Abstract
Mechanisms controlling endothelial cell survival during angiogenesis were investigated. Stimulation of quiescent endothelial cells with mitogens, including vascular endothelial growth factor and basic fibroblast growth factor, induced up to ∼16-fold up-regulation of the cell cycle-regulated apoptosis inhibitor survivin. Mitogen stimulation rapidly increased survivin RNA expression in endothelial cells, which peaked after 6 to 10 hours in culture and decreased by 24 hours. Inflammatory cytokines, tumor necrosis factor α, and interleukin-1 did not induce survivin expression in endothelial cells. Formation of three-dimensional vascular tubes in vitro was associated with strong induction of survivin in endothelial cells, as compared with two-dimensional cultures. By immunohistochemistry, survivin was minimally expressed in endothelium of nonproliferating capillaries of normal skin, whereas it became massively up-regulated in newly formed blood vessels of granulation tissue in vivo. Recombinant expression of green fluorescent protein survivin in endothelial cells reduced caspase-3 activity and counteracted apoptosis induced by tumor necrosis factor α/cycloheximide. These findings identify survivin as a novel growth factor-inducible protective gene expressed by endothelial cells during angiogenesis. Therapeutic manipulation of survivin expression and function in endothelium may influence compensatory or pathological (tumor) angiogenesis.
Apoptosis, the genetic control of cell death and viability, preserves tissue and organ homeostasis by eliminating senescent or damaged cells. 1 This process involves different gene families of inhibitors and stimulators of cell death and culminates with activation of intracellular cysteine proteases, caspases. 2 Aberrations of apoptosis are known to contribute to human diseases, including cancer 3 and vascular disorders. 4 Specifically, aberrantly increased cell death has been shown to influence atherosclerotic plaque instability, 5 congestive heart failure, 6 coronary disease, 7 and ischemic neuronal loss. 8
The endothelium is one of the most critical sites for the control of apoptosis in vascular injury and vascular remodeling. 9 In inflammation, a heterogeneous group of protective genes activated by nuclear factor κB opposes cell death and proinflammatory changes in endothelial cells (EC) induced by cytokines, ie, tumor necrosis factor α (TNFα). 10 Inhibition of apoptosis may also be required during vascular remodeling and new blood vessel formation, angiogenesis. 11 In this context, EC-specific mitogens, including vascular endothelial cell growth factor (VEGF) or basic fibroblast growth factor (bFGF), transduce survival signals critically maintaining EC viability in vivo. 12-14 However, the downstream effector genes coupling mitogen-dependent survival to the anti-apoptotic machinery in EC have not been completely elucidated.
In this study, we sought to investigate a potential role of the cell cycle-regulated apoptosis inhibitor survivin 15,16 on EC viability. We found that mitogen stimulation strongly induced survivin expression in endothelium during vascular remodeling and angiogenesis, in vitro and in vivo, and that this pathway counteracted caspase-3 activity and apoptosis induced by TNFα.
Materials and Methods
Cells and Cell Culture
Human umbilical vein EC were maintained in M199 medium supplemented with 20% fetal calf serum (FCS), 50 μg/ml endothelial cell growth supplement (ECGS), 100 μg/ml heparin, 100 μg/ml penicillin, and 100 μg/ml streptomycin (all from Life Technologies, Grand Island, NY) in 5% CO2 at 37°C. Bovine aortic EC were isolated and maintained in culture as described. 17 Subconfluent EC were rendered quiescent by 24 hours’ culture in M199 plus 0.1% FCS. Cells were detached with 0.05% trypsin/0.02% EDTA, seeded in C6-well plates (Costar Corp., New Bedford, MA), grown to 70% confluency, and used between passages 2 and 3.
