Abstract
Collagenase-3 (MMP-13) is a human matrix metalloproteinase specifically expressed by invading tumor cells in squamous cell carcinomas (SCCs) of the head and neck. Here, we have further elucidated the role of MMP-13 in tumor invasion by examining its expression in invasive malignant tumors of the female genital tract. Using in situ hybridization, expression of MMP-13 mRNA was detected in 9 of 12 vulvar SCCs, primarily in tumor cells, but not in intact vulvar epithelium, in cervical SCCs (n = 12), or in endometrial (n = 11) or ovarian adenocarcinomas (n = 8). MMP-13 expression was especially abundant in vulvar carcinomas showing metastasis to lymph nodes and was associated with expression of membrane type 1 MMP by tumor cells and gelatinase-A (MMP-2) by stromal cells, as detected by immunohistochemistry. MMP-13 mRNAs were detected in 9 of 11 cell lines established from vulvar carcinomas and in 4 of 6 cell lines from cervical carcinomas, whereas endometrial (n = 10) and ovarian (n = 9) carcinoma cell lines were negative for MMP-13 mRNA. No correlation was detected between MMP-13 expression and p53 gene mutations in vulvar SCC cell lines. However, MMP-13 expression was detected in 5 of 6 vulvar and cervical SCC cell lines harboring HPV 16 or 68 DNA. These results show that MMP-13 is specifically expressed by malignantly transformed squamous epithelial cells, including vulvar SCC cells, and appears to serve as a marker for their invasive capacity.
Matrix metalloproteinases (MMPs) 1 are a family of zinc-dependent endopeptidases collectively capable of degrading essentially all extracellular matrix components. At present, the MMP gene family contains 16 human members, which can be classified into subgroups of collagenases, gelatinases, stromelysins, membrane-type MMPs (MT-MMPs), and novel MMPs according to their substrate specificity and structure. 1,2 Degradation of matrix components by MMPs plays an important role in normal tissue remodeling, eg, during fetal development, tissue repair, angiogenesis, and endometrial cycling, as well as in pathological conditions such as osteoarthritis, rheumatoid arthritis, atherosclerosis, periodontitis, autoimmune blistering disorders of the skin, and tumor invasion and metastasis. 1-3 Members of the collagenase subgroup of MMPs, ie, collagenase-1 (MMP-1), collagenase-2 (MMP-8), and collagenase-3 (MMP-13), are the principal neutral proteinases capable of degrading native fibrillar collagens in the extracellular space. They all cleave type I, II, and III collagens at a specific site, generating 3/4 N-terminal and 1/4 C-terminal fragments, which then denature in physiological temperature and are degraded by other MMPs, eg, gelatinases. 2,3 MMP-13 also cleaves native type I collagen in the N-terminal nonhelical telopeptide. 4 Because fibrillar collagens are the most abundant structural components of human connective tissues, it is conceivable that the ability to degrade collagenous extracellular matrix is crucial for invasion of neoplastic cells. 2,3
Human collagenase-3 (MMP-13), originally cloned from breast carcinoma tissue, 5 is characterized by exceptionally wide substrate specificity compared to other MMPs. MMP-13 cleaves fibrillar collagens, preferring type II collagen over types I and III, and displays over 40-fold stronger gelatinase activity than MMP-1 and MMP-8. 6-8 In addition, MMP-13 degrades type IV, IX, X, and XIV collagens, tenascin, fibronectin, and aggrecan core protein. 8,9 Apparently due to its ability to degrade a wide range of matrix components, the physiological expression of human MMP-13 is limited to situations in which rapid and effective remodeling of collagenous extracellular matrix is required, ie, fetal bone development and postnatal bone remodeling. 10,11 On the other hand, MMP-13 apparently plays a role in excessive degradation of collagenous matrix in osteoarthritic cartilage, 7,12,13 rheumatoid synovium, 11,13,14 chronic cutaneous ulcers, 15 intestinal ulcerations, 16 and periodontitis. 17 The wide substrate specificity of MMP-13 also makes it a potent proteolytic tool for invading tumor cells and the expression of MMP-13 has been documented in breast carcinomas, 5,18,19 squamous cell carcinomas (SCCs) of the head and neck, 20,21 cutaneous basal cell carcinomas, 21 and chondrosarcomas. 22 Our recent observations show that in SCCs of the skin, oral cavity, and larynx, MMP-13 is expressed mainly by tumor cells at the invading margin of the tumor (and also in some cases by stromal fibroblasts), 20,21 whereas no MMP-13 expression is noted in intact or re-epithelializing epidermis, healthy oral mucosa, or normal keratinocytes in culture. 15,20,21,23
To further elucidate the role of MMP-13 in tumor cell invasion, we have examined its expression in invasive malignant tumors of the female genital tract. Previous studies have demonstrated expression of gelatinase-A (MMP-2) and gelatinase-B (MMP-9) in endometrial carcinomas 24-26 and expression of MMP-2, MMP-9, and MT1-MMP in cervical 25,27-29 and ovarian 30-34 carcinomas. At present, neither the level of expression of MMP-13 in any of these neoplastic tumors nor the role of MMPs in the invasion of vulvar carcinoma is known. In this study we show that MMP-13 mRNA is abundantly expressed by tumor cells of vulvar SCCs in vivo, whereas no expression of MMP-13 is detected in cervical SCCs or endometrial or ovarian adenocarcinomas in vivo. In addition, MMP-13 expression was noted in vulvar and cervical carcinoma cells in culture, especially by 5 of 6 vulvar and cervical SCC cell lines harboring human papilloma virus (HPV) 16 or 68 DNA, whereas no correlation was detected between MMP-13 expression and p53 gene mutations in vulvar SCC cell lines. These results provide evidence that MMP-13 is specifically expressed by malignantly transformed squamous epithelial cells, including vulvar SCC cells, and may serve as a marker for their invasive capacity.
Materials and Methods
In Situ Hybridizations
Formalin-fixed, paraffin-embedded specimens of vulvar SCCs (n = 12, Table 1 ▶ ), cervical SCCs (n = 12; 1 in situ carcinoma, 6 T1N0M0, 2 T2N0M0, 1 T3N1M0,1 T4N0M0 and 1 T4N0M1; 5 grade II, 6 grade III), endometrial adenocarcinomas (n = 11), and ovarian serous adenocarcinomas (n = 8) were obtained from the Department of Pathology, University of Turku, Finland. In vitro transcribed antisense and sense RNA probes were labeled with [α-35S]UTP as described previously. 35 A 491-bp ApaI fragment from the 5′ end of the human MMP-13 cDNA 23 was used to transcribe antisense and sense RNAs, respectively. The specificity of these probes has been shown previously. 10,15-17,20,21 Generation and specificities of human MMP-1 and stromelysin-1 (MMP-3) antisense and sense RNA probes have been described previously. 36,37 Tissue sections 5 μm thick were treated with proteinase K, washed in 0.1 mol/L triethanolamine buffer containing 0.25% acetic anhydride, and covered with 50–100 μl of hybridization buffer containing 2.5–5 × 10 4 cpm/μl of 35S-labeled antisense or sense RNA probe. After hybridization at 50–55°C for 18 hours in a humidified chamber, the slides were washed under stringent conditions, including RNase A treatment, to remove unhybridized probe. 36 After autoradiography for 25 to 35 days, the photographic emulsion was developed and the slides were stained with hematoxylin and eosin. Samples of tissues previously shown to express MMP-13 mRNA (breast carcinoma) 5 and MMP-1 and MMP-3 mRNA (chronic dermal ulcers) 15 were used as positive controls and a labeled sense probe was used as a negative control in each in situ hybridization.
Table 1.
