Abstract
Renal inflammatory conditions are characterized by mononuclear cell recruitment to sites of inflammation. We have developed a modified Stamper-Woodruff assay system to analyze mechanisms of functional T cell adhesion to cryostat sections of renal biopsy material from patients with vasculitic glomerulonephritis (GN) and acute allograft rejection. Peripheral blood T cells adhered to intraglomerular, periglomerular, and tubulointerstitial regions of the cortex. Blocking monoclonal antibodies against tissue expressed ICAM-1, VCAM-1, and the CS-1 domain of fibronectin (CS-1Fn) differentially attenuated T cell adhesion. Glomerular adhesion in vasculitic GN and tubulointerstitial adhesion in acute rejection were particularly sensitive to both anti-ICAM-1 and anti-VCAM-1 antibodies, indicating a prominent role for ICAM-1 and VCAM-1 at glomerular sites in vasculitis and at tubulointerstitial sites in rejection. Furthermore, using KL/4 cells (LFA-1 expressing) and Jurkat cells (VLA-4 expressing), we demonstrated specific LFA-1/ICAM-1- and VLA-4/VCAM-1-mediated interactions within glomerular and tubulointerstitial compartments. Jurkat cells also adhered to VCAM-1-free sites, and binding was inhibitable by anti-CS-1Fn antibody, thereby demonstrating a role for VLA-4/fibronectin interactions especially at intraglomerular sites in acute rejection where VCAM-1 is notably absent. We therefore propose a prominent functional role for ICAM-1, VCAM-1, and CS-1 domain fibronectin in T cell recruitment to the inflamed kidney.
Leukocyte infiltration is a key event in the pathogenesis of renal inflammatory states such as vasculitic glomerulonephritis (GN) and acute allograft rejection. 1,2 The migration of T lymphocytes to inflammatory sites is dependent on coordinated leukocyte-endothelial cell interactions. 3 In vasculitic GN, glomerular capillary thrombosis with characteristic focal segmental necrotizing lesions progresses to a proliferative crescentic glomerulonephritis. There is associated T lymphocyte infiltration in intraglomerular, periglomerular, and tubulointerstitial regions. 4,5 In contrast, in acute allograft rejection, T cell infiltrates are localized to the interstitium and peritubular areas and typically spare the glomerulus. 6,7
Leukocyte adhesion is an early event for the subsequent migration into extravascular tissues. 8 T lymphocyte interactions are mediated by the β1 integrin VLA-4 (α4β1) and the β2 integrin LFA-1 (CD11a/CD18). Integrins can convert between low- and high-affinity states, depending on the local environment (ie, presence of cytokines and chemokines), to mediate leukocyte adhesion. 9 LFA-1 interacts with tissue-expressed ICAM-1, whereas VLA-4 can interact with both VCAM-1 and an alternatively spliced 25-mer CS-1-peptide-containing isoform of the extracellular matrix glycoprotein fibronectin (CS-1Fn). 10
VCAM-1 and ICAM-1 are constitutively present in the normal kidney. There is distinct VCAM-1 expression on the glomerular parietal epithelium and also on occasional tubular epithelial cells, whereas ICAM-1 expression is prominent in the glomeruli and sparse in peritubular regions. In vasculitic GN and acute allograft rejection, both ICAM-1 and VCAM-1 are heavily expressed in peritubular regions and on tubular epithelial cells 11-14 ; however, glomerular expression of VCAM-1 associated with areas of segmental necrosis is a discerning feature of vasculitic GN. 15,16 Increased expression of fibronectin has been reported in experimental immune complex glomerulonephritis and rejecting renal allografts. 17-19
Here, we describe the first study using peripheral blood T lymphocytes in a modified Stamper-Woodruff adhesion assay 20 to investigate the functional roles of ICAM-1, VCAM-1, and CS-1Fn in human renal inflammatory states. In previous studies, this technique has been applied to demonstrate adhesion molecule interactions in a variety of inflammatory conditions, including ICAM-1-dependent T cell adhesion in a rat model of acute renal allograft rejection, 21 VCAM-1- and ICAM-1-mediated adhesion of T cell and macrophage-derived cell lines in murine lupus nephritis, 22 CS-1Fn-mediated T cell adhesion in rheumatoid arthritis, 23 ICAM-1- and VCAM-1-dependent T cell adhesion in cervical intraepithelial neoplasia, 24 VCAM-1-mediated monocyte adhesion in simian encephalitis, 25 and P-selectin-dependent eosinophil adhesion in asthma and nasal polyposis, 26 and a role for multiple adhesion molecules in mediating monocyte adhesion to human atherosclerotic plaques. 27
We have studied the adhesive properties of peripheral blood T lymphocytes to renal biopsies taken from patients with vasculitic GN and acute allograft rejection. Furthermore, we have used an LFA-1-expressing KL/4 cell line 28 and a VLA-4-expressing Jurkat cell line in this assay system to demonstrate differential roles of ICAM-1/LFA-1-, VCAM-1/VLA-4-, and CS-1Fn/VLA-4-mediated interactions within the kidney in these disease states.
Materials and Methods
Tissue Collection and Preparation
Renal biopsy specimens from 18 patients with anti-neutrophil cytoplasmic autoantibody (ANCA)-positive vasculitis (Wegener’s granulomatosis or microscopic polyangiitis with appearance of segmental glomerular lesions) and 18 patients with acute cellular allograft rejection (scoring 4(IA) to 4(IIA) on the 1997 Banff diagnostic categorization system) were used in these studies. Studies with leukocytic cell lines were performed on biopsies from 8 patients with vasculitis and 7 patients with acute rejection, whereas studies with peripheral blood T lymphocytes were performed on an additional 10 vasculitis and 11 acute rejection patient biopsies. Each biopsy was embedded in Brights cryo-m-bed (Merck, Dorset, UK), snap-frozen in liquid nitrogen, and stored at −70°C. Control tissue comprised cortical fragments from the unaffected pole of five kidneys removed for renal carcinoma. Serial 7 to 10-μm cryostat sections collected onto Superfrost Plus slides (Merck), air dried and fixed in acetone (Merck) for 10 minutes, were used in all subsequent studies. Approval for the study of human material was obtained from the local research ethics committee.
Cell Culture
A Jurkat human T lymphoblastoid cell line (a gift from the Department of Cancer Studies, University of Birmingham) and the K562 human erythroleukemic cell line transfected with LFA-1, KL/4 (a gift from Dr. M. Robinson, Celltech, Slough, UK) were used in these studies. Both cell lines were maintained in RPMI-1640 supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mmol/L glutamine. For the KL/4 cells the medium was additionally supplemented with 0.2 mg/ml G418 (GIBCO, Paisley, UK).
Antibodies
The following mouse monoclonal antibodies were used: anti-human ICAM-1, RR1 (a gift from Dr. R. Rothlein; Boehringer-Ingelheim R&D, Ridgefield, CT); anti-human VCAM-1 cell culture supernatant, Ig11 (a gift from Professor D. Haskard; Imperial College, London, UK); anti-human VCAM-1, BBIG-VI (R&D Sytems, Oxon, UK); anti-human CD11a, BCA1 (R&D Systems); anti-human VLA-4, Max68, and anti-human LFA-1, KIM185 (gifts from Dr. M. Robinson); anti-human CS-1Fn cell culture supernatant, 90.45 (gift from Dr. M. Elices; Cytel Corp. San Diego, CA); and anti-human CD3 (DAKO, High Wycombe, UK) conjugated to horseradish peroxidase (HRP; conjugate synthesized within the laboratory). All monoclonal antibodies to adhesion molecules were blocking except KIM185 (β2 integrin activating antibody). RR1 and BBIG-VI were suitable for immunohistochemistry. We also used biotinylated rabbit anti-mouse IgG (DAKO) and HRP-conjugated streptavidin ABC complex (DAKO). Irrelevant mouse IgG (Serotec, Oxford, UK) and mouse IgM (Binding Site, Birmingham, UK) were used as controls.
Tissue Preparation
All incubations were performed at room temperature in a humidified box. Tissue endogenous peroxidase activity was blocked by treating the sections with Tris-buffered saline (TBS; pH 7.4) containing 0.3% H2O2 and 0.1% NaN3, for 10 minutes.