Modulation of Survivin Expression in EC
Quiescent subconfluent EC were incubated with VEGF (Collaborative Biomedical Products, Bedford, MA; 10–100 ng/ml), basic fibroblast growth factor (bFGF; Calbiochem Corp., La Jolla, CA; 5 ng/ml), 10% FCS, or recombinant interleukin-1 (IL-1; R&D Systems, Minneapolis, MN; 2 ng/ml, 200 U/ml) or TNFα (10 ng/ml, Endogen, Woburn, MA) for 14 hours at 37°C in M199 plus 0.1% FCS. Cells were washed, harvested by trypsin/EDTA, and extracted in 4% sodium dodecyl sulfate plus protease inhibitors. Protein-normalized aliquots of cell extracts were electrophoresed on a 13.5% sodium dodecyl sulfate polyacrylamide gel, transferred to nylon membranes (Millipore Corp.) for 1 hour at 1 A, and immunoblotted with 1 μg/ml of a rabbit antibody to survivin followed by chemiluminescence (Amersham, Arlington Heights, IL). 18 Samples were analyzed for equal protein loading by immunoblotting with a mouse antibody to β-actin. For Northern blot hybridization, serum-deprived EC were stimulated with 100 ng/ml VEGF and harvested at increasing time intervals between 1.5 and 24 hours culture at 37°C. Total RNA was extracted using the TRI Reagent (10 6 cells/0.2 ml, Molecular Research Center, Cincinnati, OH), and further processed for Northern blot hybridization with a 32Pα-dCTP random-primed labeled survivin cDNA or control β-actin probe, as described. 15
Three-Dimensional EC Culture
EC were suspended at a density of 3 × 106/ml in a liquefied matrix of rat-tail type I collagen (1.5 mg/ml) and human plasma-derived fibronectin (0.15 mg/ml) in M199, pH 7.5. One milliliter of the EC suspension was transferred into each well of rat-tail type I-coated C6 wells and warmed to 37°C to allow polymerization of the matrix. After a 24 hour incubation at 37°C in M199 plus 20% FCS, 50 μg/ml ECGS, 100 μg/ml heparin, 100 μg/ml penicillin, and 100 μg/ml streptomycin, the three-dimensional culture was placed in OCT and paraffin-embedded for immunohistochemical analysis. Alternatively, two- or three-dimensional EC cultures were homogenized in a tissue grinder and immunoblotted for survivin expression. During the incubation period, EC throughout the gel were observed to elongate and form multicellular tubular structures, as described. 19
Immunohistochemistry
Four skin biopsies, containing granulation tissue or normal, non-inflamed skin by hematoxylin-eosin staining, were collected from the archives of Yale-New Haven Hospital. Five-micron sections were prepared from paraffin-embedded tissues, deparaffinized in xylene, and rehydrated in graded alcohol with quenching of endogenous peroxidase in 2% H2O2 in methanol. Immunolocalization of survivin was carried out as described previously, 15 after antigen retrieval by pressure cooking for 5 minutes in 0.01 mol/L citrate buffer, pH 6.0. Binding of the primary antibody was revealed by addition of 3,3′-diaminobenzidine, or, alternatively, 3-amino-9-ethylcarbazol (AEC, Vector), as a substrate. Control experiments were carried out in the absence of primary antibody or in the presence of preimmune rabbit IgG.
EC Protection by Survivin
The cDNA of wild-type survivin 15 was inserted in-frame in the EcoRI site of green fluorescence protein (GFP)-encoding plasmid, pEGFPc1 (Clontech, San Francisco, CA). The correct orientation and reading frame of pEGFPc1 fusion plasmid were confirmed by DNA sequencing. Bovine aortic EC were seeded in C6-well plates at 40 to 50% confluency and transfected with GFP vector or GFP survivin by lipofectin for 6 hours at 37°C. After removal of the DNA-lipid mixture, the EC monolayer was placed in complete growth medium for 35 hours at 37°C and incubated with 5 ng/ml TNFα plus 5 μg/ml cycloheximide for an additional 8 hours at 37°C. Cells (floaters plus attached cells) were fixed in 70% ethanol, stained with 10 μg/ml propidium iodide plus 100 μg/ml RNase A and 0.05% Triton X-100 in phosphate-buffered saline, pH 7.4, and GFP-expressing cells were analyzed for DNA content by flow cytometry. In other experiments, bovine EC transfected with GFP vector or GFP survivin were treated with control medium or 5 to 10 ng/ml TNFα plus 10 μg/ml cycloheximide for 8 hours at 37°C. Cells were harvested and analyzed for caspase-3 activity by hydrolysis of the fluorogenic substrate Ac-DEVD-AMC (N-Acetyl-Asp-Glu-Val-Asp-aldehyde, Pharmingen, San Diego, CA), in the presence or in the absence of the caspase-3 inhibitor Ac-DEVD-CHO. Fluorescence emissions were quantitated on a spectrofluorometer with excitation wavelength of 360 nm and emission wavelength of 460 nm.
