Abstract
Microglia constitute the primary resident immune surveillance cell in the brain and are thought to play a significant role in the pathogenesis of several neurodegenerative disorders such as Alzheimer's disease, multiple sclerosis, Parkinson's disease and HIV-associated dementia. Measuring microglial activation in vivo in patients suffering from these diseases may help chart progression of neuroinflammation as well as assess efficacy of therapies designed to modulate neuroinflammation. Recent studies suggest that activated microglia in the CNS may be detected in vivo using Positron Emission Tomography (PET) utilizing pharmacological ligands of the mitochondrial peripheral benzodiazepine receptor (PBR). Beginning with the molecular characterization of PBR and regulation in activated microglia, we examine the rationale behind using PBR ligands to image microglia with PET. Current evidence suggests these findings might be applied to the development of clinical assessments of microglial activation in neurological disorders.
Keywords: Positron Emission Tomography, microglia, PK11195, Peripheral Benzodiazepine Receptor, neuroinflammation
1.0 MICROGLIA IN HEALTH AND DISEASE
1.1.1 Microglia: Origin and function
Microglia constitute up to 10% of the total cell population of the brain. As resident macrophages (histiocytes), microglia phagocytose cellular debris, present foreign antigens and presumably serve many other vital functions in the brain (Minghetti and Levi, 1998). Del Rio Hortega first recognized the pathological importance of microglia in the central nervous system (CNS), and he also coined their name (Del Rio Hortega, 1932). Microglia are derived from cells of the monocyte lineage. During development embryonic mesodermal cells migrate from the bone marrow into the CNS during the midgestational period (Perry and Gordon, 1991). Based on tissue culture experiments, however, some assign a neuroectodermal origin to microglia (Fedoroff et al., 1997).
Microglia change from a resting to an activated state in response to CNS insults that stimulate them to function as phagocytes (reviewed in (Gehrmann et al., 1995)). This morphological change has been best documented in the facial-nerve transection model, where microglial activation can be assessed in an environment without blood-brain barrier disruption nor migration of new bone marrow-derived macrophages. This model demonstrates the capacity of resident microglia to undergo morphological changes and activation with expression of new surface markers and to proliferate around motor neurons of the facial nucleus (reviewed in (Kreutzberg, 1996)).
In addition to activation of resident brain microglia, monocytes migrate from the vascular compartment into the CNS during CNS-inflammation and there differentiate to form macrophages (reviewed in (Guillemin and Brew, 2004)). Such cells are termed perivascular macrophages. Since no reliable histologic markers exist that differentiate these cells we use the word microglia to include both these cell types for simplicity.
Microglia were previously thought to be quiescent and non-motile in a resting state. However, recent in vivo imaging of fluorescently tagged microglia in transgenic mice using two-photon microscopy showed that these cells were far from static (Davalos et al., 2005; Nimmerjahn et al., 2005). They possess highly ramified processes that exhibit cycles of formation, extension and withdrawal. These cell processes in turn show foot-like appendages that form and withdraw repetitively. These authors hypothesize that microglia serve housekeeping functions whereby they sample and maintain homeostasis of local environments. Following laser-induced injury, time-lapse imaging shows rapid movement of ramified processes into the site of injury. Microglial processes fuse to form an area of containment separating healthy and injured tissues within about 30 seconds, suggesting that microglia may represent a first line of defense in CNS injury (Davalos et al., 2005; Fetler and Amigorena, 2005; Nimmerjahn et al., 2005).
The phagocytic function of microglia is mediated in a receptor-dependent fashion. Microglia express several receptors including Fc (constant fragment of antibodies) and complement, which enable them to engulf antibody-coated cells and opsonized antigen (Chan et al., 2003; Ulvestad et al., 1994; Webster et al., 2001). Microglia also express MHC II, which enables them to present antigen to CD4-T cells (Gehrmann et al., 1995). In addition, microglia express costimulatory substances such as B7-1, B7-2 and CD40 that allow them to stimulate T cells and initiate immune reactions (Gonzalez-Scarano and Baltuch, 1999). The actions of phagocytosis and antigen presentation enable microglia to serve an immune surveillance function in the CNS.
1.1.2 Microglia in neurodegeneration
Microglia undergo changes from a resting phenotype to an activated phenotype in response to a wide variety of CNS insults. Various degrees of microglia activation are seen in neurodegenerative disorders. Neuritic plaques, which constitute the central pathology in Alzheimer's disease (AD), are surrounded by microglia (McGeer et al., 1988b). In multiple sclerosis, areas of demyelination are rich in activated microglia (Bauer et al., 1994). HIV-dementia is characterized by viral infection of microglia (Wiley et al., 1986). Activation of microglia in other neurodegenerative diseases such as Parkinson's disease (McGeer et al., 1988a), Creutzfeldt-Jakob disease (Muhleisen et al., 1995) and Amyotrophic Lateral Sclerosis (Sargsyan et al., 2005) is known but less well characterized.
A wealth of literature suggests that activated microglia, in addition to their phagocytic role, synthesize and secrete potential neurotoxins that may cause neuronal damage or aggravate underlying pathology. These neurotoxins include free radicals (Chao et al., 1995a) ; nitric oxide (Chao et al., 1992) ; proteinases (Colton et al., 1993); eicosanoids (Heyes et al., 1996) and excitotoxins (Giulian et al., 1990; Piani et al., 1992). In addition, activated microglia also secrete substances that influence neuronal function and viability such as the cytokines interleukin-1 (Giulian et al., 1986), interleukin-6 (Righi et al., 1989) tumor necrosis factor α (Chao et al., 1995b); and chemokines such as MIP-1α (Murphy et al., 1995), MIP-1β (McManus et al., 1998) and MCP-1 (D'Aversa et al., 2002). Although some of these studies are based on tissue culture systems and remain to be confirmed in vivo, it is generally accepted that activated microglia can produce a large array of neurotoxins. The evidence supporting these wide array of neurotoxins suggests that they may act synergistically to promote neurodegeration.
Inhibition of microglial activation using non-steroidal anti-inflammatory drugs or minocycline has been hypothesized to reduce neuronal damage in animal models of Parkinson's disease (Du et al., 2001) and AD (Lim et al., 2000). Activation of microglia also inhibits neurogenesis in the hippocampus in rats that are irradiated or injected with lipopolysaccharide (LPS). Hippocampal regeneration was restored in these conditions by blocking microglial activation with either indomethacin (Monje et al., 2003) or minocycline (Ekdahl et al., 2003). These studies suggest that activation of microglia could perpetrate neurodegeneration through several mechanisms.
Recent evidence suggests that activated microglia may also be protective. For example, in therapeutic immunization protocols in AD and Tg mouse models of AD, activation of microglia promotes phagocytosis and clearance of Aβ from the CNS (Masliah et al., 2005; Nicoll et al., 2003; Schenk et al., 1999). The importance of phagocytosis is implied by the correlation of antibody affinity for microglial Fc receptor and the antibody efficacy in clearance of plaques from the brain (Bard et al., 2003). It has also been suggested that microglia can exist in different states of activation depending on the microenvironment, with some states favoring the secretion of substances damaging neurons and other states favoring a protective phagocytic role (Morgan et al., 2005).
While it is well accepted that microglial activation contributes to the pathogenesis of several neurodegenerative diseases, the detection of activated microglia is predominantly restricted to histopathological techniques utilizing postmortem CNS tissues. Non-invasive CNS imaging techniques such as computerized tomography (CT) and magnetic resonance imaging (MRI) technologies enable morphometric documentation of late stage changes in brain volumes, but are generally not considered sensitive or early detectors of brain damage (Jack et al., 2003). It is also possible to measure changes in metabolites in the brain using magnetic resonance spectroscopy (MRS). Alterations in the N-acetyl aspartate to choline ratios are thought to reflect neuronal damage, but are not specific for microglial activation (De Stefano et al., 2005; Jenkins and Kraft, 1999). Recent studies indicate that it may be possible to image activated microglia in CNS disorders using Positron Emission Tomography (PET) with ligands targeted to the peripheral benzodiazepine receptor. In the following section we examine the rationale behind these studies and examine some of the initial findings suggesting the utility of this technique to quantify CNS microglial activation.
2.0 THE PERIPHERAL BENZODIAZEPINE RECEPTOR IN IMAGING MICROGLIA WITH POSITRON EMISSION TOMOGRAPHY
2.1.1 The peripheral benzodiazepine receptor: -structure and function
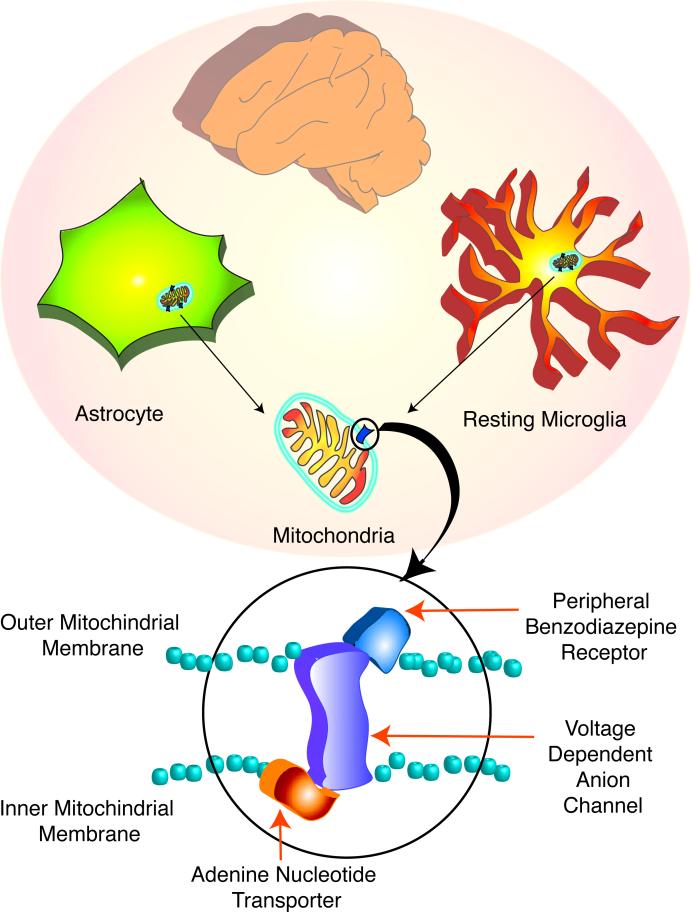
Evidence indicating that diazepam bound with high affinity in the rat kidney led to the postulation and later characterization of the peripheral benzodiazepine receptor (PBR) (Braestrup et al., 1977), named to differentiate it from the previously described diazepam binding sites in the CNS (central benzodiazepine receptor). Within the CNS, two pharmacologically distinct benzodiazepine receptors exist: the central and the peripheral benzodiazepine receptors. The central benzodiazepine receptor is a part of the ionotropic GABA receptor located on the plasma membrane of GABA-ergic neurons (Stephenson, 1995). PBR is in contrast located on the outer membrane of mitochondria, and mainly in glial cells (Casellas et al., 2002) (figure 1). It is part of a hetero-oligomeric complex comprised of the voltage-dependent anion channel and an adenine nucleotide carrier forming the mitochondrial permeability transition pore (McEnery et al., 1992) (figure 1).