Expression of MMPs in Vulvar Carcinomas
| Patient | Age | Type of lesion | Tumor grade | TNM at diagnosis | Survival in months* | MMP-13 | MMP-1 | MMP-2 | MMP-9 | MMP-3 | MT1-MMP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | S | T | S | T | S | T | S** | T | S | T | S | ||||||
| 1 | 70 | primary | I | T1N0M0 | 12 | + | + | − | ++ | + | + | + | + | − | − | N.D. | N.D. |
| 2 | 74 | recurrent | II | T1N0M0 | 168 | − | − | − | − | + | + | − | + | − | − | + | − |
| 2 | 74 | recurrent | III | T1N0M0 | 168 | +++ | − | ++ | + | + | + | − | + | + | − | + | − |
| 3 | 73 | primary | II | T3N2M0 | 3 | +++ | − | +++ | + | + | ++ | ++ | + | ++ | + | ++ | + |
| 4 | 71 | primary | I | T2N0M0 | 37 | ++ | − | + | − | ++ | + | + | + | − | − | ++ | + |
| 5 | 73 | primary | II | T2N0M0 | 36 | + | − | + | ++ | − | + | + | + | ++ | + | ++ | + |
| 6 | 75 | recurrent | I | T1N0M0 | 123+ | ++ | − | + | − | ++ | + | N.D. | N.D. | − | − | ++ | − |
| 6 | 75 | recurrent | I | T1N0M0 | 123+ | + | − | ++ | − | + | + | − | + | − | − | N.D. | N.D. |
| 7 | 77 | primary | I | T2N1M0 | 40+ | ++++ | + | + | ++ | + | + | + | + | ++ | + | +++ | + |
| 8 | 73 | recurrent | I | T3N0M0 | 44+ | − | − | − | − | − | + | N.D. | N.D. | − | − | + | − |
| 9 | 35 | recurrent | II | T3N2M0 | 9 | ++++ | + | − | + | + | ++ | + | + | + | − | +++ | + |
| 10 | 45 | primary | I | T3N0M0 | 19+ | − | − | + | − | − | + | N.D. | N.D. | − | − | ++ | + |
Expression of MMP-13, MMP-1, and MMP-3 was determined by in situ hybridization. Expression of MMP-2, MMP-9, and MT1-MMP was determined by immunostaining. Expression estimated in range from − to ++++. T, tumor; S, stroma; N.D., not determined.
*Patients marked with + after survival in months are still alive.
**Detected in inflammatory cells of stromal infiltrate.
Immunohistochemistry
Immunostainings of sections parallel to those used for in situ hybridization were performed by the avidin-biotin-peroxidase complex technique 37 using diaminobenzidine as chromogenic substrate. Monoclonal antibodies anti-MMP-9 (GE213, dilution 1:500; Diabor, Oulu, Finland), 38 anti-MMP-13 (181–15A12, dilution 1:30) (Oncogene Research Products, Cambridge, MA), anti-MT1-MMP (114–6G6, dilution 1:10) (Oncogene Research) 39 and anti-MMP-2 (42–5D11, dilution 1:200) (Oncogene Research) were used. Affinity-purified polyclonal antiserum against matrilysin (MMP-7) 40 was diluted 1:800. For staining with MMP-9 and MMP-7 antibodies, sections were pretreated with 10 μg/ml trypsin. The tissues were counterstained with hematoxylin. Negative control stainings were performed with preimmune mouse ascites fluid for mouse monoclonal antibodies and with rabbit preimmune serum for polyclonal antibodies.
Cell Cultures
The establishment and characterization of the vulvar SCC cell lines (Table 2) ▶ , 41-43 cervical SCC cell lines (CaSki, Me-180, Hx-151, Hx-156, and UT-CxC-1), 44 cervical glassy cell carcinoma cell line (UM-GCC-1), 44 and endometrial carcinoma cell lines (KLE, RL95-2, UM-EC-1, -2, UT-EC-2A, -2B, -2C, -3, -5, and -6) 45 have been described previously and their p53 and HPV status has been documented. 43-47 In addition, ovarian carcinoma cell lines (n = 9) (SK-OV-3, Ca-OV-3, UT-OV-2, -3, -4, -5, -6, -9, and -10) were used. All cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 6 mmol/L glutamine, nonessential amino acids, and 10% fetal calf serum (FCS). Cell lines UT-DEC-1 and UT-DEC-2, established from premalignant vaginal intraepithelial neoplasias 48,49 were cultured in Keratinocyte-Serum Free Medium (Gibco BRL, Gaithersburg, MD) supplemented with bovine pituitary extract (50 μg/ml) and recombinant epidermal growth factor (5 ng/ml) in early subcultures. UT-DEC-1 cells in passage 89 were cultured in DMEM supplemented with 6 mmol/L glutamine, nonessential amino acids, and 5% FCS.
Table 2.
Expression of MMPs by Cell Lines Established from Vulvar Carcinomas
| Cell line | Age | Type of lesion | Tumor grade | TNM at diagnosis | HPV status | p53 gene status | mRNA levels | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MMP-13 | MMP-1 | MMP-7 | MT1-MMP | MMP-9 | |||||||
| UM-SCV-1A | 62 | primary | II–III | T3N2M1 | − | WT | 1.3 | − | 5.6 | 0.7 | − |
| UM-SCV-1B | 62 | metastasis | III | T3N2M1 | − | WT | 4.0 | 3.0 | 4.5 | 5.0 | − |
| UM-SCV-2 | 86 | recurrent | III | T3N1M0 | − | + | 4.3 | 3.0 | 0.9 | 1.0 | − |
| UM-SCV-3 | 66 | primary | II | T2N0M0 | − | + | − | − | − | 0.7 | − |
| UM-SCV-4 | 41 | primary | I | T2N2M0 | − | + | − | − | − | 0.1 | − |
| UM-SCV-5 | 60 | recurrent | II | N.A. | − | + | 1.0 | − | 0.4 | 0.2 | 0.3 |
| UM-SCV-6 | 43 | recurrent | II | T1N1M0 | HPV16 | WT | 10 | − | − | 0.5 | − |
| UM-SCV-7 | 77 | primary | II–III | T2N2M0 | − | + | 0.2 | 1.0 | 0.9 | 0.5 | − |
| UT-SCV-1 | 70 | metastasis | I | T1N0M0 | − | + | 0.3 | − | − | 0.1 | − |
| UT-SCV-2 | 74 | recurrent | II | T1N0M0 | − | WT | 15 | − | 0.7 | 0.1 | − |
| UT-SCV-3 | 73 | primary | II | T3N2M0 | N.D. | N.D. | 6.8 | 12 | 0.2 | 0.1 | − |
mRNA levels were determined by Northern blot hybridizations, quantitated by densitometry, and corrected for the levels of GAPDH mRNA. UT-SCV-1 cell line is derived from patient # 1, UT-SCV-2 cell line from patient # 2 and UT-SCV-3 cell line from patient # 3.
N.D., not determined; N.A., not available; WT, wild-type p53 gene; +, mutated.
Cytokines and Growth Factors
Human recombinant tumor necrosis factor-α (TNF-α) and transforming growth factor-β1 (TGF-β1) were obtained from Sigma Chemical (St. Louis, MO). Human recombinant transforming growth factor-α (TGF-α) was obtained from PeproTech EC (Rocky Hill, NJ).
RNA Analysis
Total cellular RNA was isolated from cell cultures using the single step method. 50 Northern blot hybridizations were performed as described previously 23 with cDNAs labeled with [α-32P]dCTP by random priming. Human MMP-13 cDNA fragments covering the coding region and part of the 3′-untranslated region, totaling 1931 bp, were used as probes. 23 In addition, a 2-kb human MMP-1 cDNA, 51 a 1.5-kb human MMP-3 cDNA, 52 a 2.7-kb MMP-2 cDNA, 53 a 2-kb human MMP-9 cDNA, 54 a 0.7-kb human MT1-MMP (MMP-14) cDNA, 55 a 1-kb human MMP-7 cDNA, 56 and a 1.3-kb rat cDNA for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) 57 were used for Northern blot hybridizations. [32P]-cDNA/mRNA hybrids were visualized by autoradiography and the mRNA levels were quantitated by densitometry and corrected for the levels of GAPDH mRNA in the same RNA samples.
Results
Expression of MMP-13 in Vulvar Carcinomas
Our recent observations show that MMP-13 is expressed by tumor cells in SCCs of the head and neck, as well as by cell lines established from corresponding tumors. 20 In contrast, MMP-13 is not expressed by normal human epidermal keratinocytes in culture, in intact skin, or in re-epithelializing cutaneous wounds, indicating that the ability to express MMP-13 is specific to transformed keratinocytes. 15,20,21,23 In the present study, we have further elucidated the role of MMP-13 in tumor invasion by examining its expression in invasive malignant tumors of the female genital tract with distinct histogenetic origin, ie, SCCs of vulva and uterine cervix and adenocarcinomas of endometrium and ovary. Initially, we examined the expression of MMP-13 mRNAs in these tumors by in situ hybridization. Signal for MMP-13 mRNA was detected in 9 of 12 vulvar SCC samples (5 of 6 primary SCCs and 4 of 6 recurrent tumors) (Table 1) ▶ . In contrast, no MMP-13 mRNAs were detected in any of the cervical SCCs (n = 12) representing different stages of the tumor, nor in the endometrial (n = 11) or ovarian (n = 8) adenocarcinomas examined (not shown). In comparison, expression of MMP-1 mRNA was detected in 6 cervical SCCs, in tumor cells in 2 carcinomas, and in stromal cells in 4 tumors. In addition, MMP-1 was detected in stromal cells in 2 endometrial carcinomas and 1 ovarian carcinoma (not shown).
