Abstract
Thrombospondin-1 is an extracellular matrix protein that inhibits endothelial cell proliferation, migration, and angiogenesis. This study was performed to investigate the role of thrombospondin-1 in ischemic retinal neovascularization. In a murine model of retinal neovascularization, thrombospondin-1 mRNA was increased from postnatal day 13 (P13), with a threefold peak response observed on P15, corresponding to the time of development of retinal neovascularization. Prominent expression of thrombospondin-1 was observed in neovascular cells, specifically, cells adjacent to the area of nonperfusion. It has been suggested that vascular endothelial growth factor (VEGF) plays a major role in ischemia-induced retinal neovascularization of this model, so we studied the effects of VEGF on thrombospondin-1 expression. In bovine retinal microcapillary endothelial cells, VEGF induced a biphasic response of thrombospondin-1 expression; VEGF decreased thrombospondin-1 mRNA 0.41-fold after 4 hours, whereas it increased, with a threefold peak response, after 24 hours. VEGF-induced endothelial cell proliferation was completely inhibited by exogenous thrombospondin-1 and increased by 37.5% with anti-thrombospondin-1 antibody. The present findings suggest that, in the ischemic retina, retinal neovascular cells increase thrombospondin-1 expression, and VEGF may stimulate endogenous thrombospondin-1 induction, which inhibits endothelial cell growth. VEGF-mediated thrombospondin-1 induction in ischemia-induced angiogenesis may be a negative feedback mechanism.
Pathological growth of new blood vessels is characteristic of most eye diseases that cause catastrophic loss of vision, such as diabetic retinopathy, central retinal vein occlusion, retinopathy of prematurity, and age-related macular degeneration. One primary stimulus for ocular neovascularization is hypoxia, and recent studies have implicated vascular endothelial growth factor (VEGF) 1-3 as a mediator of such ocular neovascular diseases. Suppression of VEGF has been shown to inhibit neovascularization in animal models of retinal ischemia. 4-6 VEGF itself is able to produce many of the vascular abnormalities common to diabetic retinopathy and other ischemic retinopathies, 7 and levels of VEGF are elevated in patients with active ocular neovascularization. 8-11
Thrombospondin-1 (TSP-1) is an extracellular matrix protein described initially as a major component of platelet α-granules and that functions in aggregation and clot formation. 12 TSP-1, which is secreted by numerous cell types, including endothelial cells, fibroblasts, macrophages, and smooth muscle cells, 13-17 enhances the growth of smooth muscle cells and fibroblasts, 18-22 whereas it inhibits endothelial cell proliferation, migration, and angiogenesis in vitro and in vivo. 23-31 Although TSP-1 seems to promote substrate adhesion of a variety of cells, 32-36 it reduces focal adhesion of endothelial cells and fibroblasts. 34,37 TSP-1 binds to and activates transforming growth factor (TGF)-β1 38,39 and exhibits increased expression in response to serum, platelet-derived growth factor, basic fibroblast growth factor, and TGF-β1. 18,40-42 The kinetics of induction of TSP-1 by platelet-derived growth factor are similar to those of other inducible, immediate early response genes. 40,42,43 The p53 tumor suppressor gene regulates the expression of TSP-1. 44 These features are consistent with a role for TSP-1 in the regulation of cell cycle progression. Although regulation of TSP-1 in vascular wound healing 17 and tumor-induced angiogenesis 45,46 has been studied, the regulation of TSP-1 in VEGF- and hypoxia-induced neovascularization has not (to our knowledge) been investigated.
In the study described herein, we investigated the expression of TSP-1 in a mouse model of ischemia-induced retinal neovascularization, and determined whether VEGF regulates TSP-1 expression in bovine retinal microcapillary endothelial cells.
Materials and Methods
Materials
VEGF was purchased from Genzyme (Cambridge, MA). Anti-human TSP-1 antibody was obtained from Athens Research and Technology (Athens, GA). Plasma-derived horse serum (PDHS) and 10% calf serum were obtained from Wheaton (Pipersville, PA) and Gibco BRL (Gaithersburg, MD), respectively. 32 P-dATP was obtained from Amersham (Buckinghamshire, UK). TSP-1, fibronectin, sodium pyrophosphate, sodium fluoride, sodium orthovanadate, aprotinin, leupeptin, and phenylmethylsulfonyl fluoride were obtained from Sigma Chemical Co. (St. Louis, MO). Restriction enzymes were obtained from TaKaRa Biomedicals (Tokyo, Japan). Other chemicals were of analytical grade and were purchased from Sigma.
Animal Model
The study adhered to the ARVO Standards for the Use of Animals in Ophthalmic and Vision Research. To produce ischemia-induced retinal neovascularization, litters of 7-day-old (postnatal day 7, P7) C57BL/6J mice and their nursing mothers were exposed to 75 ± 2% oxygen for 5 days and then returned to ambient air at age P12 as described. 47,48 Mice of the same age maintained in ambient air served as controls. After sacrifice and enucleation, flat-mounted, fluorescein-conjugated dextran-perfused retinas were examined to assess the retinal vasculature. 48 As in previous studies, 47,48 examination of the flat-mounted, fluorescein-conjugated dextran-perfused retinas and hematoxylin and eosin (H&E)-stained sections of tissue obtained after 5 days of hypoxia showed neovascular tufts, particularly in the mid-periphery, extending above the internal limiting membrane into the vitreous. These neovascular tufts were most prominent on P17 to P19, but after P23 the neovascularization regressed, and the vascular pattern normalized by P26.
Tissue Preparation
Mice at different time points (P12 immediately after return to room air, P13, P15, P17, P19, P21, P23, and P26; n = 3 for each time point) were deeply anesthetized with pentobarbital sodium (100 mg/kg) and sacrificed by cardiac perfusion with 4% paraformaldehyde in phosphate-buffered saline. Eyes were enucleated and fixed in 4% paraformaldehyde at 4°C overnight and embedded in paraffin. Serial sections (5 μm) of the whole eyes were placed on microscope slides, and the slides were stored at 4°C. Several slides from each eye were also stained with H&E to assess the retinal vasculature.
In Situ Hybridization
Human TSP-1 49 and mouse TSP-2 cDNA 50 were obtained from the American Type Culture Collection (Rockville, MD). A TSP-1 cDNA fragment corresponding to 693 nucleotides encoding amino acids 149 to 379 and a TSP-2 cDNA fragment corresponding to 633 nucleotides encoding amino acids 201 to 411 were subcloned into the pBluescript II vector (Stratagene, La Jolla, CA). Sense and antisense RNA probes were transcribed in vitro from the linearized plasmid by the use of digoxigenin-labeled UTP and T7 or T3 RNA polymerase, according to the manufacturer’s manual (DIG RNA labeling kit SP6/T7, Boehringer Mannheim, Indianapolis, IN). The probes were reduced in size by alkaline treatment to an average of 150 bp. 51
In situ hybridization was performed as previously described, 52 with minor modification. All incubation steps were performed in a moist chamber, and all of the buffers and glassware used for the detection of mRNA had been made RNAse-free. Paraffin was removed from the sections with xylene, and the sections were rehydrated through a graded series of alcohol and then rinsed with phosphate-buffered saline (PBS). They were immersed in 0.2 N HCl for 20 minutes and then incubated in proteinase K (10 μg/ml) for 15 minutes at 37°C. After washing in PBS, the specimens were re-fixed in freshly prepared 4% paraformaldehyde in PBS for 5 minutes and then immersed two times (15 minutes each) in glycine (2 mg/ml) in PBS. They were then immersed in 2X SSC/50% deionized formamide. The specimens were incubated in the hybridization mixture for 18 hours at 45°C. The composition of the hybridization mixture was as follows: 1 mg/ml Escherichia coli tRNA, 20 mmol/L Tris/HCl buffer (pH 8.0), 10 mmol/L EDTA, 1X Denhardt’s solution, 300 mmol/L NaCl, 50% deionized formamide, and 10% dextran sulfate. The final concentration of the probe was 250 ng/ml. After hybridization, the specimens were treated as follows: 2X SSC/50% formamide for 1 hour at 45°C; 0.5 mol/L NaCl/10 mmol/L Tris/HCl buffer (pH 8.0), twice for 5 minutes each; RNAse (20 μg/ml) for 30 minutes at 37°C; 2X SSC/50% formamide for 1 hour at 45°C; 1X SSC/50% formamide for 1 hour at 45°C; and 1X SSC/50% formamide for 1 hour at room temperature.