Results
Mitogen-Stimulated Induction of Survivin in EC
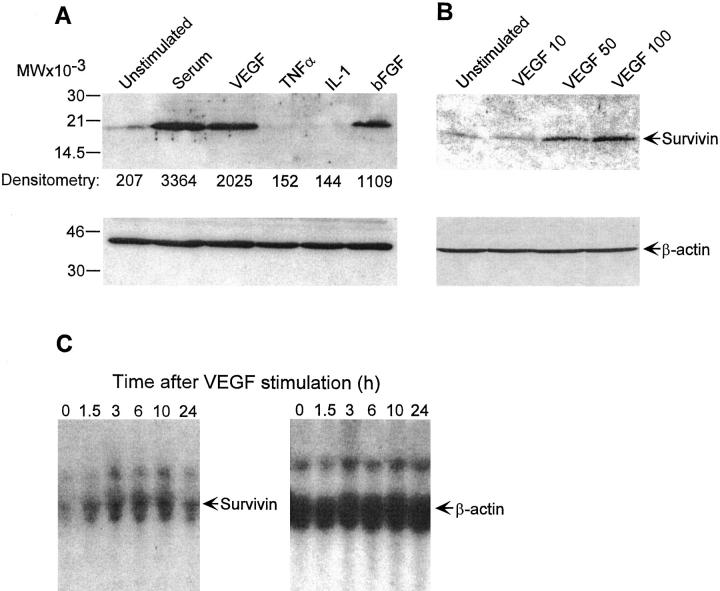
Expression of ∼16.5-kd endogenous survivin in quiescent, serum-deprived endothelium was minimally detectable by immunoblotting (Figure 1A) ▶ , in agreement with previous observations. 15 EC stimulation with serum or the specific mitogens VEGF or bFGF resulted in an 8- to 16-fold up-regulation of survivin expression, by immunoblotting (Figure 1A) ▶ . Survivin induction by VEGF was concentration-dependent and maximal at ∼50 ng/ml (Figure 1B) ▶ . EC stimulation with cytokines TNFα or IL-1 did not increase survivin expression, which was reduced below background levels of untreated cells (Figure 1A) ▶ . In control experiments by flow cytometry, TNFα or IL-1 stimulated strong up-regulation of intercellular adhesion molecule-1 in EC, whereas VEGF was ineffective (not shown). By Northern blot hybridization, a main 1.9-kb survivin message and a fainter 3.4-kb survivin transcript were minimally detected in quiescent EC (Figure 1C) ▶ . VEGF treatment resulted in rapid up-regulation of survivin RNA in EC, in a response that peaked 6 to 10 hours after stimulation and decreased to approach background levels 24 hours after treatment (Figure 1C) ▶ .
Figure 1.
Modulation of survivin expression in EC. A: Quiescent EC were incubated with medium or serum (10% FCS), VEGF (100 ng/ml), bFGF (5 ng/ml), TNFα (10 ng/ml), or IL-1 (2 ng/ml) for 16 hours at 37°C. Cells were harvested, sodium dodecyl sulfate-extracted, and analyzed for expression of survivin or β-actin by immunoblotting. B: Control or EC were stimulated with the indicated increasing concentrations of VEGF for 16 hours at 37°C and analyzed for expression of survivin or β-actin by immunoblotting. C: Total RNA was extracted from EC stimulated with 100 ng/ml VEGF at the indicated time intervals, separated on agarose-formaldehyde denaturing gels, and hybridized with probes to survivin or control β-actin.