In the majority of MMP-13-positive vulvar SCCs, MMP-13 mRNA expression was confined to tumor cells (Figures 1, A ▶ -C, E, and F, and 2, A, B, D, and E). However, in three tumors MMP-13 expression was also noted in stromal cells (Table 1) ▶ . No specific signal was detected in parallel tissue sections hybridized with the sense probe (Figure 1D) ▶ . No MMP-13 mRNA could be detected in normal intact vulvar epithelium (Figure 1H) ▶ .
Figure 1.
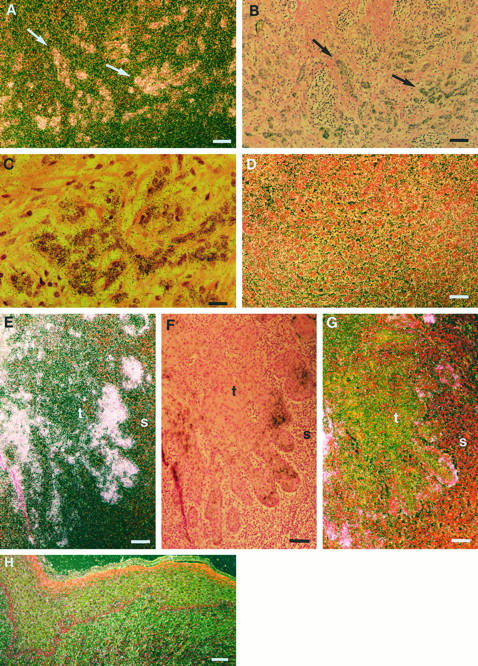
Expression of MMP-13 and MMP-1 in vulvar SCCs. A and B: Corresponding dark-field and bright-field exposures of in situ hybridization showing MMP-13 mRNA positive tumor cell islands (arrows) in primary vulvar SCC (patient 4). C: Higher magnification of B showing expression of MMP-13 mRNA in tumor cells. D: No signal is detected in a parallel section hybridized with the sense MMP-13 probe. E: Expression of MMP-13 mRNAs by tumor cells at the invasive front of a primary vulvar SCC (patient 3). F: Bright-field exposure of panel E. G: Dark-field exposure of a section parallel to that shown in panel F hybridized with MMP-1 antisense probe, showing MMP-1 mRNA in isolated tumor cells. H: Dark-field exposure of normal vulvar epithelium hybridized with MMP-13 antisense probe shows no signal. t, tumor; s, stroma. Bars: 24 μm (A, B, and D-H) and 6 μm (C).
High levels of MMP-13 mRNA expression were noted in three vulvar SCCs that showed metastasis to lymph nodes (stage N1 or N2) at the time of diagnosis, suggesting a role for MMP-13 in the high invasion capacity of these tumors (Figures 1, E and F, and 2, A, B, D, and E ▶ ▶ ; Table 1 ▶ ). Abundant MMP-13 expression was detected in both small (stage T1) and larger (stages T2 and T3) tumors, indicating that expression of MMP-13 is activated early in vulvar SCC development (Table 1) ▶ . In general, expression of MMP-13 was detected in well differentiated (grade 1) tumors, as well as in moderately (grade 2) and poorly (grade 3) differentiated tumors (Table 1) ▶ . However, differentiating tumor cells were devoid of MMP-13 mRNA, whereas expression of MMP-13 mRNA was detected in undifferentiated tumor cells (Figure 2, D and E) ▶ . Expression of MMP-13 was also examined by immunohistochemistry in five vulvar SCCs expressing high levels of MMP-13 mRNA, ie, from patients 2, 3, 4, 7, and 9. Staining for MMP-13 was noted in all these vulvar SCCs, primarily in tumor cells and occasionally in stromal fibroblasts (Figure 2H) ▶ . No staining was detected in negative controls using preimmune mouse ascites fluid instead of primary antibody (Figure 2I) ▶ .
Figure 2.
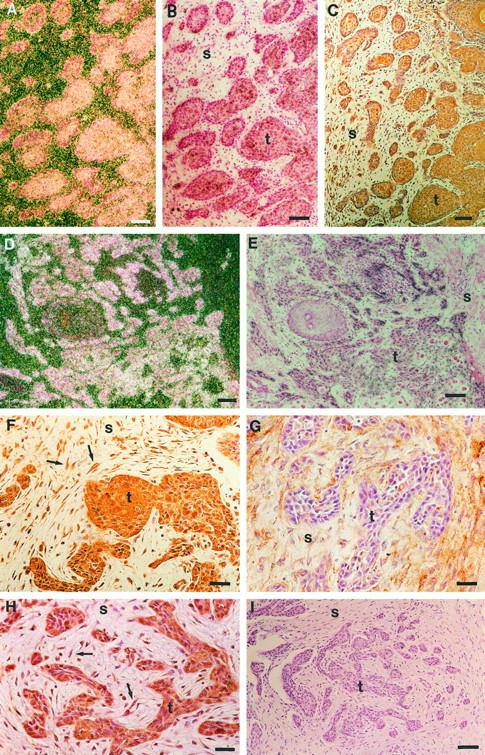
Colocalization of MMP-13, MT1-MMP, and MMP-2 expression in vulvar SCCs. A: Dark-field exposure of an in situ hybridization showing MMP-13 mRNA positive tumor cells in a primary vulvar SCC (patient 7). B: Bright-field photomicrograph corresponding to panel A. C: Immunostaining of a section parallel to that shown in panel B with anti-MT1-MMP antibody, showing MT1-MMP-positive tumor cells and stromal fibroblasts. D: Dark-field exposure showing expression of MMP-13 mRNA by tumor cells in a recurrent vulvar SCC (patient 9). E: Bright-field exposure corresponding to panel D. F: Immunostaining of a section parallel to that shown in panel E with anti-MT1-MMP antibody showing MT1-MMP-positive tumor cells and stromal fibroblasts (arrows). G: Immunostaining of a section parallel to that shown in panel E with anti-MMP-2 antibody showing MMP-2 expression in stromal cells and in tumor cells in the periphery of tumor islands. H: Immunostaining of a section parallel to that shown in panel E with anti-MMP-13 antibody showing MMP-13-positive tumor cells and stromal fibroblasts (arrows). I: Control immunostaining of section parallel to that shown in panel E using preimmune mouse ascites fluid in place of primary antibody shows no positive staining. t, tumor; s, stroma. Bars: 24 μm (A-E, I) and 10 μm (F-H).
Signal for collagenase-1 (MMP-1) mRNA was observed in 10 of 12 vulvar SCCs examined, 8 samples of tumor cells, and 6 samples of stromal cells (Table 1) ▶ . Of these, in two tumors MMP-1 mRNA was detected only in stromal cells. In many of the tumors examined, expression of MMP-1 in tumor cells was lower than MMP-13 expression (Figure 1, E ▶ -G). Of the 12 vulvar SCCs examined, two were entirely negative for both MMP-13 and MMP-1 mRNA (Table 1) ▶ . Intact vulvar epithelium was negative for MMP-1 mRNA (not shown).
Coordinate Expression of MMP-13 and MT1-MMP by Tumor Cells in Vulvar SCCs
Latent MMP-13 is activated by stromelysin-1 (MMP-3), membrane type 1 MMP (MT1-MMP), and gelatinase-A (MMP-2). 6,58 In this context, we also examined the expression of these MMPs in vulvar SCCs. Expression of MT1-MMP was detected by immunohistochemistry in all 10 vulvar SCCs examined (Table 1) ▶ . Interestingly, the expression of MT1-MMP was abundant in tumor cells, often noted in the regions with MMP-13-positive tumor cells in parallel sections (Figure 2, A ▶ -F and H). In addition, in certain tumors, stromal fibroblasts stained positive for MT1-MMP, although less intensely than tumor cells (Figure 2, C and F) ▶ . Expression of MMP-2 was also detected by immunohistochemistry in stromal cells of all 12 vulvar SCCs examined and in tumor cells in 9 SCCs (Table 1) ▶ . In contrast to MT1-MMP, staining for MMP-2 was often more pronounced in stromal fibroblasts than in tumor cells (Figure 2G) ▶ . MMP-2-positive cells were also detected in the vicinity of tumor cells expressing MMP-13 in parallel sections (Figure 2, D, E, and G) ▶ . These results show that the expression of MMP-13 is associated with the expression of MT1-MMP or MMP-2 in vulvar SCCs, providing an optimal environment for pericellular activation of tumor cell-derived latent MMP-13. Using in situ hybridization, expression of MMP-3 mRNA was detected in five tumors, all of which also expressed MMP-13 (Table 1) ▶ . However, the signal for MMP-3 mRNA was generally observed in isolated tumor cells and it did not seem to colocalize with that of MMP-13 (not shown).
