeared after Ca2+ was added back to the bath (arrow 3). Similar results were observed in 10 cells.
( B ). Requirement of HCN channels for hyperpolarization-induced Ca2+ influx. Left panel: there were no time-dependent inward HCN current (lower left) and Ca influx signals (upper left) in response to a 50 s hyperpolarization pulse (inset shows the pulse protocol) in a non-transfected HEK293 cell. Right panel: in a HEK293 cell transfected with GFP-HCN2, a 10 s hyperpolarization pulse induced a time-dependent inward HCN2 current (lower inset) and Ca influx (upper right) simultaneously.
Fractional Ca2+ current through HCN2 and HCN4 channels in HEK293 cells
If Ca2+ ion indeed pass through the HCN channels, the changes in fura-2 Ca2+ signals should be directly associated with the time- and voltage-dependent properties of HCN channels. To test this hypothesis, IHCN2 was elicited by a step to −120 mV for 3s (#1), 10s (#2), and 20s (#3) (protocol shown in the insets of Fig.2B) from a holding potential of −40mV. Measurement of Ca2+ fluorescence (Figs. 2A, 2C) showed a rise in [Ca2+]i (arrows #1 in Fig. 2A and 2C), and this rise was increased with longer pulse durations of 10s (arrow #2) and 20s (arrow #3). These data demonstrate a correlation of increasing Ca2+ influx with the prolonged (time-dependent) activation of HCN2 channels.
Gating of HCN channels is also voltage dependent. A hyperpolarizing step to −70mV for 3 seconds did not activate HCN4 channels (middle in Fig. 3B) and induced no Ca2+ signal (Fig. 3A, upper in Fig. 3B), whereas a step to −120 mV for 3 seconds activated the channels (middle in Fig. 3B) and simultaneously induced calcium influx (Fig. 3A, upper in Fig. 3B). The pulse protocol is shown in the lower panel of figure 3B. In figure 3A, the peaks between −120mV/3s and −70mV/3s marks correspond to hyperpolarizing steps to −120mV, −110mV, −100mV, −90mV, and −80mV. Decreasing amplitudes of these peaks at various potentials suggest a voltage dependent change in calcium influx, which simultaneously accompanies the voltage dependent activation of the channels. Fig. 3C elaborates the relationship between normalized ΔFd and total ion inflow (Q) for HCN4 channels at tested pulses. The superimposed traces indicates a correlation between the HCN4 currents and the Ca2+ influx through HCN4 channels.
Figure 3.
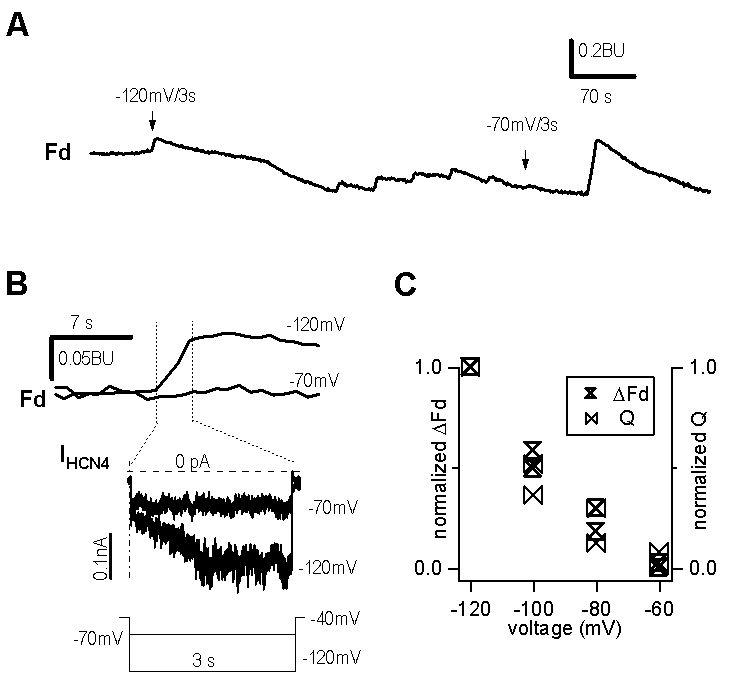
Ca2+ influx through HCN4 channels in HEK293 cells
( A ). Calcium signals in response to different hyperpolarizing pulses. Arrows indicate changes in Fd in response to steps to −120mV and −70mV for 3s, respectively. The peaks between arrows represent calcium signals in response to −120mV, −110mV, −100mV, −90mV, and −80mV for 3 seconds. The last peak is the calcium signal in response to −120mV for 10 seconds.
