Abstract
Four members of the carcinoembryonic antigen (CEA) family, CEA, CEACAM1 (BGP), CEACAM6 (NCA-50/90), and CEACAM7 (CGM2), are coexpressed in normal colorectal epithelia but are deregulated in colorectal cancers, where they could play a role in tumorigenesis. As a basis for functional studies, their expression patterns in normal tissues first need to be clarified. This is well documented for CEACAM1 and CEA but not for CEACAM6 or CEACAM7. We have now carried out immunohistochemical expression studies on 35 different organs, using CEACAM6-specific (9A6) and CEACAM7-specific (BAC2) monoclonal antibodies. CEACAM7 was only found on the apical surface of highly differentiated epithelial cells in the colorectal mucosa and on isolated ductal epithelial cells within the pancreas. CEACAM6 was expressed in granulocytes and epithelia from various organs. CEACAM6 and CEACAM7 expression correlated with apoptosis at the table region of the normal colon, and both were absent from highly proliferating cells at the base of colonic crypts. CEACAM6 revealed a broader expression zone in proliferating cells in hyperplastic polyps and adenomas compared with normal mucosa, whereas CEACAM7 was completely absent. Down-regulation of CEACAM7 and up-regulation of CEACAM6 expression in hyperplastic polyps and early adenomas represent some of the earliest observable molecular events leading to colorectal tumors.
The lumen of the normal colon has an enlarged surface area, in the form of crypts whose main functions are the resorption of water and salts as well as mucous secretion. These crypts are coated with a single layer of epithelial cells, which are continuously being regenerated from stem cells located at the base of each crypt. 1 After division, some daughter cells regenerate stem cells, whereas the rest differentiate into four main epithelial cell types: colonocytes, goblet cells, M-cells, and endocrine cells. During their differentiation, these cells migrate up the crypts to the table region between the crypts and are finally exfoliated into the intestinal lumen. Although the turnover of epithelial cells in the small intestine is relatively similar, one major difference becomes apparent. In humans, colorectal epithelia are much more prone to malignant transformation, and for this reason it is important to understand their normal regulation and to determine any differences that become apparent in transformed cells. Colorectal tumor development is a multistep process that is known to depend on the deregulation or mutation of certain critical genes. It is generally accepted that adenomas represent the first stage of neoplasia, and one of the earliest molecular changes leading to the development of adenomas is mutation in the tumor suppressor gene APC. 2 Certain oncogenes are also often mutated in adenomas (eg, K-RAS). Although the mechanism has not yet been elucidated and their role in tumorigenesis is unclear, members of the carcinoembryonic antigen (CEA) gene family also become deregulated in adenomas. 3,4 From a clinical view, it would be of paramount interest to be able to recognize neoplastic growth as early as possible. For example, hyperplastic polyps may be precursors of neoplasia. Indeed, patients with hyperplastic polyps have been reported to have an increased risk of developing adenomas. 5 Among the hyperplastic polyps analyzed from 22 patients, none were found to contain APC mutations, but five carried a K-RAS mutation. 6 Thus hyperplastic epithelial cells may already be primed for neoplastic transformation. It would be of particular clinical interest to clarify the existence of a neoplastic predisposition in hyperplastic polyps. This could be approached by documenting molecular differences in hyperplastic polyps and normal colorectal epithelial cells. In this context, it would be interesting to see if other markers of neoplasia (eg, members of the CEA family) are deregulated in hyperplastic polyps.
CEA is a classical tumor marker for several types of adenocarcinoma, especially those of colorectal origin. 7 Although it is much more prevalent in colorectal tumors compared to the corresponding normal tissue, this may be due to differences in clearance rather than to differential expression. 8 CEA is the name-giving member of a family of molecules, three members of which are coexpressed with CEA in the normal colorectal mucosa. 9 According to the new official nomenclature system decided at the 9th International CEA/PSG Workshop (Ratzeburg, Germany, September 1998), these three family members are CEACAM1 (formerly BGP), CEACAM6 (formerly NCA-50/90), and CEACAM7 (formerly CGM2). 10 In colorectal cancers, these family members become deregulated. CEACAM1 is down-regulated, 11 whereby both mouse (formerly Bgp) and rat CEACAM1 (formerly C-CAM) have been shown to block proliferation after transfection into colorectal and prostatic tumor cell lines. 12,13 CEACAM7 reveals an expression pattern similar to that of CEACAM1 in the normal colonic mucosa and is down-regulated in colorectal tumors; however, nothing is known about its function and the protein is not well characterized to date. Interestingly, CEACAM6 expression is up-regulated in colorectal tumors compared to the normal mucosa, 11 suggesting a role opposite to that of CEACAM1 and/or CEACAM7 during colorectal tumorigenesis. Although it is known that CEACAM6 is expressed in different tissues, 14 no systematic analysis has been carried out to date to determine its expression pattern in the human body. Apart from its putative role in colorectal tumorigenesis, CEACAM6, along with other CEA family members, has also been reported to serve as a receptor for mediating adherence and entry of Neisseria bacteria into human tissues. 15 Thus knowledge of their expression in a given tissue should give an indication of susceptibility to Neisserial infections.
In this study we developed and tested a new monoclonal antibody against CEACAM7 (BAC2), as a previously developed CEACAM7 monoclonal antibody named CAC2 3 has since been found to cross-react with CEACAM6. We then used this specific BAC2 monoclonal antibody to analyze CEACAM7 expression and have compared it to that of CEACAM6 in 35 normal organs and during development in the fetal colon by immunohistochemical staining. Their expression patterns were also compared in hyperplastic colorectal polyps and adenomas of various histological types. Finally, CEACAM6 and CEACAM7 expression in the normal colon has been compared to the expression of Ki67 (a marker for cell proliferation) and CD95 (a marker for apoptotically sensitive cells), as well as cells that have undergone apoptosis, which has been assessed by a DNA degradation (TUNEL) test.
Materials and Methods
Tissue and Tumor Source
All adult and fetal organs were from the Institute of Pathology, University Hospital, Freiburg. Adenomas and hyperplastic colorectal epithelia were provided by the Department of Surgery, University Hospital, Freiburg. The tissues were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) (10 mmol/L NaH2PO4, 43 mmol/L K2HPO4, and 123 mmol/L NaCl) for 24–48 hours, followed by a 24-hour incubation in 0.5 mol/L sucrose in PBS before being embedded in Jung Freeze medium (Leica Instruments, Nussloch, Germany), which was diluted with an equal volume of water. This was followed by freezing on dry ice or direct freezing in a cryomicrotome at −40°C. Alternatively, unfixed tissues were transported in Dulbecco’s modified Eagle’s medium (DMEM) and directly embedded and frozen. Frozen blocks were stored at −70°C before cryosectioning.
Cryosectioning, Immunohistochemistry, and Determination of Apoptotic Cells
Cryosections of 7 μm thickness were made on a Frigocut 2700 cryotome (Reichert and Jung/Leica Instruments, Nussloch, Germany) and transferred to Superfrost slides (Roth, Karlsruhe, Germany). After drying under a hood at ambient temperature for 30 minutes, the sections were stored at −70°C before immunostaining.