Immunohistochemistry
The sections were treated sequentially with 0.1% avidin and 0.01% biotin to block endogenous biotin, followed by a 10-minute blocking stage with 10% rabbit serum. Three-stage indirect immunohistochemistry was performed with primary anti-human monoclonal antibodies (ICAM-1 (RR1) or VCAM-1 (BBIG-VI)), a biotin-labeled rabbit anti-mouse secondary antibody and streptavidin ABC/HRP complex. Binding of the tertiary complex was visualized by the addition of 3,3′-diaminobenzidine (DAB; Sigma, Dorset, UK). The sections were counterstained with Mayer’s hematoxylin (Merck), mounted in dibutyl polystyrene xylene (DPX) (Merck), and coverslipped.
Adhesion Studies
Before the adhesion assay, cells in suspension were stimulated (peripheral blood T lymphocytes and Jurkat cells with 1 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma) and KL/4 cells with 25 μg/ml KIM185 for 30 minutes at 37°C. Acetone-fixed serial sections and preactivated cell suspensions were co-incubated in an agitated system in the presence of irrelevant control antibody or blocking monoclonal antibody. The binding of peripheral blood T lymphocytes isolated from patients showing evidence of acute rejection to their own biopsies was assesed. Due to the lower incidence of vasculitis as compared with acute rejection and the limited availability of blood before initiation of therapy in patients with vasculitis, T lymphocytes obtained from control volunteers were used to address peripheral blood T cell adhesion processes in vasculitic GN. We also investigated specific LFA-1- and VLA-4-mediated interactions using an LFA-1-transfected KL/4 cell line and a VLA-4-expressing Jurkat cell line, respectively. The cell numbers (see below) used in the studies were carefully optimized to delineate specific localization of adherent cells while obtaining an appreciable level of adhesion to determine the effect of blocking antibodies.
Peripheral Blood T Lymphocyte Adhesion
Peripheral blood from patients with acute cellular allograft rejection and control volunteers was collected and heparinized. Peripheral blood mononuclear cells were isolated by centrifugation over Ficoll/Hypaque. Mononuclear cells were collected from the interface, washed, and incubated in RPMI-1640 with 10% FCS in a petri dish (20 × 10 6 cells in 10 ml per dish) for 1 hour at 37°C in 5% CO2. T lymphocytes (nonadherent cells) were collected by gentle washing with assay medium.
Preactivated T lymphocytes suspended at 5 × 10 6 cells per ml were overlaid on the tissue sections in a volume of 100 μl and incubated on a rotating platform at 70 revolutions per min (rpm) for 30 minutes at room temperature. Nonadherent cells were washed off with cold TBS, and sections were fixed in acetone for 10 minutes. Bound cells were labeled with the anti-CD3 HRP conjugate, and the cells were visualized by developing their peroxidase activity with DAB for 30 minutes. The sections were counterstained with Mayer’s hematoxylin, mounted in DPX, and coverslipped.
KL/4 Cell Adhesion
Preactivated KL/4 cells, suspended at 5 × 10 6 cells per ml in RPMI-1640 containing 10% FCS, were overlaid on tissue sections in a volume of 100 μl and incubated on a rotating platform at 70 rpm for 30 minutes at 37°C. The slides were then washed in cold TBS to remove any nonadherent cells. Adherent cells were fixed in acetone for 10 minutes. Bound KL/4 cells were visualized by incubation with DAB for 30 minutes, using the endogenous peroxidase activity of K562 cells. The sections were counterstained with Mayer’s hematoxylin, mounted in DPX, and coverslipped.
Jurkat Cell Adhesion
Preactivated Jurkat cells suspended at 5 × 10 6 cells per ml in RPMI-1640 containing 10% FCS were overlaid on the tissue sections in a volume of 100 μl and incubated on a rotating platform at 70 rpm for 30 minutes at room temperature. Nonadherent cells were washed off with cold TBS, and sections were fixed in acetone for 10 minutes. Bound cells were labeled with the anti-CD3 HRP conjugate, and the cells were visualized by developing their peroxidase activity with DAB for 30 minutes. The sections were counterstained with Mayer’s hematoxylin, mounted in DPX, and coverslipped.
Blocking Studies
Before the co-incubation, serial sections and the cell suspension were incubated for 30 minutes with neutralizing antibodies directed against VCAM-1 and ICAM-1 at room temperature and LFA-1 or VLA-4 at 37°C. In preliminary studies, a concentration of 20 μg/ml consistently provided maximal blocking for all antibodies tested.
Combined Tissue Adhesion Assay and Immunohistochemistry
To co-localize leukocyte adhesion and tissue adhesion molecule expression, after the adhesion assay, sections were fixed in acetone for 10 minutes and stained for VCAM-1 and ICAM-1 using the immunoperoxidase staining technique described above. This order of performing the combined assay precluded any intervention by the antibodies in the initial adhesion assay.
Data Analysis
We analyzed glomerular and tubulointerstitial adhesion in turn. For each section the number of adherent cells in 5 × 625-μm 2 fields of tubulointerstitium were counted using a graticule and expressed as number of adherent cells per field. The total number of glomeruli in each section was counted, and the total number of adherent cells on the glomerular tuft and the parietal epithelium was expressed as number of adherent cells per glomerulus.
Data are quoted as mean and standard error of mean (mean ± SEM). Significance of differences in adhesion between control and neutralizing antibodies was determined using a one-tailed two-sample t-test. Nonparametric statistical analyses using the Mann-Whitney two-sample test confirmed the results of the t-test. Statistical advice was supplied by Dr. Roger L. Holder from the Department of Mathematics and Statistics at the University of Birmingham.
Results
Immunohistochemical Studies
Immunohistochemical (IHC) analysis confirmed the presence and up-regulation of the tissue ligands VCAM-1 and ICAM-1 in both disease states. In both vasculitic GN and acute rejection there was marked VCAM-1 expression in the parietal epithelium and tubular epithelium and at peritubular regions of the interstitium. In addition, there was distinct intraglomerular expression of VCAM-1 in vasculitic GN, which was notably absent in acute allograft rejection (Figure 1, A and B) ▶ .
Figure 1.
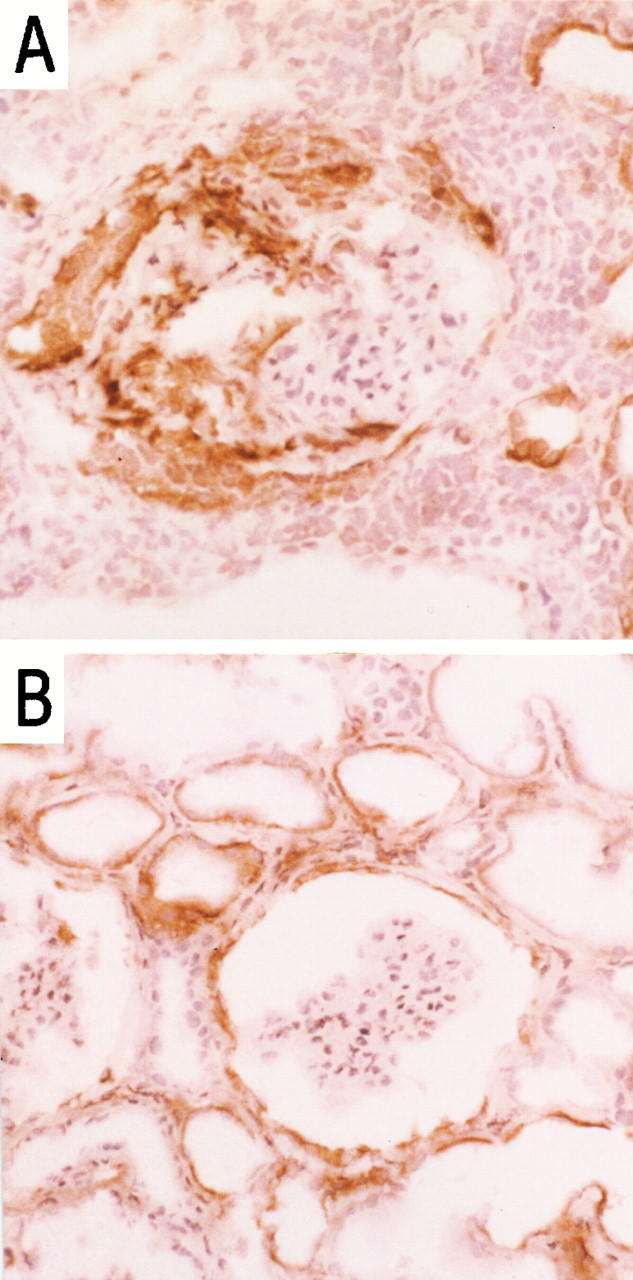
Immunohistochemical localization of VCAM-1 in vasculitic GN (A) and acute rejection (B) in sections of renal biopsies. Magnification, ×50.
