Abstract
Vascular endothelial growth factor (VEGF) is an endothelial cell mitogen and permeability factor that is potently angiogenic in vivo. We report here studies that suggest that VEGF potentiates angiogenesis in vivo and prolongs the survival of human dermal microvascular endothelial cells (HDMECs) in vitro by inducing expression of the anti-apoptotic protein Bcl-2. Growth-factor-enriched and serum-deficient cultures of HDMECs grown on collagen type I gels with VEGF exhibited a 4-fold and a 1.6-fold reduction, respectively, in the proportion of apoptotic cells. Enhanced HDMEC survival was associated with a dose-dependent increase in Bcl-2 expression and a decrease in the expression of the processed forms of the cysteine protease caspase-3. Cultures of HDMECs transduced with and overexpressing Bcl-2 and deprived of growth factors showed enhanced protection from apoptosis and exhibited a twofold increase in cell number and a fourfold increase in the number of capillary-like sprouts. HDMECs overexpressing Bcl-2 when incorporated into polylactic acid sponges and implanted into SCID mice exhibited a sustained fivefold increase in the number of microvessels and a fourfold decrease in the number of apoptotic cells when examined 7 and 14 days later. These results suggest that the angiogenic activity attributed to VEGF may be due in part to its ability to enhance endothelial cell survival by inducing expression of Bcl-2.
VEGF is a potent endothelial-cell-specific mitogen and angiogenic factor that has been shown to play a central role in neovascular responses that accompany a number of physiological and pathological processes. 1-4 VEGF is produced by a variety of cell types, including keratinocytes, 5 macrophages, 6 mast cells, 7 and smooth muscle cells. 8 VEGF is also produced by several types of tumors 2,3,9 where it has been shown to influence both tumor neovascularization and tumor dissemination. 10
The angiogenic property of VEGF has been attributed to several distinct functions associated with this cytokine. VEGF is an endothelial cell mitogen and permeability-enhancing factor that influences the egress of plasma proteins and cells that both directly and indirectly stimulate angiogenesis. 11-13 Recent studies suggest that VEGF may also function as a survival factor for endothelial cells. 14-16 Spyridopoulos reported that VEGF is able to support the survival of endothelial cells exposed to the apoptosis-inducing cytokine tumor necrosis factor (TNF)-α. 15 Watanabe and Dvorak 16 have shown that endothelial cells exposed to VEGF used vitronectin and α5β5 to sustain their survival when grown on a nonsupportive (hydrophobic polystyrene) surface. In both instances, VEGF was able to enhance the survival of endothelial cells by inducing endothelial cells to produce a scaffold of matrix molecules that maximized cell adherence and proliferation.
Apoptosis is a genetically controlled, morphologically unique form of cell death that plays a central role in reinforcing appropriate cellular patterns and in regulating cell number by eliminating cells that are harmful or no longer needed. Conversely, disruption of this program has been shown to contribute to the pathogenesis of several developmental, inflammatory and degenerative diseases, including cancer. 17,18 The Bcl-2 gene family consists of a group of homologous proteins that function to either promote or suppress cell death. 17,19-21 A role for Bcl-2 in endothelial cell survival has recently been proposed by Kondo et al. 22 They showed that aortic endothelial cells overexpressing Bcl-2 were protected from the apoptogenic effects of basic fibroblast growth factor withdrawal. More recently, Gerber et al 23 have shown that VEGF is able to protect serum-starved human umbilical vein endothelial cells from apoptosis in association with a significant up-regulation in the anti-apoptotic proteins Bcl-2 and A1.
To further define the mechanisms, by which VEGF promotes angiogenesis and the significance of enhanced endothelial cell survival in this process, we examined the effects of VEGF on the expression of several Bcl-2 family members during the development of angiogenic responses in vitro and in vivo. We report here that growth-factor-enriched and serum-deprived cultures of human dermal microvascular endothelial cells (HDMECs) grown on collagen type I gels in the presence of VEGF exhibited a significant reduction in apoptosis. Enhanced HDMEC survival was associated with a dose-dependent increase in Bcl-2 expression and a decrease in the expression of the processed forms of the cysteine protease caspase-3. HDMECs transduced with and overexpressing Bcl-2 exhibited enhanced protection from apoptosis induced by growth-factor deprivation and showed an increased responsiveness to growth stimuli and an enhanced ability to form spontaneous sprout-like structures in culture. Furthermore, we show that HDMECs overexpressing Bcl-2 exhibit a sustained increase in the number of functioning microvessels that developed at 7 and 14 days after implantation into SCID mice. Our results indicate that angiogenic responses triggered by VEGF may be due in part to its ability to enhance the survival of endothelial cells by up-regulating expression of the anti-apoptotic protein Bcl-2.
Materials and Methods
Cell Proliferation and Capillary Tube Assay
The ability of VEGF to induce endothelial cells to proliferate and organize into capillary-like sprouts was examined using HDMECs grown on type I collagen gels as previously described. 24 Briefly, 3.0 × 10 5 HDMECs (Cell Systems Corp., Kirkland, WA) were seeded in 60-mm tissue culture dishes (Corning Costar Corp., Cambridge, MA) that were coated with a 1.5 ml of gelled solution of bovine dermal type I collagen (Vitrogen 100, Collagen Biomaterials, Palo Alto, CA). Gelation was achieved by exposing the collagen solution to ammonia vapors for 15 minutes. Cells were suspended in MCDB 131 media supplemented with epidermal growth factor (10 ng/ml), hydrocortisone (1 μg/ml), bovine brain extract containing 10 μg/ml heparin, fetal bovine serum (FBS; 5%), 50 μg/ml gentamicin, and 50 ng/ml amphotericin-B (Endothelial Cell Growth Medium, EGM, Clonetics Corp., San Diego, CA), allowed to attach for 24 hours, and washed to remove nonadherent cells. Beginning on day 1 and at 2-day intervals thereafter, cells were fed with either 50 ng/ml recombinant human VEGF165 (Intergen Co., Purchase, NY) or 50 ng/ml recombinant human interleukin (IL)-8 (R&D Systems, Minneapolis, MN). At daily intervals, the number of cells in 10 random high-power fields (×200) as well as the number of capillary-like sprouts (×100) were counted as previously described. 25 The data were obtained from triplicate dishes per condition at each time point.