The following monoclonal antibodies were used for immunohistochemical staining: 9A6, which has been shown elsewhere to be specific for CEACAM6 16 ; 4/3/17, which recognizes CEACAM1 and CEA 17 ; 26/3/13, which has specificity for CEA 18 ; CAC2 3 and BAC2 (see below), which, respectively, recognize and are specific for CEACAM7; Mib-1 (Dako, Hamburg, Germany), which recognizes Ki-67; and Ab-1 (Pharmingen, Hamburg, Germany), which is specific for the human FAS receptor (CD95). In all cases, peroxidase-labeled, rabbit anti-mouse IgG or IgM, with minimal cross-reactivity to human serum proteins (Dianova, Hamburg, Germany), was used as the second antibody. Immunostaining was as described previously. 19 Briefly, the sections were thawed for 15 minutes at ambient temperature under a hood and rehydrated for 3 minutes in PBS. Endogenous peroxidase activity was blocked by incubation in 0.3% H2O2 in methanol for 30 minutes, and the sections were washed in PBS. The sections were preincubated in 3% ovalbumin/PBS (Sigma, Deisenhofen, Germany) for 30 minutes in a moist chamber, washed in PBS, and incubated at 4°C for 12–16 hours with the monoclonal antibodies at a concentration of 5–10 μg/ml PBS, or using undiluted hybridoma supernatants for BAC2 (see below). As negative controls, sections were incubated in PBS or cell culture medium. After washing in PBS, the peroxidase-coupled second antibody was used at a concentration of 5–10 μg/ml PBS for 1–2 hours at ambient temperature. The color reaction was achieved using diaminobenzidine as substrate. Counterstaining was omitted or was very brief (1 s) with Mayer’s hemalaun. After dehydration, the sections were mounted in VitroClud (Leica Instruments) for microscopy.
Apoptotic cells were visualized with the Cell Death Detection Kit POD (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer’s instructions.
Development of a CEACAM7-Specific Monoclonal Antibody and Specificity Testing
The development of monoclonal antibodies that recognize CEACAM7 has already been described. 3 The same procedure was used to immunize a second CEA-transgenic mouse for the establishment of hybridoma cells that produce antibodies that recognize a CEACAM7/human IgG-Fc fusion protein but not a Ceacam10 (formerly Cea10)/human IgG-Fc fusion protein, proving specificity of the antibodies for the CEACAM7 moiety. One clone (BAC2) was expanded after the first subcloning step. Specificity testing was carried out by FACScan analyses against various HeLa or Chinese hamster ovary (CHO) cell lines that had been stably transfected with cDNA expression vectors for individual members of the CEA family, as described previously. 3 The PSG1 transfectant and the monoclonal antibody BAP1 have been described elsewhere. 20 A newly developed CHO transfectant was included that expresses CEACAM7 and was recently developed in our laboratory with the use of CEACAM7 cDNA that was described elsewhere. 21 This cDNA was subcloned into the pBHE expression vector before transfection using lipofectamine, according to the manufacturer’s instructions (Life Technologies, Eggenstein, Germany). Stable transfectants expressing CEACAM7 were identified by FACScan analyses using the CEACAM7/CEACAM6-recognizing monoclonal antibody CAC2. 3
Results
BAC2 Monoclonal Antibody Reveals Specificity for CEACAM7
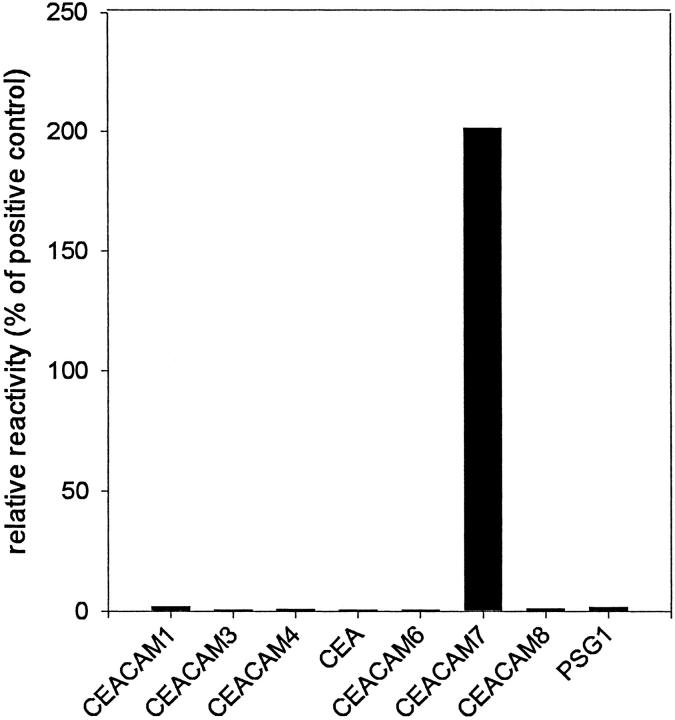
The specificity of the newly developed BAC2 monoclonal antibody was determined in FACScan analyses against HeLa transfectants that each stably express CEA, CEACAM3 (formerly CGM1), CEACAM6, CEACAM8 (formerly CGM6/NCA-95), and a recombinant membrane-bound PSG1 on their surfaces and against CHO transfectants that each stably express CEACAM1, CEACAM4 (formerly CGM7), and CEACAM7. As a negative control, transfectants that have stably integrated the empty expression vector (HeLa-neo) or the parental cells (CHO-K1) were tested. Positive controls were carried out for each transfectant, using a monoclonal antibody (D14HD11) that recognizes CEACAM1, CEACAM3, CEACAM4, CEACAM6 and CEA, or a monoclonal antibody (80H3) that recognizes CEACAM8, a monoclonal antibody that recognizes PSG (BAP1), as well as the CAC2 monoclonal antibody that recognizes CEACAM7. These results are summarized in Figure 1 ▶ . It is obvious that BAC2 only reacts with the CEACAM7 transfectant and recognizes no other CEA family transfectants.
Figure 1.
Determination of specificity of the BAC2 monoclonal antibody for CEACAM7 by FACScan analyses. Stable transfectants expressing individual CEA family members were incubated with BAC2 to determine its specificity. Stable transfectants expressing CEACAM1, CEACAM4, and CEACAM7 are CHO cells and CEA, CEACAM3, CEACAM6, CEACAM8, and a recombinant membrane-bound PSG1 are HeLa cells. As negative controls, the parental CHO-K1 and HeLa-neo cells were both negative with all antibodies (not shown). For each transfectant, an isotype-matched murine IgG was also included as a negative control, and the mean fluorescence values (ranging from 2 to 10), were subtracted from the values obtained with BAC2 or the positive control antibodies. The D14HD11 antibody was used as a positive control for the CEA, CEACAM1, CEACAM3, CEACAM4, and CEACAM6 transfectants, 80H3 was used for CEACAM8, BAP1 was used for the PSG1 transfectant, and CAC2 was used for CEACAM7. The ratio of the mean fluorescences obtained with BAC2 and the respective positive control antibody (×100) was calculated (relative reactivity). The mean fluorescence values of the control antibodies were between 260 and 880.
