Abstract
p27kip1 (p27) is a member of the universal cyclin-dependent kinase inhibitor (CDKI) family. p27 expression is regulated by cell contact inhibition and by specific growth factors, such as transforming growth factor (TGF)-β. Since the cloning of the p27 gene in 1994, a host of other functions have been associated with this cell cycle protein. In addition to its role as a CDKI, p27 is a putative tumor suppressor gene, regulator of drug resistance in solid tumors, and promoter of apoptosis; acts as a safeguard against inflammatory injury; and has a role in cell differentiation. The level of p27 protein expression decreases during tumor development and progression in some epithelial, lymphoid, and endocrine tissues. This decrease occurs mainly at the post-translational level with protein degradation by the ubiquitin-proteasome pathway. A large number of studies have characterized p27 as an independent prognostic factor in various human cancers, including breast, colon, and prostate adenocarcinomas. Here we review the role of p27 in the regulation of the cell cycle and other cell functions and as a diagnostic and prognostic marker in human neoplasms. We also review studies indicating the increasingly important roles of p27, other CDKIs, and cyclins in endocrine cell hyperplasia and tumor development.
Recent studies have shown that cyclins and cyclin-dependent kinase (CDK) complexes have important regulatory roles during cell cycle progression 1-7 (Figure 1 ▶ ). Cyclin-CDK complexes are in turn regulated by the cyclin-dependent kinase inhibitors (CDKIs), which generally inhibit cell cycle progression (Table 1 ▶ ). These proteins fall into two families based on their structural and functional properties. The INK4 group includes p16/INK4A (p16), p15/INK4B (p15), p18/INK4C (p18), and p19/INK4D (p19). They all have four ankyrin repeats and form complexes with CDK4 and/or CDK6 and the D-type cyclins. They have functional activities that are dependent on the presence of a normal retinoblastoma protein. 8-10 Maximal expression of the INK4 proteins occurs during the middle of the S phase in proliferating cells. Both p15 and p16 show a high frequency of gene deletions, and various human tumors and cell lines have mutations of the p16 gene, suggesting that these genes may function as tumor suppressors. 11-13
Figure 1.
Schematic view of mammalian cyclin-dependent kinase (CDK) inhibitors and cyclin-CDK complexes in the cell cycle. Members of the INK4 group (p15/INK4B, p16/INK4A, p18/INK4C, and p19/INK4D) and the Cip/Kip group (p21, WAF/CIPI, p27/Kip1, and p57/Kip2) have inhibitory roles in G1 to S progression.
Table 1.
Members of the Cyclin-Dependent Kinase Inhibitors of the INK4 and Cip/Kip Families
| Family | Chromosome Location |
|---|---|
| INK4 | |
| p15 (INK4B) | 9 p21 |
| p16 (INK4A) | 9 p21 |
| p18 (INK4C) | 1 p32 |
| p19 (INK4D) | 19 p13 |
| Cip/Kip | |
| p21 Waf1/Cip1 | 6 p21 |
| p27 Kip1 | 12 p13 |
| p57 Kip2 | 11 p15 |
The second group of CDK inhibitors, the Cip/Kip family, includes p21/WAF1/CIP1 (p21), p27/kip1 (p27) and p57/kip2 (p57). 14-22 These proteins inhibit kinase activities of pre-activated G1 cyclin E-CDK2, cyclin D-CDK4/6, and other cyclins. The Cip/Kip proteins are designated as universal CDKIs because they interact with various CDK complexes, with cyclins A, E, D1, D2, and D3, and CDKs. 15 Overexpression of the kip proteins leads to cell cycle arrest. Members of the kip proteins share a great deal of homology. p27 protein has a 42% amino acid homology with p21 and a 47% homology with p57 at the amino-terminal domain, the region that mediates inhibition of CDK. Kip proteins all have a nuclear localization signal at their carboxyl-terminal domain. Unlike the INK4 group, which inhibits CDK4/6 only, the Cip/Kip inhibitors can also target CDK2 in complexes
The p27 gene is located on chromosome 12p13 at the junction of 12p12–12p13.1. 21 This gene was cloned by several groups in 1994. 14,16,23 Structural analysis of the p27 protein was recently reported. 24 Examination of the crystal structure of the 69-amino-acid amino-terminal inhibitory domain of p27 bound to the phosphorylated cyclin A-CDK2 showed that p27 binding causes large conformational changes in and around the catalytic cleft of CDK2 24 and that p27 has separate binding sites on the cyclin and CDK subunits. This explains how p27 and other Kip/Cip inhibitors can bind isolated subunits. 15,24 Binding of the p27 cyclin-CDK complex is significantly tighter than binding to the isolated CDK and cyclin subunits, which is consistent with cooperative binding of the two subunits.
p27 was first identified in cells treated with transforming growth factor (TGF)-β or by stimulation of contact inhibition where p27 was found as an inactive form bound to CDK2-cyclin E. 18,25 The protein was purified from a cyclin E-CDK2 affinity column and characterized by its strong inhibitory activity toward cyclin E-CDK2. p27 can directly inhibit the enzymatic activity of CDK-cyclin complexes and arrest cells in G1. 18 The association of p27 with CDK-4 cyclin D or with CDK2-cyclin E complexes blocks phosphorylation of CDK4 on Thr 172 and CDK2 on Thr 160 via a CDK activation kinase. 22,26 p27 can be induced by cyclic AMP and other negative regulators of the cell cycle 22 and can be down-regulated by interleukin 2. 26
The levels of p27 protein are increased in quiescent cells and rapidly decrease after stimulation with mitogens. Constitutive expression of p27 in cultured cells causes cell cycle arrest in the G1 phase. 15,16 When murine BALB/c-3T3 fibroblasts are deprived of serum mitogens, p27 accumulates in these cells. 27 This finding was correlated with inactivation of G1-cyclin-CDK complexes and with cell cycle arrest in G1. Inhibition of p27 expression with antisense oligonucleotides prevents cell cycle arrest in response to mitogen depletion, indicating that p27 is an essential component of the pathway that connects mitogenic signals to the cell cycle. 27
Although p27 inhibits cyclin E-CDK2, recent studies have also shown that p27 can serve as a substrate for cyclin E-CDK2. 27 Using a murine fibroblast model, it was shown that cyclin E-CDK2 can directly phosphorylate p27 (Figure 2) ▶ , and the cyclin E-CDK2-dependent phosphorylation of p27 results in elimination of p27 from the cell, allowing transition from G1 to S phase. 28
Figure 2.
Effect of p27 on cyclin E-CDK2 complex. p27 binds to cyclin E-CDK2 complexes and prevent its activation. Although some p27 is present in proliferating cells, it is sequestered and unavailable to interact with cyclin E-CDK2. p27 can be regulated by the same enzyme it targets for inhibition by becoming a cyclin E-CDK2 substrate, leading to its phosphorylation and proteolysis by the ubiquitin-proteasome pathway. Phosphorylation of retinoblastoma (Rb) protein leads to G1 to S progression.
Other investigators demonstrated Ras-mediated down-regulation of p27 that involves suppression of synthesis leading to an increase in the degradation of the p27 protein. 29 It is postulated that Ras function is required in late G1 for down-regulation of p27 and passage of the cell through the restriction point. 29
The role of TGF-β in regulating p27 has been investigated in many systems. 18,30-32 Using a C3H 10T1/2 mouse fibroblast model it was shown that cyclin E-CDK2 inhibits p27 in the growth-arrested state and that TGF-β down-regulates the steady-state level of the p27 protein. 30 Mal et al 31 showed that mink lung epithelial cells arrested in G1 by TGF-β could be rescued from this arrest by disabling of p27 via adenovirus oncoprotein E1A. Qian et al demonstrated that TGF-β down-regulates p27 protein and mRNA levels in cultured rat anterior pituitary cells. 32
Functions of p27
There are many putative functions attributed to p27 (Table 2) ▶ . Extensive investigations have been performed to elucidate the role of p27 as a CDKI in normal and neoplastic cells. 14,16,18,27-30 Some studies suggest a putative role as a tumor suppressor gene. Loss of p27 protein expression may result in tumor development and/or progression; however, this loss of expression does not appear to result from gene mutations. 33-36 More than 500 tumors have been examined for p27 mutations, and less than 5 of these have shown specific mutations. 34 These have included a stop codon at position 76 of an adult T-cell leukemia and hemizygous deletion of the p27 gene in a B-cell non-Hodgkin’s lymphoma 33 in addition to 2 point mutations in an analysis of 36 primary breast carcinomas. 34 One of the mutations in the breast carcinomas was a polymorphous mutation at codon 142 and the other a nonsense mutation at codon 104. 34
Table 2.
