Abstract
The intermediate filament proteins nestin, vimentin, and desmin show a specific temporal expression pattern during the development of myofibers from myogenic precursor cells. Nestin and vimentin are actively expressed during early developmental stages to be later down-regulated, vimentin completely and nestin to minimal levels, whereas desmin expression begins later and is maintained in mature myofibers, in which desmin participates in maintaining structural integrity. In this study we have analyzed the expression levels and distribution pattern of nestin in intact and denervated muscle in rat and in human. Nestin immunoreactivity was specifically and focally localized in the sarcoplasm underneath neuromuscular junctions (NMJs) and in the vicinity of the myotendinous junctions (MTJs), ie, in regions associated with acetylcholine receptors (AChRs). This association prompted us to analyze nestin in neurogenically and myogenically denervated muscle. Immunoblot analysis disclosed a marked overall increase of accumulated nestin protein. Similar to the extrajunctional redistribution of AChRs in denervated myofibers, nestin immunoreactivity extended widely beyond the NMJ region. Re-innervation caused complete reversion of these changes. Our study demonstrates that the expression levels and distribution pattern of nestin are regulated by innervation, ie, signal transduction into myofibers.
Intermediate filaments (IFs) are cytoskeletal filamentous structures with a diameter of approximately 10 nm. On the basis of the molecular structure of their constituent proteins, IFs are divided into six main classes, 1,2 and the number of individual IFs exceeds 40. Three IF proteins, vimentin, desmin, and the more recently discovered nestin, 3 are expressed in skeletal muscle cells. Their differentiation-state-specific expression pattern indicates that all three proteins may play pivotal roles during the development of myofibers from myogenic precursor cells. Vimentin and nestin are expressed during early developmental stages of the prenatal period, whereas desmin expression is initiated at later stages. 4 The specific functions of these IF proteins are largely unknown. However, during myogenesis these three molecules co-localize closely in the filamentous cytoskeletal network, as demonstrated in vitro in G6-derived myoblasts and myotubes, 4,5 which suggests that these molecules have complementary functions in determining the structure and properties of IFs and, thereby, also in the formation of differentiated myofibers.
During development, desmin expression (eg, in rat 4 and chicken6) increases continuously with advancing maturation. Furthermore, during differentiation, the intracellular distribution of desmin undergoes a major change from a diffuse sarcoplasmic pattern in immature myogenic cells to a banded pattern corresponding to the sarcomeric striations of mature myofibers. 6 The importance of desmin in maintaining the structural integrity of the adult muscle was confirmed by recent studies using selective gene targeting in mice. Desmin knock-out mice showed severe degeneration especially of the cardiac myocytes, but skeletal muscles were also affected. 7,8 The temporal distribution of vimentin during development shows an inverse relationship to that of desmin, as vimentin expression, both at mRNA and protein levels, has been reported to decrease until it appears to be completely terminated in fully developed myofibers. 4 The expression of nestin in intact myofibers also occurs almost exclusively during early developmental stages, as the overall nestin mRNA level decreases to a hardly detectable level in adult myofibers, and only very weak nestin immunoreactivity was discernible in longitudinal sections. 4 Interestingly, the immunoreactivity pattern of nestin-specific antibodies has in some sections been reported to show a similar banded pattern as desmin in longitudinal sections of myofibrils.
In a separate regeneration study (S. Vaittinen et al, manuscript in preparation), we observed in untreated control sections in mature myofibers a novel nestin immunoreactivity pattern, which had obviously gone unnoticed in previous studies. Prompted by this observation, we examined in detail the distribution and expression of nestin in normal myofibers as related to those of desmin and vimentin. In the present study, we report on an accentuated nestin pattern in the sarcoplasm adjoining both NMJs and MTJs in tibialis anterior muscle of mature rat. Our study shows that the distribution and expression levels of nestin show a clear dependence of the innervation status of myofibers.
Materials and Methods
Animals
Twenty-one outbred HSD:SD male specific-pathogen-free rats supplied by the Central Animal Laboratory of the University of Turku were used in this study. At the time of denervation they were 13 to 14 weeks old, weighing 300 to 391 g. The experiments were approved by the ethical committee for animal experiments at the University of Turku.
Denervation Procedure
Neurogenic Denervation
The tibialis anterior muscle of the left hind limb was denervated by freezing the deep peroneal nerve. Denervation was performed under a combined, intraperitoneally dosed anesthesia of ketamine, 7.5 mg/kg (Ketalar, 50 mg/ml; Parke Davis, Barcelona, Spain) and 0.25 mg/kg medetomidine (Domitor, 1 mg/ml; Orion-Farmos, Turku, Finland). A longitudinal skin and fascia incision of ∼1.5 cm was made ∼1 cm from the lateral condyle of the left femur. The deep peroneal nerve, situated on the lateral head of the gastrocnemius muscle, was exposed by bluntly dissecting the biceps femoralis muscle with scissors and injured by squeezing it for a few seconds with forceps chilled in liquid nitrogen (−196°C). The neurial sheaths of the nerve remained unsevered during the cold injury, which allows optimal circumstances for re-innervation. The tibialis anterior muscle was not touched during the denervation procedure. Fascia and skin were closed with absorbable catgut and nonabsorbable nylon sutures, respectively. On recovery, animals were allowed to move freely in their cages.