TUNEL Assay and Flow Cytometry
HDMECs were seeded in collagen or on the surface of plastic culture dishes and exposed for 3 days to either 50 ng/ml VEGF165 or 50 ng/ml IL-8 added to either EGM or MCDB 131 medium devoid of growth factors (Endothelial Cell Basal Medium, EBM, Clonetics Corp.) supplemented only with 1% FBS. HDMECs were then retrieved from collagen gels with a solution of 2.5 mg/ml collagenase (Sigma Chemical Co., St. Louis, MO) and from the surface culture dishes with a solution of 0.5% trypsin/5.3 mmol/L EDTA (Gibco BRL, Gaithersburg, MD), fixed in 1% paraformaldehyde for 15 minutes, and stored in 70% ethanol at −20°C. The percentage of apoptotic cells was evaluated using the APO-BRDU terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end-labeling (TUNEL) assay 26 according to the manufacturer’s instructions (Phoenix Flow Systems, Phoenix, AZ). Apoptotic cells were quantitated by flow cytometry using an argon laser excited at 488 nm (Epics XL, Coulter Corp., Hialeah, FL). A total of 10,000 cells were examined for each condition. The data shown are representative of three independent experiments.
DNA Ladder Analysis
The ability of VEGF to protect endothelial cells from DNA fragmentation was evaluated using a DNA ladder assay. 27 Briefly, HDMEC cultures grown in the presence of either 50 ng/ml VEGF165 or 50 ng/ml IL-8 in EBM (Clonetics) with 1% FBS were retrieved from gels as previously described. Cells were fixed in 70% ethanol at −20°C overnight, centrifuged, and resuspended in 40 μl of phosphate-citrate buffer at room temperature for 30 minutes. Supernatants were concentrated in a SpeedVac (Savant Instruments, Farmingdale, NY) and resuspended in 3 μl of 0.25% Nonidet P-40 (Sigma), followed by 3 μl of 1 mg/ml RNAse (Sigma). After 30 minutes of incubation at 37°C, 3 μl of 1 mg/ml proteinase K (Sigma) was added to the extract and incubated for an additional 30 minutes at 37°C. The DNA was resolved in a 1.5% agarose gel and visualized under ultraviolet light after staining with ethidium bromide. Positive controls consisted of HDMECs cultured in suspension in a solution of 1.68% methylcellulose (Sigma) for 72 hours. 28
Northern and Western Analyses
Total HDMEC RNA was extracted with TRIzol (Gibco BRL), subjected to electrophoresis in 1% agarose gel, and transferred to a Hybond nylon membrane (Amersham Corp., Arlington Heights, IL). 32P-radiolabeled cDNA probes were prepared using the random primed DNA labeling kit (Boehringer Mannheim, Indianapolis, IN). After hybridization, blots were washed and exposed to X-Omat AR film (Eastman Kodak Corp., Rochester, NY). The membranes were rehybridized with a β-actin probe to control for equal loading. For Western analysis, whole-cell lysates were prepared as described 29 and run in a 12% Tris-glycine gel at 125 V, and the protein was transferred to a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). Blots were blocked for 2 hours in PBS/0.1% Tween containing 5% nonfat dried milk and then probed overnight at 4°C with the appropriate primary antibody. The primary antibodies and the concentrations used were as follows: monoclonal hamster anti-Bcl-2, 0.1 μg/ml (15131A, Pharmingen, San Diego, CA); monoclonal mouse anti-flag, 10 μg/ml (M2, Eastman Kodak); monoclonal mouse anti-caspase-3, 1 μg/ml (AM20, Oncogene Research Products, Cambridge, MA); polyclonal rabbit anti-Bax antibody, 1/1000, and rabbit polyclonal anti-Bcl-xL/S 1/1000 (a gift from G. Nuñez). Blots were exposed for 2 hours to the appropriate peroxidase-coupled secondary antibodies (Amersham) and washed, and the bound antibody was detected with the ECL system (Amersham). The membranes were reprobed with a monoclonal mouse anti α-tubulin antibody (CP06, Calbiochem-Novabiochem Corp., San Diego, CA) to control for equal gel loading. The relative band densities were calculated with NIH Image 1.61b7 software.
Retroviral Vector Construction and HDMEC Transduction
A 753-bp cassette containing human bcl-2 from the expression plasmid pcDNA3-hu bcl-2-flag (a gift from G. Nuñez) was inserted in the EcoRI cloning site of a retroviral vector (LXSN, gift from D. Miller). 30 The Bcl-2 construct or the vector alone was transfected into PA317 amphotropic packaging cells with Superfect transfection reagent (Qiagen, San Clarita, CA). Viral supernatants from confluent cultures of vector-producing cells were collected after 24 hours, centrifuged, filtered, and stored at −70°C. HDMECs were transfected with either Bcl-2 or vector alone by incubating 1 × 10 6 HDMECs overnight with a 1/10 dilution of the viral supernatant in the presence of 4 μg/ml Polybrene (Sigma). The viral supernatant was aspirated, cells were washed with Hanks’ balanced salt solution (Gibco BRL), and EGM-MV medium containing 250 μg/ml G418 (Gibco BRL) was used to select for resistant clones. Bcl-2 expression was confirmed by examining total RNA and protein by Northern and Western blot analysis, respectively.
Sponge Implants in SCID Mice
Porous poly (l-lactic acid) (PLA) sponges were fabricated as previously described. 31 Briefly, PLA (Aldrich Chemical Co., Milwaukee, WI) was dissolved in chloroform to yield a solution of 10% polymer (w:v), and 0.12 ml of this solution was loaded into Teflon cylinders packed with 0.4 g of sodium chloride particles. The solvent was allowed to evaporate, and then the sponges were immersed for 16 hours in an aqueous solution containing 10 mg/ml polyvinyl alcohol (Aldrich Chemical) in PBS. The sponges (measuring approximately 6 mm × 6 mm × 1 mm) with an average pore diameter of 180 μm were dried, lyophilized, and sterilized by exposure to γ-radiation. The sponges were then soaked in 100% ethanol for 2 hours, washed 1 hour in PBS, and then left overnight in fresh PBS. Just before implantation, 1 × 10 6 Bcl-2-transduced HDMECs (HDMEC-Bcl-2), vector control (HDMEC-LXSN), or parental HDMEC (untransduced) were resuspended in a 1:1 mixture of EBM-MV/Matrigel (Collaborative Biomedical Products, Cambridge, MA) and allowed to adsorb into the sponges. Male SCID mice (CB.17.SCID, Taconic, Germantown, NY), 3 to 4 weeks old, were anesthetized with ketamine and xylazine, and two sponges were implanted subcutaneously in the dorsal region of each mouse. At 7 and 14 days after transplantation, mice were sacrificed, and the sponges were retrieved, fixed overnight in 10% buffered formalin, dehydrated through graded ethanol, embedded in paraffin, and mounted on Superfrost (Fisher Scientific, Pittsburgh, PA) glass slides for histological examination. Three sponges from four to five mice were evaluated for cell type at each time point.