Putative Functions of p27Kip1
| “Universal” cyclin-dependent kinase inhibitor that regulates progression through the cell cycle. 1-7,14,16,18 |
|---|
| Potential tumor suppressor gene. 44-47 |
| Promoter of apoptosis. 37,38 |
| Regulator of drug resistance in solid tumors. 35 |
| Role in cell differentiation in muscle, oligodendrocytes, osteoblasts, and granulosa cells. 39-42 |
| Safeguard against inflammatory injury. 43 |
St. Croix et al 35 reported that p27 has a role in regulating drug resistance in solid tumors. Human and mouse tumor cells grown as multicellular spheroids in three-dimensional culture show a consistent up-regulation of p27 (up to 15-fold). 35 When a mammary tumor cell line (EMT-6) was treated with antisense p27 oligonucleotides, there was increased cell proliferation, with restoration of the drug- or radiation-induced cell cycle perturbations that were repressed in spheroid culture. 35
Recent studies have implicated p27 as a promoter of apoptosis. 37,38 Using the MDA-MB-231 breast carcinoma cell line to overexpress p27, Katayose et al 37 found that apoptosis was increased when measured by several techniques. 37 Similar results were observed in other cell lines. Interestingly, p27 did not induce apoptosis in the total cell population. As cells in G1 did not undergo apoptosis, a possible explanation for this observation was that the cells arrested in G1 may be protected in some way from induction of apoptosis. 37
Many studies have shown that p27 has a role in regulating differentiation in some tissues. 39-42 In a study of mouse embryo skeletal muscle, functional assays showed that ectopic p27 expression enhances the efficiency of MyoD-initiated muscle differentiation. It was proposed that p27 acts as a trigger for CDKI while myoblasts are exiting the cell cycle and initiating differentiation. In a study using oligodendrocyte cell precursors from rats, Durand et al 40 showed that p27 accumulates progressively in the precursor cells as they proliferate and that p27 is present at high levels in mature oligodendrocytes, implicating p27 accumulation in the differentiation of oligodendroglial cells.
In a study of the effects of CDKIs on the regulation of the G1 phase cyclin-dependent kinases, it was noted that parathyroid hormone increases p27 levels but not p21 levels in osteoblasts. The data implicate parathyroid hormone in blocking entry of cells into the S phase and inhibiting cell proliferation when p27 accumulates within these cells. The effect of parathyroid hormone is mediated through the protein kinase A pathway. 41 Robker and Richards reported that follicle-stimulating hormone and estradiol regulate granulosa cell proliferation during the development of preovulatory follicles by increasing the levels of cyclin D2 relative to p27, whereas luteinizing hormone terminates follicular growth by down-regulating cyclin D2 while up-regulating p27 as well as p21. 42 Thus, the LH surge with high cyclic AMP levels induces the granulosa cells to enter a nonproliferative or more differentiated stage as they enter the luteal phase.
p27 has been implicated in the protection of some cells against inflammatory injury. 43 Using mice with an engineered deletion of the p27 gene, 44-46 a model of experimental glomerulonephritis was used to analyze immune-mediated inflammation. Renal function decreases in p27 null mice compared with controls with wild-type p27, and this is associated with increased glomerular cell proliferation, apoptosis, and matrix protein accumulation. 43 Both tubular epithelial cell proliferation and apoptosis are increased in p27 null mice after ureteral obstruction. The authors concluded that p27 may have a general role in protecting cells and tissues from inflammatory injury. Interestingly, this in vivo effect of p27 on apoptosis in p27 null mice is the opposite effect observed in vitro where p27 enhanced apoptosis. 38
There are two lines of evidence indicating that p27 suppresses cell proliferation in vivo. Malignant human brain tumor cell transfection with the p27 gene leads to inhibition of proliferation and cell cycle arrest in G1. 47 Ectopic overexpression of p27 is associated with a striking decrease in aneuploid cells, loss of anchorage-independent growth in soft agar, and failure to induce tumor development in a xenograft model. Mice lacking the p27 gene show an increase in body weight, thymic hypertrophy, and hyperplasia of pituitary intermediate lobe adrenocorticotrophic hormone cells, adrenal glands, and gonadal organs. 44-46 Analysis of CDK2 in the thymocytes of these knockout mice show a 10-fold increase in the activity of this enzyme. A surprising finding was that the effects of TGF-β, rapamycin, and contact inhibition on cell proliferation remained unchanged in p27 null mice, indicating that the presence of p27 is not an absolute requirement for this pathway. 44
Several recent studies have implicated p27 in inhibition of cell cycle progression by homophilic cell-cell interaction and in tumor metastasis. 48,49 St. Croix et al examined the role of the homophilic cell-cell adhesion molecule E-cadherin in contact-dependent growth inhibition using a mouse mammary carcinoma cell line, EMT 16, in a multicellular spheroid model in vitro. They observed that E-cadherin expression after transfection with an E-cadherin expression vector resulted in an increase in the level of p27 and showed that E-cadherin was a major growth suppressor as well as an invasion suppressor. 48 Studies of p27 protein expression in primary colorectal carcinomas and their metastatic foci showed a marked reduction in p27 expression in the metachronous metastases compared with the corresponding primary tumor, suggesting that down-regulation of p27 in circulating tumor cells may confer the ability to grow in an environment of altered extracellular matrix or intercellular adhesion properties that may facilitate tumor metastasis. 49
Regulation of p27 Expression
p27 protein levels increase in cells treated with cyclic AMP, lovastatin, rapamycin, and tamoxifen, 22,23,50,51 and this increase is probably related to the G1 block produced by these agents. Cells undergoing differentiation also have increased levels of p27 protein. 52,53 Several studies have shown that the human papilloma virus can regulate p27 activity. 31 Some studies have shown that p27 levels are regulated by alterations of protein stability; thus, the half-life of p27 is much longer in quiescent cells compared with proliferating cells. 50
Ubiquitination is the principal mechanism regulating p27 protein degradation. 54-57 Ubiquitin is a small protein of 7000 MW that is covalently linked to a target protein. 58-63 The ubiquitin-target protein complex is specified by the ubiquitinating enzymes E1, E2, and E3. The ubiquitin-activating enzymes (E1s) are the first enzymes involved in protein ubiquitination. These enzymes form a thioester bond between the carboxy terminus of ubiquitin and an internal cysteine residue. E2s are designated as ubiquitin-conjugating enzymes, or Ubcs, and form a thioester bond between the internal systemic residue and the carboxy terminus of a molecule of ubiquitin. E2 transfers the ubiquitin to ε-amino groups of lysine in the target protein. The ubiquitin ligases (E3s) are not as well characterized. They act as substrate recognition factors. The proteasome, which is a multimeric protein complex, recognizes the covalent adduction between ubiquitin and the target protein such as p27, which leads to degradation of the target protein with recycling of ubiquitin.
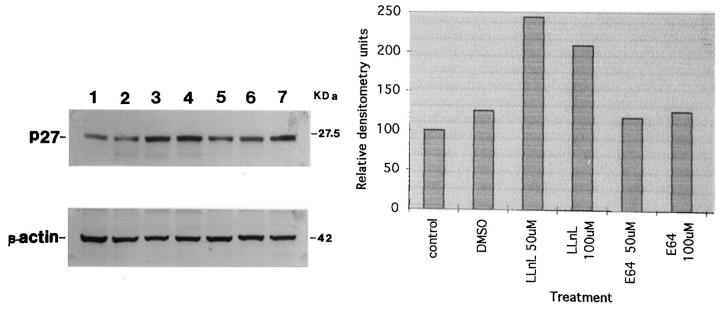
Pagano et al 54 showed that the peptide-aldehyde N-acetyl-leucinyl-leucinyl-norleucinal-H (LLnL), an inhibitor of the chymotryptic site on the protease, leads to the accumulation of p27 protein and its ubiquinated forms of approximating Mr 70,000 and Mr 100,000, indicating that p27 was polyubiquitinated in vivo. Our laboratory has also observed ubiquitin-proteasome regulation of p27 protein in a human pituitary cell line (Figure 3) ▶ .
Figure 3.
Analysis of the effect of inhibition of the chymotryptic site of the ubiquitin-proteasome pathway on cellular p27. The HP75 pituitary cell line (produced in our laboratory) was treated with the peptide-aldehyde N-acetyl-leucinyl-leucinyl-norleucinal-H (LLnL), an inhibitor of the chymotryptic site on the proteasome, or with the cysteine protease inhibitor l-trans-expoxysuccinic acid (E64) as a control for 16 hours in culture. The cells were homogenized and analyzed for p27 by Western blotting using a monoclonal antibody (Transduction Laboratories, Lexington, KY) and enhanced chemiluminescence (Amersham, Arlington Heights, IL). The samples include the following: lane 1, control cells with culture media only; lane 2, cells treated with dimethylsulfoxide (100-μl volume equivalent to the LLNL and E64 vehicle volume); Lane 3, 50 μmol/L LLnL; lane 4, 100 μmol/L LLnL; lane 5, 50 μmol/L E64; lane 6, 100 μmol/L E64; lane 7, HeLa cells used as a p27-positive control. LLnL, but not E64, increased p27 protein in the HP75 cells. β-Actin was used to normalize for protein loading. The graph on the right was generated by densitometric analysis of the film. These results indicate that the ubiquitin-proteasome pathway is one of the mechanisms regulating the expression of p27 protein in pituitary tumor cells.