Immunological detection of hybridized probes was performed as previously described 53 using a nucleic acid detection kit (Boehringer Mannheim). Alkaline-phosphatase-conjugated F(ab) fragments of anti-digoxigenin antibody were applied overnight at 4°C. After development of the color reagent, the specimens were fixed in 10% formalin for 20 minutes to avoid precipitation of crystallized chromogenic substance. Finally, the slides were dehydrated through a graded series of alcohol, cleared with xylene, and coverslipped with xylene-based permanent mounting medium.
Confocal Microscopy
For immunofluorescence analysis, all incubation steps were performed in a moist chamber, and rinses were performed by immersing slides in a PBS bath. Paraffin was removed from the sections by treatment with xylene, after which they were rehydrated through a graded series of alcohol and rinsed with PBS. Each section was incubated for 20 minutes with blocking reagent (Dako, Glostrup, Denmark), incubated overnight at 4°C with anti-TSP-1 antibody, and then washed for 30 minutes with PBS. The sections were incubated for 30 minutes with anti-rabbit IgG labeled with fluorescein isothiocyanate (Dako), washed for 30 minutes with PBS, and coverslipped with VECTASHIELD with propidium iodide (Vector Laboratories, Burlingame, CA). Normal rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA) was used as a negative control.
All specimens were examined with a Zeiss scanning laser confocal microscope (LSM 410 invert laser scan microscope, Zeiss, Oberkochen, Germany). Digitized images were captured by computer and stored on an optical disk for subsequent display. Photographic images were taken from the computer with a digital printer (Pictrography, Fuji Photo Film, Tokyo, Japan).
Cell Cultures
Primary cultures of bovine retinal endothelial cells (BRECs) were isolated by homogenization and a series of filtration steps as described previously. 54,55 Primary BRECs were grown on fibronectin-coated dishes (Iwaki Glass, Tokyo, Japan) containing Dulbecco’s modified Eagle’s medium (DMEM) with 5.5 mmol/L glucose, 10% PDHS, 50 mg/L heparin, and 50 U/L endothelial cell growth factor (Boehringer Mannheim). The cells were incubated at 37°C in 5% CO2; the medium was changed every 3 days. Endothelial cell homogeneity was confirmed by immunoreactivity with anti-factor VIII antibodies. After the cells reached confluence, the medium was changed every 3 days, and cells from passages 3 to 10 were used for the experiments. Endothelial cell homogeneity and cellular characteristics, such as shape and growth rate, were carefully observed and remained constant through passage 15.
Hypoxia Studies
Confluent cell monolayers were exposed to 1 ± 0.5% oxygen using a water-jacketed mini-CO2/multigas incubator with reduced oxygen control (model BL-40M, Jujikagaku, Tokyo, Japan). All cells were maintained at 37°C in a constant 5% carbon dioxide atmosphere with oxygen deficit induced by nitrogen replacement. Cells cultured under these conditions showed no morphological changes by light microscopy after exposures exceeding 72 hours, excluded Trypan blue dye (>98%), and could subsequently be passaged normally. Cells incubated under standard normoxic conditions (95% air, 5% CO2) from the same batch and passage were used as controls.
Northern Blot Analysis
For the animal study, total RNA was isolated from mouse retinas at different time points (10 retinas from five mice at each time point: P12 immediately after return to room air, P13, P15, P17, P19, P21, P23, and P26) using guanidium thiocyanate. For the culture study, total RNA was isolated from individual tissue culture dishes. Northern blot analysis was performed on 15 μg of total RNA after 1% agarose/2 mol/L formaldehyde gel electrophoresis and subsequent capillary transfer to Biodyne nylon membranes (Pall BioSupport, East Hills, NY) and ultraviolet cross-linking using a FUNA-UV-LINKER (FS-1500, Funakoshi, Tokyo, Japan). Radioactive probes were generated using Amersham Megaprime labeling kits (Amersham) and [32P]dATP. Blots were prehybridized, hybridized, and washed in 0.5X SSC, 5% SDS at 65°C with four changes over 1 hour in a rotating hybridization oven (TAITEC, Koshigaya, Japan). All signals were analyzed using a densitometer (BAS-2000II, Fuji Photo Film), and lane loading differences were normalized using the 36B4 cDNA probe. 56
Analysis of TSP-1 mRNA Half-Life
TSP-1 mRNA half-life experiments were carried out using BRECs. The cells were exposed to vehicle or VEGF (25 ng/ml) for the indicated periods of time before mRNA stability measurements. Transcription was inhibited by the addition of actinomycin D (5 μg/ml). For inhibition of protein synthesis, BRECs were treated with cycloheximide (10 μg/ml) for the time periods indicated.
Western Blot Analysis
Cells were washed three times with cold PBS and then solubilized in 100 μl of lysis buffer (1% Triton X-100, 50 mmol/L HEPES, 10 mmol/L EDTA, 10 mmol/L sodium pyrophosphate, 100 mmol/L sodium fluoride, 1 mmol/L sodium orthovanadate, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 2 mmol/L phenylmethylsulfonyl fluoride). After centrifugation at 12,000 rpm for 10 minutes, the protein concentration was determined by BCA protein assay reagent (Pierce, Rockford, IL). An equal amount of protein from each sample was subjected to 7.5% SDS gel electrophoresis and transferred to nitrocellulose membrane (Schleicher & Schuell, Keene, NH). After blocking with PBS containing 0.1% Tween 20 (PBS-T; Bio-Rad, Hercules, CA) with 3% bovine serum albumin for 1 hour at room temperature, the membrane was incubated with anti-TSP-1 antibody for 1.5 hours. After washing with PBS-T, the signals were detected by the ECL Western blot analysis system (Amersham).
Retinal Endothelial Cell Growth Assay
BRECs were plated (approximately 2500 cells per well) onto 24-well culture plates (Iwaki Glass) and incubated overnight in DMEM containing 10% calf serum. The cells were treated with vehicle, VEGF (25 ng/ml), human TSP-1 (5 μg/ml), anti-TSP-1 antibody (10 μg/ml), or combinations thereof. After incubation for 4 days at 37°C, the cells were lysed in 0.1% SDS, and the DNA content was measured by means of Hoechst-33258 dye and a fluorometer (model TKO-100, Hoefer Scientific Instruments, San Francisco, CA). It has been shown that total cellular DNA content measured in this manner correlates closely with actual cell number as determined by hemocytometer counting of trypsinized cells. 57
Statistical Analysis
Determinations were performed in triplicate, and the experiments were repeated at least three times. Results are expressed as the mean ± SD, unless otherwise indicated. Statistical analysis used Student’s t-test or analysis of variance to compare quantitative data populations with normal distributions and equal variance. Data were analyzed using the Mann-Whitney rank sum test or the Kruskal-Wallis test for populations with non-normal distributions or unequal variance. A P value of <0.05 was considered statistically significant.