Immunolocalization of CD34 Antigen and in Situ TUNEL Assay
Deparaffinized tissue sections were prepared as described earlier, and antigen retrieval was achieved by microwaving tissue sections for 14 minutes in citrate buffer (2.1 g/L citric acid, pH 6.0). Tissue sections were incubated with 2 μg/ml monoclonal mouse anti-human CD34 (Serotec, Raleigh, NC) for 1 hour at 37°C. The sections were processed using a Vectastain Elite ABC kit, (Vector Laboratories, Burlingame, CA), and a solution of 0.014 g of 3-amino-9-ethyl carbazole (Sigma) in 2.5 ml of N,N-dimethylformamide (Sigma) was used to visualize the bound antibody. An in situ TUNEL assay (ApopTag peroxidase In situ apoptosis detection kit, Oncor, Gaithersburg, MD) was used according to manufacturer’s instructions to determine the percentage of apoptotic cells in the sponge implants. The number of CD34+ blood vessels and TUNEL-positive cells was counted blindly in 10 random fields per sponge using an optical microscope (×400).
Statistical Analyses
The statistical analyses were performed with SigmaStat (Sigma). A descriptive analysis was performed initially, followed by z-test or one-way ANOVA, according to experimental design.
Results
VEGF and IL-8 Induce Proliferation and Sprouting of HDMECs in Culture
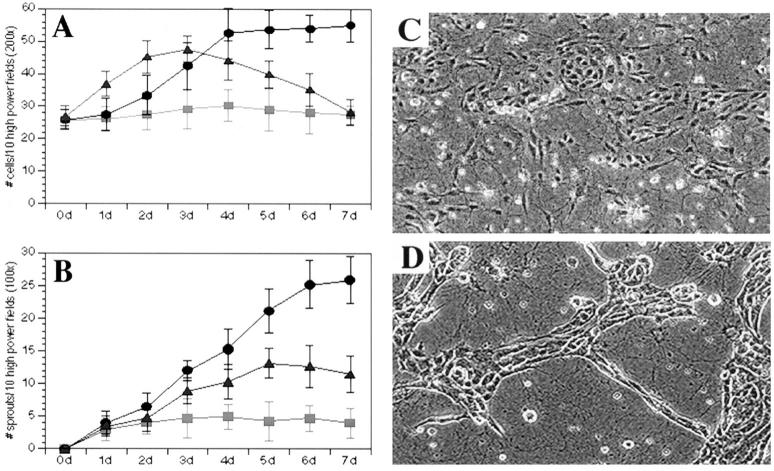
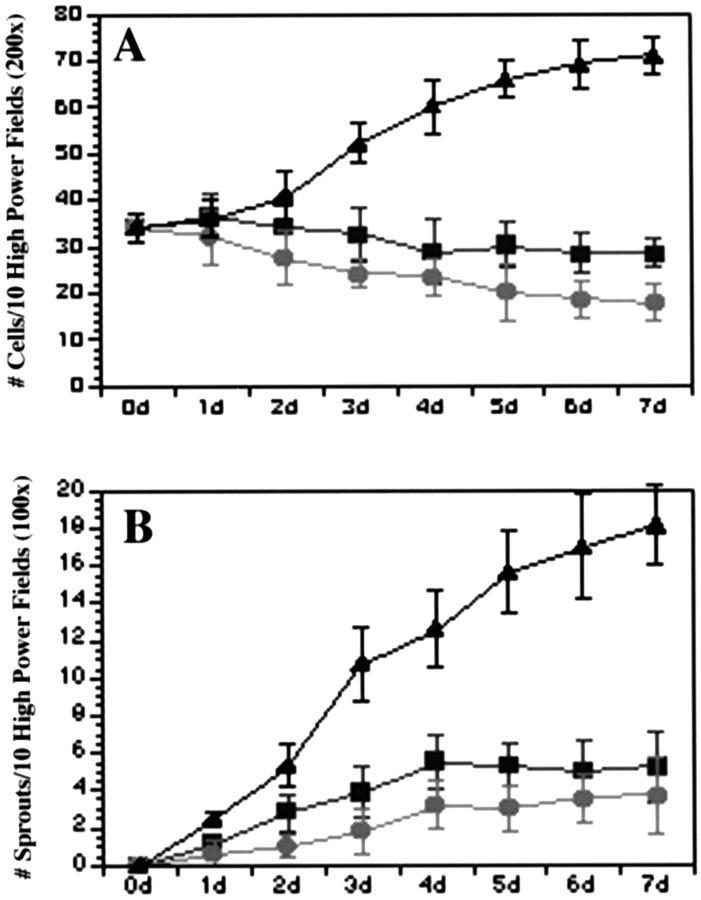
To assess the relationship between endothelial survival and the development of the angiogenic phenotype we examined the ability of VEGF and IL-8, two well described pro-angiogenic mediators, to induce HDMECs grown on collagen gels to proliferate and form sprout-like structures. 32 In this model system, endothelial cells when exposed to angiogenic factors proliferate, migrate, and organize into sprout-like structures that mimic stages in the development of microvessels in vivo. 25 For these experiments HDMECs were grown in the presence of 50 ng/ml of either VEGF or IL-8, and the total number of endothelial cells as well as the number of capillary-like sprouts that developed were counted each day for 7 days. After 7 days exposure to VEGF, HDMECs exhibited a twofold increase in cell number (P ≤ 0.01) as compared with untreated HDMECs (Figure 1A) ▶ . We then compared the mitogenic potency of VEGF to the pro-angiogenic chemokine IL-8. 32 Cultures of HDMECs treated with IL-8 exhibited a rapid increase in cell number that gradually declined to levels that were at or below levels encountered in untreated HDMEC cultures (Figure 1A) ▶ . In addition to their mitogenic effect, both VEGF and IL-8 were able to induce HDMECs to organize and differentiate into capillary-like structures. On the addition of VEGF to HDMEC cultures, a significant increase in the number of sprout-like structures was observed (Figure 1B) ▶ . By day 7, the number of HDMECs exposed to IL-8 developed far fewer sprouts when compared with VEGF-treated HDMECs (P ≤ 0.01). Cultures of untreated HDMECs showed little or no capacity to form spontaneous sprout-like structures.
