Abstract
Although young mice homozygous for the osteopetrosis (op) mutation usually developed prominent osteopetrosis, its severity was markedly reduced in aged op/op mice. This age-associated reversal of osteopetrosis was accompanied by the expansion of bone marrow cavities and increased numbers of tartrate-resistant acid phosphatase (TRAP)-positive cells and of macrophages in the bone marrow. The TRAP-positive cells were mononuclear and developed ruffled borders and numerous vesicles, vacuoles, and granules. Enzyme-linked immunosorbent assay demonstrated a significant elevation of serum granulocyte/macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-3 levels in the aged op/op mice. To examine whether GM-CSF and/or IL-3 could correct osteopetrosis in young op/op mice, 5 ng of recombinant murine (rm)GM-CSF and/or 100 ng of rmIL-3 were injected daily into young op/op mice. In these treated young op/op mice, the bone marrow cavities were expanded significantly at 2 weeks after administration, associated with significantly increased numbers of TRAP-positive cells and bone marrow macrophages. TRAP-positive cells increased in number with days after injection. These results suggest that GM-CSF and IL-3 induce the development of osteoclasts to correct osteopetrosis in the op/op mice with aging.
Osteoclasts are a physiological polykaryon and the major if not exclusive resorptive cells of bone and are thought to be a member of the monocyte/macrophage family. 1-4 Osteoclasts are derived from bone marrow progenitors, differentiated from granulocyte/macrophage colony-forming cells (GM-CFCs) via the stage of tartrate-resistant acid phosphatase (TRAP)-positive mononuclear osteoclasts (so-called preosteoclasts), and polarized, forming a unique ruffled membrane at the osteoclast-bone interface. Finally, polykaryonic cells are formed by fusion of mononuclear osteoclasts with each other. 5-8 In the process of osteoclast development and differentiation, the myeloid and B lymphoid transcription factor PU.1 (also called Spi-1 or Sfpi-1), macrophage colony-stimulating factor (M-CSF or colony-stimulating factor (CSF)-1), c-fos, c-src, carbonic anhydrase II, or H+-ATPase are known to be involved in its different stages. 8-10 The involvement of these factors is based on the results obtained in studies of mice deleted of PU.1, 8,9 c-fos, 11 or c-src, 7,12 mice bearing a naturally occurring Csfmop/Csfmop (op/op) gene mutation, 7,13 or humans lacking functional H+-ATPase 14 or carbonic anhydrase II genes. 15 All of these mice and humans develop osteopetrosis. 8-15 In op/op mice, the production of functional M-CSF protein is impaired due to a defect in the coding region of Csfm gene, 13 and M-CSF deficiency results in widespread defects of the monocytic cell development, including monocyte/macrophage differentiation, and osteoclast ontogeny during their early life. 7,13,16,17 The failure of osteoclast development and differentiation in op/op mice results in impaired bone resorption and remodeling, leading to systemic osteopetrosis. 7,13,16 In op/op mice, however, there are immature macrophages in many tissues such as spleen, lungs, or brain, although their numbers are reduced. 16,17 For the development and survival of tissue macrophages, GM-CSF and IL-3, which are major macrophage growth factors other than M-CSF, are essential. 13,17,18
In our previous studies, it was shown that M-CSF administration into op/op mice induced the proliferation of GM-CFCs, their differentiation into mononuclear osteoclasts, formation of multinuclear osteoclasts by fusion of mononuclear osteoclasts, development and differentiation of monocyte/macrophages, and proliferation and differentiation of immature tissue macrophages. 7,16 It is known that aged op/op mice undergo a hematopoietic recovery encompassing progressive increases in numbers of osteoclasts and macrophages, resolution of osteopetrosis, and expansion of bone marrow cavities. 19-21 These findings suggest that the hematopoietic system has the capacity to use alternative mechanisms to compensate for the absence of functional M-CSF activity. In op/op mice, as M-CSF is totally deficient, GM-CSF and IL-3 are suggested as candidates for the major cause to induce the alternative mechanisms. 13,17,18 In previous in vitro studies, GM-CSF and/or IL-3 were demonstrated to be critical factors for osteoclast formation. 5,22-30 However, the effects of GM-CSF or IL-3 on osteoclast development in op/op mice in the previous in vitro studies were controversial; some investigators reported that GM-CSF or IL-3 stimulated osteoclast development, 5,22-30 whereas other researchers showed inhibitory effects of GM-CSF or IL-3 on osteoclast formation in vitro. 30-35 In a previous in vivo study, Jedrzejczak et al reported that administration of GM-CSF in large doses was unable to correct osteopetrosis of op/op mice in vivo. 36 Mice homozygous for targeted mutations in the GM-CSF structural gene do not develop osteopetrosis and are known not to impair the development of osteoclasts and macrophages. 37,38 M-CSF/GM-CSF-deficient mice do not impair the recovery of osteopetrosis and hematopoietic deficiencies with aging. 39 In this way, there are great discrepancies of data among the previous in vitro and in vivo studies on the roles of GM-CSF or IL-3 on osteopetrosis of op/op mice. Thus, the mechanisms of these growth factors for bone remodeling of the aged op/op mice remained unsolved.
In the present study, serum levels of GM-CSF or IL-3 in aged (more than 60-week-old) op/op mice were measured by enzyme-linked immunosorbent assay (ELISA) and compared with those of young (4-week-old) op/op mice. In the aged op/op mice, x-ray findings and histological changes of the femurs were examined, compared with those of the young op/op mice. The relative sizes of the bone marrow cavities in the femurs of the aged op/op mice were calculated, compared with those of the young op/op mice. To clarify whether or not GM-CSF or IL-3 induces correction of osteopetrosis of op/op mice in vivo, GM-CSF and/or IL-3 were injected into young op/op mice, their femurs were examined roentgenologically or histologically, and the percentages of bone marrow cavity against bone were calculated and compared with those of young or aged op/op mice. In all of these mice, osteoclasts were examined by TRAP staining and ultrastructurally, and their numbers were determined to elucidate the effects of GM-CSF and/or IL-3 on osteoclast development.
Materials and Methods
Animals
(C57BL/6J × C3HeB/FeJ) F2 op/op mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and maintained under routine conditions at the Laboratory Animal Center, Kumamoto University School of Medicine. The op/op mice were produced from matings of +/op heterozygotes. Normal littermates (+/?) consisted of two-thirds +/op and one-third +/+. Homozygous op/op mice develop a small body, a domed skull, absence of incisors, and a short tail at approximately 10 days after birth, whereas the heterozygotes and wild-type mice do not show any abnormalities and were indistinguishable phenotypically. Discrimination of wild-type (+/+) from heterozygotes (+/op) was done by reverse transcriptase nested polymerase chain reaction (RT-nested PCR) as described below. Young (4-week-old) and aged (more than 60-week-old) op/op mice as well as young (4-week-old) wild-type mice were examined. The young op/op and wild-type mice were injected subcutaneously with 5 ng of recombinant mouse (rm)GM-CSF (Genzyme, Cambridge, MA) per day according to the method reported previously 40 and killed at 3, 7, or 14 days after initial injection. In the present study, we injected 5, 10, 50, or 100 ng of rmIL-3 (Genzyme) subcutaneously into BALB/c mice daily for 5 days, and the most effective dose of the cytokine was determined in these IL-3-treated mice by examining numerical increases of F4/80-positive Kupffer cells in the liver. We found that 100 ng of rmIL-3 was the most effective dose to induce numerical increases in macrophages. In the present study, 100 ng of rmIL-3 per day was thus injected into young op/op and wild-type mice subcutaneously, the mice were killed at 3, 7, or 14 days after injection, and the recovery of bone marrow was examined in the femurs at 2 weeks after daily injection. Additional groups of other young op/op and wild-type mice were subcutaneously injected with both 5 ng of rmGM-CSF and 100 ng of rmIL-3 per day, killed at 3, 7, or 14 days after daily administration, and examined. Besides the wild-type mice, BALB/c and C3H/HeN mice were purchased from Nihon SLC Co. (Hamamatsu, Japan) and used for controls. Three or more mice were examined in each experiment.
X-Ray Examination
X-ray examination of bones in the mice was performed with the digital microradiographic system μFX-1000 (Fujifilm, Tokyo, Japan) and analyzed by using a Fujifix bioimage analyzer BAS-2000 (Fujifilm).
