Abstract
Matrix metalloproteinases (MMPs) are believed to contribute to the complex process of cancer progression. They also exhibit an α1-proteinase inhibitor (αPI)-degrading activity generating a carboxyl-terminal fragment of ∼5 kd (αPI-C). This study reports that overexpression of αPI-C in S2–020, a cloned subline derived from the human pancreas adenocarcinoma cell line SUIT-2, potentiates the growth capability of the cells in nude mice. After stable transfection of a vector containing a chimeric cDNA encoding a signal peptide sequence of tissue inhibitor of metalloproteinase-1 followed by cDNA for αPI-C into S2–020 cells, three clones that stably secrete αPI-C were obtained. The ectopic expression of αPI-C did not alter in vitro cellular growth. However, subcutaneous injection of the αPI-C-secreting clones resulted in tumors that were 1.5 to 3-fold larger than those of control clones with an increased tendency to invasiveness and lymph node metastasis. These effects could be a result of modulation of natural killer (NK) cell-mediated control of tumor growth in nude mice, as the growth advantage of αPI-C-secreting clones was not observed in NK-depleted mice, and αPI-C-secreting clones showed decreased NK sensitivity in vitro. In addition, production of αPI and generation of the cleaved form of αPI by MMP were observed in various human tumor cell lines and in a highly metastatic subline of SUIT-2 in vitro. These results provide experimental evidence that the αPI-degrading activity of MMPs may play a role in tumor progression not only via the inactivation of αPI but also via the generation of αPI-C.
It is generally accepted that a family of structurally related neutral metalloproteinases called matrix metalloproteinases (MMPs) have an important role in tumor progression, particularly in invasive/metastatic events via their degrading activity against various extracellular matrix (ECM) proteins. 1 Recently, a number of researchers have reported that serine proteinase inhibitors (serpins) are also good substrates for MMPs. 2-6 We have previously reported that many tumor cell lines produce and secrete serpins such as α1-proteinase inhibitor (αPI). 7 αPI, usually called α1-antitrypsin, is a major plasma serpin that has a broad inhibitory spectrum against serine proteinases and is the primary physiological inhibitor of leukocyte elastase. Among MMPs, interstitial collagenase (MMP-1, EC 3.4.24.7), neutrophil collagenase (MMP-8, EC 3.4.24.34), stromelysin-1 (MMP-3, EC 3.4.24.17), matrilysin (MMP-7, EC 3.4.24.23), and stromelysin-3 (MMP-11) effectively cleave αPI. 2-6 Of these, matrilysin exhibits the most efficient αPI-degrading activity, 4, 5 and stromelysin-3 is more potent than collagenases and stromelysin-1. 6 Moreover, a matter of importance is that the mature forms of human stromelysin-3 exhibit a very limited substrate specificity and appear unable to degrade any major ECM component. 6 The cleavages of αPI by these MMPs occur at peptide bonds within αPI’s active-site loop, 2-6 resulting in the inactivation of the inhibitory activity and release of a carboxyl-terminal (C-terminal) fragment of ∼5 kd (αPI-C) that is thought to be largely concealed in a native αPI. We have found that the tumor-cell-derived αPI and its cleaved form, which is ∼5 kd smaller than the noncleaved αPI, were concomitantly present in the serum-free culture conditioned media of the tumor cell lines. 7 These observations led to the postulation that the MMP-dependent hydrolysis of αPI and the subsequent generation of αPI-C may occur in a tumor-cell microenvironment.
To date, little is known about the biological significance of αPI and the αPI-degrading activity of MMPs such as matrilysin and stromelysin-3 in tumors, and rather surprisingly, patients with immunohistochemically αPI-positive adenocarcinomas had worse prognosis than the negative ones. 8-10 On the other hand, the ectopic expression of human stromelysin-3 in MCF-7 breast carcinoma cells resulted in enhanced tumorigenicity of the cells in nude mice. 11 Similarly, overexpression of matrilysin in a colon carcinoma cell line was found to increase its tumorigenicity in nude mice without modulation of the invasive property in vitro, 12 and intestinal tumorigenesis was suppressed in mice lacking matrilysin. 13 This evidence suggests that MMPs such as stromelysin-3 and matrilysin somehow favor the survival and growth of the cancer cells in a tissue microenvironment in vivo, possibly by functioning in an undefined capability independent of ECM degradation. 14
One of the key events in the survival and growth of cancer cells in vivo is a resistance to the host immune system. Natural cytotoxicity, mediated by natural killer (NK) cells and lymphokine-activated killer (LAK) cells, plays an important role in the host defense mechanism against cancer cells. 15 As NK activity within tumors of patients with cancer is lower than that found in the peripheral blood, the presence of tumor-derived suppressor factors has been suggested. 15 Cercek et al 16,17 have purified a peptide that could have immunosuppressive effects from sera of patients bearing various solid cancers. This peptide, designated as CRISPP (cancer recognition, immune defense suppression, and serine protease protection peptide), was reported to have unique reversible suppressive effects on NK and LAK cells in vitro. 17 Of particular interest is that the amino acid sequence of the CRISPP is highly homologous (83% to 100%) to that of the C-terminal part of αPI (Met358-Gln393). 16 As this C-terminal part overlaps in αPI-C generated by the MMP-dependent hydrolysis of αPI, it can be hypothesized that αPI-C may have suppressive activity against NK and LAK cells and that MMPs, particularly matrilysin and stromelysin-3, may contribute to the survival of cancer cells in vivo through the generation of αPI-C from the tumor-cell-derived and/or host-derived αPI. However, the biological significance of αPI-C in vivo is yet to be clarified. To confirm the above hypothesis in vivo, we have attempted to construct an αPI-C expression/secretion vector to examine the effects of αPI-C in vivo. The generation of αPI-C by tumor cells enhanced the growth and invasiveness of the tumor cells in nude mice.
Materials and Methods
Cell Lines and Culture
All cultured cells are derived from cells of human origin. Cloned sublines S2–020, S2–007 and S2–028 were derived from a single pancreas adenocarcinoma cell line, SUIT-2. 18 S2–020 formed poorly differentiated adenocarcinoma in the nude mouse. 18 It was low metastatic in a spontaneous metastasis assay in nude mice but was highly invasive in vitro. 19,20 S2–007 formed moderately differentiated adenocarcinoma and was highly metastatic in nude mice but was low invasive in vitro. 18-20 S2–028 formed well differentiated adenocarcinoma and was nonmetastatic in nude mice and noninvasive in vitro. 19,20 These clones were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 5% fetal bovine serum (FBS). S2–020 was used for the transfection study described below. In addition to the above-described SUIT-2 subclones, a normal fetal intestinal cell line (FHs74 int), a fibroblast cell line (Flow 2000), 7 colorectal adenocarcinoma cell lines (RCM-1, -2, and -3, CoCM-1, SW837, WiDr, and Colo 205), and 2 gastric adenocarcinoma cell lines (MKN-45 and -28), another SUIT-2 subline (S2–013), a renal cell carcinoma line (MRT-1), lung adenocarcinoma and squamous-cell carcinoma lines (LC-2/ad and LC-1/sq), 3 urinary bladder transitional-cell carcinoma lines (UMK-1 and -2 and T-24), a fibrosarcoma cell line (HT1080), an osteosarcoma cell line (OST), a primary culture of chordoma, and a glioblastoma cell line (MGM-1) were used. FHs74 int, WiDr, SW837, and Colo 205 were obtained from Dainihon Seiyaku (Osaka, Japan). MKN-45 and -28 were from IBL (Fujioka, Japan). Flow 2000 was from Japanese Cancer Research Resources Bank (Osaka, Japan). T-24 and OST were from RIKEN Cell Bank (Tsukuba, Japan). HT1080 was a kind gift from Dr. J. Suzumiya, Fukuoka University, Fukuoka, Japan. The other cell lines were established in our laboratory. To obtain serum-free conditioned medium (SFCM), confluent cells were washed three times with serum-free DMEM and cultured in it for 24 hours. To determine effects of tissue inhibitor of metalloproteinase-1 (TIMP-1), 2 μg/ml recombinant human TIMP-1 (rTIMP-1, Fuji Chemical Industries, Toyama, Japan) was added into the serum-free culture medium. Alternatively, the cells were transiently transfected with an expression plasmid pSG5 (Stratagene, La Jolla, CA) harboring a full-length cDNA for human TIMP-1 (pSG-TIMP), using lipofectamine reagent (Gibco-BRL, Gaithersburg, MD). Twenty-four hours after the transfection procedure, the cells were washed three times with serum-free medium, and SFCM was collected as described above. The enhanced secretion of TIMP-1 was confirmed by immunoblot analysis with anti-human TIMP-1 antibody (Fuji Chemical Industries) and by a TIMP-1 ELISA system (Amersham, Little Chalfont, UK). To examine in vitro growth characteristics, replicate 35-mm dishes were seeded at 2 × 10 5 cells/3 ml of growth medium. The number of viable cells was counted daily, and doubling time was determined during the log-phase of growth.