( B ). Comparison of enlarged calcium signals (upper traces) and HCN4 currents (middle traces) at −70mV and −120mV for 3 seconds. Lower panel is the voltage protocol. A leak current of −80 pA was subtracted for optimal comparison of two current traces.
( C ). Voltage dependence of normalized total ion inflow (Q) and Ca2+ influx (ΔFd) through HCN4 channels.
To quantify the Ca2+ influx through HCN channels, we employed a widely used calibrating approach: quantitating Ca2+ flux through the voltage-dependent Ca2+ channel expressed in rat adrenal chromaffin cells, which passes 100% Ca2+ (ref 28, also see eq. 4 in Methods for details) as calibration for fura-2 signals. A depolarizing step to 0 mV for 500ms from a holding potential of −70 mV (lower panel, Fig. 4A) activated a voltage-dependent Ca2+ current (ICa) and simultaneously induced an increase in Fd (ΔFd2, Fig. 4B, upper panel). The total ion influx charge was calculated from the time integral of ICa current trace (Fig. 4B, middle panel, shaded region). In Fig. 4A we show that in a HEK293 cell expressing HCN2 channels, a hyperpolarizing step to −120 mV for 10 s (inset) activated the HCN2 current (middle panel) and simultaneously induced an increase in Fd (ΔFd1, upper panel). The time integral of ion flux through HCN2 channels was calculated from the current trace (Fig. 4A, middle panel, shaded area). The relationship between total ion influx and the corresponding increase in Fd (ΔFd) obtained with different durations of stimulation (Fig. 4C) was best fitted by a linear equation, indicating a correlation of the increased ΔFd with the increased ion flux through voltage-dependent calcium channels (Fig. 4B) and HCN2 channels (Fig. 4A). Using equation 1 in Methods, we determined Pf for HCN2 to be 0.47 ± 0.02% (n=6) (Fig. 4D).
Figure 4.
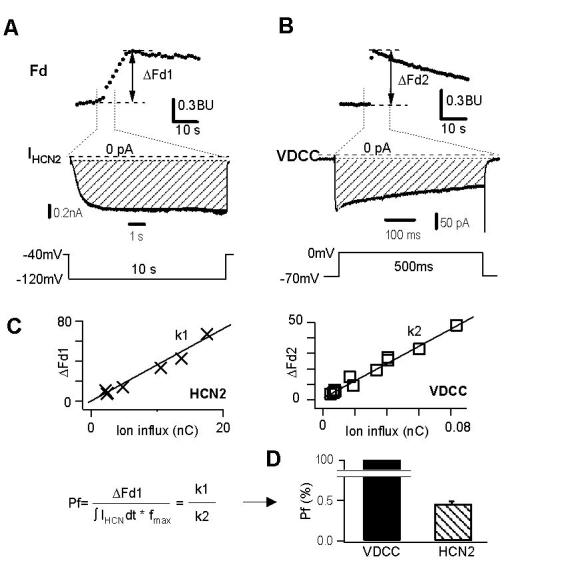
Protocol to determine fractional Ca2+ current through HCN2 channels.
( A ). Ca2+ signal changes in response to a hyperpolarizing pulse to −120 mV for 10 s (lower trace) in a HEK293 cell expressing HCN2 channels. The change in fluorescence induced by the stimulus is shown as ΔFd1 (upper trace). Dashed line of middle trace represents zero current. The shaded area indicates total ion influx charge through the HCN2 channels.
( B ). Fluorescence changes in response to a depolarizing pulse (lower panel) in a rat adrenal chromaffin cell. The change in fluorescence induced by the stimulus is shown as ΔFd2 (upper trace). Dashed line of middle trace represents zero current. The shaded area indicates total ion influx charge through voltage-dependent calcium channels (VDCC).