When purified p27 was incubated with Ubc2 or Ubc3, a mono-ubiquitinated form of p27 was generated, suggesting that polyubiquinated p27 requires additional factors such as E3. Extracts from proliferating or S phase cells contain more p27 ubiquinating and degradation activities than extracts from quiescent cells. 61,63 Half-life studies have shown that p27 in proliferating cells is sixfold less stable than in quiescent cells, 54 which explains why p27 is expressed at much higher levels in quiescent cells than in proliferating cells and highlights the translational control of p27 expression by ubiquitin-mediated degradation.
Phosphorylation appears to be an important mechanism for p27 degradation. 55,56,64 p27 phosphorylation is cell cycle dependent and peaks during the late G1 phase. The amount of p27 protein is inversely correlated with its phosphorylation. Using human fibroblasts, Monsaki et al 58 reported that cyclin E/CDK2 phosphorylated p27 on threonine 187 in vitro, and phosphorylation of p27 affected the stability of the p27 protein. It has ben shown that p27 must be phosphorylated by CDK2 on a conserved carboxyl-terminal CDK target site to be degraded by the proteasome. 56
The importance of phosphorylation and the ubiquitin ligase complex in the degradation of cell cycle proteins such as p27 is derived in part from experiments in yeast. 65 A specific ubiquitin ligase complex, SCFcdc4P, when mixed with the ubiquitin-activating enzymes E1, the ubiquitin-conjugating enzyme E2, Cdc34p, and ubiquitin led to the reconstitution of ubiquination of the phosphorylated CdK inhibitor Sic1p, providing a molecular basis for the G1/S transition, which may be a general mechanism for ubiquitination after phosphorylation in eukaryotes. 65
Although protein degradation by the ubiquitin-proteasome pathway is the principal method of proteolysis of p27, recent studies have shown that methylation may be another mechanism regulating p27 expression. Using various pituitary tumor cell lines, Qian et al 66 showed that GH3 and GHRH-CL1 cell lines, both of which produce prolactin and growth hormone, express very little p27 protein or mRNA. 33 Analysis of exon I and part of exon II of the p27 gene using bisulfite genomic sequencing showed that both GH3 and GHRHCL1 cell lines are hypermethylated whereas the control rat pituitary p27 gene is hypomethylated. 66 By changing the methylation status of the p27 gene with 5-aza-2′-deoxycytidine they were able to show that methylation and methyltransferase activity regulated expression of p27 in some pituitary cell lines. The importance of methylation of the p27 gene in regulating its function is not known.
p27 as a Diagnostic and Prognostic Marker
During the past 2 years a large number of studies have examined the diagnostic and prognostic significance of p27 expression in various tumors. Almost all studies report decreased p27 expression in more aggressive tumors 67-75 76-90 (Figure 4) ▶ . p27 expression is reported to be an independent prognostic factor or potentially useful in the diagnosis of a broad spectrum of tumors. According to Steeg and Abrams, 67 for a new prognostic marker to enter into routine clinical use at least three criteria must be met. 1) The marker provides information independent of and better than conventional pathological criteria. 2) The marker provides information that can alter treatment decisions. 3) Studies with the marker are reproducible.
Figure 4.

Immunohistochemical staining for p27 expression in normal and neoplastic tissues. A: Lymph node tissue with p27 staining on left showing strong immunoreactivity in quiescent cells and less staining in proliferating cells. The germinal center (GC) with proliferating cells had few cells positive for p27. Staining with a Ki67 antibody MIB-1, which recognizes proliferating cells, showed the opposite pattern of staining compared with p27 with strong staining of most cells in the GC. Magnification, ×200. B: There is selective localization of p27 in normal anterior pituitary cells. Immunostaining of normal anterior pituitary for p27 (nuclear purple staining) and for thyroid stimulating hormone (TSH; brown cytoplasmic staining) shows localization of p27 in some TSH cells (arrows). Magnification, ×300. C: Normal and neoplastic breast tissue with strong staining for p27 in normal mammary ducts whereas the invasive carcinoma cells are mostly negative. Magnification, ×300. D: Normal and neoplastic prostate tissue showing strong staining for p27 in normal prostatic ducts while the invasive carcinoma cells are weakly positive or negative. Magnification, ×300. E: Parathyroid tissue showing strong nuclear staining for p27 in the normal cells on the left whereas the adenoma on the right stains weakly, indicating low expression of p27. Magnification, ×250. F: The normal thyroid on the left shows strong nuclear staining for p27 in the follicular cells. The papillary carcinoma on the right shows low expression of p27 protein. Magnification, ×250.
Many reports have validated the utility of p27 as a prognostic and/or diagnostic marker (Table 3) ▶ .
Table 3.
Role of p27 as a Diagnostic/Prognostic Marker in Human Cancers
| Type of tumor | Role of p27 | Reference |
|---|---|---|
| Breast carcinoma | Decreased p27 in carcinoma and it is associated with tumor progression. | Catzavelos et al 68 |
| Breast carcinoma | Decreased p27 contributes to tumor progression. | Porter et al 69 |
| Breast carcinoma | Lack of p27 associated with poor prognosis. | Tan et al 70 |
| Breast and colon carcinoma | Inverse correlation of p27 and tumor malignancy. | Fredersford et al 71 |
| Colon carcinoma | p27 is an independent prognostic marker. | Loda et al 72 |
| Colon carcinoma | Significant correlation between p27 and tumor grade. | Ciapoorone et al 73 |
| Esophageal Barretts-associated adenocarcinoma | Loss of p27 confers poor prognosis. | Singh et al 74 |
| Gastric carcinoma | Correlation of p27 and tumor aggressiveness. | Mori et al 75 |
| Lung non-small-cell carcinoma | p27 is a prognostic factor, correlates with survival. | Esposito et al 76 |
| Lung non-small-cell carcinoma | p27 decreased in carcinoma compared with non-neoplastic lung tissues. | Kawana et al 77 |
| Lung non-small-cell carcinoma | p27 is a prognostic factor for survival. | Yatabe et al 78 |
| Prostate adenocarcinoma | Low p27 is an independent prediction of treatment failure. | Tsihlias et al 79 |
| Prostate adenocarcinoma | Absent or low p27 is an adverse prognostic factor. | Yang et al 80 |
| Prostate adenocarcinoma | Low p27 correlates with lymph node metastasis and higher Gleason scores. | Cheville et al 81 |
| Prostate adenocarcinoma | Prostate carcinoma with low p27 more biologically aggressive. | Cordon-Cardo et al 82 |
| Malignant melanoma | Loss of p27 is a prognostic indicator of early relapse. | Florenes et al 83 |
| Oral cavity carcinoma | Low p27 association with oral dysplasia and carcinoma. | Jordan et al 84 |
| Endocrine tumors | Low p27 associated with higher-grade tumors. | Lloyd et al 85 |
| Thyroid tumors | Low p27 associated with more aggressive tumors. | Erickson et al 86 |
| Parathyroid tumors | Low p27 associated with carcinoma. | Erickson et al 88 |
| Pituitary tumors | Decreased p27 with tumor progression. | Jin et al 89 |
| Pituitary corticotroph tumors | Low p27 associated with more aggressive ACTH tumors. | Dahia et al 90 |
| Lymphomas | Low p27 in tumors with a higher growth fraction. | Sanchez-Beato et al 93 |
| Lymphomas | p27 expression inversely related to proliferation rate in all lymphomas, except mantle cell type. | Quintanilla-Martinez et al 94 |
Studies of breast carcinoma 68-70 showed that p27 protein expression is lower in more aggressive tumors. The studies used a single immunohistochemical assay for the protein, indicating the reliability of this technique. Catzavelos et al 68 showed that p27 is a predictor of reduced disease-free survival by Kaplan-Meier analysis. Porter et al 69 combined analyses of p27 and cyclin E and showed that both of these cell cycle regulators are prognostic markers of tumor behavior. Tan et al 70 analyzed p27 expression in breast cancers less than 1 cm in size in 202 patients and found that nodal status and low p27 expression are independent prognostic parameters by both univariate and multivariate analyses. They concluded that p27 identified node-negative patients with small invasive breast carcinomas that were at high risk for tumor progression and therefore might benefit from adjuvant therapy.