To rule out any cross-reactivity of BAC2 with CEA, CEACAM1, CEACAM6, or PSGs in immunohistochemical analyses, its staining pattern was compared with that of other CEA family-recognizing monoclonal antibodies with known specificities. These results are summarized in Table 1 ▶ . CEACAM1 was found in the liver, kidney, submandibulary gland, and placenta, using the 4/3/17 monoclonal antibody, which recognizes CEACAM1 and CEA (the latter is absent from these organs). BAC2 did not stain these organs and thus does not cross-react with CEACAM1. Negativity of BAC2 in the placenta also rules out cross-reactivity with PSG subgroup members of the CEA family. A CEACAM6-specific monoclonal antibody (9A6) and CAC2 stained granulocytes in the bone marrow, whereas BAC2 did not. Finally, cross-reactivity against CEA and CEACAM6 could be ruled out because CEA and CEACAM6-positive colorectal tumors were not stained using BAC2, whereas CAC2 did reveal cross-reactivity. Cross-reactivity of CAC2 was not recognized in previous immunohistological analyses under the conditions used. 3 Only colonic epithelial cells were stained with BAC2 in these tests, confirming the specificity of this newly developed monoclonal antibody for CEACAM7.
Table 1.
Immunohistochemical Specificity Test for BAC2 Monoclonal Antibody
| Monoclonal antibody | BAC2 | CAC2 | 9A6 | 26-3-13 | 4-3-17 |
|---|---|---|---|---|---|
| Antigen recognized | CEACAM7 | CEACAM6+ CEACAM7 | CEACAM6 | CEA | CEA+ CEACAM1 |
| Colon | + | + | + | + | + |
| Colon carcinoma | − | + | + | + | + |
| Liver | − | − | − | − | + |
| Kidney | − | − | − | − | + |
| Submandibulary gland | − | + | + | − | + |
| Placenta | − | − | − | − | + |
| Granulocytes | − | + | + | − | + |
+, positively staining cells.
Expression of CEACAM6 and CEACAM7 in Normal Adult and Fetal Tissues
To gain more insight into the possible functions of CEACAM6 and CEACAM7, their expression was investigated in a variety of normal tissues. For these analyses, 35 different organs and tissues were tested with the antibody 9A6, which has been shown elsewhere to be specific for CEACAM6 16 and the CEACAM7-specific antibody BAC2. The results of these immunohistochemical analyses are summarized in Table 2 ▶ , and chosen examples are depicted in Figure 2 ▶ . Tissues from between two and five individuals were tested in most cases, apart from thyroid, adrenal gland, heart, urether, bladder, and bone marrow, which were only tested from one individual each. Maximum staining intensity was achieved on nonfixed cryosections.
Table 2.
Immunohistochemical Analysis of CEACAM6 and CEACAM7 Expression in Different Normal Organs and Tissues
| Monoclonal antibody antigen recognized | 9A6 | BAC2 | Expression pattern |
|---|---|---|---|
| CEACAM6 | CEACAM7 | ||
| Tongue | + | − | Upper third of squamous epithelia |
| Esophagus | + | − | Squamous epithelia |
| Stomach body | + | − | Single crypts, luminal cells |
| Duodenum | − | − | |
| Jejunum | − | − | |
| Ileum | − | − | |
| Appendix | + | + | Epithelia, upper fifth of crypt for each antigen |
| Colon | + | + | Epithelia, upper fifth of crypt for each antigen |
| Thyroid gland | − | − | |
| Submandibular salivary gland | + | − | Mucous epithelia most prominent |
| Anterior lingual gland | + | − | Mucous epithelia most prominent |
| Pancreas | + | + | Small ducts, with BAC2 only individual cells |
| Breast | + | − | Some breast ducts |
| Adrenal gland | − | − | |
| Liver | + | − | Granulocytes |
| Gall bladder | + | − | Apical epithelia |
| Lung | + | − | Pneumocytes, bronchiole epithelia, granulocytes |
| Kidney | − | − | |
| Bladder | − | − | |
| Skin | + | − | Eccrine sweat glands, hair follicles, squamous epithelia |
| Bone marrow | + | − | Myeloid cells |
| Tonsils | + | − | Epithelia and granulocytes |
| Spleen | + | − | Myeloid cells |
| Prostate gland | + | − | Single ducts |
| Fallopian tube | − | − | |
| Cervix | + | − | Squamous epithelia |
| Uterus | − | − | |
| Ovary | − | − | |
| Testis | − | − | |
| Seminal vesicle | − | − | |
| Epididymus | − | − | |
| Heart | − | − | |
| Urether | − | − | |
| Umbilical cord | + | − | Amnion epithelia |
| Placenta | + | − | Amnion epithelia |
Figure 2.
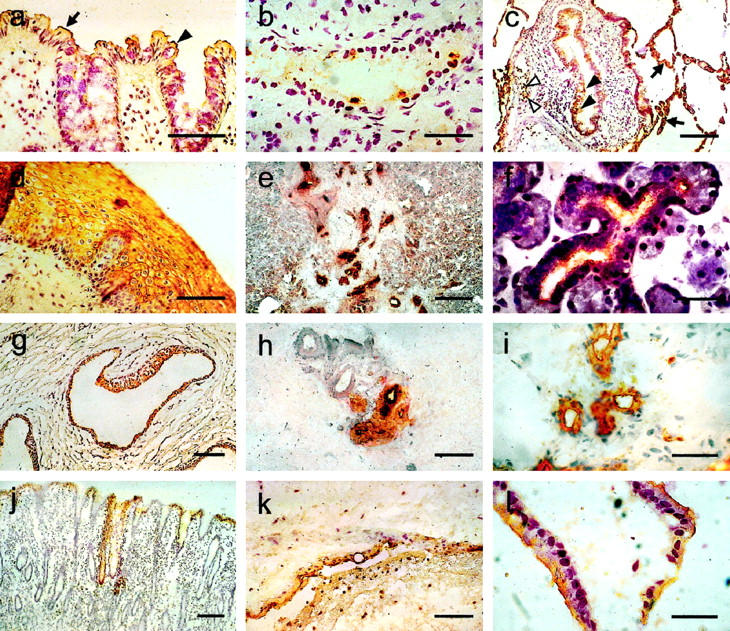
Immunohistochemical staining of different human tissues, using monoclonal antibodies to determine the expression patterns of CEACAM6 and CEACAM7. Cryosectioned tissues were incubated with BAC2 to locate CEACAM7 (a and b) or with 9A6 to locate CEACAM6 (c–l), followed by a peroxidase-labeled anti-mouse antibody, staining with diaminobenzidine and counterstaining with Mayer’s hemalaun. The following tissues are shown: a: colon, showing CEACAM7 on absorptive epithelial cells (arrow) and goblet cells (arrowhead); b: pancreatic duct; c: lung, showing CEACAM6 expression in pneumocytes (arrows), bronchiolar epithelia (filled arrowheads), and granulocytes (open arrowheads); d: esophagus; e: pancreas; f: submandibular salivary gland; g: prostate gland; h: breast; i: eccrine sweat gland; j: stomach; k: placenta, amnion epithelia; and l: gall bladder. Magnification bars, 100 μm (a, d, g, h, and k), 50 μm (b, f, i, and l), and 200 μm (c, e, and j).