( C ) & ( D) .Quantitative determination of the fractional Ca2+ current through HCN2 channels. In C, ΔFd1 for HCN2 is plotted against the corresponding ion influx, Q1. In D, Pf for HCN2 is determined by Pf = (Fd1/Q1) / (Fd2/Q2) = 0.47 ± 0.02% (n=6), with Fd2 and Q2 corresponding to ΔFd2 and ion influx of voltage-gated Ca2+ channels, respectively (n = 7). The fractional Ca2+ current in Ca2+ channels is assumed to be 100% (refs. 26,30,32).
Ca2+ influx through If channels in ventricular myocytes
Given HCN2 and HCN4 as the two isoforms that encode If channels in rat ventricle (25), we hypothesized similar Ca2+ flux through If channels in rat ventricular myocytes. In response to hyperpolarizing pulses ranging from −70mV to −150mV (inset of Fig. 5A) the If current traces were shown in fig. 5A. In this cell, If began to activate around −80mV, close to the previously reported values (5, 21).
Figure 5.
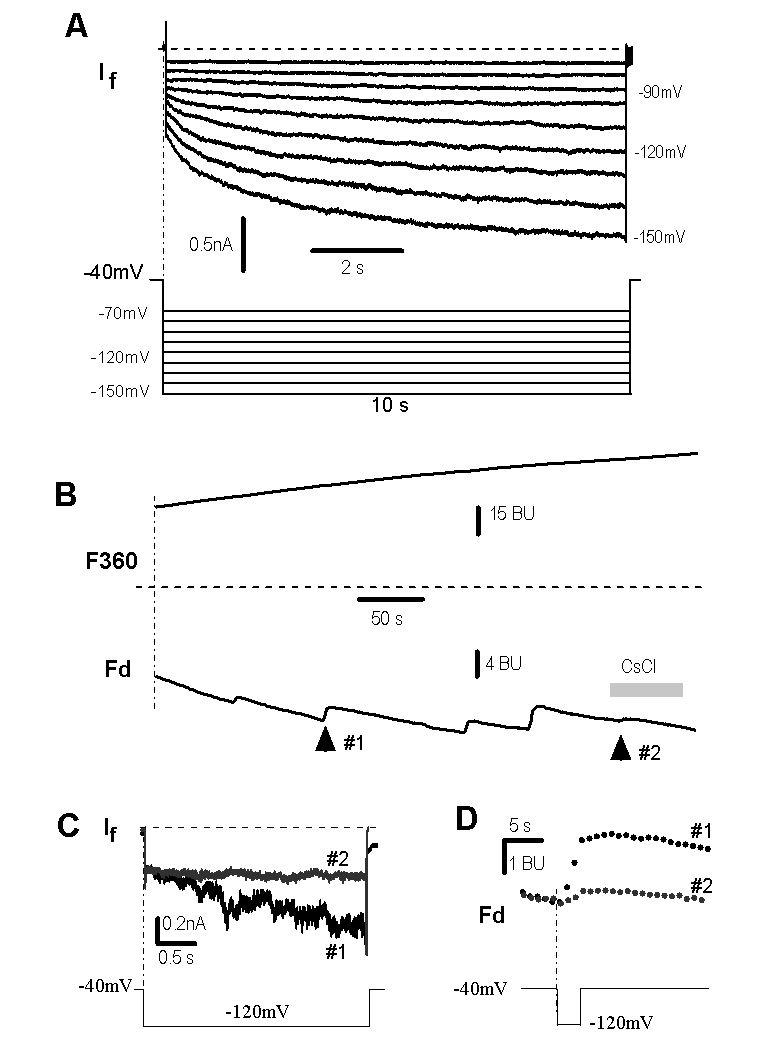
If in rat ventricular myocytes.
( A ).If was recorded in steps ranging from −70 mV to −150 mV with 10mV increments (protocol in lower panel) in a rat ventricular myocyte.
( B ). Ca2+ signals in response to a step to −120 mV in the absence (arrow #1) and presence of 2 mM CsCl (arrow #2).