Myogenic Denervation
We have previously shown that transection of the extensor digitorum longus (EDL) muscle below the most distal NMJ region leaves the distal (abjunctional) muscle stumps without innervation. These stumps are later re-innervated as follows. Axons sprout from the nerve terminals innervating the proximal stumps, and they pierce through the scar tissue formed between the stumps and abut on the NMJs formed de novo on the abjunctional stumps. 9 Specimens from the above mentioned publication were used in the present study. EDL muscle was transected in the following way. Under ether anesthesia a longitudinal skin and fascia incision of ∼1 cm in length was made just above the lateral malleolus of the left fibula. The tibialis anterior and EDL muscles were visualized within their common fascial envelope, which was opened over a distance of ∼3 mm. A curved stitch cutter was then inserted under the EDL muscle belly ∼10 mm above the level of the lateral malleolus, and the muscle was transected leaving its medial aponeurosis intact. The wound was closed with two nylon sutures. Animals were allowed to move freely immediately after surgery. 9
Sampling
Animals were killed by CO2 ventilation 1, 3, 7, 14, 21, 28, and 56 days (three rats in each experimental group) after denervation. The tibialis anterior muscle of each rat was sampled as follows. The muscle was exposed by careful removal of the skin and fascia. It was then divided in situ sagittally in the midline. The medial half of the muscle was further divided into a superior and inferior part, and then both samples were frozen immediately in liquid nitrogen (−196°C) and stored at −70°C until Western blotting was performed. The lateral half of the muscle was also divided into two parts, and the superior part was frozen in isopentane chilled with liquid nitrogen and stored at −70°C until processed further. The inferior part was left intact, and the whole hind limb (pinned on a piece of cork to maintain both the stifle (knee) and hock (ankle) at a straight angle) was then fixed in 4% paraformaldehyde in 0.1 mol/L phosphate buffer. After 2 to 3 days of fixation, the inferior part was detached and routinely embedded in paraffin. The tibialis anterior muscles of the opposite intact hind limb from seven rats were used as control samples. Animals with myogenic denervation were killed by cervical dislocation under ether anesthesia 1, 4, 5, 7, 10, 15, 30, 42, and 56 days (two animals per time period) after the trauma, and EDL muscle was either frozen in freon cooled with liquid nitrogen or fixed in 4% buffered paraformaldehyde for paraffin embedding (for details see Rantanen et al). 9
Muscle Samples from Cadavers
Muscle samples from the abductor pollicis brevis muscle were taken from three male cadavers of ages 42, 42, and 41 years. The causes of death were ketoacidosis, head injury (by gun shot), and strangulation, respectively. Samples were divided in two; one part was frozen in isopentane chilled with liquid nitrogen and stored at −70°C until processed further, and the other part was fixed by 4% paraformaldehyde in 0.1 mol/L phosphate buffer, followed by routine embedding in paraffin.
Histology and Immunohistochemistry
The following antisera and antibodies were used: rabbit polyclonal anti-nestin antiserum 130 2,10 or mouse monoclonal antibody to rat nestin (Pharmingen, San Diego, CA), mouse monoclonal antibodies to human vimentin and desmin (Zymed Laboratories, San Francisco, CA), and mouse monoclonal antibody to neurofilament (Euro-Diagnostica, Malmö, Sweden). NMJs were demonstrated by both enzyme and toxin histochemistry. The former was performed by reacting frozen sections for acetylcholinesterase (AChE). 11 The latter was performed by incubating frozen sections overnight in 1:200 biotinylated α-bungarotoxin (BTX; Molecular Probes, Eugene, OR), which specifically binds to AChRs. The bound primary antibodies were visualized by using an appropriate avidin-biotin-peroxidase kit (Vector Laboratories, Burlingame, CA), which was also used for visualization of BTX. Diaminobenzidine was used as the chromogen. Statistical significance was tested by one-way ANOVA with Newman-Keuls post-test, using the GraphPad Prism program (Intuitive Software for Science, San Diego, CA).
Immunoblotting
Pieces of frozen muscle, derived from rats sacrificed at different time points after the denervation procedure, were pulverized in liquid nitrogen, dissolved in Laemmli sample buffer, sonicated, and centrifuged to remove insoluble material. The muscle extracts were separated on 6% SDS-polyacrylamide gels, and for immunoblotting, proteins were then electrotransferred from the gels to nitrocellulose membranes using a semi-dry transfer apparatus (Bio-Rad, Hercules, CA). Immunoblotting was performed using the polyclonal rabbit antibodies against vimentin 264 12 and nestin 130. 10 Binding of the primary antibodies to the proteins was detected using horseradish-peroxidase conjugated goat anti-rabbit immunoglobulins and the enhanced chemiluminescence (ECL) Western blotting detection kit (Amersham, Little Chalfont, UK). Proteins remaining on gels after transfer, or on parallel gels loaded with the same samples, were stained with Coomassie Brilliant blue to control for equal loading of proteins.
Results
Specific Distribution of Nestin, Vimentin, and Desmin in Intact Adult Myofibers
We first examined in detail the normal distribution of nestin in adult rat muscle fibers as compared with the distribution of desmin and the localization of muscular junctions. The distribution of muscular IFs was optimally visualized in longitudinal paraffin sections.
Nestin
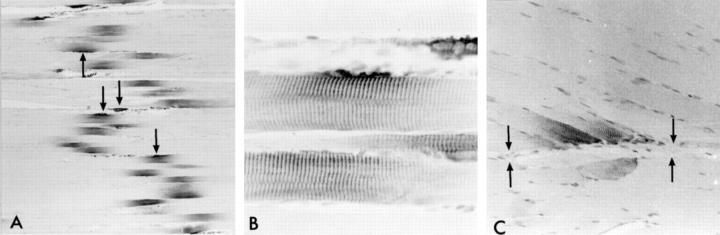
Nestin immunoreactivity was observed in the midbelly parts of virtually all myofibers. These immunopositive segments ranged from 130 to 230 (mean 180) μm in length and formed an obliquely oriented band across the muscle, which first gave an artifactual impression (Figure 1A) ▶ . Many of these segments were associated with a focal protrusion of the muscle membrane, with subsarcolemmal accentuation of the immunoreactivity as well as immunostaining of some cells lying outside the sarcolemmal protrusions, a pattern suggestive of NMJs (Figure 1B) ▶ . In addition, similar though somewhat weaker focal nestin immmunoreactivity of 100- to 200-μm strips of the sarcoplasm was observed at the ends of myofibers attaching to tendons or to thick fasciae within the muscle, ie, next to MTJs (Figure 1C) ▶ . The sarcoplasm outside the segments next to NMJ and MTJ was negative for nestin.