Figure 1.
Effect of VEGF on HDMEC proliferation and sprout formation in culture. VEGF was more potent than IL-8 and untreated controls in inducing HDMEC proliferation (A) and sprout formation (B to D). HDMECs were plated on type I collagen and cultured in complete EGM-MV in the presence of 50 ng/ml VEGF (•) or 50 ng/ml IL-8 (▴) or in the absence of additional cytokines (▪). C and D: Representative microscopic field (×200) of HDMECs seeded in collagen for 5 days in the absence of additional cytokines (C) or fed with EGM-MV supplemented with 50 ng/ml VEGF (D). At daily intervals, the number of cells and sprouts was counted in 10 random fields from three independent experiments.
VEGF but Not IL-8 Protects Endothelial Cells from Apoptosis in Vitro
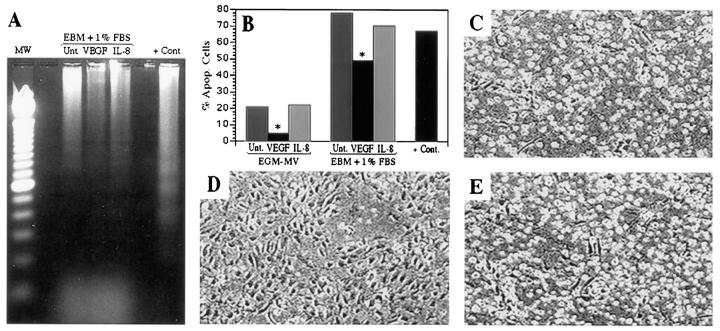
DNA ladder analysis and TUNEL staining were performed to evaluate the ability of VEGF and IL-8 to protect HDMECs from apoptosis when grown in nutrient-rich endothelial growth medium and under conditions of growth factor deprivation. In untreated HDMEC cultures, DNA ladder analysis showed a typical pattern of DNA fragmentation (Figure 2A) ▶ . Flow analysis of TUNEL-stained cells revealed a baseline level of apoptosis of 19.8%. Cultures treated with IL-8 showed a DNA ladder profile similar to untreated HDMEC cultures with a comparable percentage (20.4%) of TUNEL-positive cells. However, when VEGF was added to HDMEC cultures, DNA fragmentation was barely detectable. Under these conditions, less than 5% (P ≤ 0.01) of the cells were apoptotic (Figure 2B) ▶ . A further test of the protective effect of VEGF on HDMEC apoptosis was undertaken by growing HDMECs in basal medium devoid of exogenous growth factors and supplemented only with 1% FBS. DNA ladder analysis revealed that VEGF was able to confer substantial protection on HDMECs from apoptosis in this experimental setting (Figure 2A) ▶ . This result was confirmed by flow cytometry which demonstrated that when HDMECs were grown in presence of VEGF, less than 50% of endothelial cells were apoptotic (P ≤ 0.01), as compared with 78.1% in the untreated group and 70.5% in the IL-8-treated group (Figure 2B) ▶ . Microscopically, the proportion of cells that rounded up and detached from the collagen was higher in the untreated (Figure 2C) ▶ and IL-8-treated groups (Figure 2E) ▶ , as compared with the VEGF-treated HDMECs (Figure 2D) ▶ . VEGF also protected HDMECs from undergoing apoptosis when grown in nutrient-rich endothelial growth medium. Under these conditions, 19.8% and 20.4% of the cells were apoptotic in untreated and IL-8-treated cultures, respectively. However, when VEGF was added, less than 5% (P ≤ 0.01) of the cells were apoptotic (Figure 2B) ▶ . Flow cytometry also revealed that neither VEGF nor IL-8 was able to rescue HDMECs grown in surface culture from apoptotic stimuli. We observed (data not shown) that 8.5% of HDMECs were apoptotic in cultures fed for 3 days with EGM-MV supplemented with 50 ng/ml VEGF and 10.2% in the group supplemented with IL-8, as compared with 9.8% for untreated controls. Taken together, these data indicate that VEGF, but not IL-8, protected HDMECs cultured in type 1 collagen gels from baseline levels of apoptosis as well as growth-factor-deficiency-induced apoptosis.
Figure 2.
Effect of VEGF and IL-8 on HDMEC apoptosis in culture. VEGF prevents DNA fragmentation, as shown in the DNA ladder assay (A) and in TUNEL assay followed by flow cytometry (B) and prevents cell detachment from collagen (C to E). DNA ladder assay (A) was performed with DNA extracted from HDMECs cultured 3 days on type I collagen and fed with EBM supplemented with 1% FBS in the presence of 50 ng/ml VEGF or 50 ng/ml IL-8 or untreated (ie, in the absence of additional cytokines). Flow cytometry (B) was performed with HDMECs cultured 3 days on type I collagen and fed either with complete EGM-MV (first three columns) or EBM supplemented with 1% FBS (second three columns) in the presence of 50 ng/ml VEGF, 50 ng/ml IL-8, or untreated. *Statistically different (P ≤ 0.01). The positive controls for apoptosis (A and B) were HDMECs cultured in suspension in 1.68% methylcellulose for 72 hours. Microscopic appearance of HDMECs cultured 3 days on type I collagen and fed with EBM supplemented with 1% FBS in absence of additional angiogenic factors (C) or in the presence of 50 ng/ml VEGF (D) or 50 ng/ml IL-8 (E).
VEGF Induces Expression of Bcl-2 in HDMECs
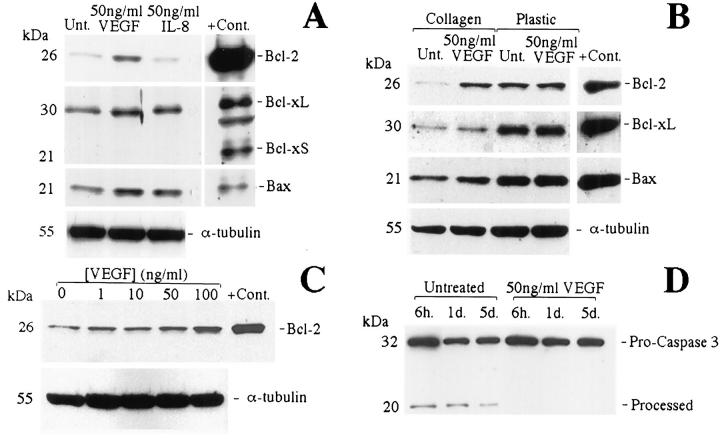
Western blots were used to evaluate the effect of VEGF on the expression of several members of the Bcl-2 family of proteins. VEGF when added to cultures of HDMECs grown in complete medium induced an 11.2-fold increase (measured by relative band density in Figure 3A ▶ ) in the level of expression of Bcl-2, which was dose dependent for concentration up to 100 ng/ml (Figure 3C) ▶ . However, VEGF had no detectable effect on the expression of Bax, Bcl-xL, and Bcl-xS (Figure 3A) ▶ . IL-8 had no detectable effect on the expression of any of the proteins examined (Figure 3A) ▶ . Interestingly, induction of Bcl-2 expression by VEGF was observed only when the cells were seeded in collagen (Figure 3B) ▶ , indicating an apparent substrate requirement for expression of Bcl-2. 15
Figure 3.