ICAM-1 was up-regulated in both disease states with heavy glomerular, peritubular, and tubular positivity (Figure 2, A and B) ▶ .
Figure 2.
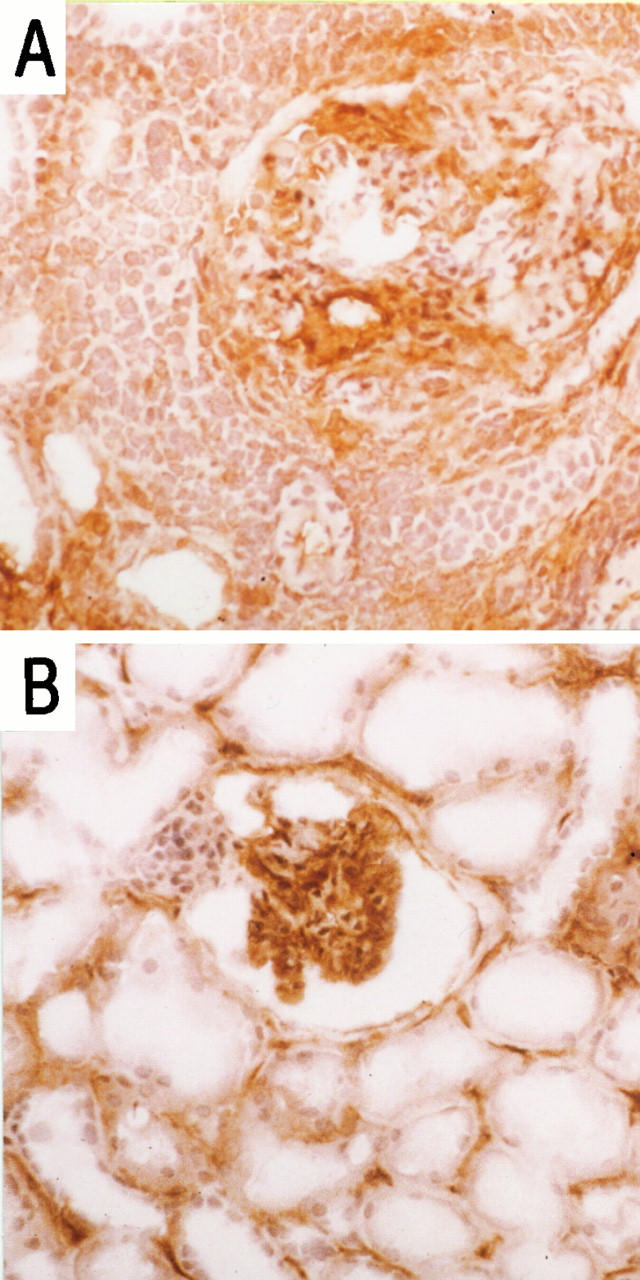
Immunohistochemical localization of ICAM-1 in vasculitic GN (A) and acute rejection (B) in sections of renal biopsies. Magnification, ×50.
Adhesion Studies
Peripheral Blood T Lymphocyte Adhesion in Vasculitic Glomerulonephritis
Adhesion of resting T lymphocytes isolated from peripheral blood to cryostat sections of renal biopsy material was sparse at both glomerular and tubulointerstitial sites. Hence, T cell integrin molecule activation with PMA was necessary to achieve optimal levels of adhesion and evaluate the inhibitory effects of blocking monoclonal antibodies. T lymphocytes did not adhere to normal tissue sections irrespective of their activation state.
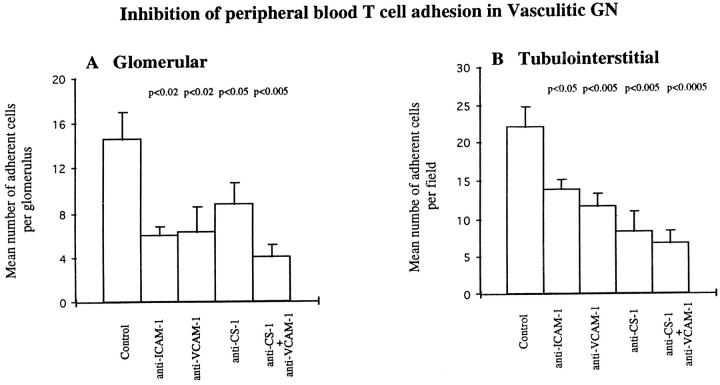
T lymphocyte adhesion at glomerular and tubulointerstitial sites was differentially attenuated using the anti-ICAM-1 monoclonal antibody (Figure 3) ▶ . Inhibition at glomerular sites was greater than at tubulointerstitial sites, reducing T cell adhesion by 58% (from 14.55 ± 2.4 to 6 ± 0.79) compared with 37% (from 22.1 ± 2.67 to 13.76 ± 1.4), respectively (Figure 4) ▶ . In additional studies, the combination of anti-LFA-1 with anti-ICAM-1 had no effect over and above those seen with anti-ICAM-1 alone. Inhibitory effects of anti-VCAM-1 monoclonal antibody were also more prominent within the glomerular compartment. Glomerular T cell adhesion was reduced by 56% (from 14.55 ± 2.4 to 6.31 ± 2.29) as compared with 47% (from 22.1 ± 2.67 to 11.7 ± 1.56) at tubulointerstitial sites (Figures 4 and 5) ▶ ▶ . In additional studies using biopsies from an additional five patients, anti-VCAM-1 combined with anti-VLA-4 produced a small increase in inhibition of adhesion at both glomerular and tubulointerstitial sites. Thus, in these studies anti-VCAM-1 showed 63% and 46% inhibition at glomerular and tubulointerstitial sites, respectively, anti-VLA-4 showed 50% and 30% inhibition at each site, whereas the antibodies in combination inhibited by 73% and 54% at each site.
Figure 3.
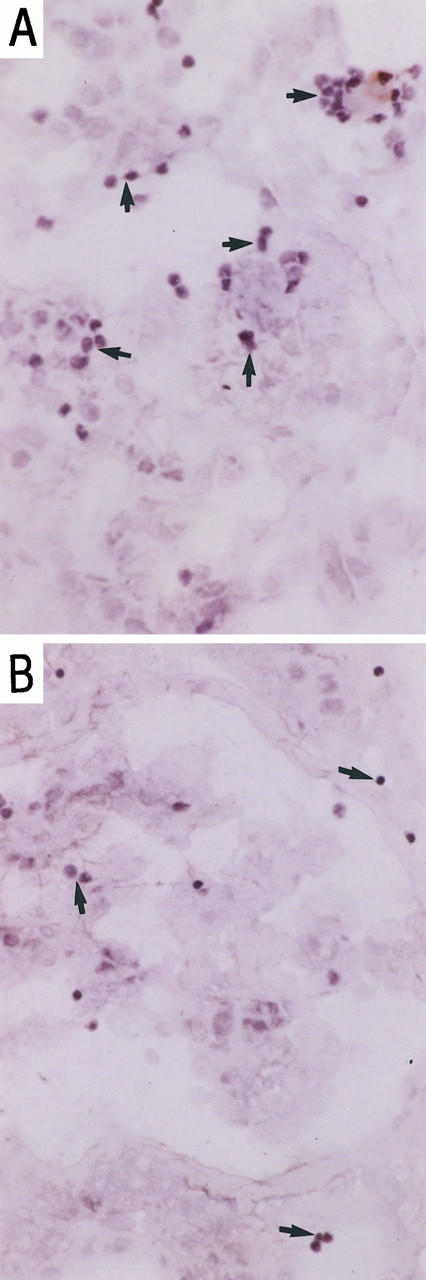
Peripheral blood T cell adhesion to serial sections of renal biopsies in vasculitic GN in the presence of irrelevant control antibody (A) and blocking anti-human ICAM-1 monoclonal antibody; RR1 (B). Magnification, ×100. Adherent cells were visualized using HRP-conjugated anti-CD3 and DAB, and the section was counterstained with Mayer’s hematoxylin as described in Materials and Methods.