RNA Analysis
Total RNA was extracted from the tail of animals using the acid guanidium phenol chloroform method. The RT reaction was performed, using a random primer, and then nested PCR was done by using the following primers. The primer pairs for the mouse M-CSF were designed based on the published cDNA sequence. 13 The sequences of outer and inner primers and sizes of amplified products were as follows: outer primer sense, 5′ -CCAGGAACAGCTGGATGATC-3′ and antisense, 5′-AGTTGCAATCAGGC TTGGTC-3′ (size of amplified products, 359 bp); inner primer sense, 5′-CTGTTTGCT ACCTAAA GAAGGC-3′ and antisense, 5′-CATCTCGGCTAGAGCACTTAGC-3′ (size of amplified products, 315 bp). After the RT-nested PCR, sequencing was performed, using a PCR product presequencing kit US 70995 (Amersham Life Science, Cleveland, OH), a Thermo Sequenase fluorescent-labeled primer cycle sequencing kit RPN 2436/RPN 2536 (Amersham Life Science, Little Chalfont, UK), and an ALF express DNA sequencer (Pharmacia Biotech, Uppsala, Sweden). For sequencing, we used cy5-labeled primer GAGTCTCATGGAAAGTTCGG. By the sequence analysis, wild-type mice (+/+) were distinguished from heterozygotes (+/op), because the sequence pattern at 443 bp from the 5′ end of the Csfm gene was shown to be disordered in the heterozygous mutant mice.
Tissue Preparation and Fixation
Femurs were removed from the mice, cut through their central portion in a longitudinal direction along their long axis, and fixed in 10% neutral formalin fixative for light microscopy. For electron microscopy, the bone tissues were obtained from the femurs, decalcified in 3% EDTA (pH 7.0) at 4°C for 7 days, and then cut into small tissue specimens. The specimens were fixed in 2.5% glutaraldehyde for 2 hours and post-fixed with 1.0% osmium tetroxide for 2 hours. Liver, spleen, thymus, mesenteric lymph nodes, kidneys, lungs, brain, uterus, and testis were removed, and specimens were sampled from each tissue. Tissue specimens were fixed in 10% neutral formalin at room temperature or in 1% periodate-lysine-paraformaldehyde (PLP) solution at 4°C for 6 hours. The other tissue specimens were frozen in liquid nitrogen, cut by a cryostat, and fixed in acetone for 10 minutes.
Light Microscopy and Histochemistry
The formalin-fixed bone tissues were embedded in paraffin. Paraffin sections were cut at 3 μm thickness and stained with hematoxylin and eosin (H&E) for routine histological examination. To detect osteoclasts and their mononuclear precursor cells, 6-μm-thick serial paraffin sections were prepared and processed for the histochemical localization of TRAP as described previously. 7,41
Immunohistochemistry
F4/80 and BM8, rat anti-mouse monoclonal antibodies against macrophages, were purchased from BMA Biomedicals AG (August, Switzerland). As both antibodies can be applied to paraffin sections, we used both monoclonal antibodies to detect macrophages in paraffin-embedded bone tissues. Paraffin sections, 6 μm thick, were prepared and then treated with 1% trypsin. In addition, PLP-fixed tissue specimens for frozen sections were washed for 4 hours with phosphate buffer solution containing 10%, 15%, and 20% sucrose, embedded in OCT compound (Miles, Elkhart, IN), and frozen in liquid nitrogen. From these specimens, 6-μm-thick frozen sections were prepared by a cryostat. After inhibition of endogenous peroxidase activity by the method of Isobe et al, 42 PLP- or acetone-fixed frozen sections, as well as paraffin sections, were stained by the indirect immunoperoxidase method with F4/80 and BM8 as described previously. 16,17 Anti-rat immunoglobulin/horseradish-peroxidase-linked F(ab′)2 fragment (Amersham, Little Chalfont, UK) was used as secondary antibody. After visualization with 3,3′-diaminobenzidine, the sections were stained with hematoxylin and mounted with Malinol. For negative controls, the same procedures were performed, omitting the monoclonal antibodies.
Electron Microscopy
Three mice from each group were used, and one femur was sampled from one mouse for electron microscopy. After fixation, the tissue specimens were dehydrated in a graded series of ethanols, processed through propylene oxide, and embedded in Epon 812 (E. Fullan, Lathan, NY). Five Epon-embedded femoral specimens were made to observe the entire proximal femoral metaphysis. Ten 1-μm-thick serial sections were cut by an ultratome Nova (LKB, Uppsala, Sweden), stained with toluidine blue, and observed by a light microscope to determine whether osteoclasts are mononuclear or multinuclear. Then, ultrathin sections were cut, and 20 or more osteoclasts were observed with a JEM-2000EX electron microscope (JEOL, Tokyo, Japan).
ELISA
Serum GM-CSF and IL-3 were measured with murine GM-CSF and IL-3 ELISA kits according to the protocols supplied with the kits from Endogen (Boston, MA).
Measurements of Bone Marrow Cavity Areas
According to the method previously described, 43-45 bone marrow cavity areas were measured. Briefly, the portion between epiphyseal plates was selected in the H&E-stained serial sections of femurs and photographed under a light microscope at a magnification of ×2.5. Bone marrow cavity areas were measured on each photomicrograph, and their percentage relative to bone, including bone cortex, was calculated with a personal computer Power Macintosh 9500/200 using the NIH image 1.61/ppc.
Cell Enumeration
The numbers of TRAP-positive cells were counted per 10 mm on the endosteal surfaces in the diaphysis and metaphysis of femurs in the animals according to the method described elsewhere. 16 In 6-μm-thick serial sections, TRAP-positive multinuclear cells (osteoclasts) were discriminated from TRAP-positive mononuclear cells, and their numbers were counted. Although TRAP-positive cells with more than three nuclei were judged as osteoclasts in previous studies, 46,47 binuclear TRAP-positive cells were also included in multinuclear osteoclasts in the present study as described elsewhere. 48 In the present study, TRAP-positive mononuclear cells were called mononuclear osteoclasts. This nomenclature was originally proposed in recent studies and was based on fundamental ultrastructural, cytochemical, and functional similarities to TRAP-positive multinuclear cells (multinuclear osteoclasts). 49-52 Numbers of F4/80-positive cells per 1-mm 2 sections were also counted in the bone marrow, spleen, liver, mesenteric lymph nodes, kidneys, uterus, or testis in young and aged op/op mice and in GM-CSF-, IL-3-, or GM-CSF- plus IL-3-treated op/op mice. The percentages of F4/80-positive cells relative to the wild-type mice were determined.
Statistics
The significance of differences between means was evaluated by the Student t-test. P values <0.05 were considered significant.
Results
X-Ray and Histopathological Changes of Femurs in Young and Aged op/op Mice
The marrow cavity of the femurs in the 4-week-old wild-type mice showed normal width and contained few bone trabeculae in the central portion of the diaphysis, whereas primary and secondary spongiosa were developed in the metaphysis (Figure 1A) ▶ . In contrast, 4-week-old op/op mice abundantly developed bone trabeculae and fine cartilaginous bars and showed a marked reduction of marrow cavity in the femurs due to prominent osteopetrosis and osteosclerosis (Figure 1B) ▶ . These bony trabeculae and cartilaginous bars were mostly thin and fused randomly with each other to show a lattice-like pattern. The compact cortical bones were thin in the young op/op mice, particularly in the metaphysis (Figure 1B) ▶ , whereas the bone cortex was thick in the wild-type mice (Figure 1A) ▶ and was similar to that of normal BALB/c or C3H/HeN mice (data not shown). In more than 60-week-old op/op mice, the bone marrow cavity was expanded and was accompanied by reduced numbers of bone trabeculae and cartilaginous bars (Figure 1C) ▶ . The bone trabeculae and cartilaginous bars in the marrow cavity and compact cortical bones were thickened.
Figure 1.