Construction of αPI-C Expression/ Secretion Vector
In an attempt to generate an αPI-C expression/secretion vector, a chimeric cDNA encoding a signal peptide sequence of TIMP-1 followed by cDNA for αPI-C, which is the C-terminal region of αPI (Met358-Arg394), with a stop codon (TAA) at the 3′ terminus was constructed. First, cDNA corresponding to αPI-C including the stop codon was obtained by a reverse transcription polymerase chain reaction (RT-PCR) using poly A+ RNA obtained from cultured RCM-1 human rectal adenocarcinoma cell line that synthesizes and secretes αPI in vitro. 21 A 37-mer forward primer (Atim 1), composed of a 17-mer that corresponded to the 3′ terminus of the signal sequence of TIMP-1 followed by a 20-mer corresponding to the 5′ terminus of αPI-C, and a 29-mer reverse primer (Atim 2), composed of a 9-mer carrying a SalI site followed by a 20-mer corresponding to the 3′ terminus of αPI-C, including the stop codon, were used. Sequences of Atim 1 and Atim 2 are 5′-ATAGCCCCAGCAGGGCCATGTCTATCCCCCCAGAGGT-3′ and 5′-GCGGTCGACTTATTTTTGGGTGGGATTCA-3′, respectively. Second, cDNA corresponding to the signal peptide sequence of TIMP-1 was amplified. A plasmid, pSG-TIMP, was used as the template. A 29-mer forward primer (Atim 3), composed of a 9-mer carrying an XhoI site followed by a 4-mer corresponding to the untranslated region upstream from the translational start site compatible with Kozak’s rule and a 16-mer corresponding to the 5′ terminus of the signal peptide sequence of TIMP, and a 20-mer reverse primer (Atim 4) corresponding to the 3′ terminus of the signal peptide sequence of TIMP. Sequences of Atim 3 and Atim 4 are 5′-CCGCTCGAGCCACCATGGCCCCCTTTGAG-3′ and 5′-GGCCCTGCTGGGGGCTATCA-3′, respectively. Third, the gel-purified products from both of the above reactions were used as templates for fusion by overlap extension using PCR. For this reaction, Atim 3 and Atim 2 were used. The 206-bp DNA product, designated as ATIM, that was generated in this PCR was ethanol precipitated and agarose gel purified. In the fourth and final reaction, the product was double digested by SalI and XhoI, ethanol precipitated, agarose gel purified, and subcloned into pCI-neo (Promega, Madison, WI) expression vector to create plasmid pCI-neoATIM. The inserted sequence was confirmed by a double-strand DNA sequencing of the plasmid (see Figure 3B ▶ ).
Figure 3.
Construction of αPI-C expression/secretion vector (pCI-neoATIM) and selection of αPI-C secreting clones. A: Schematic representation of the MMP cleavage sites in αPI. 2-6 B: Sequence of chimeric DNA, designated as ATIM, consisting of signal sequence of TIMP-1 (underline) and cDNA for αPI-C corresponding to Met358-Lys394 of αPI. This DNA was subcloned into the XhoI-Sal I site of an expression vector, pCI-neo, to generate pCI-neoATIM. The immediate-early enhancer/promoter region of the human cytomegalovirus controls the expression of the inserted DNA. C: Secretion of αPI-C into the serum-free conditioned medium of each clone. Representative results for 10 clones, including parent S2–020, are shown. Ten mock-transfected clones (pCI 1 to 10) and 20 pCI-neoATIM transfected clones (ATIM 1 to 20) were cloned after the selection using geneticin. Among ATIM clones, three clones (11, 1, and 8) secreted notable levels of αPI-C compared with the parent S2–020 and mock-transfected clones. *P < 0.05; **P < 0.0001, compared with parent and pCI clones (one-way ANOVA, Fisher’s PLSD test). D: RNA blot analysis of poly(A)+-enriched RNA from cultured cells (2 μg/lane). Hybridization was performed with αPI-C and GAPDH probes. Duration of autoradiographic exposure was 24 and 6 hours, respectively.
Selection of αPI-C-Secreting Stable Transfectants
S2–020 cells were transfected with pCI-neoATIM linearized by BamHI using lipofectamine reagent. Stable transfectants were selected with geneticin (0.5 mg/ml; Gibco-BRL), and isolated clones were obtained by ring cloning. For control, S2–020 cells were similarly transfected with linearized pCI-neo carrying no exogenous DNA, and the stable transfectants were cloned. Twenty clones of pCI-neoATIM-transfected (ATIM 1 to 20), and 10 clones of the mock-transfected control (pCI 1 to 10) were isolated. Each clone was cultured, and SFCM was harvested. The amount of αPI-C peptide in SFCM was measured by ELISA as described below. Three clones (ATIM 1, 8, and 11) secreted a notable amount of αPI-C and were used in the subsequent experiment.
Preparation of αPI-C Antiserum and Immunoassay
Polyclonal anti-αPI-C rabbit serum was obtained by immunizing synthetic peptide corresponding to Met358-Ile375 of αPI synthesized as multiple antigen peptide resin (Sawady Technology, Tokyo, Japan). The immunoglobulin fraction was purified (E-Z-SEP purification kit, Pharmacia, Uppsala, Sweden), and the antibody was further affinity purified. A 13-mer synthetic peptide corresponding to 12 amino acid residues of the amino terminus of αPI-C with an additional cystein residue at the C terminus (αPI-C13) was conjugated to activated 2-fluoro-1-methylpyridinium toluene-4-sulfonate cellulofine (Seikagaku Kogyo, Tokyo, Japan) and used for the affinity column preparation. The secreted αPI-C in the conditioned medium was measured by an antibody capture immunoassay. Briefly, 96-well microtiter plates (MaxiSorp, Nunc, Naperville, IL) were coated with samples or standard peptide (αPI-C13) solution in 20 mmol/L sodium carbonate buffer (pH 9.6) at 4°C overnight. The presence of adsorbed αPI-C was detected with an enzyme immunoassay using the rabbit anti-αPI-C IgG prepared as described above and peroxidase-conjugated swine anti-rabbit IgG (Dakopatts, Glostrup, Denmark). O-Phenylenediamine was used as the color reagent according to the standard technique. 7 Absorbance at 492 nm was measured using the microplate spectrophotometer, and values were analyzed using SOFT max PRO software (Molecular Devices, Sunnyvale, CA). Sandwich ELISA for human αPI was done according to the method described previously. 7 With this system, bovine αPI did not cross-react.
RNA Blot Analysis, Gelatin Zymography, and Immunoblot Analysis
Poly(A)+-enriched RNA was extracted using the Fast Track mRNA isolation kit (Invitrogen, San Diego, CA) from the cultured cells. Two micrograms of poly(A)+-enriched RNA was electrophoresed on 1% formaldehyde agarose gel and transblotted onto Hybond-N+ nylon membrane (Amersham), and RNA was ultraviolet cross-linked onto the membrane. Hybridization was performed in a mixed solution of 50% formamide, 5X Denhardt’s solution, 25 mmol/L phosphate buffer (pH 6.5), 0.1% SDS, 100 μg/ml sonicated and heat-denatured salmon sperm DNA, and 5X standard saline citrate (SSC) at 42°C for 16 hours. The blots were washed as follows: three times in 0.1% SDS in 1X SSC for 15 minutes at room temperature and twice in the same solution for 20 minutes at 65°C. The membranes were autoradiographed with Kodak XR-5 film at −80°C for 6 hours or 24 hours. The αPI-C cDNA corresponding to the C-terminal region of αPI (Met358-Lys394) was synthesized by PCR using the pCI-neoATIM plasmid as a template. The 5′ end of the reverse primer was radiolabeled with [γ-32P]ATP, and the corresponding PCR product was gel purified and used as a probe. For internal control of loading, the blots were subsequently hybridized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe (Clontech, Palo Alto, CA). The GAPDH probes were radiolabeled by random priming with [α-32P]CTP.