Figure 1.
The Peripheral Benzodiazepine Receptor is expressed in astrocytes and microglia in the CNS
In the resting CNS, PBR expression is thought to be restricted to astrocytes and microglia. PBR is present in the mitochondria of these cells in association with the voltage dependent anion channel and the adenine nucleotide transporter, forming the mitochondrial permeability transition pore. Note that PBR is situated on the outer mitochondrial membrane.
Outside the CNS, PBR is ubiquitously expressed and is particularly enriched in steroidogenic cells. Several functions have been attributed to PBR. It is thought to aid in the transport of cholesterol from the outer to the inner mitochondrial membranes and thus be vital in steroid synthesis (Papadopoulos, 2004; Papadopoulos et al., 1997). As a constituent of the mitochondrial permeability transition pore, PBR is believed to regulate cell death (McEnery et al., 1992) and mitochondrial respiration (Hirsch et al., 1989). PBR is also thought to play a role in cell proliferation, differentiation and protein and ion transport (reviewed in (Casellas et al., 2002; Gavish et al., 1999))
Less is known about the functions of this receptor within the CNS more specifically. It is thought to be involved in neurosteroid synthesis (Papadopoulos et al., 2006), regulating mitochondrial function (Casellas et al., 2002) and modulating neuroinflammation in microglial cells (discussed below). Although the role of this receptor in the CNS is not yet entirely clear, several studies have focused on changes in PBR expression in CNS diseases.
2.1.2 PBR in CNS diseases
Pharmacological ligands that bind PBR have been used extensively to study receptor-binding parameters in brain tissues. These studies mainly utilize specific ligands that bind PBR with high affinity, such as the isoquinoline carboxamide derivative PK11195 (PBR antagonist) that binds with nanomolar affinity, and also R05-4864 (PBR agonist), a 4'-chloro derivative of diazepam that binds with micromolar affinity. In the resting CNS, PBR was first located in ependymal cells lining the ventricles, the olfactory bulb and the choroid plexus (Weissman et al., 1984). Subsequently, PBR was detected in glial cells including astrocytes and microglia.
Several studies have subsequently focused on changes in PBR expression in CNS disease. [3H]-PK11195 autoradiography was first used to label glioma cell lines implanted into mice brains (Starosta-Rubinstein et al., 1987). Several binding studies using homogenized brain tissue and autoradiography studies have now shown increased [3H]-PK11195 binding (reflecting increased in PBR protein expression) in a wide variety of neurological diseases (summarized in Table 1) and animal models. This includes conditions such as multiple sclerosis (Banati et al., 2000; Vowinckel et al., 1997), experimental autoimmune encephalitis (Vowinckel et al., 1997), stroke (Stephenson et al., 1995), brain trauma (Raghavendra Rao et al., 2000), facial nerve transaction (Banati et al., 1997; Gehlert et al., 1997), and SIVE (Mankowski et al., 2003) (Venneti et al., 2004). As discussed below, in the majority of these studies, cellular localization of increased PBR expression is specific to activated microglial elements.
Table 1.
Summary of [11C]-PK11195 imaging studies in the CNS using PET.
| Reference | Disease | Finding | Comment |
|---|---|---|---|
| (Charbonneau et al., 1986) | Not applicable | Detected [11C]-PK11195 binding in the myocardium of dogs and humans |
First study with [11C]- PK11195 |
| (Pappata et al., 1991) | Glioblastoma | Two fold higher binding of [11C]-PK11195 in human glioblastomas |
no clinical pathological correlation possible |
| (Petit-Taboue et al., 1991) | Not applicable | Characterization of [11C]-PK11195 kinetics in the brains of human baboons |
|
| (Sette et al., 1993) | Baboon model of focal cerebral ischemia |
Increased [11C]-PK11195 in peri-infarct areas of baboons with focal cerebral ischemia |
Relative contributions to PK11195 binding from astrocytes versus microglia not assessed. |
| (Vowinckel et al., 1997) | Multiple sclerosis |
Increased [11C]-PK11195 in MRI-defined lesions in one MS patient |
Increased [3H]-PK11195 binding corresponded to microglial staining in experimental autoimmune encephalitis (CD11b) and MS tissue (CD68) |
| (Banati et al., 1999) | Rasmussen's encephalitis (RE) |
Increased [11C]- PK11195 in the affected hemispheres of two patients with RE |
Did not see changes in [11C]- PK11195 in three patients with hippocampal sclerosis. RE brain tissue showed increased staining of CR3/CR4. |
| (Gerhard et al., 2000) | Ischemic stroke |
Increased [11C]-PK11195 retention in five patients with ischemic stroke |
no clinical pathological correlation possible |
| (Banati et al., 2000) | Multiple sclerosis (MS) |
Increased [11C]-PK11195 retention in eleven cases with MS |
Increased [3H]-PK11195 binding corresponded to microglial staining in EAE (OX-42) and different MS tissue (EBM11) but no clinical pathological correlation possible |
| (Cagnin et al., 2001b) | Herpes Encephalitis |
Increased [11C]-PK11195 retention in two cases with Herpes Encephalitis |
no clinical pathological correlation possible |
| (Goerres et al., 2001) | Cerebral Vasculitis |
Case report of increased [11C]-PK11195 retention in one patients with cerebral vasculitis |
no clinical pathological correlation possible |
| (Cumming et al., 2001) | Animal model of Parkinson's disease with intrastriatal graft. |
Pigs treated with MPTP when grafted with porcine fetal mesencephalic neurons show increased [11C]- PK11195 retention in region of graft |
Relative contributions to PK11195 binding from astrocytes versus microglia not assessed. |
| (Cagnin et al., 2001a) | AD | Increased [11C]-PK11195 retention in eight patients with AD |
no clinical pathological correlation possible |
| (Cicchetti et al., 2002) | Rat model of Parkinson's disease injected with 6-OHDA |
Increased [11C]-PK11195 retention at the site of lesion |
Increased staining for microglia using CR3 observed, but not correlated with increased PK11195 binding |
| (Debruyne et al., 2003) | MS | [11C]-PK11195 retention was higher in MS patients (total assessed=22) depending on the sate of disease progression |
no clinical pathological correlation possible |
| (Turner et al., 2004) | Amyotrophic lateral sclerosis (ALS) |
Increased [11C]-PK11195 retention in ten patients with ALS |
no clinical pathological correlation possible |
| (Venneti et al., 2004) | Macaque model of HIV- encephalitis |
Increased [11C]-PK11195 retention in six macaques with SIV-encephalitis |
Increased PK11195 binding corresponded to brain macrophage staining (CD68) but not astrocytes (GFAP) |
| (Henkel et al., 2004) | Corticobasal degeneration |
Increased [11C]-PK11195 retention in one patients with corticobasal degeneration |
no clinical pathological correlation possible |
| (Gerhard et al., 2004) | Corticobasal degeneration |
Increased [11C]-PK11195 retention in four patients with corticobasal degeneration |
no clinical pathological correlation possible |
| (Cagnin et al., 2004) | Frontotemporal dementia |
Increased [11C]-PK11195 retention in five patients with frontotemporal dementia |
no clinical pathological correlation possible |
| (Gerhard et al., 2005) | Ischemic stroke | Serial PET scans showed [11C]-PK11195 around lesion as early as 3 days. Five patients assessed |
no clinical pathological correlation possible |
| (Ouchi et al., 2005) | Parkinson's disease |
Increased [11C]-PK11195 retention in ten early- stage drug-naïve patients with Parkinson's disease |
no clinical pathological correlation possible |
| (Versijpt et al., 2005) | MS | 22 MS patients assessed with [11C]-PK11195. Increased uptake correlated with brain atrophy |
no clinical pathological correlation possible |
| (Turner et al., 2005) | Upper Motor Neuron Syndromes |
Varying [11C]-PK11195. Retention in three cases with Upper Motor Neuron syndromes |
no clinical pathological correlation possible |
| (Hammoud et al., 2005) | HIV infection | Increased [11C]-PK11195 retention in 10 patients with HIV |
No differences between demented and non-demented patients |
| (Gerhard et al., 2006) | Parkinson's disease |
Increased [11C]-PK11195 retention in 18 patients with Parkinson's disease |
no clinical pathological correlation possible |
| (Cagnin et al., 2006) | Hepatic encephalopathy |
Increased [11C]-PK11195 retention in five HE |
no clinical pathological correlation possible |
| (Cumming et al., 2006) | Pig model of Parkinson's disease |
[11C]-PK11195 was not different in pigs treated with MPTP and controls |
Relative contributions to PK11195 binding from astrocytes versus microglia not assessed. |
| (Chen and Guilarte, 2006) | Demyelination model in mice |
Mice treated with cuprizone show increased [11C]-PK11195 retention |
Increased PK11195 binding corresponded better to microglia staining (Mac-1) in earlier stages of remyelination but corresponded better with astrocytes (GFAP) during latter stages of remyelination. |
| (Price et al., 2006) | Ischemic Stroke | Four patients showed progressive [11C]- PK11195 retention when imaged at various time points upto 30 days after ischemic stroke |
no clinical pathological correlation possible |
| (Pavese et al., 2006) | Huntington's disease |
Increased [11C]-PK11195 retention in 11 patients with Huntington's disease correlated with decreased striatal [11C]- Raclopride (D2 receptor ligand) uptake compared to 10 healthy controls |
no clinical pathological correlation possible |
| (Wiley et al., 2006) | HIV infection | No increased [11C]- PK11195 retention in 12 patients with minor cognitive impairment and HIV infection compared to 5 non-HIV infected controls |
no clinical pathological correlation possible |
2.1.3 PBR in CNS diseases: Astrocytes versus Microglia
The relative contributions of astrocytes versus microglia to PBR-ligand binding in neuroinflammation is a subject of continuing debate. Astrocyte PBR expression is mostly supported by studies that have focused on [3H]-PK11195 binding in cell culture model systems. Others report increased [3H]-PK11195 binding corresponding to microglia in brain tissues based on data from a rat model of stroke (Myers et al., 1991) and ischemia (Stephenson et al., 1995), experimental autoimmune encephalitis (Vowinckel et al., 1997), multiple sclerosis (Banati et al., 2000; Vowinckel et al., 1997), facial nerve axotomy in rats (Banati et al., 1997), brain trauma in rats (Raghavendra Rao et al., 2000), SIV encephalitis in macaques (Mankowski et al., 2003; Venneti et al., 2004) and hippocampal axonal lesions in rodents (Pedersen et al., 2006). Some of these studies also report a stronger correlation of [3H]-PK11195 binding with microglia versus astrocytes in brain tissues (Mankowski et al., 2003; Raghavendra Rao et al., 2000; Venneti et al., 2004). However, some studies report increased astrocyte expression of PBR following an initial increase in microglia in rodents treated with the neurotoxins trimethyltin (Kuhlmann and Guilarte, 2000) and cuprizone (Chen et al., 2004). In vitro studies also suggest that neurons may express PBR (Jayakumar et al., 2002), but these studies have not been confirmed in brain tissues.