Expression of MMP-9 by Tumor and Inflammatory Cells in Vulvar SCCs
As MMP-13 has been show to activate latent MMP-9, 59 we also examined the expression of MMP-9 in vulvar SCCs by immunostaining. Interestingly, MMP-9 expression was detected in mononuclear and polymorphonuclear inflammatory cells in stromal infiltrate adjacent to tumor in all nine SCCs examined (Table 1) ▶ . In addition, MMP-9-positive tumor cells were detected at the invading edge of six tumors (Figure 3A ▶ , Table 1 ▶ ). In certain vulvar SCCs, MMP-9-positive tumor cells or inflammatory cells colocalized with MMP-13-expressing tumor cells (Figure 3, A and B) ▶ . However, MMP-9-positive tumor and inflammatory cells were also detected in areas devoid of MMP-13 mRNA (not shown). Expression of matrilysin (MMP-7) was also detected by immunostaining in tumor cells of 3 of 5 vulvar SCCs examined (not shown).
Figure 3.
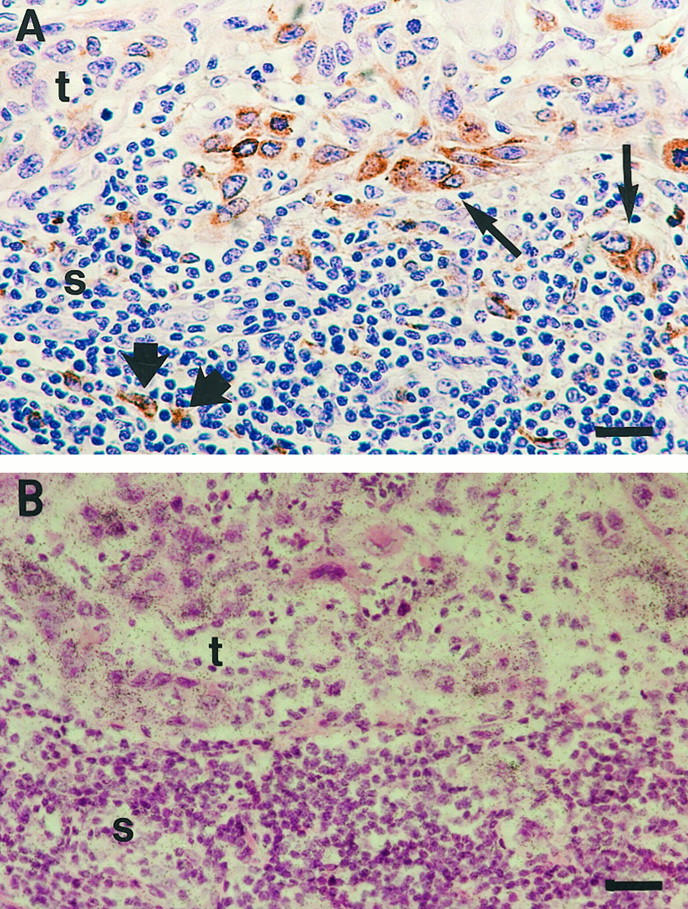
Colocalization of MMP-13- and MMP-9-expressing cells in vulvar SCCs. A: Immunostaining of a vulvar SCC (patient 3) with anti-MMP-9 antibody, showing MMP-9-positive tumor cells (thin arrows) and mononuclear inflammatory cells (thick arrows). B: In situ hybridization of a section parallel to that shown in A with MMP-13 antisense probe showing MMP-13 expression by tumor cells. Bars: 6 μm.
Expression of MMP-13 by Vulvar SCC Cell Lines
We also examined the expression of MMP-13 in 11 cell lines established from primary or recurrent vulvar SCCs or their metastases (Table 2) ▶ . As shown in Figure 4A ▶ and Table 2 ▶ , basal expression of MMP-13 mRNAs was detected in 9 of 11 vulvar SCC cell lines examined (3 of 5 cell lines from primary tumors and all 6 cell lines from recurrent tumors or metastases) (Table 2) ▶ . Basal expression of MMP-1 mRNA was detected in 4 of 11 SCC cell lines (Figure 4A ▶ , Table 2 ▶ ). Only 2 of 11 vulvar SCC cell lines were negative for both MMP-13 and MMP-1 (Table 1) ▶ . Interestingly, expression of MT1-MMP was detected in all vulvar SCC cell lines examined (Figure 4A ▶ , Table 2 ▶ ). Expression of MMP-9 was detected at basal level in one vulvar SCC cell line examined (Table 2) ▶ , whereas expression of MMP-7 was detected in 7 cell lines (Figure 4A ▶ , Table 2 ▶ ). In contrast, expression of MMP-3 or MMP-2 mRNA was not detected in any vulvar SCC cell line examined (Table 2) ▶ .
Figure 4.
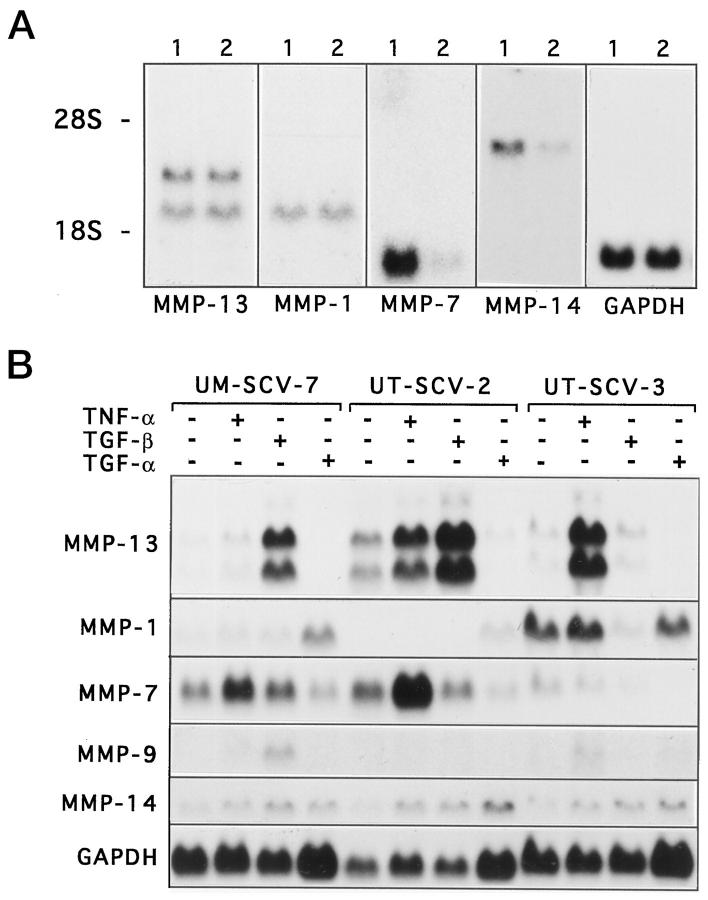
Expression of MMP-13 in cell lines from vulvar SCCs. A: Vulvar SCC cell lines UM-SCV-1B 1 and UM-SCV2 2 were cultured in DMEM supplemented with 6 mmol/L glutamine, non-essential amino acids, and 10% FCS. B: Cell lines UM-SCV-7, UT-SCV-2, and UT-SCV-3 derived from vulvar SCCs were incubated for 24 hours in serum-free medium alone (control) or in the presence of TNF-α (20 ng/ml), TGF-β1 (5 ng/ml), or TGF-α (10 ng/ml), as indicated. A, B: Total RNA was extracted and 20-μg aliquots were analyzed by Northern blot hybridizations for expression of MMP-13, MMP-1, MMP-7, MMP-9, MT1-MMP (MMP-14), and GAPDH mRNAs, as indicated. For details of the cell lines, see Table 2 ▶ .