For gelatin zymography, each SFCM sample derived from the same cell number (3.6 × 10 4 cells) was applied to SDS-PAGE (10% separating gel) containing 0.1% gelatin as a substrate under nonreducing conditions without boiling. After electrophoresis, the gel was washed in 2.5% (v/v) Triton X-100 at room temperature for 60 minutes with two changes of the detergent solution to remove SDS. The gel was rinsed once with incubation buffer (5 mmol/L CaCl2 and 0.02% NaN3 in 50 mmol/L Tris/HCl, pH 7.6), incubated in the same buffer overnight at 37°C, and then stained with 2.5% Coomassie brilliant blue in 30% methanol and 10% acetate. Enzyme activity was detected as a clear band on the resulting blue background of undigested gelatin.
Immunoblot analyses for matrilysin and αPI were done according to the method described previously. 7,22 For the detection of αPI, samples were pretreated with or without peptide N-glycosidase F (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer’s instructions. 7 In addition, purified human αPI (12 μg/ml; Sigma Chemical Co., St. Louis, MO) treated with or without human recombinant matrilysin (0.3 μg/ml; Oriental Yeast Co., Shiga, Japan) was also subjected to the immunoblot analysis using rabbit anti-human αPI IgG (Dakopatts) to confirm the cleavage of αPI by matrilysin.
In Vitro Invasion Assay
The In vitro matrigel invasion assay was done according to the method described previously. 20 Briefly, a Chemotaxicell containing an 8-μm pore size polyvinylpyrrolidone-free polycarbonate filter (Kurabo, Osaka, Japan) was coated with Matrigel (Collaborative Research, Bedford, MA; 63.3 μg/cm2) and allowed to dry. The assay was done in the serum-free culture medium containing 0.1% bovine serum albumin, and 50 μg/ml fibronectin (Collaborative Research) was used as a chemoattractant. The cells (2 × 10 5 cells/well) were placed in the Chemotaxicell and cultured at 37°C for 24 hours. After the incubation, the cells were fixed, and the cells on the upper surface of the filter were wiped off. Then the cells on the lower surface were stained with hematoxylin and counted.
In Vivo Tumorigenicity, Growth, and Invasion/Metastasis Assays
A total volume of 0.15 ml containing 5 × 10 6 cells was subcutaneously injected into 6-week-old male nude mice (BALB/c-nu/nu). Tumor volume was estimated by the formula V = L × W 2 × 0.5, where V is volume, L is length, and W is width. Mice were sacrificed 40 days after the injection. For an intraperitoneal injection, 1 × 10 6 cells/0.15 ml were injected, and the body weight of each injected mouse was examined daily. Mice were sacrificed 28 days after the injection, and mean gain of the body weight (g)/day of each mouse during the last 2 weeks was calculated. All mice were necropsied, and each organ and tumor was examined histologically after fixation in phosphate-buffered formalin (4%) followed by embedding in paraffin. Immunohistochemical localization of αPI-C was performed using anti-αPI-C rabbit IgG prepared as described above using the avidin-biotin complex method.
Anti-Asialo GM1 Antibody Treatment
Mice were given intraperitoneally a 100-μl aliquot (500 μg) of anti-asialo GM1 antibody (Wako Pure Chemical Industries, Osaka, Japan) twice a week from 3 days before until 28 days after the subcutaneous injection of the tumor cells (5 × 10 5 cells/mouse). Tumor volume was estimated as described above. The mice were sacrificed 28 days after the injection.
NK Sensitivity Assay
Tumor cell targets in the subconfluent culture in 96-well microtiter plates (1 × 10 4 cells/well) were labeled with Na51CrO4 (400 μCi/10 7 cells) for 3 hours at 37°C in DMEM with 5% FBS. Then they were washed twice in serum-free RPMI 1640 medium and cultured in this medium for 30 minutes. They were washed once again and cultured in 100 μl/well of the serum-free medium for 2 hours to ensure the secretion of αPI-C before addition of effector cells. The effector cells were prepared from nude mouse spleen cells and added into the targets at different concentrations. The total volume per well was 200 μl. The assays were performed in quadruplicate at effector to target ratios (E/T) ranging from 25:1 to 200:1. Controls included labeled target cells incubated in culture medium alone (spontaneous release) and labeled target cells incubated in 1% HCl (maximal release). After 5 hours of incubation at 37°C, the plates were centrifuged for 10 minutes at 700 × g, and 100 μl of sample was harvested from each well and counted in a gamma counter. The percentage of specific chromium release was calculated as follows: (experimental release − spontaneous release)/(maximal release − spontaneous release) × 100. Results were expressed as percentage of specific chromium release at different E/T ratios.
Statistical Analysis
Comparison between groups was determined by one-way ANOVA or Mann-Whitney U test. The P values lower than 0.05 were considered as significant.
Results
Synthesis and Secretion of αPI by SUIT-2 Subclones with Different Invasive/Metastatic Properties
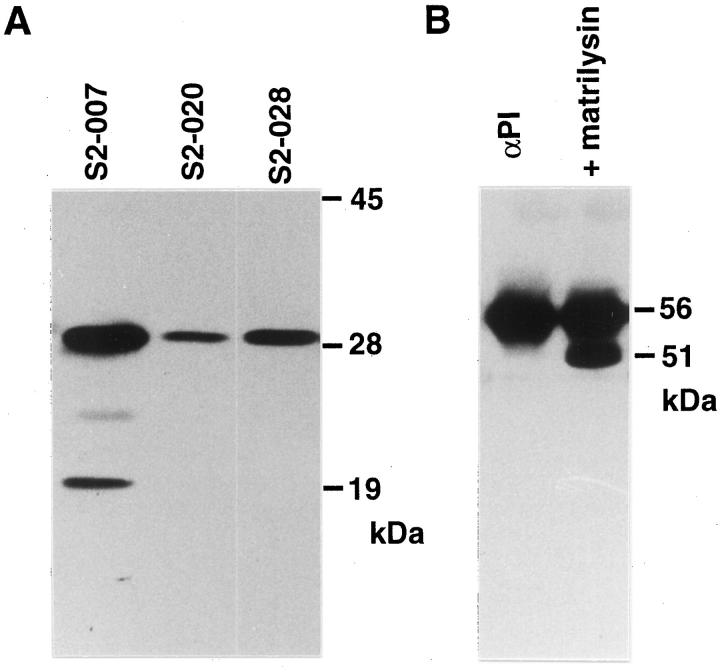
Before we started our study, the in vivo effects of αPI-C, synthesis and the properties of αPI were analyzed in the subclones with different metastatic and invasive potentials, derived from a single pancreatic adenocarcinoma cell line, SUIT-2. A metastatic subclone, S2–007, secreted larger (20-fold or more) amounts of αPI than low (S2–020) or nonmetastatic (S2–028) clones (Table 1 ▶ and Figure 1A ▶ ). Expression of the αPI gene was also confirmed by RT-PCR in S2–007 (data not shown). With immunoblot analysis, the S2–007-derived αPI showed a very broad band compared with the normal serum αPI. S2–028-derived αPI showed a little higher molecular weight (MW) than the normal serum form, which may represent an altered heavy N-glycosylation of the tumor-cell-derived αPI as we have reported previously. 7 In fact, after N-glycosidase treatment, S2–028-derived αPI showed the same molecular size (45 kd) as the normal deglycosylated form (Figure 1A) ▶ . S2–020-derived αPI also showed a very weak signal similar to that of S2–028 (data not shown).
Table 1.