CEACAM7 revealed a very narrow expression pattern, being restricted to highly differentiated epithelial cells in the upper fifth of the colorectal crypts (Figures 2a and 3j) ▶ ▶ and to the apical surface of isolated epithelial cells lining small ducts within the pancreas (Figure 2b) ▶ . All other tissues and organs tested were negative for CEACAM7 (Table 2) ▶ . In contrast, CEACAM6 revealed a comparatively broad expression pattern, being found in granulocytes (Figure 2c) ▶ and in epithelial cells in a variety of organs (Figure 2, d–l) ▶ . Positively staining squamous epithelia reveal nonpolarized cell surface localization and cytoplasmic staining (Figure 2d) ▶ In simple epithelia, CEACAM6 was present on the apical surface. Organs rich in granulocytes (eg, bone marrow and spleen) revealed CEACAM6 positivity in those cells (data not shown).
Figure 3.
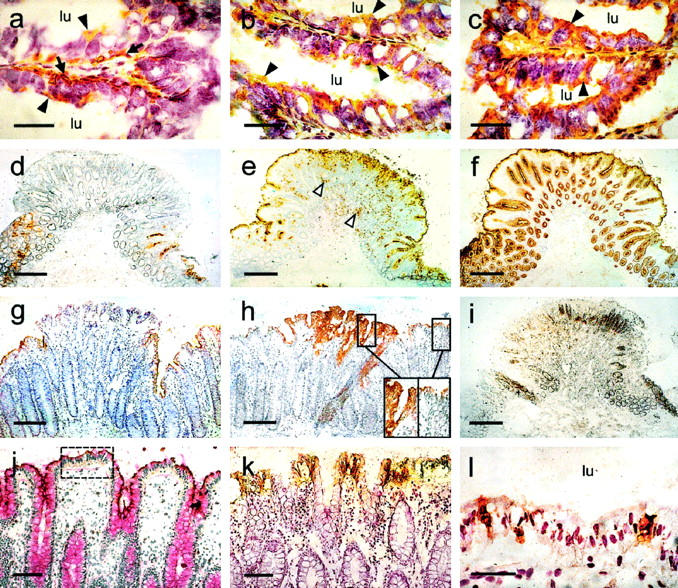
Immunohistochemical analysis of CEA family expression in fetal and adult colon, a tubular adenoma, and a hyperplastic polyp and comparison with cellular proliferation and apoptosis. Cryosectioned tissues were incubated with BAC2, which recognizes CEACAM7 (a, d, g, and j); 9A6, which is specific for CEACAM6 (b, e, and h); 26/3/13, which locates CEA (c and f); Ki-67 to identify highly proliferating cells (i); and Ab-1, which recognizes the human FAS receptor CD95 (k). Peroxidase-labeled anti-mouse antibody and diaminobenzidine staining were used to visualize labeling. Apoptotic cells in the table region of colonic crypts were identified with the TUNEL method to determine DNA degradation (l, brown staining). For counterstaining, Mayer’s hemalaun was used in all cases. The following tissues are shown: fetal colon from the 24th week of pregnancy (a–c), where arrowheads indicate apical cell staining and arrows show perinuclear staining (lu = lumen), a tubular adenoma (d–f, i), a hyperplastic polyp (g and h), including 2:1 magnifications of sections of the polyp and adjacent mucosa (h, insets) and normal colon (j–l), including PAS staining (j). The table region between crypts (j, inset) was studied by TUNEL analysis (l). Magnification bars, 25 μm (a–c), 500 μm (d–f, i), 200 μm (g and h), 100 μm (j and k), and 50 μm (i).
In addition, the expression of CEACAM6 and CEACAM7 was also investigated during fetal development of the colon and compared to that of CEA. Six fetuses ranging from the 18th to the 35th weeks of pregnancy as well as colon from a 2-day-old child, a 10-day-old child, and an adult were used for this study. All three CEA family members revealed an apical expression throughout development (Figure 3, a–c) ▶ , which was continued post partum. Whereas CEA was expressed on the apical surface of all epithelial cells down to the base of the crypt, CEACAM6 was only found in the upper half and CEACAM7 in the upper third of the developing crypts. In contrast to CEA and CEACAM6, CEACAM7 also revealed a strong perinuclear staining in the epithelial cells of the upper crypt throughout fetal development (Figure 3a) ▶ , which became strongly reduced at day 2 and was no longer seen at day 10 post partum. Chromogranin staining was also carried out on a serial section at the 24th week of pregnancy to identify enterochromaffin cells. The CEACAM7 perinuclear staining pattern did not match that of the enterochromaffin cells, which were few in number and were located at various positions throughout the crypts (data not shown).
CEACAM6 and CEACAM7 Are Oppositely Deregulated in Hyperplastic Polyps and Adenomas
To determine when CEACAM6 and CEACAM7 become deregulated during tumorigenesis, their expression patterns were investigated in 25 colorectal polyps. In five cases, only biopsy specimens were studied, otherwise whole adenomas with adjacent mucosa were analyzed. These results are summarized in Table 3 ▶ . Eight polyps were hyperplastic, and the rest represented adenomas of various sizes and morphological types (12 tubular, four tubulovillous, and one adenoma with atypia). CEACAM6 was expressed in all polyps (25/25) and often revealed a broader expression zone in the upper crypt epithelia than in the adjacent mucosa (21/25; Figure 3h ▶ ). In parallel, a loss of polarity was observed, with expression seen not only on the apical surface but throughout the cytoplasm and on the basolateral cell membranes (Figure 3h ▶ , inset). In one tubular adenoma, no broader expression zone was found for CEACAM6, but many granulocytes within the polyp stained positive, indicating inflammation (Figure 3e) ▶ . In comparison, CEA is shown to be located down to the base of the crypts and throughout the same tubular adenoma (Figure 3f) ▶ . In contrast, CEACAM7 was absent from all 16 polyps that could be analyzed for CEACAM7 expression. The adjacent mucosa was positive for CEACAM7 in all but one case (15/16). The loss of expression of CEACAM7 is shown in a tubular adenoma (Figure 3d) ▶ and in a hyperplastic polyp (Figure 3g) ▶ .
Table 3.