Three studies have examined p27 expression in colorectal cancers. 71-73 Loda et al 72 showed that the absence of p27 protein expression was a powerful negative prognostic marker in colorectal carcinomas, particularly in stage II tumors, and suggested that this marker may help in the selection of patients who would benefit from adjuvant therapy. These investigators also showed that carcinomas with low or absent p27 protein showed enhanced proteolytic activity for p27, suggesting that the low p27 expression resulted from increased protease-mediated degradation rather than from altered gene expression. In another study of p27 in multistage colorectal carcinogenesis, Ciaparrone et al 73 found a significant correlation between p27 expression and tumor grade with well and moderately differentiated carcinomas expressing higher p27, while the poorly differentiated carcinomas had significantly lower expression.
Other areas of the gastrointestinal tract have also been studied for p27 expression and tumor behavior. In esophageal adenocarcinomas, low p27 protein correlated with higher histological grade, depth of invasion, presence of lymph node metastasis, and patient survival. 74 Interestingly, in the study of Singh et al, both cytoplasmic as well as nuclear localization of p27 were associated with decreased patient survival. 74 p27 has also been found to be an independent prognostic factor for patients with gastric carcinomas. 75
In a study of p27 expression in non-small-cell lung carcinoma, Esposito et al 76 showed that p27 was a prognostic factor correlating with patient survival. Kawana et al 77 also examined a group of non-small-cell carcinomas and showed that the p27 labeling index decreased in carcinomas compared with non-neoplastic lung tissues and was inversely related to the proliferation marker Ki-67. Yatabe et al 78 also found that p27 was a significant prognostic factor in non-small-cell lung carcinoma; however, in the more aggressive small cell carcinoma, they identified an increased p27 expression when compared with the corresponding normal lung epithelium. This variable expression of p27 in endocrine tumors will be discussed below.
Several studies have analyzed p27 expression in prostate adenocarcinoma. 79-82 Tsihlias et al 79 and Yang et al 80 showed that low p27 expression was an independent predictor of treatment failure and an independent prognostic factor for disease recurrence. Yang et al 81 suggested that patients with low or absent p27 protein expression may be candidates for novel adjuvant therapies. Cheville et al 81 observed that p27 expression correlated with a higher mean Gleason score, lymph node metastasis, and aneuploid cancers, but it did not correlate with subclinical biochemical failure. Cordon-Cardo et al 82 observed that primary prostate carcinomas with lower levels of p27 protein were more biologically aggressive. In addition, they observed that p27 protein and mRNA were almost undetectable in both epithelial and stromal cells of benign prostatic hyperplasia (BPH), supporting the concept that BPH is not a precursor to prostate carcinoma.
In the first reported study of p27 expression in malignant melanoma, Florenes et al 83 observed that this CDKI was correlated with tumor thickness in nodular melanoma but not in superficial spreading melanomas. Although p27 did not appear to influence overall survival for either subgroup, a complete loss of p27 expression had potential importance as a prognostic indicator of early relapse in patients with nodular melanomas.
Analysis of p27 protein expression in squamous cell lesions of the oral cavity showed that p27 was significantly reduced in oral dysplasias and carcinomas compared with that in normal squamous epithelium. 84 There is also a significant reduction in p27 protein between low- and high-grade dysplasias, suggesting that changes in p27 expression may be an early change in oral squamous cell carcinogenesis.
Analysis of p27 protein expression in endocrine tumor was first reported by Lloyd et al. 85 They observed decreased expression of p27 in endocrine adenomas and carcinomas compared with normal tissues. However, the changes are not as striking as identified in some non-endocrine tissues, such as breast, prostate, and colonic carcinomas. For example, in a study of thyroid carcinomas, Erickson et al 86 found similar levels of p27 in high- and low-grade cancers, ie, anaplastic carcinomas and papillary carcinomas. Similar findings were observed in a comparison of different types of papillary carcinomas by others. 87 In a large series of parathyroid tumors, there was significantly decreased p27 expression in carcinomas compared with adenomas. 88 Jin et al 89 reported that in pituitary tumors, the p27 protein levels decreased during progression from adenomas to carcinomas, but the differences were moderate compared with other non-endocrine tumors. Jin et al 89 showed that regulation in the pituitary was post-translational as the mRNA levels were similar in normal and tumorous pituitaries. Similar findings were reported in parathyroid tumors by Erickson et al. 88 In ACTH-secreting pituitary tumors, Dahia et al 90 reported that corticotroph adenomas express p27 protein, although one carcinoma in their series showed loss of p27 protein expression. Analysis of three other pituitary carcinomas by the same investigators showed two tumors with loss of expression, although one case showed moderate expression.
p27 protein expression has been used as a diagnostic marker in some endocrine tumors. Erickson et al found significant differences in the p27 labeling indices between follicular adenomas and follicular carcinomas. 86 They suggested that immunostaining for p27 might be useful in distinguishing between these two tumors. Similarly, they reported that p27 immunostaining could be used to distinguish between parathyroid adenomas and carcinomas. 88
The relationship of decreased levels of p27 protein and tumor progression is variable in different endocrine tumors. Our studies of adrenal cortical and medullary tumors showed only slight differences between normal benign and malignant tumors with respect to p27 protein expression. 85 Yatabe et al 78 also reported a higher level of p27 in small-cell neuroendocrine lung carcinomas compared with non-small-cell tumors. As small-cell lung carcinomas have a high proliferation rate, one would predict low levels of p27 protein, as in many tissues there is an inverse relationship between tumor proliferation and p27 expression. However, small-cell carcinoma is known to have genetic defects, such as in the Rb and p53 tumor suppressor genes and over expression of c-myc, 91 that might allow this neoplasm to proliferate despite high p27 levels. Recent reports suggest that c-myc could overcome p27-induced growth arrest by allowing cyclin E-CDK2 to function in the presence of elevated levels of p27. 92
Analysis of p27 expression in lymphomas has shown that p27 protein is present in quiescent lymphocytes within lymphoid tissues and peripheral blood. 93 Lymphomas with a low proliferative rate are mostly positive, whereas tumors with a higher growth fraction have low p27 protein levels. However, p27 expression in some high-grade, mitotically active tumors were increased, such as Burkitt’s lymphoma and large B-cell. These tumors also have mutations in the p53 pathway that might allow the cells to escape the inhibitory effects of p27. Quintanilla-Martinez et al 94 found that in lymphomas p27 expression was inversely related to cell proliferation. Interestingly, all mantle cell lymphomas lacked p27 protein. They postulated that the uncoupling of p27 protein expression from proliferation rate may be related to the high levels of cyclin D1 found in these mantle cell lymphomas.
Role of CDKIs and Cyclins in Endocrine Cell Hyperplasia and Neoplasia
With a few exceptions, endocrine tumors are usually slow growing. Compared with other types of carcinomas, endocrine tumors, such as papillary and follicular thyroid carcinomas, carcinoid tumors, islet cell carcinomas and parathyroid carcinomas, continue to grow slowly even after metastasizing. 95 These observations suggest that there is significant inhibitory control of cell proliferation in most endocrine tumors and possibly a greater role of CDKIs in endocrine cell proliferation. In addition to genetic alterations in suppressor genes and oncogenes, 96-104 several lines of evidence point to an increasing importance of cell cycle protein dysregulation during the development of endocrine tumors 104-111 (Figure 5) ▶ .
Figure 5.
Schematic model showing the influence of p27, other CDKIs and cyclins on the development of endocrine cell hyperplasia and neoplasia. Dysregulation of various CDKIs, including p27, p16, p21, and p57 and of the D-type cyclins have been observed in various proliferating endocrine tissues (see text). Dysregulation of CDKIs and cyclins leading to increased cell proliferation in adult endocrine tissues increases the likelihood of developing genetic alterations resulting in tumor development.
