Abstract
Fibronectin is secreted from the cell as a soluble protein that must then polymerize to regulate cell function. To elucidate the process of fibronectin matrix assembly in vascular disease, we immunostained sections of balloon-injured rat carotid artery for the fibronectin-binding α5β1 integrin. Whereas α5β1 integrin was not evident in the normal carotid artery, its expression was induced after a vascular injury. By 14 days, the α5β1 integrin was localized exclusively to the less differentiated smooth muscle cells (SMCs) at the luminal surface of the neointima. Platelet-derived growth factor-BB, dominant in neointimal formation, selectively increased the expression of the α5β1 integrin by human SMCs in culture. To track the assembly of fibronectin fibers, fluorescence-labeled soluble fibronectin protomers were added to cultured SMCs and to fresh segments of normal and balloon-injured rat carotid arteries. Fibronectin fiber formation in cultured SMCs could be detected within 10 minutes, and was blocked by an RGD peptide, an anti-β1 integrin antibody, and an anti-α5β1 integrin antibody, but not by an anti-β3 integrin antibody. En face confocal microscopy of arterial segments revealed that soluble fibronectin had polymerized on the α5β1 integrin-expressing SMCs of the luminal surface of the injured arterial neointima, but not on the α5β1 integrin-negative neointimal SMCs below this or on the endothelial cells of uninjured arteries. Furthermore, in situ fibronectin assembly by the neointimal SMCs was inhibited by an RGD peptide and by an anti-β1 integrin antibody. These studies indicate that a subpopulation of SMCs in the repairing artery wall orchestrates integrin-mediated fibronectin assembly.
Fibronectin is an extracellular glycoprotein that has important roles in cell adhesion, migration, growth, and differentiation. 1 In the normal adult artery wall, fibronectin comprises a small fraction of the extracellular matrix (ECM); however, in the diseased artery wall, fibronectin is abundant. 2 An increased expression of arterial fibronectin is observed in the vascular lesions of atherosclerosis, 2,3 restenosis after angioplasty, 4 and transplant arteriopathy, 5 suggesting an important pathophysiological role for fibronectin in these conditions. In vitro studies suggest that such roles may include the regulation of the vascular smooth muscle cell (SMC) phenotype, 6 SMC and endothelial-cell migration and proliferation, 7-9 and leukocyte trafficking. 10
After balloon-mediated arterial injury, there is rapid accumulation of fibronectin at the site of the injury, in association with neointimal formation. 2,11-13 There are two potential sources for this newly deposited fibronectin. Some fibronectin may be derived from the circulatory system, where it exists as a plasma protein originally synthesized by the liver. In addition, fibronectin is synthesized locally, as a specific response to an injury, by resident vascular SMCs. 2,13 Locally derived or cellular fibronectin is distinct from plasma fibronectin by virtue of the unique fibronectin domains that arise through alternative splicing. Cellular fibronectin contains the type III fibronectin modules ED-A and AD-B, whereas plasma fibronectin does not contain either of these splice variants.
Regardless of its origin, fibronectin is initially secreted from the cell as a soluble dimeric protein. Within the ECM, soluble fibronectin protomers polymerize to form insoluble, multimeric fibronectin. This assembly process is of paramount importance because only insoluble, fibrillar fibronectin can act as an adhesive ligand and regulate cell function. Moreover, fibronectin polymerization is not a spontaneous process, but requires specific cellular interaction. 14 This contrasts with other ECM components, such as fibrillar collagen, basement membrane collagen, and laminin, which are capable of self-polymerization. Therefore, in the vessel wall, it is likely that a coordinated interplay between vascular cells and soluble fibronectin must occur to generate a fibronectin-rich ECM favorable to neointimal formation and growth.
