Abstract
In Stevens-Johnson syndrome, pathological keratinization of the ordinarily nonkeratinized corneal and conjunctival mucosal epithelia results in severe visual loss. We examined conjunctiva covering cornea in five eyes in the chronic cicatricial phase of Stevens-Johnson syndrome. Normal conjunctiva from five age-matched individuals was studied also. The number of epithelial cells in Stevens-Johnson syndrome conjunctiva that were immunoreactive with a monoclonal antibody, Ki-67, to a nuclear antigen found only in proliferating cells was greater than normal (93.8 ± 19.8 cells above 100 basal cells versus 12.8 ± 0.5 cells above 100 basal cells; P = 0.009). In addition, although clinical inflammation was mild, massive lymphocytic infiltration was seen in the substantia propria of conjunctiva covering cornea. In situ hybridization documented transglutaminase 1 (keratinocyte transglutaminase) mRNA in suprabasal cells of the abnormally thickened conjunctival epithelium in all Stevens-Johnson syndrome patients. In contrast, no message was detected in normal conjunctival or corneal epithelia. Transglutaminase 1 is expressed during the terminal differentiation of keratinocytes where it helps synthesize cornified cell envelopes. We speculate that in Stevens-Johnson syndrome, epithelial hyperproliferation, and transglutaminase 1 gene expression lead to the pathological keratinization of ocular surface mucosal epithelia.
Stevens-Johnson syndrome (SJS), erythema multiforme major, is an acute inflammatory disease that affects mucosal membranes and skin. 1-5 It is a self-limited disease, and after the acute phase has passed, skin and most mucosa recover without significant scarring. However, long-term ocular consequences are devastating. During the chronic phase of the disease, for example, most SJS patients experience numerous ocular surface problems, including symbrepharon, entropion, ectropion, trichiasis, dry eye, persistent conjunctival inflammation, corneal vascularization (conjunctivalization), and keratinization. Some of these problems can be managed by the use of antibiotics, corticosteroids, and/or artificial tears, however, the pathological keratinization of the ordinarily nonkeratinized corneal and conjunctival mucosal epithelia is a serious and potentially debilitating problem that is difficult to manage pharmacologically.
The general term given to the pathological transition of a nonkeratinized, stratified epithelium into a keratinized epithelium is squamous metaplasia, 6,7 and in the human eye, it is a process that is accompanied by the loss of conjunctival goblet cells, an increase in epithelial stratification, and an enlargement of the superficial epithelial cells. 8,9 Squamous metaplasia has been described in numerous disorders of the ocular surface, 6,7 including dry-eye disorders in which the aqueous layer of the tear film is deficient, eg, Sjögren syndrome, 10 as well as disorders such as SJS and ocular cicatricial pemphigoid (OCP) in which the mucous layer is deficient. 8 Despite the effort that has gone into understanding squamous metaplasia, the pathogenesis behind the abnormal differentiation of cells remains unknown.
In this study, we first wanted to discover whether or not the abnormally thick conjunctiva in SJS might be a result of increased cellular proliferation, as is thought to be the case in squamous metaplasia associated with OCP. 11 To investigate this we initiated a immunohistochemical study using the murine monoclonal antibody Ki-67, an antibody that reacts with a human nuclear antigen that is present in proliferating cells but absent from quiescent cells. 12 Previous work has shown that the Ki-67 nuclear antigen is expressed in the G1, S, G2, and M phases but not in the G0 phase. 13 Furthermore, when compared with flow cytometry, 3H-thymidine labeling and BrdU labeling, Ki-67 immunohistochemistry allows a readier evaluation of the growth fraction of a given human cell population. 14 The second feature of SJS studied was prompted by the observation that in severe cases, diseased conjunctival epithelium covering the cornea often resembles the keratinized epidermis of skin (Figure 1A) ▶ . This led us to wonder whether transglutaminase 1 (keratinocyte transglutaminase; TGase1) might be involved in the pathological keratinization of ocular surface mucosal epithelia in SJS. TGase1 is an enzyme expressed during the terminal differentiation of keratinocytes to form the highly insoluble, cross-linked cell envelope at the periphery of cornified cells. 15 We used in situ hybridization to investigate the expression of the TGase1 gene in normal and SJS conjunctiva.