Studies of parathyroid tumors showed that the PRAD1 oncogene represented a rearrangement of cyclin D1 with a pericentric inversion on chromosome 11. 99,100 Point mutations in PRAD1 are not needed for tumorigenesis. 107 Dysregulation of CDKI in parathyroid adenomas and carcinomas has been reported, especially with p27. 88 Pituitary adenomas and carcinomas were found to have dysregulation of various CDKIs, including p16 and p27. Woloshak et al 105,106 observed decreased expression of p16 in pituitary adenomas compared with normal pituitaries, and they showed that this decreased expression is due to increased methylation of the p16 gene. Similarly, Jin et al 89 and Dahia et al 90 showed dysregulation of p27 during progression from normal pituitary to adenomas and carcinomas. Dahia et al 90 studied corticotroph adenomas and carcinomas and reported loss of p27 expression in most pituitary carcinomas. Our laboratory has shown that two mechanisms of regulation of p27 occur in endocrine cells and tumors, the ubiquitin-proteasome pathway 54,57 and p27 gene methylation 65 with the former being the more common mechanism. Our studies have also shown cyclin D expression in pituitaries with a shift from cyclin D2 in normal pituitary to cyclin D3 in immortalized pituitary tumors such as GH3. 108
In the adrenal cortex another CDKI, p57kip2 (p57), is implicated in adrenal cortical tumorigenesis. Mice lacking p57 develop adrenal cortical hyperplasia and cytomegaly. 103,104 The levels of p57 mRNA are high in normal adrenal cortex and very low in some adrenal cortical adenomas and carcinomas, indicating decreased expression of this CDKI with tumorigenesis. Although the mechanism of regulation of p57 has not been elucidated in adrenal cortical tumors, the changes in mRNA indicate that post-translational regulation may not be as important as methylation or other epigenetic changes. 109
Numerous abnormalities in oncogenes and tumor suppressor genes have been identified in thyroid tumors and are implicated in tumor progression. 101,110 However, dysregulation of CDKIs and cyclins also play important roles in thyroid tumor progression, as p27 protein levels are much higher in thyroid adenomas and normal thyroids compared with carcinomas. The CDKI p21, which is a downstream mediator of p53, has been implicated in thyroid tumorigenesis. Zedenius et al showed that thyroid tumors with p53 mutations have markedly reduced p21 expression. 111,112 Cyclin D1 overexpression has also been observed in Hurthle cell carcinomas compared to adenomas (L. A. Erickson, L. Jin, J. R. Goellner, L. R. Zukerberg, R. V. Lloyd, unpublished observations).
These data highlight the increasing importance of CDKIs and cyclins in regulating endocrine tumor development and progression and should provide models to study the mechanisms involved in differentiation and tumor development in endocrine tissues.
Future Challenges
Although a great deal of knowledge about the role of p27 in cell cycle progression and tumor development has accumulated, there are still many unanswered questions. Preliminary evidence suggests that c-myc may regulate p27 levels. 92 Other studies indicate that cyclin E, 28 Stat proteins (signal transducer and activator of transcription 6) in lymphoid cells, 113 and cyclin D 114 can also regulate p27 levels in some cells. A recent study using cultured astrocytes indicated that multiple CDKIs are necessary to maintain cell cycle progression in this system. 115 Some tumors, such as mantle cell lymphomas, overexpress cyclin D1 but have very little p27. 94 Other studies have shown an interaction of p27 and p21 in some tumors with the cleavage of both p27 and p21 resulting in activation of CDK2, leading to increased apoptosis in some cells. 38 More experimental data about the interaction of p27 with other cell cycle regulatory proteins are needed.
The rarity of mutations and other genetic alterations in the p27 gene during tumor development is not consistent with its role as a tumor suppressor gene. Investigations into whether there are mutations or other genetic alterations in the ubiquitin-proteasome system leading to increased degradation of p27 and other cell cycle proteins during tumor progression are needed, and the experimental tools are available to address these questions. The recent observations of increased p27 in several human breast cancer cell lines compared with cell lines from normal mammary epithelial cells were surprising and difficult to explain. 116 Additional experiments done by transfecting normal and neoplastic mammary lines with a vector containing p27 showed that the increased expression of p27 was associated with decreased cyclin D1 in the neoplastic MCF7 cell line, but not in the normal cell line, and slightly increased levels of cyclin E protein in both cell lines 117 indicated that the role of multiple interacting CDKIs and cyclins in regulating G1 to S progression and their synchronous dysregulation during tumor development requires additional studies. With the complex interactions of CDKIs, CDKs, and cyclins, there is an increased likelihood of alterations of these genes and/or their protein products. Finally, analysis of the roles of specific growth factors, hormones, and other influences on p27, other CDKI, CDKs, and cyclins should provide new insights into the mechanisms underlying the molecular changes leading to cellular differentiation or tumorigenesis. 118
Note Added in Proof
Since submission of this review, two significant studies have been published that provide new insights into the role of p27 in tumorigenesis. Franklin et al 119 showed that p27 and p18 mediate two separate pathways to collaboratively suppress pituitary tumorigenesis, possibly by controlling the function of Rb. Fero et al 120 showed that p27 is haplo-insufficient for tumor suppression, belonging to a new class of tumor suppressor genes.
Footnotes
Address reprint requests to Dr. R. V. Lloyd, Department of Laboratory Medicine and Pathology, Mayo Clinic, 200 First Street, SW, Rochester, MN 55905. E-mail: lloyd.ricardo@mayo.edu.
Supported in part by NIH grants CA42951 and CA37238.
References
- 1.Sherr CJ: G1 phase progression: cycling on cue. Cell 1994, 79:551-555 [DOI] [PubMed] [Google Scholar]
- 2.Hunter T, Pines J: Cyclins and cancer. II. Cyclin D and CDK inhibitors come of age. Cell 1994, 79:573-582 [DOI] [PubMed] [Google Scholar]
- 3.Sherr CJ, Roberts JM: Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev 1995, 9:1149-1163 [DOI] [PubMed] [Google Scholar]
- 4.Kamb A: Cell-cycle regulators and cancer. Trends Genet 1995, 11:136-140 [DOI] [PubMed] [Google Scholar]
- 5.Massague J, Polyak K: Mammalian antiproliferative signals and their targets. Curr Opin Genet Dev 1995, 5:91-96 [DOI] [PubMed] [Google Scholar]
- 6.Clurman BE, Roberts JM: Cell cycle and cancer. J Natl Cancer Inst 1995, 87:1499-1501 [DOI] [PubMed] [Google Scholar]
- 7.Sherr CJ: Cancer cell cycles. Science 1996, 274:1672-1677 [DOI] [PubMed] [Google Scholar]
- 8.Guan KL, Jenkins CW, Li Y, Nichols MA, Wu X, O’Keefe CL, Matera AG, Xiong Y: Growth suppression by p18, a p16INK4/MTS1- and p14 INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev 1994, 8:2939-2952 [DOI] [PubMed] [Google Scholar]
- 9.Hirai H, Roussel MF, Kato JY, Ashmun RA, Sherr CJ: Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol Cell Biol 1995, 15:2672-2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan FK, Zhang J, Cheng L, Shapiro DN, Winoto A: Identification of human and mouse p19, a novel CDK4 and CDK6 inhibitor with homology to p16ink4. Mol Cell Biol 1995, 15:2682-2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirama T, Koeffler HP: Role of the cyclin-dependent kinase inhibitors in the development of cancer. Blood 1995, 86:841-854 [PubMed] [Google Scholar]