The molecular basis of fibronectin assembly has been studied in culture and found to involve cell surface fibronectin receptors, the actin cytoskeleton, microtubule dynamics, and the Rho family of small GTPases. 14-16 Little is known however about fibronectin matrix assembly in intact tissue, including the vessel wall. Based on in vitro studies of nonvascular cells, the process can be expected to depend on one or more members of the integrin superfamily of heterodimeric adhesion receptors. 15,17 Of these, α5β1 integrin is a dominant fibronectin receptor, and we, as well as others, have shown that the α5β1 integrin is abundantly expressed on the surface of human SMCs in culture. 18,19 However, there is no information on the expression of this integrin in the injured or diseased adult artery wall. This is noteworthy in light of growing evidence that integrin expression in vitro may not predict the same expression in vivo. 20
This study elucidates the process by which soluble fibronectin is converted to insoluble fibronectin fibers during neointimal formation. We first established the α5β1 integrin expression profile in normal and balloon-injured rat carotid artery and related these findings to the SMC phenotype, as indicated by the expression of smooth muscle α-actin. The effect of platelet-derived growth factor(PDGF)-BB on the integrin expression in SMCs was also determined, recognizing the central role of this growth factor in neointimal formation. We subsequently tracked and characterized fibronectin assembly, using fluorescently labeled fibronectin protomers, in cultured SMCs and in fresh segments of normal and injured carotid arteries. Our findings indicate that a specialized subpopulation of SMCs in the injured artery wall orchestrates integrin-mediated fibronectin assembly during vascular repair.
Materials and Methods
Integrin Antibodies
The monoclonal antibodies (mAbs) used included the human α1β1 integrin-specific mAb Ts2/7, 21 the human α2β1 integrin-specific mAb BHA2.1 22 (Chemicon Inc., Temecula, CA), the human α3β1 integrin-specific mAb P1B5 (Life Technologies, Inc., Gaithersburg, MD), a human α5β1 integrin-specific mAb HA5 (Chemicon Inc., Temecula, CA), a function-blocking human α5β1 integrin-specific mAb JBS5, a human β1 integrin subunit-specific mAb HB1.1 (Chemicon), and a human αvβ3 integrin-specific mAb 23C6 (Serotec). To study rat integrin expression, we used a rat α5β1 integrin-specific mAb HMα5–1(PharMingen), a rat β1 integrin subunit-specific mAb Ha2/5 (PharMingen), and a rat β3 integrin subunit-specific mAb F11 (PharMingen). Control antibodies for immunostaining, flow cytometry, and blocking experiments included mouse immunoglobulin G (IgG; P3, ATCC), hamster IgG (G235–2356, PharMingen), and hamster IgM (G235–1, PharMingen). The peptides GRGDNP, RGES, and GPenGRGDSPCA were purchased from Life Technologies.
SMC Culture
Primary cultures of rat and human arterial SMCs were initiated by explant outgrowth from aortic segments and from segments of internal thoracic artery retrieved at the time of coronary artery bypass surgery, respectively. 23,24 The identity of vascular SMCs was confirmed morphologically and by positive immunostaining with a mAb to smooth muscle α-actin (1A4, DAKO). Cells were grown in M199 (Life Technologies),and supplemented with the designated concentration of fetal bovine serum. All experiments were performed using rat SMCs in the fourth to sixth subculture and human SMCs in the third to sixth subculture.
Balloon Injury to the Rat Carotid Artery
The left carotid artery of male Sprague-Dawley rats was injured using a 2F Fogarty catheter, as previously described. 25,26 The uninjured right carotid artery was used as a control. At 0, 4, and 14 days after injury, rats were anesthetized, and the carotid arteries were perfused in situ with phosphate-buffered saline (PBS). Vessels were then harvested, embedded in OCT compound, frozen in liquid nitrogen, and cut into 6-μm cryostat sections. For some experiments, arteries were perfused in situ with methanol-Carnoy’s fixative (methanol:chloroform:glacial acetic acid, 6:3:1), immersed in the same fixative overnight, embedded in paraffin, and then sectioned at 6 μm thickness.