In Vitro and In Vivo Characteristics of Subclones Derived from a Human Pancreas Adenocarcinoma Cell Line, SUIT-2
| Subline | In vitro doubling time | In vitro Matrigel invasion* | Secretion of αPI in vitro† | In vitro NK sensitivity‡ | Histology in nude mouse§ | In vivo doubling time¶ | Spontaneous lung metastasis in nude mouse∥ | |
|---|---|---|---|---|---|---|---|---|
| E/T 50 | E/T 200 | |||||||
| S2-028 | 35.3 hours | 1.2 ± 0.4 | 28.7 ± 1.3 | 1.11 ± 0.28 | 2.55 ± 1.30 | Well differentiated, papillary | 21.9 days | None (0 /11) |
| S2-007 | 41.8 hours | 7.2 ± 2.1 | 637.5 ± 8.2 | 0.09 ± 0.00 | 0.90 ± 0.06 | Moderately differentiated, tubular | 9.7 days | High (10 /14) |
| S2-020 | 35.8 hours | 32.1 ± 4.6 | 25.5 ± 1.2 | 5.21 ± 0.27 | 11.76 ± 0.67 | Poorly differentiated | 10.8 days | Low (3 /11) |
*Values are means (per 200× field) of triplicate assay ± SE.
†Values are ng/106 cells/24 hours; means of triplicate assay ± SE.
‡Values are percentage of specific lysis at different effector to target (E/T) ratio; means of quadruplicate assay ± SE.
§Histology of the xenografted tumors. 18
¶Volume doubling time in nude mice. 19
∥Incidence of lung metastasis after subcutaneous injection of the tumor cells in nude mice. 19
Figure 1.
Immunoblot analysis of SUIT-2 subline-derived αPIs. Before the analysis, samples were pretreated without (non-treated) or with (+ PNG-F) peptide N-glycosidase F. Electrophoresis was done under reducing conditions, and 10% separating gel was used. A: One microliter of normal human serum (serum) or concentrated SFCM equivalent to 5 × 10 5 cells (S2–007 or S2–028) was used, and the blots were stained with rabbit anti-human αPI IgG. B: Immunoblot analysis of S2–007-derived αPI after peptide N-glycosidase F treatment. Each SFCM, derived from S2–007 cells without TIMP-1 treatment (control S2–007), treated with 2 μg/ml rTIMP-1 (+ TIMP-1), transfected with a control vector carrying no insert (mock), or transfected with TIMP-1 expression vector (pSG-TIMP), was analyzed. After the N-glycosidase treatment, the presence of a cleaved αPI that is ∼5 kd smaller than the ordinary form is evident in SFCM of S2–007, and the cleaved form was decreased in the presence of exogenous or endogenous TIMP-1.
MMP-Dependent Hydrolysis of S2–007-Derived αPI in Vitro
Peptide N-glycosidase F treatment of SFCM from S2–007 revealed the presence of a cleaved αPI of 40 kd as well as a normal 45-kd deglycosylated αPI (Figure 1B) ▶ . Therefore, the very broad band of S2–007-derived αPI observed in Figure 1A ▶ seems to represent not only the altered N-glycosylation but also a co-presence of the cleaved form. The generation of the cleaved form was inhibited by 30% in the presence of exogenously added rTIMP-1 (2 μg/ml) in the medium, or was significantly inhibited (85% inhibition) in S2–007 transfected with human TIMP-1 cDNA in the expression vector pSG5 (Figure 1B) ▶ . The transfection of TIMP-1 cDNA resulted in markedly increased (∼10-fold: 5.65 ± 0.31 μg/ml, mean ± SD) TIMP-1 secretion by S2–007 cells. Endogenous TIMP-1 levels of untreated S2–007, S2–020, and S2–028 sublines were 0.60 ± 0.27, 0.52 ± 0.01, and 0.42 ± 0.04 μg/ml, respectively. By contrast, addition of 1 μg/ml aprotinin did not cause detectable inhibition (data not shown). These observations suggested that MMPs were involved, at least partly, in the generation of the cleaved αPI. Indeed, S2–007 secreted high levels of both latent and active forms of matrilysin (Figure 2A) ▶ , which has a potent αPI-degrading activity (Figure 2B) ▶ . 4,5 Moreover, an active form of interstitial collagenase, which also exhibits the αPI-degrading activity, was also secreted by S2–007. 19 As the cleavages of αPI by these MMPs occur at peptide bonds within αPI’s active-site loop (Figure 3A) ▶ , resulting in a release of the C-terminal fragment of ∼5 kd (αPI-C), it is reasonable to hypothesize that αPI-C can be generated in pericellular microenvironments of certain tumor cells, through the cleavages of tumor-cell-derived or host-derived αPI. In addition to the 40-kd αPI fragment, a 34-kd fragment was observed in the TIMP-1 transfection study. However, the generation of the 34-kd fragment was not affected by the expression of TIMP-1 (Figure 1B) ▶ , and the nature of this fragment is uncertain at present.
Figure 2.
Secretion of matrilysin by SUIT-2 sublines (A) and effects of matrilysin on purified human αPI (B). A: Immunoblot analysis of matrilysin secreted by SUIT-2 sublines. Each sample derived from the same cell number (5 × 10 4 cells) was analyzed. Both 29-kd proenzyme and 19-kd active form are present in SFCM from S2–007. A 12% separating gel was used. B: Effect of recombinant human matrilysin on human plasma αPI. A total of 400 ng of purified human αPI was incubated without (αPI) or with (+ matrilysin) 10 ng of recombinant matrilysin for 1 hour at 37°C and analyzed by immunoblot analysis using anti-human αPI antibody. A cleaved fragment that is ∼5 kd smaller than the normal αPI was generated after the matrilysin treatment.
Although S2–020 subline was most invasive in vitro, it formed rather well-demarcated tumors and grew relatively slowly in nude mice, and the incidence of spontaneous lung metastasis was low compared with S2–007 (Table 1) ▶ , suggesting that additional phenotypes were lacking in this subline to sufficiently exhibit its aggressive potential in nude mice. For instance, S2–020 was most sensitive to nude mouse NK cells (Table 1) ▶ and secreted very low levels of matrilysin without detectable active forms (Figure 2A) ▶ as compared with S2–007. Therefore, we decided to use the S2–020 subline for the following transfection study to search for a possible role of αPI-C in vivo.
Generation of αPI-C Secreting Clones of S2–020
S2–020 cells were transfected with a mammalian expression vector pCI-neo carrying a chimeric cDNA encoding of a signal peptide sequence of TIMP-1 followed by cDNA for αPI-C, ie, the C-terminal region of αPI (Met358-Lys394) that can be generated by MMP-dependent hydrolysis of αPI (pCI-neoATIM, Figure 3, A and B ▶ ). Among 20 clones of the stable transfectant, 3 clones (ATIM 1, 8, and 11) secreted notable amounts of αPI-C into SFCM compared with the parent and mock-transfected clone (pCI; Figure 3C ▶ ). Low levels of immunoreactivity observed in SFCMs of the control clones may represent a cross-reactivity of the antibody to the endogenous αPI, as S2–020 secreted low levels of αPI (Table 1) ▶ , and the immunoreactivity was decreased when ultrafiltrates (cutoff of 10 kd) of SFCM were used. The ectopic expression of αPI-C mRNA in the transfected clones was also confirmed by RNA blot analysis (Figure 3D) ▶ .
Effects of Ectopic αPI-C Expression on Cellular Characteristics in Vitro
The secretion of αPI-C did not alter the growth of the cells in vitro significantly, and only ATIM 8 grew a little more slowly than other clones. In vitro doubling time of S2–020 (parent), pCI 1, pCI 2, ATIM 11, ATIM 1, and ATIM 8 were 35.8, 35.2, 37.3, 38.5, 35.0, and 45.0 hours, respectively. Culture morphology of the cells was not altered also as judged by a phase-contrast microscopy (data not shown). To confirm that the transfection procedure did not affect the secretion of MMPs, gelatin zymography of SFCM derived from each clone was done. As shown in Figure 4A ▶ , patterns of the gelatinolytic bands were similar between the clones, and several bands of between 45 and 55 kd were observed as the main activities. In addition, secretion of matrilysin was not significantly altered by the transfection (Figure 4B) ▶ . Both parent S2–020 cells and transfectants secreted low levels of matrilysin, and the active forms were not detectable.