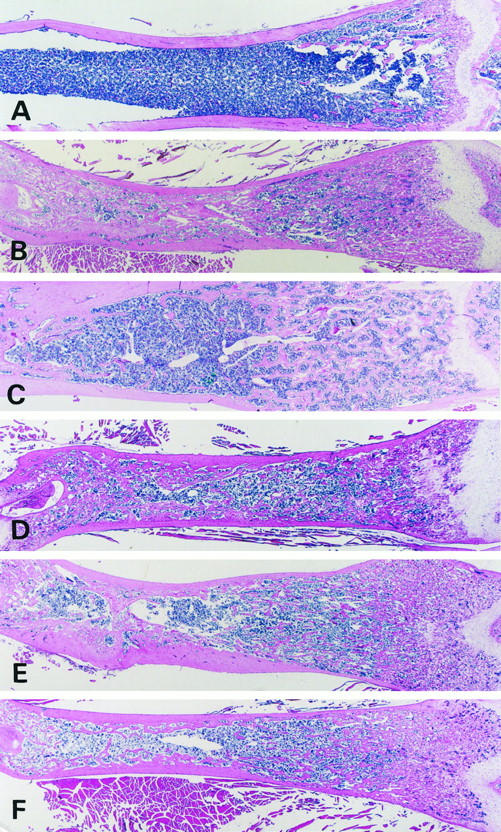
Histological changes of the femurs of a young (4-week-old) wild-type mouse (A), a young (4-week-old) op/op mouse (B), an aged (60-week-old) op/op mouse (C), and GM-CSF- (D), IL-3- (E), or GM-CSF- plus IL-3-treated young (4-week-old) op/op mice (F). A: The femur of the wild-type mouse has a wide marrow cavity. B: Prominent osteosclerosis and marked narrowing of bone marrow with increased fine cartilaginous bars are seen in the metaphysis and diaphysis of the femur in the young op/op mouse. C: A bone marrow cavity is formed in the diaphysis, and cartilaginous bars become thickened and are decreased in amount in the metaphysis of femurs in the aged op/op mouse. D to F: A bone marrow cavity is seen in the diaphysis of femurs in the GM-CSF- (D), IL-3- (E) and GM-CSF- plus IL-3-treated young op/op mice (F). In all these mice, cartilaginous bars are reduced in amount in the diaphysis and metaphysis of the bones, compared with the untreated young op/op mouse (B). H&E stain; magnification, ×4.
Figure 2 ▶ shows the percentages of marrow cavity areas relative to bone areas in the femurs in the young or aged op/op and wild-type mice. In the wild-type mice, the marrow cavity of the femurs was approximately 70% of bone and was reduced to approximately 20% in young op/op mice, whereas the bone marrow cavity of the aged op/op mice was approximately 30% wider than that of the young op/op mice. These data indicated that op/op mice showed a recovery of osteopetrosis and expansion of bone marrow cavity with aging and were consistent with the x-ray findings of femurs in these animals (Figure 3, A–C) ▶ .
Figure 2.
Percentages of bone marrow cavity areas between the epiphyseal plates in the femur shaft of wild-type mice, young and aged op/op mice, and GM-CSF- and/or IL-3-treated young op/op mice. Compared with the wild-type mice, the marrow cavity of femurs in the untreated young op/op mice is significantly reduced. Compared with the young op/op mice, the bone marrow cavities are significantly expanded in the aged op/op mice and GM-CSF- and/or IL-3-treated young op/op mice. Each value is representative of three animals; bars indicate mean ± SD.
Figure 3.
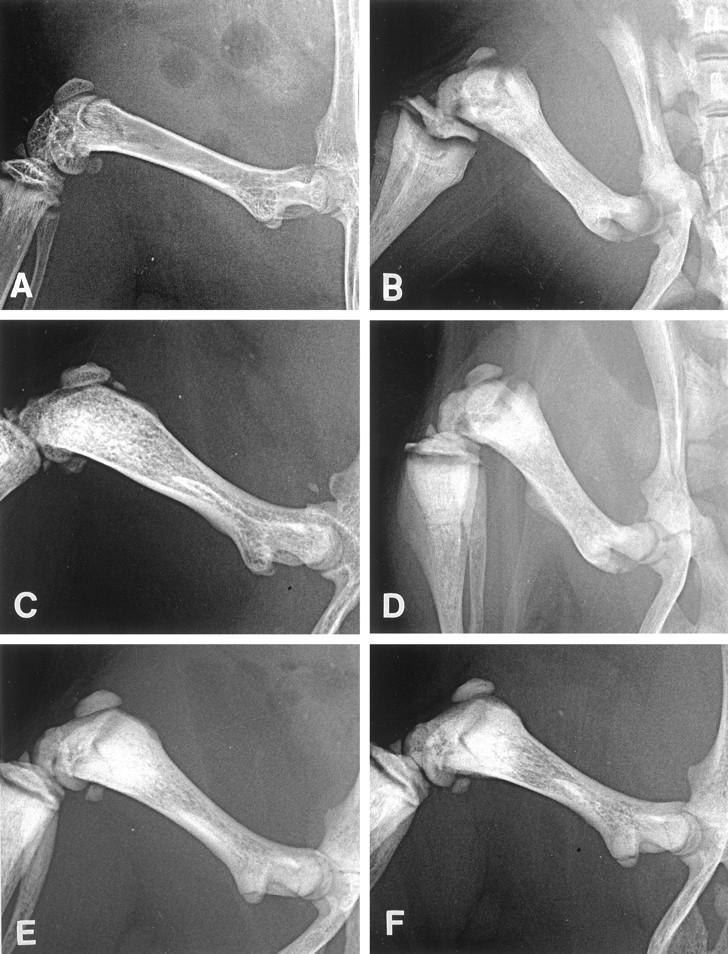
X-ray findings of femurs in a young wild-type mouse (A), a young op/op mouse (B), an aged op/op mouse (C), and a GM-CSF-treated (D), an IL-3-treated (E), and a GM-CSF- plus IL-3-treated (F) young op/op mouse. A: The femur of the wild-type mouse has a wide marrow cavity and clearly distinguishable cortical bones. B: The femur of the young op/op mouse is short and thick, associated with diffuse bone density in the medullary cavity and indistinguishable cortical bones. C: In the aged op/op mouse, the medullary cavity is formed in the femur, is distinguished from the bone cortex, and shows a lacy pattern of bone density. D to F: In the treated op/op mice, the bone marrow cavity is formed, and the cortical bones are distinguished from the cavity. Slight lacy patterns of density are found in the medullary cavity.
Serum GM-CSF or IL-3 Levels in the Young and Aged op/op Mice by ELISA
Table 1 ▶ shows GM-CSF and IL-3 levels in serum of young or aged op/op mice, wild-type mice, and GM-CSF-, IL-3-, or GM-CSF- plus IL-3-treated young op/op mice measured by ELISA. GM-CSF and IL-3 were undetectable in serum of the young op/op and wild-type mice. In the aged op/op mice, serum GM-CSF and IL-3 levels were elevated significantly. Among GM-CSF-, IL-3-, or GM-CSF- plus IL-3-treated young op/op mice, serum IL-3 levels were elevated significantly in GM-CSF- plus IL-3-treated animals. In the aged and treated young op/op mice, however, we could find no significant relationship between the serum GM-CSF or IL-3 levels and the degrees of osteopetrosis recovery (data not shown).
Table 1.
Serum GM-CSF and IL-3 Levels in Young or Aged op/op Mice, GM-CSF-, IL-3-, or GM-CSF- plus IL-3-Treated Young op/op Mice and Wild-Type Mice
| Mice | GM-CSF (pg/ml) | IL-3 (pg/ml) |
|---|---|---|
| op/op mice | ||
| Young | ND (3) | ND (3) |
| Aged | 24.90± 15.69* (11) | 26.72± 5.84* (3) |
| Young GM-CSF-treated | ND (3) | ND (7) |
| Young IL-3-treated | ND (3) | ND (3) |
| Young GM-CSF- plus IL-3-treated | ND (3) | 84.16 ± 30.08* (3) |
| Wild type | ND (3) | ND (3) |
Results are expressed as mean ± SD. Young mice were 4 weeks old; aged mice were >60 weeks old. Mice were treated with GM-CSF, IL-3, or GM-CSF plus IL-3 for 2 weeks. ND, undetectable. Numbers of mice examined are shown in parentheses.
*P < 0.05.