- 12.Jin X, Nguyen D, Zhang WW, Kyritsis AP, Roth JA: Cell cycle arrest and inhibition of tumor cell proliferation by the p16/INK4 gene mediated by an adenovirus vector. Cancer Res 1995, 55:3250-3253 [PubMed] [Google Scholar]
- 13.Reed JA, Loganzo F, Jr, Shea CR, Walker GJ, Flores JF, Glendening JM, Bogdany JK, Shiel MJ, Haluska FG, Fountain JW: Loss of expression of the p16/cyclin-dependent kinase inhibitor 2 tumor suppressor gene in melanocytic lesions correlates with invasive stage of tumor progression. Cancer Res 1995, 55:2713-2718 [PubMed] [Google Scholar]
- 14.Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J: Cloning of p27kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 1994, 78:59-66 [DOI] [PubMed] [Google Scholar]
- 15.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D: p21 is a universal inhibitor of cyclin kinases. Nature 1993, 366:701-704 [DOI] [PubMed] [Google Scholar]
- 16.Toyoshima H, Hunter T: p27, a novel inhibitor of G1 cyclin-cdk protein kinase activity, is related to p21. Cell 1994, 78:67-74 [DOI] [PubMed] [Google Scholar]
- 17.El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B: WAF1, a potential mediator of p53 tumor suppression. Cell 1993, 75:817-825 [DOI] [PubMed] [Google Scholar]
- 18.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A: p27 kip1, a cyclin-Cdk inhibitor links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev 1994, 8:9-22 [DOI] [PubMed] [Google Scholar]
- 19.Matsuoka S, Edwards MC, Bai C, Parker S, Zhang P, Baldini A, Harper JW, Elledge SJ: p57/Kip2, a structurally distinct member of the p21/CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev 1995, 9:650-662 [DOI] [PubMed] [Google Scholar]
- 20.Lee MH, Reynisdottir L, Massague J: Cloning of p57 Kip2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev 1995, 9:639-649 [DOI] [PubMed] [Google Scholar]
- 21.Ponce-Castaneda MV, Lee M-H, Latres E, Polyak K, Lacombe L, Montgomery K, Mathew S, Krauter K, Sheinfeld J, Massague J, Cordon-Cardo C: p27kip1 chromosomal mapping to 12p 12–12p 13.1 and absence of mutations in human tumors. Cancer Res 1995, 55:1211-1214 [PubMed] [Google Scholar]
- 22.Kato JY, Matsuoka M, Polyak K, Massagué J, Sherr CJ: Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27kip1) of cyclin-dependent kinase 4 activation. Cell 1994, 79:487-496 [DOI] [PubMed] [Google Scholar]
- 23.Slingerland J, Hengst L, Pan C, Alexander D, Stampfer M, Reed S: A novel inhibitor of cyclin-Cdk activity detected in TGF-β-arrested epithelial cells. Mol Cell Biol 1994, 14:3683-3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo AA, Jeffrey PD, Patten AK, Massague J, Pavletich NP: Crystal structures of the p27kip1 cyclin dependent-kinase inhibitor bound to the cyclin A-cdk 2 complex. Nature 1996, 382:325-331 [DOI] [PubMed] [Google Scholar]
- 25.Koff A, Ohtsuki ME, Polyak K, Roberts J, Massagué J: Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF β. Science 1993, 257:1689-1694 [DOI] [PubMed] [Google Scholar]
- 26.Firpo EJ, Koff A, Solomon MJ, Roberts JM: Inactivation of a Cdk2 inhibitor during interleukin-2 induced proliferation of human T lymphocytes. Mol Cell Biol 1994, 14:4889-4901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coats S, Flanagen M, Nourse J, Roberts JM: Requirement of p27kip1 for restriction point control of the fibroblast cell cycle. Science 1996, 272:877-880 [DOI] [PubMed] [Google Scholar]
- 28.Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE: Cyclin E-CDK2 is a regulator of p27kip1. Genes Dev 1997, 11:1464-1478 [DOI] [PubMed] [Google Scholar]
- 29.Takuwa N, Takuwa Y: Ras activity late in G1 phase required for p27kip1 down regulation, passage through the restriction point and entry into S phase in growth factor-stimulated NIH 3T3 fibroblasts. Mol Cell Biol 1997, 17:5348-5358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravitz MJ, Yan S, Herr KD, Wenner CE: Transforming growth factor β-induced activation of cyclin E-CDK2 kinase and down-regulation of p27kip1 in C3H 10 T1/2 mouse fibroblasts. Cancer Res 1995, 55:1413-1416 [PubMed] [Google Scholar]
- 31.Mal A, Poon RY, Howe PH, Toyoshima H, Hunter T, Harter ML: Inactivation of p27kip1 by the viral E1A oncoprotein in TGF-β treated cells. Nature 1996, 380:262-265 [DOI] [PubMed] [Google Scholar]
- 32.Qian X, Jin L, Grande JP, Lloyd RV: Transforming growth factor-β and p27 expression in pituitary cells. Endocrinology 1996, 137:3051-3060 [DOI] [PubMed] [Google Scholar]
- 33.Morosetti R, Kawamata N, Gombart AF, Miller CW, Hatta Y, Hirama T, Said JW, Tomonaga M, Koeffler HP: Alterations of the p27/kip1 gene in non-Hodgkin’s lymphomas and adult T-cell leukemia/lymphoma. Blood 1995, 86:1924-1930 [PubMed] [Google Scholar]
- 34.Spirin KS, Simpson JF, Takeuchi S, Kawamata N, Miller CW, Koeffler HP: p27/kip1 mutation found in breast cancer. Cancer Res 1996, 56:2400-2404 [PubMed] [Google Scholar]
- 35.St Croix B, Florenes VA, Rak JW, Flanagan M, Bhattacharya N, Slingerland JM, Kerbel RS: Impact of the cyclin-dependent kinase inhibitor p27kip1 on resistance of tumor cells to anti-cancer agents. Nature Med 1996, 2:1204-1210 [DOI] [PubMed] [Google Scholar]
- 36.Tanaka C, Yoshimoto K, Yang P, Kimura T, Yamada S, Moritani M, Saro T, Itakura M: Infrequent mutations of p27kip1 gene and trisomy 12 in a subset of human pituitary adenomas. J Clin Endocrinol Metab 1997, 82:3141-3147 [DOI] [PubMed] [Google Scholar]
- 37.Katayose Y, Kim M, Rakkar ANS, Li Z, Cowan KH, Seth P: Promoting apoptosis: a novel activity associated with the cyclin-dependent kinase inhibitor p27. Cancer Res 1997, 57:5441-5445 [PubMed] [Google Scholar]
- 38.Levkau B, Koyama H, Raines EW, Clurman BE, Herren B, Orth K, Roberts JM, Ross R: Cleavage of p21cipl/wafl and p27kipl mediates apoptosis in endothelial cells through activation of Cdk2: role of a caspace cascade. Mol Cell 1998, 1:553-563 [DOI] [PubMed] [Google Scholar]
- 39.Zabludoff SD, Csete M, Wagner R, Yu X, Wold BJ: p27kip1 is expressed transiently in developing myotomes and enhances myogenesis. Cell Growth Differ 1998, 9:1-11 [PubMed] [Google Scholar]
- 40.Durand B, Gao FB, Raff M: Accumulation of the cyclin-dependent kinase inhibitor p27/kip1 and the timing of oligodendrocyte differentiation. EMBO J 1997, 16:306-317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onishi T, Hruska K: Expression of p27kip1 in osteoblast-like cells during differentiation with parathyroid hormone. Endocrinology 1997, 138:1995-2004 [DOI] [PubMed] [Google Scholar]
- 42.Robker RL, Richards JS: Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin-D2 and p27kip1. Mol Endocrinol 1998, 12:924-940 [DOI] [PubMed] [Google Scholar]
- 43.Ophascharoensuk V, Fero ML, Hughes J, Roberts JM, Shankland SJ: The cyclin-dependent kinase inhibitor p27kip1 safeguards against inflammatory injury. Nature Med 1998, 4:575-580 [DOI] [PubMed] [Google Scholar]
- 44.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY, Nakayama KI: Mice lacking p27kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 1996, 85:707-720 [DOI] [PubMed] [Google Scholar]
- 45.Kiyokawa H, Kinerman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A: Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27 kip1. Cell 1996, 85:721-732 [DOI] [PubMed] [Google Scholar]