Figure 1.
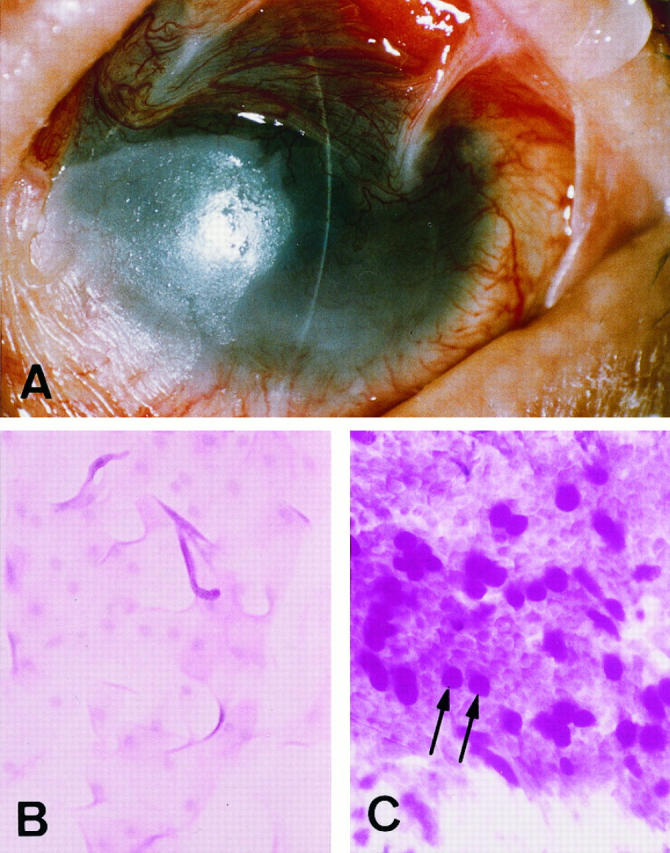
A: The left eye of Stevens-Johnson syndrome patient 5 with the eyelids eased open. Vascularized conjunctiva covers the cornea, seriously impairing vision. B: Impression cytology of the bulbar inferior conjunctiva from this individual is indicative of squamous metaplasia with few or no mucin-producing goblet cells identified. C: Goblet cells (arrows) are present in normal conjunctiva.
Materials and Methods
Tissue Samples
With informed consent we obtained conjunctiva-covering cornea from five patients with SJS (Table 1) ▶ at the time of a lamellar keratoplasty (surgical removal of the outer layer of the cornea) to improve vision. All eyes were in the chronic cicatricial phase, and the corneal surfaces were totally covered by conjunctival tissue (Figure 1A) ▶ . In general, the ocular surfaces were relatively quiet, though some mild episodic inflammation was occasionally seen in four of the five patients. Impression cytology found very few goblet cells in the bulbar inferior conjunctiva of the SJS eyes (Figure 1B) ▶ , a characteristic of squamous metaplasia. Again with proper informed consent, normal tissue was obtained during ocular surgery from the bulbar inferior conjunctiva from five age-matched individuals without any history of ocular surface disease. Impression cytology confirmed the presence of numerous mucin-producing goblet cells in this tissue (Figure 1C) ▶ , a normal feature of conjunctiva.
Table 1.
Patient Profile
| Patient number | Age/gender | Episodic inflammation | Visual acuity | Age (yrs.) at onset | Time (yrs.) since acute episode |
|---|---|---|---|---|---|
| 1 | 57 /M | Mild | HM | 55 | 2 |
| 2 | 57 /M | None | 4/200 | 47 | 10 |
| 3 | 24 /M | Mild | 20/40 | 13 | 11 |
| 4 | 74 /F | Mild | 2/200 | 44 | 30 |
| 5 | 27 /M | Mild | HM | 7 | 20 |
HM, hand motion.