Inactivation of p53 tumor suppressor gene by either mutation or oncogenic HPV E6 protein is often detected in vulvar SCC cells. 43,46,47 In our material, six of the vulvar SCC cell lines examined have mutations in p53 gene, four cell lines have wild-type p53 gene, and one cell line with wild-type p53 harbors HPV 16 DNA (Table 2) ▶ . 43,46,47 Interestingly, MMP-13 expression was detected in four vulvar SCC cell lines with p53 mutation and in one cell line that harbors HPV 16 DNA and has wild-type p53 gene (Table 2) ▶ . However, lack of MMP-13 expression in two cell lines with mutated p53 shows that inactivation of p53 is not alone sufficient for induction of MMP-13 expression (Table 2) ▶ . Furthermore, basal expression of MMP-13 mRNA was detected in all three cell lines with wild-type p53 gene and no HPV DNA, indicating that inactivation of p53 is not essential for induction of MMP-13 expression in vulvar SCC cells (Table 2) ▶ .
Enhancement of MMP-13 Expression in Vulvar SCC Cells by TNF-α and TGF-β
The expression of MMP-13 in cell lines from SCCs of the head and neck is enhanced by TNF-α, TGF-β, and TGF-α. 20 In this context we examined the regulation of MMP-13 gene expression in selected vulvar SCC cell lines by Northern blot hybridizations. Expression of MMP-13 mRNAs was enhanced by 2.5-fold and 17.5-fold by a 24-hour treatment with TNF-α (20 ng/ml) in cell lines UT-SCV-2 and UT-SCV-3, respectively (Figure 4B) ▶ . TGF-β1 (5 ng/ml), in turn, up-regulated expression of MMP-13 mRNAs in UM-SCV-7 and UT-SCV-2 cells by 32-fold and 6.3-fold, respectively (Figure 4B) ▶ . Interestingly, the expression of MMP-1 was not stimulated by TNF-α or TGF-β in any of the cell lines, and was even suppressed by 83% with TGF-β1 in cell line UT-SCV-3. In contrast, treatment of UM-SCV-7 and UT-SCV-2 cell lines by TGF-α (10 ng/ml) enhanced MMP-1 mRNA expression threefold and 3.9-fold, respectively (Figure 4B) ▶ . In comparison, MMP-7 mRNA abundance was up-regulated by TNF-α in UM-SCV-7 (2.1-fold) and UT-SCV-2 (1.9-fold) cells, and MMP-9 mRNA levels were stimulated by TGF-β1 in UM-SCV-7 cells (5.8-fold) and by TNF-α in UT-SCV-3 cells (4.6-fold) (Figure 4B) ▶ . Interestingly, MT1-MMP mRNA abundance was enhanced by TGF-β (up to 4.6-fold), in all three cell lines and by TNF-α (up to 3.7-fold) and TGF-α (up to 2.5-fold) in UT-SCV-2 and 3 cell lines (Figure 4B) ▶ .
Expression of MMP-13 in Cervical Carcinoma Cells
The expression of MMP-13 was also examined in cell lines established from cervical, endometrial, and ovarian carcinomas. Of the six cervical carcinoma cell lines examined, one (UM-GCC-1) was derived from glassy cell carcinoma and the remaining five from SCCs. None of the cervical carcinoma cell lines examined has mutations in p53 gene, but ME-180 cell line harbors HPV 68 DNA and other cell lines contain HPV 16 DNA. 43,44 Interestingly, 4 of 5 cervical SCC cell lines expressed clearly detectable basal levels of MMP-13 mRNAs, suggesting an association between oncogenic HPV infection and the ability to express MMP-13 (Figure 5) ▶ . However, no basal expression of MMP-13 gene was detected in cell lines Hx-156 and UM-GCC-1 harboring HPV 16 DNA, indicating that transformation by oncogenic HPV is not alone sufficient to turn on MMP-13 expression in cervical carcinoma cells (Figure 5) ▶ .
Figure 5.
Expression of MMP-13 mRNA in cell lines derived from cervical carcinomas. Cervical SCC cell lines and a glassy cell carcinoma cell line, UM-GCC-1, were cultured in DMEM supplemented with 6 mmol/L glutamine, non-essential amino acids, and 10% FCS. Total RNA was extracted and 15-μg aliquots were analyzed for expression of MMP-13 and GAPDH mRNA by Northern blot hybridizations.
In accordance with the lack of MMP-13 mRNA expression in endometrial and ovarian carcinomas in vivo, no MMP-13 mRNAs were detected in any endometrial (n = 10) or ovarian (n = 9) carcinoma cell line, either at basal level or after treatment with TNF-α, TGF-β, or TGF-α (not shown).
Expression of MMP-13 by Cell Lines from Vaginal Premalignant Lesions
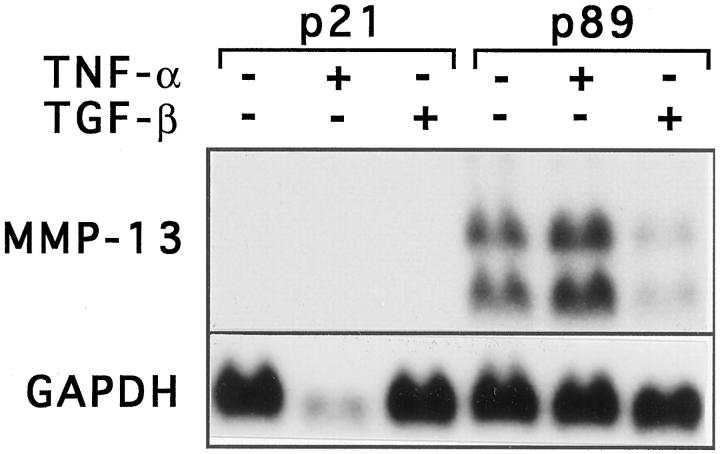
To further elucidate the role of oncogenic HPV in MMP-13 expression by transformed squamous epithelial cells, we examined two immortalized cell lines established from premalignant vaginal intraepithelial neoplasias. UT-DEC-1 and UT-DEC-2 express HPV 33 and HPV 16 E6/E7 mRNA, respectively, and contain wild-type p53 and retinoblastoma genes. 48,49 Neither cell line expressed MMP-13 mRNAs in passages 8 and 21 (UT-DEC-1) or in passages 10, 26, and 40 (UT-DEC-2), either at basal level or after treatment with TNF-α or TGF-β (Figure 6 ▶ and data not shown). These observations show that immortalization of squamous epithelial cells by oncogenic HPV does not alone render them capable of expressing MMP-13. UT-DEC-1 cell line was further examined at late subculture (passage 89), at which stage abundant expression of MMP-13 mRNAs was detected at basal level or after treatment with TNF-α (Figure 6) ▶ . In contrast, treatment of cells with TGF-β suppressed MMP-13 mRNA expression by 60% (Figure 6) ▶ . Interestingly, UT-DEC-1 cells at subculture 89 grow anchorage-independently in the presence of glucocorticoid and epidermal growth factor, 49 indicating that the ability to express MMP-13 is associated with selection of a malignant phenotype of these transformed vaginal epithelial cells.
Figure 6.
Expression of MMP-13 mRNA in a cell line derived from vaginal premalignant lesion. UT-DEC-1 cells established from a premalignant vaginal intraepithelial dysplasia were maintained in Keratinocyte Serum Free Medium (passage 21, p21) or serum-free DMEM (passage 89, p89) without or with TNF-α (20 ng/ml) or TGF-β1 (5 ng/ml), as shown, for 24 hours. Total RNA was extracted and 15-μg aliquots were analyzed for expression of MMP-13 and GAPDH mRNA by Northern blot hybridizations.
Discussion
In the present study, we show for the first time that the recently discovered human collagenase-3, MMP-13, is specifically expressed in tumor cells of vulvar SCCs, whereas no MMP-13 expression can be detected in intact vulvar epithelium, cervical SCCs, or endometrial or ovarian adenocarcinomas in vivo. Expression of MMP-13 mRNA was detected in vulvar and cervical SCC cell lines, but not in endometrial or ovarian carcinoma cell lines, suggesting that the ability to express MMP-13 is specific for malignant squamous epithelial cells. The expression of MMP-1 mRNA was also detected in tumor cells of vulvar SCCs in vivo and in culture, although the expression of MMP-1 in vivo was more often detected in stromal cells. It is likely that the combined collagenolytic activity of tumor-derived MMP-13 and MMP-1 produced by tumor or stromal cells plays an important role in SCC invasion. However, our results provide evidence that MMP-13 is the principal collagenase expressed by tumor cells in vulvar SCCs. Furthermore, in our material of 12 vulvar SCCs, all three tumors with lymph node metastases at the time of diagnosis showed high levels of MMP-13 mRNA, strongly suggesting a crucial role for MMP-13 in invasion and metastasis of vulvar carcinomas.