Results
TSP-1 mRNA Levels in Hypoxic Retina
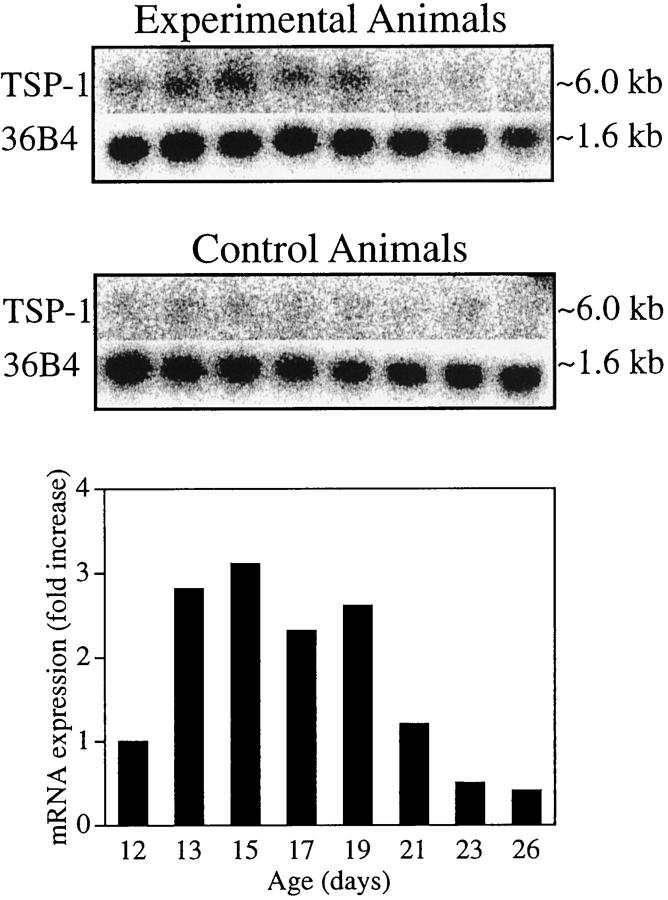
Similar to previous studies, 47,48 histological examination of H&E-stained sections of tissue obtained after 5 days of hypoxia showed neovascular tufts, particularly in the mid-periphery, extending above the internal limiting membrane into the vitreous. These neovascular tufts were most prominent on P17 to P19, but after P23 the neovascularization regressed, and the vascular pattern normalized by P26. To investigate mRNA expression of TSP-1 in retinal neovascularization, Northern blot analysis was performed using total RNA. Figure 1 ▶ shows a prominent increase in TSP-1 mRNA levels from P13 to P19. RNA from age-matched control animals raised in room air demonstrated a comparatively constant and low level of TSP-1 mRNA. The fold increase in TSP-1 mRNA at each time point compared with age-matched controls, after normalization to the 36B4 signal in each lane, showed a maximal 3.1-fold increase in TSP-1 mRNA on P15. To investigate mRNA expression of TSP-2 in retinal neovascularization, we also performed Northern blot analysis. However, TSP-2 mRNA was not detected (data not shown).
Figure 1.
TSP-1 mRNA expression during hypoxia and the development of neovascularization. Results of Northern blot analysis of total RNA (15 μg) isolated from animals after various durations of hypoxia and from age-matched normal controls. Northern blots and control 36B4 (top) and quantification (bottom). For these calculations, the amount of TSP-1 mRNA at each time point was first normalized to its own 36B4 signal. The fold increase over the normalized value for the corresponding age-matched normal control was then calculated. A maximal 3.1-fold increase of TSP-1 mRNA was observed on P15 compared with normal age-matched controls. Similar data were obtained from another Northern blot analysis (data not shown).
Localization of TSP-1 mRNA and Protein in Hypoxic Retina
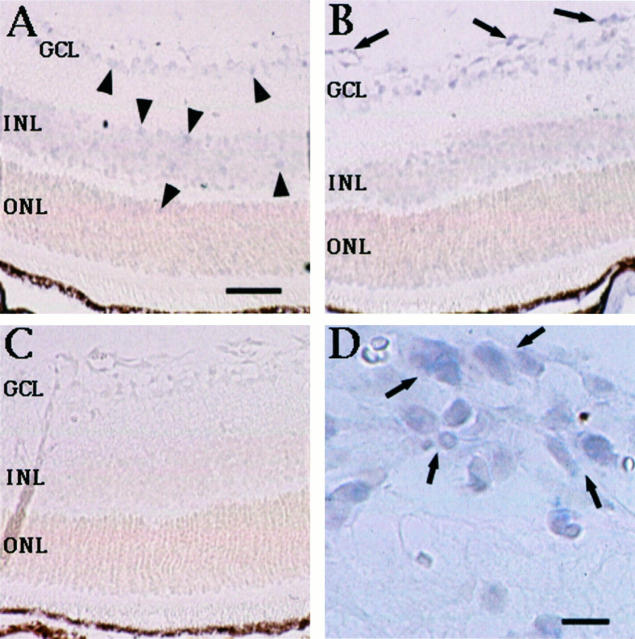
To determine the site of TSP-1 mRNA expression during the development of neovascularization, in situ hybridization was performed. TSP-1 mRNA was observed in vascular cells in retinas from both hypoxic P17 mice (Figure 2B) ▶ and normal control P17 mice (Figure 2A) ▶ . Weak TSP-1 signals were also detected in the ganglion cell layer and the inner and outer nuclear layers. No signal was seen in the negative control hybridized with sense probe (Figure 2C) ▶ . Analysis of the pattern of TSP-1 mRNA expression in vascular cells demonstrated that, in hypoxic retina, the signal of TSP-1 was increased both in intensity and in number of vessels involved near the avascular area, particularly at the neovascular tufts (Figure 2D) ▶ . To investigate mRNA localization of TSP-2 in retinal neovascularization, we also performed in situ hybridization. However, TSP-2 mRNA was not detected (data not shown).
Figure 2.
Distribution of TSP-1 mRNA during hypoxia and the development of neovascularization. TSP-1 mRNA was detected in the vascular cells in retinas from both hypoxic P17 mice (B) and normal control P17 mice (A). TSP-1 signals were also weakly detected in the ganglion cell layer (GCL), and the inner and outer nuclear layers (INL and ONL). No signal was seen in the negative control hybridized with sense probe (C). Analysis of the pattern of TSP-1 mRNA expression demonstrated that, in the hypoxic retina, the signal of TSP-1 was increased both in intensity and in number of vessels involved near the avascular area, particularly at the neovascular tufts (B and D, arrows). A, B, and C are at the same magnification: bar, 50 μm (A) and 10 μm (D).
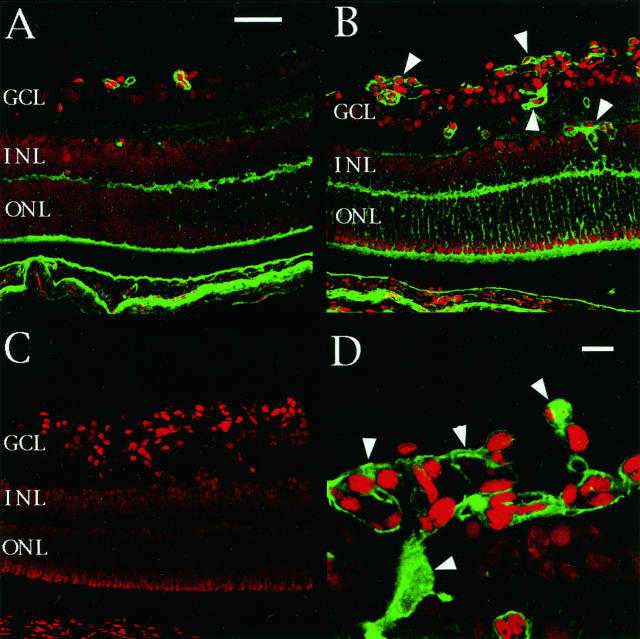
To investigate TSP-1 protein expression, indirect immunofluorescence was performed with confocal microscopy. Figure 3 ▶ demonstrates the distribution of TSP-1 protein, consistent with the results of the in situ hybridization studies described above. TSP-1 immunoreactivity was observed in vascular cells, the outer plexiform layer, and the photoreceptors of retinas from both hypoxic P17 mice (Figure 3B) ▶ and normal control P17 mice (Figure 3A) ▶ . TSP-1 protein was also weakly detected in the inner and outer nuclear layers. No TSP-1 immunoreactivity was seen in the negative control (Figure 3C) ▶ . The immunoreactivity of TSP-1 was increased both in intensity and in number of vessels involved near the avascular area, particularly at the neovascular tufts (Figure 3D) ▶ . In retinas from other time points, TSP-1 was expressed prominently in vascular cells near the avascular area and was observed in the same pattern as at P17 (data not shown).
Figure 3.
Localization of TSP-1 protein expression during hypoxia and the development of neovascularization. TSP-1 immunoreactivity was observed in the vascular cells, outer plexiform layer, and photoreceptors in retinas from both hypoxic P17 mice (B) and normal control P17 mice (A). TSP-1 protein was also weakly detected in the inner and outer nuclear layers (INL and ONL). No TSP-1 immunoreactivity was seen in the negative control (C). The immunoreactivity of TSP-1 was increased both in intensity and in number of vessels involved near the avascular area, particularly at the neovascular tufts (B and D, arrowheads). A, B, and C are at the same magnification: bar, 50 μm (A) and 10 μm (D).