Immunohistochemistry for Ki-67 Nuclear Antigen
Samples of conjunctiva were snap frozen, and 7-μm thick sections were cut. To minimize endogenous peroxidase activity, the tissue sections were incubated with 1% H2O2 for 1 hour, and to block nonspecific binding they were incubated with 10% goat serum at room temperature for 1 hour. Subsequently, the sections were incubated at 4°C for 12 hours with the murine monoclonal antibody Ki-67 (Immunotech, Marseille, France), diluted to yield an IgG concentration of 100 μg/ml. Control sections were incubated with the same concentration of normal mouse IgG (DAKO, Kyoto, Japan). Samples were next incubated with a biotinylated antiserum directed against mouse IgG (Vector Laboratories, Burlingame, CA; working dilution 1:200) for 2 hours at room temperature, and then with an avidin-biotin-peroxidase complex (Vector Laboratory; 1:100) for 50 minutes at room temperature. Sections were then exposed to 200 μg/ml of 3,3′-diaminobenzidine in 0.05 mol/L Tris-HCl buffer (pH 7.6) containing 50 μg/ml H2O2 (10 minutes at room temperature). Between each of these steps, sections were rinsed thoroughly with 0.1 mol/L phosphate-buffered saline. As has been done previously, 11 to compare the number of proliferating cells in SJS conjunctival epithelium with the number in normal conjunctival epithelium, we counted the Ki-67-labeled cells in all epithelial layers overlying 100 basal epithelial cells. Cells in three sections of each conjunctiva were counted independently by two investigators and the data averaged.
TGase1 in Situ Hybridization
Paraffin-embedded tissues were sectioned into 4-μm thick sections for in situ hybridization. The RNA probes were synthesized according to our previous reports. 16,17 Briefly, a 2-kb KpnI fragment of human TGase1 cDNA was inserted into plasmid pGEM4Z to construct pdM-K2. After linearizing pdM-K2 with EcoRI and BamHI, the antisense and sense cRNAs were transcribed in vitro using digoxigenin (DIG)-labeled UTP by T7 and SP6 RNA polymerase, respectively, according to the manufacturer’s manual (DIG RNA Labeling Kit SP6/T7; Boehringer Mannheim, Mannheim, Germany). The RNA probes were fragmented by limited alkaline hydrolysis. In situ hybridization was performed according to our previous protocol. 17 First, the sections were deparaffinized in xylene and rehydrated through a graded ethanol series. After proteinase K digestion (18 μg/ml), the sections were postfixed with 4% (w/v) paraformaldehyde in phosphate-buffered saline for 10 minutes and treated with 0.1 mol/L triethanolamine-HCl (pH 8.0) for 1 minute. Following acetylation for 10 minutes, the sections were dehydrated, air-dried then incubated overnight at 45°C in hybridization buffer composed of 50% formamide, 10 mmol/L Tris-HCl (pH 7.5), 1 mg/ml yeast tRNA (Sigma), 1× Denhardt’s solution (Sigma), 10% PEG6000, 600 mmol/L NaCl, 0.25% sodium dodecyl sulfate, 1 mmol/L EDTA, and 0.2 μg/ml probe. After hybridization, the sections were washed at 45°C for 1 hour in 50% formamide and 2× SSC, then digested with 20 μg/ml RNase (Sigma) in 10 mmol/L Tris-HCl (pH 8.0), and 500 mmol/L NaCl at 37°C for 10 minutes. Hybridized DIG-labeled probes were visualized with a Nucleic Acid Detection Kit (Boehringer Mannheim).
