Abstract
It was previously shown in the PC-295 xenograft that the number of chromogranin A (CgA)-positive neuroendocrine (NE) cells increased after androgen withdrawal. NE cells did not proliferate and differentiated from G0-phase-arrested cells. Here we further characterized NE differentiation, androgen receptor status, and apoptosis-associated Bcl-2 expression in the PC-295 model after androgen withdrawal to assess the origin of NE cells. PC-295 tumor volumes decreased by 50% in 4 days. Intraperitoneal bromodeoxyuridine (BrdU) incorporation and MIB-1 labeling decreased to 0%, and the apoptosis was maximal at day 4. Androgen receptor expression and prostate-specific antigen (PSA) serum levels decreased rapidly within 2 days. The number of NE cells increased 6-fold at day 4 and 30-fold at day 7. Five and ten percent of the CgA-positive cells were BrdU positive after continuous BrdU labeling for 2 and 4 days, respectively. However, no MIB-1 expression was observed in CgA-positive cells. NE cells expressed the regulated secretory pathway marker secretogranin III but were negative for androgen receptor and Bcl-2. Bcl-2 expression did increase in the non-NE tumor cells. In conclusion, androgen withdrawal leads to a rapid PC-295 tumor regression and a proliferation-independent induction of NE differentiation. The strictly androgen-independent NE cells that were still present after 21 days differentiated mainly from G0-phase-arrested cells.
Neuroendocrine (NE) cells form an androgen-independent subfraction of the prostate. 1 NE cells have been localized in nearly all clinical prostatic adenocarcinoma with different expression patterns. 2-8 These cells produce various growth-modulating neuropeptides in a paracrine or autocrine way. 9-15
Possible roles for NE cells in the prostate may be regulation of homeostasis and secretion of prostatic fluid, either actively or passively. NE cells can be identified by routine electron microscopy (dense core granules) or by immunohistochemistry with specific antibodies against secreted products, for example, serotonin, 16 or secretion-associated proteins, such as chromogranin A (CgA), 17-19 which is a marker for neuroendocrine differentiation. NE cells are considered to be nonproliferating cells and do not express the androgen receptor 20 and are therefore probably unaffected by androgen deprivation. Consequently, they will not undergo apoptosis under such circumstances. Therefore, it is relevant to assess whether or not CgA-positive cells co-express the anti-apoptotic oncogene Bcl-2. 21 Moreover, NE cells show a heterogeneous cytokeratin expression pattern as there are basal, luminal, and intermediate NE cell types 16,22,23 and are often found near Bcl-2-positive prostate cancer cells. 24,25
The single expression of CgA is not the only requisite for the determination of NE differentiated cells as there exists a regulated secretory pathway (RSP) in NE cells 26 next to the lysosomal and an exocrine constitutive pathway. Along the RSP pathway, secretion and processing of bioactive neuropeptides and growth hormones, such as insulin and glucagon in the pancreas, 27,28 are regulated. The RSP consists of a sequence of processes linked from transcription/translation of various factors to final secretion of neuropeptides at the plasma membrane from secretory granules. 29 Different markers can be identified, such as granular markers secretogranin III (SGIII) and secretogranin V (7B2), 30-32 peptidylglycine α-amidating monooxygenase (PAM), 33,34 the processing enzymes prohormone convertase 1 and 2 (PC-1 and -2), and carboxy peptidase E. Evaluation of these specific markers of the RSP is ongoing in Xenopus laevis, lung and prostate cancer. 35,36
The number of prostate cancer cases still increases, and the search for a curative therapy for metastasized cancer continues. NE cells have been found in most prostatic adenocarcinomas, and the role of NE cells in the progression to androgen-independent growth is still unclear. Fundamental questions related to a possible role for NE differentiation in the progression of prostate cancer can be solved only by using representative prostate cancer models with NE differentiation. Several groups have recently been developing tumor models as both in vitro cell lines 37 and in vivo xenografts. 38-40 NE differentiation has been studied in a panel of 11 in vivo human prostate cancer xenograft models. Some models do not express the NE phenotype, and PC-324 and PC-346 lose their NE phenotype after a few passages in nude mice. 41 The PC-295 and PC-310 models are androgen-dependent models of which a part of the cells constitutively show the NE phenotype. Therefore, these two models are very suitable for studying NE differentiation in prostate cancer and the role that NE cells may play in the progression of prostate cancer. In both the PC-295 and PC-310 models, androgen deprivation leads to an increased number of NE cells. In a previous study with the androgen-dependent PC-295 model, 41 tumors continuously labeled with the S-phase marker bromodeoxyuridine (BrdU) for 2 days before castration showed no double labeling of NE cells after castration with BrdU. This shows that the increased NE phenotype is not induced before castration. Absence of BrdU and another proliferation marker, MIB-1, shows that NE cells are not proliferating and are, thus, G0-phase-arrested cells before androgen withdrawal. However, it cannot totally be excluded that proliferation occurring after androgen withdrawal is responsible for the increase in NE differentiation in the PC-295 model.
In this study, we further looked for the origin of NE cells by focusing on the kinetics of the process of NE differentiation. Furthermore, we characterized changes in apoptosis and subpopulations of prostatic epithelial cells as marked by their expression of CgA, SGIII, growth-modulating neuropeptides, androgen receptor, and Bcl-2 after androgen withdrawal in the PC-295 model both shortly after castration and after prolonged androgen deprivation.
Materials and Methods
PC-295 Xenograft Model
The nude mouse human prostate cancer xenograft model PC-295 was established from a pelvic lymph node metastasis. 40,42 The tumors usually grow with a doubling time of approximately 14 days and a lag phase of 2 to 3 months. The model represents a strictly androgen-dependent and moderately differentiated tumor, histologically organized in solid sheets and micro-acini. In short, PC-295 tumors were implanted subcutaneously at both shoulders of intact nude NMRI males, obtained from the breeding colony of the Erasmus University Center for Animal Research. Optimal growth conditions were reached by supplementation of PC-295-transplanted mice with testosterone implants, as previously described. 43 The subcutaneous tumors developed within 2 to 3 months and were grown up to a maximal volume of 2000 mm3. Tumor volume changes were followed weekly by two perpendicular diameter measurements (D1 and D2), after which the volume was calculated from the formula: V = (Π/6)(D1 × D2)3/2.
Castration Experiments with the PC-295 Human Prostate Cancer Xenograft Model
Two consecutive castration experiments were performed with 39 testosterone-supplemented PC-295-bearing male NMRI mice in total. Androgen withdrawal was performed by castrating the mice under hypnorm anesthesia (Janssen Pharmaceuticals, Oxford, UK) and by removing the silastic implant. The mice received BrdU to check the effect of androgen withdrawal on the proliferative activities in the PC-295 tumor tissue. One group of mice received BrdU (1 mg/ml) intraperitoneally (IP) as standard labeling for 1 hour before sacrifice. The second group received BrdU (30 mg/ml) via subcutaneously inserted osmotic Alzet micropumps (Alzet 1007D, Alza Corp., Palo Alto, CA) having a flow rate of 0.5 μl/hour as of the moment of castration until sacrifice. Three mice were sacrificed at each time point after castration. For the first experimental set-up, these time points were 0, 0.5, 1, 1.5, 2, 4, 7, 14, and 21 days, whereas in the second set-up, mice received BrdU for 0, 1.5, 2, 4, and 7 days after castration.