( C ). 2 mM Cs+ (trace #2, corresponding to arrow #2 in panel A) blocked If at −120mV (trace #1, corresponding to arrow #1 in panel A).
( D ). Enlargement of fluorescent signals marked by arrows #1 and #2 in panel B. The fractional Ca2+ current of If was 0.6 ± 0.1% (n = 3). Dashed lines in A and C represent zero currents.
To examine the fractional Ca2+ current through If channels, we applied a 3 s hyperpolarizing pulse to −120mV from a holding potential of −40mV, and detected a rise of Ca2+ signal (arrow #1 in Fig. 5B, 5D) concomitant with activation of If (Fig. 5C, #1). When If was blocked by 2 mM cesium (trace #2 in Fig. 5C), the increased Ca2+ signal was blocked as well (mark #2 in Figs. 5B and 5D). These results indicate that Ca2+ indeed passes through If channels in rat ventricular myocytes, which is consistent with Ca2+ influx through HCN2 and HCN4 channels (Figs. 2 and 3). Quantitative analysis revealed a Pf of 0.6 ± 0.1% (n = 3) for If channels, similar to HCN2 and HCN4 channels expressed in HEK293 cells (Figs. 2 and 4, ref. 28).
Ca2+ influx through If channels in SHR ventricular myocytes
Although we have demonstrated the Ca2+ flux through HCN2 and HCN4 channels in HEK293 cells and If channels in rat ventricular myocytes, we thought the evidence supporting calcium permeation through If channels would be stronger if we can find the altered change in Ca2+ flux at membrane hyperpolarization in an animal model in which If is naturally altered. In spontaneously hypertensive rat (SHR) ventricle If current density is significantly increased (5). Fig. 6 shows a typical example in that in response to a 10s pulse to −150mV from the holding potential of −70mV, both If (upper panel) and Ca2+ influx (lower panel) through If channels are significantly larger in SHR myocytes than in normal rat myocytes (Fig. 6A). The averaged If current density at −150mV is increased by 55% in SHR compared to the control (Fig. 6B). The increase in If is associated with an increase in Ca2+ influx (69%) (Fig. 6C).
Figure 6.
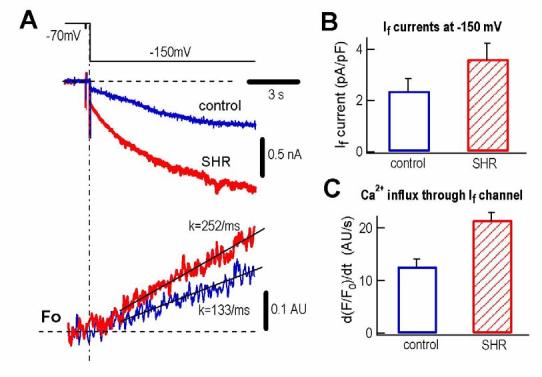
Ca2+ influx through If channels in SHR ventricular myocytes.
( A ). If currents (middle traces) and Ca2+ signals (lower traces, shown as F/F0) in ventricular myocytes from control rat and SHR rat upon a 10-s hyperpolarization pulse to −150 mV from a holding potential of −70 mV (protocol in upper panel).
( B ). Comparison of If current density in control (2.34 ± 0.49 pA/pF, n = 8) and in SHR (3.63 ± 0.63 pA/pF, n = 12) at −150 mV, (p < 0.05).
( C ). Comparison of the rates of Ca2+ signal changes in control (1.27 ± 0.15 AU/s, n = 7) and in SHR (2.15 ± 0.14 AU/s, n = 5) in response to a pulse to −150 mV (p < 0.001).
Shortening of action potential by Ca2+ influx through If channels
As an initial effort to investigate the functional role of Ca2+ flux through If channels in cardiac myocytes, we took the advantage of the established role of Ca2+ in cardiac action potential.
Upon membrane depolarization, L-type calcium channels are activated, allowing Ca2+ to enter the cell, which provides a major inward current contributing to the plateau phase of the action potential in ventricular myocytes (2). It is well documented that Ca2+ entry through L-type Ca2+ channels causes the channel inactivation (11, 12). On the other hand, we were wondering if Ca2+ can enter the cell through If channels upon hyperpolarization, they should also be able to inhibit the subsequent gating of L-type Ca2+ channels which produces less inward current and, in turn, would shorten the action potential duration.