Figure 4.
A: Gelatin zymography of SFCM. Each sample derived from the same cell number (3.6 × 10 4 cells) of parent S2–020, mock transfected clone (pCI) or ATIM-transfected clone (ATIM), was electrophoresed on a gelatin-containing gel under nonreducing condition. For a control, SFCM from MGM-1 cells, which secrete gelatinase A, was also electrophoresed (control lane). B: Immunoblot analysis of matrilysin in SFCM of each clone. Each sample derived from the same cell number (3.6 × 10 4 cells) was analyzed.
Effects of αPI-C Expression in Nude Mice
In contrast to in vitro study, subcutaneous injection of the αPI-C-secreting clones in nude mice (5 × 10 6 cells/mouse) resulted in a tendency of enhanced tumor growth. The enhancement was statistically significant in two clones (ATIM 1 and 8) but not in ATIM 11 (Figure 5A) ▶ . Forty days after the injection, mice were sacrificed and autopsied. Expression of the αPI-C peptide in vivo was confirmed immunohistochemically (Figure 5B) ▶ . Although the anti-αPI-C antibody could cross-react to the endogenous human αPI in the clones, the amount of αPI secreted by each clone was very low and almost the same (data not shown), and thus the differences of immunoreactivity observed would be derived from αPI-C synthesized by the ATIM clone. Histologically, the αPI-C-secreting clones formed tumors having more infiltrative margins than controls with a tendency of decreased infiltration of mononuclear leukocytes (Figure 6) ▶ . Neutrophils were variably infiltrated, and it may be a reaction to necrotic changes. Metastases to the axillary lymph nodes were present only in the αPI-C-secreting clones (Table 2) ▶ . Moreover, intraperitoneal or intrapleural dissemination, mostly due to direct invasion into the peritoneal or pleural cavity, was observed in 6 of 21 mice in which the αPI-C-secreting clones were injected, whereas neither parent (0/6) nor controls (0/13) formed any dissemination (Table 2) ▶ . These aggressive behaviors were even noted in ATIM 11, which did not show significant growth enhancement. Although the αPI-C-secreting clones may exhibit a vague tendency of increased lung metastases, it was not a statistically significant level compared with the parent or mock-transfected controls, and numbers of the metastatic foci were low. In addition, when the mice (n = 3) carrying ATIM-1-derived tumor were sacrificed 18 days after the injection (mean tumor size, 535 mm3, which is comparable with the sizes of control tumors on the 40th day shown in Table 2 ▶ ), no lung metastasis could be found, whereas lymph node metastases were present in one mouse (1/3).
Figure 5.

Enhanced growth and aggressiveness of ATIM clones in nude mice. A: Growth curve of the in vivo tumors. A total of 5 × 10 6 cells/mouse was injected subcutaneously. *P < 0.05 compared with parent and mock-transfected clones (one-way ANOVA, Fisher’s PLSD test). B: Immunohistochemical staining of αPI-C. a, parent; b, ATIM 8. Positive reactivity was observed in the cytoplasm of ATIM 8 but not in the parent cells in vivo.
Figure 6.

Histology of the xenograft tumors. a: Mock-transfected clone (pCI 2); b: parent; c: ATIM 1; d: ATIM 8. The αPI-C-secreting clones (ATIMs) show more infiltrative margin with decreased mononuclear leukocyte infiltration compared with parent and mock-transfected clones. The arrow indicates a bone marrow metastasis. H&E stain; magnification, ×65 (a and c) and ×130 (b and d).
Table 2.
Autopsy Findings of Nude Mice Injected with S2-020 Clones
| Subcutaneous injection (5 × 106 cells/mouse) | Intraperitoneal injection (1 × 106 cells/mouse) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tumor volume (mm3) | Metastasis | Peritoneal/ pleural dissemination | Ascites (ml) | BW gain‡ (g/day) | Icterus | Lung metastasis | |||
| Lungs | Axillary LN | ||||||||
| Parent (n = 6) | 648 ± 146 | 2/6 (1, 16)* | 0 /6 | 0 /6 | |||||
| pCI 1 (n = 6) | 550 ± 62 | 1/6 (3) | 0 /6 | 0 /6 | pCI 1 (n = 4) | 1.00 ± 0.47 | 0.15 ± 0.01 | 0 /4 | 0 /4 |
| pCI 2 (n = 7) | 582 ± 173 | 0/7 | 0 /7 | 0 /7 | pCI 2 (n = 8) | 0.66 ± 0.47 | 0.16 ± 0.02 | 0 /8 | 0 /8 |
| ATIM 11 (n = 7) | 959 ± 142 | 3/7 (5, 3, 1) | 1 /7 | 3 /7 | ATIM 11 (n = 7) | 1.07 ± 0.25 | 0.10 ± 0.02a | 0 /7 | 0 /7 |
| ATIM 1 (n = 7) | 2357 ± 602 | 4/7 (8, 7, 4, 6) | 3 /7 | 1 /7 | ATIM 1 (n = 8) | 1.40 ± 0.42 | 0.02 ± 0.05b | 4 /8 | 1 /8 (7)* |
| ATIM 8 (n = 7) | 1868 ± 502 | 3/7 (1, 3, 27) | 4 /7 | 2/ 7 | ATIM 8 (n = 7) | 0.97 ± 0.19 | 0.03 ± 0.06c | 1 /8 | 0 /8 |
*Number of metastatic foci in both lungs.
‡Gain of body weight (g)/day during last 2 weeks. a, b and c: p = 0.064, 0.009, and 0.021, respectively, compared to pCI 2 (Mann Whitney U-test).
Values for tumor volume, ascites and BW gain are means ± SE.
After intraperitoneal injection (1 × 10 6 cells/mouse) of the cells, peritoneal dissemination and accumulation of ascites were observed in all clones. Mice were sacrificed and necropsied 28 days after the injection. Body weight gain per day was low in the mice receiving the αPI-C-secreting clones (Table 2) ▶ , and these mice were more cachexic. Invasion into the liver and/or pancreas was more evident in the αPI-C-secreting clones (data not shown), resulting in jaundice in five mice. However, the lung metastases were observed in only one mouse (ATIM 1) in this experiment, and this may be due to the shorter experimental period and the lower number of the cells injected compared with the subcutaneous injection study.
Effects of αPI-C Expression on the Growth of S2–020 Cells in NK-Depleted Nude Mice
As in vitro analysis of cell proliferation did not show obvious differences between the αPI-C secreting clones and the control clones, one possible explanation for the enhanced growth of the αPI-C secreting clones in vivo is that αPI-C may modulate host-tumor interaction in favor of the tumor cells to survive and to grow in the tissue microenvironment. To obtain more information on the mechanism of the αPI-C-induced tumor growth and/or aggressiveness in nude mice, we injected the cells into nude mice treated with or without anti-asialo GM-1 antibody. This antibody causes marked depletion of NK activity in the mice. 23 In nude mice, NK-cell-mediated control of tumor growth is an important defense mechanism against the tumor, and in fact, anti-asialo GM-1 treatment enhances the growth of tumor cells in nude mice. 23,24 When 5 × 10 5 cells (1/10 of the first experiment) were injected subcutaneously, the αPI-C-secreting clone again showed enhanced tumor growth compared with the control clone in untreated mice. This effect was particularly evident at the early stage of the tumor development (Figure 7A) ▶ . However, this growth advantage of the αPI-C-secreting clone over the control clone was abolished by anti-asialo GM-1 treatment. The treatment resulted in an enhanced growth of S2–020 cells in vivo (Figure 7A) ▶ accompanied by a tendency of reduced infiltration of mononuclear leukocytes (data not shown), indicating that NK-cell-mediated control of tumor growth is in fact important in the growth of S2–020 cells in nude mice. Under the NK-depleted condition induced by the antibody, tumor growth did not show any obvious differences between αPI-C-secreting clone and nonsecreting clone (Figure 7A) ▶ . This finding suggests that NK activity is involved, at least partly, in the effects of αPI-C on the tumor growth in vivo, and αPI-C may modulate the host NK-cell activity in favor of the tumor cells.
Figure 7.