- 46.Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai LH, Broudy V, Perlmutter RM, Kaushansky K, Roberts JM: A syndrome of multiorgan hyperplasia with features of gigantism, tumor genesis and female sterility in p27kip1-deficient mice. Cell 1996, 85:733-744 [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Willingham T, Shuford M, Nisen PD: Tumor suppression and inhibition of aneuploid cell accumulation in human brain tumor cells by ectopic overexpression of the cyclin-dependent kinase inhibitor p27kip1. J Clin Invest 1996, 97:1983-1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.St Croix B, Sheehan C, Rak JW, Florenes VA, Slingerland JM, Kerbel RS: E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27kip1. J Cell Biol 1998, 142:557-571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas GV, Szigeti K, Murphy M, Draetta G, Pagano M, Loda M: Down regulation of p27 is associated with development of colorectal adenocarcinoma metastases. Am J Pathol 1998, 153:681-687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poon RY, Toyoshima H, Hunter T: Redistribution of the CDK inhibitor p27 between different cyclin-CDK complexes in the mouse fibroblast cell cycle and in cells arrested with lovastatin or ultraviolet irradiation. Mol Biol Cell 1995, 6:1197-1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nourse J, Firpo E, Flanagan UM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts J: Interleukin-2-mediated elimination of p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature 1994, 372:570-573 [DOI] [PubMed] [Google Scholar]
- 52.Watts CK, Brady A, Sarcevic B, deFazio A, Musgrove EA, Sutherland RL: Anti-estrogen inhibition of cell cycle progression in breast cancer cells is associated with inhibition of cyclin-dependent kinase activity and decreased retinoblastoma protein phosphorylation. Mol Endocrinol 1995, 9:1804-1813 [DOI] [PubMed] [Google Scholar]
- 53.Halevy O, Novitch BG, Spicer DB, Skapek SX, Rhee J, Hannon GJ, Beach D, Lassar AB: Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science 1995, 267:1018-1021 [DOI] [PubMed] [Google Scholar]
- 54.Pagano M, Tam SW, Theodoras AM, Romero-Beer P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M: Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 1995, 269:682-685 [DOI] [PubMed] [Google Scholar]
- 55.Morisaki H, Fujimoto A, Ando A, Nagata Y, Ikeda K, Nakanishi M: Cell cycle-dependent phosphorylation of p27 cyclin-dependent kinase (cdk) inhibitor of cyclin E Cdk2. Biochem Biophy Res Commun 1997, 240:386-390 [DOI] [PubMed] [Google Scholar]
- 56.Vlach J, Hennrecke S, Amati B: Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27kip1. EMBO J 1997, 16:5334-5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hengst L, Reed SI: Translational control of p27kipl accumulation during the cell cycle. Science 1996, 271:1861-1864 [DOI] [PubMed] [Google Scholar]
- 58.Alessandrini A, Chiaur DS, Pagano M: Regulation of the cyclin-dependent kinase inhibitor p27 by degradation and phosphorylation. Leukemia 1997, 11:342-345 [DOI] [PubMed] [Google Scholar]
- 59.Goldberg AL: Functions of the proteasome: the lysis at the end of the tunnel. Science 1995, 268:522-523 [DOI] [PubMed] [Google Scholar]
- 60.Rubin DM, Finley D: Proteolysis. The proteasome: a protein-degrading organelle? Curr Biol 1995, 5:854-858 [DOI] [PubMed] [Google Scholar]
- 61.Brandeis M, Hunt T: The proteolysis of mitotic cyclins in mammalian cells persists from the end of mitosis until the onset of S phase. EMBO J 1996, 15:5280-5289 [PMC free article] [PubMed] [Google Scholar]
- 62.Uren A, Jaksu J, de Mara JF, Yeudall A, Sontos E, Gutkind S, Heidaran MA: Carboxyl-terminal domain of p27kip1 activates CDC2. J Biol Chem 1997, 272:21669-21672 [DOI] [PubMed] [Google Scholar]
- 63.Millard SS, Yan JS, Nguyen H, Pagano M, Kiyokawa H, Koff A: Enhanced ribosomal association of p27Kip1 mRNA is a mechanism contributing to accumulation during growth arrest. J Biol Chem 1997, 272:7093-7098 [DOI] [PubMed] [Google Scholar]
- 64.Hochstrasser M: Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Curr Opin Cell Biol 1995, 7:215-223 [DOI] [PubMed] [Google Scholar]
- 65.Feldman RMR, Correll CC, Kaplan KB, Deshaies RJ: A complex of Cdc4p, Skp1p and Cdc53p/Cullin catalyzes ubiquination of the phosphorylated CDK inhibitor Sic1p. Cell 1997, 91:221-230 [DOI] [PubMed] [Google Scholar]
- 66.Qian X, Jin L, Kulig E, Lloyd RV: DNA methylation regulates p27kipl expression in rodent pituitary cell lines. Am J Pathol 1998, 153:1475-1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steeg PS, Abram JS: Cancer prognostics: post, present and p27. Nature Med 1997, 3:152-154 [DOI] [PubMed] [Google Scholar]
- 68.Catzavelos C, Bhattacharya N, Ung YC, Wilson JA, Roncari L, Sandhu C, Shaw P, Yeger H, Marava-Protzner I, Kapuska L, Franssen E, Pritchard KI, Slingerland JM: Decreased levels of the cell-cycle inhibitor p27kip1 protein: prognostic implications in primary breast cancer. Nature Med 1997, 3:227-230 [DOI] [PubMed] [Google Scholar]
- 69.Porter PL, Malone KE, Heagerty PJ, Alexander GM, Gatti LA, Firpo EJ, Daling JR, Roberts JM: Expression of cell-cycle regulators p27kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nature Med 1997, 3:222-225 [DOI] [PubMed] [Google Scholar]
- 70.Tan P, Cady B, Wanner M, Worland P, Cukor B, Magi-Galluzzi C, Lavin P, Draetta G, Pagano M, Loda M: The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a,b) invasive breast carcinoma. Cancer Res 1997, 57:1259-1263 [PubMed] [Google Scholar]
- 71.Fredersdorf S, Burns J, Milne AM, Packham G, Fallis L, Gillett CE, Royds JA, Peston D, Hall PA, Hanby AM, Barnes DM, Shousha S, O’Hare MJ, Lu X: High level expression of p27kip1 and cyclin D1 in some human breast cancer cells: inverse correlation between the expression of p27kip1 and degree of malignancy in human breast and colorectal cancers. Proc Natl Acad Sci USA 1997, 94:6380-6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M: Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinoma. Nature Med 1997, 3:231-234 [DOI] [PubMed] [Google Scholar]
- 73.Ciaparrone M, Yamamoto H, Yao Y, Sgambato A, Cattoretti G, Tomita N, Monden T, Rotterdam H, Weinstein IB: Localization and expression of p27kip1 in multistage colorectal carcinogenesis. Cancer Res 1998, 58:114-122 [PubMed] [Google Scholar]
- 74.Singh SP, Lipman J, Goldman H, Ellis FH, Jr, Aizenman L, Cangi MG, Signoretti S, Chiaur DS, Pagano M, Loda M: Loss or altered subcellular localization of p27 in Barrett’s associated adenocarcinoma. Cancer Res 1998, 58:1730-1735 [PubMed] [Google Scholar]
- 75.Mori M, Mimori K, Shiraishi T, Tanaka S, Ueo H, Susimachi K, Akiyoshi T: p27 expression and gastric carcinoma. Nature Med 1997, 3:593. [DOI] [PubMed] [Google Scholar]
- 76.Esposito V, Baldi A, DeLuca A, Groger AM, Loda M, Giordano GG, Caputi M, Baldi F, Pagano M, Giordano A: Prognostic role of the cyclin-dependent kinase inhibitor p27 in non-small cell lung cancer. Cancer Res 1997, 57:3381-3385 [PubMed] [Google Scholar]
- 77.Kawana H, Tamaru J, Tanaka T, Hirai A, Saito Y, Kitagawa M, Mikata A, Harigaya K, Kuriyama T: Role of p27Kip1 and cyclin-dependent kinase 2 in the proliferation of non-small cell lung cancer. Am J Pathol 1998, 153:505-513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yatabe Y, Masuda A, Koshikawa T, Nakamura S, Kuroishi T, Osada H, Takahashi T, Mitsudomi T, Takahashi T: p27kip1 in human lung cancers: differential changes in small cell and non-small cell carcinomas. Cancer Res 1998, 58:1042-1047 [PubMed] [Google Scholar]
- 79.Tsihlias J, Kapusta LR, DeBuer G, Morava-Protzner I, Zbieranowski I, Bhattacharya N, Catzavelos GC, Klotz LH, Slingerland JM: Loss of cyclin-dependent kinase inhibitor p27kip1 is a novel prognostic factor in localized human prostate adenocarcinoma. Cancer Res 1998, 58:542-548 [PubMed] [Google Scholar]
- 80.Yang RM, Naitoh HJ, Murphy M, Wang H-J, Phillipson J, DeKernion JB, Loda M, Reiter RE: Low p27 expression predicts poor disease-free survival in patients with prostate cancer. J Urol 1998, 159:941-945 [PubMed] [Google Scholar]
- 81.Cheville JC, Lloyd RV, Sebo TJ, Cheng L, Erickson L, Bostwick DG, Lohse CM, Wollan P: Expression of p27kipl in prostatic adenocarcinoma. Mod Pathol 1998, 11:324-328 [PubMed] [Google Scholar]
- 82.Cordon-Cardo C, Koff A, Drobnjak M, Capodieci P, Osman I, Millard SS, Gaudin PB, Fazzari M, Zhang Z-F, Massague J, Scher HI: Distinct altered patterns of p27Kip1 gene expression in benign prostatic hyperplasia and prostatic carcinoma. J Natl Cancer Inst 1998, 90:1284-1291 [DOI] [PubMed] [Google Scholar]