Figure 1.
Nestin immunoreactivity in normal muscle of adult rat. A: The specific reactivity in the sarcoplasm forms an oblique band across the muscle. The reactivity is especially accentuated at sarcolemmal protrusions (four marked with an arrow). B: A detailed view of the nestin immunoreactivity associated with NMJ. Note the cross-striated pattern of staining. C: Nestin reactivity in the MTJ regions. The fascia to which the myofibers attach is indicated by arrows. Magnification, ×71 (A), ×429 (B), and ×156 (C).
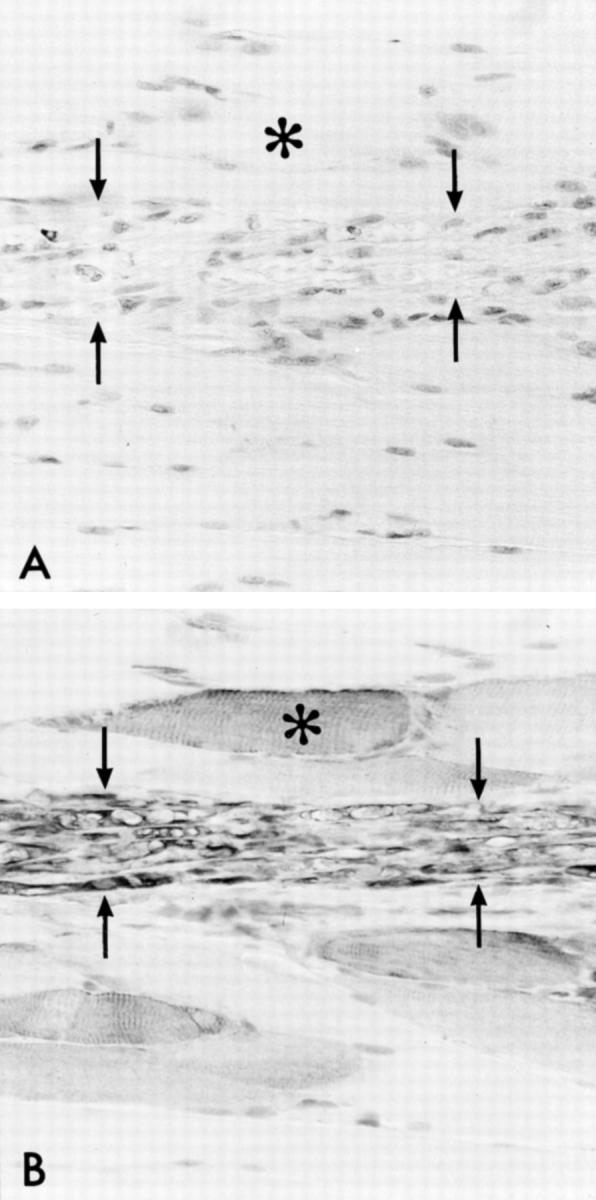
The nestin immunoreactivity associated with sarcolemmal protrusions was examined in greater detail in consecutive frozen sections to confirm the assumed connection to NMJs. The protrusions stained positively for AChE and BTX, both of which react specifically with regions containing AChRs. Furthermore, neurofilament-positive axons were found converging toward the protrusions (Figure 2, A–D) ▶ . Intramuscular nerves were also detectable by nestin-immunoreactive Schwann cells (Figures 2, A and D, and 3B) ▶ ▶ .
Figure 2.
Demonstration of NMJ-associated nestin immunoreactivity in serial cryostat sections. Distribution of nestin (A), AChE (B), BTX (C), and neurofilaments (D) in consecutive sections of the same fibers reveal that the specific nestin immunoreactivity is specifically associated with NMJ. The asterisk indicates an intramuscular nerve branch in A and D. Magnification, ×150 to ×170.
Figure 3.
Verification of successful denervation of the rat tibialis anterior muscle. Immunostaining of an intramuscular branch of the severed deep peroneal nerve (between the arrows) on day 7 after denervation. A: Neurofilament staining of the nerve branch shows the absence of axons. B: In a consecutive section, Schwann cells of the degenerated nerve are clearly immunoreactive for nestin. Asterisks indicate the corresponding myofiber. Magnification, ×247.
Vimentin
The sarcoplasm of myofibers, including the regions next to NMJs or MTJs, was entirely negative for vimentin immunoreactivity, whereas connective tissue cells, mainly fibroblasts, in the endomysium, perimysium, and epimysium were strongly positive. Endothelial cells in the vessels were negative, but the connective tissue cells in the outer layers of larger vessels were immunopositive. Schwann cells in the intramuscular nerve twigs were also positive with anti-vimentin antibody (data not shown).
Desmin
Desmin immunostaining rendered all myofibers regularly striated with strong immunopositivity corresponding to the sarcomeres. In addition, there was some topographic variation in the intensity of the immunoreactivity, the folds of NMJs (but not the sarcoplasm underlying NMJs) and the sarcoplasm in the vicinity of MTJ being somewhat accentuated. Furthermore, there was some subsarcolemmal perinuclear diffuse staining and seemingly random variation between different areas of sarcoplasm. The smooth muscle cells of small arterioles also stained positively for desmin (data not shown).