A: Effects of anti-asialo GM1 on the growth of each clone. A total of 5 × 10 5 cells were injected subcutaneously. Without treatment, ATIM 8 (n = 3) showed enhanced tumor growth, particularly in the early stage compared with pCI 2 (n = 3). However, this phenomenon was abolished by anti-asialo GM1 treatment (n = 4, for each clone). B: In vitro sensitivity to NK cytotoxicity of each clone using nude mouse non-activated spleen lymphocytes. Bar 1, parent; bar 2, pCI 1; bar 3, pCI 2; bar 4, ATIM 11; bar 5, ATIM 1; bar 6, ATIM 8. Shown are the means ± SE of quadruplicate assays at effector to target ratios (E/T) ranging from 25:1 to 200:1. pCI 2 was not tested at E/T 200:1. *P < 0.05; **P < 0.01, compared with parent and mock-transfected clones (one-way ANOVA, Fischer’s PLSD test).
Effects of αPI-C Synthesis on NK Cytotoxicity in Vitro
Finally, NK sensitivities of the clones were compared in vitro. As shown in Figure 7B ▶ , all αPI-C-secreting clones showed decreased sensitivity to NK cytotoxicity of nonactivated nude mouse spleen cells. It should be emphasized that the level of NK sensitivity is inversely correlated with the in vivo aggressiveness of the cells shown in Table 2 ▶ . Although ATIM 1 secreted similar or a little less αPI-C compared with ATIM 8 (Figure 3C) ▶ , ATIM 1 was more resistant to NK cytotoxicity than ATIM 8. The reason for this finding is uncertain at present, and there may exist an additional deviation of the phenotype in ATIM 1
Synthesis of αPI by Adenocarcinoma Cell Lines in Vitro
To determine whether human tumor cells other than SUIT-2 synthesize αPI, we have analyzed αPI antigen levels in culture-conditioned media of various human tumor cell lines by enzyme immunoassay. As shown in Table 3 ▶ , αPI was frequently secreted by adenocarcinoma cell lines. A sarcoma cell line (HT1080), a glioblastoma cell line (MGM-1), and a primary culture of chordoma also secreted αPI. By contrast, normal intestinal epithelial (FHs74int) and a fibroblast (Flow 2000) cell line did not synthesize detectable amounts of αPI.
Table 3.
Secretion of αPI by Human Cell Lines in Vitro
| Cell line | Origin | αPI |
|---|---|---|
| FHs74 int | Small intestine, fetus | <2.0 |
| Flow 2000 | Fibroblast, fetus | <2.0 |
| RCM-1 | Rectum, Ad. | 46.5 ± 4.0 |
| RCM-2 | Rectum, Ad. | <2.0 |
| RCM-3 | Rectum, Ad. | 10.1 ± 1.2 |
| SW837 | Rectum, Ad. | 41.6 ± 2.2 |
| CoCM-1 | Colon, Ad. | 8.2 ± 0.5 |
| WiDr | Colon, Ad. | 350.8 ± 24.6 |
| Colo205 | Colon, Ad. | <2.0 |
| MKN-45 | Stomach, Ad. | <2.0 |
| MKN-28 | Stomach, Ad. | <2.0 |
| S2-013 | Pancreas, Ad. | 55.0 ± 3.5 |
| MRT-1 | Kidney, Ad. | 47.8 ± 2.7 |
| LC-2/ad | Lung, Ad. | 763.4 ± 29.0 |
| LC-1/sq | Lung, SCC | <2.0 |
| UMK-1 | Urinary bladder, TCC | <2.0 |
| UMK-2 | Urinary bladder, TCC | <2.0 |
| T-24 | Urinary bladder, TCC | <2.0 |
| HT1080 | Soft tissue, fibrosarcoma | 35.9 ± 4.9 |
| OST | Bone, osteosarcoma | <2.0 |
| MGM-1 | Brain, glioblastoma | 6.2 ± 0.2 |
| Chordoma | Soft tissue, chordoma | 214.0 ± 14.2 |
Values are ng/106 cells/24 hours in confluent culture, containing 10% FBS (mean ± SE). Ad, adenocarcinoma; SCC, squamous cell carcinoma; TCC, transitional cell carcinoma.
Discussion
The results of this study show that overexpression of αPI-C, a C-terminal fragment of αPI generated by MMPs, can enhance the growth and aggressiveness of the invasive behavior and lymph node metastasis of S2–020 human pancreas adenocarcinoma cells in vivo. Although the precise mechanism by which αPI-C increases tumor growth in vivo remains to be clarified, the results described here suggest that the phenomenon is possibly due to its modulatory effects on NK-cell activity against the tumor cells. S2–020 is a highly invasive subline in the in vitro matrigel invasion assay but exhibits less invasiveness and fewer metastases in nude mice as compared with S2–007. Therefore, although S2–020 has capability of invading ECM in vitro, additional phenotypes to sufficiently express its aggressive potential are lacking in this subline of nude mice. As S2–020 was NK sensitive and the ectopic expression of αPI-C in S2–020 resulted in decreased sensitivity of the cells to NK cytotoxicity as well as the increased in vivo growth and invasiveness, one of the lacking phenotypes required for in vivo aggressive behavior of this subline may be the resistance to NK cytotoxicity. In fact, the metastatic subline S2–007 was more resistant to NK cytotoxicity than S2–020. However, the ectopic expression of αPI-C in S2–020 did not significantly improve its capability of the spontaneous lung metastasis, and it is possible that the differences of lung metastases observed between the clones may simply be derived from the differences in tumor sizes. Thus, one or more other phenotypes to establish a sufficient number of metastatic colonies in the lung may still be lacking in this subline. Secretion of a MMP, such as matrilysin, may be a candidate for the lacking phenotype, as S2–020 secreted much less matrilysin than S2–007.
Importantly, the findings described in this report could be in accordance with the previous findings of a CRISPP peptide that is highly homologous or identical to αPI-C and is a suppressor of NK and LAK cytotoxicity in vitro. 16,17 Therefore, this report confirms the effects of the CRISPP peptide in vivo for the first time. The CRISPP peptides were reported to be frequently found in blood plasma of cancer patients but not of healthy individuals. 16 The origin of the CRISPP peptide is uncertain at present. 25 However, theoretically, MMP-dependent cleavage of αPI can also generate a CRISPP-like peptide, αPI-C, in the tumor-cell microenvironment and thus may contribute to tumor progression. Although we do not have any direct evidence that a sufficient amount of αPI-C is generated by MMPs in human tumor tissues in vivo, a tumor-cell-derived αPI and its cleaved fragment were concomitantly present in SFCM of some human adenocarcinoma cell lines 7 and the metastatic subline (S2–007) of SUIT-2. Furthermore, in S2–007, the experimental evidence suggested that MMPs were involved in the generation of the cleaved αPI. In addition to the tumor-cell-derived αPI, interstitial fluid also contains αPI that can be bound to ECM. 26 Such an ECM-bound αPI, as well as a soluble form, can also be a substrate for MMPs. 26 These lines of evidences suggest that local production of αPI-Cs would be expected in vivo in invading tumor tissues with high levels of MMPs, such as matrilysin, stromelysin-1 and 3, neutrophil collagenase, and interstitial collagenase. Therefore, together with the fact that the CRISPP peptide (αPI-C homologue) is found in the blood plasma of most cancer patients, 16 the in vivo relevance of the experimental system described in this report to human cancer seems to be reasonably supported.