Correction of Osteopetrosis and Expansion of Bone Marrow Cavity in Young op/op Mice by Daily Administration of GM-CSF, IL-3, and GM-CSF plus IL-3
GM-CSF and IL-3 are major cytokines for macrophage growth and differentiation in op/op mice totally lacking M-CSF. As elevated GM-CSF and IL-3 levels were found in the aged op/op mice that showed correction of osteopetrosis, we injected daily rmGM-CSF (5 ng/day), rmIL-3 (100 ng/day), or rmGM-CSF plus rmIL-3 into 4-week-old op/op mice to examine the effects of these cytokines on the correction of osteopetrosis. Expansion of bone marrow cavity, reduced amounts of bone trabeculae or cartilaginous bars, and bone thickening were observed in the young op/op mice at 2 weeks after daily administration of GM-CSF, IL-3, or GM-CSF plus IL-3 (Figure 1, D–F) ▶ . In the GM-CSF-, IL-3-, or GM-CSF- plus IL-3-treated op/op mice, osteopetrosis of the femurs was corrected, and bone marrow cavity was expanded significantly (P < 0.01) (Figure 2) ▶ . Although significant expansion of bone marrow cavity was not demonstrated between GM-CSF-treated and GM-CSF- plus IL-3-treated op/op mice (P > 0.05), bone marrow expansion in the GM-CSF- plus IL-3-treated op/op mice was significant, compared with the IL-3-treated op/op mice (P < 0.05). These results were consistent with the x-ray findings of femurs in these treated mutant mice (Figures 3, D–F) ▶ . However, correction of osteopetrosis was not confirmed in young op/op mice daily injected for 2 weeks with 0.71 μg of IL-3, the dose used for intestinal epithelial cell growth in a previous in vivo study 53 (data not shown).
Changes in the Number of TRAP-Positive Cells and Their Cytological and Ultrastructural Features
Table 2 ▶ shows numbers of TRAP-positive cells per 10 mm on the endosteal surfaces in the femurs of young op/op, aged op/op, and young wild-type mice. In the wild-type mice, there were approximately 45 TRAP-positive cells in the endosteal surface of the diaphysis and approximately 500 TRAP-positive cells in the metaphysis, and two-thirds of them were multinuclear (Figure 4A) ▶ . The TRAP-positive multinuclear cells (osteoclasts) had heterochromatic nuclei and their cell cytoplasm was large, pleomorphic, and irregularly shaped. The numbers of TRAP-positive multinuclear cells in the wild-type mice were similar to those in BALB/c and C3H/HeN mice (data not shown). TRAP-positive mononuclear cells possessed a spindle or oval nucleus with a slender cytoplasm. In the op/op mice at the age of 4 weeks, a few TRAP-positive cells were detected in the metaphysis and diaphysis of the femurs (Figure 4B) ▶ , almost all of them were mononuclear, and multinuclear osteoclasts were absent. In the aged op/op mice, the numbers of TRAP-positive cells, as shown in Table 2 ▶ , were significantly increased in the diaphysis and metaphysis of the femurs, compared with those of young op/op mice (P < 0.05), and most of them were mononuclear (Figure 4C) ▶ . In the op/op mice given GM-CSF, IL-3, or GM-CSF plus IL-3 daily for 2 weeks, the numbers of TRAP-positive mononuclear cells were significantly increased, compared with those of the young op/op mice (P < 0.05) (Figure 4, C–F ▶ ; Table 2 ▶ ). In addition, the number of multinuclear osteoclasts was increased significantly in the diaphysis and metaphysis of femurs in GM-CSF- plus IL-3-treated young op/op mice, compared with the young op/op mice. However, multinuclear osteoclast numbers in the metaphysis were smaller than in the wild-type mice (Table 2) ▶ .
Table 2.
Numbers of TRAP-Positive Cells per 10 mm on the Endosteal Surfaces in the Diaphysis and Metaphysis of Femurs in Young or Aged op/op Mice, GM-CSF-, IL-3- or GM-CSF- plus IL-3-Treated Young op/op Mice and Wild-Type Mice
| Mice | Number of TRAP+ cells (mean ± SD) | |||
|---|---|---|---|---|
| Mononuclear | Multinuclear | |||
| Metaphysis | Diaphysis | Metaphysis | Diaphysis | |
| Wild type | 115.00 ± 36.06 | 29.60 ± 12.52 | 406.67 ± 32.15 | 14.00 ± 2.00 |
| op/op mice | ||||
| Young | 6.83 ± 2.48 | 8.33 ± 3.40 | 0.83 ± 2.04 | 0.17 ± 0.41 |
| Aged | 44.00 ± 6.99* | 25.40 ± 4.01* | 2.50 ± 3.54 | 0.60 ± 0.52 |
| Young GM-CSF-treated | 52.50 ± 8.22* | 37.14 ± 11.94* | 3.33 ± 2.58 | 2.67 ± 2.73 |
| Young IL-3-treated | 22.60 ± 6.43* | 36.00 ± 7.07* | 0.40 ± 0.55 | 1.00 ± 1.00 |
| Young GM-CSF- plus IL-3-treated | 37.50 ± 6.61* | 36.77 ± 8.78* | 17.50 ± 2.50* | 12.33 ± 3.93* |
Young mice were 4 weeks old; aged mice were >60 weeks old. Mice were treated with GM-CSF, IL-3, or GM-CSF plus IL-3 for 2 weeks.
*P < 0.05.
Figure 4.

Appearance of TRAP-positive cells in the femur of a young wild-type mouse (A), a young op/op mouse (B), an aged op/op mouse (C), or a GM-CSF- (D), an IL-3- (E), or a GM-CSF- plus IL-3-treated (F) young op/op mouse. A and C to F: TRAP-positive cells are seen on the endosteal surfaces of the femur diaphysis in the young wild-type mouse, the aged op/op mouse, and the treated young op/op mouse. Arrowheads show TRAP-positive cells, and arrows indicate TRAP-positive resorption surfaces where no osteoclasts are detected. Cells in the center of a black square are shown in the inset at a higher magnification. A: There are numerous TRAP-positive osteoclasts in a young wild-type mouse. Inset: A high magnification of a multinucleated osteoclast. B: TRAP-positive cells are absent in the osteopetrotic femur bone of the young op/op mouse. C to F: Increased numbers of osteoclasts are seen in an aged op/op mouse (C) and a GM-CSF- (D), an IL-3- (E), and a GM-CSF- plus IL-3-treated (F) young op/op mouse. Inset: Mononuclear TRAP-positive cells in the aged (C) and GM-CSF-treated young (D) op/op mouse and a multinucleated osteoclast in GM-CSF- plus IL-3-treated young op/op mouse (F). TRAP staining; magnification, ×66 and ×100 (inset).
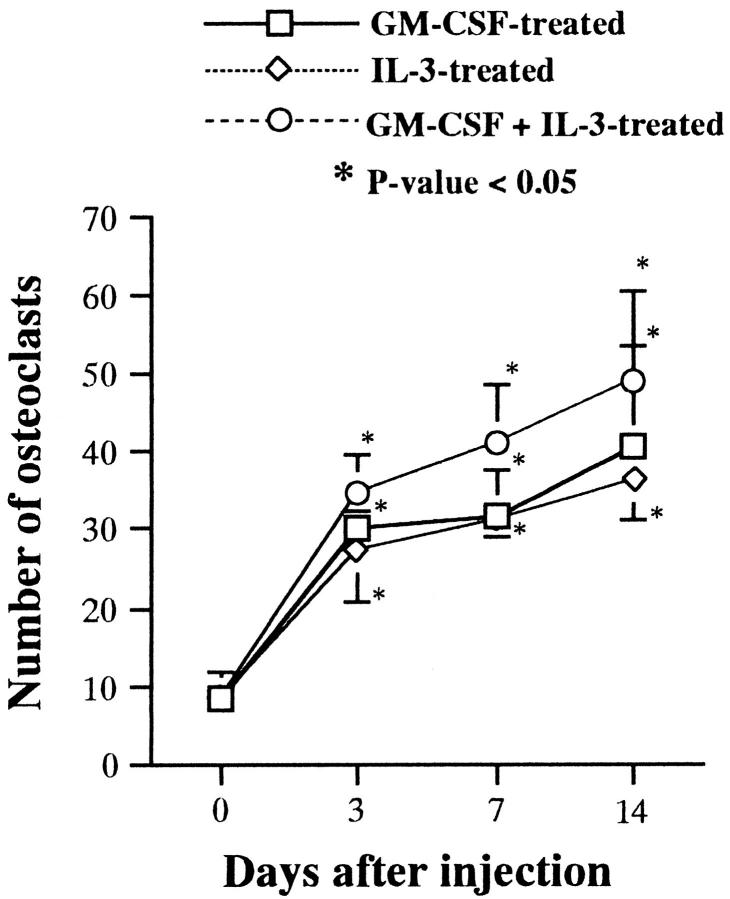
Figure 5 ▶ shows changes in the total numbers of TRAP-positive cells in the femurs of young op/op mice at 3, 7, and 14 days after daily subcutaneous injection of GM-CSF, IL-3, or GM-CSF plus IL-3. The total number of TRAP-positive cells in the femurs of GM-CSF-, IL-3-, or GM-CSF- plus IL-3-treated op/op mice increased with days after injection. Among three groups of the treated op/op mice, significant numerical increments of TRAP-positive cells were the highest in the GM-CSF- plus IL-3-treated ones during the period examined (P < 0.05).