Figure 4.
Inhibition of peripheral blood T cell adhesion to glomerular (A) and tubulointerstitial (B) regions of cryostat sections of renal biopsy material from five patients with vasculitic GN using monoclonal antibodies against ICAM-1, VCAM-1, and CS-1Fn as described in Materials and Methods. Data are presented as mean number ± SEM of adherent cells per glomerulus or per field of tubulointerstitium. P values indicate statistical significance of inhibition.
Figure 5.
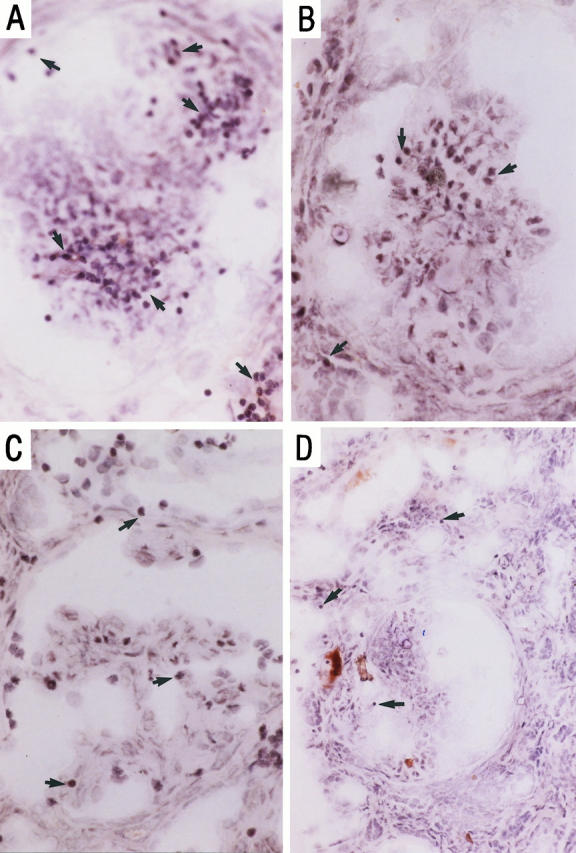
Peripheral blood T cell adhesion to serial sections of renal biopsies in vasculitic GN in the presence of irrelevant control antibody (A) blocking anti-human VCAM-1 monoclonal antibody, Ig11 (B), anti-CS-1Fn, 90.45 (C), and both antibodies (D). Tissue illustrated here was from a different patient from that in Figure 3 ▶ . Adherent cells were visualized using HRP-conjugated anti-CD3 and DAB, and the section was counterstained with Mayer’s hematoxylin as described in Materials and Methods. Magnification, ×100 (A to C) and ×50 (D).
In contrast, T lymphocyte adhesion inhibition by anti-CS-1Fn monoclonal antibody was more pronounced at tubulointerstitial sites producing 62% inhibition (from 22.1 ± 2.67 to 8.2 ± 2.86) as compared with 39% (from 14.55 ± 2.4 to 8.78 ± 1.88) at glomerular sites (Figures 4 and 5) ▶ ▶ . T cell adhesion was further reduced at both glomerular and tubulointerstitial regions when anti-CS-1Fn and anti-VCAM-1 monoclonal antibodies were used in combination, producing maximal inhibition of up to 72% (Figures 4 and 5) ▶ ▶ . This level of inhibition was similar to that observed with combined anti-VLA-4 and anti-VCAM-1.
KL/4 Cell Adhesion in Vasculitic Glomerulonephritis
We used an LFA-1-transfected K562 cell line (KL/4) that was 97.1% LFA-1 positive (determined by FACS analysis), to isolate LFA-1-mediated adhesion processes. Adhesion of KL/4 cells was β2 integrin activation dependent as no adhesion was observed by resting KL/4 cells in the absence of integrin activation by KIM185. Although normal tissue sections were able to support activated KL/4 cell adhesion (7.2 ± 3.7 and 4.7 ± 2 at glomerular and tubulointerstitial sites, respectively), this was significantly greater in the inflamed tissue (Table 1) ▶ .
Table 1.
Inhibition of KL/4 Cell Adhesion
| Blocking antibody | Glomerular | Tubulointerstitial | ||
|---|---|---|---|---|
| Vasculitic GN | Acute rejection | Vasculitic GN | Acute rejection | |
| Control | 14.61 ± 3.9 | 13.26 ± 4.7 | 13.7 ± 2.14 | 23.71 ± 12.4 |
| anti-LFA-1 | 8.6 ± 1.8 | 6.4 ± 3.7 | 10.2 ± 2.2 | 9 ± 2.27 |
| anti-ICAM-1 | 7.5 ± 2.2 (p<0.1) | 9.9 ± 4.5 | 9.48 ± 2.2 (p<0.1) | 15.56 ± 8.37 (p<0.1) |
Inhibition of KL/4 all adhesion to glomeruler and tubulointerstitial regions of cryostat sections of renal biopsy material from seven patients with vasculitic GN and five patients with acute rejection using monoclonal antibodies against LFA-1 and ICam-1 as described in Methods. Inhibition is expressed as mean number of adherent cell ± SEM; p values indicate statistical significance of inhibition.
Anti-ICAM-1 significantly attenuated KL/4 cell adhesion at both glomerular and tubulointerstitial sites by 48% (from 14.61 ± 3.9 to 7.5 ± 2.2) and by 31% (from 13.7 ± 2.14 to 9.48 ± 2.2), respectively. This pattern of inhibition reflected that seen with anti-ICAM monoclonal antibody and peripheral blood T lymphocytes (58% and 37% inhibition in glomerular and tubulointerstitial compartments). Anti-LFA-1 produced variable levels of inhibition of KL/4 cell adhesion ranging between 25% and 60%, which was not statistically significant (Table 1) ▶ .
Jurkat Cell Adhesion in Vasculitic Glomerulonephritis
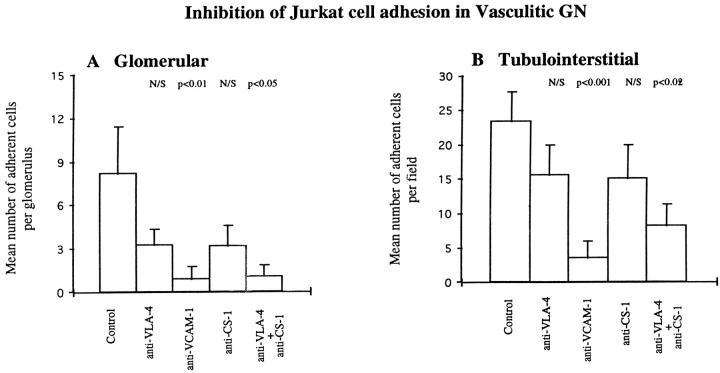
We used a functional VLA-4-expressing Jurkat cell line, which was 97.66% VLA-4 positive (determined by FACS analysis) to isolate VLA-4- from LFA-1-mediated interactions. As with KL/4 cells, resting Jurkat cells were unable to adhere to either normal or inflamed tissue sections. PMA-activated Jurkat cell adhesion to normal tissue was negligible, but there was substantial adhesion in vasculitic GN. There was strong adhesion at intraglomerular, periglomerular, and tubulointerstitial sites. Anti-VCAM-1 significantly inhibited glomerular (from 8.19 ± 3.2 to 0.89 ± 0.89) and tubulointerstitial (from 23.34 ± 4.37 to 3.54 ± 2.38) Jurkat cell adhesion by up to 89% in both compartments (Figure 6) ▶ . Thus, using this naturally VLA-4-expressing cell line to isolate VLA-4-mediated adhesion, effects of the anti-VCAM-1 blocking monoclonal antibody were more marked than with peripheral blood T lymphocytes where 56% and 47% inhibition of adhesion was achieved in glomerular and tubulointerstitial compartments, increasing to 73% and 54%, respectively, with combined anti-VCAM-1 and anti-VLA-4.