Expression of MT1-MMP was detected by immunostaining in tumor cells of all vulvar SCCs examined and MT1-MMP mRNA was also detected in all cell lines derived from vulvar SCCs, suggesting that MT1-MMP plays an important role in their invasion capacity. However, less intense immunostaining for MT1-MMP was also observed in stromal fibroblasts in a subset of vulvar SCCs. Interestingly, MT1-MMP-positive tumor cells often colocalized with SCC cells expressing MMP-13 mRNA. Staining for MMP-2 was also detected in all vulvar carcinomas examined, but it appeared to be more pronounced in stromal fibroblasts often detected in the vicinity of MMP-13-expressing tumor cells. In SCCs of the head and neck, expression of MMP-2 and MT1-MMP mRNA has previously been reported in stromal cells 60,61 and expression of MT1-MMP by tumor cells as well. 62 It is likely that colocalization of the cells expressing MT1-MMP and MMP-2 with tumor cells producing MMP-13 creates optimal conditions for pericellular activation of tumor cell-derived latent MMP-13. In addition, expression of MMP-3 and MMP-7 by some SCC tumor cells provides further activity for a tumor-driven proteolytic cascade, in which latent MMP-13 can be activated by MT1-MMP or MMP-3, both of which are expressed by tumor cells. Furthermore, in analogy with cutaneous SCCs, 60,63 expression of MMP-9 by invading tumor cells and adjacent tumor infiltrating inflammatory cells adds a further link to this MMP cascade, as MMP-13 can activate latent MMP-9. 59 Together all these observations provide evidence that efficient breakdown of the extracellular matrix surrounding invasive SCCs involves interplay between tumor cells, stromal cells, and inflammatory cells. Because there are differences in the substrate specificity of the MMPs present in the peritumoral environment of vulvar SCCs, it can be proposed that each of these MMPs has a distinct role in SCC invasion. Therefore, it is possible that specific inhibition of the expression or activity of even one of these MMPs, eg, MMP-13, could disturb the balance in this complex network of proteases and markedly inhibit invasion by SCC tumor cells.
Expression of MMP-13 was also detected in 4 of 5 cell lines derived from cervical SCCs, although no expression of MMP-13 could be detected in vivo in any of the cervical carcinomas in our material, which contained moderately and poorly differentiated tumors representing different stages of invasion. It is therefore possible that MMP-13 does not play a crucial role in invasion of cervical SCCs in vivo, but that selection of an MMP-13-expressing cell population may occur during subculturing of cervical SCC cells as a consequence of genomic instability. This notion is also supported by our observation that UT-DEC-1, a cell line derived from premalignant vaginal dysplasia, expressed MMP-13 only after it had been selected by subculturing to grow anchorage-independently.
Transformation of vulvar or cervical epithelial cells is often associated with inactivation of p53 tumor suppressor gene, either by mutation or by oncogenic HPV E6 protein. 43,46,47 In the present study, comparison of the p53 and HPV status of the vulvar SCC cell lines with their MMP-13 expression revealed detectable basal levels of MMP-13 mRNAs in cell lines with intact p53 gene and no HPV DNA, indicating that p53 inactivation is not essential for induction of MMP-13 expression in transformed vulvar squamous epithelial cells. In addition, no MMP-13 expression was detected in two vulvar SCC cell lines with mutated p53 gene, showing that inactivation of p53 does not alone render SCC cells capable of expressing MMP-13. Interestingly, one vulvar SCC cell line harboring HPV 16 DNA and wild-type p53 gene expressed abundant levels of MMP-13 mRNA. Furthermore, all cervical SCC cell lines expressing MMP-13 harbored HPV 16 or 68 DNA, suggesting an association between oncogenic HPV infection and induction of MMP-13 expression in vulvar and cervical SCC cells. However, no MMP-13 expression was detected either in two cervical carcinoma cell lines harboring HPV 16 DNA or in early subcultures of two HPV-positive cell lines from vaginal premalignant intraepithelial lesions, indicating that oncogenic HPV alone is not sufficient to induce MMP-13 expression. However, it is likely that loss of cellular growth control due to p53 inactivation and subsequent genomic instability plays an important role in the selection of an invasive, transformed squamous epithelial cell population capable of expressing MMP-13. In summary, these observations provide further evidence that MMP-13 expression is not simply an indicator for transformation of squamous epithelial cells, but may also serve as a marker for the invasive capacity of SCCs.
Expression of MMP-13 mRNAs in selected vulvar SCC cell lines, as in SCCs of the head and neck, is stimulated by TNF-α or TGF-β, which may play a role in stimulating vulvar SCC cells in vivo during ulceration and inflammation of the tumors. However, the response of the vulvar SCC cell lines to TNF-α and TGF-β appears variable, indicating heterogeneity between these cell lines. Because TGF-β is a potent inhibitor of epithelial cell growth, it is not surprising that malignant epithelial cells may lose their response to TGF-β in the process of malignant transformation. Nevertheless, these observations show that MMP-13 expression by vulvar SCC cells is susceptible to stimulation by TNF-α and TGF-β, both of which also enhance expression of MMP-1 by normal keratinocytes. 1,2,23 However, expression of MMP-1 mRNA by vulvar SCC cells was not enhanced by TNF-α or TGF-β, indicating differential regulation of MMP-13 and MMP-1 in vulvar SCC cells. Interestingly, expression of MT1-MMP mRNA in these vulvar SCC cells was stimulated by TGF-β and, in two cell lines, also by TNF-α and TGF-α, providing evidence that these inflammatory cell-derived modulators may also play a role in up-regulation of MT1-MMP expression in vulvar SCCs in vivo.
Invasion and metastasis of malignant cells is a multistep process involving detachment of cells from primary tumor, degradation of structural barriers such as basement membrane and collagenous extracellular matrix, and migration of cells through the degraded matrix. 1-3 It is evident that invasion of SCC cells in vivo involves interplay between tumor cells, stromal cells, and inflammatory cells, all of which can express a variable set of MMPs and contribute to degradation of stromal matrix components of SCCs. In this context, it is interesting that MMP-13 is specifically expressed by transformed squamous epithelial cells. Furthermore, lack of MMP-13 expression in normal keratinocytes both in vitro 23 and in vivo 15,20,21 provides strong evidence that MMP-13 is specifically activated in SCC cells as the primary tumor progresses toward an invasive phenotype. This notion is supported by our observation that cell lines established from premalignant vaginal intraepithelial neoplasias do not express MMP-13 until selection of a late subculture cell population capable of anchorage-independent growth. This hypothesis is also supported by our recent findings showing lack of MMP-13 expression in cutaneous premalignant tumors or in SCCs in situ. 21 These observations provide further evidence that MMP-13 expression is a marker for the invasion capacity of SCC cells in vivo. Finally, based on the observations of this study, it is conceivable that unveiling the regulatory mechanisms of MMP-13 gene expression in SCC cells may prove beneficial in developing novel therapeutic modalities to combat invasion and metastasis of these neoplastic cells.
Acknowledgments
The expert technical assistance of Ms. Marita Potila, Ms. Eeva Virtanen, and Ms. Alli Tallqvist is gratefully acknowledged. We thank Drs. E. Bauer, G. Goldberg, M. Kurkinen, and P. Fort for plasmids.
Footnotes
Address reprint requests to Veli-Matti Kähäri M.D., Ph.D., University of Turku, MediCity Research Laboratory, Tykistökatu 6, FIN-20520 Turku, Finland. E-mail: veli-matti.kahari@utu.fi.
Supported by grants from the Academy of Finland, Sigrid Jusélius Foundation, Cancer Research Foundation of Finland, Turku University Foundation, Paulo Foundation, and the Turku and Helsinki University Central Hospital Research Foundations.