After blood samples were taken for determining serum prostate-specific antigen (PSA) levels, mice were sacrificed. Tumor volumes were measured, and tumors were removed. The tumors were cut into small pieces that were either fixed in 4% buffered formalin and paraffin embedded for immunohistochemical analysis or snap-frozen in liquid nitrogen and stored at −80°C for biochemical analysis. The paraffin-embedded material was processed routinely for hematoxylin and eosin (H&E) staining.
Immunohistochemistry
To identify the fraction of cells expressing the NE phenotype, paraffin-embedded tissue sections of the PC-295 xenografts were stained immunohistochemically with antibodies against CgA (clone LK2H10, ICN Pharmaceuticals, Aurora, OH) and SGIII (rabbit polyclonal antibody; Department of Animal Physiology, University of Nijmegen). 44 For identification of the proliferative capacity, tissue sections were stained with antibodies against BrdU (clone IIB5, Eurodiagnostics, Apeldoorn, The Netherlands) and against the proliferation-associated Ki-67 antigen (MIB-1, Immunotech, Marseille, France). In addition, apoptotic cells were identified by the TUNEL method (TdT-Kit, Boehringer Technologies, Mannheim, Germany). Other antibodies used were directed against the androgen receptor (clone F39.4, kindly provided by Dr. A. O. Brinkmann, Department of Endocrinology and Reproduction, Erasmus University), the cytoplasmic Bcl-2 antigen (clone 124, Dako, Glostrup, Denmark), and the neuropeptides bombesin (MoAb 2A11; kindly provided by Dr. F. Cuttita, National Cancer Institute, Bethesda, MD), serotonin (5HT; rabbit polyclonal antibody), vasoactive intestinal polypeptide (VIP, rabbit polyclonal), and calcitonin (rabbit polyclonal).
Paraffin-embedded xenograft tissues were cut at 4-μm sections for single immunostaining and 2 μm for double immunostaining. The sections were mounted on 3-ami-nopropyltriethoxysilane-coated glass slides and incubated overnight at 60°C. The slides were deparaffinized through xylene and 100% ethanol, and endogenous peroxidase activity was blocked with 3.3% hydrogen peroxide (H2O2) in methanol for 10 minutes. After rinsing with tap water and dest, the slides were placed in 10 mmol/L citrate buffer (pH 6.0). Antigen retrieval was then performed in a microwave at 700 W for an initial 12.5 minutes and a subsequent 5.5 minutes. 45 The slides were allowed to cool down to room temperature and then rinsed with phosphate-buffered saline (PBS). The tissue slides were then put into the Sequenza immunostaining system (Shandon, Uncorn, UK). All slides were preincubated with normal goat serum (DAKO) diluted 1:10 in PBS, which yields for all primary antibodies used hereafter, for 15 minutes. The primary antibody was incubated at the appropriate concentration for 2 hours at room temperature or overnight at 4°C. The secondary antibody was incubated for 30 minutes, being either horseradish-peroxidase-conjugated goat anti-mouse or goat anti-rabbit (1:50), or biotinylated goat anti-mouse and goat anti-rabbit (1:400) for monoclonal and polyclonal antibodies, respectively. In case of biotinylated goat anti-mouse and goat anti-rabbit, a horseradish peroxidase/streptavidin/biotin complex diluted 1:1:200 in PBS, prepared at least 30 minutes before use, was incubated for a subsequent 30 minutes. Between the subsequent steps, the slides were rinsed four times with PBS. The bound horseradish peroxidase was visualized in 10 minutes with diaminobenzidine (Fluka, Neu-Ulm, Germany) in PBS containing 0.075% H2O2 as substrate. Slides were rinsed extensively in tap water and finally counterstained in Mayer’s hematoxylin, dehydrated through a series of alcohol, and embedded in malinol.
To assess the properties of the NE cells, double staining of CgA, respectively, with BrdU, MIB-1, androgen receptor, and Bcl2 was performed on the PC-295 xenograft tumors. The double staining consists of two consecutive stainings with two primary antibodies. The first staining was always a horseradish-peroxidase-related stable diaminobenzidine complex, whereas the second staining was performed with an alkaline-phosphatase-conjugated goat anti-mouse secondary antibody. The alkaline phosphatase was visualized after a 30-minute incubation of the slides with AS-MX-phosphate (0.3 mg/ml; Sigma, St. Louis, MO) mixed with new fuchsin (2.5 μl/ml; Sigma), NaNO2 (1.45 mmol/L; Sigma), and levamisole (0.5 mg/ml; Sigma) in the dark. All compounds were diluted in 0.2 mol/L Tris buffer (Gibco, Life Technologies, Breda, The Netherlands) adjusted to pH 8.0. In between both stainings, the slides were rinsed with PBS for 1 hour and again boiled in a microwave in 10 mmol/L citrate buffer (pH 6.0) for 10 minutes. For immunostaining of incorporated BrdU, the slides were treated with 2 N HCl at 37°C for 30 minutes to uncoil DNA and subsequently neutralized with a 0.1 mmol/L sodium tetraborate buffer (pH 8.5). As negative control, PBS replaced the primary antibody in all stainings. Radical prostatectomies containing normal prostatic tissue were used as positive control for CgA, SGIII, androgen receptor, Bcl-2, and Ki-67 expression.
For all markers, except androgen receptor, the number of positive cells was determined by quantitative counts of all cells in tumor squares at ×310 magnification from which the number of positive cells per square millimeter was calculated. In total, positive cells were scored as percentage of the total cell number, in ≥25 squares. For the androgen receptor, the level of immunostaining was assessed semiquantitatively.
Western Analysis
We further confirmed the expression patterns of immunohistochemically determined CgA, SGIII, and androgen receptor in our castration series of PC-295 by Western immunoblotting. As positive controls, we used material of human pheochromocytoma for CgA, of rat pituitary for SGIII, and of the human in vitro cell line LNCaP for androgen receptor expression. The procedure of protein extraction was used as previously described. 41 Frozen tissues of the PC-295 xenografts were crushed in a liquid-nitrogen-chilled metal cylinder. The tissue homogenates were transferred into a lysis buffer (10 mmol/L Tris (pH 7.4), 150 mmol/L NaCl (Sigma), 1% Triton X-100 (Merck, Darmstadt, Germany), 1% deoxycholate (Sigma), 0.1% SDS (Gibco), 5 mmol/L EDTA (Merck), and protease inhibitors (1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L aprotinin, 50 mg/L leupeptin, 1 mmol/L benzamidine, and 1 mg/L pepstatin; all from Sigma). After centrifugation of the mixture at 100,000 rpm at 4°C for 10 minutes, the protein content of the supernatants was measured by the Bradford method (Bio-Rad protein assay, München, Germany).