Figure 5.
Total numbers of TRAP-positive cells in the femurs of young op/op mice at 3, 7, or 14 days after daily administration of GM-CSF, IL-3, or GM-CSF plus IL-3.
In the wild-type mice, the multinuclear cells showed the ultrastructure of osteoclasts characterized by many heterochromatic nuclei, markedly developed ruffled borders with an ample clear zone, and numerous clear vesicles, vacuoles, or granules of variable sizes, rough endoplasmic reticulum, Golgi complexes, and abundant mitochondria. In the young op/op mice, the osteoclasts were mononuclear and showed a poor development of ruffled borders, indistinct clear zone, reduced numbers of vesicles, vacuoles, or granules in the cytoplasm, and a smooth cell membrane with few microvilli facing the capillary lumen (Figure 6A) ▶ . In the mononuclear osteoclasts, the nucleus was oval or elongated, often with a prominent nucleolus (Figure 6A) ▶ . In the aged op/op mice, the mononuclear osteoclasts predominated, showing the same fundamental ultrastructure as in the young op/op mice. However, the cells were slightly larger with a more copious cytoplasm, contained more numerous vesicles, vacuoles, and granules, and developed ruffled borders more markedly than in the young op/op mice (Figure 6B) ▶ . In the GM-CSF-treated op/op mice, the mononuclear osteoclasts were larger in size with a round nucleus with indistinct nucleoli and developed more marked ruffled borders, extended more numerous microvilli from the cell surface facing the lumen of blood capillaries, and developed more numerous vesicles, vacuoles, and granules in the cytoplasm than in young op/op mice (Figure 6C) ▶ . In the IL-3 or GM-CSF- plus IL-3-treated op/op mice, mononuclear osteoclasts showed similar ultrastructural features to those in the GM-CSF-treated op/op mice (Figure 6D) ▶ . In GM-CSF- plus IL-3-treated op/op mice, multinuclear osteoclasts showed similar ultrastructural features to those of the wild-type mice (data not shown).
Figure 6.

Ultrastructural features of osteoclasts in a young (A) or an aged (B) op/op mouse and a GM-CSF-treated (C) or an IL-3-treated (D) young op/op mouse. A: Osteoclast in the young op/op mouse has a slender nucleus, contains cytoplasmic vesicles, vacuoles, and granules, and develops poor ruffled borders (arrow). B: Mononuclear osteoclast in the aged op/op mouse possesses an elongated nucleus, and its cytoplasm is more copious, contains much more numerous vesicles, vacuoles, and granules, and develops ruffled borders more markedly than in the young op/op mouse. An arrow indicates the development of ruffled borders. C: Mononuclear osteoclast has a large round nucleus with a copious cytoplasm, developing ruffled borders (arrow) and containing numerous vesicles, vacuoles, and granules in the GM-CSF-treated young op/op mouse. D: Mononuclear osteoclast in the IL-3-treated young op/op mouse is similar to that in the GM-CSF-treated one and developing ruffled borders (arrow). Magnification, ×4000 (A and B) and ×3500 (C and D).
Numerical and Cytological Changes of Macrophages in the Aged and GM-CSF-, IL-3-, or GM-CSF- plus IL-3-Treated Mutant Mice
Figure 7 ▶ shows changes in the percentage of macrophages in the bone marrow and other various tissues of young and aged op/op, GM-CSF-, IL-3-, or GM-CSF- plus IL-3-treated young op/op mice, relative to the wild-type mice. In agreement with the data reported in previous studies, 19-21 the percentages of macrophages in the bone marrow of femur bones in the aged op/op mice, relative to the wild-type mice, were increased significantly, compared with the young op/op mice. However, the percentages of macrophages in the other tissues of aged op/op mice, relative to the wild-type mice, were not increased, compared with the young op/op mice. In the GM-CSF-, IL-3-, or GM-CSF- plus IL-3-treated mice, the percentages of macrophages relative to the total numbers of macrophages in the wild-type mice were increased significantly in the bone marrow, spleen, liver, and lymph nodes. However, the percentages of macrophages in the endometrium, testis, or kidneys remained low in these treated mutant mice.
Figure 7.
Percentages of F4/80-positive macrophages relative to those of wild-type mice in various tissues of untreated young or aged op/op mice and GM-CSF- and/or IL-3-treated young op/op mice.
Discussion
In agreement with the data reported in previous studies, 19-21 the present investigation has revealed that osteopetrosis is corrected in aged op/op mice, accompanied by the expansion of bone marrow cavity and hematopoietic recovery. In the aged op/op mice, the numbers of TRAP-positive cells and F4/80-positive macrophages were increased significantly in the femurs, compared with the young op/op mice. Most TRAP-positive cells were mononuclear. Multinuclear osteoclasts were rare in the aged op/op mice as in the young ones. By scanning electron microscopy, Begg et al demonstrated that resorptive pits formed by osteoclasts were distinguishable on the endosteal surfaces of femurs in aged op/op mice, suggesting the resorptive activity of osteoclasts. 19,20 In agreement with the previous ultrastructural study, 21 we also confirmed that the mononuclear osteoclasts have ruffled borders in the interfaces to bone matrix, although the development of the ruffled borders are not so prominent as multinuclear osteoclasts. Also, numerous vesicles, vacuoles, and granules were observed in mononuclear osteoclasts in the aged op/op mice, suggesting the endocytic, digesting, and remodeling processes of bone matrix by these cells. Mononuclear osteoclasts have been recently considered as functional osteoclasts irrespective of nuclear numbers, based on their ultrastructural, cytochemical, and functional similarities to multinuclear osteoclasts. 49-52
In young op/op mice, the numbers of macrophages are reduced in various organs and tissues. 16,17,54,55 The numerical reductions are tissue dependent, 16,54,56 and these macrophages are small and round and showed immature ultrastructure, often with distinct phagocytic activities. 16,17 As shown in the present study, macrophages are increased in number in the bone marrow of aged op/op mice, in agreement with the data shown in previous studies. 19-21 These macrophages are ultrastructurally immature as in young op/op mice. In normal mice, studies showed conflicting results about IL-3 production, which increased 57 or decreased with age. 58,59 The production of GM-CSF was unchanged in male and virgin female mice with aging, whereas GM-CSF levels were enhanced in aged multiparous female mice. 59 In our aged op/op mice, in which multiparous female mice were excluded in our present study, serum GM-CSF and IL-3 levels were elevated significantly. In our previous study, Northern blot analysis demonstrated the expression of GM-CSF and IL-3 mRNA in various tissues of op/op mice. 17 Taking all of the previous information and our present data together, in op/op mice, which totally lack functional M-CSF activity, GM-CSF and IL-3 are considered the major growth factors to support the maintenance and differentiation of immature macrophages and to induce numerical increases of immature macrophages in the bone marrow of aged op/op mice. To clarify the roles of GM-CSF and IL-3, we injected subcutaneously 5 ng of rmGM-CSF, 100 ng of rmIL-3, or both daily for 2 weeks into young op/op mice. These treatments resulted in numerical increases of macrophages in the bone marrow of GM-CSF-, IL-3-, or GM-CSF- plus IL-3-treated mutant mice, in agreement with the results in a study of Wiktor-Jedrzejczak et al, 36 showing that GM-CSF induces variable increases in number of macrophages in various tissues of op/op mice. In the aged op/op mice, however, the numbers of macrophages were not increased in tissues other than the bone marrow, as in young mutant mice. Based on the present data, there is a difference in action of the growth factors introduced exogenously or produced in situ, and the locally produced GM-CSF and/or IL-3 in tissues other than bone marrow of aged op/op mice seem insufficient for correction of tissue macrophage deficiencies.