Figure 6.
Inhibition of Jurkat cell adhesion to glomerular (A) and tubulointerstitial (B) regions of cryostat sections of renal biopsy material from eight patients with vasculitic GN using monoclonal antibodies against VLA-4, VCAM-1, and CS-1Fn as described in Materials and Methods. Data are presented as mean number ± SEM of adherent cells per glomerulus or per field of tubulointerstitium. P values indicate statistical significance of inhibition.
Anti-VLA-4 and anti-CS-1Fn monoclonal antibodies alone produced variable degrees of inhibition in glomerular and tubulointerstitial compartments (Figure 6) ▶ . However, when anti-VLA-4 and anti-CS-1Fn were used in combination, Jurkat cell adhesion was significantly reduced by 88% (from 8.19 ± 3.2 to 1 ± 0.8) and by 65% (from 23.34 ± 4.37 to 8.15 ± 3.12) at glomerular and tubulointerstitial sites, respectively (Figure 6) ▶ .
Peripheral Blood T Lymphocyte Adhesion in Acute Rejection
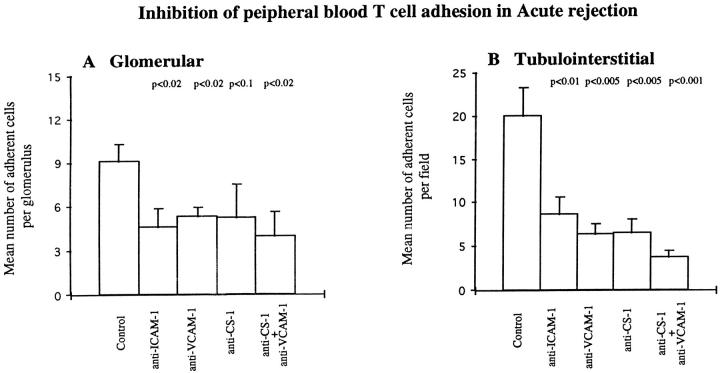
Adhesion of autologous resting T lymphocytes to cryostat sections of acute allograft rejection renal biopsies was sparse at both glomerular and tubulointerstitial sites, necessitating previous treatment with PMA for the adhesion studies. Activated T cells bound to glomerular and tubulointerstitial sites. Compared with vasculitic GN, there was equivalent T cell adhesion at tubulointerstitial sites but less adhesion at glomerular sites (Figures 4 ▶ and 7).
In allograft rejection, 57% inhibition of T lymphocyte adhesion was achieved at tubulointerstitial sites (from 20.02 ± 3.3 to 8.58 ± 2.04) by anti-ICAM-1 monoclonal antibody compared with 49% (from 9.08 ± 1.22 to 4.59 ± 1.25) at glomerular sites (Figure 7) ▶ . The inhibition by anti-ICAM-1 at tubulointerstitial sites was greater than the 37% seen in vasculitic GN, but inhibition at glomerular sites was less than the 58% seen in vasculitic GN (Figure 4) ▶ . Combining anti-ICAM-1 and anti-LFA-1 did not further increase inhibition over that seen with anti-ICAM-1 alone.
Figure 7.
Inhibition of peripheral blood T cell adhesion to glomerular (A) and tubulointerstitial (B) regions of cryostat sections of renal biopsy material from five patients with acute rejection using monoclonal antibodies against ICAM-1, VCAM-1, and CS-1Fn as described in Materials and Methods. Data are presented as mean number ± SEM of adherent cells per glomerulus or per field of tubulointerstitium. P values indicate statistical significance of inhibition.
Inhibition of T cell adhesion using the anti-VCAM-1 monoclonal antibody was 68% (from 20.02 ± 3.3 to 6.4 ± 1.12) at tubulointerstitial sites and 42% (from 9.08 ± 1.22 to 5.27 ± 0.69) at glomerular sites (Figure 7) ▶ , similar to the pattern seen with the anti-ICAM-1 monoclonal antibody. Tubulointerstitial inhibition was greater and glomerular inhibition less than seen in vasculitis (Figure 4) ▶ , reflecting the differences in the glomerular and tubulointerstitial distribution of VCAM-1 in the two inflammatory conditions. Combining anti-VCAM-1 and anti-VLA-4 reduced T cell adhesion by a further 7% at glomerular sites but had no further inhibitory effect at tubulointerstitial sites.
T cell adhesion was significantly reduced in the presence of anti-CS-1Fn by 43% (9.08 ± 1.22 to 5.19 ± 2.3) in glomerular compartments and by 67% (20.02 ± 3.3 to 6.5 ± 1.6) in tubulointerstitial compartments (Figure 7) ▶ . These levels of inhibition were comparable to the 39% and 62% seen in the glomerular and tubulointerstitial compartments in vasculitis (Figure 4) ▶ . Anti-CS-1Fn and anti-VCAM-1 used in combination further reduced adhesion in tubulointerstitial regions to 81% (from 20.02 ± 3.3 to 3.72 ± 0.72). In the glomerulus, however, there was less marked enhancement of inhibition of T cell adhesion using the two antibodies; anti-CS-1Fn and anti-VCAM-1 inhibited by 56% (9.08 ± 1.22 to 3.98 ± 1.6) compared with 43% (9.08 ± 1.22 to 5.19 ± 2.3) with anti-CS-1Fn used alone (Figure 7) ▶ , indicating a lesser role for VCAM-1 at glomerular sites in acute rejection where VCAM-1 is mainly expressed along the glomerular parietal epithelium.
KL/4 Cell Adhesion in Acute Rejection
Adhesion of activated KL/4 cells at glomerular sites was comparable between acute rejection and vasculitic GN. However, there was enhanced adhesion of KL/4 cells to the tubulointerstitium in acute rejection (Table 1) ▶ . Only modest inhibition of adhesion was achieved at both glomerular and tubulointerstitial sites by blocking antibodies against ICAM-1 and its leukocyte-expressed ligand LFA-1 (Table 1) ▶ ; only anti-ICAM-1 achieved marginally significant inhibition of 34% at tubulointerstitial sites.
Jurkat Cell Adhesion in Acute Rejection
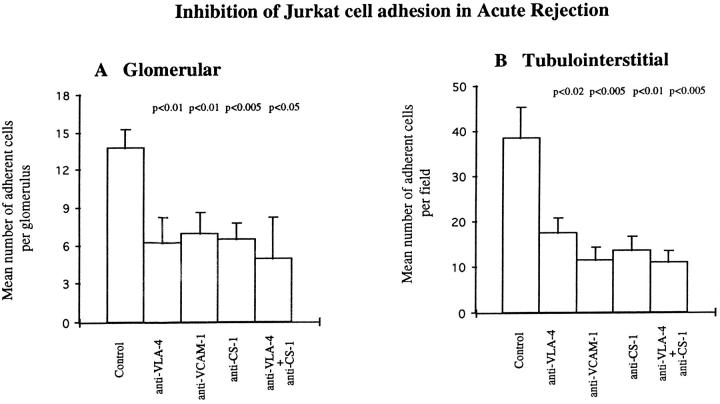
Adhesion of Jurkat cells was of similar magnitude to vasculitic GN at glomerular sites (Figures 6, A and 8 ▶ ▶ , A), whereas at tubulointerstitial sites adhesion was nearly twice as great as in vasculitic GN (Figures 6, B and 8 ▶ ▶ , B). Tubulointerstitial adhesion was significantly inhibited by anti-VCAM-1 monoclonal antibody by 70% (from 38.4 ± 6.9 to 11.4 ± 2.8; Figure 8 ▶ ). Inhibition of glomerular adhesion with anti-VCAM-1 was 49% (13.71 ± 1.5 to 6.5 ± 1.8), which was considerably less pronounced than at tubulointerstitial regions (Figure 8) ▶ ; this represented inhibition of VCAM-1-dependent adhesion to the parietal epithelium as there was little or no VCAM-1 expression at intraglomerular sites. (Figure 8) ▶ . Anti-VLA-4 and anti-CS-1Fn monoclonal antibodies alone produced significant inhibition of similar magnitudes in glomerular and tubulointerstitial compartments. Additional increases in inhibition were observed when the two antibodies were used together, reaching maximal inhibitions of 64% (13.71 ± 1.5 to 4.9 ± 3.3) and 72% (38.4 ± 6.9 to 10.8 ± 2.5) at glomerular and tubulointerstitial sites, respectively (Figure 8) ▶ .