Translation of these in vitro studies to in vivo settings is difficult. For example, in our hands filtration-binding studies using [3H]-PK11195 (reflective of the total number of PBR binding sites) show a Bmax value in the range 843 ± 216 fmols/mg tissue in normal human frontal cortex. Bmax values of primary human embryonic astrocytes in culture are in the range 2487 ± 319 fmols/mg tissue, while in primary human embryonic microglia the range is 5276 ± 2710 fmols/mg tissue (unpublished results), similar to values obtained by others in other tissue culture models (Itzhak et al., 1995; Park et al., 1996). While Bmax values seem to be higher in microglia, how much of the Bmax in normal human frontal cortex is derived from microglia versus astrocytes? Further, how does one measure astrocytes and microglia in brain tissue to determine their relative contributions toPK11195 binding? Several investigators have tried to answer these questions by combining [3H]-PK11195 autoradiography with immunohistochemistry for both astrocytes and microglia. This approach is limited by the antibodies used to detect specific cell types, as well as by sampling biases restricted to the brain sections used in these analyses. Moreover, PET imaging in vivo adds further complexities that make it difficult to extrapolate these studies. Despite these limitations, a majority of the evidence currently suggests that PK11195 binding is increased in microglia in neurological diseases with lower or less significant contributions from astrocytes.
2.1.4 Potential mechanisms for PBR upregulation in microglia
The mechanisms responsible for increased PBR expression in microglia in the CNS are not known. In pancreatic islet cells, the cytokines TNF-α, IFN-γ and IL-1β cause an increase in PBR mRNA and [3H]-PK11195 binding in a transcription dependent fashion (Rey et al., 2000). Similar results are seen in testicular Leydig cell exposed to TNF-α (Trincavelli et al., 2002). In rats injected intracerebrally with IL-1, TNF-, or LPS, increased [3H]-PK11195 binding was attributed to microglia (Biegon et al., 2002; Bourdiol et al., 1991). IL-1 and TNF-α also increase [3H]-PK11195 binding in cultured astrocytes (Oh et al., 1992). In experimental autoimmune encephalitis, IL-6 and TNF-α expression profiles correlate with increase in spinal cord [3H]-PK11195 binding (Agnello et al., 2000). These data suggest that cytokines increase PBR expression in various cell types including microglia. However, the mechanisms mediating the functional consequences of PBR-ligand binding increases are not known. It has been proposed that cellular upregulation of PBR in these systems may serve as a protective strategy against cytokine toxicity. Since activated microglia themselves are sources of cytokines, increase in PBR expression would be an autocrine-paracrine phenomenon.
Microglia change from a ramified morphology to an amoeboid morphology on activation (Kreutzberg, 1996). These changes may also be accompanied by morphological alterations in the mitochondria (Banati et al., 2004). However, the subcellular changes in PBR in microglia on activation are not known. Increases in cell size due to evolution to an ameboid morphology upon activation may result in an increase in the mitochondrial population in each microglial cell without change in the number of PBR per mitochondrion. Hence it is possible that increased PBR in activated microglia actually reflect an increase in mitochondrial number. On the other hand, the number of mitochondria may not change significantly, but the number of PBR per mitochondrion may increase. However, the most likely possibility is that the number of mitochondria as well as the number of PBR per mitochondria increases with activation in each microglial cell. While these possibilities need to be confirmed experimentally, it is important to note that activation of microglia may result in mitochondrial changes that may influence the functioning of these cells.
2.1.5 Potential functions for PBR in microglia
The functional consequences of increased PBR expression in microglia are unknown. PBR is thought to play a role in modulating the activation of microglia. For example, treating primary human embryonic microglia with PK11195 decreases expression of COX2 and TNF-α and decreases intracellular calcium levels (Choi et al., 2002). Further, PK11195 decreases microglial activation, iNOS, IL-1β, IL-6, TNF-α levels and the extent of neuronal damage in quinolinic acid-injected rats (Ryu et al., 2005). Diazepam is also speculated to inhibit HIV-tat induced microglial chemotaxis by acting on PBR (Lokensgard et al., 2001). Since PBR may play a role in regulating cell division, PBR may also play a role in the regulation of microglial proliferation upon neuronal injury.
The association of PBR with the mitochondrial permeability transition pore suggests a role in the regulation of cell survival in microglia. At the mitochondrial permeability transition pore, PBR interacts with several resident mitochondrial proteins including the voltage dependent anion channel and the adenine nucleotide carrier (figure 1) that in turn interact with proteins regulating apoptosis (McEnery et al., 1992). Interestingly, forced macrophage-PBR overexpression in myxoma poxvirus-infected macrophages blocks apoptosis (Everett et al., 2002; Everett and McFadden, 2001). Forced PBR expression in neurons in vivo and Jurkat cells in vitro also protects these cells from apoptosis (Johnston et al., 2001; Stoebner et al., 2001). PBR upregulation in testicular Leydig cells protects them from cytokine-induced toxicity (Rey et al., 2000; Trincavelli et al., 2002). This is also seen in blood phagocytic cells where PBR protects against oxidant-induced cell death (Carayon et al., 1996). PBR expression in microglia may thus protect them from various toxins, thereby contributing to longer microglia life spans in the brain and perpetuating neuroinflammation (figure 2).
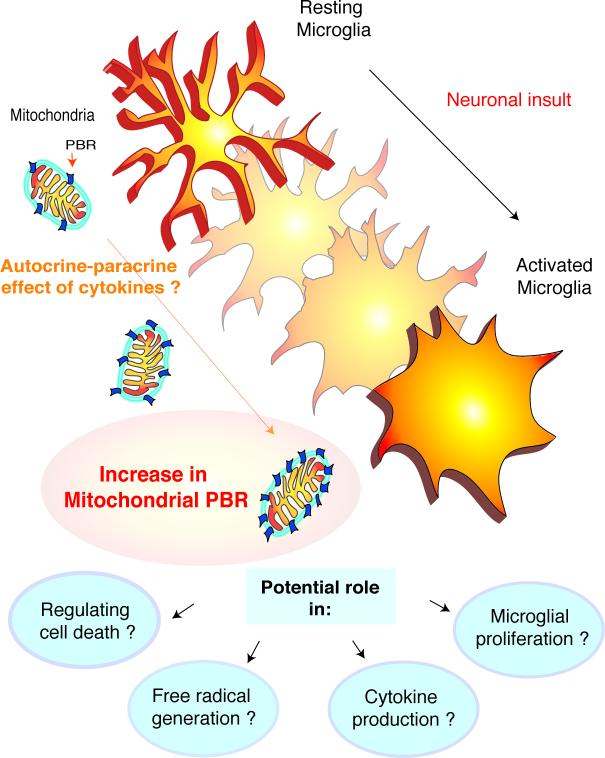
Figure 2.
Potential functions and mechanisms of Peripheral Benzodiazepine Receptor increases in activated microglia
This figure illustrates potential mechanisms of PBR increases in microglia. The process of activation, in response to neuronal insults, involves cytokine production by microglia. Since, pro-inflammatory cytokines may increase PBR (see text), it is possible that these cytokines act in an autocrine-paracrine fashion to increase PBR expression in microglia that are undergoing activation.
The functions of PBR in microglia are unknown. It is possible that PBR plays a yet undefined role in several aspects of microglial function such as the regulation of cell death, proliferation, cytokine and free radical generation.
2.1.6 PBR ligands may be used to image activated microglia in vivo
Several ligands have been synthesized that bind specifically to PBR. Labeling these ligands with 3H and 11C has enabled their use in autoradiography and PET respectively. For these ligands and PET studies in general, the concentrations used in vivo are at tracer levels and are thought not to alter receptor function or produce pharmacological effects. Of these ligands PK11195, a lipid soluble isoquinoline carboxamide, the R-enantiomer of which has a high affinity for PBR (Shah et al., 1994), has been the most extensively characterized (Banati, 2002). [11C]-PK11195 has been used in animal models and human subjects with various CNS diseases (summarized in Table 1). These studies predominantly support the hypothesis that [11C]-PK11195 can label microglia using PET; however, the sensitivity and specificity of this PET ligand remain to be fully evaluated.
To fully appreciate the state of the art, the limitations of the published studies should be considered. For example, the determination of the cell type responsible for increased PK11195 binding may not be possible due to inadequacies of human postmortem tissues. This is an important limitation as [11C]-PK11195 binding seen in regions traditionally not associated with disease pathology cannot be presumed to arise from activated microglia. For instance, a study in AD patients showed high [11C]-PK11195 binding in the entorhinal, temporoparietal and cingulate cortices, brain regions that show a high degree of AD pathology (Cagnin et al., 2001a). However, this study also reported high levels of [11C]-PK11195 binding in regions not involved in AD, such as the thalamus and the brainstem (Cagnin et al., 2001a). [11C]-PK11195 thalamic binding is also reported in ischemic stroke (Pappata et al., 2000), normal subjects with increasing age (Cagnin et al., 2001a), Alzhemimer's disease (Cagnin et al., 2001a), Parkinson's disease (Ouchi et al., 2005) and Huntington's disease (Pavese et al., 2006).Similarly, a recent study using [11C]-PK11195 to image activated microglia in amyotrophic lateral sclerosis (ALS) patients showed high binding in the occipital cortex and thalamus, areas that are not traditionally implicated in ALS pathology (Turner et al., 2004). The authors interpret these findings as a result of microglial activation and “synaptic stripping” in regions connected to areas of primary pathology. Since it is not possible to confirm the histological presence of activated microglia in these regions in human studies, it is possible that these increases reflect regional variations in the constitutive PBR population that are independent of the disease pathology or a non-uniform element of non-specific [11C](R)-PK11195 binding. In such cases it is vital that PET findings be compared to histopathology to confirm the presence of microglia. It is obviously not possible to conduct such comparisons in human in vivo PET studies. This emphasizes the need to compare human histopathologic data with ligand binding studies to determine if given neuropathologies correlate with ligand binding.
2.1.7 Modeling [11C]-PK11195 binding in the CNS: Interpretations and limitations
A distinct advantage of PET over other medical imaging modalities is the ability to extract quantitative information regarding physiologic parameters that are relevant to a disease process. After corrections for physical and engineering factors such as radioactive decay, photon attenuation and scattering, variations in PET detector efficiency, and counting losses resulting from electronics dead-time, PET images represent the spatial and temporal distribution of radioactivity in terms of absolute units of radioactivity concentration,. Mathematical models are applied to dynamic PET image data in order to derive estimates of physiologic parameters directly from observations of the kinetic behavior of the radiotracer, which may be influenced by a plurality of factors such as perfusion, tissue clearance, peripheral metabolism, and receptor binding phenomena. These mathematical models provide estimates of one or more outcome measures that indicate or are related to the functional state of the tissue or physiological process under study (i.e., density of PBR binding sites).