Immunostaining of Rat Tissues
Tissue sections of the skin, heart, large intestine, thoracic aorta, uninjured carotid artery and injured carotid artery were harvested from rats and examined for expression of the α5β1 integrin. Frozen sections were dipped in acetone, pretreated with 10% goat serum, and then incubated with the hamster anti-α5β1 integrin antibody HMa5–1 (1:50 dilution) or isotype-matched control antibody (G235–1) overnight at 4°C. The bound primary antibody was detected with a biotinylated goat anti-hamster IgM antibody, an avidin-biotin-peroxidase complex, and 3,3′-diaminobenzidine chromogen (Sigma Chemical Co., St. Louis, MO). Staining for the smooth muscle α-actin was performed in paraffin-embedded sections of arteries fixed in methanol-Carnoy’s solution. These sections were blocked with 10% horse serum and endogenous peroxidase activity blocked with 3.0% hydrogen peroxide in methanol. Sections were incubated for 2 hours at room temperature with the anti-smooth muscle α-actin mAb 1A4.The bound primary antibody was detected with bio- tinylated horse anti-mouse IgG antibody and color-developed as described above. Sections were lightly counterstained with Harris’ hematoxylin. To double-immunostain for the α5β1 integrin and smooth muscle α-actin, frozen sections were first incubated with HMα5–1, as described above, and developed using a diaminobenzidine-nickel chloride (0.4% in PBS) solution to yield a gray color. The same slide was then blocked with 10% normal horse serum and immunostained for smooth muscle α-actin, using mAb 1A4. This reaction was developed using diaminobenzidine alone to yield a brown color. Double-immunostained sections were not counterstained. Immunostaining for leukocyte markers was performed on frozen sections with mouse anti-rat CD4 mAb (MCA55, Serotec; reactive against helper T-lymphocytes and cells of monocyte/macrophage lineage), mouse anti-rat CD45RA (CLO33AP, Cedarlane; reactive against B lymphocytes), and a mouse anti-rat granulocyte mAb (HIS48; Pharmingen). The sections were color developed and counterstained as for α5β1 integrin staining.
Flow Cytometry
Flow cytometry for the detection of integrin expression was carried out by indirect immunofluorescence staining as described previously. 22,27 Early confluent SMCs were trypsinized and washed in cold PBS with 1% bovine serum albumin. Cells were incubated for 30 minutes on ice with the control or integrin-specific mAbs at predetermined saturating concentrations. The washed cells were incubated with a fluorescein isothiocyanate (FITC)-labeled anti-mouse F(ab)′2 fragment (Becton-Dickinson, Ontario, Canada), and fluorescence staining was analyzed using a B.D. FacScan (Mountainview, CA).
Fibronectin Assembly by Cultured SMCs
Human plasma fibronectin was isolated by gelatin-sepharose chromatography as described. 28 The purity of the isolate was verified by the presence of a single band on gel electrophoresis, which was confirmed to be fibronectin by Western blot analysis (data not shown). The fibronectin was then dialyzed against borate-buffered saline (170 mmol/L boric acid, 170 mmol/L sodium tetraborate, 75 mmol/L NaCl, pH 9.3) at 4°C overnight and then labeled with FITC by transferring the dialysate to a solution of borate-buffered saline containing 30 μg/ml FITC and mixing in the dark at room temperature for 1.5 hours. The physiological pH was restored, and unbound FITC was removed by dialysis against PBS at 4°C for 4 days. The concentration of FITC-labeled fibronectin was measured spectrophotometrically by measuring absorption at 280- and 493-nm wavelengths. The ratio of FITC to protein in the FITC-fibronectin conjugate was determined to be between 3.2 and 3.8, based on the approach of Mishell and Shiigi. 29
Labeled soluble fibronectin was added to rat or human SMCs that were cultured on glass coverslips in M199 supplemented with fibronectin-free fetal bovine serum. The latter was obtained by passing fetal bovine serum through a gelatin-sepharose chromatography column and collecting the effluent. FITC-labeled fibronectin (100 nmol/L) was added to cultures for designated intervals, after which the cells were washed extensively with PBS and fixed with 4% paraformaldehyde. Cells were mounted in glycerol/PBS (9:1) containing Hoechst 33258 (2.5 μg/ml, Sigma) to identify the cell nuclei, and the presence of insoluble, polymerized fibronectin was evaluated by fluorescence microscopy. Blocking peptides or antibodies were added 16 hours after the SMCs were seeded onto coverslips and 30 minutes before the addition of FITC-labeled fibronectin protomers.