In a previous study, Wiktor-Jedrzejczak et al reported that osteopetrosis in op/op mice was not corrected by exogenous administration of GM-CSF in a high dose. 36 Furthermore, we could not confirm any significant osteopetrotic changes in femurs in young op/op mice daily injected with IL-3 in such a large dose, as used in a previous study. 53 These data indicate that long-term administration of GM-CSF or IL-3 in high doses does not correct osteopetrosis of op/op mice. In our recent study, however, we found that 5 ng of rmGM-CSF is the most effective dose for the induction of increases in number of macrophages. 40 In addition, the present study demonstrated that 100 ng of rmIL-3 was the most effective for inducing numerical increases of Kupffer cells in the liver of normal mice, when subcutaneously injected. In such a low dose, we succeeded in inducing a significant correction of osteopetrosis, expansion of bone marrow cavity, and hematopoietic recovery in the femurs of all GM-CSF-, IL-3-, and GM-CSF- plus IL-3-treated young op/op mice. Similar to the aged op/op mice, we found numerical increases of TRAP-positive cells in the femurs of all of these treated mutant mice. These data indicate that GM-CSF and/or IL-3 can correct osteopetrosis in M-CSF-deficient op/op mice. In previous studies, it was established that M-CSF administration rapidly induced the correction of osteopetrosis, formation and expansion of bone marrow cavity, and recovery of bone marrow hematopoiesis in young op/op mice. 7,17 As in the aged op/op mice, GM-CSF- or IL-3-treated young op/op mice showed correction of osteopetrosis by increased numbers of mononuclear osteoclasts and their implication in bone resorption. In the GM-CSF- plus IL-3-treated op/op mice, mature multinucleated osteoclasts developed in the metaphysis and diaphysis of femur bones. These results indicate that GM-CSF or IL-3 alone is sufficient to induce the development, differentiation, and survival of mononuclear osteoclasts and do not participate in their fusion, differentiation, and maturation into multinuclear osteoclasts. Administration of both GM-CSF and IL-3 appears to induce a synergistic effect on the fusion of mononuclear osteoclasts and their differentiation and maturation into multinuclear osteoclasts.
In previous in vitro studies, data regarding the effects of GM-CSF or IL-3 on osteoclast formation were controversial; some researchers reported that both growth factors had stimulatory effects, 5,22-30 and other investigators described inhibitory biological actions of these factors. 30-35 These discrepancies may have resulted from various factors, including the culture system used, the cell types studied or the differentiation stages of cultured cells, the concentrations of the growth factors added to culture media, or the amounts of endogenous GM-CSF or IL-3 production from cultured cells. Among these factors, the differentiation stages of the cultured cells used and the endogenous production of GM-CSF or IL-3 by them are the most important critical factors in osteoclast formation. The processes of osteoclast formation are divided into two phases: 1) the early proliferative phase and 2) the late differentiation phase. 33,60 In the early proliferative phase, GM-CSF is thought to function as a stimulator for expansion of osteoclast precursors, 5,29,61 whereas it acts as an inhibitory factor in the differentiation phase of osteoclast formation. 33 The precise amounts of GM-CSF or IL-3 endogenously produced by cultured cells were not determined in most of the previous in vitro studies. 5,22-35 A recent study, using osteoblastic or spleen cells from GM-CSF-deficient mice or GM-CSF/IL-3/IL-5 β-chain-deficient mice clearly demonstrated that IL-18 is produced by osteoblasts and acts via GM-CSF to inhibit osteoclast formation. 35 This in vitro system is very useful for studying effects of GM-CSF exogenously added to medium, because the osteoblastic or spleen cells from GM-CSF-deficient mice used for co-culture do not produce GM-CSF. However, the cultured cells used in this study were predominantly macrophages (more than 95%), which had differentiated from bone marrow mononuclear cells of normal mice in the presence of M-CSF. Therefore, the inhibitory effects of GM-CSF may result from its action in the late differentiation phase of osteoclast formation. Because our in vivo study suggests that GM-CSF or IL-3 acts on more immature cells than mononuclear osteoclasts, it is difficult to compare our data with those in the previous in vitro studies.
It is known that IL-3 induces the production of GM-CSF, G-CSF, and IL-6 by bone marrow cells and bone marrow stromal cells, showing hierarchical regulation of cytokine production. 62-64 Osteoblasts, macrophages, and endothelial cells produce and secrete GM-CSF and IL-3 in bone tissues and bone marrow. IL-3 is predominantly produced by antigen- or mitogen-activated T cells. 65 In a preliminary study, we observed the expression of GM-CSF and/or IL-3 mRNA in young, aged, or GM-CSF- and/or IL-3-treated op/op mice by in situ hybridization (data not shown). Taking these data together, our present study suggests that endogenously produced or exogenously provided GM-CSF and/or IL-3 function as a stimulator for osteoclast formation. In contrast, Nilsson et al 39 demonstrated that GM-CSF-deficient op/op mice showed hematopoietic recovery with aging at 40 weeks of age, suggesting that GM-CSF is not required for the age-related correction of the hematopoietic deficiencies in op/op mice. However, it was previously known that the correction of hematopoietic deficiencies in aged op/op mice becomes evident at approximately 22 weeks of age. 19 Taking this information together, it rather appears likely that GM-CSF deficiency in GM-CSF-deficient op/op mice induces a marked delay in the age-related correction of hematopoietic deficiencies in op/op mice. Supporting this notion, the present study demonstrated that daily administration of GM-CSF or IL-3 in low doses corrected the osteopetrosis in op/op mice and induced increases in the numbers of mononuclear osteoclasts. These data indicate that GM-CSF or IL-3 has similar stimulatory effects for the osteoclast formation in vivo. In addition, the present study showed that daily administration of GM-CSF and IL-3 into young op/op mice induced increased numbers of multinuclear osteoclasts. However, additional studies are required to elucidate whether GM-CSF or IL-3 directly stimulate osteoclast development and to determine the mechanism of development of multinuclear osteoclasts after administration of both GM-CSF and IL-3 in young op/op mice.
In conclusion, GM-CSF and/or IL-3 support the development and differentiation of mononuclear and/or multinuclear osteoclasts to correct osteopetrosis in op/op mice. Similar mechanisms appear to participate in the recovery of osteopetrosis in the mutant mice with aging.
Acknowledgments
We thank Dr. Masatake Araki, Gene Technology Center, for giving his advice, and Mr. Takenobu Nakagawa and Miss Emi Miyata, Second Department of Pathology, Kumamoto University School of Medicine, for their skillful technical assistance.
Footnotes
Address reprint requests to Dr. Kiyoshi Takahashi, Second Department of Pathology, Kumamoto University School of Medicine, 2-2-1 Honjo, Kumamoto, Japan.
Supported by grants-in-aid for scientific research from the Ministry of Education, Science, and Culture, Japan (09877047) and National Institutes of Health grant CA20408.