To investigate the effects of pre-activating If on the action potential duration, we compared the action potential duration measured at the 15% of amplitude with and without pre-activating If (Fig. 7A). A hyperpolarizing pulse to −120mV for 5s was applied to open If channels prior to initiating an action potential (Fig. 7B). Comparing to the control (without a preceding hyperpolarizing pulse that opens If channels), the action potential duration was shortened by 32 ± 7 % (n=5, p<0.05) and returned to control when the hyperpolarizing pulse was removed (Fig. 7A, 7C). In addition, if the intracellular Ca2+ was buffered by adding 10mM EGTA to the pipette solution, the effect of pre-activation of If on the shortening of action potential duration was eliminated (99 ± 3 %, n=8, Fig. 7C). This indicates that Ca2+ influx through If channels was functionally involved in the shortening of action potential duration. This conclusion was further supported by two additional experiments. In the first experiment, when the cell was stimulated by a 50 ms depolarization from −40mV to 0 mV to activate L-type Ca2+ channels (leading to a Ca2+ influx similar to that of 1 nA If for 5 s), the action potential duration was shortened to a similar degree (27 ± 4 %, n=4, p<0.05, Fig. 7C). In the second experiment, ZD7288 (30 μM), which is a specific antagonist of If channels (28), was able to eliminate the shortening effect of If activation on action potential duration (91 ± 5 %, n=3, Fig. 7C). Taken together, all these data support the conclusion that Ca2+ influx through If channels can contribute to the action potential duration.
Figure 7.
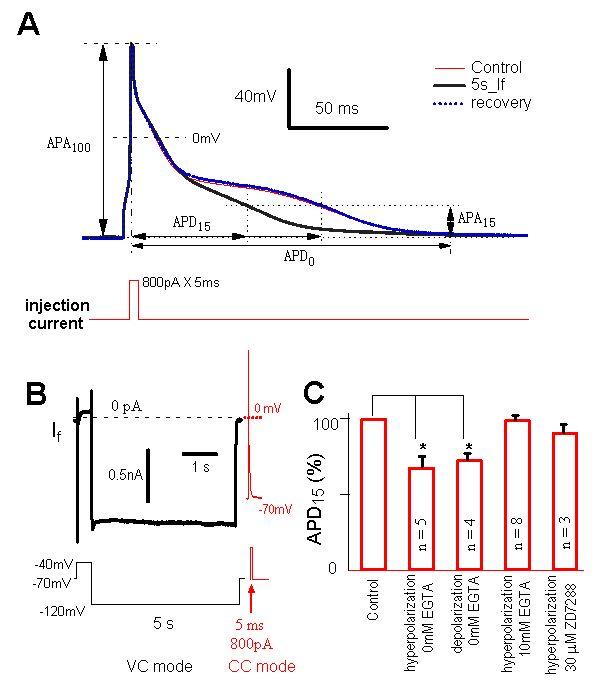
If-induced shortening in action potential duration.
( A ). Action potentials (APs, upper traces) in a ventricular myocyte before (thin line, control) and after (thick line) a 5 s hyperpolarizing pulse to −120 mV. Action potentials were induced by 800 pA depolarizing current for 5 ms (lower trace). Action potential duration, APD15, starts at the peak of the AP, or 100% of AP-amplitude (APA100), and ends at the time when the APA has decayed to 15% of APA100. A pre-hyperpolarization pulse for 5 s shortened APD15. This effect was reversible (dashed line).
( B ). Hyperpolarization-induced current in the same ventricular myocyte of panel A. Immediately before the AP recording under current clamp (CC), the cell was stimulated by a 5 s hyperpolarizing pulse (inset) under voltage-clamp (VC). Lower panel shows the pulse protocol.
( C ). Statistic analysis. Compared with control, APD15 was shortened by 32 ± 7% (n = 5) by pre-activation of If at −120mV for 5 s without EGTA in the pipette solution. Pre-activation of Ca2+ channels for 50 ms also shortened APD15 by 27 ± 4% (n = 4). With 10 mM EGTA in the pipette, pre-activation of If for 5 s had no effect on APD (99 ± 3%; n = 8). ZD7288 blocked the effect (91 ± 5%; n = 3).