VEGF induces Bcl-2 expression in HDMECs grown on collagen and prevents caspase-3 cleavage. Western blots of whole-cell lysates from HDMECs fed with complete EGM-MV and cultured 3 days on type I collagen in the presence of VEGF or IL-8 or untreated (A), cultured 3 days on type I collagen or plastic surface in the presence of VEGF or untreated (B), cultured 3 days on type I collagen in the presence of increasing concentrations of VEGF (C), or cultured on type I collagen in the presence of VEGF or untreated (D). Positive controls were whole-cell lysates from cells transduced with Bcl-2, Bcl-xL/S, or Bax.
Bcl-2 Enhances Endothelial Cell Survival
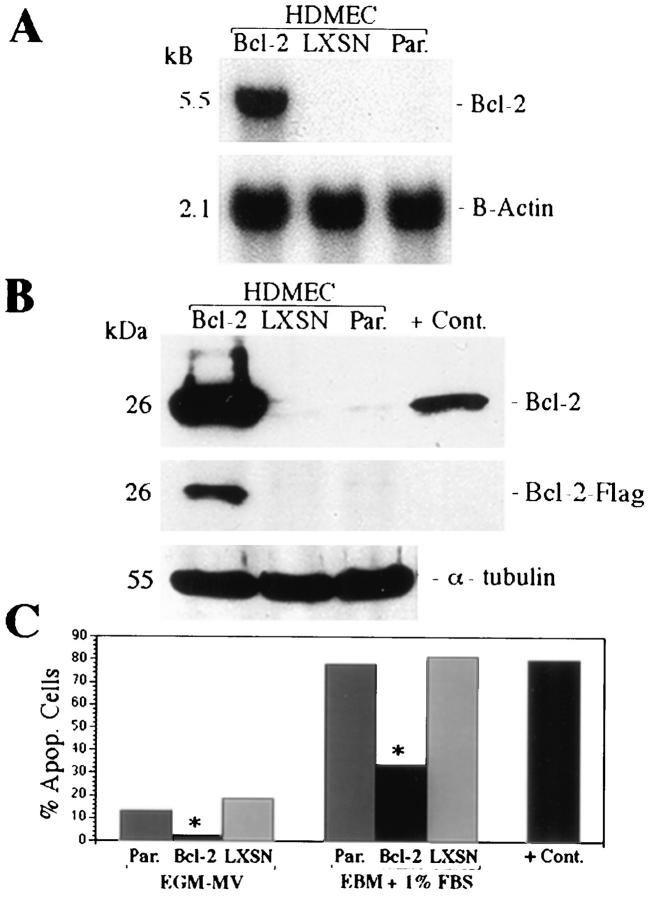
Western blots were also used to evaluate the relationship between VEGF-induced expression of Bcl-2 and cleavage of the interleukin-1 β-converting enzyme-like aspartate-specific protease caspase 3. Activation of this caspase results in downstream activation of endonucleases and DNA fragmentation. 33-35 Cultures of HDMECs exposed to VEGF showed an approximately threefold reduction in the activated forms of the protease at all time periods examined (Figure 3D) ▶ . To further demonstrate the ability of Bcl-2 to protect endothelial cells from apoptosis, HDMECs were stably transduced with the bcl-2 gene in a retrovirus vector. Northern (Figure 4A) ▶ and Western (Figure 4B) ▶ analyses confirmed that transduced HDMECs exhibit a marked increase in the expression of Bcl-2 as compared with HDMECs transduced with the vector alone or untransduced HDMECs. This was also confirmed using anti-flag antibody, which showed a positive band only for HDMEC-Bcl-2. Western blot analysis confirmed the stability of transduction with Bcl-2 for at least eight passages (data not shown). Flow cytometry was then performed to evaluate the effect of overexpression of Bcl-2 on survival of HDMECs. When HDMECs were grown in complete EGM-MV medium, only 2.7% of the HDMEC-Bcl-2 cells were apoptotic after 3 days as compared with 19.1% of the HDMEC-LXSN and 13.2% of the parental HDMECs (Figure 4C) ▶ . The same protective effect of Bcl-2 was observed for cells grown in medium deprived of growth factors. In this setting, only 33.9% of HDMEC-Bcl-2 were apoptotic compared with 81.6% of the HDMEC-LXSN or 78.1% of the parental HDMECs (Figure 4C) ▶ .
Figure 4.
Overexpression of Bcl-2 in endothelial cells increases survival. Northern (A) and Western (B) blot analyses of HDMECs stably transduced with Bcl-2 (HDMEC-Bcl-2), vector only (HDMEC-LXSN), or parental HDMEC (untransduced) were performed to confirm expression of Bcl-2. TUNEL assay followed by flow cytometry (C) was performed with cells cultured 3 days on type I collagen and fed either with complete EGM-MV (first three columns) or EBM supplemented with 1% FBS (second three columns). *Statistically different (P ≤ 0.01). Positive controls were HDMECs cultured in suspension in 1.68% methylcellulose for 72 hours (C).