Results
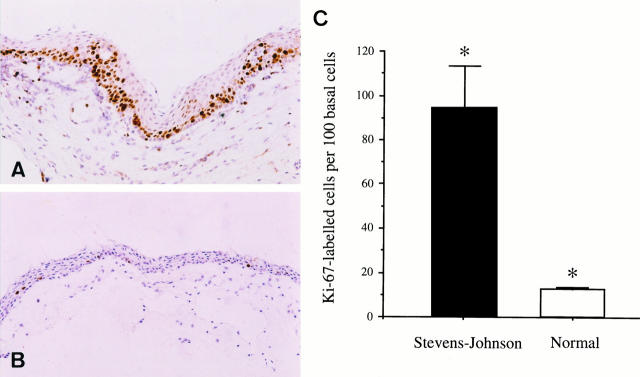
Immunohistochemistry documented numerous Ki-67-positive cells in SJS conjunctiva, all of which were located in the basal epithelial cell layer or the one or two layers of cells immediately above this (Figure 2A) ▶ . Occasional localized groups of Ki-67-positive cells seen more superficially (Figure 2A) ▶ were due to in-folds of the basement membrane. Normal conjunctival epithelium contained fewer Ki-67-positive cells than SJS conjunctiva (Figure 2B) ▶ . This difference was statistically significant (Mann-Whitney U-test; P = 0.009), SJS conjunctiva containing 93.8 ± 19.8 (mean ± SE) Ki-67-positive cells above 100 basal epithelial cells compared with 12.8 ± 0.5 in normal conjunctiva (Figure 2C) ▶ . The number of proliferating cells in normal conjunctiva counted in our experiment (12.8 ± 0.5 Ki-67-labeled cells above 100 basal cells) is larger than the number of proliferating cells (1.6 ± 0.2 tritiated thymidine-labeled cells per 100 basal cells) reported by Thoft and associates. 11 Presumably, this is because the Ki-67 antibody labels proliferating cells in more stages of the cell cycle (G1, S, G2, and M phases) 13 than does tritiated thymidine (S phase only). 11
Figure 2.
Conjunctiva covering cornea in Stevens-Johnson syndrome patient 5 (A) and normal conjunctiva (B). A: Immunohistochemistry using the Ki-67 antibody that recognizes a nuclear antigen found only in proliferating cells shows high numbers of Ki-67-positive cells (dark brown cells) mainly in the basal and suprabasal regions of Stevens-Johnson conjunctiva. B: In contrast only very few dark Ki-67 immunolabeled cells are seen in normal conjunctival epithelium. Some goblet cells are also present (arrows). C: The difference between the number of proliferating epithelial cells in Stevens-Johnson syndrome and normal is statistically significant (*Mann-Whitney U-test; P = 0.009). Original magnification, ×50 (A and B).
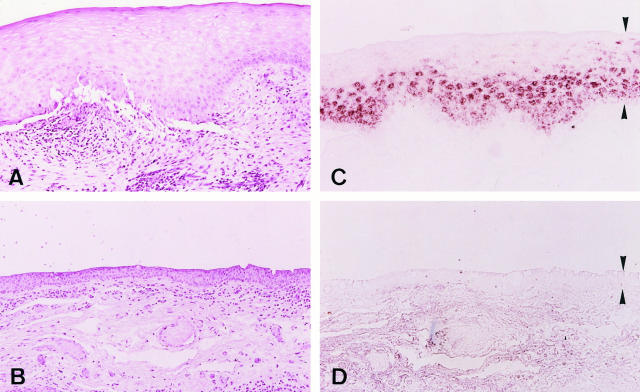
Although clinically observable conjunctival inflammation in our SJS patients was either absent or mild, massive lymphocytic infiltration was seen in the substantia propria of the invading conjunctiva in all cases (Figure 3A) ▶ . This was not the case in normal tissue in which only few lymphocytes were found subepithelially in conjunctiva (Figure 3B) ▶ . It is worth noting that the lymphocyte infiltration in SJS was often especially prominent around vessels (Figure 3A) ▶ .
Figure 3.