A 20-μg aliquot of each sample was transferred to an SDS-polyacrylamide gel, and gel electrophoresis was performed with prestained markers as size standards (Novex, San Diego, CA). The gels were blotted to a 0.45-μm cellulose nitrate membrane (Schleicher & Schuell, Dassel, Germany). The immunoblot was blocked with PBS (pH 7.7) containing 0.1% Tween-20 (Sigma) and 5% dry milk for 1 hour. The CgA, androgen receptor, or SGIII antibodies were added in their optimal concentration and incubated overnight on an orbital shaker at 4°C. After rinsing four times for 15 minutes each with PBS, incubation for 1 hour was performed with the secondary horseradish-peroxidase-conjugated antibodies and goat anti-mouse for mouse monoclonal and goat anti-rabbit for rabbit polyclonal antibodies, respectively. Subsequently, a short incubation with a mixture of 10 ml of luminol and 100 μl of oxidizing agent (BM chemiluminescense kit, Boehringer Mannheim, Mannheim, Germany) followed, after washing four times for 15 minutes each with PBS. Excess reagent was removed, and antibodies were visualized by exposure of the blots to an x-ray film.
Reverse Transcriptase-Polymerase Chain Reaction
RNA was isolated by using the single-step RNAzol B method (Campro Scientific, Veenendaal, The Netherlands). 46 Frozen tissue (100 mg) was homogenized in 1 ml of RNAzol. Chloroform (0.1 ml) was added, and the mixture was vortexed for 15 seconds followed by a 5-minute incubation on ice. The homogenate was then centrifuged at 4°C at 12,000 × g for 15 minutes. The upper water phase containing the RNA was removed and mixed with an equal volume of isopropanol. This mixture was then kept at 4°C for 15 minutes and after that centrifuged at 4°C at 12,000 × g for 15 minutes. The supernatant was removed, and the RNA pellet was washed twice with 75% ethanol by vortexing and centrifugation at 4°C at 12,000 × g. The pellet was then dried and resuspended in sterile H2O. The concentration was determined at OD 260, and solutions of 1 μg/μl were prepared for further use in reverse transcriptase polymerase chain reaction (RT-PCR). The quality of the isolated RNA was checked by determining the 260/280 ratio and by formaldehyde gel electrophoresis to check the ribosomal (28 S and 18 S) bands.
RT-PCR was performed for CgA, SGIII, PAM, PC1 and -2, and ß2-microglobulin (ß2MG) with a standard protocol. The reverse transcriptase reaction was performed with a master mix containing 5 mmol/L MgCl2, PCR buffer, 10 mmol/L dNTPs, RNAse inhibitor (10 U), reverse transcriptase (25 U), 2.5 mmol/L random hexamer primers, and 0.5 μg of RNA in a total volume of 10 μl covered with 50 μl of mineral oil. The master mix was then processed at 42°C for 60 minutes followed by a 15-minute incubation at 99°C, and the reaction was stopped at 4°C for 5 minutes. The cDNA mix that was formed was then used totally with the master mix of the PCR protocol. In this protocol, the master mix contained reaction buffer, Goldstar Red DNA polymerase (Eurogentec, Seraing, Belgium; 1 U), 15 μmmol/L sense and antisense primer in a total volume of 40 μl. All samples were first denatured at 94°C for 10 minutes, and then amplification was performed for 35 cycles of 1 minute at 94°C, 1 minute at 60°C, and 1 minute at 72°C and a final extension at 72°C for 10 minutes. The PCR product was checked on a 1% agarose gel and, if necessary, followed by Southern blotting.
Results
A decline in proliferative activity (Figure 1A) ▶ , a rapid and persistent loss of androgen receptor expression (Figure 1C) ▶ , as previously shown for the PC-82 xenograft model, 47 and an increase of apoptosis in the PC-295 human prostate cancer xenograft is associated with a rapid tumor volume decrease (Figure 1B) ▶ after androgen withdrawal. The percentage of BrdU-incorporating cells after 1 hour of IP administration decreased rapidly from a basal percentage of 10 in the controls to nearly zero at day 4 after castration. Accordingly, the MIB-1 expression decreased from an initial percentage of 20 in the controls to near zero at day 4 after castration. Within a period of 2 days, the expression of androgen receptor in ∼90% of PC-295 cells disappeared. The percentage of apoptotic cells increased from the moment of castration to a maximum at day 4, when ∼20% of the tumor cells were apoptotic. The PSA serum levels paralleled the dramatic tumor volume decrease and decreased rapidly after castration to zero after 7 days (Figure 1C) ▶ .
Figure 1.
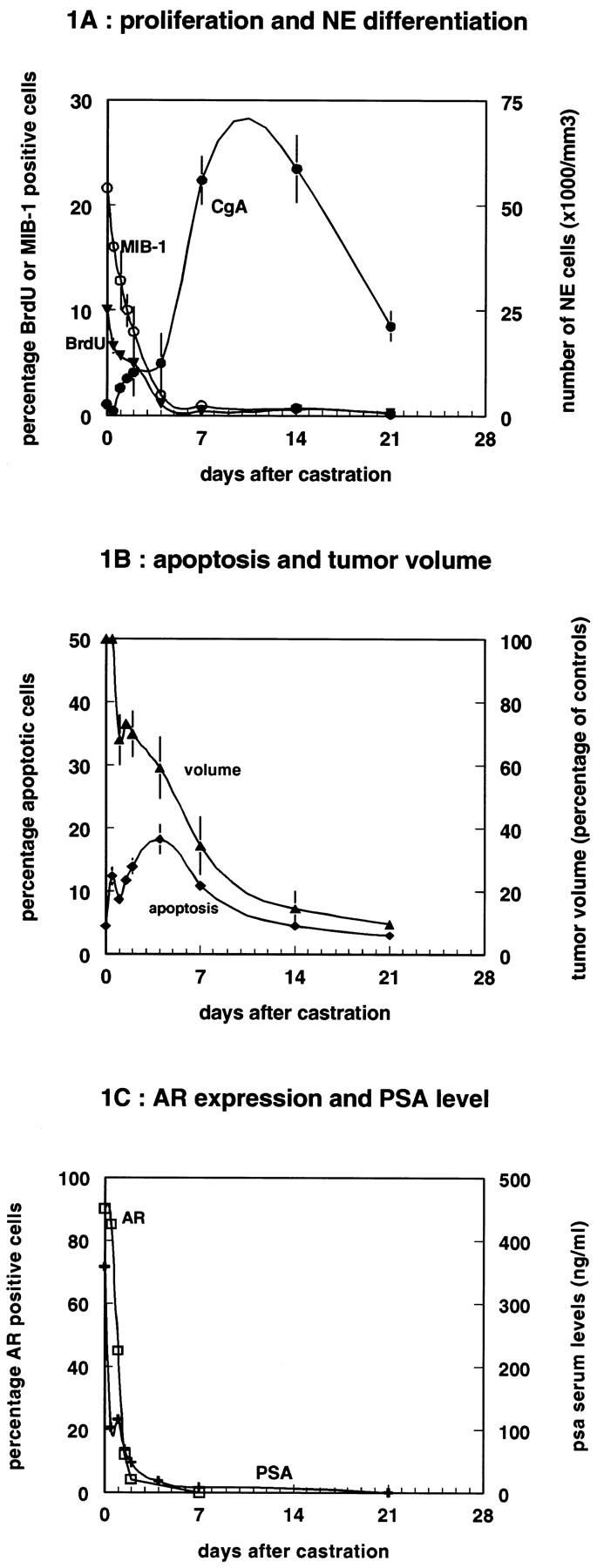
Monitoring of proliferation, apoptosis, NE differentiation, and tumor volume changes in the PC-295 model after androgen withdrawal. A: Results of the immunohistochemical staining for proliferation (BrdU, ▾ mice received BrdU IP 1 hour before sacrifice) and MIB-1, ○) and neuroendocrine differentiation (CgA, •). Proliferation is shown in mean percentage of positive cells scored per 1000 cells (n = 3), whereas the NE differentiation is shown as the number of CgA-positive cells per mm3. One-way ANOVA showed that both BrdU and MIB-1 decreased in time (Kruskal-Wallis, P < 0.05). At each time point mean values are given (n = 3). B: Results from volume measurements (▴) after castration and the immunohistochemical staining for apoptosis by TUNEL (⧫). Apoptosis is shown as the percentage of positive cells scored per 1000 cells. Tumor volume decrease was significantly different from controls (t-test, P < 0.05). At each time point mean values are given (n = 3).C: Results from prostate-specific antigen serum measurements (PSA, +) and immunohistochemical staining for the androgen receptor (AR, □; scored as percentage/1000 tumor cells) for the different time points after castration.