References
- 1.van Furth R, Cohn ZA, Hirsh JG, Spector WG, Langevoort HL: The mononuclear phagocyte system: a new classification of macrophages, monocytes and their precursor cells. Bull World Health Org 1972, 46:845-852 [PMC free article] [PubMed] [Google Scholar]
- 2.van Furth R: Cells of the mononuclear phagocyte system: nomenclature in terms of sites and conditions. van Furth R eds. Mononuclear Phagocytes, Functional Aspects, Part I. 1980, :pp 1-30 Martinus Nijhoff Publishers The Hague [Google Scholar]
- 3.van Furth R: Origin and turnover of monocytes and macrophages. Curr Top Pathol 1989, 79:125-150 [PubMed] [Google Scholar]
- 4.van Furth R: Production and migration of monocytes and kinetics of macrophages. van Furth R eds. Mononuclear Phagocytes: Biology of Monocytes and Macrophages. 1992, :pp 3-12 Kluwer Academic Publishers, Dordrecht, The Netherlands, [Google Scholar]
- 5.Kurihara N, Suda T, Miura Y, Nakauchi H, Kodama H, Hiura K, Hakeda Y, Kumegawa M: Generation of osteoclasts from isolated hematopoietic progenitor cells. Blood 1989, 74:1295-1302 [PubMed] [Google Scholar]
- 6.Kurihara N, Chenu C, Miller M, Civin C, Roodman GD: Identification of committed mononuclear precursors for osteoclast-like cells formed in long-term human marrow cultures. Endocrinology 1990, 126:2733-2741 [DOI] [PubMed] [Google Scholar]
- 7.Umeda S, Takahashi K, Naito M, Shultz LD, Takagi K: Neonatal changes of osteoclasts in osteopetrosis (op/op) mice defective in production of functional macrophage colony-stimulating factor (M-CSF) protein and effects of M-CSF on osteoclast development and differentiation. J Submicrosc Cytol Pathol 1996, 28:13-26 [PubMed] [Google Scholar]
- 8.Teitelbaum SL, Tondravi MM, Ross FP: Osteoclasts, macrophages, and the molecular mechanisms of bone resorption. J Leukocyte Biol 1997, 61:381-388 [DOI] [PubMed] [Google Scholar]
- 9.Felix R, Hofstetter W, Cecchini MG: Recent developments in the understanding of the pathophysiology of osteopetrosis. Eur J Endocrinol 1996, 134:143-156 [DOI] [PubMed] [Google Scholar]
- 10.Tondravi MM, McKercher SR, Anderson K, Erdmann JM, Quiroz M, Maki R, Teitelbaum SL: Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature 1997, 386:81-84 [DOI] [PubMed] [Google Scholar]
- 11.Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleish HA, Wagner EF: c-fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 1994, 266:443-448 [DOI] [PubMed] [Google Scholar]
- 12.Soriano P, Montgomery C, Gesek R, Bradley A: Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 1991, 64:693-702 [DOI] [PubMed] [Google Scholar]
- 13.Yoshida H, Hayashi S-I, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S-I: The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 1990, 345:442-443 [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T, Kurihara N, Yamaoka K, Ozono K, Okada M, Yamamoto K, Matsumoto S, Michigami T, Ono J, Okada S: Bone marrow-derived osteoclast-like cells from a patient with craniometaphyseal dysplasia lack expression of osteoclast-reactive vacuolar proton pump. J Clin Invest 1993, 91:362-367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sly WS, Hewett-Emmett D, Whyte MP, Yu YS, Tashian RE: Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci USA 1983, 80:2752-2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naito M, Hayashi S-I, Yoshida H, Nishikawa S-I, Shultz LD, Takahashi K: Abnormal differentiation of tissue macrophage populations in ‘osteopetrosis’ (op) mice defective in the production of macrophage colony-stimulating factor. Am J Pathol 1991, 139:657-667 [PMC free article] [PubMed] [Google Scholar]
- 17.Umeda S, Takahashi K, Shultz LD, Naito M, Takagi K: Effects of macrophage colony-stimulating factor on macrophages and their related cell populations in the osteopetrosis mouse defective in production of functional macrophage colony-stimulating factor protein. Am J Pathol 1996, 149:559-574 [PMC free article] [PubMed] [Google Scholar]
- 18.Nishikawa S-I, Hayashi S-I, Yoshida H, Naito M, Takahashi K, Shultz LD: A model mouse defective in M-CSF production: molecular biology and pathology of osteopetrosis mouse (op/op). Imai Y Tew JG Hoefsmit ECM eds. Dendritic Cells in Lymphoid Tissues. 1991, :pp 225-231 Elsevier Science Publishers, Amsterdam [Google Scholar]
- 19.Begg SK, Radley JM, Pollard JW, Chisholm OT, Stanley ER, Bertoncello I: Delayed hematopoietic development in osteopetrotic (op/op) mice. J Exp Med 1993, 177:237-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Begg SK, Bertoncello I: The hematopoietic deficiencies in osteopetrotic (op/op) mice are not permanent, but progressively correct with age. Exp Hematol 1993, 21:493-495 [PubMed] [Google Scholar]
- 21.Takatsuka H, Umezu H, Hasegawa G, Usuda H, Ebe Y, Naito M, Shultz LD: Bone remodeling and macrophage differentiation in osteopetrotsis (op) mutant mice defective in the production of macrophage colony-stimulating factor. J Submicrosc Cytol Pathol 1998, 30:239-247 [PubMed] [Google Scholar]
- 22.MacDonald BR, Mundy GR, Clark S, Wangf EA, Kuehl TJ, Stanley ER, Roodman GD: Effects of human recombinant CSF-GM and highly purified CSF-1 on the formation of multinucleated cells with osteoclast characteristics in long-term bone marrow cultures. J Bone Miner Res 1986, 1:227-233 [DOI] [PubMed] [Google Scholar]
- 23.Liggett W, Jr, Shevde N, Anklesaria P, Sohoni S, Greenberger J, Glowacki J: Effects of macrophage colony stimulating factor and granulocyte-macrophage colony stimulating factor on osteoclastic differentiation of hematopoietic progenitor cells. Stem Cells 1993, 11:398-411 [DOI] [PubMed] [Google Scholar]
- 24.Schneider GB, Relfson M: A bone marrow fraction enriched for granulocyte-macrophage progenitors gives rise to osteoclasts in vitro. Bone 1988, 9:303-308 [DOI] [PubMed] [Google Scholar]
- 25.Kurihara N, Chenu C, Miller M, Civin C, Roodman GD: Identification of committed mononuclear precursors for osteoclast-like cells formed in long term human marrow cultures. Endocrinology 1990, 126:2733-2741 [DOI] [PubMed] [Google Scholar]
- 26.Scheven BAA, Visser JWM, Nijweide PJ: In vitro osteoclast generation from different bone marrow fractions, including a highly enriched haematopoietic stem cell population. Nature 1986, 321:79-81 [DOI] [PubMed] [Google Scholar]
- 27.Perkins SL, Gibbons R, Kling S, Kahn AJ: Age-related bone loss in mice is associated with an increased osteoclast progenitor pool. Bone 1994, 15:65-72 [DOI] [PubMed] [Google Scholar]
- 28.Bertolini DR, Strassmann G: Differential activity of granulocyte-macrophage and macrophage colony-stimulating factors on bone resorption in fetal rat long bone organ cultures. Cytokine 1991, 3:421-427 [DOI] [PubMed] [Google Scholar]
- 29.Takahashi N, Udagawa N, Akatsu T, Tanaka H, Shionome M, Suda T: Role of colony-stimulating factors in osteoclast development. J Bone Miner Res 1991, 6:977-985 [DOI] [PubMed] [Google Scholar]
- 30.Hattersley G, Chambers TJ: Effects of interleukin-3 and of granulocyte-macrophage and macrophage colony stimulating factors on osteoclast differentiation from mouse hemopoietic tissue. J Cell Physiol 1990, 142:201-209 [DOI] [PubMed] [Google Scholar]
- 31.Shinar DM, Sato M, Rodan GA: The effect of hemopoietic growth factors on the generation of osteoclast-like cells in mouse bone marrow cultures. Endocrinology 1990, 126:1728-1735 [DOI] [PubMed] [Google Scholar]
- 32.Shuto T, Kukita T, Hirata M, Jimi E, Koga T: Dexamethasone stimulates osteoclast-like cell formation by inhibiting granulocyte-macrophage colony-stimulating factor production in mouse bone marrow cultures. Endocrinology 1994, 134:1121-1126 [DOI] [PubMed] [Google Scholar]
- 33.Shuto T, Jimi E, Kukita T, Hirata M, Koga T: Granulocyte-macrophage colony-stimulating factor suppresses lipopolysaccharide-induced osteoclast-like cell formation in mouse bone marrow cultures. Endocrinology 1994, 134:831-837 [DOI] [PubMed] [Google Scholar]