Conjunctiva covering cornea in Stevens-Johnson syndrome patient 1 (A and C), and normal conjuctiva (B and D). A: Lymphocytic infiltration is seen in the substantia propria of Stevens-Johnson conjunctiva beneath a thick epithelium with numerous cells adjacent to vessels. B: Few lymphocytes are seen in normal conjunctiva. C: TGase1 mRNA located in suprabasal cells in conjunctival epithelium in Stevens-Johnson syndrome. D: The epithelium in normal conjunctiva contains no TGase1 mRNA. The epithelium in C and D is delineated by arrowheads. Original magnification, ×50.
Importantly, in situ hybridization clearly demonstrated the presence of TGase1 mRNA in SJS conjunctival epithelium (Figure 3C) ▶ . This message was invariably located in a band of epithelial cells located either in or above the suprabasal region. TGase1 mRNA was detected in the conjunctiva of all five SJS patients studied, however, the intensity of the message varied; it was strong in patients 1, 2, and 4, but weaker in patients 3 and 5. In contrast to SJS, the TGase1 gene was not expressed in any of the normal, nonkeratinized conjunctival (Figure 3D) ▶ or corneal (not shown) epithelia examined here. It was noteworthy that in SJS conjunctiva the TGase1 signal was often particularly strong in the suprabasal epithelium near high concentrations of subepithelial inflammatory cells. This relationship was especially clear in patient 1.
Discussion
We have shown that, in SJS, conjunctiva that covers cornea contains significantly more Ki-67 immunopositive cells than normal (Figure 2) ▶ . Our results indicate that these Ki-67 positive cells exist mainly in the basal and suprabasal regions of the conjunctival epithelium. As the Ki-67 monoclonal antibody detects a nuclear cell antigen that is present only in proliferating cells, 12-14 we conclude that conjunctival epithelial cell hyperproliferation is a feature of SJS. Previous investigations have shown that conjunctival hyperproliferation is also a feature of OCP, as indicated by the increased mitotic rate found by tritiated thymidine autoradiography. 11 Goblet cell frequency is also less in OCP conjunctiva. 11 This inverse relationship between mitotic rate and epithelial goblet cell content has been demonstrated in animal experiments also, 18 leading to the hypothesis that hypermitosis interferes with the normal differentiation of conjunctival epithelial cells in conditions like OCP, and results in epithelial abnormalities such as the lack of goblet cells. 11 Similarly in SJS conjunctiva, the goblet cell deficiency (Figure 1B) ▶ might be a consequence of epithelial hyperproliferation (Figure 2A) ▶ .
Pathological keratinization of ocular surface mucosal epithelia, the end stage of squamous metaplasia, is often observed in cicatricial ocular surface diseases such as SJS and OCP. Where it occurs it represents a serious clinical problem because it is invariably associated with a worsening of vision and an instability of the tear film. At present, the disease mechanism behind this keratinization is not understood. The present results, however, suggest that, in SJS at least, a link between TGase1 gene expression and conjunctival epithelial cell keratinization might exist. TGase1, keratinocyte transglutaminase, is an enzyme coded for by a gene sublocalized to chromosome 14q11.2 19 that catalyzes ε-(γ-glutamyl) lysine cross-links of proteins to form the cell envelope at the periphery of cornified cells. 15 It is present in the epidermis of skin, 15,20,21 and, along with TGase3, 22 is involved with epidermal keratinization. Specifically, TGase3 catalyzes small oligomer formation of loricrin, a component of the cornified cell envelope, by intrachain cross-linking, 23 whereas TGase1 forms very large oligomeric loricrin complexes by interchain cross-linking. Indeed, experiments with knockout mice have revealed that TGase1 is essential for the distribution of the cell envelope precursor protein at the cell periphery and that the function of TGase1 cannot be compensated for by TGase3 nor other TGase isozymes. 24 In view of this, the present study used TGase1 as a representative marker of keratinization. We did not detect TGase1 gene expression in the mucous epithelia of normal conjunctiva (Figure 3D) ▶ and cornea (not shown). In contrast, TGase1 mRNA was clearly present in suprabasal cells in the conjunctival epithelium in SJS. The obvious implication is that the pathological keratinization of the conjunctival epithelium in SJS might be caused by the expression of TGase1, an enzyme whose mRNA is not ordinarily present in mucosal ocular surface epithelia.