Expression of CEACAM6 and CEACAM7 in Polyps and Adjacent Mucosa
| Polyp | Polyp size (mm) | CEACAM7 expression* | CEACAM6 expression† | ||
|---|---|---|---|---|---|
| Polyp | Normal mucosa | Polyp | Normal mucosa | ||
| Hyperplastic polyps | |||||
| 1‡ | 0.5 × 1.0 | − | − | ++ | + |
| 2 | 1.5 × 0.5 | − | + | +/− | + |
| 3‡ | 1.0 × 1.0 | − | − | ++ | + |
| 4‡ | 1.5 × 1.0 | − | − | ++ | + |
| 5 | 2.0 × 0.5 | − | + | ++ | + |
| 6 | 2.0 × 2.0 | − | + | + | + |
| 7‡ | 2.0 × 2.0 | − | − | ++ | + |
| 8‡ | 2.0 × 2.0 | − | − | ++ | + |
| Tubular adenomas | |||||
| 9‡ | 1.5 × 1.0 | − | − | ++ | + |
| 10‡ | 2.0 × 1.5 | − | − | ++ | + |
| 11 | 2.0 × 1.5 | − | + | + | + |
| 12 | 2.5 × 2.0 | − | + | ++ | + |
| 13 | 3.0 × 2.0 | − | + | ++ | + |
| 14‡ | 3.0 × 3.0 | − | − | ++ | + |
| 15 | 4.0 × 2.0 | − | + | + | + |
| 16‡ | 5.0 × 5.0 | − | − | ++ | + |
| 17 | >5 | − | + | ++ | + |
| 18 | <5 | − | + | ++ | + |
| 19 | <10 | − | + | ++ | + |
| 20 | 14.0 × 12.0 | − | − | ++ | + |
| Tubulovillous adenomas | |||||
| 21 | 4.0 × 4.0 | − | + | ++ | + |
| 22 | <5 | − | + | ++ | + |
| 23 | >5 | − | + | ++ | + |
| 24 | 11.0 × 11.0 | − | + | ++ | + |
| Adenoma with atypia | |||||
| 25 | 14.0 × 10.0 | − | + | ++ | + |
*Expression of CEACAM7 was found on the apical surface of epithelial cells from the upper fifth of the crypts (+) or was absent (−); it was always absent from polyps.
†Expression of CEACAM6 in polyps revealed either a broader expression zone (++), or was comparable (+), or was reduced (+/−) compared to the adjacent mucosa (+).
‡These tissue samples were fixed with formaldehyde for more than 4 days; all other samples were unfixed or fixed for <2 days.
CEACAM6 and CEACAM7 Expression Correlate with CD95 Expression and Apoptosis in Normal Colon
The FAS receptor CD95 can induce apoptosis after interacting with its ligand, FAS, and is therefore a marker for cells that are predisposed to apoptosis. With the use of the CD95-specific monoclonal antibody Ab-1 for immunostaining, the FAS receptor was found to be expressed basolaterally on epithelial cells from the table region at the top of the crypts in the normal colon (Figure 3k) ▶ . Therefore, in normal colon the expression of CD95 appears to overlap with that of CEACAM6 and CEACAM7, which are also expressed in the upper parts of the crypt (Figure 3, g, h, and j) ▶ .
To determine which cells have undergone apoptosis, DNA fragmentation was investigated on sections with the terminal deoxynucleotidyl transferase-mediated biotin-dUTP nick end labeling (TUNEL) method. In the normal colon, two regions within the crypt revealed the presence of apoptotic cells. One region was toward the base of the crypts (data not shown), and the other was in the table region between adjacent crypts (Figure 3l) ▶ . In the latter but not the former case, this overlapped with CEACAM6 and CEACAM7 expression.
Loss of CEACAM7 Expression in Adenomas Correlates with a Shift in the Localization of the Zone of Highly Proliferating Cells
Ki-67 is a marker of proliferating cells. Its expression was studied in normal colonic tissue and in polyps, using a specific monoclonal antibody (Mib-1). Ki-67 was localized in epithelial cells at the base of the crypts in the lower fifth in the normal colon (Figure 3i) ▶ . In the transition region from normal colon to a hyperproliferative polyp, Ki-67 localization moved toward the upper part of the crypts, and the basally located epithelial cells became negative (Figure 3i) ▶ . In the two adenomas studied, when Ki-67 expression started to move up the crypts, CEACAM7 expression at the top of the crypts disappeared (cf. Figure 3, i and d ▶ ).
Discussion
In a previous publication we described the development of monoclonal antibodies CAC1 and CAC2, which specifically recognized the CEACAM7 protein in immunohistochemical staining of colonic mucosa and did not stain tumors or cross-react with other family members under the conditions applied. 3 For those analyses, hybridoma supernatants were used. Under these conditions, a weak but definite cross-reactivity with CEACAM6 was observed in FACScan analyses. In the meantime, these antibodies have been purified, and now immunohistochemical cross-reactivity has also been found against CEACAM6 (see Table 1 ▶ ). For this reason, we have developed a new monoclonal antibody (BAC2), using the same procedure as described previously. 3 BAC2 revealed CEACAM7 specificity in both FACScan (Figure 1) ▶ and immunohistochemical (Table 1) ▶ analyses. With the help of this reagent, we have now analyzed the expression pattern of CEACAM7 in a large variety of normal tissues.
Interestingly, CEACAM7 has a very narrow expression spectrum, being found only on the apical surface of highly differentiated epithelial cells of the rectum and colon and on single epithelial cells lining pancreatic ducts. In the colon, both absorptive and goblet epithelial cells were positively stained (Figure 2a) ▶ , which we previously reported CEACAM7-positive at the mRNA level by in situ hybridization analyses. 3 CEACAM7 was absent from all other tissues and organs that were tested, including all other regions of the intestine. These data correlate well with results gained from comparing the CEACAM7 cDNA sequence with data available in the dbEST gene bank (www.ncbi.nlm.nih.gov/dbEST; 22 ), an expression data bank that has been compiled using a collection of 1.8 × 10 6 partial cDNA sequences from over 50 human adult and fetal tissues. In this data bank, CEACAM7 sequences were found only in cDNA libraries from human colon, colonic tumors, pancreas, and pancreatic islets. The existence of CEACAM7 mRNAs in colonic tumors and pancreatic islets is thought to be due to contamination from normal mucosa and pancreatic ducts, respectively. Based on this assumption, it can be concluded that the transcriptional and translational expression patterns of CEACAM7 are identical in normal tissues. This narrow expression pattern indicates a highly specialized function for CEACAM7 in the pancreatic and colonic epithelial cells, where it is expressed on the apical surface in both cases.
Despite the fact that the expression pattern of CEACAM6 is broader than that of CEACAM7 with respect to its presence in a variety of organs, it is generally restricted to two cell types, ie, epithelial cells and myeloid cells. Our studies confirmed the expression of CEACAM6 in the following tissues and cells, which has already been described elsewhere: granulocytes, macrophages, and monocytes 23,24 ; colonic epithelial cells 18 ; pneumocytes and bronchiole epithelia 25 ; pancreatic ducts and tonsil epithelia 26 ; sweat glands 27 ; and skin and hair follicles. 28 In addition, we have found CEACAM6 in squamous epithelia of the esophagus, cervix, and tongue. Single crypts in the main part of the stomach expressed CEACAM6 on the luminal surface. Gall bladder epithelia revealed apical staining. In the salivary glands, the mucous epithelial cells were more strongly positive than the serous epithelial cells. Some breast ducts expressed CEACAM6, whereas others were negative. In the prostate gland, CEACAM6 was found in single tubuli as well as in hyperplastic or neoplastic tubuli.