Androgen withdrawal also leads to an immediate increase in the proportion of NE cells. The number of NE cells, as marked by the expression of CgA, increased from an initial 1,000 cells/mm 2 to 60,000 NE cells/mm 2 at day 7 after castration (Figure 1A) ▶ . The course of CgA expression in time is given in Figure 2 ▶ in which the immunostainings of the different time points are shown. The typical granular staining for CgA increased clearly, and positive cells were still observed 21 days after castration. The NE cell was the main tumor cell type found already from day 7 after castration on, as determined by immunohistochemistry for both CgA and SGIII.
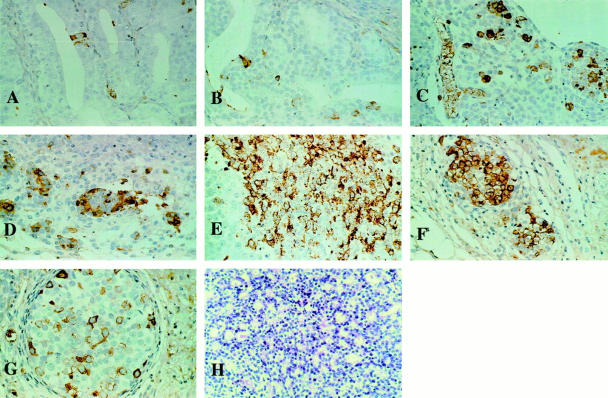
Figure 2.
Immunohistochemical staining for CgA in the PC-295 model at different time points post-castration. A: Control tissue of a poorly differentiated PC-295 tumor with one NE cell. B: PC-295 tumor 1 day after castration with one NE cell (open type). C: PC-295 tumor 2 days after castration with dispersed NE cells. D: PC-295 tumor 4 days after castration with a cluster of NE cells. E: PC-295 tumor 7 days after castration with a large number of NE cells. F: PC-295 14 days after castration. G: PC-295 tumor 21 days after castration. H: H&E staining of the PC-295 tumor in the control situation.
It was clear that the characteristic cribriform growth pattern of untreated PC-295 tumors disappeared ∼4 days after castration, finally resulting in a solid tumor largely composed of islets of CgA-positive cells surrounded by murine stromal cells. Increased expression of the neuropeptides serotonin and bombesin was detected in part of the CgA-positive cells at 7, 14, and 21 days after castration (data not shown). Increased expression of somatostatin receptors was seen 4 and 7 days after castration, whereas expression of calcitonin or VIP was not detected. The PC-295 tumor cells showed increased expression of Bcl-2 from 4 days after castration on. Bcl-2 expression was found in non-NE cells at days 14 and 21 after castration in predominantly CgA- and SGIII-immunostained tumor fields.
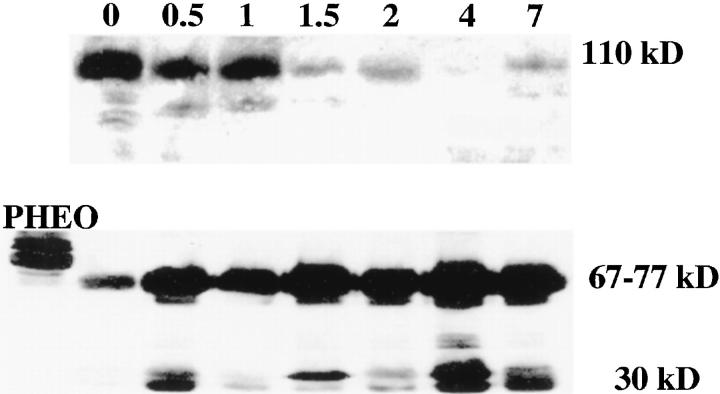
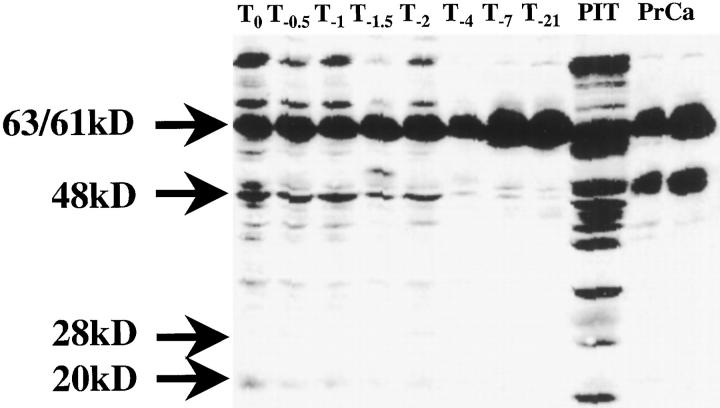
Western blot analysis confirmed the immunohistochemical results, showing the increased CgA expression associated with decreased androgen receptor expression (Figure 3) ▶ . The specific CgA 68-kd signal and the amount of smaller 30-kd CgA-derived peptide clearly increased after androgen withdrawal, whereas 112-kd AR expression was clearly lost 2 days after castration. Increased expression of 63/61-kd SGIII (Figure 4) ▶ was observed from 7 days after castration on. The amounts of the processed forms of SGIII, as indicated by the 48-, 28-, and 20-kd fragments, decreased after androgen withdrawal.