As stated, this study suggests that conjunctival epithelial hyperplasia and keratinization in SJS are based on increased proliferation coupled with TGase1 gene expression. What might cause the hyperproliferation and unusual expression of the TGase1 gene? After considering possible disease mechanisms, we suspect that inflammatory processes might be involved. Previously, other investigators have speculated that conjunctival inflammation might influence goblet cell loss in diseases like SJS. 9 We now speculate that epithelial keratinization in SJS might also be related to inflammatory activity. This is based on the notion that epithelial keratinization in SJS is a consequence of TGase1 gene expression, along with the hypothesis that the expression of the TGase1 gene might be induced by cytokines released by infiltrating cells. In support of this we point out that TGase1 mRNA expression can be induced by a number of factors, including Ca2+, 25,26 ganglioside GQ1b, 26 12-O-tetradecanoyl-phorobol-13-acetate, 19,27 and interferon-γ (IFN-γ). 28 We feel that of these inducers of the TGase1 gene, IFN-γ is the most likely to be involved with the pathogenesis of SJS, especially in light of our previous immunohistochemical work (unpublished data) that has indicated that the subepithelial infiltrating cells in SJS conjunctiva are immunoreactive with antibodies to IFN-γ.
Our proposed pathogenesis for SJS, ie, conjunctival epithelial hyperfroliferation accompanied by subepithelial cellular infiltration and the release of IFN-γ leading to TGase1 gene expression and subsequent keratinization, is similar in some respects to the disease mechanism behind the keratinizing skin disease, psoriasis. For example, epidermal hyperproliferation and incomplete epidermal differentiation are hallmarks of psoriasis. We also know that, in psoriasis, TGase1 gene expression is altered, 17 and the activity of the enzyme in the psoriatic epidermis is increased. 29 Moreover, Th1 type CD4-lymphocytes and numerous inflammatory cytokines are present in the lesional skin in psoriasis. 30 Our previous work has indicated that the subepithelial infiltrating cells in SJS conjunctiva are also Th1-type CD4-lymphocytes. The cytokines found in psoriatic epidermis include IFN-γ, as stated earlier, a potent inducer of squamous differentiation and TGase1 expression 28 that we have found previously in SJS conjunctiva. It is worth pointing out that if inflammatory events do indeed cause hyperproliferation and TGase1 gene expression in SJS conjunctiva, it is likely that, as is the case in psoriasis, 30 several cytokines, not just IFN-γ, would be involved.
We propose that epithelial hyperproliferation and TGase1 gene expression underlie conjunctival hyperplasia and pathological keratinization in SJS, and possibly other forms of dry eye as well. This identifies the down-regulation of TGase1 gene expression as a potential treatment. Furthermore, if, as we suspect, inflammatory cytokines such as IFN-γ are found to induce TGase1 gene expression, anti-inflammatory medication might prove effective in controlling SJS even in its chronic cicatrical phase.
Acknowledgments
We thank Dr. Chie Sotozono for help with patient care.
Footnotes
Address reprint requests to Kohji Nishida, M.D., Ph.D., Department of Ophthalmology, Kyoto Prefectural University of Medicine, Kawaramachi Hirokoji, Kamigyo-ku, Kyoto 602-0841, Japan. E-mail: knishida@eyeeye.ophth.kpu-m.ac.jp.
Supported by Grants-in-Aid for Scientific Research (09771460, 09877336, 10470365, and 08457467) from the Ministry of Education, Culture and Science, Japan, by a Health Sciences Research Grant (Research on Eye and Ear Sciences) from the Ministry of Health and Welfare, Japan, by a research grant from the Kyoto Foundation for the Promotion of Medical Science, and by the Intramural Research Fund of Kyoto Prefectural University of Medicine.