Survivin Expression in Three-Dimensional EC Cultures
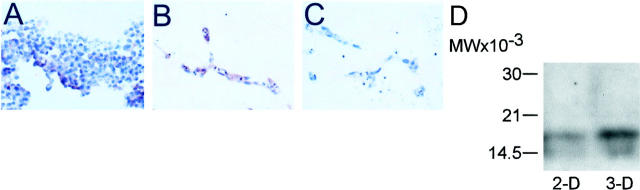
Survivin was expressed at very low levels in two-dimensional EC cultures, by immunohistochemistry (Figure 2A) ▶ . In contrast, formation of three-dimensional vascular tubes in collagen/fibronectin matrix resulted in strong expression of survivin in EC (Figure 2B) ▶ . No staining of three-dimensional EC cultures was observed with control nonbinding antibody (Figure 2C) ▶ . By immunoblotting, a prominent ∼16.5-kd survivin band was prominently induced in EC extracts of three-dimensional vascular tubes, as compared with two-dimensional EC cultures (Figure 2D) ▶ .
Figure 2.
Expression of survivin in three-dimensional EC culture. EC were grown in three-dimensional fibronectin-collagen gels, paraffin-embedded, and analyzed for survivin expression (red staining) by immunohistochemistry. A: Survivin expression in control, two-dimensional EC culture. B: Survivin expression in three-dimensional EC culture. C: Control staining of three-dimensional EC culture with preimmune antibody. D: Two- (2-D) or three-dimensional (3-D) EC cultures were harvested, homogenized in a tissue grinder, and analyzed for survivin expression by immunoblotting.
Survivin Expression in Proliferating EC in Vivo
In four out of four cases, survivin was strongly expressed in the cytoplasm of EC of newly formed capillaries of skin granulation tissue, by immunohistochemistry (Figure 3A) ▶ . Abundant expression of survivin was also demonstrated in EC of large vessels of granulation tissue at the dermis/hypodermis junction (Figure 3C) ▶ . By contrast, no staining of granulation tissue was observed in the absence of primary antibody (Figure 3, B and D) ▶ , or with control preimmune antibody (not shown). Analysis of nonproliferating capillaries of noninflamed normal skin revealed minimally detectable expression of survivin in EC (Figure 3E) ▶ , as compared with control staining with preimmune IgG (Figure 3F) ▶ .
Figure 3.
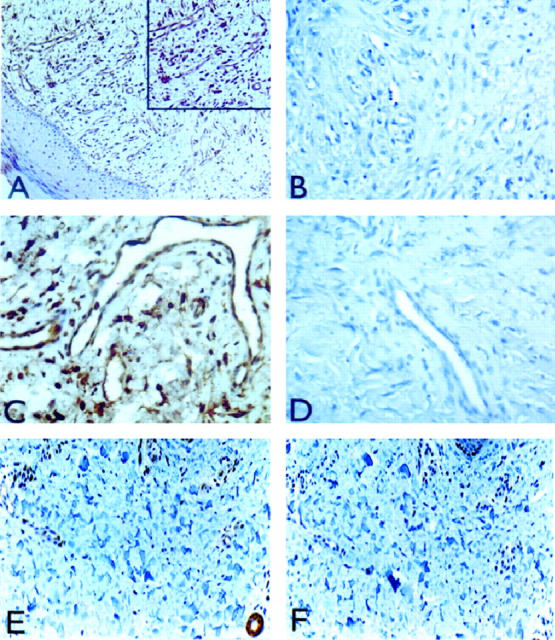
Expression of survivin in proliferating and nonproliferating skin capillaries. Five-micron sections of formalin-fixed, paraffin-embedded skin biopsies containing granulation tissue and normal skin were analyzed for survivin expression by immunohistochemistry after antigen retrieval by pressure-cooking. A: Strong cytoplasmic expression of survivin in EC of dermal capillaries in granulation tissue. Inset: Detail representation of dermal capillaries stained for survivin expression. C: Expression of survivin in endothelium of large vessel in granulation tissue at the dermis/hypodermis junction. B and D: Control staining for A and C, respectively, in the absence of primary antibody. E: Expression of survivin in nonproliferating capillaries of noninflamed normal skin. F: Control incubation for panel E in the presence of preimmune antibody. Original magnifications, ×200 (A, E, F) and ×400 (B, C, D).