In Situ Fibronectin Assembly by Rat Carotid Artery
To study the assembly of fibronectin fibers by neointimal SMCs of the intact artery wall, we developed an in situ fibronectin assembly protocol. The left carotid artery of rats was balloon-injured and the middle one-third was harvested, unfixed, at 12 days after injury. Arterial segments were opened longitudinally and incubated with 2.5 mmol/L ethylenediaminetetraacetic acid in PBS for 15 minutes. After rinsing with Ca2+- and Mg2+-free PBS, the arterial segments were incubated for 36 hours with FITC-labeled soluble fibronectin (250 nmol/L) in the presence of 1.8 mmol/L Ca2+. After three washes with PBS, the artery fragments were pinned onto dental wax and then fixed with 3% formaldehyde. Segments were mounted whole in glycerol/PBS (9:1), containing Hoechst 33258, and imaged en face by confocal microscopy using a Zeiss LSM 410 microscope. An argon ion UV laser, emitting at 351 nm for the detection of Hoechst 33258, was used to establish nuclear morphology. These images could be used to distinguish endothelial cells, medial SMCs, and neointimal SMCs, based on their distinct nuclear morphology and alignment in situ. A krypton/argon laser emitting at 488 nm was used to detect FITC-fibronectin. Optical sectioning of samples was used to establish the relative position of cells and fibronectin fibers.
Results
α5β1 Integrin Is Expressed in Injured, but Not Normal, Rat Carotid Artery and Localizes to the Neointimal SMCs Subjacent to the Lumen
To establish an expression profile of the α5β1 integrin in normal rat tissues, we immunostained frozen sections of the large intestine, skin, thoracic aorta, and carotid artery. As shown in Figure 1, A and B ▶ , SMCs in the muscle layers of the large intestine were strongly positive, whereas the luminal epithelial cells were negative. SMCs of the arrector pili muscle in the skin were also positive (Figure 1C) ▶ . In contrast to the SMCs in these nonvascular tissues, SMCs in the media of the thoracic aorta and normal carotid artery did not express immunodetectable α5β1 integrin (Figure 1D) ▶ . Only rare adventitial cells, presumably fibroblasts, were positive (Figure 1D) ▶ .
Figure 1.
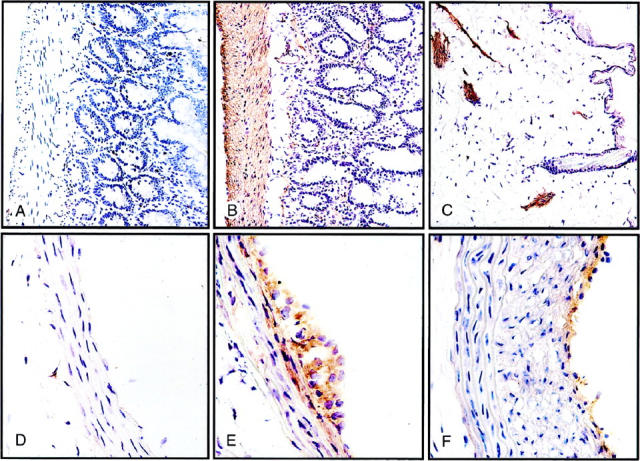
Immunohistochemical identification of α5β1 integrin in rat tissues containing nonvascular and vascular SMCs. Frozen sections were incubated with either control antibody or mAb HA5, and bound primary mAb was detected as described in Materials and Methods. A: Section of colon incubated with hamster IgG control antibody, showing no detectable muscle staining. B: Near adjacent section of colon immunostained for α5β1 integrin, showing extensive staining in SMCs of the muscularis externa and more weakly in the muscularis mucosa and microvasculature of the lamina propria. C: Section of skin showing pronounced α5β1 integrin expression in arrector pili muscles. D: Uninjured rat carotid artery with no staining for α5β1 integrin with the exception of occasional adventitial cells. E: Left carotid artery 4 days after balloon injury, showing induction of a5β1 integrin expression in SMCs of the inner medial layers and the developing neointima. F: Left carotid artery 14 days after balloon injury, showing highly localized α5β1 integrin expression at the luminal surface.