Compartmental models are the most general models applied to PET image data and are based upon a principal assumption that each unit of the injected radiotracer can at any given time be assigned to one of several compartments representing possible physiologic states or locations of the tracer (e.g.; metabolized tracer, specifically bound tracer). Compartmental models describe the exchange of tracer between compartments in terms of rate constants (parameters) that represent the fractional rate of influx or efflux from one compartment to another per unit of time (i.e., min−1). Generally, a compartmental model consists of a single compartment representing the arterial plasma concentration of authentic free tracer and one or more compartments representing tissue tracer pools. The arterial plasma compartment is typically measured directly by rapid sampling of arterial blood via a catheter placed in the radial artery and subsequent radioisotope counting of plasma samples to determine the time-varying plasma radioactivity concentration. Peripheral metabolism may result in the cleavage of the radiotracer molecule to produce one or more species of radiolabeled metabolites, which radioisotope counting is unable to distinguish from parent radiotracer. Additional blood samples must be obtained throughout the course of the study to determine the time-varying fraction of unmetabolized radiotracer using analytical techniques such as high-performance liquid chromatography (HPLC). After correction for metabolism, the discrete arterial blood samples define the time-varying concentration of a delivery and removal compartment that serves as the “input function” that drives the tissue response. While compartmental models can be configured with an arbitrary number of compartments and parameters, statistical noise and the coarse temporal sampling of PET data does not typically support the application of model configurations with more than four compartments or six estimated parameters.
In general, the selection of a specific model configuration and outcome measure is largely predicated on some a priori knowledge of the in vivo tracer kinetics. Often, this a priori knowledge is gained from preclinical studies that seek to elucidate some important characteristics of a prospective radioligand. These preclinical studies may include in vitro or ex vivo binding experiments to investigate the specificity, selectivity and reversibility of radioligand binding, studies of the metabolic fate of the tracer in plasma, toxicological investigations, and whole body bio-distribution studies for dosimetry estimates. Often, imaging studies are conducted in non-human primates to characterize the in vivo kinetic behavior of the ligand in a species that is phylogenetically proximal to human, which are particularly useful in the process of model formulation and the selection of an appropriate outcome measure. For protein-ligand binding studies, a commonly used outcome measure is the distribution volume (DV, mL/mL), which is defined as the ratio of the concentration of radiotracer in tissue to that in the blood at equilibrium. It is often desirable to correct DV estimates for non-specific binding of radiotracer by using the DV estimate of a reference region believed to be devoid of specific binding as a normalizing factor. This ratio of DV estimates is termed the distribution volume ratio (DVR) and is closely related to yet another outcome measure, the binding potential (BP), which is proportional to the ratio of the free receptor pool Bmax and the ligand dissociation constant KD (Mintun et al., 1984). Radiotracer specific factors such as the tissue and plasma free fractions are challenging to accurately assess and limit the interpretation of BP to one of proportionality of Bmax/KD. Another limitation of the method is the inability to individually estimate Bmax and KD, although it is generally assumed that KD remains constant and that observed changes in BP in vivo largely reflect changes in Bmax. Nevertheless, the DVR and BP outcome measures are often chosen as indices of neuroreceptor populations, but it is important to note that these indices are only defined under steady-state conditions. Methods of analysis estimating such equilibrium parameters cannot be employed judiciously in the analysis of radiotracer kinetics involving radiotracers which do not achieve steady-state between tissue and plasma compartments (Koeppe, 2002)
PK11195 has been well characterized pharmacologically (Benavides et al., 1988; Benavides et al., 1983a; Benavides et al., 1983b; Le Fur et al., 1983a; Le Fur et al., 1983b) and has been shown to readily enter brain tissue and distribute uniformly in the normal baboon brain (Cremer et al., 1992). However, difficulties in the interpretation of [11C]-PK11195 PET studies were immediately obvious. In order to demonstrate the specific binding of a radioligand in vivo, it is a common practice to use a pharmacologically significant dose of the unlabeled ligand or another competing ligand to block or displace specific binding of the radioligand. The expectation is that an excess of the unlabeled competitor will block specific binding of the radioligand if administered as a pretreatment, or result in an accelerated clearance of radiotracer if administered after the radiotracer injection as a displacing agent. In the case of [11C]-PK11195, a consistent increase in brain radioactivity concentrations was observed following the administration of 1 mg/kg of unlabeled PK11195 when it was administered as either a blocking or a displacing agent (Petit-Taboue et al., 1991). This observation was attributed to the blockade of far more abundant PBR sites in peripheral tissues by unlabeled PK11195 resulting in increased plasma concentrations of [11C]-PK11195 causing increased availability of the radioligand to the brain. As a result, it was not possible to demonstrate specific binding of [11C]-PK11195 to brain PBR receptors in vivo using conventional techniques. Nevertheless, the inability to demonstrate specific binding in vivo did not prevent [11C]-PK11195 from being applied to study neuroinflammation in a variety of diseases with inflammatory CNS pathology. The utility of [11C]-PK11195 as a marker for activated microglia was therefore evaluated based on its ability to localize in the brain in a manner consistent with the known pathology of a disease rather than on any direct empirical evidence of specific binding.
The development and validation of a compartmental model for [11C]-PK11195 in the brain was hindered until recently by the reported instability of PK11195 in plasma, which confounded accurate determination of the arterial input function and radiolabeled metabolites (Cleij MC, 2003). It was later shown that these difficulties arose as a result of base-promoted dechlorination of the precursor of PK11195 during the radiosynthesis, which resulted in a radiolabeled impurity with similar chromatographic properties as authentic [11C]-PK11195. Adjustments to the radiosynthetic procedure were later shown to eliminate the dechlorination side-chain reaction that resulted in the production of this radiolabeled impurity (Cleij MC, 2003).
In the absence of a validated compartmental model to describe the kinetic behavior of [11C]-PK11195 in the brain, simplified methods of analysis have been employed, including the simplified reference tissue model (SRTM) described by Lammertsma et al., (Lammertsma and Hume, 1996) to make estimates of the specific binding of [11C]-PK11195 in vivo. The SRTM analysis is a model based-method that assumes a specific underlying physiologic model and involves simplifications of the model equations that, under certain circumstances, allow DVR or BP to be directly estimated from the image data using a reference tissue (e.g., a brain region devoid of PBR) as an implicit representation of the input function in place of the arterial data. A criticism of this approach is that the application of SRTM assumed an underlying model configuration to describe the kinetics of [11C]-PK11195 that was never validated by the application of a plasma-input model. A second complication is the assumption that a discrete reference region can be consistently defined on the image that is devoid of specific binding of [11C]-PK11195. In the case of a disease with widespread or unpredictable patterns of pathological involvement, this may not be feasible. To circumvent this problem, a cluster analysis technique has been employed to extract a reference ligand kinetic from the segmentation of the dynamic PET image into clusters of voxels with indistinguishable kinetic behavior, which may or may not define a circumscribable anatomic brain region (Cagnin et al., 2001a). The reference ligand kinetic is then selected from the available clusters as the one with the most rapid clearance and by its similarity to a ligand kinetic derived from other studies in normal brain. Subsequently, this reference ligand kinetic is used to drive the SRTM analysis to generate a parametric image whereby the value of each image element (voxel) is a representation of BP. There are many limitations and criticisms of this approach. One obvious problem is that the output from the cluster analysis is being used as an input to an analysis method (SRTM) that until very recently was not validated for [11C]-PK11195 (Kropholler et al., 2006). Another problem is that in most cases the majority of identified clusters arise from extracerebral structures, with the majority of brain tissue voxels arising from one of a few clusters that primarily differentiate gray and white matter. A third problem that has not been satisfactorily addressed is the impact of voxel-level noise on the determination of BP and the robustness of the BP measure determined from parametric image analysis. As it is often a challenge to distinguish differences in kinetics between brain voxels representing affected and reference tissues, the data inherently suffers from poor signal-to-noise characteristics. Perhaps the most serious criticism of this method is that, in the case of the PBR, the assumption that the reference ligand kinetic is devoid of specific binding and therefore can be described by a single tissue compartment representing non-specific binding is violated in every case. A constitutive population of PBR receptors exists throughout the brain (Kurumaji et al., 1997; Miyazawa et al., 1998; Rao and Butterworth, 1997), which may not be an insignificant fraction of the concentration of PBR receptors found in the disease state. Indeed, Banati et al., (Banati et al., 2000) estimated the ratio of PBR receptors in a multiple sclerosis lesion to constitutive levels to be approximately 2:1. It is thus possible that the reference region defined by either discrete anatomical definition (e.g., a region-of-interest) or by cluster analysis inherently contains a significant fraction of specific binding of [11C]-PK11195 to constitutive PBR receptors, and may not be consistent with a principal assumption of the SRTM.
Recently, an optimal compartmental model of the brain kinetics of [11C]-PK11195 has been identified and validated using a metabolite corrected arterial input function (Kropholler et al., 2005). The authors evaluated several compartmental model configurations which allowed for reversible and irreversible binding of [11C]-PK11195, and based upon statistical criterion concluded that a compartmental model which allowed for exchange of tracer between two tissue compartments best described the brain kinetics of [11C]-PK11195 in vivo. The two tissue compartments in this optimal model configuration represent the time-varying concentrations of free [11C]-PK11195 in brain tissue and specifically bound [11C]-PK11195 to PBR, and the fully free model results in the estimation of five parameters:
K1: representing tracer influx to brain from blood, or the product of the extraction of the ligand and blood flow (ml/min/ml of tissue);
k2: representing tracer removal from tissue to blood (min−1);
k3: the pseudo-first order specific binding association rate constant, or the product of the bimolecular association constant kon, the tissue free fraction of tracer f2, and the free receptor concentration Bmax;
k4: the first order bimolecular dissociation rate constant, koff; and Vvasc: a constant representing the vascular volume of tissue.
The authors investigated variations of the reversible two tissue compartment model that involved imposing constraints of specific model parameters, including the constraint of the ratio K1/k2, k4, or Vvasc to whole cortex values, in order to determine whether or not any of these constraints improved the bias and variability of the outcome measures DV and BP. The authors concluded that constraining the K1/k2 ratio to a whole cortex value resulted in improved estimates of regional DV and BP values. Interestingly, whole cortex BP values determined using this plasma-input compartmental model were significantly higher than those determined using SRTM and cluster analysis. The authors suggest that this discrepancy can be explained by the plasma-input model's failure to account for exchange of tracer between non-specifically bound and bound states, resulting in an overestimation of BP. The model that the authors identify as optimal for the analysis of [11C]-PK11195 assumes that [11C]-PK11195 exchanges infinitely fast between free and non-specifically bound states, which may not accurately reflect the behavior of the tracer in vivo. Indeed, it seems that non-specific binding of [11C]-PK11195 is significant as indicated by preclinical studies in rat brain (Shah et al., 1994) and the lack of demonstrable blockade or displacement of [11C]-PK11195 brain radioactivity. A second possible explanation is the inclusion of a component of specific binding in the reference kinetic in the SRTM. This would result in an underestimation of the reference tissue model BP. This explanation may be supported by estimates of PBR Bmax in normal human brain, which range from 346±51 fmol/mgP in the cerebellum to 923±69 fmol mgP in the occipital cortex (Rao and Butterworth, 1997). Other studies have confirmed these observations (Kurumaji et al., 1997; Sauvageau et al., 2002). It is likely the case that both factors influence the estimation of BP, but more preclinical experiments aimed at characterizing the specific and non-specific binding of [11C]-PK11195 in the brain are needed to further refine the model of [11C]-PK11195 brain kinetics and to optimize [11C]-PK11195 brain imaging studies.