Endothelial Cells Overexpressing Bcl-2 Show Enhanced Sprout Formation in Vitro and Angiogenesis in Vivo
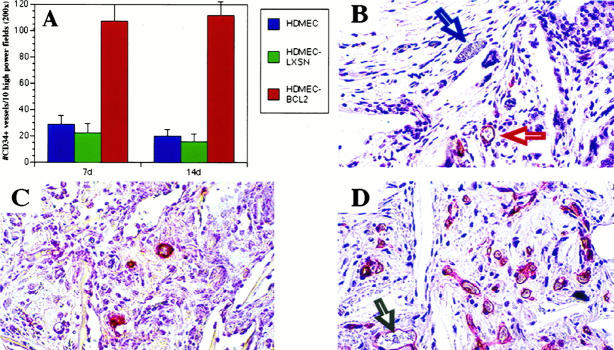
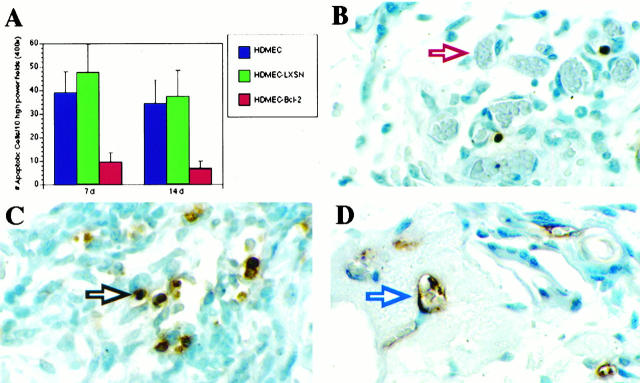
The effect of overexpression of Bcl-2 on the ability of HDMECs to form sprouts in vitro and microvessels in vivo was also evaluated. After 7 days, the number of cells (Figure 5A) ▶ and the number of sprout-like structures (Figure 5B) ▶ that developed in cultures of HDMEC-Bcl-2 were significantly higher (P ≤ 0.01) as compared with HDMEC-LXSN and parental HDMECs. This tendency for enhanced growth and spontaneous sprout formation by HDMEC-Bcl-2 persisted throughout the duration of the assay (7 days). To see whether overexpression of Bcl-2 influenced capillary growth in vivo, we examined the ability of HDMEC-Bcl-2 to organize into functional microvessels after implantation into SCID mice. CD34, a marker that exhibits high specificity for endothelial cells, was used to identify blood vessels populated by human endothelial cells. 36 When sponges were examined 7 and 14 days after implantation, the number of CD34+ blood vessels in the SCID mice was significantly higher (P = 0.01) in sponges populated with HDMEC-Bcl-2 than in sponges containing either HDMEC-LXSN or parental HDMECs (Figure 6, A, C, and D) ▶ . The increased vascularization encountered in sponges populated by HDMEC-Bcl-2 was associated with a decrease in the number of cells undergoing apoptosis (P ≤ 0.01) at both 7 and 14 days after implantation (Figure 7) ▶ . Histological analysis revealed that only blood vessels in the interior of the sponges were stained with the anti-human CD34 antibody and not the mouse blood vessels in the connective tissue surrounding the sponge implant (Figure 6B) ▶ . Immunostaining with anti-human CD31 antibody and anti-flag antibody confirmed that the blood vessels in the interior of the sponges were of human origin (data not shown). These findings demonstrate that transplanted HDMECs were able to organize into capillary structures, connect with mouse microvessels, and become functional blood vessels. Moreover, our results indicate that HDMECs overexpressing Bcl-2 are able to participate in a vigorous and sustained angiogenic response in vivo (Figure 6D) ▶ .
Figure 5.
Effect of Bcl-2 on endothelial cell proliferation and number of sprouts in culture. HDMECs overexpressing Bcl-2 show enhanced cell proliferation (A) and sprout formation (B). Endothelial cells transduced with Bcl-2 (▴), vector only (•), or parental (untransduced) cells (▪) were plated on type I collagen and cultured in complete EGM-MV. At daily intervals, the number of cells and sprouts was counted in 10 random fields from three independent experiments.
Figure 6.
HDMECs overexpressing Bcl-2 show enhanced angiogenesis in SCID mice. HDMEC-Bcl-2, HDMEC-LXSN, or HDMEC were seeded in PLA sponges and implanted in SCID mice. After 7 or 14 days, the sponges were retrieved and stained with anti-CD34, and the number of CD34+ blood vessels was counted in 10 random fields from three independent sponges per time point and cell type (A). Photomicrograph from histological sections show anti-CD34 staining of HDMECs in the interior of the sponge (red arrow) and the absence of staining of mouse blood vessels (blue arrow) in the surrounding connective tissue (B), sponges seeded with HDMECs had fewer CD34+ blood vessels after 14 days (C), compared with sponges seeded with HDMEC-Bcl-2 after the same time period (D). Human blood vessels in the interior of the sponge showing blood-filled lumens (gray arrow). All photomicrographs were at ×200 magnification.
Figure 7.
Apoptosis in sponges implanted in SCID mice. HDMEC-Bcl-2, HDMEC-LXSN, or HDMECs were seeded in PLA sponges and implanted subcutaneously in the back of the SCID mice. After 7 or 14 days, the sponges were retrieved, stained with the ApopTag peroxidase in situ kit. The number of TUNEL-positive cells was counted in 10 random fields from three independent sponges per time point and experimental condition (A). Photomicrographs from histological sections show sponges seeded with HDMEC-Bcl-2 had fewer TUNEL-positive cells (apoptotic cells) 14 days after implantation (B), compared with sponges seeded with HDMECs (C and D) after the same time period. The red arrow in B points to a TUNEL-negative microvessel populated by HDMEC-Bcl-2 cells. The black arrow in C points to a TUNEL-positive stromal cell in the sponge interior, and the blue arrow in D points to a TUNEL-positive microvessel populated by untransduced HDMECs. All photomicrographs were at ×1000 magnification.
Discussion
VEGF is an endothelial-cell-specific mitogen and permeability factor that potently induces angiogenesis in vivo. We report here that the ability of VEGF to potentiate and sustain angiogenesis is associated with its ability to prolong the survival of endothelial cells and induce expression of Bcl-2. We showed that HDMECs when grown on collagen gels under optimal growth conditions exhibit a baseline level of apoptosis of approximately 19%. This is likely a reflection of the periodic endothelial cell proliferation and apoptosis that is required to maintain the integrity of the endothelial monolayer. However, when VEGF was added to the culture medium, fewer cells underwent apoptosis (5%), indicating that this growth factor enhances the survival of endothelial cells in this model system. The addition of VEGF led to a rapid induction of endothelial growth that plateaued around day 4 despite the continued presence of VEGF. We also found that VEGF induced endothelial cells to rapidly organize into capillary-like sprouts. These events coincided with a reduction in HDMEC apoptosis and induction of Bcl-2 expression. These results were in marked contrast to events that occurred in response to the angiogenic chemokine IL-8. Like VEGF, IL-8 was able to stimulate proliferation and sprouting of HDMECs but was unable to sustain either phenotype. However, unlike VEGF, IL-8 was unable to enhance the survival of HDMECs and did not induce expression of Bcl-2. This suggests that Bcl-2 functions synergistically with the mitogenic and other angiogenesis-associated functions of VEGF to generate a potent and sustained angiogenic response. These data also suggest that the ability of growth factors to promote and sustain angiogenesis may depend on whether they are also able to promote endothelial cell survival.