Four days after the carotid injury, however, there was substantial expression of the α5β1 integrin in SMCs forming the early neointima, as well as those in the innermost layers of the media (Figure 1E) ▶ . By 14 days, the medial SMCs were no longer positive for the α5β1 integrin. Most of the neointimal SMCs were also negative, with the notable exception of a rim at the luminal surface of one or two cell layers (Figure 1F) ▶ . This corresponds to the population of SMCs well known to maintain proliferative activity during lesion formation. 25
α5β1 Integrin Expression in the Rat Carotid Artery Is Inversely Correlated with That of Smooth Muscle α-Actin
The temporal and spatial pattern of α5β1-integrin expression after an arterial injury suggested that this integrin was selectively expressed in a subpopulation of SMCs that was actively contributing to lesion formation. One of the hallmarks of SMC activation during lesion formation is a fall in the expression of smooth muscle α-actin. 30,31 To examine this, we immunostained sections of normal and injured carotid arteries for smooth muscle α-actin. As illustrated in Figure 2 ▶ , the temporal and spatial pattern of smooth muscle α-actin expression was inversely related to that of the α5β1 integrin. Medial SMCs of the normal carotid artery expressed smooth muscle α-actin, but SMCs in the neointima 4 days after injury were negative. At 14 days, most of the neointimal SMCs had become positive for smooth muscle α-actin, with the important exception of those SMCs composing the luminal edge. None of the cells at this edge expressed mononuclear inflammatory cell markers, (CD4, CD45RA; Figure 2, D and E ▶ ), nor did they react with a pan-granulocyte mAb (data not shown). These findings exclude the possibility that the smooth muscle-α-actin-negative cells subjacent to the lumen were colonized inflammatory cells. To verify the reciprocal relationship between the α5β1 integrin expression and that of the smooth muscle α-actin, we double-immunostained frozen sections for both proteins. As shown in Figure 2F ▶ , α5β1 integrin expression was localized to the luminal-edge SMCs, whereas smooth muscle α-actin expression predominated below this.
Figure 2.
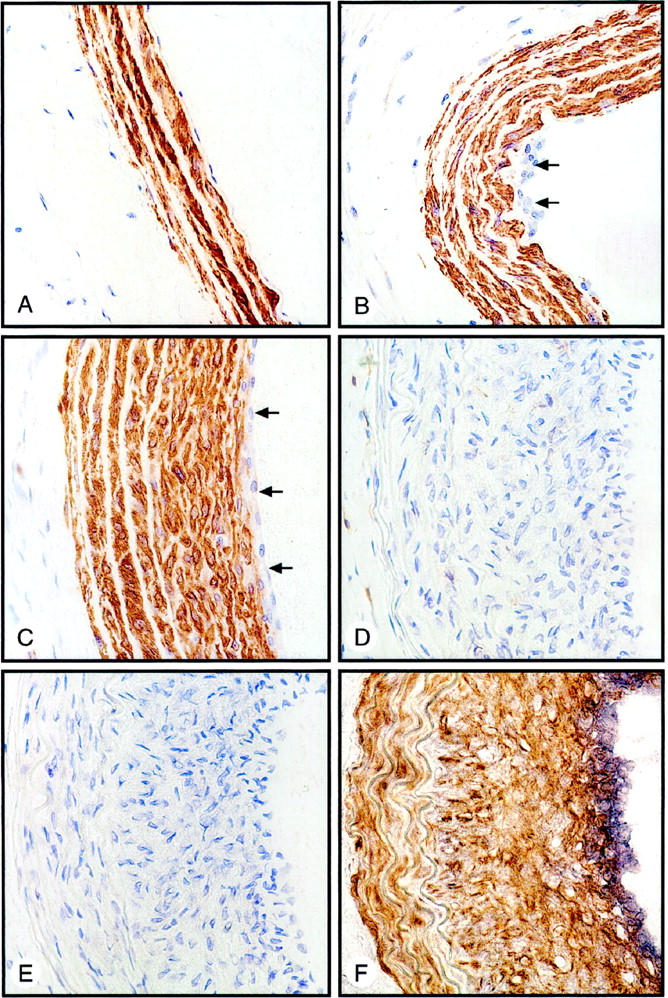
Expression of smooth muscle α-actin after balloon injury to the rat carotid artery. A: Paraffin-embedded section of uninjured rat carotid artery, showing extensive expression of smooth muscle α-actin in medial SMCs. B: Carotid artery 4 days after balloon injury, showing an absence of smooth muscle α-actin in SMCs of the developing neointima (arrows). C: Carotid artery 14 days after balloon injury. Most of the neointimal SMCs now express smooth muscle α-actin with the exception of cells forming the luminal edge (arrows). D: Frozen section of rat carotid artery 14 days after balloon injury, immunostained for CD4-expressing cells, showing staining of some adventitial cells but no luminal neointimal cells. E: Frozen section of 14-day-injured rat carotid artery, adjacent to that shown in E, showing lack of immunostaining for B lymphocytes. F: Frozen section of injured rat carotid artery, near or adjacent to those sections in D and E, double-immunostained for smooth muscle α-actin (brown) and α5β1 integrin (blue-black), showing a predominantly reciprocal relationship between these two proteins.