Anti-Apoptotic Effect of Survivin in EC
Treatment with TNFα/cycloheximide induced EC apoptosis and generation of a hypodiploid population by propidium iodide staining and flow cytometry (Figure 4A) ▶ . Expression of GFP survivin inhibited TNFα-induced apoptosis in EC and reduced the percentage of hypodiploid cells to control levels of untreated cultures (Figure 4A) ▶ . In contrast, transfection of GFP vector alone did not affect TNFα-induced EC apoptosis (Figure 4A) ▶ . Moreover, expression of GFP survivin in EC strongly inhibited caspase-3 activity in TNFα-treated EC, as determined by DEVD hydrolysis, whereas GFP vector alone was ineffective (Figure 4B) ▶ . In control experiments, preincubation of TNFα-treated EC extracts with the caspase-3 inhibitor DEVD-CHO abrogated DEVD hydrolysis (Figure 4B) ▶ .
Figure 4.
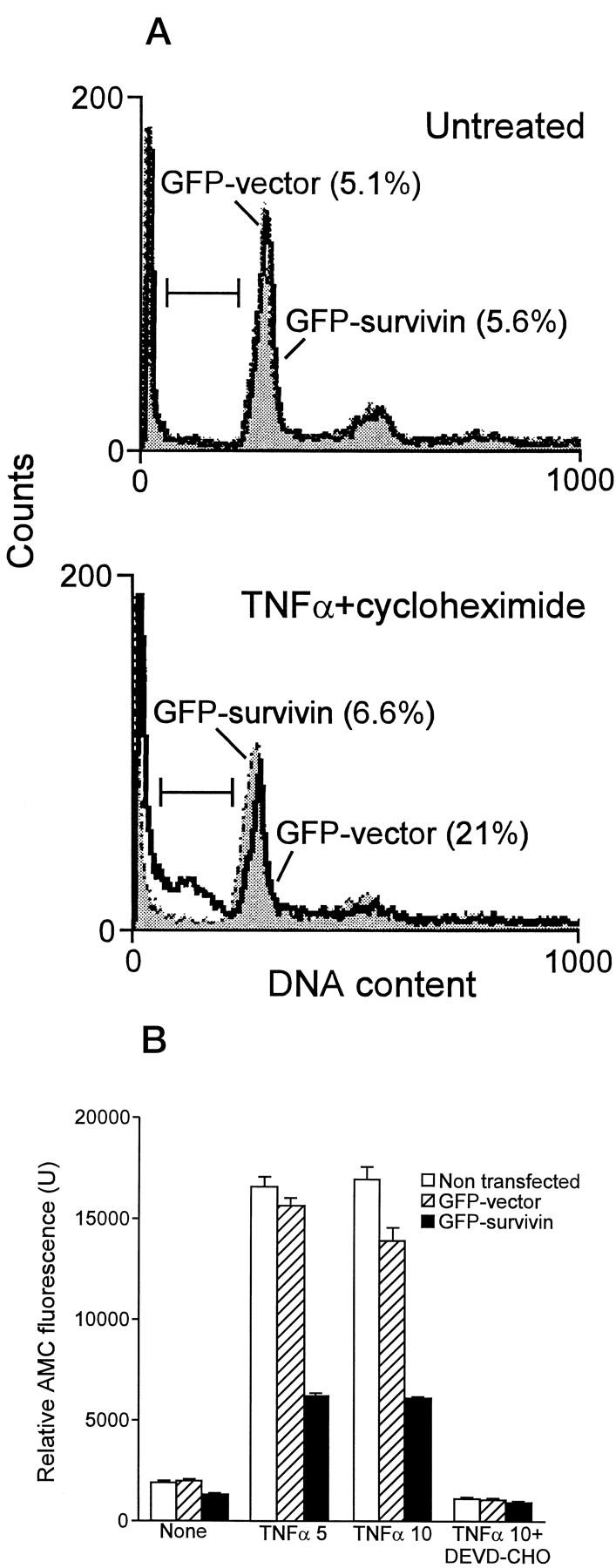
Anti-apoptotic function of survivin in EC. A: Subconfluent bovine aortic EC were transfected with GFP vector or GFP survivin by lipofectin, cultivated for 35 hours at 37°C, and treated with 5 ng/ml TNFα/5 μg/ml cycloheximide for additional 8 hours at 37°C. GFP-expressing cells (green fluorescence) were analyzed for DNA content by propidium iodide staining (red fluorescence) and flow cytometry. The percentage of cells with hypodiploid DNA content (sub-G1 fraction) is indicated in parentheses for each condition tested. B: EC, untreated or transfected with GFP vector or GFP survivin, were incubated with the indicated concentrations of TNFα/10 μg/ml cycloheximide, harvested, and analyzed for caspase-3 activity by hydrolysis of the fluorogenic substrate DEVD-AMC in the presence or absence of the caspase-3 inhibitor DEVD-CHO. Data are the mean ± SD of replicates of a representative experiment.
Discussion