The Distribution of Nestin, Vimentin, and Desmin Is Altered in Denervated Muscle
The denervation was confirmed by the signs of paralysis of the tibialis anterior muscle. In tissue sections, it was verified by the disappearance of neurofilament immunoreactivity in intramuscular nerves by day 7 after denervation with simultaneous appearance of macrophages (Figure 3, A and B) ▶ .
Nestin Distribution after Neurogenic Denervation
The length of the nestin-positive segments in myofibers began to increase already by day 3 after denervation, and it had more than doubled by day 7. By day 21 there were several fibers in which the length of immunopositive segments exceeded 1 mm, and the mean length was over threefold in comparison with controls (Figure 4, A and C) ▶ . By that time the myofibers had also become clearly atrophic. The intensity of the nestin-immunoreactivity seemed to be high over the whole length of the nestin-positive segments. The normal nestin distribution was restored on re-innervation. Neurofilament-positive axons became visible within the regenerating nerves in a proximo-distal sequence around day 28, and the length of the nestin-positive segments in myofibers began to decrease, also in a proximo-distal sequence corresponding to the reappearance of the axons. By 56 days the length of nestin-positive segments was approximately the same as in intact animals (Figure 4, B ▶ and inset). In addition, at this stage the reversal of the neurogenic atrophy of the myofibers seemed to be proceeding well. A quantitative presentation of the lengths of the nestin-positive segments at different stages of denervation and re-innervation reveals the transitory character of the altered nestin distribution after denervation (Figure 4C) ▶ . In contrast to the marked increase in the nestin immunoreactivity associated with NMJs, denervation had no significant effects on that associated with MTJs (data not shown).
Figure 4.
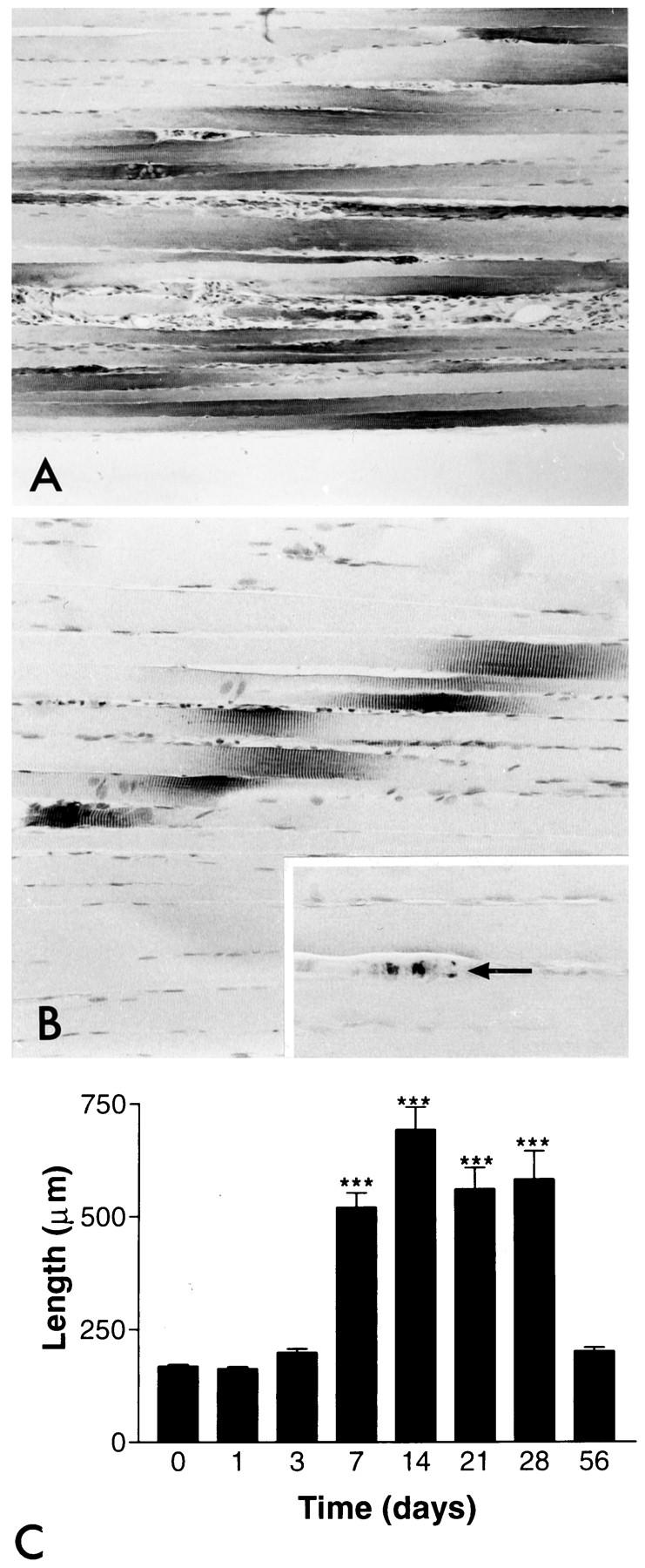
The pattern and intensity of nestin immunoreactivity after denervation and re-innervation. A: Nestin immunoreactivity 28 days after denervation. The length of immunoreactivity has clearly increased. Note that the denervated myofibers have become atrophic. B: Restored nestin distribution after complete re-innervation 56 days after denervation. The length of the nestin immunoreactivity has returned to the same as in intact myofibers (cf Figure 1A ▶ ). Inset: Regenerated axons have also become detectable with neurofilament immunostaining (arrow). Magnification, ×79 (A), ×158 (B), and ×247 (inset). C: The length of nestin immunoreactivity in the NMJ regions on each group is shown as a time course after denervation. Numbers are mean values ± SEM; ***P < 0.001.