Figure 3.
Expression of the androgen receptor (AR) and Chromogranin A (CgA) in the PC-295 model following androgen withdrawl analyzed by Western blotting. Both panels show the time points T0, T−0.5, T−1,T−1.5, T−2, T−4, and T−7. In the upper panel the decreasing signal of androgen receptor expression is found at 110-112 kd. The CgA signal can be found between 66 and 75 kd in the lower panel, which are the lowest bands in the control Pheochromocytoma (Pheo). Both panels also show the processed proteins or degradation products.
Figure 4.
Expression of Secretogranin III in the PC-295 model following androgen withdrawl analyzed by Western blotting. Secretogranin III is expressed as 63/61 kd (upper arrow) double band in prostate carcinoma (PrCa), pituitary (rat), and in the PC-295 models at all indicated time points. The processed forms of the protein are also visible at the lower bands of 48 kd, 28 kd, and 20 kd (compare the pituitary lane).
Immunohistochemical double labeling of PC-295 tumors clearly showed absence of MIB-1 expression in CgA-positive cells. For BrdU labeling, however, a different result was observed. Tumors of mice injected with BrdU 1 hour before sacrifice did not show BrdU-labeled CgA-positive NE cells. Continuously BrdU-labeled tumors, respectively, showed 5% and 10% double-labeled NE cells at days 2 and 4 after castration, whereas 40% and 70% of the whole cell population was BrdU positive, respectively.
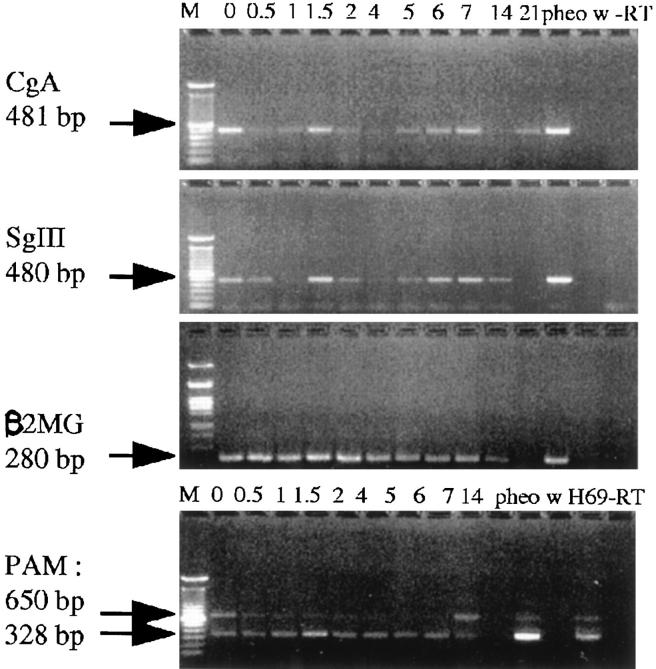
RT-PCR with human specific CgA and SGIII primers demonstrated a clear increase of the NE phenotype in PC-295 after 4 days of androgen withdrawal by using β2MG expression as internal human control (Figure 5) ▶ . No significant variation in PAM expression was noted after castration.
Figure 5.
Results of RT-PCR for NE markers in the PC-295 model post castration. Expression patterns of CgA, SGIII, β2MG, and PAM are shown for different time points in the tumor after castration. Lengths of the PCR products are given. H69 is a small cell lung cancer cell line, and −RT and w are controls for reverse transcriptase and water, respectively.
Discussion
In this report, we extend the previous observations done in the PC-295 human prostate cancer xenograft model and describe in more detail the characteristics of NE differentiation in prostate cancer under hormonal regulation. The growth and regression of the PC-295 human prostate cancer xenograft in the presently described castration experiment was comparable to the result of earlier studies performed with this model. 40 The tumor doubling time was 13 days, and the half-life time of tumors after castration was 4.3 days, which was within the same range as found in earlier experiments. After 21 days of androgen withdrawal, only 10% of the initial tumors (200 instead of 2000 mm3) was left, so the PC-295 tumor model regressed rapidly after androgen withdrawal. Both Western blot analysis and immunohistochemistry showed a rapid decrease in androgen receptor expression in the PC-295 tumors, 41 comparable to the behavior of the fully androgen-dependent human PC-82 xenograft after castration. 47 This was paralleled by a clear and rapid decrease of the PSA serum levels, clearly demonstrating the androgen-dependent character of the PC-295 model.
The immunohistochemical results showed a rapid decrease of BrdU- and MIB-1-positive cells from 10% and 20% in the controls to ∼0% in tumors at days 4 and 7, respectively. Both nuclear proliferation markers showed a 50% decrease 2 days after castration. The TUNEL staining for apoptosis showed a maximum of apoptotic cells at 4 days after castration. The NE phenotype was clearly and rapidly induced at 4 days after androgen withdrawal, at which time point there were only a few proliferating cells left.
Analysis for CgA by Western and RT-PCR confirmed the increased expression of the CgA protein and RNA in time from a basal expression level in the controls to a maximum at 7 and 14 days after castration. There is considerable variation in the basal level of NE cells in the androgen-supplemented situation, which could be explained by an inter-tumor variation between different mice, which seems to be associated with environmental, eg, host-derived factors, rather than due to an intrinsic tissue heterogeneity. Expression levels of the NE phenotype in the different tumors were confirmed by comparing the immunohistochemical picture with the Western and RT-PCR data at the different time points. Despite inter-tumor differences in CgA expression levels at the moment of castration, a clear increase in the mean value of NE differentiation was observed 4, 7, and 14 days after castration. RT-PCR analysis showed that expression of CgA mRNA initially decreased after androgen withdrawal, after which it increased again after day 4 when the tumor cells did not proliferate. The difference between immunohistochemistry and RT-PCR from day 0 to day 4 is most likely caused by inter-tumor variation in basal CgA expression levels. Another explanation is that the levels of CgA mRNA are higher in NE cells of the controls compared with NE cells directly after androgen withdrawal.
The induction of NE differentiation after androgen withdrawal has now clearly been shown. To further characterize NE cells and the process of NE differentiation we are testing other and possible early markers of NE differentiation. In the PC-295 model, we detected increased expression of SGIII at both the protein level by Western blotting and immunohistochemistry and at mRNA level as determined by RT-PCR analysis. This clearly indicates that the regulated secretory pathway (RSP) is active in these prostatic NE cells. Holthuis et al were able to clone the various components of the genetic route of the RSP in Xenopus laevis 29 and found several NE markers that are co-expressed with SGIII, such as Ac45 and X7365 for the RSP. The expression of CgA is not a requisite for regulated secretion of bioactive neuropeptides, as CgA is just a general marker for NE cells and not functionally involved. It will be relevant to study RSP-related proteins, such as SGIII and PC1, which are currently being studied, to establish whether neuropeptides can be actively secreted by NE cells in the PC-295 model.