References
- 1.Birkedal-Hansen H, Moore WGI, Bodden MK, Windsor LJ, Birkedal-Hansen B, De Carlo A, Engler JA: Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 1993, 4:197-250 [DOI] [PubMed] [Google Scholar]
- 2.Kähäri V-M, Saarialho-Kere U: Matrix metalloproteinases in skin. Exp Dermatol 1997, 6:199-213 [DOI] [PubMed] [Google Scholar]
- 3.Stetler-Stevenson WG, Aznavoorian S, Liotta L: Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 1993, 9:541-573 [DOI] [PubMed] [Google Scholar]
- 4.Krane SM, Byrne MH, Lemaitre V, Henriet P, Jeffrey JJ, Witter JP, Liu X, Wu H, Jaenisch R, Eeckhout Y: Different collagenase gene products have different roles in degradation of type I collagen. J Biol Chem 1996, 271:28509-28515 [DOI] [PubMed] [Google Scholar]
- 5.Freije JMP, Díez-Itza I, Balbín M, Sánchez LM, Blasco R, Tolivia J, López-Otín C: Molecular cloning, and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem 1994, 269:16766-16773 [PubMed] [Google Scholar]
- 6.Knäuper V, López-Otín C, Smith B, Knight G, Murphy G: Biochemical characterization of human collagenase-3. J Biol Chem 1996, 271:1544-1550 [DOI] [PubMed] [Google Scholar]
- 7.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE: Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest 1996, 97:761-768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knäuper V, Cowell S, Smith B, López-Otín C, O’Shea M, Morris H, Zardi L, Murphy G: The role of the C-terminal domain of human collagenase-3 (MMP-13) in activation of procollagenase-3, substrate specificity, and tissue inhibitor of metalloproteinase interaction. J Biol Chem 1997, 272:7608-7616 [DOI] [PubMed] [Google Scholar]
- 9.Fosang AJ, Last K, Knäuper V, Murphy G, Neame PJ: Degradation of cartilage aggrecan by collagenase-3 (MMP-13). FEBS Lett 1996, 380:17-20 [DOI] [PubMed] [Google Scholar]
- 10.Johansson N, Saarialho-Kere U, Airola K, Herva R, Nissinen L, Westermarck J, Vuorio E, Heino J, Kähäri V-M: Collagenase-3 (MMP-13) is expressed by hypertrophic chondrocytes, periosteal cells, and osteoblasts during human fetal bone development. Dev Dyn 1997, 208:387-397 [DOI] [PubMed] [Google Scholar]
- 11.Ståhle-Bäckdahl M, Sandstedt B, Bruce K, Lindahl A, Jimenez MG, Vega JA, López-Otín C: Collagenase-3 (MMP-13) is expressed during human fetal ossification and re-expressed in postnatal bone remodeling and in rheumatoid arthritis. Lab Invest 1997, 76:717-728 [PubMed] [Google Scholar]
- 12.Reboul P, Pelletier J-P, Tardif G, Cloutier J-M, Martel-Pelletier J: The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not synoviocytes. A role in osteoarthritis. J Clin Invest 1996, 97:2011-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wernicke D, Seyfert C, Hinzmann B, Gromnica-Ihle E: Cloning of collagenase 3 from synovial membrane and its expression in rheumatoid arthritis and osteoarthritis. J Rheumatol 1996, 23:590-595 [PubMed] [Google Scholar]
- 14.Lindy O, Konttinen YT, Sorsa T, Ding Y, Santavirta S, Ceponis A, López-Otín C: Matrix metalloproteinase-13 (collagenase-3) in human rheumatoid synovium. Arthritis Rheum 1997, 40:1391-1399 [DOI] [PubMed] [Google Scholar]
- 15.Vaalamo M, Mattila L, Johansson N, Kariniemi A-L, Karjalainen-Lindsberg M-L, Kähäri V-M, Saarialho-Kere U: Distinct populations of stromal cells express collagenase-3 (MMP-13) and collagenase-1 (MMP-1) in chronic ulcers but not in normally healing wounds. J Invest Dermatol 1997, 109:96-101 [DOI] [PubMed] [Google Scholar]
- 16.Vaalamo M, Karjalainen-Lindsberg ML, Puolakkainen P, Kere J, Saarialho-Kere U: Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13), macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinases-3 (TIMP-3) in intestinal ulcerations. Am J Pathol 1998, 152:1005-1014 [PMC free article] [PubMed] [Google Scholar]
- 17.Uitto V-J, Airola K, Vaalamo M, Johansson N, Putnins E, Firth JD, Salonen J, López-Otín C, Saarialho-Kere U, Kähäri V-M: Collagenase-3 (matrix metalloproteinase-3) expression is induced in oral mucosal epithelium during chronic inflammation. Am J Pathol 1998, 152:1489-1499 [PMC free article] [PubMed] [Google Scholar]
- 18.Heppner KJ, Matrisian LM, Jensen RA, Rodgers WH: Expression of most matrix metalloproteinases family members in breast cancer represents a tumor-induced host response. Am J Pathol 1996, 149:273-282 [PMC free article] [PubMed] [Google Scholar]
- 19.Uría A, Ståhle-Bäckdahl M, Seiki M, Fueyo A, López-Otín C: Regulation of collagenase-3 expression in human breast carcinomas is mediated by stromal-epithelial cell interactions. Cancer Res 1997, 57:4882-4888 [PubMed] [Google Scholar]
- 20.Johansson N, Airola K, Grénman R, Kariniemi A-L, Saarialho-Kere U, Kähäri V-M: Expression of collagenase-3 (matrix metalloproteinase-13) in squamous cell carcinomas of the head and neck. Am J Pathol 1997, 151:499-508 [PMC free article] [PubMed] [Google Scholar]
- 21.Airola K, Johansson N, Kariniemi A-L, Kähäri V-M, Saarialho-Kere U: Human collagenase-3 is expressed in malignant squamous epithelium of the skin. J Invest Dermatol 1997, 109:225-231 [DOI] [PubMed] [Google Scholar]
- 22.Uría JA, Balbin M, López JM, Alvarez J, Vizoso F, Takigawa M, López-Otín C: Collagenase-3 (MMP-13) expression in chondrosarcoma cells and its regulation by basic fibroblast growth factor. Am J Pathol 1998, 153:91-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson N, Westermarck J, Leppä S, Häkkinen L, Koivisto L, López-Otín C, Peltonen J, Heino J, Kähäri V-M: Collagenase-3 (MMP-13) gene expression by HaCaT keratinocytes is enhanced by tumor necrosis factor-α and transforming growth factor-β. Cell Growth Differ 1997, 8:243-250 [PubMed] [Google Scholar]
- 24.Inoue Y, Abe K, Obata K, Yoshioka T, Ohmura G, Doh K, Yamamoto K, Hoshiai H, Noda K: Immunohistochemical studies on matrix metalloproteinase-9 (MMP-9) and type IV collagen in endometrial carcinoma. J Obstet Gynecol Res 1997, 23:139-145 [DOI] [PubMed] [Google Scholar]
- 25.Tamakoshi K, Kikkawa F, Nawa A, Ishikawa H, Mizuno K, Tamakoshi A, Yamagata S, Suganuma N, Tomoda Y: Characterization of extracellular matrix-degrading proteinase and its inhibitor in gynecological cancer tissues with clinically different metastatic form. Cancer 1995, 76:2565-2571 [DOI] [PubMed] [Google Scholar]
- 26.Soini Y, Alarakkola E, Autio-Harmainen H: Expression of messenger RNAs for metalloproteinases 2 and 9, Type IV collagen, and laminin in nonneoplastic and neoplastic endometrium. Hum Pathol 1996, 28:220-226 [DOI] [PubMed] [Google Scholar]
- 27.Gilles C, Polette M, Piette J, Munaut C, Thompson EW, Birembaut P, Foidart JM: High level of MT-MMP expression is associated with invasiveness of cervical cancer cells. Int J Cancer 1996, 65:209-213 [DOI] [PubMed] [Google Scholar]
- 28.Nuovo GJ, MacConnell PB, Simsir A, Valea F, French DL: Correlation of the in situ detection of polymerase chain reaction-amplified metalloproteinase complementary DNAs and inhibitors with prognosis in cervical carcinoma. Cancer Res 1995, 55:267-275 [PubMed] [Google Scholar]
- 29.Nuovo GJ: In situ detection of PCR-amplified metalloproteinase cDNAs, their inhibitors, and human papillomavirus transcripts in cervical carcinoma cell lines. Int J Cancer 1997, 71:1056-1060 [DOI] [PubMed] [Google Scholar]
- 30.De Nictolis M, Garbisa S, Lucarini G, Goteri G, Masiero L, Ciavattini A, Garzetti GG, Stetler-Stevenson WG, Fabris G, Biagini G, Prat J: 72-kilodalton type IV collagenase, type IV collagen, and Ki 67 antigen in serous tumors of the ovary: a clinicopathologic, immunohistochemical, and serological study. Int J Gynecol Pathol 1996, 15:102-109 [DOI] [PubMed] [Google Scholar]