In this study, we have shown that mitogen stimulation of EC results in strong up-regulation of the cell cycle-regulated apoptosis inhibitor, survivin. 15,16 From minimally detectable levels in quiescent endothelium, survivin became abundantly expressed in EC of three-dimensional vascular tubes in vitro and in newly formed capillaries during angiogenesis in vivo. Recombinant expression of survivin efficiently reduced caspase-3 activity in EC and blocked EC apoptosis induced by TNFα/cycloheximide.
Recent experimental evidence has suggested that inhibition of EC apoptosis may be an essential prerequisite to maintain angiogenesis in vivo. 11 Accordingly, disruption of αvβ3 integrin-matrix interaction 20 or interference with VEGF-dependent survival signals 12-14 triggered EC apoptosis and involution of newly formed capillaries in vivo. Despite the up-regulation of anti-apoptotic bcl-2 and A1 molecules in VEGF-stimulated endothelium, 21,22 alternative mechanisms of cytoprotection have been postulated, 23 prompting the search for additional effector genes contributing to VEGF-dependent EC survival. Here, the dramatic up-regulation of survivin in mitogen-stimulated endothelium is consistent with the cell cycle-dependent expression of the survivin gene in G2/M 16 and suggests that this pathway may maintain a critical anti-apoptotic threshold at cell division. Consistent with the spontaneous induction of apoptosis resulting from survivin targeting in model cell types, 18,24 these data suggest that VEGF induction of survivin may provide a critical prerequisite to maintain EC viability during angiogenesis, whereas loss of survivin may facilitate involution of newly formed capillaries in vivo. 12-14 On the other hand, survivin expression in VEGF-stimulated endothelium may not be simply a consequence of cell proliferation, since survivin was not detected in other normal proliferating tissues, including the basal layer of epidermis. 18 This suggests that VEGF induction of survivin may provide a unique paradigm of regulation of this anti-apoptotic pathway in a normal, terminally differentiated cell type.