Although CEACAM7 is apparently coexpressed with CEACAM6 and CEA in the fetal colon, one major difference is obvious. Whereas CEA and CEACAM6 are apically located throughout development, with some cytoplasmic staining also seen in fetal gut, CEACAM7 is primarily located at the base of the epithelial cells, in what appears to be a perinuclear location. Shortly after birth (day 2) the location of CEACAM7 is much less pronounced in the perinuclear region, and at day 10 it is only found on the apical surface. The reason for this shift is not understood, but as it correlates with birth, it indicates that CEACAM7 may be stored within the epithelial cells until the intestine begins its normal functions. It also indicates a unique function for CEACAM7 compared to CEA and CEACAM6 in the colon. Interestingly, there are no novel features recognizable in the primary structure of the derived CEACAM7 protein that would offer an explanation for this phenomenon. All three CEA family members are apparently GPI-linked to the plasma membrane, 9 although sequence differences from the other CEA family members in the hydrophobic GPI-signal region could have some influence in holding CEACAM7 back in epithelial cells of the fetal gut.
Despite the fact that CEACAM6 and CEACAM7 reveal similar expression patterns in the normal colonic mucosa, being coexpressed in highly differentiated epithelial cells, they become oppositely deregulated in hyperproliferating mucosa, adenomas, and carcinomas. Whereas CEACAM7 is completely down-regulated, CEACAM6 becomes up-regulated, with a broader expression zone at the top of the hyperproliferating polyps or adenomas, where the polarity of its expression is often lost. Rather than being limited to the apical surface, CEACAM6 was often seen on the whole of the outer membrane. It has been reported elsewhere that CEACAM6 is up-regulated in colonic 11,29 and other tumors, 30 where it could play a role in promoting tumor progression, as has been shown for CEA. 31,32 Indeed, it is known that both CEA and CEACAM6 can function in vitro as cellular adhesion molecules, and this property has been suggested to influence tissue architecture. 33 It has been suggested that loss of polarity of CEA and CEACAM6 might favor tumor development by causing a reversion of the monolayered adult colonic epithelium to an embryonic multilayered configuration. Interestingly, we found that squamous epithelia often express CEACAM6 on the whole of their cell surface, and they, too, reveal a multilayered arrangement. It has also been reported that both CEA and CEACAM6, but not CEACAM1, inhibit cell differentiation when expressed ectopically in L6 myoblasts and adipocytes and when overexpressed in colonocytes. 33 This property would also favor tumor development. CEACAM7 might play an opposing role in tumor development, by suppressing colorectal tumor growth. This is in analogy with the tumor suppressor activity that has been shown for rat and mouse CEACAM1 in prostatic 13 and colonic tumors. 12 Indeed, the expression pattern of CEACAM7 in the normal colon mucosa and its down-regulation in adenomas/carcinomas are very similar to those of CEACAM1. 3 We previously postulated that these two CEA family members, along with CEA and CEACAM6, could form a functional molecular complex in the epithelial cell membrane, as suggested for CEA family members in granulocytes 34 and similar to those found for other immunoglobulin superfamily members (eg, B- and T-cell receptors). 35 Signal transduction via such a CEA family, multimolecular complex could be modulated by the relative stoichiometries of the individual components. It has been shown that CEACAM1 or its rodent equivalents can interact with serine/threonine protein kinases, 36,37 tyrosine kinases, 38,39 and tyrosine phosphatases, 40 so that different signaling pathways via this molecule are plausible. Furthermore, the GPI-linked CEA family members have also been shown to be involved in signal transduction, although the mechanism still remains unclear. 41 Thus, depending on the relationship of its components to each other, such a CEA family complex may execute either a tumor suppressor or a tumor-promoting effect through different signaling pathways.
We have found apoptosis in epithelial cells located in the upper crypt region of the colon, which confirms similar data reported by others. 42-44 Furthermore, expression of the FAS receptor, a putative initiator of apoptosis in colonocytes, has been found in the same region, by us and by other groups. 45,46 It has been reported elsewhere that after incubation of colon carcinoma cells with an antibody that binds the FAS receptor, the tumor cells detached from their substrate. 47 Thus stimulation of the FAS receptor could also lead to exfoliation of the epithelial cells. Our data not only confirm the expression of the FAS receptor and the presence of apoptotic cells, but also reveal an overlap with CEACAM6 and CEACAM7 expression in those epithelial cells located in the intercryptal table region of normal crypts. It has been shown that expression of the FAS receptor is down-regulated in most colorectal adenocarcinomas. 48 This correlates with the down-regulation of CEACAM7 in adenocarcinomas. Indeed, we already find down-regulation of CEACAM7 in hyperplastic polyps coupled with a slight reduction in the number of apoptotic cells, compared with the neighboring normal mucosa. In the adenomas analyzed, there is a more pronounced decrease in the number of apoptotic cells compared with the surrounding mucosa (data not shown). Therefore, the loss of CEACAM7 expression in polyps seems to parallel the reduction of apoptotic events. Similar results for a reduction of apoptosis in adenomas have been reported elsewhere, 49 whereas others describe an increase in apoptosis in hyperplastic polyps and adenomas compared with normal colonic mucosa. 50,51 Based on our observation that CEACAM7 is only expressed on fully differentiated epithelial cells in the upper crypt region, the loss of CEACAM7 expression suggests that the epithelial cell in the hyperplastic polyps cannot fully differentiate. This loss of expression also coincides with a shift in the proliferation zone in two adenomas studied (determined by Ki-67 expression), which moves from the base of neighboring normal crypts progressively to the upper cryptal regions of the adenoma (Figure 3i) ▶ . This Ki67 expression shift has also been reported by others in colonic adenomatous polyps. 49 Despite these correlations it still remains to be proved that CEACAM7 expression has any influence on apoptosis or cell proliferation in colonic epithelial cells from the table region, but experiments are currently under way in our laboratory to test this and other possible functions.
Expression of CEACAM6 in the colon overlaps with FAS receptor expression and apoptotic events in normal crypts but not in adenomas. This apparent paradox may be explained through the dysregulation of the putative CEA family molecular complex discussed above, assuming that these molecules are involved in tumorigenesis, which, however, still remains to be proved. Although further investigations are necessary to confirm these speculations, based on the results gained thus far, it can be stated that deregulation of CEACAM6 and CEACAM7 in hyperplastic polyps and adenomas represents some of the earliest molecular changes in colonic cells that are primed for neoplasia.
Acknowledgments
We acknowledge Prof. Sabine von Kleist for her continuous support. We also thank Dirk Schillinger and Sabine Lutz for their excellent technical assistance.
Footnotes
Address reprint requests to Dr. John Thompson, Institute of Molecular Medicine and Cell Research, University of Freiburg, Stefan-Meier-Strasse 8, D-79104 Freiburg, Germany. E-mail: joth@uni-freiburg.de.
Supported by the Dr. Mildred Scheel Stiftung für Krebsforschung.