Figure 8.
Inhibition of Jurkat cell adhesion to glomerular (A) and tubulointerstitial (B) regions of cryostat sections of renal biopsy material from seven patients with acute rejection using monoclonal antibodies against VLA-4, VCAM-1, and CS-1Fn as described in Materials and Methods. Data are presented as mean number ± SEM of adherent cells per glomerulus or per field of tubulointerstitium. P values indicate statistical significance of inhibition.
Combined Adhesion and Immunohistochemical Studies
We further analyzed the co-localization of VLA-4- and LFA-1-mediated adhesion to their respective tissue ligands, VCAM-1 and ICAM-1. LFA-1-transfected KL/4 cells specifically adhered to ICAM-1-positive regions only (Figure 9A) ▶ . Jurkat cells, however, adhered to both VCAM-1-positive and -negative sites (Figure 9B) ▶ . Co-localization of Jurkat cells and VCAM-1 confirmed VLA-4/VCAM-1 interactions, whereas adhesion at VCAM-1-free sites supports the involvement of other VLA-4-mediated interactions, such as VLA-4 binding to CS-1-containing fibronectin.
Figure 9.
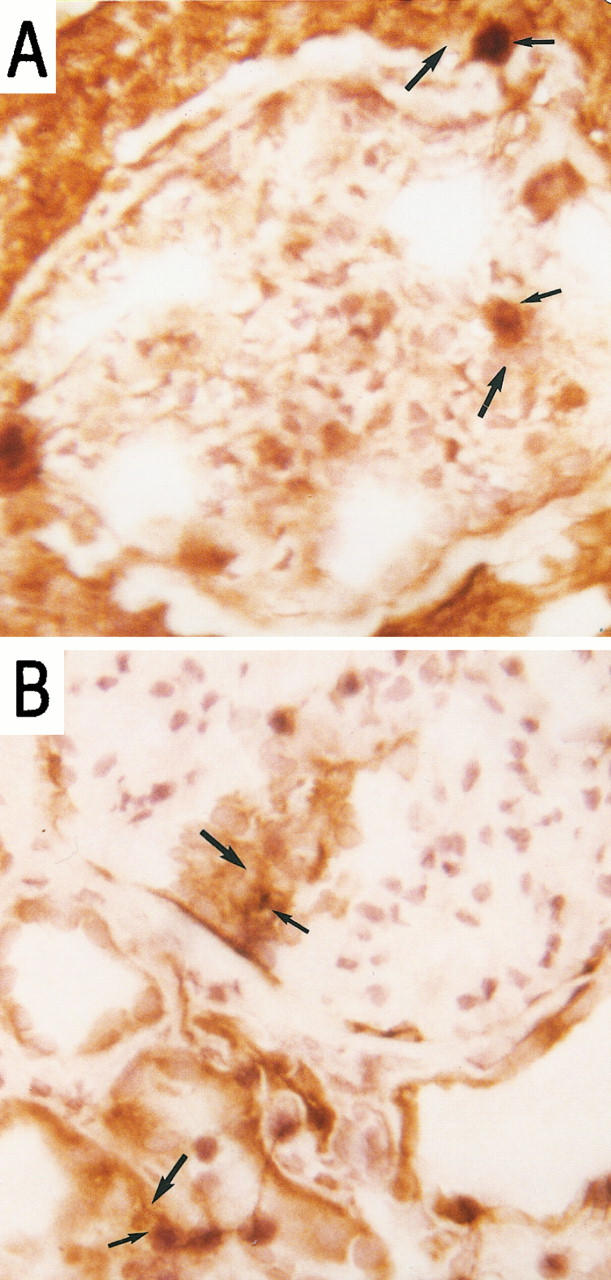
Co-localization of KL/4 cell adhesion (thin arrows) and ICAM-1 expression (thick arrows) (A) and Jurkat cell adhesion (thin arrows) and VCAM-1 expression (thick arrows) (B), using a combined adhesion and immunohistochemical technique. Magnification, ×100 and focused in plane of the leukocyte.
Discussion
To our knowledge this is the first report of the use of the Stamper-Woodruff assay to demonstrate that the adhesion molecules ICAM-1 and VCAM-1 have a functional role in mediating adhesion of human peripheral blood T lymphocytes and certain integrin-expressing leukocytic cell lines to cryostat sections of renal biopsies taken from patients with vasculitic GN and acute allograft rejection.
Immunohistochemical studies on serial sections confirmed previously reported differential patterns of VCAM-1 and ICAM-1 staining in the two disease states. 9-14 The T-lymphocyte-expressed integrins LFA-1 and VLA-4 mediate T cell adhesion to tissue-expressed ICAM-1 and VCAM-1, respectively. The activation state of the integrins is a critical determinant of leukocyte adhesion processes. 29-31 In our system, low levels of peripheral blood T cell adhesion was possible to inflamed tissues in the absence of any previous activation, but to achieve optimal adhesion for subsequent evaluation of inhibition, the cells had to be pretreated with PMA. In contrast, activation of both the Jurkat and KL/4 cell lines was an absolute prerequisite for any leukocyte adhesion.
Up-regulation of tissue-expressed adhesion molecules and activation of their leukocyte-expressed integrin ligands promotes T lymphocyte recruitment to sites of inflammation. This process is closely regulated by local stimuli such as cytokines and chemokines. In vitro studies have shown that pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, and interferon (IFN)-γ, stimulate expression and up-regulation of adhesion molecules such as ICAM-1 and VCAM-1. 32,33 Expression of TNF-α and IL-1 has been demonstrated in vasculitic GN, 34 and cytokines are up-regulated to varying extents in allograft rejection. 35,36 In addition, locally produced chemotactic mediators or chemokines, such as MCP-1, RANTES, and MIP-1α, regulate the rapid switching of integrins from a low-affinity to a high-affinity state. 37,38 We have shown expression of these chemokines as well as MIP-1β in vasculitic glomerulonephritis. 39 In vivo, these chemokines may be the true physiological trigger for integrin activation, and in future studies our system could be adapted to test this by replacing PMA or the KIM185 antibody with a relevant chemokine.
Activated peripheral blood T lymphocytes did not adhere to normal tissue sections, but adhesion was seen in both disease states to capillary tuft and parietal regions of the glomerulus as well as to tubular and interstitial regions. Adhesion to glomerular sites was greater in vasculitic GN compared with acute rejection, whereas comparable levels of adhesion were seen to tubulointerstitial sites in both diseases. Adhesion was inhibitable by blocking tissue-expressed adhesion molecules ICAM-1, VCAM-1, and CS-1 domain fibronectin. The pattern and degree of inhibition was variable between disease states and between glomerular and tubulointerstitial compartments within the kidney. T cell adhesion at glomerular sites in vasculitic GN and at tubulointerstitial sites in acute rejection was most sensitive to inhibition by both anti-ICAM-1 and anti-VCAM-1. This observation suggests a major role for ICAM-1 and VCAM-1 in mediating T cell adhesion at glomerular sites in vasculitis and at tubulointerstitial sites in acute rejection. In both diseases, CS-1Fn appeared to play a greater adhesive role within the tubulointerstitium than within glomeruli.