VEGF Regulates TSP-1 Gene Expression in BRECs
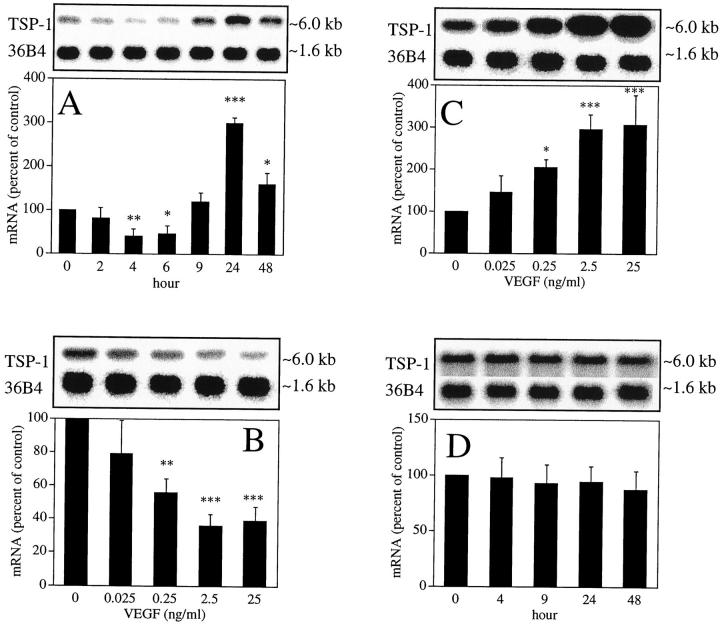
As VEGF is expressed prominently in our mouse model, 47 and has been suggested to play a central role in retinal neovascularization, 4,5 we determined gene regulation of TSP-1 by VEGF using Northern blot analysis in BRECs. As shown in Figure 4 ▶ >A,25 ng/ml VEGF stimulation decreased TSP-1 mRNA 0.41 ± 0.17-fold (P < 0.01) after 4 hours and increased after 9 hours, with a 3.0 ± 0.13-fold (P < 0.0001) peak response observed after 24 hours. To investigate whether VEGF would regulate mRNA expression of TSP-2 in BRECs, we also performed Northern blot analysis. However, TSP-2 mRNA was not detected (data not shown).
Figure 4.
TSP-1 mRNA expression by VEGF in BRECs. A: Time-dependent induction of TSP-1 mRNA after stimulation by 25 ng/ml VEGF. B: Dose-dependent decrease of TSP-1 mRNA 4 hours after stimulation by VEGF. C: Dose-dependent increase of TSP-1 mRNA 24 hours after stimulation by VEGF. D: Time-dependent changes of TSP-1 by hypoxia (1% oxygen). The size of the mRNAs corresponded to ∼6.0 kb (TSP-1) and ∼1.6 kb (36B4). Representative Northern blots and control 36B4 (top) and quantitation of multiple experiments after normalization to the control signals are shown (bottom). *P < 0.05; **P < 0.01; ***P < 0.001.
We also studied the dose-related response of VEGF-induced TSP-1 mRNA expression at 4 and 24 hours after VEGF stimulation. As shown in Figure 4B ▶ , levels of TSP-1 mRNAs were down-regulated in a dose-dependent manner 4 hours after VEGF stimulation, with significant decreases observed at concentrations as low as 0.25 ng/ml (0.57 ± 0.084 times, P < 0.01). Maximal decreases were observed at concentrations of 2.5 ng/ml (0.36 ± 0.069 times, P < 0.001). Figure 4C ▶ shows that TSP-1 mRNA was up-regulated in a dose-dependent manner 24 hours after VEGF stimulation, with significant increases observed at concentrations as low as 0.25 ng/ml (2.1 ± 0.19 times, P < 0.05). Maximal increases were observed at concentrations of 25 ng/ml (3.1 ± 0.72 times, P < 0.001).
To investigate whether hypoxic conditions affect TSP-1 and TSP-2 gene expression in BRECs, we performed Northern blot analysis using BRECs exposed to 1 ± 0.5% oxygen. As shown in Figure 4D ▶ , TSP-1 mRNA levels were not affected under hypoxic conditions. TSP-2 mRNA was not detected (data not shown).
Effects of VEGF on the Half-Life of TSP-1 mRNA in BRECs
To investigate whether the VEGF-induced TSP-1 mRNA level is mediated through the regulation of transcription or mRNA stability, we examined the effect of inhibition of de novo gene transcription. Northern blot analyses were performed after administration of actinomycin D (5 μg/ml) after 4 hours and 24 hours of stimulation of VEGF (25 ng/ml). The half-life of TSP-1 mRNA was 1.4 hours in unstimulated controls and 1.6 hours after 4 hours of treatment with 25 ng/ml VEGF (Figure 5A) ▶ . However, the half-life of TSP-1 mRNA was 1.4 hours in unstimulated controls and 2.5 hours after 24 hours of treatment with 25 ng/ml VEGF (Figure 5B) ▶ .
Figure 5.
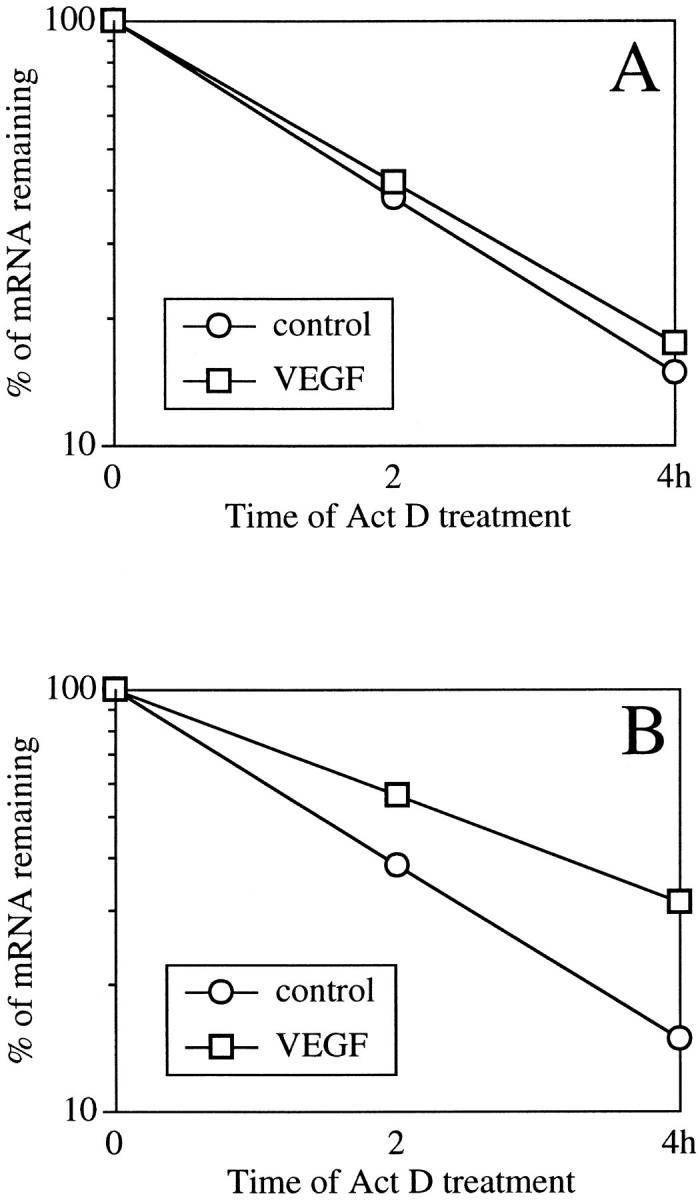
Decay of TSP-1 mRNA in the presence of actinomycin D in BRECs after 4 hours (A) and 24 hours (B) of treatment. The graphs show the decay of TSP-1 mRNA in the presence or absence of 25 ng/ml VEGF supplemented with 5 μg/ml actinomycin D. Total RNA was analyzed by Northern blotting, and the mRNA hybridization signals were quantified using densitometric scanning. The values in the graph indicate the percentage of initial TSP-1 mRNA signal remaining in the specific conditions.