DISCUSSION
In this study, we have provided several lines of evidence to demonstrate the permeation of Ca2+ in If channels in rat ventricular myocytes. First, we used HEK293 cells expressing HCN2 and HCN4 channels, the two HCN channel isoforms encoding If channels. Using HEK293 cells allow us to avoid potential contamination of Ca2+ flux measurement since P(f) is small under our experimental conditions. There are no endogenous HCN channels in HEK293 cells (Fig. 1). Activation of HCN2 and HCN4 is accompanied by Ca2+ influx (Figs. 1,2 and 4). Second, longer (Fig. 2) and stronger (Fig. 3) hyperpolarizing pulses enhanced Ca2+ influx. Third, Ca2+ influx cannot be observed either at less hyperpolarization at which HCN channels are closed (Fig. 3B) or in the presence of Cs+ (Fig. 5). Finally, in HEK293 cells that were not transfected with HCN channels or in the absence of extracellular Ca2+, no Ca2+ influx was detected (Fig. 1). All these evidence point to the direction that the fractional Ca2+ current may present in If in cardiac myocytes.
To strengthen the link between the Ca2+ flux and If channel activation at very negative potential, we need cardiac cells that natively express either higher or no If channels. We chose spontaneously hypertensive rat (SHR) ventricular myocytes in which If channel expression is significantly highercompared to the normal rat ventricle (5). Using SHR cells allows us to compare the Ca2+ flux under two native conditions in the same species. The results shown in fig. 6 provide additional evidence supporting our hypothesis that calcium indeed permeate If channels, although at −150mV we cannot exclude the possible contribution of other ionic mechanisms such as Na/Ca exchanger current and Ca2+ release from sarcoplasmic reticulum. The higher percent increase in Ca influx (69%) than that in If current (55%) may also reflect the possibility of involving other ionic mechanism. Nonetheless, these data point to a potential role of the fractional Ca2+ influx through If channels during diastole in pathophysiologic ventricles where If channel expression is significantly increased (6,9,13).
It is well understood that the plateau phase of a ventricular action potential is maintained by a fine balance of outward and inward currents. The major time-dependent inward current that determines the duration of the plateau phase is the L-type calcium current, generated by calcium influx through L-type channels upon membrane depolarization. The contribution of this calcium inward current to the action potential duration is limited by the inaction of L-type calcium channels partially caused by calcium influx (2). Within every heartbeat, the amount of calcium enters the cell during depolarization will have to get out of cell when the membrane repolarizes to the resting potential via Na/Ca exchanger and calcium pump (2). That means that at negative membrane potentials, what we have learned is the mechanisms that extrude intracellular calcium to set the heart at relax (diastolic) stage ready for next action potential. The calcium influx through If channels that are open at negative potentials raised the possibility that calcium can still “leak” into the cell at resting or diastolic stage.
Compared with the fractional Ca2+ currents of other cation channels, such as nAChR (2.5%) (32), glutamate channels (10% for NMDA (17), 0.5-5% for AMPA/kainate receptors (4,24), CNG channels (10-80%) (8), and voltage-dependent calcium channels (100%) (30), the Pf of HCN/If channels is relatively small (0.47% in HCN2 and 0.6% in If channels). However, given the nature of local calcium signaling, this small Ca2+ flux through If channels may be sufficient to increase the local calcium concentration near the intracellular side of L-type calcium channels, effectively accelerates ICa inactivation which, in turn, shortens the action potential duration.
Although we have demonstrated the permeation of Ca2+ through If channels in rat ventricular myocytes, more experiments are needed to illustrate the physiological role of fractional calcium current via If channels in cardiac myocytes. Such evidence can only be achieved in a pacing tissue in which If is activated in the physiological voltage range. For example, If appears around −50mV in the cardiac pacemaker sinoatrial (25) and atrial myocytes (18) and −70mV in the neonatal rat ventricular cells (21). In addition, recent studies have shown that under dynamic conditions the activation of HCN1 and HCN2 channels can be dramatically shifted to rather positive voltages (1,16). Under those conditions, many mechanistic aspects and physiological implications of Ca2+ permeation in If channels would be tested. This can finally establish the physiological relevance of fractional Ca2+ current through HCN.