When cultured endothelial cells were deprived of growth factors and essential nutrients, the anti-apoptotic effects of VEGF were also pronounced. Under these conditions, large numbers of endothelial cells were dead or dying after 3 days in culture. The addition of VEGF to the medium increased the survival of cultured HDMECs by 30%, as measured by flow cytometry. These results were confirmed by DNA ladder analysis where a marked reduction in DNA fragmentation was observed. VEGF consistently induced expression of the Bcl-2 in HDMECs seeded in collagen; however, this phenomenon was not observed when cells were grown directly on the surface of tissue culture dishes. This suggests that conditions that lead to enhanced survival of endothelial cells require that these cells interact with a suitable substrate. Recently, Gerber et al reported that Bcl-2 and A1 mediated the survival of human umbilical vein endothelial cells (HUVECs) in vitro. 23 In contrast to the survival-enhancing effects of VEGF, IL-8 did not potentiate endothelial cell survival nor did it up-regulate Bcl-2. IL-8 is a potent mediator of angiogenesis that is produced by many different cell types, including endothelial cells and macrophages. 32,37 Although both VEGF and IL-8 have been implicated in angiogenic responses, the mechanism by which IL-8 induces angiogenesis appears different from VEGF. This suggests that inducers of angiogenesis may employ a different mechanism to induce a similar phenotypic response in endothelial cells. Overexpression of Bcl-2 is sufficient to block apoptosis by retaining cytochrome c in the mitochondria, thereby inhibiting caspase-3 activation and subsequent downstream apoptotic events. 33-35 We investigated the ability of VEGF to limit the proteolytic cleavage of caspase-3 into its smaller derivatives in endothelial cells. We found that endothelial cells exposed to VEGF demonstrated a threefold reduction in cleaved forms of caspase-3. These data further corroborate our finding that VEGF when added to the culture medium protects endothelial cells from apoptosis.
To investigate the role of Bcl-2 in angiogenesis, we generated a clonal population of HDMECs that stably overexpressed Bcl-2. The HDMEC-Bcl-2 proliferated at a rate comparable to parental cells exposed to VEGF and demonstrated the ability to align and differentiate into sprouts in vitro without the addition of exogenous VEGF. These cells were also remarkably resistant to apoptosis when grown in either complete EGM-MV medium and in growth-factor-deficient medium. Perhaps the most informative analysis of the role of Bcl-2 in angiogenesis was obtained from the implantation of HDMECs overexpressing Bcl-2 (HDMEC-Bcl-2) in SCID mice. The number of human microvessels that developed in sponges populated with HDMEC-Bcl-2 was significantly higher than in sponges containing parental HDMECs or HDMECs transfected with vector alone. In addition, the number of apoptotic cells in sponges containing HDMEC-Bcl-2 was significantly lower as compared with the controls. This suggests that overexpression of Bcl-2 in endothelial cells has at least one of the following functions in vivo, neither of which is mutually exclusive. First, Bcl-2 may protect endothelial cells from apoptosis induced by inhibitors of angiogenesis resulting in a net gain in new blood vessels. Indeed, HDMECs overexpressing Bcl-2 are refractory to the apoptotic and angiosuppressive effects of the angiogenesis inhibitor thrombospondin 1 (J. E. Nör, R. J. Mitra, M. M. Sutorik, D. J. Mooney, V. P. Castle, and P. J. Polverini, in preparation). Second, Bcl-2 might potentiate the ability of endothelial cells to differentiate into functional blood vessels. Our SCID mouse model of angiogenesis would suggest that HDMECs overexpressing Bcl-2 were able to participate in a vigorous and sustained angiogenic response through at least 14 days.
In conclusion, we have demonstrated that the angiogenic activity of VEGF is mediated in part by its ability to induce expression of Bcl-2 in endothelial cells. In addition, VEGF was able to enhance the endothelial cell survival when grown in a growth-factor-deficient environment. Several studies have demonstrated increased expression of VEGF adjacent to sites of necrosis in solid tumors. This is an environment that under normal circumstances imposes severe constraints on endothelial cell growth and survival. Our findings suggest a mechanism whereby VEGF-induced expression of Bcl-2 may function to enhance the survival of endothelial cells in the toxic, oxygen-deficient environment of tumors and ensure the continuous, uninterrupted flow of nutrients to the tumor.
Acknowledgments
We thank G. Nuñez and D. Miller for generously supplying reagents and V. Castle for insightful discussions. We also thank R. Mitra, J. Liu, M. Peters, M. Sutorik, S. Nabai, F. Chen, and M. Kukuruga for technical assistance.
Footnotes
Address reprint requests to Dr. Peter J. Polverini, University of Michigan School of Dentistry, Ann Arbor, MI 48109-1078. E-mail: neovas@umich.edu.
Supported by National Institutes of Health grants HL39926 and CA64416 (P.J. Polverini) and CAPES grant 2889/92-3 (J.E. Nör).