References
- 1.Stevens AM, Johnson FC: A new eruptive fever associated with stomatitis and ophthalmia. Am J Dis Child 1922, 24:526-533 [Google Scholar]
- 2.Patz A: Ocular involvement in erythema multiforme. Arch Ophthalmol 1950, 43:244-256 [DOI] [PubMed] [Google Scholar]
- 3.Howard GM: The Stevens-Johnson syndrome. Am J Ophthalmol 1963, 55:893-900 [PubMed] [Google Scholar]
- 4.Arstikaitis MJ: Ocular aftermath of Stevens-Johnson syndrome. Arch Ophthalmol 1973, 90:376-379 [DOI] [PubMed] [Google Scholar]
- 5.Foster CS, Fong LP, Azar D, Kenyon KR: Episodic conjunctival inflammation after Stevens-Johnson syndrome. Ophthalmology 1988, 95:453-462 [DOI] [PubMed] [Google Scholar]
- 6.Nelson JD, Havener VR, Cameron D: Cellulose acetate impression of the ocular surface. Arch Ophthalmol 1983, 101:1869-1872 [DOI] [PubMed] [Google Scholar]
- 7.Tseng SCG: Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology 1985, 92:728-733 [DOI] [PubMed] [Google Scholar]
- 8.Kinoshita S, Kiorpes TC, Friend J, Thoft RA: Goblet cell density in ocular surface disease. Arch Ophthalmol 1983, 101:1284-1287 [DOI] [PubMed] [Google Scholar]
- 9.Tseng SCG, Hirst LW, Maumenee AE, Kenyon KR, Sun T-T, Green R: Possible mechanism for the loss of goblet cells in mucin-deficient disorders. Ophthalmology 1984, 91:545-552 [DOI] [PubMed] [Google Scholar]
- 10.Pflugfelder SC, Huang AJW, Feuer W, Chuchovski PT, Pereira IC, Tseng SCG: Conjunctival cytologic features of primary Sjögren’s syndrome. Ophthalmology 1990, 97:985-991 [DOI] [PubMed] [Google Scholar]
- 11.Thoft RA, Friend J, Kinoshita S, Nikolic L, Foster S: Ocular cicatricial pemphigoid associated with hyperproliferation of the conjunctival epithelium. Am J Ophthalmol 1984, 98:37-42 [DOI] [PubMed] [Google Scholar]
- 12.Gerdes J, Schwab U, Lemke H, Stein H: Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 1983, 31:13-20 [DOI] [PubMed] [Google Scholar]
- 13.Gerdes J, Lemke H, Baisch H, Wacker H-H, Schwab U, Stein H: Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 1984, 133:1710-1715 [PubMed] [Google Scholar]
- 14.Gerdes J: Ki-67 and other proliferation markers useful for immunohistological diagnostic and prognostic evaluation in human malignancies. Osborn M eds. Seminars in Cancer Biology, 1990, vol 1.:pp 99-206 Saunders Scientific Publications, London [PubMed] [Google Scholar]
- 15.Thacher SM, Rice RH: Keratinocyte-specific transglutaminase of cultured human epidermal cells: relation to cross-linked envelope formation and terminal differentiation. Cell 1985, 40:685-695 [DOI] [PubMed] [Google Scholar]
- 16.Yamada K, Matsuki M, Morishima Y, Ueda E, Tabata K, Yasuno H, Suzuki M, Yamanishi K: Activation of the human transglutaminase 1 promoter in transgenic mice: terminal differentiation-specific expression of the TGM1-lacZ transgene in keratinized stratified squamous epithelia. Hum Mol Genet 1997, 6:2223-2231 [DOI] [PubMed] [Google Scholar]