Nestin Distribution after Myogenic Denervation
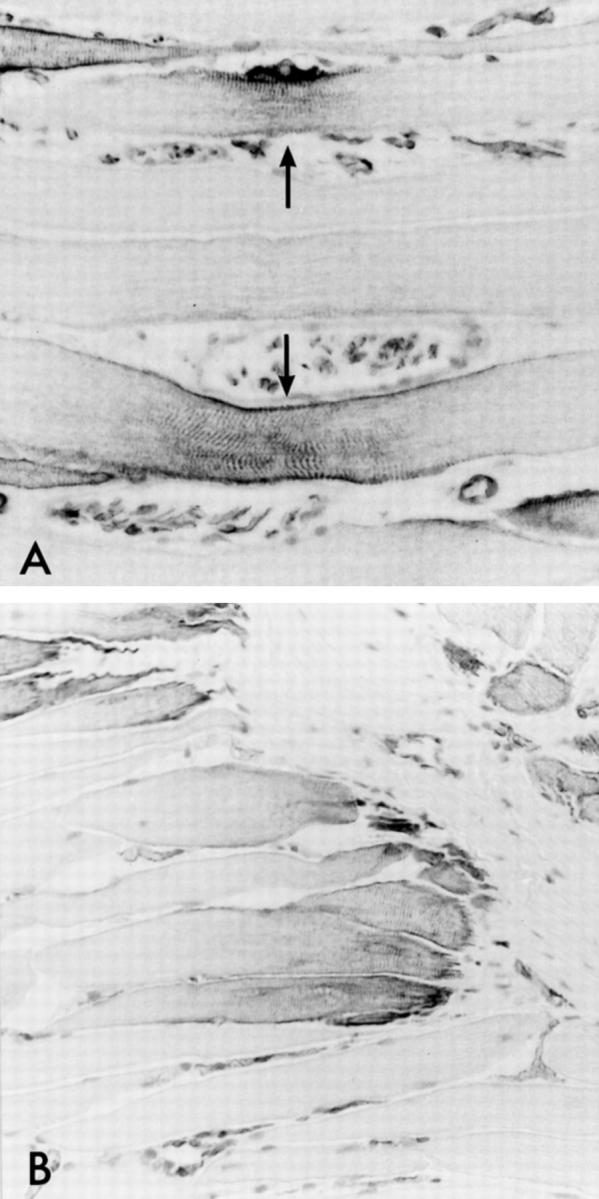
After the transection, the regenerating parts of the myofibers next to their cut ends became immunoreactive for nestin, which also occurs after necrotizing muscle injury (S. Vaittinen et al, in preparation). This immunoreactivity was later reduced but remained at the tips of the stumps, next to the newly formed MTJs, which bind the regenerated stumps to the intervening connective tissue. In addition, the sarcoplasm next to the newly formed NMJs (identified by BTX positivity and by presence of an axon nearby) 9 was immunoreactive for nestin (Figure 5, A–C) ▶ .
Figure 5.
Nestin immunoreactivity in myogenically denervated and re-innervated myofibers. A: The sarcoplasm next to a newly formed NMJ in an abjunctional myofiber stump is immunoreactive for nestin. Immunoreactivity for BTX (B) and neurofilament (C) verify the identity of the NMJ. Magnification, ×244.
Vimentin Distribution
A vast majority of the myofibers remained immunonegative for vimentin during the denervation and re-innervation. However, in very few scattered fibers definite vimentin immunoreactivity was detected. Remarkably, in these fibers nestin immunopositivity was very strong and extended over a considerable length, and the vimentin and nestin immunoreactivities co-localized partly in a NMJ-centered pattern (Figure 6, A and B) ▶ , In the connective tissue around the fibers, there was a clear increase in the number of vimentin-positive cells (Figure 6A) ▶ .
Figure 6.

Concerted increase of nestin and vimentin immunoreactivity in a subpopulation of myofibers. A: Most of the increase in vimentin immunoreactivity on day 21 after denervation is due to connective tissue cells, although a minority of the nestin-positive myofibers is also positive for vimentin (eg, fibers 1 and 2). B: Nestin immunostaining in a consecutive section. Magnification, ×99.
Desmin Distribution
Desmin immunostaining was cross-striated as in controls with random accentuation in the sarcoplasm. Furthermore, the intensity of the immunoreactivity seemed to vary, following the same time sequence as for nestin and vimentin. Again, the folds of NMJs (but not the sarcoplasm underlying NMJs) and the sarcoplasm in the vicinity of MTJ were accentuated (data not shown).
Increased Accumulation of Vimentin and Nestin in Denervated Muscle
To relate the immunohistochemically observed increases in nestin and vimentin reactivity to the amounts of protein present in the whole tissue, we performed Western blot analysis of extracts from tissue sections.
An increase in the accumulated nestin protein on the Western-blot-based analysis was detectable 7 days after denervation. Nestin accumulation was further increased on sampling days 14 and 21. On day 28 the amount of nestin had markedly decreased and was attenuated to nondetectable levels by day 56 (Figure 7, A and B) ▶ . Nestin reactivity on the Western blots showed a typically multibanded pattern, which is characteristic especially of tissue samples. 10,13 One major band can be observed at >200 kd with a number of lower molecular weight bands appearing below the major band.
Figure 7.