No changes in the PC-295 model were found in PAM mRNA expression levels as determined by RT-PCR. PAM was expressed as three splice variants, which was similar to the expression pattern of PAM in lung cancer cell lines. 33 PAM is not necessarily expressed in NE cells, as it is a protein that activates neuropeptides by amidation after their secretion from NE cells. It is therefore not surprising that the expression of PAM did not increase in the PC-295 model after castration.
Serotonin and bombesin, but not VIP and calcitonin, were expressed in the PC-295 model. Using somatostatin (ss) autoradiography by [125I-TYR0]SS-28, which binds with high affinity to the five known somatostatin-receptor subtypes, specific binding was demonstrated for ss receptors type sst1 and sst2 in PC-295 tumor tissue (L. Hofland, Department of Internal Medicine, Erasmus University, unpublished results). These results indicate that a number of growth-modulating neuropeptides and some of their receptors can play a role in the PC-295 model.
From the double stainings of CgA with BrdU and MIB-1, it was concluded that the greater part of the NE cells was in the G0 phase of the cell cycle, which was in agreement with other studies on prostate cancer. 22,23 From the double-labeling studies with MIB-1 and CgA it was seen that the few proliferating cells at 7 days after castration were surrounding the predominantly CgA-positive islets of the PC-295 human prostate cancer tissue. Subsequently, comparing the Western blot results for CgA and androgen receptor clearly showed that the NE cells did not express the androgen receptor. NE cells thus seem to be an androgen-independent, nonproliferating part of the prostatic epithelium.
Bonkhoff et al predominantly found in benign prostatic glands that proliferation was restricted to the prostatic basal cell layer and that basal cells did not express the androgen receptor. In prostate cancer specimens, androgen-receptor-negative NE cells expressed cytokeratin 18 and 5, and these cells were found in the proximity of proliferating cells. In the PC-295 experimental tumor model, clustered NE cells are thus also found in the proximity of proliferating cells, indicating a possible interaction between NE cells or their products and neighboring proliferating cells. The fact that basal, proliferating cells and CgA-positive NE cells are the only cells surviving androgen deprivation suggests that the absence of androgen receptor expression plays a key role in their survival. Another factor for cellular survival is Bcl-2, which protects cells from undergoing apoptosis. This is also shown in our model as Bcl-2 expression increases after androgen withdrawal. After castration, Bcl-2 is not expressed in NE cells but in a proportion of non-NE putative androgen-independent cells in the PC-295 xenograft. In a recent publication, 16 Xue et al showed a specific keratin expression pattern for most NE cells in prostate cancer patient material by using serotonin as a NE marker combined with the luminal epithelial cell marker (cytokeratin 18) and a typical basal cell marker (cytokeratin 5, RCK 103). A minority of the prostatic NE cells expressed only cytokeratins for basal cells or luminal cells. NE cells thus form a heterogeneous population. However, no expression of Bcl-2 in NE cells in patient material was found, suggesting that Bcl-2 expression is lost during NE differentiation of Bcl-2-positive basal cells. 16 In the in vivo model LuCaP, cell lineage markers PSA, neuron-specific enolase (NSE), and Bcl-2 were used to mark the different epithelial cell types found in prostate cancer tissue. 39 They proposed a model in which Bcl-2-positive proliferating epithelial cells differentiated into either a lineage of NSE-expressing NE cells or a lineage of PSA-expressing exocrine secretory cells. In this latter population, the expression of NSE is heterogeneous. These amphicrine, ie, both NSE- and PSA-positive, cells may be induced to express Bcl-2, which might result in tumor cells surviving hormonal manipulation. These results partly support the stem cell model for benign prostatic hyperplasia proposed by Isaacs and Coffey. 48 They propose a model of prostatic stem cells, which divide into a limited number of amplifying, basal cells followed by a limited number of cell divisions to finally form transit cells that differentiate into the different epithelial cell subpopulations. In prostate cancer, most probably the amplifying step in this model is not limiting, resulting in numerous types of exocrine and endocrine cells expressing different combinations of cytokeratins.
In the PC-295 model, we found BrdU-positive NE cells. As we did not find MIB-1 expression in NE cells, we believe that the NE cells did not proliferate, but rather that a fraction of CgA-positive cells entered S phase shortly after castration. It is supposed that such cells are arrested before mitosis. Another more plausible explanation is that the BrdU-labeled cells were amplified after castration and differentiated into NE cells in the time that followed between BrdU labeling and sacrifice at day 2 or 4 after castration. Considering Xue et al and the present results, there are possibly two routes of NE differentiation in androgen-dependent epithelia: a regular route from proliferating cells via an intermediate cell type to NE cells and an alternative route of induced NE differentiation from exocrine luminal cells by androgen withdrawal.
The CgA and SGIII staining, the increased apoptosis, and the nonproliferative status of most of the NE cells seen in the controls (T0) and at 7 days after castration in the currently described PC-295 xenograft clearly show the process of neuroendocrine differentiation as being induced by androgen withdrawal. From the histological picture it is also clear that the changes in NE expression are not due to a selection of NE cells. The BrdU-positive NE cells are probably the best indicators of the post-castration amplified cells that differentiate into a NE cell. The greater part of the NE cells found after 4 days of androgen withdrawal differentiated from G0-phase-arrested luminal or intermediate cells. Clearly, the induction of the NE phenotype is a proliferation-independent process in the PC-295 model. PC-295 tumors did not grow without androgens either in female mice or after long-term castration of PC-295 tumor-bearing mice (data not shown). This may be explained by the rapid decrease in proliferation and the rapid tumor regression of the PC-295 tissue after castration. Probably, the decreased tumor vascularity in PC-295, observed after prolonged androgen withdrawal, caused the death of surviving nonproliferating cells. All together, neither the induction of the NE phenotype nor Bcl-2 expression of non-NE cells after castration in PC-295 led to androgen-independent growth or maintenance of the tumor vascularity.
As the prognostic value of CgA expression or occurrence of NE differentiation in prostate cancer is still under debate, additional testing of SGIII and PAM as possible better NE-related prognostic markers for the progression of prostate cancer is still relevant. Possible changes in expression of components of the regulated secretory pathway or PAM might have effects on, for example, overproduction of bioactive growth-stimulating neuropeptides.
The PC-295 in vivo model is a potential model for additional studies of the induction of NE differentiation by androgen withdrawal, the regulated secretory pathway in particular. In addition, PC-310, another androgen-dependent, NE differentiated prostate cancer model, is currently under investigation and might provide us with additional knowledge on NE differentiation. Differentiation of NE cells from basal or luminal cells and the role of NE cells in progression of prostate cancer are important aspects to be studied as well. SGIII, PAM, or other markers for NE differentiation are to be tested retrospectively for their prognostic value in the survival of patients undergoing radical prostatectomies. Human prostate cancer xenograft models with NE differentiation will be used to answer fundamental questions on prostate cancer.