References
- 1.Potten CS, Booth C, Pritchard DM: The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol 1997, 78:219-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinzler KW, Vogelstein B: Lessons from hereditary colorectal cancer. Cell 1996, 87:159-170 [DOI] [PubMed] [Google Scholar]
- 3.Thompson J, Seitz M, Chastre E, Ditter M, Aldrian C, Gespach C, Zimmermann W: Down-regulation of carcinoembryonic antigen-family member 2 expression is an early event in colorectal tumorigenesis. Cancer Res 1997, 57:1776-1784 [PubMed] [Google Scholar]
- 4.Nollau P, Scheller H, Kona-Horstmann M, Rohde S, Hagenmüller F, Wagener C, Neumaier M: Expression of CD66a (human C-CAM) and other members of adhesion molecules in human colorectal adenomas. Cancer Res 1997, 57:2354-2357 [PubMed] [Google Scholar]
- 5.Croizet O, Moreau J, Arany Y, Delvaux M, Rumeau JL, Escourrou J: Follow-up of patients with hyperplastic polyps of the large bowel. Gastrointest Endosc 1997, 46:119-123 [DOI] [PubMed] [Google Scholar]
- 6.Jen J, Powell SM, Papadopoulos N, Smith KJ, Hamilton SR, Vogelstein B, Kinzler KW: Molecular determinants of dysplasia in colorectal lesions. Cancer Res 1994, 54:5523-5526 [PubMed] [Google Scholar]
- 7.Ballesta AM, Molina R, Filella X, Jo J, Gimenez N: Carcinoembryonic antigen in staging and follow-up of patients with solid tumors. Tumor Biol 1995, 16:32-41 [DOI] [PubMed] [Google Scholar]
- 8.Kuroki M, Arakawa F, Yamamoto H, Shimura H, Ikehara Y, Matsuoka Y: Active production and membrane anchoring of carcinoembryonic antigen observed in normal colon mucosa. Cancer Lett 1988, 43:151-157 [DOI] [PubMed] [Google Scholar]
- 9.Thompson JA, Grunert F, Zimmermann W: Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal 1991, 5:344-366 [DOI] [PubMed] [Google Scholar]
- 10.Beauchemin N, Draber P, Dveksler G, Gold P, Gray-Owen S, Grunert F, Hammarström S, Holmes KV, Karlson A, Kuroki M, Lin SH, Lucka L, Najjar SM, Neumaier M, Öbrink B, Shively JE, Skubitz KM, Stanners CP, Thomas P, Thompson JA, Virji M, von Kleist S, Wagener C, Watt S, Zimmermann W: Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res 1999, 252:243–249 [DOI] [PubMed]
- 11.Neumaier M, Paululat S, Chan A, Matthaes P, Wagener C: Biliary glycoprotein, a potential human cell adhesion molecule, is down-regulated in colorectal carcinomas. Proc Natl Acad Sci USA 1993, 90:10744-10748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunath T, Ordonez-Garcia C, Turbide C, Beauchemin N: Inhibition of colonic tumor cell growth by biliary glycoprotein. Oncogene 1995, 11:2375-2382 [PubMed] [Google Scholar]
- 13.Hsieh J-T, Luo W, Song W, Wang Y, Kleinerman DI, Van NT, Lin S-H: Tumor suppressive role of an androgen-regulated epithelial cell adhesion molecule (C-CAM) in prostate carcinoma cell revealed by sense and antisense approaches. Cancer Res 1995, 55:190–197 [PubMed]
- 14.Thompson JA: Molecular cloning and expression of carcinoembryonic antigen gene family members. Tumor Biol 1995, 16:10-16 [DOI] [PubMed] [Google Scholar]
- 15.Bos MP, Grunert F, Belland RJ: Differential recognition of members of the carcinoembryonic antigen family by Opa variants of Neisseria gonorrhoeae. Infect Immun 1997, 65:2353-2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grunert F, Soubt M, Jantscheff P, Nagel G: Specificity and epitope localization of CD66/CD67 mAb. Leucocyte Typing. V. White Cell Differentiation Antigens, vol 1. Edited by SF Schlossman, L Boumsell, W Gilks, JM Harlan, T Kishimoto, C Morimoto, J Ritz, S Shaw, R Silverstein, T Springer, TF Tedder, RF Todd. New York, Oxford University Press, 1995, pp 907–911
- 17.Daniel S, Nagel G, Johnson JP, Lobo FM, Hirn M, Jantscheff P, Kuroki M, von Kleist S, Grunert F: Determination of the specificities of monoclonal antibodies recognizing members of the CEA family using a panel of transfectants. Int J Cancer 1993, 55:303-310 [DOI] [PubMed] [Google Scholar]
- 18.Grunert F, AbuHarfeil N, Schwarz K, von Kleist S: Two CEA, and three NCA species, although distinguishable by monoclonal antibodies, have nearly identical peptide patterns. Int J Cancer 1985, 36:357-362 [PubMed] [Google Scholar]
- 19.Thompson J, Mössinger S, Reichardt V, Engels U, Beauchemin N, Kommoss F, von Kleist S, Zimmermann W: A polymerase chain reaction assay for the specific identification of transcripts encoded by individual carcinoembryonic antigen (CEA) gene family members. Int J Cancer 1993, 55:311-319 [DOI] [PubMed] [Google Scholar]
- 20.Zhou G-Q, Baranov V, Zimmermann W, Grunert F, Erhard B, Mincheva-Nilsson L, Hammarström S, Thompson J: Highly specific monoclonal antibody demonstrates that pregnancy-specific glycoprotein (PSG) is limited to syncytio-trophoblast in human early and term placenta. Placenta 1997, 18:491-501 [DOI] [PubMed] [Google Scholar]
- 21.Thompson J, Zimmermann W, Nollau P, Neumaier M, Weber-Arden J, Schrewe H, Craig I, Willcocks T: CGM2, a member of the carcinoembryonic antigen gene family is down-regulated in colorectal carcinomas. J Biol Chem, 1994, 269:32924–32931 [PubMed]
- 22.Boguski MS: The turning point in genome research. Trends Biochem Sci 1995, 20:295-296 [DOI] [PubMed] [Google Scholar]
- 23.Burtin P, Quan PC, Sabine MC: Nonspecific cross-reacting antigen as a marker for human polymorphs, macrophages and monocytes. Nature 1975, 255:714-716 [DOI] [PubMed] [Google Scholar]
- 24.Bordes M, Knobel S, Martin F: Carcinoembryonic antigen (CEA) and related antigens in blood cells and haematopoietic tissues. Eur J Cancer 1975, 11:783-786 [DOI] [PubMed] [Google Scholar]
- 25.Abbona GC, Papotti M, Gugliotta P, Pecchio F, Rapellino M: Immunohistochemical detection of carcinoembryonic antigen (CEA) in non-neoplastic lung disease. Int J Biol Markers 1993, 8:240-243 [DOI] [PubMed] [Google Scholar]
- 26.Skubitz KM, Grunert F, Jantscheff P, Kuroki M, Skubitz APN: CD66 Family Workshop Panel Report. Leucocyte Typing. VI. White Cell Differentiation Antigens. Edited by T Kishimoto, H Kikutani, AEG von dem Borne, SM Goyert, DY Mason, M Miyasaka, L Moretta, K Okumura, S Shaw, TA Springer, K Sugamura, H Zola. New York and London, Garland Publishing, Taylor and Francis Group, 1997, pp 992–1000