Accumulation of nestin and vimentin in denervated muscle. Western blot analysis shows a clear increase in the amount of nestin (A) and vimentin from day 7 to 28. B and C: This is clearly illustrated by image-analysis-based densitometry of the nestin (B) and vimentin (C) protein bands on the Western blots. The even protein loading of the gels was controlled by Coomassie blue staining of the proteins remaining on the gels after electrotransfer and by Coomassie blue staining of parallel gels.
A small increase in accumulated vimentin protein could be observed already 1 to 3 days after denervation, as compared with the protein amount in the control sample (Figure 7, A and C) ▶ . The accumulation of vimentin protein continued to increase up to day 21 after the denervation procedure, after which the vimentin levels were attenuated and reached normal levels by day 56.
Nestin in Human Muscle
In the human abductor pollicis brevis muscle, a similar nestin staining pattern as in rat muscles could be observed (Figure 8, A and B) ▶ . Nestin was positive in both the NMJ and MTJ regions, the former being identified by enzyme histochemical staining for AChE (data not shown).
Figure 8.
Nestin in adult human muscle shows a distribution similar to that observed in adult rat. A and B: Nestin immunostaining of NMJ regions (A, arrows) and MTJ regions (B). Magnification, ×272 (A) and ×192 (B).
Discussion
Nestin, Vimentin, and Desmin in Developing Skeletal Muscle
Three IF proteins, nestin, vimentin, and desmin, are expressed during the development of skeletal muscle. Although the exact function of these three IF proteins during myogenesis has not been clarified, they are likely to have complementary functions during this process. During differentiation of G6 myoblasts into multinucleate myotubes, nestin, vimentin, and desmin show identical intracellular distribution. 5 This has been interpreted to indicate that nestin co-assembles with desmin and vimentin into the same cytoplasmic filamentous network and that nestin most likely forms heteropolymers with vimentin and desmin. 5 When differentiation extends beyond the myotube stage, vimentin expression ceases and desmin becomes the predominant IF protein. The results of the present study demonstrate that the limited nestin expression remaining at the terminally differentiated stages is highly specific and may be associated with some specialized IF functions.
Nestin, Vimentin, and Desmin in Intact Mature Myofibers
As the differentiation of myofibers proceeds, the expression of nestin and vimentin becomes strongly reduced, whereas that of desmin is maintained, and desmin becomes a major cytoskeletal component with cross-striated sarcomeric orientation. A down-regulation of nestin and vimentin expression was observed in rat thigh muscle after postnatal day 14, and mRNA transcripts were strongly reduced in 5-month-old rats. 4 However, as that study focused only on the overall presence of nestin mRNA transcripts, no systematic analysis of the subcellular distribution of nestin protein distribution was performed. The present study showed that although the overall nestin expression in mature myofibers is low, nestin protein is definitely present in two separate restricted loci in the sarcoplasm, ie, adjacent to NMJs and MTJs, having a similar cross-striated sarcomeric pattern as that of desmin. Immunoreactivity in these two areas can easily go unnoticed, as these specialized focal regions are infrequently included in transverse histological sections or in longitudinal sections of limited size.
NMJs were identified by the visualization of two molecules with predominant NMJ-associated localization, AChE and AChR, the former demonstrated by enzyme histochemistry and the latter identified by BTX, which specifically binds to the α-subunit of the nicotinic AChR molecule. Furthermore, terminal axons could often be discerned by neurofilament immunostaining in the vicinity of NMJs. The pattern of nestin immunoreactivity in the microscopic sections results from the anatomy of the skeletal muscle innervation. Each myofiber is innervated by a single axon terminal, which abuts on each myofiber at a single NMJ, usually located in the middle third of the myofiber. Because the neighboring myofibers are of similar length and the axons of intramuscular nerve branches ramify into their individual terminals more or less in a single plane, the NMJs of adjacent fibers become located next to each other. Consequently, in longitudinal microscopic sections NMJs form a band running obliquely across the long axis of the whole muscle belly. The specific distribution of nestin at the NMJs could be connected to neuromuscular signaling. This possibility is discussed below together with the alterations in nestin immunoreactivity during denervation.
Based on the intensity of the immunohistochemical reaction, the amount of accumulated nestin protein at the MTJs seemed to be almost as high as that associated with NMJs. The MTJs were identified by their location at the ends of myofibers, which often adhere to tendinous fasciae running obliquely within the muscle belly. The abundance of nestin in the vicinity of MTJs could relate to the suggested role of nestin as an IF protein associated with dynamic phases of cellular organization, as evidenced by results demonstrating active nestin expression especially during development of muscle cells 4 and regenerative phases after muscle injury in both human 14 and rat (S. Vaittinen et al, manuscript in preparation). A continuous regeneration process may occur near MTJs, where myofibers are most easily damaged under mechanical stress. 15 Furthermore, AChRs, with which nestin appears to associate, are also present in the MTJ region, where the presence of AChRs has been proposed to facilitate muscle repair. 16
Our results on expression of vimentin and desmin in intact muscle were identical to those previously reported; ie, adult myofibers were negative for vimentin and the clearly positive immunoreactivity for desmin had the cross-striated sarcomeric pattern. Desmin was also clearly accentuated in the sarcolemmal foldings of NMJs, a finding previously reported by Sealock et al. 17 Accentuation of desmin immunoreactivity close to MTJs could have the same explanation discussed above in connection with nestin, ie, reinforcement of myofiber structure close to a region where the myofiber is exposed to greatest stress.