2.1.8 Newer PBR ligand in PET imaging
For much of the last 23 years since the initial investigations of [11C]-PK11195 were conducted, there has been a paucity of ligands with high affinity and demonstrated selectivity for PBR. In the last five years, however, there has been a relative explosion of new compounds that are potent and selective ligands for PBR and may be suitable for PET imaging. One such compound is DAA1106 [N-(2,5-dimethoxybenzyl)-N-(4-uoro-2-phenoxyphenyl) acetamide], an aryloxyanilide derivative, is a recently synthesized ligand that binds selectively and with high affinity to PBR (Chaki et al., 1999). DAA1106 shows a higher affinity to PBR compared to PK11195 suggested by the differences in the dissociation constants. The Kd (dissociation constant) of PK11195 ranges between 4nM to 20nM, while the Kd of DAA1106 is around 0.1nM (dissociation constant being inversely proportional to binding affinity) (Chaki et al., 1999; Okuyama et al., 1999). Due to its high affinity, DAA1106 may serve as a better ligand to label PBR both ex vivo and in vivo (Maeda et al., 2004; Zhang et al., 2003). This may also address some of the issues related to nonspecific binding seen in studies with PK11195.
In addition to some fluorinated derivatives of DAA1106, specifically the fluoroethyl derivative [18F]-FEDAA1106, which has been recently characterized in normal human brain (Fujimura et al., 2006), other promising compounds that are still in preclinical stages of development include deuterium substituted analogs of [18F]-FEDAA1106 that are less susceptible to in vivo defluorination (Zhang et al., 2005; Zhang et al., 2004), high-affinity quinoline-carboxamides ([11C]-VC195) and halogenated 2-quinolinecarboxamides that are structurally similar to PK11195 (Belloli et al., 2004; Cappelli et al., 2006), [11C]-vinpocetine (Gulyas et al., 2005) and the pyrazolopyrimidine [11C]-DPA-713 (James et al., 2005). While the utility of all of these novel ligands for in vivo PET imaging of neuroinflammation remains to be demonstrated, they represent several new classes of ligands that are potent and selective for PBR.
3.0 SUMMARY
The low levels of mitochondrial PBR present in the normal CNS increase dramatically with injury and neurodegeneration, and predominantly in microglia, as suggested by several studies using animal models and human postmortem tissues. The mechanisms of PBR regulation and its functions in microglia are not known. In this review, we discuss the evidence supporting preferential increases in PBR in microglia. We also discuss hypothesized mechanisms of PBR regulation at the cellular level as well as possible functions of PBR in microglia. Finally, we review the motivation for, results of, and current methodology behind using PBR ligands targeted to microglial cells for the purpose of assessing neuroinflammation in vivo using PET in neurological disorders.
Several neurodegenerative disorders are accompanied by increases in activated microglia that are thought to contribute to disease progression. Modulating neuroinflammation in many of these diseases is an attractive therapeutic target. To design therapies for neurodegenerative disorders, it is critical to be able to monitor their success in arresting the progression of neurological disease. But monitoring therapeutic efficacy is complicated by the fact that one can only assess absence of disease progression and not recovery of function. Any attempt to develop therapy targeted at neuroinflammation for diseases such as AIDS dementia, Alzheimer's disease or multiple sclerosis will require some means of monitoring the inflammatory pathogenic process, activated microglia. Imaging microglia may provide an index of disease progression and in turn help assess the therapeutic efficacy of antiinflammatory and other potentially neuroprotective drugs. PET imaging of microglia with PBR ligands may be able to assist the early detection of neuroinflammation, monitor the severity and progression of the disease, and help evaluate the effectiveness of CNS therapies aimed at decreasing neuroinflammation.
ABBREVIATIONS
- [11C]
Crabon-11 isotope
- [3H]
Tritium isotope
- AD
Alzheimer's Disease
- AIDS
Acquired Immunodeficiency Syndrome
- ANT
Adenine Nucleotide Transporter
- Aβ
Amyloid Beta
- Bmax
Maximal bound Receptors
- BP
Binding Potential
- CD 4+ T cell
Cluster of Differentiation type 4 T lymphocyte
- CD68
Lysosomal marker for activated macrophages
- CNS
Central Nervous System
- COX2
Cyclooxygenase
- CSF
Cerebrospinal Fluid
- DAA1106
(N-(2,5-Dimethoxybenzyl)-N-(5-fluoro-2-phenoxyphenyl)acetamide)
- DV
Distribution Volume
- DVR
Distribution Volume Ration
- Fc
Constant Fragment of Antibody
- GFAP
Glial Fibrillary Acidic Protein (marker for astrocytes)
- HIV
Human Immunodeficiency Virus
- HIVE
Human Immunodeficiency Virus Encephalitis
- HPLC
High Performance Liquid Chromatography
- IL
Interleukin
- INF-γ
Interferon-gamma
- iNOS
Inducible Nitric Oxide Synthase
- KD
Dissociation Constant
- LPS
Lipopolysaccaride
- MCP-1
Monocyte Chemoattractant Protein-1
- MDM
Monocyte Derived Macrophages
- min
Minute
- MIP-1a
Macrophage Inflammatory Protein-1alpha
- MIP-1b
Macrophage Inflammatory Protein-1beta
- MRI
Magnetic Resonance Imaging
- MRS
Magnetic Resonance Spectroscopy
- NSAID
Non-Steroidal Anti-inflammatory Drugs
- PBR
Peripheral Benzodiazepine Receptor
- PET
Positron Emission Tomography
- PK11195
[1-(2-chlorophenyl)-N-methyl-N-(1methylpropyl)-3-isoquinolinecarboxamide]
- SIV
Simian Immunodeficiency Virus
- SIVE
Simian Immunodeficiency Virus Encephalitis
- SRTM
Simplified Reference Tissue Model
- T cell
T (thymic)-lymphocyte Cell
- Tg
Transgenic mice
- TNF-α
Tumor Necrosis factor alpha
- VDAC
Voltage Dependent Anion Channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agnello D, Carvelli L, Muzio V, Villa P, Bottazzi B, Polentarutti N, Mennini T, Mantovani A, Ghezzi P. Increased peripheral benzodiazepine binding sites and pentraxin 3 expression in the spinal cord during EAE: relation to inflammatory cytokines and modulation by dexamethasone and rolipram. J Neuroimmunol. 2000;109:105–11. doi: 10.1016/s0165-5728(00)00279-4. [DOI] [PubMed] [Google Scholar]
- Banati RB. Visualising microglial activation in vivo. Glia. 2002;40:206–17. doi: 10.1002/glia.10144. [DOI] [PubMed] [Google Scholar]
- Banati RB, Egensperger R, Maassen A, Hager G, Kreutzberg GW, Graeber MB. Mitochondria in activated microglia in vitro. J Neurocytol. 2004;33:535–41. doi: 10.1007/s11068-004-0515-7. [DOI] [PubMed] [Google Scholar]
- Banati RB, Goerres GW, Myers R, Gunn RN, Turkheimer FE, Kreutzberg GW, Brooks DJ, Jones T, Duncan JS. [11C](R)-PK11195 positron emission tomography imaging of activated microglia in vivo in Rasmussen's encephalitis. Neurology. 1999;53:2199–203. doi: 10.1212/wnl.53.9.2199. [DOI] [PubMed] [Google Scholar]
- Banati RB, Myers R, Kreutzberg GW. PK (‘peripheral benzodiazepine’)--binding sites in the CNS indicate early and discrete brain lesions: microautoradiographic detection of [3H]PK11195 binding to activated microglia. J Neurocytol. 1997;26:77–82. doi: 10.1023/a:1018567510105. [DOI] [PubMed] [Google Scholar]
- Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, Price G, Wegner F, Giovannoni G, Miller DH, Perkin GD, Smith T, Hewson AK, Bydder G, Kreutzberg GW, Jones T, Cuzner ML, Myers R. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain. 2000;123(Pt 11):2321–37. doi: 10.1093/brain/123.11.2321. [DOI] [PubMed] [Google Scholar]
- Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, Guido T, Hoenow K, Hu K, Johnson-Wood K, Khan K, Kholodenko D, Lee C, Lee M, Motter R, Nguyen M, Reed A, Schenk D, Tang P, Vasquez N, Seubert P, Yednock T. Epitope and isotype specificities of antibodies to beta -amyloid peptide for protection against Alzheimer's disease-like neuropathology. Proc Natl Acad Sci U S A. 2003;100:2023–8. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Sminia T, Wouterlood FG, Dijkstra CD. Phagocytic activity of macrophages and microglial cells during the course of acute and chronic relapsing experimental autoimmune encephalomyelitis. J Neurosci Res. 1994;38:365–75. doi: 10.1002/jnr.490380402. [DOI] [PubMed] [Google Scholar]
- Belloli S, Moresco RM, Matarrese M, Biella G, Sanvito F, Simonelli P, Turolla E, Olivieri S, Cappelli A, Vomero S, GalliKienle M, Fazio F. Evaluation of three quinolinecarboxamide derivatives as potential radioligands for the in vivo pet imaging of neurodegeneration. Neurochem Int. 2004;44:433–40. doi: 10.1016/j.neuint.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Benavides J, Cornu P, Dennis T, Dubois A, Hauw JJ, MacKenzie ET, Sazdovitch V, Scatton B. Imaging of human brain lesions with an omega 3 site radioligand. Ann Neurol. 1988;24:708–12. doi: 10.1002/ana.410240603. [DOI] [PubMed] [Google Scholar]
- Benavides J, Malgouris C, Imbault F, Begassat F, Uzan A, Renault C, Dubroeucq MC, Gueremy C, Le Fur G. “Peripheral type” benzodiazepine binding sites in rat adrenals: binding studies with [3H]PK 11195 and autoradiographic localization. Arch Int PharmacodynTher. 1983a;266:38–49. [PubMed] [Google Scholar]
- Benavides J, Quarteronet D, Imbault F, Malgouris C, Uzan A, Renault C, Dubroeucq MC, Gueremy C, Le Fur G. Labelling of “peripheral-type” benzodiazepine binding sites in the rat brain by using [3H]PK 11195, an isoquinoline carboxamide derivative: kinetic studies and autoradiographic localization. J Neurochem. 1983b;41:1744–50. doi: 10.1111/j.1471-4159.1983.tb00888.x. [DOI] [PubMed] [Google Scholar]
- Biegon A, Alvarado M, Budinger TF, Grossman R, Hensley K, West MS, Kotake Y, Ono M, Floyd RA. Region-selective effects of neuroinflammation and antioxidant treatment on peripheral benzodiazepine receptors and NMDA receptors in the rat brain. J Neurochem. 2002;82:924–34. doi: 10.1046/j.1471-4159.2002.01050.x. [DOI] [PubMed] [Google Scholar]
- Bourdiol F, Toulmond S, Serrano A, Benavides J, Scatton B. Increase in omega 3 (peripheral type benzodiazepine) binding sites in the rat cortex and striatum after local injection of interleukin-1, tumour necrosis factor-alpha and lipopolysaccharide. Brain Res. 1991;543:194–200. doi: 10.1016/0006-8993(91)90028-t. [DOI] [PubMed] [Google Scholar]