ACKNOWLEDGEMENTS
We thank Drs. Zhao-Nian Zhou and Huang-Tian Yang and Mrs. Shuhua Bai for help in preparing myocytes. This work was supported by grants from the Major State Basic Research Program of P.R. China (2006CB500800), the National Natural Science Foundation of China (30330210, 30328013 and C010505). HGY is supported by a Scientist Development Award from the American Heart Association and an RO1 HL075023 from NHLBI.
REFERENCES
- 1.Azene EM, Xue T, Marbán E, Tomaselli GF, Li RA. Non-equilibrium behavior of HCN channels: Insights into the role of HCN channels in native and engineered pacemakers. Cardiovas. Res. 2005;111:11–20. doi: 10.1016/j.cardiores.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 3.Beaumont V, Zucker RS. Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nat Neurosci. 2000;3:133–141. doi: 10.1038/72072. [DOI] [PubMed] [Google Scholar]
- 4.Burnashev N, Zhou Z, Neher E, Sakmann B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J Physiol. 1995;485:403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerbai E, Barbieri M, Mugelli A. Occurrence and properties of the hyperpolarization-activated current If in ventricular myocytes from normotensive and hypertensive rats during aging. Circulation. 1996;94:1674–1681. doi: 10.1161/01.cir.94.7.1674. [DOI] [PubMed] [Google Scholar]
- 6.Cerbai E, Pino R, Porciatti F, Sani G, Toscano M, Maccherini M, Giunti G, Mugelli A. Characterization of the Hyperpolarization-Activated Current, If, in Ventricular Myocytes From Human Failing Heart. Circulation. 1997;95:568–571. doi: 10.1161/01.cir.95.3.568. [DOI] [PubMed] [Google Scholar]
- 7.Duan KL, Yu X, Zhang C, Zhou Z. Control of secretion by temporal patterns of action potentials in adrenal chromaffin cells. J Neurosci. 2003;23:11235–43. doi: 10.1523/JNEUROSCI.23-35-11235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dzeja C, Hagen V, Kaupp UB, Frings S. Ca2+ permeation in cyclic nucleotide-gated channels. EMBO J. 1999;18:131–144. doi: 10.1093/emboj/18.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Velasco M, Goren N, Benito G, Blanco-Rivero J, Bosca L, Delgado C. Regional distribution of hyperpolarization-activated current (If) and hyperpolarization-activated cyclic nucleotide-gated channel mRNA expression in ventricular cells from control and hypertrophied rat hearts. J Physiol. 2003;553:395–405. doi: 10.1113/jphysiol.2003.041954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauss R, Seifert R, Kaupp UB. Molecular identification of a hyperpolarization - activated channel in sea urchin sperm. Nature. 1998;393:583–587. doi: 10.1038/31248. [DOI] [PubMed] [Google Scholar]
- 11.Hadley RW, Lederer WJ. Ca2+ and voltage inactivate Ca2+ channels in guinea-pig ventricular myocytes through independent mechanisms. J Physiol. 1991;444:257–68. doi: 10.1113/jphysiol.1991.sp018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hess P, Tsien RW. Mechanism of ion permeation through calcium channels. Nature. 1984;309:453–56. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- 13.Hoppe UC, Jansen E, Südkamp M, Beuckelmann DJ. Hyperpolarization-Activated Inward Current in Ventricular Myocytes From Normal and Failing Human Hearts. Circulation. 1998;97:55–65. doi: 10.1161/01.cir.97.1.55. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization activated mammalian cation channels. Nature. 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig A, Zong X, Stieber J, Hullin R, Hofmann F, Biel M. Two pacemaker channels from human heart with profoundly different activation kinetics. EMBO J. 1999;18:2323–2329. doi: 10.1093/emboj/18.9.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannikko R, Pandey S, Larsson HP, Elinder F. Hysteresis in the voltage dependence of HCN channels: conversion between two modes affects pacemaker properties. J. Gen. Physiol. 