The suppression of caspase-3 activity in survivin-expressing EC shown here is consistent with the general function of IAP proteins as caspase inhibitors, either directly or through interference with caspase-9 processing. 25,26 In EC, active caspase-3 has been directly implicated in proteolysis of p125FAK 27, 28 and p27/p21 cyclin-dependent kinase inhibitors, 29 thus disassembling cell-to-matrix interactions and dysregulating cell division control mechanisms. In this context, VEGF induction of survivin is expected to provide a broad anti-apoptotic spectrum, counteracting a variety of death-inducing stimuli converging on caspase-3 activation as the executioner phase of apoptosis. 2 It is also intriguing that both bcl-2 and survivin become up-regulated in VEGF-stimulated EC. 21 This suggests that inhibition of EC apoptosis during angiogenesis may occur simultaneously through parallel and non-overlapping pathways, involving preservation of mitochondrial integrity by bcl-2 30 and suppression of caspase activity by survivin. 25,26
The findings reported here may have potentially far-reaching therapeutic implications. First, targeted inhibition of apoptosis in endothelium may be exploited to limit tissue damage in vascular diseases. 31 Accordingly, caspase antagonists 32 or overexpression of anti-apoptotic bcl-2 33 afforded increased neuronal viability in ischemia/hypoxia models. In this context, survivin gene transfer may result in improved EC viability during VEGF-stimulated compensatory angiogenesis in ischemic vascular diseases. 34 Conversely, for the selective expression of survivin in cancer, 15 molecular antagonists of this pathway may not only sensitize tumor cells to therapy-induced apoptosis, but also remove a critical EC cytoprotective mechanism exploited during tumor angiogenesis.
Footnotes
Address reprint requests to Dario C. Altieri, M.D., Yale University School of Medicine, BCMM436B, 295 Congress Avenue, New Haven, CT 06536. E-mail: dario.altieri@yale.edu.
Supported by National Institutes of Health grants CA78810 and HL 54131 (to D.C.A.), HL 51014 (to J. S. P.), and HL10112 (to M. M.), and completed during the tenure of an Established Investigatorship award to D. C. A. by the American Heart Association. C. A. was supported by a fellowship from the Lymphoma Research Foundation of America.
References
- 1.Vaux DL, Korsmeyer SJ: Cell death in development. Cell 1999, 96:245-254 [DOI] [PubMed] [Google Scholar]
- 2.Salvesen GS, Dixit VM: Caspases: intracellular signaling by proteolysis. Cell 1997, 91:443-446 [DOI] [PubMed] [Google Scholar]
- 3.Thompson CB: Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267:1456-1462 [DOI] [PubMed] [Google Scholar]
- 4.Rudin CM, Thompson CB: Apoptosis and disease: regulation and clinical relevance of programmed cell death. Annu Rev Med 1997, 48:267-281 [DOI] [PubMed] [Google Scholar]
- 5.Bjorkerud S, Bjorkarud B: Apoptosis is abundant in human atherosclerotic lesions, especially in inflammatory cells (macrophages and T cells), and may contribute to the accumulation of gruel and plaque instability. Am J Pathol 1996, 149:367-380 [PMC free article] [PubMed] [Google Scholar]
- 6.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P: Apoptosis in the failing human heart. N Engl J Med 1997, 336:1131-1141 [DOI] [PubMed] [Google Scholar]
- 7.Olivetti G, Quaini F, Sala R, Lagrasta C, Corradi D, Bonacina E, Gambert SR, Cigola E, Anversa P: Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol 1996, 28:2005-2016 [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Nagayama T, Jin K, Stetler RA, Zhu RL, Graham SH, Simon RP: Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci 1998, 18:4914-4928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karsan A, Harlan JM: Modulation of endothelial cell apoptosis: mechanisms and pathophysiological roles. J Atheroscler Thromb 1996, 3:75-80 [DOI] [PubMed] [Google Scholar]
- 10.Bach FH, Hancock WW, Ferran C: Protective genes expressed in endothelial cells: a regulatory response to injury. Immunol Today 1997, 18:483-486 [DOI] [PubMed] [Google Scholar]
- 11.Risau W: Mechanisms of angiogenesis. Nature 1997, 386:671-674 [DOI] [PubMed] [Google Scholar]
- 12.Benjamin LE, Keshet E: Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors: induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proc Natl Acad Sci USA 1997, 94:8761-8766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E: Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1995, 1:1024-1028 [DOI] [PubMed] [Google Scholar]