- Braestrup C, Albrechtsen R, Squires RF. High densities of benzodiazepine receptors in human cortical areas. Nature. 1977;269:702–4. doi: 10.1038/269702a0. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, Banati RB. In-vivo measurement of activated microglia in dementia. Lancet. 2001a;358:461–7. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Myers R, Gunn RN, Lawrence AD, Stevens T, Kreutzberg GW, Jones T, Banati RB. In vivo visualization of activated glia by [11C] (R)-PK11195-PET following herpes encephalitis reveals projected neuronal damage beyond the primary focal lesion. Brain. 2001b;124:2014–27. doi: 10.1093/brain/124.10.2014. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Rossor M, Sampson EL, Mackinnon T, Banati RB. In vivo detection of microglial activation in frontotemporal dementia. Ann Neurol. 2004;56:894–7. doi: 10.1002/ana.20332. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Taylor-Robinson SD, Forton DM, Banati RB. In vivo imaging of cerebral “peripheral benzodiazepine binding sites” in patients with hepatic encephalopathy. Gut. 2006;55:547–53. doi: 10.1136/gut.2005.075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelli A, Matarrese M, Moresco RM, Valenti S, Anzini M, Vomero S, Turolla EA, Belloli S, Simonelli P, Filannino MA, Lecchi M, Fazio F. Synthesis, labeling, and biological evaluation of halogenated 2-quinolinecarboxamides as potential radioligands for the visualization of peripheral benzodiazepine receptors. Bioorg Med Chem. 2006;14:4055–66. doi: 10.1016/j.bmc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Carayon P, Portier M, Dussossoy D, Bord A, Petitpretre G, Canat X, Le Fur G, Casellas P. Involvement of peripheral benzodiazepine receptors in the protection of hematopoietic cells against oxygen radical damage. Blood. 1996;87:3170–8. [PubMed] [Google Scholar]
- Casellas P, Galiegue S, Basile AS. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem Int. 2002;40:475–86. doi: 10.1016/s0197-0186(01)00118-8. [DOI] [PubMed] [Google Scholar]
- Chaki S, Funakoshi T, Yoshikawa R, Okuyama S, Okubo T, Nakazato A, Nagamine M, Tomisawa K. Binding characteristics of [3H]DAA1106, a novel and selective ligand for peripheral benzodiazepine receptors. Eur J Pharmacol. 1999;371:197–204. doi: 10.1016/s0014-2999(99)00118-1. [DOI] [PubMed] [Google Scholar]
- Chan A, Seguin R, Magnus T, Papadimitriou C, Toyka KV, Antel JP, Gold R. Phagocytosis of apoptotic inflammatory cells by microglia and its therapeutic implications: termination of CNS autoimmune inflammation and modulation by interferon-beta. Glia. 2003;43:231–42. doi: 10.1002/glia.10258. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992;149:2736–41. [PubMed] [Google Scholar]
- Chao CC, Hu S, Peterson PK. Modulation of human microglial cell superoxide production by cytokines. J Leukoc Biol. 1995a;58:65–70. doi: 10.1002/jlb.58.1.65. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Sheng WS, Peterson PK. Tumor necrosis factor-alpha production by human fetal microglial cells: regulation by other cytokines. Dev Neurosci. 1995b;17:97–105. doi: 10.1159/000111278. [DOI] [PubMed] [Google Scholar]
- Charbonneau P, Syrota A, Crouzel C, Valois JM, Prenant C, Crouzel M. Peripheral-type benzodiazepine receptors in the living heart characterized by positron emission tomography. Circulation. 1986;73:476–83. doi: 10.1161/01.cir.73.3.476. [DOI] [PubMed] [Google Scholar]
- Chen MK, Baidoo K, Verina T, Guilarte TR. Peripheral benzodiazepine receptor imaging in CNS demyelination: functional implications of anatomical and cellular localization. Brain. 2004;127:1379–92. doi: 10.1093/brain/awh161. [DOI] [PubMed] [Google Scholar]
- Chen MK, Guilarte TR. Imaging the Peripheral Benzodiazepine Receptor Response in Cns Demyelination and Remyelination. Toxicol Sci. 2006 doi: 10.1093/toxsci/kfj172. [DOI] [PubMed] [Google Scholar]
- Choi HB, Khoo C, Ryu JK, van Breemen E, Kim SU, McLarnon JG. Inhibition of lipopolysaccharide-induced cyclooxygenase-2, tumor necrosis factor-alpha and [Ca2+]i responses in human microglia by the peripheral benzodiazepine receptor ligand PK11195. J Neurochem. 2002;83:546–55. doi: 10.1046/j.1471-4159.2002.01122.x. [DOI] [PubMed] [Google Scholar]
- Cicchetti F, Brownell AL, Williams K, Chen YI, Livni E, Isacson O. Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. Eur J Neurosci. 2002;15:991–8. doi: 10.1046/j.1460-9568.2002.01938.x. [DOI] [PubMed] [Google Scholar]
- Cleij MC, A. F, Baron JC, Clark JC. Base-promoted dechlorination of (R)-[C-11]PK-11195. 15th International Symposium on Radiopharmaceutical Chemistry. 2003;46(Suppl 1):588. [Google Scholar]
- Colton CA, Keri JE, Chen WT, Monsky WL. Protease production by cultured microglia: substrate gel analysis and immobilized matrix degradation. J Neurosci Res. 1993;35:297–304. doi: 10.1002/jnr.490350309. [DOI] [PubMed] [Google Scholar]
- Cremer JE, Hume SP, Cullen BM, Myers R, Manjil LG, Turton DR, Luthra SK, Bateman DM, Pike VW. The distribution of radioactivity in brains of rats given [N-methyl-11C]PK 11195 in vivo after induction of a cortical ischaemic lesion. Int J Rad Appl Instrum B. 1992;19:159–66. doi: 10.1016/0883-2897(92)90003-h. [DOI] [PubMed] [Google Scholar]
- Cumming P, Danielsen EH, Vafaee M, Falborg L, Steffensen E, Sorensen JC, Gillings N, Bender D, Marthi K, Andersen F, Munk O, Smith D, Moller A, Gjedde A. Normalization of markers for dopamine innervation in striatum of MPTP-lesioned miniature pigs with intrastriatal grafts. Acta Neurol Scand. 2001;103:309–15. doi: 10.1034/j.1600-0404.2001.103005309.x. [DOI] [PubMed] [Google Scholar]
- Cumming P, Pedersen MD, Minuzzi L, Mezzomo K, Danielsen EH, Iversen P, Aagaard D, Keiding S, Munk OL, Finsen B. Distribution of PK11195 binding sites in porcine brain studied by autoradiography in vitro and by positron emission tomography. Synapse. 2006;59:418–26. doi: 10.1002/syn.20257. [DOI] [PubMed] [Google Scholar]
- D'Aversa TG, Weidenheim KM, Berman JW. CD40-CD40L interactions induce chemokine expression by human microglia: implications for human immunodeficiency virus encephalitis and multiple sclerosis. Am J Pathol. 2002;160:559–67. doi: 10.1016/S0002-9440(10)64875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Bartolozzi ML, Guidi L, Stromillo ML, Federico A. Magnetic resonance spectroscopy as a measure of brain damage in multiple sclerosis. J Neurol Sci. 2005;233:203–8. doi: 10.1016/j.jns.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Debruyne JC, Versijpt J, Van Laere KJ, De Vos F, Keppens J, Strijckmans K, Achten E, Slegers G, Dierckx RA, Korf J, De Reuck JL. PET visualization of microglia in multiple sclerosis patients using [11C]PK11195. Eur J Neurol. 2003;10:257–64. doi: 10.1046/j.1468-1331.2003.00571.x. [DOI] [PubMed] [Google Scholar]
- Del Rio Hortega P. Cytology and Cellular Pathology of the Nervous system. Hoeber; New York: 1932. [Google Scholar]
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:14669–74. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–7. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett H, Barry M, Sun X, Lee SF, Frantz C, Berthiaume LG, McFadden G, Bleackley RC. The myxoma poxvirus protein, M11L, prevents apoptosis by direct interaction with the mitochondrial permeability transition pore. J Exp Med. 2002;196:1127–39. doi: 10.1084/jem.20011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett H, McFadden G. Viruses and apoptosis: meddling with mitochondria. Virology. 2001;288:1–7. doi: 10.1006/viro.2001.1081. [DOI] [PubMed] [Google Scholar]
- Fedoroff S, Zhai R, Novak JP. Microglia and astroglia have a common progenitor cell. J Neurosci Res. 1997;50:477–86. doi: 10.1002/(SICI)1097-4547(19971101)50:3<477::AID-JNR14>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Fetler L, Amigorena S. Neuroscience. Brain under surveillance: the microglia patrol. Science. 2005;309:392–3. doi: 10.1126/science.1114852. [DOI] [PubMed] [Google Scholar]
- Gavish M, Bachman I, Shoukrun R, Katz Y, Veenman L, Weisinger G, Weizman A. Enigma of the peripheral benzodiazepine receptor. Pharmacol Rev. 1999;51:629–50. [PubMed] [Google Scholar]
- Gehlert DR, Stephenson DT, Schober DA, Rash K, Clemens JA. Increased expression of peripheral benzodiazepine receptors in the facial nucleus following motor neuron axotomy. Neurochem Int. 1997;31:705–13. doi: 10.1016/s0197-0186(97)00007-7. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. 1995;20:269–87. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Neumaier B, Elitok E, Glatting G, Ries V, Tomczak R, Ludolph AC, Reske SN. In vivo imaging of activated microglia using [11C]PK11195 and positron emission tomography in patients after ischemic stroke. Neuroreport. 2000;11:2957–60. doi: 10.1097/00001756-200009110-00025. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol Dis. 2006;21:404–12. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Schwarz J, Myers R, Wise R, Banati RB. Evolution of microglial activation in patients after ischemic stroke: a [11C](R)-PK11195 PET study. Neuroimage. 2005;24:591–5. doi: 10.1016/j.neuroimage.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Watts J, Trender-Gerhard I, Turkheimer F, Banati RB, Bhatia K, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in corticobasal degeneration. Mov Disord. 2004;19:1221–6. doi: 10.1002/mds.20162. [DOI] [PubMed] [Google Scholar]
- Giulian D, Baker TJ, Shih LC, Lachman LB. Interleukin 1 of the central nervous system is produced by ameboid microglia. J Exp Med. 1986;164:594–604. doi: 10.1084/jem.164.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Vaca K, Noonan CA. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–6. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- Goerres GW, Revesz T, Duncan J, Banati RB. Imaging cerebral vasculitis in refractory epilepsy using [(11)C](R)-PK11195 positron emission tomography. AJR Am J Roentgenol. 2001;176:1016–8. doi: 10.2214/ajr.176.4.1761016. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–40. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol. 2004;75:388–97. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- Gulyas B, Halldin C, Vas A, Banati RB, Shchukin E, Finnema S, Tarkainen J, Tihanyi K, Szilagyi G, Farde L. [11C]vinpocetine: a prospective peripheral benzodiazepine receptor ligand for primate PET studies. J Neurol Sci. 2005;229-230:219–23. doi: 10.1016/j.jns.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Hammoud DA, Endres CJ, Chander AR, Guilarte TR, Wong DF, Sacktor NC, McArthur JC, Pomper MG. Imaging glial cell activation with [11C]-R-PK11195 in patients with AIDS. J Neurovirol. 2005;11:346–55. doi: 10.1080/13550280500187351. [DOI] [PubMed] [Google Scholar]