Using VLA-4-expressing (Jurkat) and LFA-1-expressing (KL/4) cell lines the differential roles of β1 and β2 integrins were investigated in isolation. KL/4 cells, but not Jurkat cells, were able to adhere to normal kidney sections at glomerular and peritubular sites corresponding to the markedly higher levels of ICAM-1 in normal tissue as compared with VCAM-1. This suggests the potential for constitutively expressed ICAM-1 to mediate T cell trafficking in the normal kidney; however, this would be conditional on the presence of other permissive factors such as appropriate chemoattractant factors, which may not be available in the normal kidney. Adhesion of both cell types was substantial in both diseases, although there were some differences in the patterns of localization. Thus, adhesion of Jurkat cells and KL/4 cells was greater at tubulointersitial sites in acute rejection compared with vasculitic GN, which is consistent with the strong ability of antibodies to ICAM-1, VCAM-1, and CS-1Fn to block binding of peripheral blood T lymphocytes from patients with acute rejection at this site. This may reflect a greater density of VLA-4 adhesion ligands within the tubulointerstitial regions in acute rejection. There was little difference in adhesion of Jurkat cells or KL/4 cells at glomerular sites in the two diseases.
Inhibition of adhesion of both the KL/4 and Jurkat cell lines was achieved using blocking antibodies against LFA-1 and VLA-4 and their tissue ligands ICAM-1, VCAM-1, and CS-1-containing fibronectin. The Jurkat cell line proved the most susceptible to adhesion inhibition using anti-VCAM-1 monoclonal antibody or a combination of anti-VLA-4 and anti-CS-1Fn monoclonal antibodies. Inhibition of KL/4 cell adhesion was less satisfactory, but nonetheless patterns of inhibition seen with anti-ICAM-1 were similar to those seen with peripheral blood T lymphocytes. The expression of ICAM-1 on KL/4 cells 40 may contribute to KL/4 cell adhesion to LFA-1 expressed on tissue leukocyte infiltrates. This would not be inhibited by anti-LFA-1 treatment of KL/4 cells in suspension and may explain the low levels of inhibition observed with this antibody.
Furthermore, we have demonstrated adhesion-molecule-specific localization of KL/4 and Jurkat cells in our modified Stamper-Woodruff assay system. Curiously, unlike KL/4 cells, which bound solely to ICAM-1-rich areas, Jurkat cell binding was also noted at VCAM-1-free sites. This observation and the inhibitory effect of anti-CS-1Fn suggest an important role for VLA-4/CS-1Fn interactions. Jurkat cells express a second β1 integrin, VLA-5, 41 which is also a receptor for the fibronectin receptor. Another α4 integrin (α4β7) has also been reported on leukocytes. Like VLA-4, this molecule binds to both VCAM-1 and the CS-1 domain of fibronectin as well as a mucosal addressin cell adhesion molecule-1, MAdCAM-1. 42,43 It is possible that adhesion molecules such as MAdCAM-1 and VLA-5 are responsible for additional VCAM-1-independent adhesion processes, although we have not observed MAdCAM-1 expression in the kidney (unpublished).
Binding of peripheral blood T cells and Jurkat cells to the glomerular tuft in acute rejection was of particular interest for two reasons. First, the lack of glomerular VCAM-1 expression in acute rejection suggests a role for VLA-4/CS-1Fn or possibly VLA-5/fibronectin interactions. This is supported by the inhibitory effects of anti-CS-1Fn. Second, despite this in vitro evidence showing ability of peripheral blood T cells and specific integrin-molecule-expressing cell lines to bind to glomeruli in acute allograft rejection, little T cell infiltration is observed in vivo. 2 This highlights the necessity for other cofactors in the microenvironment. We have found little chemokine expression at this location (unpublished), so the ability to activate T cell integrin ligands may be limited in allograft glomeruli. The reasons for this are unclear but suggest a degree of protection of glomerular sites from allogeneic T cell attack.
To date, a number of studies on animal models have demonstrated that monoclonal antibodies against adhesion molecules can suppress T-lymphocyte-mediated immunological reactions. Anti-ICAM-1 and anti-LFA-1 monoclonal antibodies have been used to protect against the onset of acute cellular rejection and restore deteriorating renal function in experimental models of renal transplant with variable success. 44,45 Furthermore, an anti-β3-integrin antibody for the prevention of arterial restenosis is available, and several other integrin-based drugs are under development. 46 This study provides further justification for the development of targeted therapeutic agents that will modify T cell retention and recruitment within the inflamed kidney.
Footnotes
Address reprint requests to Professor Caroline O. S. Savage, Renal Immunobiology, Division of Medical Sciences, Birmingham Centre for Immune Regulation, The Medical School, University of Birmingham, Birmingham, B15 2TT, UK. E-mail: c.o.s.savage@bham.ac.uk.
Supported by the Medical Research Council of Great Britain and the Special Trustees of Former United Birmingham Hospitals.
References
- 1.Nolasco FEB, Cameron JS, Hartley B, Coelho A, Hildreth G, Reuben R: Intraglomerular T cells and monocytes in nephritis: study with monoclonal antibodies. Kidney Int 1987, 31:1160-1166 [DOI] [PubMed] [Google Scholar]
- 2.McWhinnie DL, Thompson JF, Taylor HM, Chapman JR, Bolton EM, Carter NP, Wood RFM, Morris PJ: Morphometric analysis of cellular infiltration assessed by monoclonal antibody labeling in sequential human renal allograft biopsies. Transplantation 1986, 42:352-358 [DOI] [PubMed] [Google Scholar]
- 3.Springer TA: Traffic signals for lymphocyte recirculation and leukocyte emigration: the multi-step paradigm. Cell 1994, 76:301-314 [DOI] [PubMed] [Google Scholar]
- 4.Adu D, Howie AJ: Vasculitis in the kidney. Current Diagn Pathol 1995, 2:73-77 [Google Scholar]
- 5.D’Amico G, Sinico RA, Ferrario F: Renal vasculitis. Nephrol Dial Transplant 1996, 11(suppl 9):69-74 [DOI] [PubMed] [Google Scholar]
- 6.Bishop GA, Hall BM: Expression of leucocyte and lymphocyte adhesion molecules in the human kidney. Kidney Int 1989, 36:1078-1085 [DOI] [PubMed] [Google Scholar]
- 7.Hauser IA: Expression of cell adhesion molecules in primary renal disease and renal allograft rejection. Nephrol Dial Transplant 1997, 12:1122-1131 [DOI] [PubMed] [Google Scholar]
- 8.Nikolic-Paterson DJ, Main IW, Lan HY, Hill PA, Atkins RC: Adhesion molecules in glomerulonephritis. Springer Semin Immunopathol 1994, 16:3-22 [DOI] [PubMed] [Google Scholar]
- 9.Sugimori T, Griffith DL, Arnaout MA: Emerging paradigms of integrin ligand binding and activation. Kidney Int 1997, 51:1454-1462 [DOI] [PubMed] [Google Scholar]
- 10.Wayner EA, Garcia-Pardo A, Humphries MJ, McDonald JA, Carter WG: Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol 1989, 109:1321-1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briscoe DM, Pober JS, Harmon WE, Cotran RS: Expression of vascular cell adhesion molecule-1 in human renal allografts. J Am Soc Nephrol 1992, 3:1180-1185 [DOI] [PubMed] [Google Scholar]
- 12.Andersen CB, Blaehr H, Ladefoged S, Larsen S: Expression of the intercellular adhesion molecule-1 (ICAM-1) in human allografts and cultured human tubular cells. Nephrol Dial Transplant 1992, 7:147-154 [DOI] [PubMed] [Google Scholar]