Effects of Cycloheximide on TSP-1 mRNA Regulation in BRECs
We examined the possibility that VEGF regulates TSP-1 mRNA expression through new protein synthesis by treating BRECs for 4 and 24 hours with VEGF (25 ng/ml) and the protein synthesis inhibitor cycloheximide (10 μg/ml). Figure 6A ▶ shows that cycloheximide was unable to prevent the decrease of TSP-1 mRNA; treatment by both VEGF and cycloheximide decreased TSP-1 mRNA 42 ± 15% after 4 hours compared with cycloheximide alone (P < 0.01). However, Figure 6B ▶ shows that after 24 hours, the increase of TSP-1 mRNA was abolished by cycloheximide; treatment by both VEGF and cycloheximide decreased TSP-1 mRNA 32 ± 18% after 24 hours compared with cycloheximide alone (P < 0.05).
Figure 6.
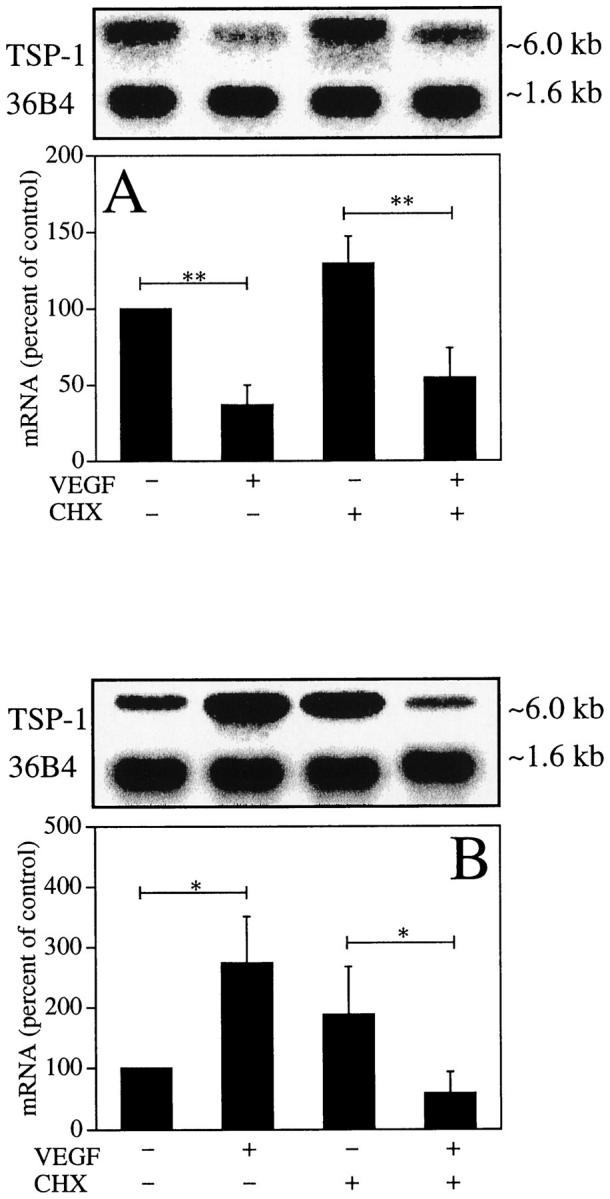
Effects of cycloheximide on TSP-1 mRNA regulation in BRECs at 4 hours (A) and 24 hours (B) of treatment. BRECs were incubated in the indicated conditions, after which total RNA was analyzed. Representative Northern blots and control 36B4 (top) and quantitation of multiple experiments after normalization to the control signal are shown (bottom). *P < 0.05; **P < 0.01.
VEGF Induction of TSP-1 Protein Production in BRECs
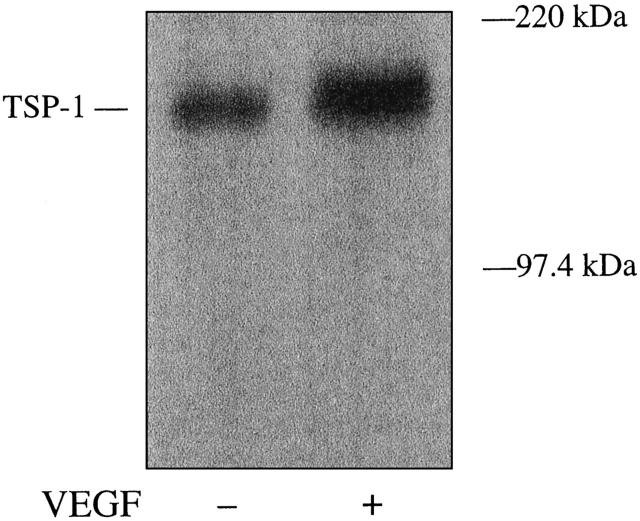
To determine whether VEGF regulation of TSP-1 mRNA is well correlated with its protein level, TSP-1 protein expression was assessed by Western blot analysis using anti-human TSP-1 antibody. As shown in Figure 7 ▶ , VEGF (25 ng/ml) increased the level of TSP-1 protein (∼150 kd) after 24 hours.
Figure 7.
Effect of VEGF on TSP-1 protein expression in BRECs. Confluent monolayers of BRECs in serum-free medium were studied after 24 hours, with and without 25 ng/ml VEGF. An equal amount of protein of each sample was subjected to 7.5% SDS gel electrophoresis and transferred to a nitrocellulose membrane. Signals were detected by Western blot analysis with anti-TSP-1 antibody. The size of the TSP-1 protein corresponded to ∼150 kd.
TSP-1 Has a Role in Inhibition of BREC Proliferation
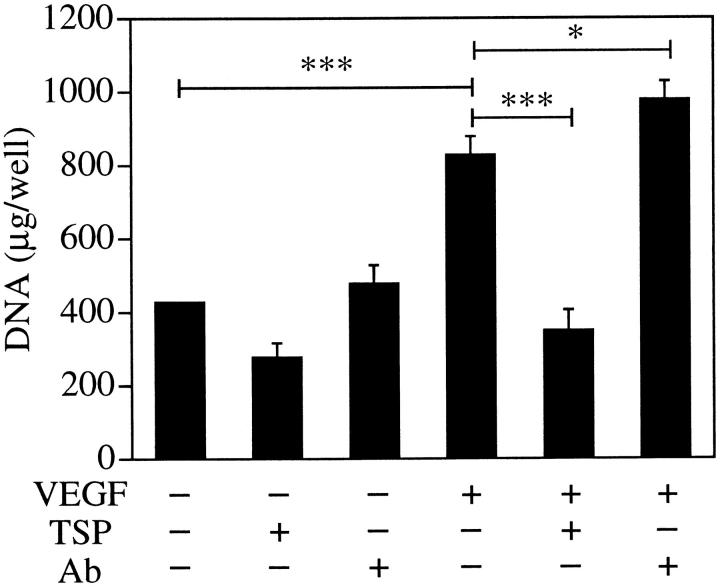
To investigate the effect of TSP-1 on VEGF-induced angiogenesis, we performed DNA content growth assay in BRECs. As shown in Figure 8 ▶ , stimulation with 25 ng/ml VEGF increased BREC growth 1.9 ± 0.12-fold (P < 0.001) in comparison with the unstimulated control. This VEGF-induced BREC proliferation was completely inhibited with exogenous TSP-1 (5 μg/ml, P < 0.001) and facilitated by 37.5% with anti-TSP-1 antibody (10 μg/ml, P < 0.05).
Figure 8.
Effect of TSP-1 on BREC growth. BRECs were plated sparsely on 24-well culture plates and incubated overnight. The cells were treated with vehicle, VEGF (25 ng/ml), human TSP-1 (5 μg/ml), anti-TSP-1 antibody (10 μg/ml), or combinations thereof. After incubation for 4 days at 37°C, the cells were lysed and the DNA content was measured. *P < 0.05; ***P < 0.001.
Discussion
TSP-1 is one of a small number of molecules found naturally in vertebrates that can inhibit angiogenesis. 23-31 Transfection of TSP-1 cDNA into human breast carcinoma cells and hemangioma cells reduces primary tumor growth and angiogenesis. 58,59 Moreover, TSP-1 specifically induces apoptosis of bovine aortic endothelial cells. 60 These characteristics of TSP-1 suggest it could play a potential role in modulating the retinal neovascularization induced by hypoxia that occurs in a variety of ischemic ocular diseases. We have now demonstrated an increased expression of TSP-1 in such ischemia-induced retinal neovascularization in vivo and have found that VEGF stimulates thrombospondin-1 expression in retinal microcapillary endothelial cells, which has an inhibitory effect on the cell growth of the endothelial cells in vitro.