- Henkel K, Karitzky J, Schmid M, Mader I, Glatting G, Unger JW, Neumaier B, Ludolph AC, Reske SN, Landwehrmeyer GB. Imaging of activated microglia with PET and [11C]PK 11195 in corticobasal degeneration. Mov Disord. 2004;19:817–21. doi: 10.1002/mds.20040. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Achim CL, Wiley CA, Major EO, Saito K, Markey SP. Human microglia convert l-tryptophan into the neurotoxin quinolinic acid. Biochem J. 1996;320(Pt 2):595–7. doi: 10.1042/bj3200595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JD, Beyer CF, Malkowitz L, Beer B, Blume AJ. Mitochondrial benzodiazepine receptors mediate inhibition of mitochondrial respiratory control. Mol Pharmacol. 1989;35:157–63. [PubMed] [Google Scholar]
- Itzhak Y, Roig-Cantisano A, Norenberg MD. Ontogeny of peripheral-type benzodiazepine receptors in cultured astrocytes and brain from rat. Brain Res Dev Brain Res. 1995;84:62–6. doi: 10.1016/0165-3806(94)00163-t. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., Slomkowski M, Gracon S, Hoover TM, Felmlee JP, Stewart K, Xu Y, Shiung M, O'Brien PC, Cha R, Knopman D, Petersen RC. MRI as a biomarker of disease progression in a therapeutic trial of milameline for AD. Neurology. 2003;60:253–60. doi: 10.1212/01.wnl.0000042480.86872.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ML, Fulton RR, Henderson DJ, Eberl S, Meikle SR, Thomson S, Allan RD, Dolle F, Fulham MJ, Kassiou M. Synthesis and in vivo evaluation of a novel peripheral benzodiazepine receptor PET radioligand. Bioorg Med Chem. 2005;13:6188–94. doi: 10.1016/j.bmc.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Panickar KS, Norenberg MD. Effects on free radical generation by ligands of the peripheral benzodiazepine receptor in cultured neural cells. J Neurochem. 2002;83:1226–34. doi: 10.1046/j.1471-4159.2002.01261.x. [DOI] [PubMed] [Google Scholar]
- Jenkins BG, Kraft E. Magnetic resonance spectroscopy in toxic encephalopathy and neurodegeneration. Curr Opin Neurol. 1999;12:753–60. doi: 10.1097/00019052-199912000-00016. [DOI] [PubMed] [Google Scholar]
- Johnston C, Jiang W, Chu T, Levine B. Identification of genes involved in the host response to neurovirulent alphavirus infection. J Virol. 2001;75:10431–45. doi: 10.1128/JVI.75.21.10431-10445.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppe RA. Data Analysis and Imaging Processing. Priniciples and Practice of Positron Emission Tomography. Lippincott, Williams, and Wilkins; Philadelphia: 2002. [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–8. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kropholler MA, Boellaard R, Schuitemaker A, Folkersma H, van Berckel BN, Lammertsma AA. Evaluation of reference tissue models for the analysis of [(11)C](R)-PK11195 studies. J Cereb Blood Flow Metab. 2006 doi: 10.1038/sj.jcbfm.9600289. [DOI] [PubMed] [Google Scholar]
- Kropholler MA, Boellaard R, Schuitemaker A, van Berckel BN, Luurtsema G, Windhorst AD, Lammertsma AA. Development of a tracer kinetic plasma input model for (R)-[11C]PK11195 brain studies. J Cereb Blood Flow Metab. 2005;25:842–51. doi: 10.1038/sj.jcbfm.9600092. [DOI] [PubMed] [Google Scholar]
- Kuhlmann AC, Guilarte TR. Cellular and subcellular localization of peripheral benzodiazepine receptors after trimethyltin neurotoxicity. J Neurochem. 2000;74:1694–704. doi: 10.1046/j.1471-4159.2000.0741694.x. [DOI] [PubMed] [Google Scholar]
- Kurumaji A, Wakai T, Toru M. Decreases in peripheral-type benzodiazepine receptors in postmortem brains of chronic schizophrenics. J Neural Transm. 1997;104:1361–70. doi: 10.1007/BF01294737. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–8. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Le Fur G, Guilloux F, Rufat P, Benavides J, Uzan A, Renault C, Dubroeucq MC, Gueremy C. Peripheral benzodiazepine binding sites: effect of PK 11195, 1-(2-chlorophenyl)-N-methyl-(1-methylpropyl)-3 isoquinolinecarboxamide. II. In vivo studies. Life Sci. 1983a;32:1849–56. doi: 10.1016/0024-3205(83)90063-2. [DOI] [PubMed] [Google Scholar]
- Le Fur G, Perrier ML, Vaucher N, Imbault F, Flamier A, Benavides J, Uzan A, Renault C, Dubroeucq MC, Gueremy C. Peripheral benzodiazepine binding sites: effect of PK 11195, 1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide. I. In vitro studies. Life Sci. 1983b;32:1839–47. doi: 10.1016/0024-3205(83)90062-0. [DOI] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA, Cole GM. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. J Neurosci. 2000;20:5709–14. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokensgard JR, Hu S, Hegg CC, Thayer SA, Gekker G, Peterson PK. Diazepam inhibits HIV-1 Tat-induced migration of human microglia. J Neurovirol. 2001;7:481–6. doi: 10.1080/135502801753170345. [DOI] [PubMed] [Google Scholar]
- Maeda J, Suhara T, Zhang MR, Okauchi T, Yasuno F, Ikoma Y, Inaji M, Nagai Y, Takano A, Obayashi S, Suzuki K. Novel peripheral benzodiazepine receptor ligand [11C]DAA1106 for PET: An imaging tool for glial cells in the brain. Synapse. 2004;52:283–91. doi: 10.1002/syn.20027. [DOI] [PubMed] [Google Scholar]
- Mankowski JL, Queen SE, Tarwater PJ, Adams RJ, Guilarte TR. Elevated peripheral benzodiazepine receptor expression in simian immunodeficiency virus encephalitis. J Neurovirol. 2003;9:94–100. doi: 10.1080/13550280390173283. [DOI] [PubMed] [Google Scholar]
- Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, Seubert P, Games D, Kirby L, Schenk D. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–31. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- McEnery MW, Snowman AM, Trifiletti RR, Snyder SH. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci U S A. 1992;89:3170–4. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988a;38:1285–91. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Tago H, McGeer EG. Occurrence of HLA-DR reactive microglia in Alzheimer's disease. Ann N Y Acad Sci. 1988b;540:319–23. doi: 10.1111/j.1749-6632.1988.tb27086.x. [DOI] [PubMed] [Google Scholar]
- McManus CM, Brosnan CF, Berman JW. Cytokine induction of MIP-1 alpha and MIP-1 beta in human fetal microglia. J Immunol. 1998;160:1449–55. [PubMed] [Google Scholar]
- Minghetti L, Levi G. Microglia as effector cells in brain damage and repair: focus on prostanoids and nitric oxide. Prog Neurobiol. 1998;54:99–125. doi: 10.1016/s0301-0082(97)00052-x. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984;15:217–27. doi: 10.1002/ana.410150302. [DOI] [PubMed] [Google Scholar]
- Miyazawa N, Hamel E, Diksic M. Assessment of the peripheral benzodiazepine receptors in human gliomas by two methods. J Neurooncol. 1998;38:19–26. doi: 10.1023/a:1005933226966. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–5. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Morgan D, Gordon MN, Tan J, Wilcock D, Rojiani AM. Dynamic complexity of the microglial activation response in transgenic models of amyloid deposition: implications for Alzheimer therapeutics. J Neuropathol Exp Neurol. 2005;64:743–53. doi: 10.1097/01.jnen.0000178444.33972.e0. [DOI] [PubMed] [Google Scholar]
- Muhleisen H, Gehrmann J, Meyermann R. Reactive microglia in Creutzfeldt-Jakob disease. Neuropathol Appl Neurobiol. 1995;21:505–17. doi: 10.1111/j.1365-2990.1995.tb01097.x. [DOI] [PubMed] [Google Scholar]
- Murphy GM, Jr., Jia XC, Song Y, Ong E, Shrivastava R, Bocchini V, Lee YL, Eng LF. Macrophage inflammatory protein 1-alpha mRNA expression in an immortalized microglial cell line and cortical astrocyte cultures. J Neurosci Res. 1995;40:755–63. doi: 10.1002/jnr.490400607. [DOI] [PubMed] [Google Scholar]
- Myers R, Manjil LG, Cullen BM, Price GW, Frackowiak RS, Cremer JE. Macrophage and astrocyte populations in relation to [3H]PK 11195 binding in rat cerebral cortex following a local ischaemic lesion. J Cereb Blood Flow Metab. 1991;11:314–22. doi: 10.1038/jcbfm.1991.64. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–52. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Oh YJ, Francis JW, Markelonis GJ, Oh TH. Interleukin-1-beta and tumor necrosis factor-alpha increase peripheral-type benzodiazepine binding sites in cultured polygonal astrocytes. J Neurochem. 1992;58:2131–8. doi: 10.1111/j.1471-4159.1992.tb10955.x. [DOI] [PubMed] [Google Scholar]
- Okuyama S, Chaki S, Yoshikawa R, Ogawa S, Suzuki Y, Okubo T, Nakazato A, Nagamine M, Tomisawa K. Neuropharmacological profile of peripheral benzodiazepine receptor agonists, DAA1097 and DAA1106. Life Sci. 1999;64:1455–64. doi: 10.1016/s0024-3205(99)00079-x. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann Neurol. 2005;57:168–75. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V. In search of the function of the peripheral-type benzodiazepine receptor. Endocr Res. 2004;30:677–84. doi: 10.1081/erc-200043971. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Amri H, Li H, Boujrad N, Vidic B, Garnier M. Targeted disruption of the peripheral-type benzodiazepine receptor gene inhibits steroidogenesis in the R2C Leydig tumor cell line. J Biol Chem. 1997;272:32129–35. doi: 10.1074/jbc.272.51.32129. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Lecanu L, Brown RC, Han Z, Yao ZX. Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience. 2006;138:749–56. doi: 10.1016/j.neuroscience.2005.05.063. [DOI] [PubMed] [Google Scholar]
- Pappata S, Cornu P, Samson Y, Prenant C, Benavides J, Scatton B, Crouzel C, Hauw JJ, Syrota A. PET study of carbon-11-PK 11195 binding to peripheral type benzodiazepine sites in glioblastoma: a case report. J Nucl Med. 1991;32:1608–10. [PubMed] [Google Scholar]