2005;125:305–326. doi: 10.1085/jgp.200409130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer ML, Westbrook GL. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michels G, Er F, Khan I, Südkamp M, Herzig S, Hoppe UC. Single-channel properties support a potential contribution of hyperpolarization-activated cyclic nucleotide-gated channels and If to cardiac arrhythmias. Circulation. 2005;111:399–404. doi: 10.1161/01.CIR.0000153799.65783.3A. [DOI] [PubMed] [Google Scholar]
- 19.Opthof T. Function and structure of the mouse sinus node: nothing you can see that isn't shown. Cardiovas. Res. 2001;52:1–4. doi: 10.1016/s0008-6363(01)00417-5. [DOI] [PubMed] [Google Scholar]
- 20.Palakodeti V, Oh S, Oh B-H, Mao L, Hongo M, Peterson KL, Ross J., Jr Force-frequency effect is a powerful determinant of myocardial contractility in the mouse. Am J Physiol. 1997;273:H1283–H1290. doi: 10.1152/ajpheart.1997.273.3.H1283. [DOI] [PubMed] [Google Scholar]
- 21.Robinson R,B, Yu HG, Chang F, Cohen IS. Developmental change in the voltage-dependence of the pacemaker current if in rat ventricle cells. Pflugers Arch. 1997;433:533–535. doi: 10.1007/s004240050309. [DOI] [PubMed] [Google Scholar]
- 22.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: From Molecules to Physiological Function. Annu Rev Physiol. 2003;65:453–80. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- 23.Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR. Identification of a gene encoding a hyperpolarization activated pacemaker channel of brain. Cell. 1998;93:717–729. doi: 10.1016/s0092-8674(00)81434-8. [DOI] [PubMed] [Google Scholar]
- 24.Schneggenburger R, Zhou Z, Konnerth A, Neher E. Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron. 1993;11:133–143. doi: 10.1016/0896-6273(93)90277-x. [DOI] [PubMed] [Google Scholar]
- 25.Shi W, Wymore R, Yu HG, Wu JY, Wymore R, Pan Z, Robinson RB, Dixon JE, McKinnon D, Cohen IS. On the distribution and prevalence of hyperpolarization-activated cation channel (HCN) mRNA expression in cardiac tissues. Circ Res. 1999;85:e1–e6. doi: 10.1161/01.res.85.1.e1. [DOI] [PubMed] [Google Scholar]
- 26.Yu HG, Chang F, Cohen IS. The Pacemaker current if exists in adult mammalian ventricular myocytes. Circ Res. 1993;72:232–236. doi: 10.1161/01.res.72.1.232. [DOI] [PubMed] [Google Scholar]
- 27.Yu HG, Chang F, Cohen IS. Pacemaker current if in adult cardiac ventricular myocytes. J Physiol. 1995;485:469–483. doi: 10.1113/jphysiol.1995.sp020743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu X, Duan KL, Shang CF, Yu HG, Zhou Z. Calcium influx through hyperpolarization - activated cation channels (Ih channels) contributes to activity-evoked neuronal secretion. Proc Natl Acad Sci U S A. 2004;101:1501–1506. doi: 10.1073/pnas.0305167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C, Zhou Z. Ca(2+)-independent but voltage-dependent secretion in mammalian dorsal root ganglion neurons. Nature Neurosci. 2002;5:425–30. doi: 10.1038/nn845. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Z, Bers DM. Ca2+ influx via the L-type Ca2+ channel during tail current and above current reversal potential in ferret ventricular myocytes. J Physiol. 2000;523:57–66. doi: 10.1111/j.1469-7793.2000.t01-2-00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Z, Misler S. Action potential-induced quantal secretion of catecholamines from rat adrenal chromaffin cells. J Biol Chem. 1995;270:3498–3505. [PubMed] [Google Scholar]
- 32.Zhou Z, Neher E. Calcium permeability of nicotinic acetylcholine receptor channels in bovine adrenal chromaffin cells. Pflugers Arch. 1993;425:511–517. doi: 10.1007/BF00374879. [DOI] [PubMed] [Google Scholar]