- 14.Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK: Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA 1996, 93:14765-14770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrosini G, Adida C, Altieri DC: A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 1997, 3:917-921 [DOI] [PubMed] [Google Scholar]
- 16.Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, Altieri DC: Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 1998, 396:580-584 [DOI] [PubMed] [Google Scholar]
- 17.De Luca LG, Johnson DR, Whitley MZ, Collins T, Pober JS: cAMP and tumor necrosis factor competitively regulate transcriptional activation through and nuclear factor binding to the cAMP-responsive element/activating transcription factor element of the endothelial leukocyte adhesion molecule-1 (E-selectin) promoter. J Biol Chem 1994, 269:19193-19196 [PubMed] [Google Scholar]
- 18.Grossman D, McNiff JM, Li F, Altieri DC: Expression of the apoptosis inhibitor, survivin, in nonmelanoma skin cancer and gene targeting in a keratinocyte cell line. Lab Invest 1999, 79:1121-1126 [PubMed] [Google Scholar]
- 19.Sierra-Honigman MR, Nath AK, Murakami C, Garcia-Cadena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR: Biological action of leptin as an angiogenic factor. Science 1998, 281:1683-1686 [DOI] [PubMed] [Google Scholar]
- 20.Brooks PC, Montgomery AMP, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA: Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994, 79:1157-1164 [DOI] [PubMed] [Google Scholar]
- 21.Gerber HP, Dixit V, Ferrara N: Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem 1998, 273:13313-13316 [DOI] [PubMed] [Google Scholar]
- 22.Nor JE, Christensen J, Mooney DJ, Polverini PJ: Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol 1999, 154:375-384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karsan A, Yee E, Poirier GG, Zhou P, Craig R, Harlan JM: Fibroblast growth factor-2 inhibits endothelial cell apoptosis by Bcl-2- dependent and independent mechanisms. Am J Pathol 1997, 151:1775-1784 [PMC free article] [PubMed] [Google Scholar]
- 24.Ambrosini G, Adida C, Sirugo G, Altieri DC: Induction of apoptosis and inhibition of cell proliferation by survivin gene targeting. J Biol Chem 1998, 273:11177-11182 [DOI] [PubMed] [Google Scholar]
- 25.Deveraux QL, Reed JC: IAP family proteins: suppressors of apoptosis. Genes Dev 1999, 13:239-252 [DOI] [PubMed] [Google Scholar]
- 26.LaCasse EC, Baird S, Korneluk RG, MacKenzie AE: The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene 1998, 17:3247-3259 [DOI] [PubMed] [Google Scholar]
- 27.Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY: Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol 1996, 134:793-799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levkau B, Herren B, Koyama H, Ross R, Raines EW: Caspase-mediated cleavage of focal adhesion kinase pp125FAK and disassembly of focal adhesions in human endothelial cell apoptosis. J Exp Med 1998, 187:579-586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levkau B, Koyama H, Raines EW, Clurman BE, Herren B, Orth KR, Roberts JM, Ross R: Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cell through activation of Cdk2: role of a caspase cascade. Mol Cell 1998, 1:553-563 [DOI] [PubMed] [Google Scholar]
- 30.Adams JM, Cory S: The Bcl-2 protein family: arbiters of cell survival. Science 1998, 281:1322-1326 [DOI] [PubMed] [Google Scholar]
- 31.MacLellan WR, Schneider MD: Death by design: programmed cell death in cardiovascular biology and disease. Circ Res 1997, 81:137-144 [DOI] [PubMed] [Google Scholar]
- 32.Cheng Y, Deshmukh M, D’Costa A, Demaro JA, Gidday JM, Shah A, Sun Y, Jacquin MF, Johnson EM, Jr, Holtzman DM: Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. J Clin Invest 1998, 101:1992-1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tagami MK, Yamagata K, Nara Y, Fujino H, Kubota A, Numano F, Yamori Y, Linnik MD, Zahos P, Geschwind MD, Federoff HJ: Expression of bcl-2 from a defective herpes simplex virus-1 vector limits neuronal death in focal cerebral ischemia. Stroke 1995, 26:1670-1674 [DOI] [PubMed] [Google Scholar]
- 34.Battegay EJ: Angiogenesis: mechanistic insights, neovascular diseases, and therapeutic prospects. J Mol Med 1995, 73:333-346 [DOI] [PubMed] [Google Scholar]