- Pappata S, Levasseur M, Gunn RN, Myers R, Crouzel C, Syrota A, Jones T, Kreutzberg GW, Banati RB. Thalamic microglial activation in ischemic stroke detected in vivo by PET and [11C]PK1195. Neurology. 2000;55:1052–4. doi: 10.1212/wnl.55.7.1052. [DOI] [PubMed] [Google Scholar]
- Park CH, Carboni E, Wood PL, Gee KW. Characterization of peripheral benzodiazepine type sites in a cultured murine BV-2 microglial cell line. Glia. 1996;16:65–70. doi: 10.1002/(SICI)1098-1136(199601)16:1<65::AID-GLIA7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Pavese N, Gerhard A, Tai YF, Ho AK, Turkheimer F, Barker RA, Brooks DJ, Piccini P. Microglial activation correlates with severity in Huntington disease: a clinical and PET study. Neurology. 2006;66:1638–43. doi: 10.1212/01.wnl.0000222734.56412.17. [DOI] [PubMed] [Google Scholar]
- Pedersen MD, Minuzzi L, Wirenfeldt M, Meldgaard M, Slidsborg C, Cumming P, Finsen B. Up-regulation of PK11195 binding in areas of axonal degeneration coincides with early microglial activation in mouse brain. Eur J Neurosci. 2006;24:991–1000. doi: 10.1111/j.1460-9568.2006.04975.x. [DOI] [PubMed] [Google Scholar]
- Perry VH, Gordon S. Macrophages and the nervous system. Int Rev Cytol. 1991;125:203–44. doi: 10.1016/s0074-7696(08)61220-6. [DOI] [PubMed] [Google Scholar]
- Petit-Taboue MC, Baron JC, Barre L, Travere JM, Speckel D, Camsonne R, MacKenzie ET. Brain kinetics and specific binding of [11C]PK 11195 to omega 3 sites in baboons: positron emission tomography study. Eur J Pharmacol. 1991;200:347–51. doi: 10.1016/0014-2999(91)90594-g. [DOI] [PubMed] [Google Scholar]
- Piani D, Spranger M, Frei K, Schaffner A, Fontana A. Macrophage-induced cytotoxicity of N-methyl-D-aspartate receptor positive neurons involves excitatory amino acids rather than reactive oxygen intermediates and cytokines. Eur J Immunol. 1992;22:2429–36. doi: 10.1002/eji.1830220936. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wang D, Menon DK, Guadagno JV, Cleij M, Fryer T, Aigbirhio F, Baron JC, Warburton EA. Intrinsic activated microglia map to the peri-infarct zone in the subacute phase of ischemic stroke. Stroke. 2006;37:1749–53. doi: 10.1161/01.STR.0000226980.95389.0b. [DOI] [PubMed] [Google Scholar]
- Raghavendra Rao VL, Dogan A, Bowen KK, Dempsey RJ. Traumatic brain injury leads to increased expression of peripheral-type benzodiazepine receptors, neuronal death, and activation of astrocytes and microglia in rat thalamus. Exp Neurol. 2000;161:102–14. doi: 10.1006/exnr.1999.7269. [DOI] [PubMed] [Google Scholar]
- Rao VL, Butterworth RF. Characterization of binding sites for the omega3 receptor ligands [3H]PK11195 and [3H]RO5-4864 in human brain. Eur J Pharmacol. 1997;340:89–99. doi: 10.1016/s0014-2999(97)01395-2. [DOI] [PubMed] [Google Scholar]
- Rey C, Mauduit C, Naureils O, Benahmed M, Louisot P, Gasnier F. Up-regulation of mitochondrial peripheral benzodiazepine receptor expression by tumor necrosis factor alpha in testicular leydig cells. Possible involvement in cell survival. Biochem Pharmacol. 2000;60:1639–46. doi: 10.1016/s0006-2952(00)00500-1. [DOI] [PubMed] [Google Scholar]
- Righi M, Mori L, De Libero G, Sironi M, Biondi A, Mantovani A, Donini SD, Ricciardi-Castagnoli P. Monokine production by microglial cell clones. Eur J Immunol. 1989;19:1443–8. doi: 10.1002/eji.1830190815. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Choi HB, McLarnon JG. Peripheral benzodiazepine receptor ligand PK11195 reduces microglial activation and neuronal death in quinolinic acid-injected rat striatum. Neurobiol Dis. 2005;20:550–61. doi: 10.1016/j.nbd.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Sargsyan SA, Monk PN, Shaw PJ. Microglia as potential contributors to motor neuron injury in amyotrophic lateral sclerosis. Glia. 2005;51:241–53. doi: 10.1002/glia.20210. [DOI] [PubMed] [Google Scholar]
- Sauvageau A, Desjardins P, Lozeva V, Rose C, Hazell AS, Bouthillier A, Butterwort RF. Increased expression of “peripheral-type” benzodiazepine receptors in human temporal lobe epilepsy: implications for PET imaging of hippocampal sclerosis. Metab Brain Dis. 2002;17:3–11. doi: 10.1023/a:1014044128845. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–7. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Sette G, Baron JC, Young AR, Miyazawa H, Tillet I, Barre L, Travere JM, Derlon JM, MacKenzie ET. In vivo mapping of brain benzodiazepine receptor changes by positron emission tomography after focal ischemia in the anesthetized baboon. Stroke. 1993;24:2046–57. doi: 10.1161/01.str.24.12.2046. discussion 2057-8. [DOI] [PubMed] [Google Scholar]
- Shah F, Hume SP, Pike VW, Ashworth S, McDermott J. Synthesis of the enantiomers of [N-methyl-11C]PK 11195 and comparison of their behaviours as radioligands for PK binding sites in rats. Nucl Med Biol. 1994;21:573–81. doi: 10.1016/0969-8051(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Starosta-Rubinstein S, Ciliax BJ, Penney JB, McKeever P, Young AB. Imaging of a glioma using peripheral benzodiazepine receptor ligands. Proc Natl Acad Sci U S A. 1987;84:891–5. doi: 10.1073/pnas.84.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson DT, Schober DA, Smalstig EB, Mincy RE, Gehlert DR, Clemens JA. Peripheral benzodiazepine receptors are colocalized with activated microglia following transient global forebrain ischemia in the rat. J Neurosci. 1995;15:5263–74. doi: 10.1523/JNEUROSCI.15-07-05263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson FA. The GABAA receptors. Biochem J. 1995;310(Pt 1):1–9. doi: 10.1042/bj3100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoebner PE, Carayon P, Casellas P, Portier M, Lavabre-Bertrand T, Cuq P, Cano JP, Meynadier J, Meunier L. Transient protection by peripheral benzodiazepine receptors during the early events of ultraviolet light-induced apoptosis. Cell Death Differ. 2001;8:747–53. doi: 10.1038/sj.cdd.4400861. [DOI] [PubMed] [Google Scholar]
- Trincavelli ML, Marselli L, Falleni A, Gremigni V, Ragge E, Dotta F, Santangelo C, Marchetti P, Lucacchini A, Martini C. Upregulation of mitochondrial peripheral benzodiazepine receptor expression by cytokine-induced damage of human pancreatic islets. J Cell Biochem. 2002;84:636–44. [PubMed] [Google Scholar]
- Turner MR, Cagnin A, Turkheimer FE, Miller CC, Shaw CE, Brooks DJ, Leigh PN, Banati RB. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis. 2004;15:601–9. doi: 10.1016/j.nbd.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Turner MR, Gerhard A, Al-Chalabi A, Shaw CE, Hughes RA, Banati RB, Brooks DJ, Leigh PN. Mills' and other isolated upper motor neurone syndromes: in vivo study with 11C-(R)-PK11195 PET. J Neurol Neurosurg Psychiatry. 2005;76:871–4. doi: 10.1136/jnnp.2004.047902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulvestad E, Williams K, Matre R, Nyland H, Olivier A, Antel J. Fc receptors for IgG on cultured human microglia mediate cytotoxicity and phagocytosis of antibody-coated targets. J Neuropathol Exp Neurol. 1994;53:27–36. doi: 10.1097/00005072-199401000-00004. [DOI] [PubMed] [Google Scholar]
- Venneti S, Lopresti BJ, Wang G, Bissel SJ, Mathis CA, Meltzer CC, Boada F, Capuano S, 3rd, Kress GJ, Davis DK, Ruszkiewicz J, Reynolds IJ, Murphey-Corb M, Trichel AM, Wisniewski SR, Wiley CA. PET imaging of brain macrophages using the peripheral benzodiazepine receptor in a macaque model of neuroAIDS. J Clin Invest. 2004;113:981–9. doi: 10.1172/JCI20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versijpt J, Debruyne JC, Van Laere KJ, De Vos F, Keppens J, Strijckmans K, Achten E, Slegers G, Dierckx RA, Korf J, De Reuck JL. Microglial imaging with positron emission tomography and atrophy measurements with magnetic resonance imaging in multiple sclerosis: a correlative study. Mult Scler. 2005;11:127–34. doi: 10.1191/1352458505ms1140oa. [DOI] [PubMed] [Google Scholar]
- Vowinckel E, Reutens D, Becher B, Verge G, Evans A, Owens T, Antel JP. PK11195 binding to the peripheral benzodiazepine receptor as a marker of microglia activation in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurosci Res. 1997;50:345–53. doi: 10.1002/(SICI)1097-4547(19971015)50:2<345::AID-JNR22>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Webster SD, Galvan MD, Ferran E, Garzon-Rodriguez W, Glabe CG, Tenner AJ. Antibody-mediated phagocytosis of the amyloid beta-peptide in microglia is differentially modulated by C1q. J Immunol. 2001;166:7496–503. doi: 10.4049/jimmunol.166.12.7496. [DOI] [PubMed] [Google Scholar]
- Weissman BA, Bolger GT, Isaac L, Paul SM, Skolnick P. Characterization of the binding of [3H]Ro 5-4864, a convulsant benzodiazepine, to guinea pig brain. J Neurochem. 1984;42:969–75. doi: 10.1111/j.1471-4159.1984.tb12698.x. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Lopresti BJ, Becker JT, Boada F, Lopez OL, Mellors J, Meltzer CC, Wisniewski SR, Mathis CA. Positron emission tomography imaging of peripheral benzodiazepine receptor binding in human immunodeficiency virus-infected subjects with and without cognitive impairment. J Neurovirol. 2006;12:262–71. doi: 10.1080/13550280600873868. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986;83:7089–93. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MR, Kida T, Noguchi J, Furutsuka K, Maeda J, Suhara T, Suzuki K. [(11)C]DAA1106: radiosynthesis and in vivo binding to peripheral benzodiazepine receptors in mouse brain. Nucl Med Biol. 2003;30:513–9. doi: 10.1016/s0969-8051(03)00016-7. [DOI] [PubMed] [Google Scholar]
- Zhang MR, Maeda J, Ito T, Okauchi T, Ogawa M, Noguchi J, Suhara T, Halldin C, Suzuki K. Synthesis and evaluation of N-(5-fluoro-2-phenoxyphenyl)-N-(2-[(18)F]fluoromethoxy-d(2)-5-methoxybenzy l)acetamide: a deuterium-substituted radioligand for peripheral benzodiazepine receptor. Bioorg Med Chem. 2005;13:1811–8. doi: 10.1016/j.bmc.2004.11.058. [DOI] [PubMed] [Google Scholar]
- Zhang MR, Maeda J, Ogawa M, Noguchi J, Ito T, Yoshida Y, Okauchi T, Obayashi S, Suhara T, Suzuki K. Development of a new radioligand, N-(5-fluoro-2-phenoxyphenyl)-N-(2-[18F]fluoroethyl-5-methoxybenzyl)acetami de, for pet imaging of peripheral benzodiazepine receptor in primate brain. J Med Chem. 2004;47:2228–35. doi: 10.1021/jm0304919. [DOI] [PubMed] [Google Scholar]