We investigated the gene expression of TSP-1 in a mouse model of ischemia-induced retinal neovascularization. The mRNA levels of TSP-1 in the retina were greatly increased in eyes with neovascularization compared with the control eyes, and the pattern of time-dependent TSP-1 mRNA induction was similar to that of VEGF. 47 Pierce et al reported a dramatic increase in VEGF mRNA levels 12 hours after the experimental P12 mice were returned to room air. 47 The VEGF mRNA levels remained elevated for several days and then decreased toward baseline with regression of the retinopathy. In our study, TSP-1 mRNA levels increased prominently from P13 to P19, with a maximal 3.1-fold increase on P15. We also performed in situ hybridization and indirect immunofluorescence to localize TSP-1 expression in ischemic retinas. We observed increases of TSP-1 expression in both retinal and intravitreally growing vascular cells. This increase was more evident in neovascular cells growing into the vitreous, and the most marked expression was observed at the neovascular tufts adjacent to areas of retinal nonperfusion. Pericytes have been shown to be rarely associated with neovascular vessels. 61 In addition, we have observed that almost all cells are positively stained with von Willebrand factor in the neovascular cells growing into the vitreous, 62 and a previous study revealed that glial cells are absent from the neovascular tuft. 48 Although we have not identified the cell type expressing TSP-1 in the neovascular cells, these data might suggest that endothelial cells are the primary cells expressing TSP-1 in neovascular vessels. The expression of TSP-1 was up-regulated mainly in vascular cells; no remarkable up-regulation was observed in nonvascular cells in the neural retina. These data might suggest that angiogenic vascular cells in ischemic conditions express more TSP-1 than do static and normoxic vascular cells. In this mouse model, the induction of VEGF is closely associated with the development of neovascularization. 47 Inhibition of VEGF results in suppression of retinal neovascularization. 4,5 The VEGF induction is concentrated just posterior to the neovascular tufts. 63 This ischemic retinal neovascularization is thought to be mediated predominantly through hypoxic VEGF induction. We observed the pattern of time-dependent TSP-1 mRNA induction to be similar to that of VEGF, 47 and the proximity of the site of production of VEGF and TSP-1 was also similar. This might further support our hypothesis. In addition, not all vascular cells were positive for TSP-1. In the neural retina, the pattern of TSP-1 protein expression in nonvascular cells was not exactly the same as that of TSP-1 mRNA expression, probably because many types of nonvascular cells in the neural retina might express TSP-1 and have various translational regulation, transport, and degradation systems. To our knowledge, the mechanism of up-regulation of TSP-1 in hypoxic retinal vascular cells has not been investigated. Accordingly, we tested whether VEGF and hypoxia regulate TSP-1 mRNA expression in BRECs.
In our study, VEGF stimulation decreased TSP-1 mRNA after 4 hours and increased it after 9 hours, and the peak response was observed after 24 hours. We also observed a dose dependency in the VEGF-induced changes of TSP-1 expression 4 and 24 hours after VEGF stimulation. Significant changes were observed with doses of VEGF as low as 0.25 ng/ml, suggesting that regulation of TSP-1 is mediated through specific receptors for VEGF and that the potency with which VEGF induces TSP-1 expression is similar to its capacity to induce proliferation of BRECs. 57 The dose required (0.25 ng/ml) is well below that observed in primate models of retinal ischemia 64 and active human retinal ischemic diseases. 8 Although comparison between the data from in vitro studies and in vivo studies involves some extrapolation, VEGF is thought to have a remarkable effect on TSP-1 expression during neovascularization in ischemic retinal disorders. We also tested whether hypoxia directly regulates TSP-1 mRNA expression with 1% oxygen and 5% carbon dioxide atmosphere, the oxygen deficit having been induced by nitrogen replacement; TSP-1 mRNA expression was not affected. These data suggest that TSP-1 up-regulation in ischemic neovascular cells is not regulated directly by hypoxia.
To further investigate the regulation of TSP-1 by VEGF, we performed experiments using metabolic inhibitors: actinomycin D to inhibit RNA synthesis and cycloheximide to inhibit protein synthesis. The half-life of TSP-1 mRNA was unchanged after 4 hours of treatment with VEGF and was prolonged after 24 hours of treatment with VEGF. Cycloheximide was unable to prevent the decrease of TSP-1 mRNA after 4 hours, and the increase of TSP-1 mRNA after 24 hours was abolished by cycloheximide. These data suggest that the early TSP-1 mRNA decreases are mediated not through new protein synthesis but mainly through down-regulation of transcription and that delayed TSP-1 mRNA increases are mediated through new protein synthesis and up-regulation of mRNA stability.
The effects of VEGF are mediated through endothelial-cell-specific, high-affinity phosphotyrosine kinase receptors: Flt-1 (VEGFR1) and KDR/Flk-1 (VEGFR2). 65-68 In vitro studies have shown that KDR/Flk-1 is expressed predominantly in microvascular endothelial cells and that Flt-1 is expressed predominantly in pericytes; 69 thus, it is suggested that VEGF-induced TSP-1 expression in BRECs is mediated mainly through KDR/Flk-1. However, so many factors are involved in signal transduction pathways of Flt-1 and KDR/Flk-1, including PLC-γ, 70,71 PKC, 70,72,73 MAP kinase, 72,74 PI3 kinase, GAP, NcK, 71 Src, 75 and FAK, 73 that additional studies are necessary to elucidate the entire mechanism of VEGF-induced TSP-1 expression.
VEGF caused BRECs to express increased levels of mRNAs encoding TSP-1, which could lead to increased expression of TSP-1 protein at the extracellular spaces. We have observed increased TSP-1 protein expression at 150 kd by Western blot analysis of BRECs stimulated with VEGF. We also investigated the effects of TSP-1 and anti-TSP-1 antibody on BREC growth. VEGF-induced BREC proliferation was completely inhibited with exogenous TSP-1 and facilitated with anti-TSP-1 antibody. Fur- thermore, the antibody had no significant effect on unstimulated cells. These data might suggest an inhibitory effect of TSP-1 on VEGF-induced BREC proliferation and an inhibitory role of endogenous TSP-1 in VEGF-stimulated BRECs. The magnitude of the enhancement of BREC growth is, however, not remarkable, and additional studies, including studies using antisense oligonucleotides against TSP-1 or TSP-1 gene-manipulated cells, are necessary to clarify the role of endogenous TSP-1. DiPietro et al reported that chemotactic activity to basic fibroblast growth factor and capillary-like cord formation were increased in bovine aortic endothelial cells in which endogenous TSP-1 production was disrupted by stable transfection with a vector expressing a TSP-1 antisense RNA. 76 Likewise, in basic fibroblast growth factor, 77 TSP-1 might bind to VEGF to affect VEGF interaction with endothelial cells, activity, and association with the extracellular matrix. Together with our results on BREC proliferation, this suggests that TSP-1 present in the extracellular spaces might contribute to down-regulation of three main neovascular steps: proliferation, migration, and cord formation.
As well as TSP-1, TSP-2 is an extracellular matrix protein and has an inhibitory effect on angiogenesis. 29 Increased VEGF expression has been reported to coincide with decreased TSP-2 expression in cells in which tissue factor is overexpressed. 46 In the present study, we have determined the expression of TSP-2 in the mouse model and BREC system. In contrast to TSP-1, the expression of TSP-2 is so low as to be undetectable by similar Northern blot analysis and an in situ hybridization method used to detect TSP-1. Our results are comparable with previous studies. It has been reported that TSP-2 mRNA was not detected in poly(A) RNA from retina of avian embryo 78 and that TSP-2 mRNA was not detected by Northern blot analysis using total RNA from cultured bovine aortic endothelial cells. 17 Although quantitative polymerase chain reaction analysis or an RNA protection assay might detect changes in TSP-2 mRNA, these data probably suggest that the role of TSP-2 is not remarkable in the retinal neovascularization.