References
- 1.Ferrara N: Vascular endothelial growth factor. Eur J Cancer 1996, 32A:2413-2422 [DOI] [PubMed] [Google Scholar]
- 2.Plate KH, Breiser G, Weich HA, Risau W: Vascular endothelial growth factor is a potential tumor angiogenesis factor in vivo. Nature 1992, 359:845-848 [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto M, Ohsawa M, Ohnishi A, Naka N, Hirota S, Kitamura Y, Aosaza K: Expression of vascular endothelial growth factor and its receptor mRNA in angiosarcoma. Lab Invest 1995, 73:859-863 [PubMed] [Google Scholar]
- 4.Benjamin LE, Keshet E: Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors: induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proc Natl Acad Sci USA 1997, 94:8761-8766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown LF, Yeo KY, Berse B, Yeo TK, Senger DR, Dvorak HF, Van de Water L: Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med 1992, 176:1375-1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berse B, Brown LF: Van de Water L, Dvorak HF, Senger DR: Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell 1992, 3:211-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grützkau A, Krüger-Krasagakes S, Baumeister H, Schwarz C, Kögel H, Welker P, Lippert U, Henz BM, Möller A: Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF206. Mol Biol Cell 1998, 9:875-884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrara N, Wilner J, Burton T: Aortic smooth muscle cells express and secrete vascular endothelial growth factor. Growth Factors 1991, 5:141-148 [DOI] [PubMed] [Google Scholar]
- 9.Brown LF, Berse B, Jackman RW, Tognazzi K, Guidi A, Dvorak HF, Senger DR, Connoly J, Schnitt S: Expression of vascular permeability factor (vascular endothelial growth factor) and its receptor in breast cancer. Hum Pathol 1995, 26:86-91 [DOI] [PubMed] [Google Scholar]
- 10.Folkman J, Shing Y: Angiogenesis. J Biol Chem 1992, 267:10931-10934 [PubMed] [Google Scholar]
- 11.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N: Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246:1306-1309 [DOI] [PubMed] [Google Scholar]
- 12.Dvorak HF, Brown LF, Detmar M, Dvorak AM: Vascular permeability factor/vascular endothelial growth factor, vascular hyperpermeability, and angiogenesis. Am J Pathol 1995, 146:1029-1039 [PMC free article] [PubMed] [Google Scholar]
- 13.Mandriota SJ, Pepper MS: Vascular endothelial growth factor-induced in vitro angiogenesis and plasminogen activator expression are dependent on endogenous basic fibroblast growth factor. J Cell Sci 1997, 110:2293-2302 [DOI] [PubMed] [Google Scholar]
- 14.Alon T, Hemo I, Itin A, Pe’ee J, Stone J, Keshet E: Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nature Med 1995, 1:1024-1028 [DOI] [PubMed] [Google Scholar]
- 15.Spyridopoulos I, Brogi E, Kearney M, Sullivan AB, Cetrulo C, Isner JM, Losordo DW: Vascular endothelial growth factor inhibits endothelial cell apoptosis induced by tumor necrosis factor-α: balance between growth and death signals. J Mol Cell Cardiol 1997, 29:1321-1330 [DOI] [PubMed] [Google Scholar]
- 16.Watanabe Y, Dvorak HF: Vascular permeability factor/vascular endothelial growth factor inhibits anchorage disruption induced apoptosis in microvessel endothelial cells by inducing scaffold formation. Exp Cell Res 1997, 233:340-349 [DOI] [PubMed] [Google Scholar]
- 17.Reed JC: Bcl-2 and the regulation of programmed cell death. J Cell Biol 1994, 124:1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson C: Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267:1456-1462 [DOI] [PubMed] [Google Scholar]
- 19.Nuñez G, Clarke MF: The Bcl-2 family of proteins: regulators of cell death and survival. Trends Cell Biol 1994, 4:399-403 [DOI] [PubMed] [Google Scholar]
- 20.Korsmeyer SJ: Regulators of cell death. Trends Genet 1995, 11:101-105 [DOI] [PubMed] [Google Scholar]
- 21.Kroemer G: The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nature Med 1997, 3:614-620 [DOI] [PubMed] [Google Scholar]
- 22.Kondo S, Yin D, Aoki T, Takahashi JA, Morimura T, Takeuchi J: Bcl-2 gene prevents apoptosis of basic fibroblast growth factor-deprived murine aortic endothelial cells. Exp Cell Res 1994, 213:428-432 [DOI] [PubMed] [Google Scholar]
- 23.Gerber HP, Dixit V, Ferrara N: Vascular endothelial growth factor induces expression of the anti-apoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem 1998, 273:13313-13316 [DOI] [PubMed] [Google Scholar]
- 24.Villaschi S, Nicosia R: Paracrine interactions between fibroblasts and endothelial cells in a serum-free coculture model: modulation of angiogenesis and collagen gel contraction. Lab Invest 1994, 71:291-299 [PubMed] [Google Scholar]
- 25.DiPietro LA, Nebgen DR, Polverini PJ: Modulation of in vitro angiogenesis by genetic manipulation of endothelial cell thrombospondin. J Vasc Res 1994, 31:178-185 [DOI] [PubMed] [Google Scholar]
- 26.Gavrieli Y, Sherman Y, Ben-Sasson SA: Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992, 119:493-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong J, Traganos F, Darzynkiewiez Z: A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Anal Biochem 1994, 218:314-319 [DOI] [PubMed] [Google Scholar]
- 28.Mitra RJ, Wrone-Smith T, Simonian P, Foreman KE, Nuñez G, Nickoloff BJ: Apoptosis in keratinocytes is not dependent on induction of differentiation. Lab Invest 1997, 76:99-107 [PubMed] [Google Scholar]
- 29.Lomo J, Smeland EB, Krajewski S, Reed JC, Blomhoff HK: Expression of the Bcl-2 homologue Mcl-1 correlates with survival of peripheral blood B lymphocytes. Cancer Res 1996, 56:40-43 [PubMed] [Google Scholar]
- 30.Miller AD, Miller DG, Garcia JV, Lynch CM: Use of retroviral vectors for gene transfer and expression. Methods Enzymol 1993, 217:581-599 [DOI] [PubMed] [Google Scholar]
- 31.Mooney DJ, Sano K, Kaufmann PM, Majahod K, Scloo B, Vacanti JP, Langer R: Long-term engraftment of hepatocytes transplanted on biodegradable polymer sponges. J Biomed Mater Res 1997, 37:413-420 [DOI] [PubMed] [Google Scholar]
- 32.Strieter RM, Polverini PJ, Arenberg DA, Walz A, Opdenakker G, van Damme J, Kunkel SL: Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. J Leukoc Biol 1995, 57:752-762 [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X: Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 1997, 275:1129-1132 [DOI] [PubMed] [Google Scholar]
- 34.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD: The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 1997, 275:1132-1136 [DOI] [PubMed] [Google Scholar]
- 35.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S: A caspase-activated DNase that degrades DNA during apoptosis and its inhibitor ICAD. Nature 1998, 391:43-50 [DOI] [PubMed] [Google Scholar]
- 36.Vermeulen PB, Gasparini G, Fox SB, Toi M, Martin L, Pezzella F, Viale G, Weidner N, Harris AL, Dirix LY: Quantification of angiogenesis in solid human tumors: an international consensus on the methodology and criteria of evaluation. Eur J Cancer 1996, 32A:2474-2484 [DOI] [PubMed] [Google Scholar]
- 37.Polverini PJ: The pathophysiology of angiogenesis. Crit Rev Oral Biol Med 1995, 6:230-247 [DOI] [PubMed] [Google Scholar]