Acknowledgments
We thank Mrs. C.M.A. de Ridder (Erasmus Center for Animal Research) for biotechnical, Mrs. J.J.C. Peekstok (Department of Pathology) for histological, and Mr. F.L. van der Panne (Department of Pathology) for photographic assistance.
Footnotes
Address reprint requests to Dr. Johan Jongsma, Erasmus University Rotterdam, Division of Experimental Urology, Department of Urology, Josephine Nefkens Institute, Room Be331, PO BOX 1738, 3000 DR Rotterdam, The Netherlands. E-mail: jongsma@uro.fgg.eur.nl.
Supported by the Dutch Cancer Society (grant EUR 95-1029).
References
- 1.Abrahamsson PA: Neuroendocrine differentiation and hormone-refractory prostate cancer. Prostate Suppl 1996, 6:3-8 [DOI] [PubMed] [Google Scholar]
- 2.Bonkhoff H, Stein U, Remberger K: Endocrine-paracrine cell types in the prostate and prostatic adenocarcinoma is postmitotic cells. Hum Pathol 1995, 26:167-170 [DOI] [PubMed] [Google Scholar]
- 3.di-Sant’Agnese PA: Neuroendocrine differentiation in human prostatic carcinoma. Hum Pathol 1992, 23:287-296 [DOI] [PubMed] [Google Scholar]
- 4.di-Sant’Agnese PA: Neuroendocrine differentiation in prostatic carcinoma. Cancer Suppl 1995, 75:1850-1859 [Google Scholar]
- 5.Noordzij MA, van Steenbrugge GJ, van der Kwast T, Schröder FH: Neuroendocrine cells in the normal, hyperplastic and neoplastic prostate. Urol Res 1995, 22:333-341 [DOI] [PubMed] [Google Scholar]
- 6.Noordzij MA, van der Kwast T, van Steenbrugge GJ, Hop WJC, Schröder FH: The prognostic influence of neuroendocrine cells in prostate cancer: results of a long-term follow-up study with patients treated by radical prostatectomy. Int J Cancer 1995, 62:252-258 [DOI] [PubMed] [Google Scholar]
- 7.Abrahamsson P-A, Wadström LB, Alumets J, Falkmer S, Grimelius L: Peptide-hormone- and serotonin-immunoreactive cells in normal and hyperplastic prostate glands. Pathol Res Pract 1986, 181:675-683 [DOI] [PubMed] [Google Scholar]
- 8.Aprikian AG, Cordon-Cardo C, Fair WR, Reuter VE: Characterization of neuroendocrine differentiation in human benign prostate and prostatic adenocarcinoma. Cancer 1993, 71:3952-3965 [DOI] [PubMed] [Google Scholar]
- 9.Abdul M, Logothetis CJ, Hoosein NM: Growth-inhibitory effects of serotonin uptake inhibitors on human prostate carcinoma cell lines. J Urol 1995, 154:247-250 [PubMed] [Google Scholar]
- 10.Seuwen K, Pouyssegur J: Serotonin as a growth factor. Biochem Pharmacol 1990, 39:985-990 [DOI] [PubMed] [Google Scholar]
- 11.Aprikian AG, Han K, Chevalier S, Bazinet M, Viallet J: Bombesin specifically induces intracellular calcium mobilization via gastrin releasing peptide receptors in human prostate cancer cells. J Mol Endocrinol 1996, 16:287-296 [DOI] [PubMed] [Google Scholar]
- 12.Mack D, Hacker GW, Hauser-Kronberger C, Frick J, Dietze O: Vasoactive intestinal polypeptide (VIP) and neuropeptide tyrosine (NPY) in prostate carcinoma. Eur J Cancer 1997, 33:317-318 [DOI] [PubMed] [Google Scholar]
- 13.Zia H, Hida T, Jakowlev S, Birrer M, Gozes Y, Reubi JC, Fridkin M, Gozes I, Moody TW: Breast cancer growth is inhibited by vasoactive intestinal peptide (VIP) hybrid, a synthetic VIP receptor antagonist. Cancer Res 1996, 56:3486-3489 [PubMed] [Google Scholar]
- 14.Solano RM, Carmena MJ, Carrero I, Cavallaro S, Roman F, Hueso C, Travali S, Lopez-Fraile N, Guyarro LG, Prieto JC: Characterization of vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptors in human benign hyperplastic prostate. Endocrinology 1996, 137:2815-2822 [DOI] [PubMed] [Google Scholar]
- 15.Iwamura M, Wu G, Abrahamsson PA, Di-Sant’Agnese PA, Cockett ATK, Deftos LJ: Parathyroid hormone-related protein is expressed by prostatic neuroendocrine cells. Urology 1994, 43:667-674 [DOI] [PubMed] [Google Scholar]
- 16.Xue Y, Verhofstad A, Lange W, Smedts F, Debruyne F, de la Rosette J, Schalken J: Prostatic neuroendocrine cells have a unique keratin expression and do not express Bcl-2. Am J Pathol 1997, 151:1759-1765 [PMC free article] [PubMed] [Google Scholar]
- 17.Abrahamsson PA, Falkmer S, FÖ K, Grimelius L: The course of neuroendocrine differentiation in prostatic carcinomas: an immunohistochemical study testing chromogranin A as an “endocrine marker”. Pathol Res Pract 1989, 185:373-380 [DOI] [PubMed] [Google Scholar]
- 18.Hendy GN, Bevan S, Mattei MG, Mouland AJ: Chromogranin A. Clin Invest Med 1995, 18:47-65 [PubMed] [Google Scholar]
- 19.Schmidt KW, Helpap B, Totsch M, Kirchmair R, Dockhorn-Dworniczak B, Bocker W, Fischer-Colbrie R: Immunohistochemical localization of chromogranins A and B and secretogranin II in normal, hyperplastic and neoplastic prostate. Histopathology 1994, 24:233-239 [DOI] [PubMed] [Google Scholar]
- 20.Krijnen JLM, Janssen PJA, Ruizeveld de Winter JA, van Krimpen H, Schröder FH, van der Kwast T: Do neuroendocrine cells in human prostate cancer express androgen receptor? Histochemistry 1993, 100:393-398 [DOI] [PubMed] [Google Scholar]
- 21.Cohen RJ, Cooper K, Haffejee Z, Robinson E, Becker PJ: Immunohistochemical detection of oncogene proteins and neuroendocrine differentiation in different stages of prostate cancer. Pathology. 1995, 27:229-2328532388 [Google Scholar]
- 22.Bonkhoff H, Stein U, Remberger K: Multidirectional differentiation in the normal, hyperplastic, and neoplastic human prostate: simultaneous demonstration of cell-specific epithelial markers. Hum Pathol 1994, 25:42-46 [DOI] [PubMed] [Google Scholar]
- 23.Bonkhoff H, Stein U, Remberger K: The proliferative function of basal cells in the normal and hyperplastic human prostate. Prostate 1994, 24:114-118 [DOI] [PubMed] [Google Scholar]