Expression of α5β1 Integrin on the Surface of Human SMCs Is Increased by PDGF-BB
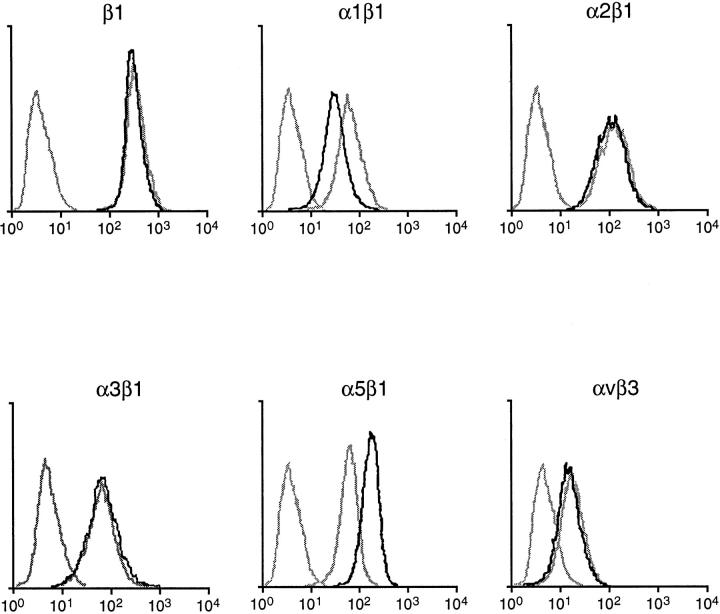
The finding of the α5β1 integrin in the luminal cells of the developing neointima prompted us to consider environmental factors that might mediate localized induction of integrin expression. PDGF is an established regulator of neointimal formation after a balloon injury of the rat carotid artery 32 and can be expected to be nearest the luminal surface, owing to its release from adherent platelets and expression by luminal SMCs. 33 To determine the effect of PDGF on the expression of the α5β1 integrin, we studied cultured SMCs by flow cytometry. Human SMCs were used for this assessment for their relevance to human disease and to facilitate comparison of α5β1 integrin expression to that of several other integrins. Monoclonal antibodies for human integrins are more readily available than those for rat integrins. As shown in Figure 3 ▶ , stimulation with 10 ng/ml PDGF-BB produced a notable increase in the expression of the cell surface α5β1 integrin. In contrast, there was no detectable change in the expression of the α2β1 integrin, α3β1 integrin, or αvβ3 integrin. There was a decline in the expression of the α1β1 integrin. The expression of the β1 integrin subunit was unchanged, indicating an overall balanced effect of PDGF-BB on the total surface pool of β1 integrins.
Figure 3.
Flow cytometry analysis showing expression of integrins by human SMCs stimulated by PDGF-BB (10 μg/ml) Tracing on the left of each graph depicts cells incubated with control IgG. Of the other 2 tracings, the dark and light curves depict findings of SMCs incubated for 48 hours with or without PDGF-BB, respectively.