The mechanism underlying the suppression of NK cytotoxicity by αPI-C is uncertain at present. It has been reported that the CRISPP peptide induces structural changes in mitochondria (SCM) of lymphocytes 16,27,28 and that the amino acid sequence of the CRISPP peptide corresponding to Phe366-Lys387 of αPI is responsible for the immunosuppresive effects in vitro. 17 This amino acid sequence of αPI-C also contains a hydrophobic pentapeptide domain, Phe370-Met374, corresponding to the SEC (serpin-enzyme complex) receptor recognition sequence, 29,30 suggesting that the sequence may somehow modulate a certain cellular function. However, the existence of SEC receptors on lymphocytes is undefined at present. A number of different cell types, including hepatocytes, mononuclear phagocytes, neutrophils, neuronal cells, and intestinal epithelial cell line Caco-2 cells express this receptor on their surface. 31 The SEC receptor recognition sequence, as well as αPI-C, is chemotactic for neutrophils and can induce an increase in the intracellular free Ca2+ concentration. 32-34 It should be noted that this sequence is exposed when αPI is complexed with its physiological target elastase or with other serine proteinases, 29,30 and these αPI-proteinase complexes can be generated in sites of inflammation or in tumor tissues. In this respect, it would be very interesting to know whether the αPI-elastase complex also exhibits NK-suppressive effects. Clearly, further efforts would be necessary to explore the biological activity of αPI-C and the precise mechanisms underlying the activity. In addition to αPI-C, an immunosuppressive effect of a noncleaved form of αPI has been reported, 35-37 and the effect seems to be independent of its proteinase inhibitory activity. 37
Previous immunohistochemical studies revealed that patients with αPI-positive adenocarcinomas of colon and lung had a worse prognosis than αPI-negative ones, particularly in the early stage. 8,9 Similar, if not identical, results were also reported in gastric adenocarcinoma. 10,38 Production of αPI by the tumor cells themselves was previously observed and, in this report, in a variety of adenocarcinomas in vitro. 7,21,39 Matrilysin is a very potent proteinase in generating αPI-C, 4,5 and an enhanced expression of matrilysin in tumor cells, including adenocarcinoma cells, has been reported by a number of groups. 1,22,40-42 Recently it appeared that the expression of matrilysin is associated with tumorigenicity, 12,13 suggesting that this enzyme may have an undefined important function in the early stage tumor progression. These lines of evidence, together with the results described in this report, may support a hypothesis that generation of αPI-C from tumor-cell-derived or host-derived αPI by matrilysin in a tumor-cell microenvironment may contribute, at least partly, to the early-stage tumor progression via modulation of tumor-host immune interactions. In addition, our results may gain insight into the biological role of stromelysin-3 in vivo, which has been largely undefined. Human stromelysin-3, in its active form, is specifically secreted by fibroblasts located in the vicinity of cancer cells. 1,42,43 It is associated with poor prognosis of cancer patients 44,45 and somehow contributes to the survival of tumor cells in nude mice, particularly in the early stage of the tumor development. 11 At present, αPI is the only known physiological substrate for the mature forms of human stromelysin-3. 6 Although a possibility of the presence of undefined specific stromelysin-3 substrates, which are critical to early-stage tumor progression, does remain, our results suggest that stromelysin-3 may have a role in cancer cell-host interaction in favor of the cancer cells, not only by inactivation of αPI but also by generating αPI-C in the pericellular microenvironment.
In conclusion, this study provides experimental evidence for an in vivo role of the carboxyl-terminal fragment of αPI, which could be generated by MMPs. Future investigations to explore a pathophysiological role of this proteolytic fragment are required. The results will add constructively to the existing body of knowledge relating to the role of MMPs in tumors and support the recently emerging concept that MMPs have a much more complex role in tumor progression than previously believed. 14
Acknowledgments
We thank Dr. J.-Y. Meng for her skillful technical assistance, Miss N. Iwakiri for preparation of histological sections, and Mrs. Y. Shiratani for her kind help in tissue culture.
Footnotes
Address reprint requests to Dr. Masashi Koono, Second Department of Pathology, Miyazaki Medical College, 5200 Kihara, Kiyotake, Miyazaki 889-1692, Japan. E-mail: koonom@post.miyazaki-med.ac.jp.
Supported by a Grant-in-Aid for Encouragement of Young Scientists 06857018 (to H. Kataoka) from the Ministry of Education, Science, and Culture and a grant from the Special Coordination Funds of the Science and Technology Agency (to M. Koono), Japan.
References
- 1.Stetler-Stevenson WG, Hewitt R, Corcoran M: Matrix metalloproteinase and tumor invasion: from correlation and causality to the clinic. Semin Cancer Biol 1996, 7:147-154 [DOI] [PubMed] [Google Scholar]
- 2.Desrochers PE, Jeffrey JJ, Weiss SJ: Interstitial collagenase (matrix metalloproteinase-1) expresses sepinase activity. J Clin Invest 1991, 87:2258-2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winyard PG, Zhang Z, Chidwick K, Blake DR, Carrell RW, Murphy G: Proteolytic inactivation of human α1 antitrypsin by human stromelysin. FEBS Lett 1991, 279:91-94 [DOI] [PubMed] [Google Scholar]
- 4.Zhang Z, Winyard PG, Chidwick K, Murphy G, Wardell M, Carrell RW, Blake DR: Proteolysis of human native and oxidised α1-proteinase inhibitor by matrilysin and stromelysin. Biochim Biophys Acta 1994, 1199:224-228 [DOI] [PubMed] [Google Scholar]
- 5.Sires UI, Murphy G, Baragi VM, Fliszar CJ, Welgus HG, Senior RM: Matrilysin is much more efficient than other matrix metalloproteinases in the proteolytic inactivation of α1-antitrypsin. Biochem Biophys Res Commun 1994, 204:613-620 [DOI] [PubMed] [Google Scholar]
- 6.Pei D, Majmudar G, Weiss SJ: Hydrolytic inactivation of a breast carcinoma cell-derived serpin by human stromelysin-3. J Biol Chem 1994, 269:25849-25855 [PubMed] [Google Scholar]
- 7.Kataoka H, Seguchi K, Inoue T, Koono M: Properties of α1-antitrypsin secreted by human adenocarcinoma cell lines. FEBS Lett 1993, 328:291-295 [DOI] [PubMed] [Google Scholar]
- 8.Karashima S, Kataoka H, Itoh H, Maruyama R, Koono M: Prognostic significance of α-1-antitrypsin in early stage of colorectal carcinomas. Int J Cancer 1990, 45:244-250 [DOI] [PubMed] [Google Scholar]
- 9.Higashiyama M, Doi O, Kodama K, Yokouchi H, Tateishi R: An evaluation of the prognostic significance of α-1-antitrypsin expression in adenocarcinomas of the lung: an immunohistochemical analysis. Br J Cancer 1992, 65:300-302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allgayer H, Babic R, Grutzner KU, Beyer BCM, Tarabichi A, Schildberg FW, Heiss MM: Tumor-associated proteases and inhibitors in gastric cancer: analysis of prognostic impact and individual risk protease patterns. Clin Exp Metastasis 1998, 16:62-73 [DOI] [PubMed] [Google Scholar]
- 11.Noël AC, Lefebvre O, Maquoi E, VanHoorde L, Chenard M-P, Mareel M, Foidart J-M, Basset P, Rio M-C: Stromelysin-3 expression promotes tumor take in nude mice. J Clin Invest 1996, 97:1924-1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witty JP, McDonnell S, Newell KJ, Cannon P, Navre M, Tressler RJ, Matrisian LM: Modulation of matrilysin levels in colon carcinoma cell lines affects tumorigenicity in vivo. Cancer Res 1994, 54:4805-4812 [PubMed] [Google Scholar]