- 31.Moser TL, Young TN, Rodriguez GC, Pizzo SV, Bast RC, Jr, Stack MS: Secretion of extracellular matrix-degrading proteinases is increased in epithelial ovarian carcinoma. Int J Cancer 1994, 56:552-559 [DOI] [PubMed] [Google Scholar]
- 32.Afzal S, Lalani EN, Poulsom R, Stubbs A, Rowlinson G, Sato H, Seiki M, Stamp GWH: MT1-MMP, and MMP-2 mRNA expression in human ovarian tumors: possible implications for the role of desmoplastic fibroblasts. Hum Pathol 1998, 29:155-165 [DOI] [PubMed] [Google Scholar]
- 33.Autio-Harmainen H, Karttunen T, Hurskainen T, Höyhtyä M, Kauppila A, Tryggvason K: Expression of 72 kilodalton type IV collagenase (gelatinase A) in benign and malignant ovarian tumors. Lab Invest 1993, 69:312-321 [PubMed] [Google Scholar]
- 34.Fishman DA, Bafetti LM, Stacks MS: Membrane-type matrix metalloproteinase expression and matrix metalloproteinase-2 activation in primary human ovarian epithelial carcinoma cells. Invasion Metastasis 1996, 16:150-159 [PubMed] [Google Scholar]
- 35.Prosser IW, Stenmark KR, Suthar M, Crouch EC, Mecham RP, Parks WC: Regional heterogeneity of elastin and collagen gene expression in intralobar arteries in response to hypoxic pulmonary hypertension as demonstrated by in situ hybridization. Am J Pathol 1989, 135:1073-1088 [PMC free article] [PubMed] [Google Scholar]
- 36.Saarialho-Kere UK, Kovacs SO, Pentland AP, Olerud JE, Welgus HG, Parks WC: Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J Clin Invest 1993, 92:2858-2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saarialho-Kere UK, Pentland AP, Birkedal-Hansen H, Parks WC, Welgus HG: Distinct populations of basal keratinocytes express stromelysin-1 and stromelysin-2 in chronic wounds. J Clin Invest 1994, 94:79-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikkari ST, Höyhtyä M, Isola J, Nikkari T: Macrophages contain 92-kd gelatinase (MMP-9) at the site of degenerated internal elastic lamina in temporal arteritis. Am J Pathol 1996, 149:1427-1433 [PMC free article] [PubMed] [Google Scholar]
- 39.Ueno H, Nakamura H, Inoue M, Imai K, Noguchi K, Sato H, Seiki M, Okada Y: Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in human invasive breast cancer. Cancer Res 1997, 57:2055-2060 [PubMed] [Google Scholar]
- 40.Busiek DF, Ross FP, McDonnell S, Murphy G, Matrisian LM, Welgus HG: The matrix metalloproteinase matrilysin (PUMP) is expressed in developing human mononuclear phagocytes. J Biol Chem 1992, 13:9087-9092 [PubMed] [Google Scholar]
- 41.Grénman SE, van Dyke DL, Worsham MJ, England B, McClatchey KD, Hopkins M, Babu VR, Grénman R, Carey T: Phenotypic characterization, karyotype analysis and in vitro tamoxifen sensitivity of new ER-negative vulvar carcinoma cell lines, UM-SCV-1A and UM-SCV-1B. Int J Cancer 1990, 45:920-927 [DOI] [PubMed] [Google Scholar]
- 42.Worsham M, van Dyke D, Grénman S, Grénman R, Hopkins M, Roberts J, Gasser K, Schwartz DR, Carey T: Consistent chromosomal abnormalities in squamous carcinoma of the vulva. Genes Chromosomes Cancer 1991, 3:420-432 [DOI] [PubMed] [Google Scholar]
- 43.Rantanen V, Grénman S, Kurvinen K, Hietanen S, Raitanen M, Syrjänen S: p53 mutations and presence of HPV DNA does not correlate with radiosensitivity of gynecological cancer cell lines. Gynecol Oncol 1998, 71:352-358 [DOI] [PubMed] [Google Scholar]
- 44.Rantanen V, Grénman S, Kulmala J, Grénman R: The intrinsic radiosensitivity and sublethal damage repair capacity of five cervical carcinoma cell lines tested with the 96-well-plate assay. J Cancer Res Clin Oncol 1995, 121:230-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rantanen V, Grénman S, Kulmala J, Grénman R: Comparative evaluation of cisplatin and carboplatin sensitivity in endometrial adenocarcinoma cell lines. Br J Cancer 1994, 69:482-486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hietanen S, Kurvinen K, Syrjänen K, Grénman S, Carey T, McClatchey K, Syrjänen S: Mutation of tumor suppressor gene p53 is frequently found in vulvar carcinoma cells. Am J Obstet Gynecol 1995, 173:1477-1482 [DOI] [PubMed] [Google Scholar]
- 47.Hietanen S, Grénman S, Syrjänen K, Lappalainen K, Kauppinen J, Carey T, Syrjänen S: Human papillomavirus in vulvar and vaginal carcinoma cell lines. Br J Cancer 1995, 72:134-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hietanen S, Auvinen E, Grénman S, Lakkala T, Sajantila A, Klemi P, Mäenpää J: Isolation of two keratinocyte cell lines derived from HPV-positive dysplastic vaginal lesions. Int J Cancer 1992, 52:391-398 [DOI] [PubMed] [Google Scholar]
- 49.Hietanen S, Syrjänen K, Syrjänen S: Characterization of keratin and cell cycle protein expression in cell lines from squamous intraepithelial lesions progressing towards a malignant phenotype. Br J Cancer 1998, 77:766-775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 51.Goldberg GI, Wilhelm SM, Kronberger A, Bauer EA, Grant GA, Eisen AZ: Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem 1986, 261:6600-6605 [PubMed] [Google Scholar]
- 52.Saus J, Quinones S, Otani Y, Nagase H, Harris ED, Jr, Kurkinen M: The complete structure of human matrix metalloproteinase-3. J Biol Chem 1988, 263:6742-6747 [PubMed] [Google Scholar]
- 53.Collier IE, Wilhelm SM, Eisen AZ, Marmer BL, Grant GA, Seltzer JL, Kronberger A, He CS, Bauer EA, Goldberg GI: H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem 1988, 263:6579-6587 [PubMed] [Google Scholar]
- 54.Wilhelm SM, Collier IE, Marmer BL, Eisen AZ, Grant GA, Goldberg GI: SV40-transformed human lung fibroblasts secrete a 92-kd type IV collagenase which is identical to that secreted by normal macrophages. J Biol Chem 1989, 264:17213-17221 [PubMed] [Google Scholar]
- 55.Lohi J, Lehti K, Westermarck J, Kähäri V-M, Keski-Oja J: Regulation of membrane type metalloproteinase-1 expression by growth factors and phorbol 12-myristate 13-acetate. Eur J Biochem 1996, 239:239-247 [DOI] [PubMed] [Google Scholar]
- 56.Quantin B, Murphy G, Breatnach R: Pump-1 cDNA codes for a protein with characteristics similar to those of classical collagenase family members. Biochemistry 1989, 28:5327-5334 [DOI] [PubMed] [Google Scholar]
- 57.Fort P, Marty L, Piechaczyk M, El Sabrouty S, Dani C, Jeanteur P, Blanchard JM: Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res 1985, 13:1431-1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knäuper V, Will H, López-Otín C, Smith B, Atkinson SJ, Stanton H, Hembry RM, Murphy G: Cellular mechanisms for human procollagenase-3 activation. Evidence that MT1-MMP (MMP-14) and gelatinase A (MMP-2) are able to generate active enzyme. J Biol Chem 1996, 271:17124-17131 [DOI] [PubMed] [Google Scholar]
- 59.Knäuper V, Smith B, López-Otín C, Murphy G: Activation of progelatinase B (proMMP-9) by active collagenase-3 (MMP-13). Eur J Biochem 1997, 248:369-373 [DOI] [PubMed] [Google Scholar]
- 60.Pyke C, Ralfkiaer E, Huhtala P, Hurskainen T, Dano K, Tryggvason K: Localization of messenger RNA for Mr 72,000 and 92,000 type IV collagenases in human skin cancers by in situ hybridization. Cancer Res 1992, 52:1336-1341 [PubMed] [Google Scholar]
- 61.Okada A, Bellocq JP, Royer N, Chenard MP, Rio MC, Chambon P, Basset P: Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Natl Acad Sci USA 1995, 92:2730-2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshizaki T, Sato H, Maruyama Y, Murono S, Furukawa M, Park CS, Seiki M: Increased expression of membrane type 1-matrix metalloproteinase in head and neck carcinoma. Cancer 1997, 79:139-144 [DOI] [PubMed] [Google Scholar]
- 63.Ståhle-Bäckdahl M, Parks WC: 92-kd gelatinase is actively expressed by eosinophils and stored by neutrophils in squamous cell carcinoma. Am J Pathol 1993, 142:995-1000 [PMC free article] [PubMed] [Google Scholar]