Nestin, Vimentin, and Desmin in Denervated and Re-Innervated Mature Myofibers
As suggested above, the specific distribution of nestin in the vicinity of NMJs could indicate that its presence is somehow related to neural transmission. This assumption is further supported by the fact that nestin IFs were observed in myofibers next to MTJs, as this region also shows abundant expression of AChE and AChR (for references see Ref. 18 ), both of which are molecules related to neural transmission. To test the hypothesis, we examined the effect of denervation on nestin expression and distribution. Two different means of denervation were used. Freezing the nerve was selected as the method for neurogenic denervation, because in that model the connective tissue sheaths of the nerve are not breached, and therefore rapid re-innervation is possible. The denervation could be verified by the visible symptoms of paresis and by the disappearance of neurofilament-positive axons in the intramuscular nerves with concomitant structural changes compatible with Wallerian degeneration. Re-innervation was ascertained by the reappearance of neurofilament-positive axons. Myogenic denervation was selected as another method, because in that case the abjunctional part of the transected muscle is completely devoid of NMJs and consequently free of the characteristic NMJ-associated AChR clusters (see below). 9
The amount and distribution of nestin immunoreactivity in the sarcoplasm seem to be specifically dependent on neural input on myofibers. After neurogenic denervation, the length of nestin immunoreactivity was increased up to fivefold, whereas inactivity alone appears to have no effect on nestin immunoreactivity (preliminary unpublished observations). The parallel significant increase in the amount of nestin in Western blotting suggests not only that the increase in the length was due to redistribution of molecules in an atrophied myofiber but also that there was an increased accumulation of nestin protein. Currently we assume that the elevated nestin levels would be due to increased protein synthesis, although inhibited protein degradation or mRNA stabilization are obviously also possible. These observations support the suggested association of nestin expression with the presence of AChR in the NMJs and MTJs. There is ample evidence that denervation of myofibers causes increased synthesis of AChRs and induces spreading of the receptors from the NMJs to the extrajunctional parts of the myofiber plasma membrane. 19 The observations from myogenically denervated muscle further support the assumption that the presence of NMJ-associated molecules is a critical determinant in nestin distribution and expression. In contrast to the neurogenic denervation, after myogenic denervation, there was no increase in nestin immunoreactivity in the abjunctional stumps (except for the parts undergoing regeneration), which have become denervated by losing their contact with the NMJs in the adjunctional stumps. This could be due to the fact that in the abjunctional stumps there are no NMJs from where AChRs could migrate to the surrounding sarcolemma. However, when the de novo formed ectopic NMJs began to appear 15 days after the transection, nestin immunoreactivity also became discernible in the sarcoplasm underlying the new NMJs.
The denervation-induced redistribution of AChRs as it occurs from the NMJs has not been described for the MTJ region. Accordingly, there were no significant changes in nestin immunoreactivity at the MTJs.
Protein synthesis in postsynaptic nerve cells has been demonstrated to be affected by the synaptic activity in a targeted manner; 20,21 ie, mRNA is specifically transported to postsynaptic sites, which allows local translation triggered by synaptic stimulus. On the other hand, in skeletal muscle the mRNA of AChRs is concentrated beneath the NMJs in innervated myofibers, whereas in denervated fibers they are more widely distributed, which indicates neurally regulated targeted expression. 19 Analogously, the expression of nestin in the myofibers could well be dependent on nerve impulses (eg, signaling by ion fluxes) across the plasma membrane of myofibers.
In accordance with previous studies, 4,22 no vimentin immunoreactivity could be detected in intact mature myofibers. The effect of denervation on vimentin distribution and expression was minimal as compared with the observed effects on nestin. The marked increase in the amount of vimentin observed in the Western blotting is likely to be mostly due to vimentin produced by cells of the mysial connective tissue sheaths.
In denervated myofibers desmin immunoreactivity retained the sarcomeric striated pattern, but its overall intensity seemed to increase. The distribution of desmin immunoreactivity differed from that of nestin, and the details of desmin expression pattern during denervation should be further examined. By immunoelectron microscopy it has been shown that desmin resides in the intermyofibrillary space next to the Z-bands of the myofibrils 23 and in the filamentous core of cellular processes at the MTJs. 24 On the basis of this topography, it has been suggested that the key function of desmin in adult muscle is adhesion to the actinin molecules at the Z-bands, which is supposed to result in keeping the myofibrils in register. 6,25 This hypothesis was later supported by Li et al. 26 They found that although skeletal muscles in desmin knockout mice appear to develop normally, the myofibrils in these muscles are fragile and become structurally abnormal under mechanical tension, indicating that desmin is important for the integrity of myofibrils and tissue cohesion.
In conclusion, we have shown that nestin is not only expressed during early phases of muscle development but also in mature myofibers in a selective manner in the sarcoplasm of the NMJ and MTJ regions, ie, in regions associated with AChRs. Furthermore, both the spatial distribution and the expression of nestin are regulated by the innervation status of myofibers. The significance of this highly characteristic expression pattern could be related to either the signaling functions or the regenerative competence of the nestin-positive regions. However, additional studies are required to elucidate these aspects.
Footnotes
Address reprint requests to Dr. Hannu Kalimo, Department of Pathology, Turku University Hospital, FIN-20520 Turku, Finland. E-mail: hannu.kalimo@utu.fi.
Supported by grants from the Ministry of Education of Finland, Turku University Hospital, Turku University Foundation, and the Academy of Finland. C. Sahlgren is supported by the Turku Graduate School of Biomedical Sciences.