- 13.Wilson CL, Heppner KJ, Labosky PA, Hogan BLM, Matrisian LM: Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci USA 1997, 94:1402-1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chambers AF, Matrisian LM: Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 1997, 89:1260-1270 [DOI] [PubMed] [Google Scholar]
- 15.Brittenden J, Heys SD, Ross J, Eremin O: Natural killer cells and cancer. Cancer 1996, 77:1226-1243 [DOI] [PubMed] [Google Scholar]
- 16.Cercek L, Cercek B: Cancer-associated SCM-recognition, immune defence suppression, and serine protease protection petide. I. Isolation, amino acid sequence, homology, and origin. Cancer Detect Prev 1992, 16:305-319 [PubMed] [Google Scholar]
- 17.Cercek L, Cercek B: Cancer-associated SCM-recognition, immune defence suppression, and serine protease protection peptide. II. Immunodefense suppressive effects of the CRISPPs peptide. Cancer Detect Prev 1993, 17:433-445 [PubMed] [Google Scholar]
- 18.Iwamura T, Taniguchi S, Kitamura N, Yamanari H, Kojima A, Hidaka K, Setoguchi T, Katsuki T: Correlation between CA19-9 production in vitro and histological grades of differentiation in vivo in clones isolated from a human pancreatic cancer cell line (SUIT-2). J. Gastroenterol Hepatol 1992, 7:512-5191391733 [Google Scholar]
- 19.Taniguchi S, Iwamura T, Katsuki T: Correlation between spontaneous metastatic potential and type I collagenolytic activity in a human pancreatic cancer cell line (SUIT-2) and sublines. Clin Exp Metastasis 1992, 10:259-266 [DOI] [PubMed] [Google Scholar]
- 20.Kataoka H, Seguchi K, Iwamura T, Moriyama T, Nabeshima K, Koono M: Reverse-zymographic analysis of protease nexin-II/amyloid β protein precursor of human carcinoma cell lines, with special reference to the grade of differentiation and metastatic phenotype. Int J Cancer 1995, 60:123-128 [DOI] [PubMed] [Google Scholar]
- 21.Kataoka H, Nabeshima K, Komada N, Koono M: New human colorectal carcinoma cell lines that secrete proteinase inhibitors in vitro. Virchows Arch B Cell Pathol 1989, 57:157-165 [DOI] [PubMed] [Google Scholar]
- 22.Kataoka H, Meng J-Y, Uchino H, Nabeshima K, Kihira Y, Matuo Y, Koono M: Modulation of matrix metalloproteinase-7 (matrilysin) secretion in coculture of human colon carcinoma cells with fibroblasts from orthotopic and ectopic organs. Oncol Res 1997, 9:101-109 [PubMed] [Google Scholar]
- 23.Habu S, Fukui H, Shimamura K, Kasai M, Nagai Y, Okumura K, Tamaoki N: In vivo effects of anti-asialo GM1. I. Reduction of NK activity and enhancement of transplanted tumor growth in nude mice. J Immunol 1981, 127:34-38 [PubMed] [Google Scholar]
- 24.Ishihara S, Okayama A, Nagatomo Y, Murai K, Yamashita R, Okamoto M, Shima T, Sasaki T, Mueller N, Tachibana N, Tsubouchi H: Enhanced engraftment of HTLV-I-infected human T cells in severe combined immunodeficiency mice by anti-asialo GM-1 antibody treatment. Microbiol Immunol 1996, 40:39-44 [DOI] [PubMed] [Google Scholar]
- 25.Cercek L, Siaw M, Cercek B, Cercek B: A DNA probe study on the origin of the cancer recognition, immune defense suppression and serine protease protection peptide. Cancer Detect Prev 1995, 19:325-330 [PubMed] [Google Scholar]
- 26.Mallya SK, Hall JE, Lee H-M, Roemer EJ, Simon SR, Golub LM: Interaction of matrix metalloproteinases with serine protease inhibitors. Ann NY Acad Sci 1994, 732:303-314 [DOI] [PubMed] [Google Scholar]
- 27.Cercek L, Cercek B: Apparent tumor specificity with the SCM test. Br J Cancer 1975, 31:252-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cercek L, Cercek B: Changes in SCM-responses of lymphocytes in mice after implantation with Ehrlich ascites cells. Eur J Cancer 1981, 17:167-171 [DOI] [PubMed] [Google Scholar]
- 29.Perlmutter DH, Glover GI, Rivetna M, Schasteen CS, Fallon RJ: Identification of a serpin-enzyme complex receptor on human hepatoma cells and human monocytes. Proc Natl Acad Sci USA 1990, 87:3753-3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joslin G, Fallon RJ, Bullock J, Adams SP, Perlmutter DH: The SEC receptor recognizes a pentapeptide neo-domain of α-1-antitrypsin-protease complexes. J Biol Chem 1991, 266:11281-11288 [PubMed] [Google Scholar]
- 31.Perlmutter DH: The SEC receptor: a possible link between neonatal hepatitis in α1-antitrypsin deficiency and Alzheimer’s disease. Pediatr Res 1994, 36:271-277 [DOI] [PubMed] [Google Scholar]
- 32.Banda MJ, Rice AG, Griffin GL, Senior RM: α1-Proteinase inhibitor is a neutrophil chemoattractant after proteolytic inactivation by macrophage elastase. J Biol Chem 1988, 263:4481-4484 [PubMed] [Google Scholar]
- 33.Joslin G, Griffin GL, Adams SP, Fallon RJ, Senior RM, Perlmutter DH: The serpin-enzyme complex (SEC) receptor mediates the neutrophil chemotactic effect of α-1-antitrypsin-elastase complexes and amyloid-β peptide. J Clin Invest 1992, 90:1150-1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takenouchi T, Munekata E: β-Amyloid peptide, substance P, and SEC receptor ligand activate cytoplasmic Ca2+ in neutrophil-like HL-60 cells: effect of chemotactic peptide antagonist BocMLF. Peptides 1995, 16:1019-1024 [DOI] [PubMed] [Google Scholar]
- 35.Arora PK, Miller HC, Aronson LD: α1-Antitrypsin is an effector of immunological stasis. Nature 1978, 274:589-590 [DOI] [PubMed] [Google Scholar]
- 36.Ades EW, Hinson A, Chapuis-Cellier C, Arnaud P: Modulation of the immune response by plasma protease inhibitors. I. Alpha2-macroglobulin and α1-antitrypsin inhibit natural killing and antibody-dependent cell-mediated cytotoxicity. Scand J Immunol 1982, 15:109-113 [DOI] [PubMed] [Google Scholar]
- 37.Laine A, Leroy A, Hachulla E, Davril M, Dessaint J-P: Comparison of the effects of purified human α1-antichymotrypsin and α1-proteinase inhibitor on NK cytotoxicity. Only α1-proteinase inhibitor inhibits natural killing. Clin Chim Acta 1990, 190:163-174 [DOI] [PubMed] [Google Scholar]
- 38.Tahara E, Ito H, Taniyama K, Yokozaki H, Hata J: Alpha1-antitrypsin, α1-antichymotrypsin and α2-macroglobulin in human gastric carcinomas: a retrospective immunohistochemical study. Hum Pathol 1984, 15:957-964 [DOI] [PubMed] [Google Scholar]
- 39.Keppler D, Markert M, Carnal B, Berdoz J, Bamat J, Sordat B: Human colon carcinoma cells synthesize and secrete α1-proteinase inhibitor. Biol Chem Hoppe-Seyler 1996, 377:301-311 [DOI] [PubMed] [Google Scholar]
- 40.McDonnell S, Navre M, Coffey RJ, Matrisian LM: Expression and localization of the matrix metalloproteinase Pump-1 (MMP-7) in human gastric and colon carcinomas. Mol Carcinog 1991, 4:527-533 [DOI] [PubMed] [Google Scholar]
- 41.Knox JD, Wolf C, McDaniel K, Clark V, Loriot M, Bowden GT, Nagle RB: Matrilysin expression in human prostate carcinoma. Mol Carcinog 1996, 15:57-63 [DOI] [PubMed] [Google Scholar]
- 42.Bolon I, Devouassoux M, Robert C, Moro D, Brambilla C, Brambilla E: Expression of urokinase-type plasminogen activator, stromelysin 1, stromelysin 3, and matrilysin genes in lung carcinomas. Am J Pathol 1997, 150:1619-1629 [PMC free article] [PubMed] [Google Scholar]
- 43.Basset P, Bellocq JP, Wolf C, Stoll I, Hutin P, Limacher JM, Podhajcer OL, Chenard MP, Rio MC, Chambon P: A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature 1990, 348:699-704 [DOI] [PubMed] [Google Scholar]
- 44.Muller D, Wolf C, Abecassis J, Millon R, Engelmann A, Bronner G, Rouyer N, Rio M-C, Eber M, Methlin G, Chambon P, Basset P: Increased stromelysin 3 gene expression is associated with increased local invasiveness in head and neck squamous cell carcinomas. Cancer Res 1993, 53:165-169 [PubMed] [Google Scholar]
- 45.Ahmad A, Hanby A, Dublin E, Poulsom R, Smith P, Barnes D, Rubens R, Anglard P, Hart I: Stromelysin 3: an independent prognostic factor for relapse-free survival in node-positive breast cancer and demonstration of novel breast carcinoma cell expression. Am J Pathol 1998, 152:721-728 [PMC free article] [PubMed] [Google Scholar]