- 27.Metze D, Bhardwaj R, Amann U, Eades-Perner A-M, Neumaier M, Wagener C, Jantscheff P, Grunert F, Luger TA: Glycoproteins of the carcinoembryonic antigen (CEA) family are expressed in sweat and sebaceous glands of human fetal and adult skin. J Invest Dermatol 1996, 106:64-69 [DOI] [PubMed] [Google Scholar]
- 28.Honda Y, Egawa K, Kuroki M, Ono T: Hair cycle-dependent expression of a nonspecific cross-reacting antigen (NCA)-50/90-like molecule on follicular keratinocytes. Arch Dermatol Res 1997, 289:457-465 [DOI] [PubMed] [Google Scholar]
- 29.Higashide T, Hinoda Y, Itoh J, Takahashi H, Satoh Y, Ibayashi Y, Imai K, Yachi A: Detection of mRNAs of carcinoembryonic antigen and nonspecific cross-reacting antigen genes in colorectal adenomas and carcinomas by in situ hybridization. Jpn J Cancer Res 1990, 81:1149-1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chi K, Jessup JM, Frazier ML: Predominant expression of mRNA coding for nonspecific cross-reacting antigen in colorectal carcinomas. Tumor Biol 1991, 12:298-308 [DOI] [PubMed] [Google Scholar]
- 31.Hostetter RB, Augustus LB, Mankarious R, Chi K, Fan D, Toth C, Thomas P, Jessup JM: Carcinoembryonic antigen as a selective enhancer of colorectal cancer metastasis. J Natl Cancer Inst 1990, 82:380-385 [DOI] [PubMed] [Google Scholar]
- 32.Hashino J, Fukuda Y, Oikawa S, Nakazato H, Nakanishi T: Metastatic potential of human colorectal carcinoma SW1222 cells transfected with cDNA encoding carcinoembryonic antigen. Clin Exp Metastasis 1994, 12:324-328 [DOI] [PubMed] [Google Scholar]
- 33.Stanners CP: Contributions of the human CEA family to malignant transformation. Stanners CP eds. Cell Adhesion and Communication Mediated by the CEA Family: Basic and Clinical Perspectives. 1998, :pp 141-154 Harwood Academic Publishers, Amsterdam [Google Scholar]
- 34.Grunert F, Kuroki M, Stocks SC: CEA family members are expressed on hematopoietic cells and their possible role in cell adhesion and signaling. Stanners CP eds. Cell Adhesion and Communication Mediated by the CEA Family: Basic and Clinical Perspectives. 1998, :pp 99-119 Harwood Academic Publishers, Amsterdam [Google Scholar]
- 35.Keegan AD, Paul WE: Multichain immune recognition receptors: similarities in structure and signaling pathways. Immunol Today 1992, 13:63-68 [DOI] [PubMed] [Google Scholar]
- 36.Sippel CJ, Fallon RJ, Perlmutter DH: Bile acid efflux mediated by the rat liver canalicular bile acid transporter/ecto-ATPase protein requires serine 503 phosphorylation and is regulated by tyrosine 488 phosphorylation. J Biol Chem 1994, 269:19535-19545 [PubMed] [Google Scholar]
- 37.Najjar SM, Boisclair YR, Nabih ZT, Philippe N, Imai Y, Suzuki Y, Ooi GT: Cloning and characterization of a functional promoter of the rat pp120 gene, encoding a substrate of the insulin receptor tyrosine kinase. J Biol Chem 1996, 271:8809-8817 [DOI] [PubMed] [Google Scholar]
- 38.Brümmer J, Neumaier M, Göpfert C, Wagner C: Association of pp60/c-src with biliary glycoprotein (CD66a), an adhesion molecule of the carcinoembryonic antigen family downregulated in colorectal carcinomas. Oncogene 1995, 11:1649-1655 [PubMed] [Google Scholar]
- 39.Skubitz KM, Campbell KD, Ahmed K, Skubitz APN: CD66 family members are associated with tyrosine kinase activity in human neutrophils. J Immunol 1995, 155:5382-5390 [PubMed] [Google Scholar]
- 40.Beauchemin N, Kunath T, Robitaille J, Chow B, Turbide C, Daniels E, Veillette A: Association of biliary glycoprotein with protein tyrosine phosphatase SHP-1 in malignant colon epithelial cells. Oncogene 1997, 14:183-190 [DOI] [PubMed] [Google Scholar]
- 41.Dráber P, Skubitz KM: Signal transduction mediated by the CEA family. Stanners CP eds. Cell Adhesion and Communication Mediated by the CEA Family: Basic and Clinical Perspectives. 1998, :pp 121-140 Harwood Academic Publishers, Amsterdam [Google Scholar]
- 42.Hall PA, Coates PJ, Ansari B, Hopwood W: Regulation of cell numbers in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci 1992, 119:3569-3577 [DOI] [PubMed] [Google Scholar]
- 43.Gavrieli Y, Sherman Y, Ben-Sasson SA: Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992, 119:493-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sträter J, Koretz K, Günthert AR, Möller P: In situ detection of enterocytic apoptosis in normal colonic mucosa and in familial adenomatous polyposis. Gut 1995, 37:819-825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwamoto M, Koji T, Makiyama K, Kobayashi N, Nakane PK: Apoptosis of crypt epithelial cells in ulcerative colitis. J Pathol 1996, 180:152-159 [DOI] [PubMed] [Google Scholar]
- 46.Sträter J, Wellisch I, Riedl S, Walczak H, Koretz K, Tandara A, Krammer PH, Möller P: CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: a possible role in ulcerative colitis. Gastroenterology 1997, 113:160-167 [DOI] [PubMed] [Google Scholar]
- 47.Günthert AR, Sträter J, von Reyher U, Henne C, Joos S, Koretz K, Moldenhauer G, Krammer PH, Möller P: Early detachment of colon carcinoma cells during CD95(APO-1/Fas)-mediated apoptosis. 1. De-adhesion from hyaluronate by shedding of CD44. J Cell Biol 1996, 134:1089-1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Möller P, Koretz KI, Leithauser F, Brüderlein S, Henne C, Quentmeier A, Krammer PH: Expression of APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in normal and neoplastic colon epithelium. Int J Cancer 1994, 57:371-377 [DOI] [PubMed] [Google Scholar]
- 49.Moss SF, Liu TC, Petrotos A, Hsu TM, Gold LI, Holt PR: Inward growth of colonic adenomatous polyps. Gastroenterology 1996, 111:1425-1432 [DOI] [PubMed] [Google Scholar]
- 50.Bedi A, Pasricha PJ, Akhtar AJ, Barber JP, Bedi GC, Giardiello FM, Zehnbauer BA, Hamilton SR, Jones RJ: Inhibition of apoptosis during development of colorectal cancer. Cancer Res 1995, 55:1811-1816 [PubMed] [Google Scholar]
- 51.Kikuchi Y, Dinjens WN, Bosman FT: Proliferation and apoptosis in proliferative lesions of the colon and rectum. Virchows Arch 1997, 431:111-117 [DOI] [PubMed] [Google Scholar]