Based on the present study, VEGF decreases TSP-1 expression in the early phase but, in contrast, increases its expression in the late phase. Although the in vivo significance of the observed response is not certain, it is interesting to speculate that the physiological significance of the biphasic TSP-1 response may be to regulate VEGF-dependent neovascularization more tightly. Initial suppression of TSP-1 might facilitate initiation of neovascularization, whereas late induction of TSP-1 probably inhibits excessive neovascularization as a negative feedback mechanism. Taken together, the present findings suggest that, in ischemic retina, retinal microvascular cells, specifically those cells adjacent to the area of nonperfusion, up-regulate TSP-1 expression and that VEGF stimulates endogenous TSP-1 expression that down-regulates BREC growth. VEGF-induced angiogenesis might have a negative feedback mechanism mediated by TSP-1 induction.
Acknowledgments
We thank Ms. Hisako Okuda for her help with the histological techniques.
Footnotes
Address reprint requests to Dr. Hitoshi Takagi, Department of Ophthalmology and Visual Sciences, Kyoto University Graduate School of Medicine, Kyoto 606, Japan. E-mail; hitoshi@kuhp.kyoto-u.ac.jp.
Supported by a grant-in-aid for scientific research from the Ministry of Education, Science, and Culture of the Japanese Government and by the Japan National Society for the Prevention of Blindness.
References
- 1.Shweiki D, Itin A, Soffer D, Keshet E: Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 359:843-845 [DOI] [PubMed] [Google Scholar]
- 2.Berse B, Brown LF, Van-de-Water L, Dvorak HF, Senger DR: Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell 1992, 3:211-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N: Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246:1306-1309 [DOI] [PubMed] [Google Scholar]
- 4.Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE: Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA 1995, 92:10457-10461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson GS, Pierce EA, Rook SL, Foley E, Webb R, Smith LE: Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc Natl Acad Sci USA 1996, 93:4851-4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamis AP, Shima DT, Tolentino MJ, Gragoudas ES, Ferrara N, Folkman J, D’Amore PA, Miller JW: Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch Ophthalmol 1996, 114:66-71 [DOI] [PubMed] [Google Scholar]
- 7.Tolentino MJ, Miller JW, Gragoudas ES, Jakobiec FA, Flynn E, Chatzistefanou K, Ferrara N, Adamis AP: Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology 1996, 103:1820-1828 [DOI] [PubMed] [Google Scholar]
- 8.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et al: Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994, 331:1480-1487 [DOI] [PubMed] [Google Scholar]
- 9.Adamis AP, Miller JW, Bernal MT, D’Amico DJ, Folkman J, Yeo TK, Yeo KT: Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 1994, 118:445-450 [DOI] [PubMed] [Google Scholar]
- 10.Pe’er J, Folberg R, Itin A, Gnessin H, Hemo I, Keshet E: Upregulated expression of vascular endothelial growth factor in proliferative diabetic retinopathy. Br J Ophthalmol 1996, 80:241-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR: Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci 1996, 37:855-868 [PubMed] [Google Scholar]
- 12.Lawler JW, Slayter HS, Coligan JE: Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J Biol Chem 1978, 253:8609-8616 [PubMed] [Google Scholar]
- 13.McPherson J, Sage H, Bornstein P: Isolation and characterization of a glycoprotein secreted by aortic endothelial cells in culture; apparent identity with platelet thrombospondin. J Biol Chem 1981, 256:11330-11336 [PubMed] [Google Scholar]
- 14.Raugi GJ, Mumby SM, Abbott-Brown D, Bornstein P: Thrombospondin: synthesis and secretion by cells in culture. J Cell Biol 1982, 95:351-354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffe EA, Ruggiero JT, Leung LK, Doyle MJ, McKeown-Longo PJ, Mosher DF: Cultured human fibroblasts synthesize and secrete thrombospondin and incorporate it into extracellular matrix. Proc Natl Acad Sci USA 1983, 80:998-1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaffe EA, Ruggiero JT, Falcone DJ: Monocytes and macrophages synthesize and secrete thrombospondin. Blood 1985, 65:79-84 [PubMed] [Google Scholar]
- 17.Reed MJ, Iruela-Arispe L, O’Brien ER, Truong T, LaBell T, Bornstein P, Sage EH: Expression of thrombospondins by endothelial cells. Injury is correlated with TSP-1. Am J Pathol 1995, 147:1068-1080 [PMC free article] [PubMed] [Google Scholar]
- 18.Majack RA, Cook SC, Bornstein P: Platelet-derived growth factor and heparin-like glycosaminoglycans regulate thrombospondin synthesis and deposition in the matrix by smooth muscle cells. J Cell Biol 1985, 101:1059-1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majack RA, Cook SC, Bornstein P: Control of smooth muscle cell growth by components of the extracellular matrix: autocrine role for thrombospondin. Proc Natl Acad Sci USA 1986, 83:9050-9054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majack RA, Goodman LV, Dixit VM: Cell surface thrombospondin is functionally essential for vascular smooth muscle cell proliferation. J Cell Biol 1988, 106:415-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phan SH, Dillon RG, McGarry BM, Dixit VM: Stimulation of fibroblast proliferation by thrombospondin. Biochem Biophys Res Commun 1989, 163:56-63 [DOI] [PubMed] [Google Scholar]
- 22.Castle VP, Ou X, O’Rourke K, Dixit VM: High level thrombospondin 1 expression in two NIH 3T3 cloned lines confers serum- and anchorage-independent growth. J Biol Chem 1993, 268:2899-2903 [PubMed] [Google Scholar]
- 23.Rastinejad F, Polverini PJ, Bouck NP: Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell 1989, 56:345-355 [DOI] [PubMed] [Google Scholar]
- 24.Good DJ, Polverini PJ, Rastinejad F, Le-Beau MM, Lemons RS, Frazier WA, Bouck NP: A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA 1990, 87:6624-6628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bagavandoss P, Wilks JW: Specific inhibition of endothelial cell proliferation by thrombospondin. Biochem Biophys Res Commun 1990, 170:867-872 [DOI] [PubMed] [Google Scholar]
- 26.Iruela-Arispe ML, Bornstein P, Sage H: Thrombospondin exerts an antiangiogenic effect on cord formation by endothelial cells in vitro. Proc Natl Acad Sci USA 1991, 88:5026-5030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taraboletti G, Roberts D, Liotta LA, Giavazzi R: Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol 1990, 111:765-772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N: Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol 1993, 122:497-511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volpert OV, Tolsma SS, Pellerin S, Feige JJ, Chen H, Mosher DF, Bouck N: Inhibition of angiogenesis by thrombospondin-2. Biochem Biophys Res Commun 1995, 217:326-332 [DOI] [PubMed] [Google Scholar]
- 30.Tolsma SS, Stack MS, Bouck N: Lumen formation and other angiogenic activities of cultured capillary endothelial cells are inhibited by thrombospondin-1. Microvasc Res 1997, 54:13-26 [DOI] [PubMed] [Google Scholar]
- 31.Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP: CD36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol 1997, 138:707-717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuszynski GP, Rothman V, Murphy A, Siegler K, Smith L, Smith S, Karczewski J, Knudsen KA: Thrombospondin promotes cell-substratum adhesion. Science 1987, 236:1570-1573 [DOI] [PubMed] [Google Scholar]
- 33.Roberts DD, Sherwood JA, Ginsburg V: Platelet thrombospondin mediates attachment and spreading of human melanoma cells. J Cell Biol 1987, 104:131-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawler J, Weinstein R, Hynes RO: Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol 1988, 107:2351-2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo NH, Krutzsch HC, Negre E, Zabrenetzky VS, Roberts DD: Heparin-binding peptides from the type I repeats of thrombospondin: structural requirements for heparin binding and promotion of melanoma cell adhesion and chemotaxis. J Biol Chem 1992, 267:19349-19355 [PubMed] [Google Scholar]