- 17.Nonomura K, Yamanishi K, Hosokawa Y, Doi H, Hirano J, Fukushima S, Yasuno H: Localization of transglutaminase 1 mRNA in normal and psoriatic epidermis by non-radioactive in situ hybridization. Br J Dermatol 1993, 128:23-28 [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita S, Friend J, Thoft RA: Biphasic cell proliferation in transdifferentiation of conjunctival to corneal epithelium in rabbits. Invest Ophthalmol Vis Sci 1983, 24:1008-1014 [PubMed] [Google Scholar]
- 19.Yamanishi K, Inazawa J, Liew F-M, Nonomura K, Ariyama T, Yasuno H, Abe T, Doi H, Hirano J, Fukushima S: Structure of the gene for human transglutaminase 1. J Biol Chem 1992, 267:17858-17863 [PubMed] [Google Scholar]
- 20.Schroeder WT, Thacher SM, Stewart-Galetka S, Annarella M, Chema D, Siciliano MJ, Davies PJA, Tang H-Y, Sowa BA, Duvic M: Type 1 keratinocyte transglutaminase: expression in human skin and psoriasis. J Invest Dermatol 1992, 99:27-34 [DOI] [PubMed] [Google Scholar]
- 21.Bernard BA, Asselineau D, Schaffar-Deshayes L, Darmon MY: Abnormal sequence expression of differentiation markers in psoriatic epidermis: inversion of two steps in the differentiation program? J Invest Dermatol 1988, 90:801-805 [DOI] [PubMed] [Google Scholar]
- 22.Kim I-G, Gorman JJ, Park SC, Steinert PM: The deduced sequence of the novel protransglutaminase E (TGase3) of human and mouse. J Biol Chem 1993, 270:12682-12690 [PubMed] [Google Scholar]
- 23.Cand E, Melino G, Giampiero M, Tarcsa E, Chung S-I, Marekov LN, Steinert PM: Biochemical, structural, and transglutaminase substrate properties of human loricrin, the major epidermal cornified cell envelope protein. J Biol Chem 1995, 270:26382-26390 [DOI] [PubMed] [Google Scholar]
- 24.Matsuki M, Yamashita F, Ishida-Yamamoto A, Yamada K, Kinoshita C, Fushiki S, Ueda E, Morishima Y, Tabata K, Yasuno H, Hashida M, Iizuka H, Ikawa M, Okabe M, Kondoh G, Kinoshita T, Takeda J, Yamanishi K: Defective stratum corneum and early neonatal death in mice lacking the gene for transglutaminase 1 (keratinocyte transglutaminase). Proc Natl Acad Sci USA 1998, 95:1044-1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Floyd EE, Jetten AM: Regulation of type I (Epidermal) transglutaminase 1 mRNA levels during squamous differentiation: downregulation by retinoid. Mol Cell Biol 1989, 9:4846-4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yada Y, Polakowska RR, Okano Y, Nozawa Y: Protein kinase C- dependent expression of type I transglutaminase mRNA in ganglioside GQ1b- and calcium-stimulated human keratinocytes. Biochem Biophys Res Commun 1993, 190:688-694 [DOI] [PubMed] [Google Scholar]
- 27.Liew F-M, Yamanishi K: Regulation of transglutaminase 1 gene expression by 12-O-tetradecanoylphorbol-13-acetate, dexamethasone, and retinoic acid in cultured human keratinocytes. Exp Cell Res 1992, 202:310-315 [DOI] [PubMed] [Google Scholar]
- 28.Saunders NA, Jetten AM: Control of growth regulatory and differentiation-specific genes in human epidermal keratinocytes by interferon γ. J Biol Chem 1994, 269:2016-2022 [PubMed] [Google Scholar]
- 29.Esmann J, Voorhees JJ, Fisher GJ: Increased membrane-associated transglutaminase activity in psoriasis. Biochem Biophys Res Commun 1989, 164:219-224 [DOI] [PubMed] [Google Scholar]
- 30.Elder JT: Cytokine and genetic regulation of psoriasis. Adv Dermatol 1995, 10:99-133 [PubMed] [Google Scholar]