References
- 1.Steinert PM, Liem RK: Intermediate filament dynamics. Cell 1990, 60:521-523 [DOI] [PubMed] [Google Scholar]
- 2.Dahlstrand J, Collins VP, Lendahl U: Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res 1992, 52:5334-5341 [PubMed] [Google Scholar]
- 3.Lendahl U, Zimmerman LB, McKay RDG: CNS stem cells express a new class of intermediate filament protein. Cell 1990, 60:585-595 [DOI] [PubMed] [Google Scholar]
- 4.Sejersen T, Lendahl U: Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci 1993, 106:1291-1300 [DOI] [PubMed] [Google Scholar]
- 5.Sjöberg G, Jiang W-Q, Ringertz NR, Lendahl U, Sejersen T: Colocalization of nestin and vimentin/desmin in skeletal muscle cells demonstrated by three-dimensional fluorescence digital imaging microscopy. Exp Cell Res 1994, 214:447-458 [DOI] [PubMed] [Google Scholar]
- 6.Gard DL, Lazarides E: The synthesis and distribution of desmin and vimentin during myogenesis in vitro. Cell 1980, 19:263-275 [DOI] [PubMed] [Google Scholar]
- 7.Li Z, Colucci-Guyon E, Pincon-Raymond M, Mericskay M, Pournin S, Paulin D, Babinet C: Cardiovascular lesions and skeletal myopathy in mice lacking desmin. Dev Biol 1996, 175:362-366 [DOI] [PubMed] [Google Scholar]
- 8.Galou M, Gao J, Humbert J, Mericskay M, Li Z, Paulin D, Vicart P: The importance of intermediate filaments in the adaptation of tissues to mechanical stress: evidence from gene knockout studies. Biol Cell 1997, 89:85-97 [PubMed] [Google Scholar]
- 9.Rantanen J, Ranne J, Hurme T, Kalimo H: Denervated segments of injured skeletal muscle fibers are re-innervated by newly formed neuromuscular junctions. J Neuropathol Exp Neurol 1995, 54:188-194 [DOI] [PubMed] [Google Scholar]
- 10.Dahlstrand J, Zimmerman LB, McKay RD, Lendahl U: Characterization of the human nestin gene reveal a close evolutionary relationship to neurofilaments. J Cell Sci 1992, 103:589-597 [DOI] [PubMed] [Google Scholar]
- 11.Karnovsky MJ, Roots L: A “direct-colouring” thiocholine method for cholinesterases. J Histochem Cytochem 1964, 12:219-221 [DOI] [PubMed] [Google Scholar]
- 12.Starger JM, Brown WE, Goldman AE, Goldman RD: Biochemical and immunological analysis of rapidly purified 10-nm filaments from baby hamster kidney (BHK-21) cells. J Cell Biol 1978, 78:93-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frojdman K, Pelliniemi LJ, Lendahl U, Virtanen I, Eriksson JE: The intermediate filament protein nestin occurs transiently in differentiating testis of rat and mouse. Differentiation 1997, 61:243-249 [DOI] [PubMed] [Google Scholar]
- 14.Sjoberg G, Edstrom L, Lendahl U, Sejersen T: Myofibers from Duchenne/Becker muscular dystrophy and myositis express the intermediate filament nestin. J Neuropathol Exp Neurol 1994, 53:416-423 [DOI] [PubMed] [Google Scholar]
- 15.Tidball JG, Salem G, Zernicke R: Site and mechanical conditions for failure of skeletal muscle in experimental strain injuries. J Appl Physiol 1993, 74:1280-1286 [DOI] [PubMed] [Google Scholar]
- 16.Bernheim L, Hamann M, Liu J-H, Fischer-Lougheed J, Bader C-R: Role of nicotinic acetylcholine receptors at vertebrate myotendinous junction: a hypothesis. Neuromusc Disord 1996, 6:211-214 [DOI] [PubMed] [Google Scholar]
- 17.Sealock R, Murnane A, Paulin D, Froehner S: Immunochemical identification on desmin in Torpedo postsynaptic membranes and at the rat neuromuscular junction. Synapse 1989, 3:315-324 [DOI] [PubMed] [Google Scholar]
- 18.Chen Q, Sealock R, Peng HB: A protein homologous to the Torpedo postsynaptic 58K protein is present at the myotendinous junction. J Cell Biol 1990, 110:2061-2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman D, Staple J: Spatial and temporal expression of acetylcholine receptor RNAs in innervated and denervated rat soleus muscle. Neuron 1989, 3:219-228 [DOI] [PubMed] [Google Scholar]
- 20.Weiler I, Greenough W: Metabotropic glutamate receptors trigger postsynaptic protein synthesis. Proc Natl Acad Sci USA 1993, 90:7168-7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang J, Schumacher E, Coulter P, Vinters H, Watson J: Dendritic translocation of RC3/neurogranin mRNA in normal aging, Alzheimer disease and fronto-temporal dementia. J Neuropathol Exp Neurol 1997, 56:1105-1118 [DOI] [PubMed] [Google Scholar]
- 22.Vater R, Cullen J, Harris JB: The expression of vimentin in satellite cells of regenerating skeletal muscle in vivo. Histochem J 1994, 26:916-928 [PubMed] [Google Scholar]
- 23.Tokuyasu KT, Dutton AH, Singer SJ: Immunoelectron microscopic studies of desmin (skeletin) localization and intermediate filament organization in chicken skeletal muscle. J Cell Biol 1983, 96:1727-1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tidball JG: Desmin at myotendinous junctions. Exp Cell Res 1992, 199:206-212 [DOI] [PubMed] [Google Scholar]
- 25.Vater R, Cullen J, Harris JB: The fate of desmin and titin during the degeneration and regeneration of the soleus muscle of the rat. Acta Neuropathol 1992, 84:278-288 [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Mericskay M, Agbulut O, Butler-Browne G, Carlsson L, Thornell L-E, Babinet C, Paulin D: Desmin is essential for the tensile strength and integrity of myofibrils but not for myogenic commitment, differentiation, and fusion of skeletal muscle. J Cell Biol 1997, 139:129-144 [DOI] [PMC free article] [PubMed] [Google Scholar]