- 24.Cohen MK, Arber DA, Coffield S, Keegan GT, McClintock J, Speights VO: Neuroendocrine differentiation in prostatic adenocarcinoma and its relationship to tumor progression. Cancer 1994, 74:1899-1903 [DOI] [PubMed] [Google Scholar]
- 25.Colombel M, Olsson CA, Ng PY, Buttyan R: Hormone-regulated apoptosis results from reentry of differentiated prostate cells onto a defective cell cycle. Cancer Res 1992, 52:4313-4319 [PubMed] [Google Scholar]
- 26.Holthuis JCM, Martens GJM: The neuroendocrine proteins secretogranin II and III are regionally conserved and coordinately expressed with proopiomelanocortin in Xenopus intermediate pituitary. J Neurochem 1996, 66:2248-2256 [DOI] [PubMed] [Google Scholar]
- 27.Smeekens SP, Montag AG, Thomas G, Albiges-Rizo C, Carrol R, Benig M, Philips LA, Martin S, Ohagi S, Gardner P, Swift H, Steiner DF: Proinsulin processing by the sutilisin-related proprotein convertases furin, PC2, and PC3. Proc Natl Acad Sci USA 1992, 89:8822-8826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tucker JD, Dhanvantari S, Brubaker PL: Proglucagon processing in islet and intestinal cell lines. Regul Peptides 1996, 62:29-35 [DOI] [PubMed] [Google Scholar]
- 29.Holthuis J, Janssen E, Van Riel M, Martens G: Molecular probing of the secretory pathway in peptide hormone producing cells. J Cell Sci 1995, 108:3295-3305 [DOI] [PubMed] [Google Scholar]
- 30.Braks JAM, Broers CAM, Danger JM, Martens GJM: Structural organization of the gene encoding the neuroendocrine chaperone 7B2. Eur J Biochem 1996, 236:60-67 [DOI] [PubMed] [Google Scholar]
- 31.Martens GJM, Bussemakers MJG, Ayoubi TAY, Jenks BG: The novel pituitary polypeptide 7B2 is a highly conserved protein coexpressed with proopiomelanocortin. Eur J Biochem 1989, 181:75-79 [DOI] [PubMed] [Google Scholar]
- 32.Sigafoos J, Chestnut WG, Merril BM, Taylor LCE, Diliberto EJ, Viveros OH: Novel peptides from adrenomedullary chromaffin vesicles. J Anat 1993, 183:253-264 [PMC free article] [PubMed] [Google Scholar]
- 33.Vos MD, Jones JE, Treston AM: Human peptidylglycine alpha-amidating monooxygenase transcripts derived by alternative mRNA splicing of an unreported exon. Gene 1995, 163:307-311 [DOI] [PubMed] [Google Scholar]
- 34.Vos MD, Scott FM, Iwai N, Treston AM: Expression in human lung cancer cell lines of genes of prohormone processing and the neuroendocrine phenotype. J Cell Biochem 1996, 27:1-12 [DOI] [PubMed] [Google Scholar]
- 35.Huttner WB, Natori S: Helper proteins for neuroendocrine secretion. Curr Biol 1995, 5:242-245 [DOI] [PubMed] [Google Scholar]
- 36.Eib DW, Martens GJM: A novel transmembrane protein with epidermal growth factor and follistatin domains expressed in the hypothalamo-hypophyssial axis of Xenopus laevis. J Neurochem 1996, 67:1047-1055 [DOI] [PubMed] [Google Scholar]
- 37.Romijn J, Erkens-Schulze S, Schröder F: Perspectives of the use of tissue culture models as an alternative to human prostate cancer xenografts in nude mice. Contrib Oncol 1996, 51:209-231 [Google Scholar]
- 38.Nagabhushan M, Miller CM, Pretlow TP, Giaconia JM, Edgehouse NL, Schwartz S, Kung HJ, de Vere White RW, Gumerlock PH, Resnick MI, Amini SB, Pretlow TG: CWR22: the first human prostate cancer xenograft with strongly androgen-dependent and relapsed strains both in vivo and in soft agar. Cancer Res 1996, 56:3042-3046 [PubMed] [Google Scholar]
- 39.Liu AY, Corey E, Bladou F, Lange PH, Vesella RL: Prostatic cell lineage markers: emergence of Bcl2+ cells of human prostate cancer xenograft LuCaP 23 following castration. Int J Cancer 1996, 65:85-89 [DOI] [PubMed] [Google Scholar]
- 40.van Weerden WM, de Ridder CMA, Verdaasdonk CL, Romijn JC, Van der Kwast TH, Schröder, Van Steenbrugge GJ: Development of seven new human prostate tumor xenograft models and their histopathological characterization. Am J Pathol 1996, 149:1055-1062 [PMC free article] [PubMed] [Google Scholar]
- 41.Noordzij MA, van Weerden WM, van der Kwast TH, Abrahamsson P-A, Gershagen S, Schröder FH, Van Steenbrugge GJ: Neuroendocrine differentiation in human prostatic tumor models. Am J Pathol 1996, 149:859-8718780390 [Google Scholar]
- 42.van Steenbrugge GJ, van Weerden WM, de Ridder CMA, van der Kwast T, Schroeder FH: Development and application of prostatic xenograft models for the study of human cancer. Sex Hormones Hormones and Antihormones in Endocrine Dependent Pathology: Basic and Clinical Aspects. Edited by Motta M, Serio M. Amsterdam, Elsevier, 1994, pp 11–22
- 43.van Steenbrugge G, Groen M, de Jong F, Schröder F: The use of steroid-containing silastic implants in male nude mice: plasma hormone levels and the effect of implantation on the weights of the ventral prostate and seminal vesicles. Prostate 1984, 5:639-647 [DOI] [PubMed] [Google Scholar]
- 44.Holthuis J, Jansen E, Martens G: Secretogranin III is a sulfated protein undergoing proteolytic processing in the regulated secretory pathway. Biol Chem 1996, 271:17755-17760 [DOI] [PubMed] [Google Scholar]
- 45.Shi SR, Key ME, Kalra KL: Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 1991, 39:741-748 [DOI] [PubMed] [Google Scholar]
- 46.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 47.Ruizeveld de Winter JA, van Weerden WM, Faber PW, Van Steenbrugge GJ, Trapman J, Brinkmann AO, Van der Kwast TH: Regulation of androgen receptor expression in the human heterotransplantable prostate carcinoma PC-82. Endocrinology 1992, 131:3045-3050 [DOI] [PubMed] [Google Scholar]
- 48.Isaacs J, Coffey D: Etiology and disease process of benign prostatic hyperplasia. Prostate Suppl 1989, 2:33-50 [DOI] [PubMed] [Google Scholar]