SMCs Can Assemble a Fibronectin Matrix in Vitro
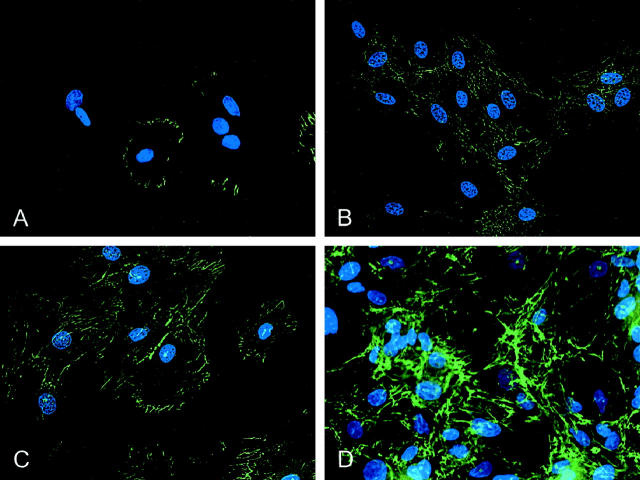
To test whether the α5β1 integrin plays a role in the assembly of a fibronectin matrix, the FITC-labeled fibronectin in its soluble, protomeric form was added to both rat and human SMCs in culture. SMCs were fixed after designated incubation periods, and fiber formation was visualized by fluorescent microscopy. As shown in Figure 4 ▶ , for rat SMCs, fibronectin fibers were clearly observed in association with cells, whereas noncellular areas were devoid of fibers. The first evidence of fiber assembly was within 10 minutes, with short, streak-like fibers primarily localized at the periphery of SMCs (Figure 4A) ▶ . The fibers became brighter and longer with an intricate network evident at 24 hours (Figure 4, B–D) ▶ . The pattern of the fibronectin matrix assembly by human SMCs was similar to that of rat SMCs, although, qualitatively, the assembly process was somewhat slower, and the fibers appeared finer (eg, Figure 5, G and I ▶ ). The addition of cycloheximide to the cultures (50 μg/ml for 24 hours) did not inhibit the assembly of exogenous fibronectin, indicating that the process was not dependent on new protein synthesis or demonstrably influenced by endogenously produced fibronectin (data not shown)
Figure 4.
Fluorescent photomicrographs illustrating the assembly of fibronectin fibers from soluble precursors by cultured rat SMCs. The fibers, assembled from FITC-labeled protomers (100 nmol/L) appear green; nuclei were labeled with Hoechst 33258 and appear blue. A: SMCs fixed 10 minutes after the addition of soluble fibronectin, showing short fibronectin fibers at the cell periphery, with no evidence of assembly in noncellular regions of the culture substrate. B−D: SMCs fixed 2 hours (B), 6 hours (C), and 24 hours (D) after incubation with FITC-labeled fibronectin protomers, showing a progressively more developed fibronectin fiber network.
Figure 5.
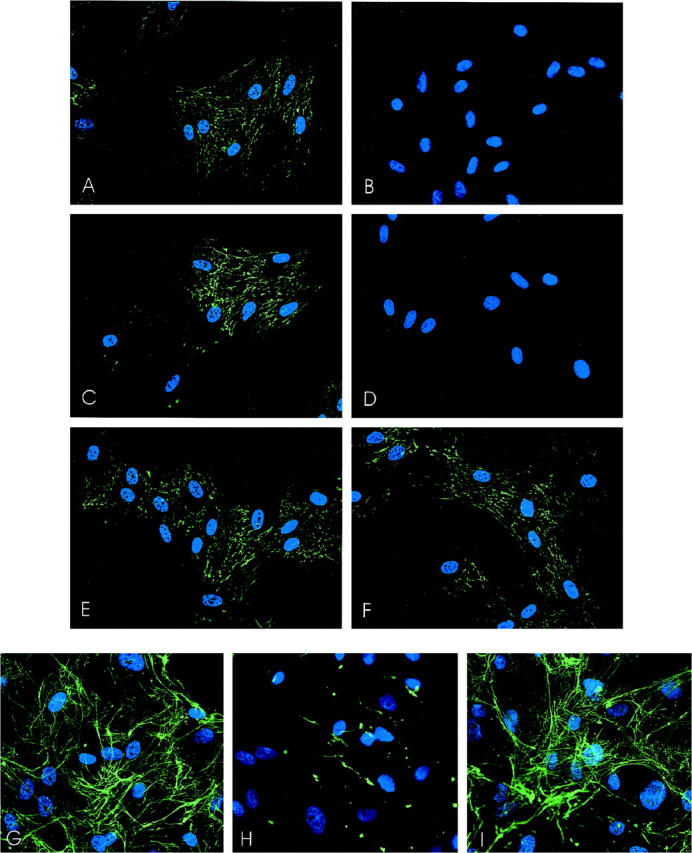
Fluorescent micrographs of fibronectin matrix assembly by rat (A−F) and human (G−I) SMCs in the presence or absence of integrin-blocking reagents. SMCs were incubated with control or blocking reagent for 15 minutes, after which FITC-labeled fibronectin protomers, in the presence of blocking reagent, were added. Fibronectin assembly on rat SMCs proceeded for 2 hours, and assembly on human SMCs proceeded for 24 hours. A: RGESP control peptide (100 μg/ml); B: GRGDNP-blocking peptide (100 μg/ml); C: control hamster anti-rat IgM (40 μg/ml); D: anti-β1 integrin mAb Ha2/5 (40 μg/ml); E: control hamster anti-rat IgG (40 μg/ml); F: anti-β3 integrin mAb (40 μg/ml). G: Control IgG (20 μg/ml); H: anti-α5β1 integrin mAb JBS51:200; I: anti-α2β1 integrin mAb BHA2.1 (20 μg/ml).