- 36.Adams JC, Lawler J: Cell-type specific adhesive interactions of skeletal myoblasts with thrombospondin-1. Mol Biol Cell 1994, 5:423-437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy-Ullrich JE, Hook M: Thrombospondin modulates focal adhesions in endothelial cells. J Cell Biol 1989, 109:1309-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy-Ullrich JE, Schultz-Cherry S, Hook M: Transforming growth factor-β complexes with thrombospondin. Mol Biol Cell 1992, 3:181-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz-Cherry S, Lawler J, Murphy-Ullrich JE: The type 1 repeats of thrombospondin 1 activate latent transforming growth factor-β. J Biol Chem 1994, 269:26783-26788 [PubMed] [Google Scholar]
- 40.Majack RA, Mildbrandt J, Dixit VM: Induction of thrombospondin messenger RNA levels occurs as an immediate primary response to platelet-derived growth factor. J Biol Chem 1987, 262:8821-8825 [PubMed] [Google Scholar]
- 41.Donoviel DB, Amacher SL, Judge KW, Bornstein P: Thrombospondin gene expression is associated with mitogenesis in 3T3 cells: induction by basic fibroblast growth factor. J Cell Physiol 1990, 145:16-23 [DOI] [PubMed] [Google Scholar]
- 42.Janat MF, Liau G: Transforming growth factor β1 is a powerful modulator of platelet-derived growth factor action in vascular smooth muscle cells. J Cell Physiol 1992, 150:232-242 [DOI] [PubMed] [Google Scholar]
- 43.Framson P, Bornstein P: A serum response element and a binding site for NF-Y mediate the serum response of the human thrombospondin 1 gene. J Biol Chem 1993, 268:4989-4996 [PubMed] [Google Scholar]
- 44.Dameron KM, Volpert OV, Tainsky MA, Bouck N: Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science 1994, 265:1582-1584 [DOI] [PubMed] [Google Scholar]
- 45.Volpert OV, Dameron KM, Bouck N: Sequential development of an angiogenic phenotype by human fibroblasts progressing to tumorigenicity. Oncogene 1997, 14:1495-1502 [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Deng Y, Luther T, Muller M, Ziegler R, Waldherr R, Stern DM, Nawroth PP: Tissue factor controls the balance of angiogenic and antiangiogenic properties of tumor cells in mice. J Clin Invest 1994, 94:1320-1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE: Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA 1995, 92:905-909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA: Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994, 35:101-111 [PubMed] [Google Scholar]
- 49.Kobayashi S, Eden-McCutchan F, Framson P, Bornstein P: Partial amino acid sequence of human thrombospondin as determined by analysis of cDNA clones: homology to malarial circumsporozoite proteins. Biochemistry 1986, 25:8418-8425 [DOI] [PubMed] [Google Scholar]
- 50.Laherty CD, O’Rourke K, Wolf FW, Katz R, Seldin MF, Dixit VM: Characterization of mouse thrombospondin 2 sequence and expression during cell growth and development. J Biol Chem 1992, 267:3274-3281 [PubMed] [Google Scholar]
- 51.Cox KH, DeLeon DV, Angerer LM, Angerer RC: Detection of mRNAs in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol 1984, 101:485-502 [DOI] [PubMed] [Google Scholar]
- 52.Ohtani H, Kuroiwa A, Obinata M, Ooshima A, Nagura H: Identification of type I collagen-producing cells in human gastrointestinal carcinomas by non-radioactive in situ hybridization and immunoelectron microscopy. J Histochem Cytochem 1992, 40:1139-1146 [DOI] [PubMed] [Google Scholar]
- 53.Tautz D, Pfeifle C: A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma 1989, 98:81-85 [DOI] [PubMed] [Google Scholar]
- 54.Takagi H, King GL, Robinson GS, Ferrara N, Aiello LP: Adenosine mediates hypoxic induction of vascular endothelial growth factor in retinal pericytes and endothelial cells. Invest Ophthalmol Vis Sci 1996, 37:2165-2176 [PubMed] [Google Scholar]
- 55.King GL, Goodman AD, Buzney S, Moses A, Kahn CR: Receptors and growth-promoting effects of insulin and insulinlike growth factors on cells from bovine retinal capillaries and aorta. J Clin Invest 1985, 75:1028-1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang P, Pardee AB: Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science 1992, 257:967-971 [DOI] [PubMed] [Google Scholar]
- 57.Thieme H, Aiello LP, Takagi H, Ferrara N, King GL: Comparative analysis of vascular endothelial growth factor receptors on retinal and aortic vascular endothelial cells. Diabetes 1995, 44:98-103 [DOI] [PubMed] [Google Scholar]
- 58.Weinstat-Saslow DL, Zabrenetzky VS, VanHoutte K, Frazier WA, Roberts DD, Steeg PS: Transfection of thrombospondin 1 complementary DNA into a human breast carcinoma cell line reduces primary tumor growth, metastatic potential, and angiogenesis. Cancer Res 1994, 54:6504-6411 [PubMed] [Google Scholar]
- 59.Sheibani N, Frazier WA: Thrombospondin 1 expression in transformed endothelial cells restores a normal phenotype and suppresses their tumorigenesis. Proc Natl Acad Sci USA 1995, 92:6788-6792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo N, Krutzsch HC, Inman JK, Roberts DD: Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res 1997, 57:1735-1742 [PubMed] [Google Scholar]
- 61.Diaz-Flores L, Gutierrez R, Varela H: Angiogenesis: an update. Histol Histopathol 1994, 9:807-843 [PubMed] [Google Scholar]
- 62.Suzuma K, Takagi H, Otani A, Suzuma I, Honda Y: Increased expression of KDR/Flk-1 (VEGFR-2) in murine model of ischemia-induced retinal neovascularization. Microvasc Res 1998, 56:183-191 [DOI] [PubMed] [Google Scholar]
- 63.Pierce EA, Foley ED, Smith LE: Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol 1996, 114:1219-1228 [DOI] [PubMed] [Google Scholar]
- 64.Miller JW, Adamis AP, Shima DT, D’Amore PA, Moulton RS, O’Reilly MS, Folkman J, Dvorak HF, Brown LF, Berse B, et al: Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol 1994, 145:574-584 [PMC free article] [PubMed] [Google Scholar]
- 65.de-Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT: The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992, 255:989-991 [DOI] [PubMed] [Google Scholar]
- 66.Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A: High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 1993, 72:835-846 [DOI] [PubMed] [Google Scholar]
- 67.Quinn TP, Peters KG, De-Vries C, Ferrara N, Williams LT: Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci USA 1993, 90:7533-7537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferrara N, Houck K, Jakeman L, Leung DW: Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev 1992, 13:18-32 [DOI] [PubMed] [Google Scholar]
- 69.Takagi H, King GL, Aiello LP: Identification and characterization of vascular endothelial growth factor receptor (Flt) in bovine retinal pericytes. Diabetes 1996, 45:1016-1023 [DOI] [PubMed] [Google Scholar]
- 70.Xia P, Aiello LP, Ishii H, Jiang ZY, Park DJ, Robinson GS, Takagi H, Newsome WP, Jirousek MR, King GL: Characterization of vascular endothelial growth factor’s effect on the activation of protein kinase C, its isoforms, and endothelial cell growth. J Clin Invest 1996, 98:2018-2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo D, Jia Q, Song HY, Warren RS, Donner DB: Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains: association with endothelial cell proliferation. J Biol Chem 1995, 270:6729-6733 [DOI] [PubMed] [Google Scholar]
- 72.Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla TA, Ways K, Jirousek M, Smith LE, King GL: Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective β-isoform-selective inhibitor. Diabetes 1997, 46:1473-1480 [DOI] [PubMed] [Google Scholar]
- 73.Abedi H, Zachary I: Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem 1997, 272:15442-15451 [DOI] [PubMed] [Google Scholar]
- 74.Seetharam L, Gotoh N, Maru Y, Neufeld G, Yamaguchi S, Shibuya M: A unique signal transduction from FLT tyrosine kinase, a receptor for vascular endothelial growth factor VEGF. Oncogene 1995, 10:135-147 [PubMed] [Google Scholar]
- 75.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH: Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem 1994, 269:26988-26995 [PubMed] [Google Scholar]
- 76.DiPietro LA, Nebgen DR, Polverini PJ: Downregulation of endothelial cell thrombospondin 1 enhances in vitro angiogenesis. J Vasc Res 1994, 31:178-185 [DOI] [PubMed] [Google Scholar]
- 77.Taraboletti G, Belotti D, Borsotti P, Vergani V, Rusnati M, Presta M, Giavazzi R: The 140-kilodalton antiangiogenic fragment of thrombospondin-1 binds to basic fibroblast growth factor. Cell Growth Differ 1997, 8:471-479 [PubMed] [Google Scholar]
- 78.Tucker RP: The in situ localization of tenascin splice variants and thrombospondin 2 mRNA in the avian embryo. Development 1993, 117:347-358 [DOI] [PubMed] [Google Scholar]