- 34.Udagawa N, Horwood NJ, Elliott J, Mackay A, Owens J, Okamura H, Kurimoto M, Chambers TJ, Martin TJ, Gillespie MT: Interleukin-18 (interferon-γ-inducing factor) is produced by osteoblasts and acts via granulocyte/macrophage colony-stimulating factor and not via interferon-γ to inhibit osteoclast formation. J Exp Med 1997, 185:1002-1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horwood NJ, Udagawa N, Elliott J, Grail D, Okamura H, Kurimoto M, Dunn AR, Martin TJ, Gillespie MT: Interleukin-18 inhibits osteoclast formation via T cell production of granulocyte macrophage colony-stimulating factor. J Clin Invest 1998, 101:595-603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiktor-Jedrzejczak W, Urbanowska E, Szperl M: Granulocyte-macrophage colony-stimulating factor corrects macrophage deficiencies, but not osteopetrosis, in the colony-stimulating factor-1-deficient op/op mouse. Endocrinology 1994, 134:1932-1935 [DOI] [PubMed] [Google Scholar]
- 37.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, Mulligan RC: Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science 1994, 264:713-716 [DOI] [PubMed] [Google Scholar]
- 38.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JAM, Maher DW, Cebon J, Sinickas V, Dunn AR: Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA 1994, 91:5592-5596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nilsson SK, Lieschke GJ, Garcia-Wijnen CC, Williams B, Tzelepis D, Hodgson G, Grail D, Dunn AR, Bertoncello I: Granulocyte macrophage colony-stimulating factor is not responsible for the correction of hematopoietic deficiencies in the maturing op/op mouse. Blood 1995, 86:66-72 [PubMed] [Google Scholar]
- 40.Takahashi K, Miyakawa K, Wynn AA, Nakayama K-I, Myint YY, Naito M, Shultz LD, Tominaga A, Takatsu K: Effects of granulocyte/macrophage colony-stimulating factor on the development and differentiation of CD5-positive macrophages and their potential derivation from a CD5-positive B-cell lineage in mice. Am J Pathol 1998, 152:445-456 [PMC free article] [PubMed] [Google Scholar]
- 41.Ibboston KJ, Roodman JD, Macmanus LM, Mundy JR: Identification and characterization of osteoclast-like cells and their progenitors in cultures of feline marrow mononuclear cells. J Cell Biol 1984, 99:471-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isobe S, Chen ST, Nakane PK, Brown WR: Studies on translocation of immunoglobulins across intestinal epithelium. I. Improvements in the peroxidase-labeled antibody method for application to study of human intestinal mucosa. Acta Histochem Cytochem 1977, 10:167-171 [Google Scholar]
- 43.Schneider GB, Relfson M, Langman CB: Effects of 1,25-dihydroxyvitamin D3 on bone resorption and natural immunity in osteopetrotic (ia) rats. J Bone Miner Res 1994, 9:585-591 [DOI] [PubMed] [Google Scholar]
- 44.Schneider GB, Relfson M: Effects of interleukin-2 on bone resorption and natural immunity in osteopetrotic (ia) rats. Lymphokine Cytokine Res 1994, 13:335-341 [PubMed] [Google Scholar]
- 45.Schneider GB, Benis KA, Flay NW, Ireland RA, Popoff SN: Effects of vitamin D binding protein-macrophage activating factor (DBP-MAF) infusion on bone resorption in two osteopetrotic mutations. Bone 1995, 16:657-662 [DOI] [PubMed] [Google Scholar]
- 46.Roodman GD, Ibboston KJ, MacDonald BR, Kuehl TJ, Mundy GR: 1,25-Dihydroxyvitamin D3 causes formation of multinucleated cells with several osteoclast characteristics in culture of primate marrow. Proc Natl Acad Sci USA 1985, 82:8213-8217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi N, Udagawa N, Akatsu T, Tanaka H, Isogai Y, Suda T: Deficiency of osteoclasts in osteopetrotic mice is due to a defect in the local microenvironment provided by osteoblastic cells. Endocrinology 1991, 128:1792-1796 [DOI] [PubMed] [Google Scholar]
- 48.Kodama H, Yamasaki A, Abe M, Niida S, Hakeda Y, Kawashima H: Transient recruitment of osteoclasts and expression of their function in osteopetrotic (op/op) mice by a single injection of macrophage colony-stimulating factor. J Bone Miner Res 1993, 8:45-50 [DOI] [PubMed] [Google Scholar]
- 49.Domon T, Wakita M: Electron microscopic and histochemical studies of mononuclear osteoclast of the mouse. Am J Anat 1991, 192:35-44 [DOI] [PubMed] [Google Scholar]
- 50.Domon T, Sugaya K, Yawaka Y, Osanai M, Hanaizumi Y, Takahashi S, Wakita M: Electron microscopic and histochemical studies of the mononuclear odontoclast of the human. Anat Rec 1994, 240:42-51 [DOI] [PubMed] [Google Scholar]
- 51.Sahara N, Toyoki A, Ashizawa Y, Deguchi T, Suzuki K: Cytodifferentiation of the odontoclast prior to the shedding of human deciduous teeth: an ultrastructural and cytochemical study. Anat Rec 1996, 244:33-49 [DOI] [PubMed] [Google Scholar]
- 52.Domon T, Osanai M, Yasuda M, Seki E, Takahashi S, Yamamoto T, Wakita M: Mononuclear odontoclast participation in tooth resorption: the distribution of nuclei in human odontoclasts. Anat Rec 1997, 249:449-457 [DOI] [PubMed] [Google Scholar]
- 53.Saxena SK, Crouse DA, Sharp JG: Effect of systemic interleukin-3 administration on epithelial cell proliferation in mouse intestine. Life Sci 1993, 53:473-477 [DOI] [PubMed] [Google Scholar]
- 54.Wiktor-Jedrzejczak W, Ratajczak MZ, Ptasznik A, Sell KW, Ahmed-Ansari A, Ostertag W: CSF-1 deficiency in the op/op mouse has differential effects on macrophage populations and differentiation stages. Exp Hematol 1992, 20:1004-1010 [PubMed] [Google Scholar]
- 55.Felix R, Cecchini MG, Hofstetter W, Elford PR, Stutzer A, Fleisch H: Impairment of macrophage colony-stimulating factor production and lack of resident bone marrow macrophages in the osteopetrotic op/op mouse. J Bone Miner Res 1990, 5:781-789 [DOI] [PubMed] [Google Scholar]
- 56.Wiktor-Jedrzejczak W, Gordon S: Cytokine regulation of the macrophage (Mø) system studied using the colony stimulating factor-1-deficient op/op mouse. Physiol Rev 1996, 76:927-947 [DOI] [PubMed] [Google Scholar]
- 57.Kubo M, Cinader B: IL-3 production as a function of age and its correlation with splenomegaly: age versus disease-related change. Immunol Lett 1990, 24:133-136 [DOI] [PubMed] [Google Scholar]
- 58.Gorczynski R, Dubiski S, Munder PG, Cinader B, Westphal O: Age-related changes in interleukin production in BALB/cNNia and SJL/J mice and their modification after administration of foreign macromolecules. Immunol Lett 1993, 38:243-251 [DOI] [PubMed] [Google Scholar]
- 59.Barrat F, Lesourd B, Boulouis HJ, Thibault D, Vincent-Naulleau S, Gjata B, Louise A, Neway T, Pilet C: Sex and parity modulate cytokine production during murine ageing. Clin Exp Immunol 1997, 109:562-568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burger EH, Nijweide PJ: Cellular origin and theories of osteoclast differentiation. Bone: The Osteoclast. Edited by Brain KH. Boca Raton, FL, CRC Press, 1991, 2:31–59
- 61.Hiura K, Sumitani K, Kawata T, Higashino K, Okawa M, Sato T, Hakeda Y, Kumegawa M: Mouse osteoblast cells (MC3T3–E1) at different stages of differentiation have opposite effects on osteoclastic cell formation. Endocrinology 1991, 128:1630-1637 [DOI] [PubMed] [Google Scholar]
- 62.Miyashita M, Sugimoto K, Suzuki J, Taniguchi S, Aramaki K, Mori KJ: Hierarchical regulation of interleukin production: induction of interleukin 6 (IL-6) production from bone marrow cells and bone marrow stromal cells by interleukin 3 (IL-3). Leuk Res 1991, 15:1125-1131 [DOI] [PubMed] [Google Scholar]
- 63.Tsuji T, Sugimoto K, Yanai T, Takashita E, Mori KJ: Induction of granulocyte-macrophage colony stimulating factor (GM-CSF) and granulocyte colony stimulating factor (G-CSF) expression in bone marrow and fractionated marrow cell populations by interleukin 3 (IL-3): IL-3-mediated positive feedback mechanisms of granulopoiesis. Growth Factors 1994, 11:71-79 [DOI] [PubMed] [Google Scholar]
- 64.Sugimoto K, Mori KJ: Cascade regulation of cytokine production in granulopoiesis. Leukemia 1997, 11(Suppl 3):464-467 [PubMed] [Google Scholar]
- 65.Morris CF, Young IG, Hapel AJ: Molecular and cellular biology of interleukin-3: colony-stimulating factors. Dexter TM Garland JM Testa NG eds. Molecular and Cellular Biology. 1990, :pp 177-214 Marcel Dekker, New York [PubMed] [Google Scholar]