- 13.Andersen CB, Ladefoged SD, Larsen S: Acute kidney graft rejection: a morphological and immunohistochemical study on “zero-hour” and follow-up biopsies with special emphasis on cellular infiltrates and adhesion molecules. APMIS 1994, 102:23-37 [PubMed] [Google Scholar]
- 14.Solez K, Racusen LC, Abdulkareem F, Kemeny E, von Willebrand E, Truong LD: Adhesion molecules and rejection of renal allografts. Kidney Int 1997, 51:1476-1480 [DOI] [PubMed] [Google Scholar]
- 15.Pall A, Howie AJ, Adu D: Glomerular vascular cell adhesion molecule-1 expression in renal vasculitis. J Clin Pathol 1996, 49:238-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rastaldi MP, Ferrario F, Tunesi S, Yang L, D’Amico G: Intraglomerular and interstitial leukocyte infiltration, adhesion molecules, and interleukin-1α expression in 15 cases of anti-neutrophil cytoplasmic autoantibody-associated renal vasculitis. Am J Kidney Dis 1996, 27:48-57 [DOI] [PubMed] [Google Scholar]
- 17.Bergijk EC, Baelde HJ, Kootstra CJ, De Heer E, Killen PD, Bruijn JA: Cloning of the mouse fibronectin V-region and variation of its splicing pattern in experimental immune complex glomerulonephritis. J Pathol 1996, 178:462-468 [DOI] [PubMed] [Google Scholar]
- 18.Paczek L, Bartlomiejczyk I, Gradowska L, Lao M, Gorski A, Morzycka-Michalik M, Gaciong Z: Increased content of fibronectin and laminin in glomeruli isolated from chronically rejected human renal allografts. Transplantation 1996, 61:654-655 [DOI] [PubMed] [Google Scholar]
- 19.Alonso J, Gomez-Chiarri M, Ortiz A, Seron D, Condom E, Lopez-Armada MJ, Largo R, Barat A, Egido J: Glomerular upregulation of EIIIA and V120 fibronectin isoforms in proliferative immune complex nephritis. Kidney Int 1996, 50:908-919 [DOI] [PubMed] [Google Scholar]
- 20.Stamper HB, Woodruff JJ: Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med 1976, 144:828-833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renkonen R, Turunen JP, Rapola J, Hayry P: Characterization of high endothelial-like properties of peritubular capillary endothelium during acute renal allograft rejection. Am J Pathol 1990, 137:643-651 [PMC free article] [PubMed] [Google Scholar]
- 22.Wuthrich RP: Vascular cell adhesion molecule-1 (VCAM-1) expression in murine lupus nephritis. Kidney Int 1992, 42:903-914 [DOI] [PubMed] [Google Scholar]
- 23.Elices MJ, Tsai V, Strahl D, Goel AS, Tollefson V, Arrhenius T, Wayner EA, Gaeta FCA, Fikes JD, Firestein GS: Expression and functional significance of alternatively spliced CS1 fibronectin in rheumatoid arthritis microvasculature. J Clin Invest 1994, 93:405-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman N, Stanley MA: Characterization and functional analysis of the expression of vascular adhesion molecules in human papillomavirus-related disease of the cervix. Cancer 1994, 74:884-892 [DOI] [PubMed] [Google Scholar]
- 25.Sasseville VG, Newman W, Brodie SJ, Hesterberg P, Pauley D, Ringler DJ: Monocyte adhesion to endothelium in simian immunodeficiency virus-induced AIDS encephalitis is mediated by vascular cell adhesion molecule-1/α4β1 integrin interactions. Am J Pathol 1994, 144:27-40 [PMC free article] [PubMed] [Google Scholar]
- 26.Symon FA, Walsh GM, Watson SR, Wardlaw AJ: Eosinophil adhesion to nasal polyp endothelium is P-selectin-dependent. J Exp Med 1994, 180:371-376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poston R, Johnson-Tidey RR: Localized adhesion of monocytes to human atherosclerotic plaques demonstrated in vitro: implications for atherogenesis. Am J Pathol 1996, 149:73-80 [PMC free article] [PubMed] [Google Scholar]
- 28.Ortlepp S, Stephens PE, Hogg N, Figdor CG, Robinson MK: Antibodies that activate β2 integrins can generate different ligand binding states. Eur J Immunol 1995, 25:637-643 [DOI] [PubMed] [Google Scholar]
- 29.Figdor CG, van Kooyk Y, Keizer GD: On the mode of action of LFA-1. Immunol Today 1990, 11:277-280 [DOI] [PubMed] [Google Scholar]
- 30.Masumoto A, Hemler ME: Multiple activation states of VLA-4. J Biol Chem 1993, 268:228-234 [PubMed] [Google Scholar]
- 31.Shimizu Y, Newman W, Gopal TV, Horgan KJ, Graber N, Beall LD, van Seventer GA, Shaw S: Four molecular pathways of T cell adhesion to endothelial cells: roles of LFA-1, VCAM-1, and ELAM-1 and changes in pathway hierarchy under activation conditions. J Cell Biol 1991, 113:1203-1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirby JA, Rajasekar MR, Lin Y, Proud G, Taylor RMR: Interaction between T lymphocytes and kidney epithelial cells during renal allograft rejection. Kidney Int 1993, 43(suppl 39):S-124-S-128 [PubMed] [Google Scholar]
- 33.Savage COS, Brooks CJ, Adu D, Richards G, Howie AJ: Cell adhesion molecule expression within human glomerular and kidney organ culture. J Pathol 1997, 181:111-115 [DOI] [PubMed] [Google Scholar]
- 34.Noronha IL, Kruger C, Andrassy K, Ritz E, Waldherr R: In situ TNF-α, IL-1β and IL-2R in ANCA-positive glomerulonephritis. Kidney Int 1993, 43:682-692 [DOI] [PubMed] [Google Scholar]
- 35.Dallman MJ: Cytokines as mediators of organ graft rejection and tolerance. Curr Opin Immunol 1993, 5:788-793 [DOI] [PubMed] [Google Scholar]
- 36.Noronha IL, Eberlein-Gonska M, Hartley B, Stephens S, Cameron JS, Waldherr R: In situ expression of tumor necrosis factor-alpha, interferon-gamma and interleukin-2 recepors in renal allograft biopsies. Transplantation 1992, 54:1017-1024 [DOI] [PubMed] [Google Scholar]
- 37.Szabo MC, Butcher EC, McIntyre BW, Schall TJ, Bacon KB: RANTES stimulation of T lymphocyte adhesion and activation: role for LFA-1 and ICAM-3. Eur J Immunol 1997, 27:1061-1068 [DOI] [PubMed] [Google Scholar]
- 38.Weber C, Alon R, Moser B, Springer TA: Sequential regulation of α4β1 and α5β1 integrin avidity by CC chemokines in monocytes: implications for transendothelial chemotaxis. J Cell Biol 1996, 134:1063-1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cockwell P, Howie AJ, Adu D, Savage COS: In situ analysis of C-C chemokine mRNA in human glomerulonephritis. Kidney Int 1998, 54:827-836 [DOI] [PubMed] [Google Scholar]
- 40.Buckley CD, Ferguson ED, Littler AJ, Bossy D, Simmons DL: Role of ligands in the activation of LFA-1. Eur J Immunol 1997, 27:957-962 [DOI] [PubMed] [Google Scholar]
- 41.Weismann M, Guse AH, Sorokin L, Broker B, Frieser M, Hallman R, Mayr GW: Integrin-mediated intracellular Ca2+ signaling in Jurkat T lymphocytes. J Immunol 1997, 158:1618-1627 [PubMed] [Google Scholar]
- 42.McMurray RW: Adhesion molecules in autoimmune disease. Semin Arthritis Rheum 1996, 25:215-233 [DOI] [PubMed] [Google Scholar]
- 43.Walsh GM, Symon FA, Lazarovits AI, Wardlaw AJ: Integrin α4β7 mediates human eosinophil interaction with MAdCAM-1, VCAM-1 and fibronectin. Immunology 1996, 89:112-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cosimi AB, Conti D, Delmonico FL, Preffer FI, Wee SL, Rothlein R, Faanes R, Colvin RB: In vivo effects of monoclonal antibody to ICAM-1 (CD54) in nonhuman primates with renal allografts. J Immunol 1990, 144:4604-4612 [PubMed] [Google Scholar]
- 45.Kelly KJ, Williams WW, Jr, Colvin RB, Bonventre JV: Antibody to intercellular adhesion molecule 1 protects the kidney against ischaemic injury. Proc Natl Acad Sci USA 1994, 91:812-816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruoslahti E: Integrins as signaling molecules and targets for tumour therapy. Kidney Int 1997, 51:1413-1417 [DOI] [PubMed] [Google Scholar]