- 83.Florenes VA, Maelandsmo GM, Kerbel RS, Slingerland JM, Nesland JM, Holm R: Protein expression of the cell-cycle inhibitor p27kip1 in malignant melanoma: inverse correlation with disease-free survival. Am J Pathol 1998, 153:305-312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jordan RC, Bradley G, Slingerland J: Reduced levels of the cell-cycle inhibitor p27kip1 in epithelial dysplasia and carcinoma of the oral cavity. Am J Pathol 1998, 152:585-590 [PMC free article] [PubMed] [Google Scholar]
- 85.Lloyd RV, Jin L, Qian X, Kulig E: Aberrant p27kipl expression in endocrine and other tumors. Am J Pathol 1997, 150:401-407 [PMC free article] [PubMed] [Google Scholar]
- 86.Erickson LA, Jin L, Wollan PC, Thompson GB, van Heerden J, Lloyd RV: Expression of p27kipl and Ki-67 in benign and malignant thyroid tumors. Mod Pathol 1998, 11:169-174 [PubMed] [Google Scholar]
- 87.Lloyd RV, Ferrero JA, Jin L, Sebo T: TGF-β, TGF-β receptors: Ki-67 and p27kipl expression in papillary thyroid carcinomas. Endocr Pathol 1997, 4:293-300 [DOI] [PubMed] [Google Scholar]
- 88.Erickson LA, Jin L, Wollan P, Thompson GB, van Heerden JA, Lloyd RV: Parathyroid hyperplasia, adenomas and carcinomas: differential expression of p27kipl protein. Am J Surg Pathol 1999 (in press) [DOI] [PubMed]
- 89.Jin L, Qian X, Kulig E, Sanno N, Scheithauer BW, Kovacs K, Young WF, Jr, Lloyd RV: Transforming growth factor-β, transforming growth factor-β receptor II, and p27kipl expression in nontumorous and neoplastic human pituitaries. Am J Pathol 1997, 151:509-519 [PMC free article] [PubMed] [Google Scholar]
- 90.Dahia PL, Aguiar RC, Honegger J, Fahlbush R, Jordan S, Lowe DG, Lu X, Clayton RN, Besser GM, Grossman AB: Mutation and expression analysis of the p27kipl gene in corticotropin-secreting tumors. Oncogene 1998, 16:69-76 [DOI] [PubMed] [Google Scholar]
- 91.Minna JB, Nau MM, Chiba I, Ihde DC: Lung cancer. Denita VT Rosenberg S Hellmen S eds. Principles and Practice of Oncology. 1989, :pp 591-705 JB Lippincott, Philadelphia [Google Scholar]
- 92.Vlach J, Hennecke S, Alevizopoulos K, Conti D, Amati B: Growth arrest by the cyclin-dependent kinase inhibitor p27kipl is abrogated by c-Myc. EMBO J 1996, 15:6595-6604 [PMC free article] [PubMed] [Google Scholar]
- 93.Sanchez-Beato M, Saez AI, Martinez-Montero JC, Sol Mateo M, Sanchez-Verde L, Villuendas R, Troncone G, Piris MA: Cyclin-dependent kinase inhibitor p27kipl in lymphoid tissue. P27kipl expression is inversely proportional to the proliferative index. Am J Pathol 1997, 151:151-160 [PMC free article] [PubMed] [Google Scholar]
- 94.Quintanilla-Martinez L, Thieblemant C, Fend F, Kumar S, Pinyal M, Campo E, Jaffe ES, Raffeld M: Mantle cell lymphomas lack expression of p27kipl, a cyclin-dependent kinase inhibitor. Am J Pathol 1998, 153:175-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kovacs K, Asa SL: Functional endocrine pathology. Malden, MA, Blackwell Science, 1998
- 96.Shirman I, Melmed S: Genetic basis of endocrine disease: pituitary tumor pathogenesis. J Clin Endocrinol Metab 1997, 82:1675-1681 [DOI] [PubMed] [Google Scholar]
- 97.Prezant TR, Levine J, Melmed S: Molecular characterization of the MEN I tumor suppressor gene in sporadic pituitary tumors. J Clin Endocrinol Metab 1998, 83:1388-1391 [DOI] [PubMed] [Google Scholar]
- 98.Clayton RN, Bogglid M, Bates AS, Bicknell J, Simpson D, Farrell W: Tumor suppressor genes in the pathogenesis of human pituitary tumors. Horm Res 1997, 47:185-193 [DOI] [PubMed] [Google Scholar]
- 99.Arnold A: Genetic basis of endocrine diseases: molecular genetics of parathyroid gland neoplasia. J Clin Endocrinol Metab 1993, 77:1108-1112 [DOI] [PubMed] [Google Scholar]
- 100.Arnold A: Molecular mechanisms of parathyroid neoplasia. Endocrinol Metab Clin North Am 1994, 23:93-107 [PubMed] [Google Scholar]
- 101.Farid NR, Shi Y, Zou M: Molecular basics of thyroid cancer. Endocr Rev 1994, 15:202-232 [DOI] [PubMed] [Google Scholar]
- 102.Gicquel C, Bertagna X, LeBouc Y: Recent advances in the pathogenesis of adrenocortical tumors. Eur J Endocrinol 1995, 133:133-144 [DOI] [PubMed] [Google Scholar]
- 103.Zhang P, Liegeois NJ, Wong C, Finegold M, Hou H, Thompson JC, Silverman A, Harper JW, DePinho RA, Elledge SJ: Altered cell differentiation and proliferation in mice lacking p57kip2 indicate a role in Beckwith-Wiedemann syndrome. Nature 1997, 387:151-158 [DOI] [PubMed] [Google Scholar]
- 104.Liu J, Kahri AI, Heikkila P, Voutilainen R: Ribonucleic acid expression of the clustered imprinted genes, p57kip2, insulin-like growth factor II, and H19, in adrenal tumors and cultured adrenal cells. J Clin Endocrinol Metab 1997, 82:1766-1771 [DOI] [PubMed] [Google Scholar]
- 105.Woloschak M, Yu A, Xiao J, Post KD: Frequent loss of the p16INK4a gene product in human pituitary tumors. Cancer Res 1996, 56:2493-2496 [PubMed] [Google Scholar]
- 106.Woloschak M, Yu A, Post KD: Frequent inactivation of the p16 gene in human pituitary tumors by gene methylation. Mol Carcinog 1997, 19:221-224 [DOI] [PubMed] [Google Scholar]
- 107.Arnold A, Kim HG, Gaz RD, Eddy RL, Fukushima Y, Byers MG, Shows TB, Kronenberg HM: Molecular cloning and chromosomal mapping of DNA rearranged with the parathyroid hormone gene in a parathyroid adenoma. J Clin Invest 1989, 83:2034-2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qian X, Kulig E, Jin L, Lloyd RV: Expression of D-type cyclins in normal and neoplastic rat pituitary. Endocrinology 1998, 139:2058-2067 [DOI] [PubMed] [Google Scholar]
- 109.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP: Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res 1998, 72:141-196 [PubMed] [Google Scholar]
- 110.Fagin JA: Genetic basis of endocrine disease. III. Molecular defects in thyroid gland neoplasia. J Clin Endocrinol Metab 1992, 75:1398-1400 [DOI] [PubMed] [Google Scholar]
- 111.Namba H, Hara T, Tukazaki T, Migita K, Ishikama N, Iro K, Nagataki S, Yamashita S: Radiation-induced G1 arrest is selectively mediated by the p53-WAF/Cip1 pathway in human thyroid cells. Cancer Res 1995, 55:2075-2080 [PubMed] [Google Scholar]
- 112.Zedenius J, Larsson C, Wallin G, Bäckdahl M, Aspenblad U, Höög A, Børresen A-L, Auer G: Alterations of p53 and expression of WAF1/p21 in human thyroid tumors. Thyroid 1996, 6:1-9 [DOI] [PubMed] [Google Scholar]
- 113.Kaplan MH, Daniel C, Schindler U, Grusby MJ: Stat protein control lymphocyte proliferation by regulating p27kipl expression. Mol Cell Biol 1998, 18:1996-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cheng M, Sexl V, Sherr CJ, Roussel MF: Assembly of cyclin D-dependent kinase and titration of p27kipl regulated by mitogen-activated protein kinase (MEK1). Proc Natl Acad Sci USA 1998, 95:1091-1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pedrem A, Razandi M, Hu R-M, Levin ER: Astrocyte progression from G1 to S phase of the cell cycle depends upon multiple protein interaction. J Biol Chem 1998, 273:13966-13972 [DOI] [PubMed] [Google Scholar]
- 116.Sgambato A, Zhang YJ, Arber N, Hibsoosh N, Doki Y, Ciaparrone M, Santella RM, Cittadini A, Weinstein IB: Deregulated expression of p27Kip1 in human breast cancers. Clin Cancer Res 1997, 3:1879-1887 [PubMed] [Google Scholar]
- 117.Dgambato A, Zhang YJ, Ciaparrone M, Soh JW, Cittadini A, Santella RM, Weinstein IB: Overexpression of p27Kip1 inhibits the growth of both normal and transformed human mammary epithelial cells. Cancer Res 1998, 58:3448-3454 [PubMed] [Google Scholar]
- 118.Jacks T, Weinberg RA: The expanding role of cell cycle regulators. Science 1998, 280:1035-1036 [DOI] [PubMed] [Google Scholar]
- 119.Franklyn DS, Godfrey VL, Lee H, Koralev GI, Schoonhoven R, Chen-Kiang S, Su L, Xiong Y: CDK inhibitors p18INK4C and p27Kip1 mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev 1998, 12:2899-2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fero ML, Rande IE, Gurley KE, Roberts JM, Kemp CJ: The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature 1998, 396:177-180 [DOI] [PMC free article] [PubMed] [Google Scholar]