Assembly of Fibronectin by SMCs Requires α5β1 Integrin
To determine whether fibronectin assembly was integrin-mediated, SMCs were pretreated with RGD peptide or function-blocking anti-integrin antibodies for 30 minutes, followed by the addition of FITC-labeled fibronectin in the presence of the respective blocking reagent. After 2 hours, cells were washed, fixed, and examined by fluorescence microscopy. The anti-integrin reagents were added 16 hours after the cells were seeded on coverslips and had no detectable effect on cell morphology. Data from rat SMC cultures are illustrated in Figure 5, A–F ▶ . Compared with cultures incubated with a control peptide (RGESP, 100 μg/ml), there was very little fibronectin in cultures incubated with the RGD blocking peptide GRGDNP (100 μg/ml) (Figure 5, A ▶ versus B). Incubation with the anti-β1 integrin antibody (Ha2/5) also substantially decreased fiber polymerization compared with an isotype-matched control IgM (Figure 5, C ▶ versus D). In comparison, the anti-β3 antibody (F11) had no demonstrable effect on fibronectin assembly compared with the isotype matched control mAb (Figure 5, E ▶ versus F). Cyclic RGD (GPenGRGDSPCA), which preferentially inhibits the avβ3 integrin, also had no detectable effect (data not shown).
We performed the same assay in human SMC cultures, using a function-blocking mAb that was specific to the human α5β1 integrin (JBS5). An analogous function-blocking antibody is not available for rat SMCs, and we specifically verified that the anti-rat mAb used for immunostaining (HMα5–1) had no effect on the adhesion of rat SMCs to fibronectin (data not shown). Incubation with mAb JBS5 (1:200 dilution of ascites fluid) led to a striking inhibition of fiber formation, compared with cultures incubated with the control IgG. Even in confluent SMC cultures incubated with fibronectin protomers for 24 hours, fibronectin fibers were barely detectable (Figure 5, G ▶ versus H). Incubation with mAb BHA2.1, an anti-α2β1 integrin mAb that we previously have shown inhibits SMC migration on type I collagen, 18 had no effect on fibronectin assembly (Figure 5I) ▶ .
Injured but Not Normal Rat Carotid Arteries Assemble a Fibronectin Matrix at Their Luminal Surface
We next determined whether the α5β1 integrin-expressing cells present on the surface of the injured rat carotid artery were capable of polymerizing soluble fibronectin in situ. Arteries were harvested 12 days after injury and subjected to a modification of the fibronectin assembly assay, as described in Materials and Methods. Figure 6A ▶ depicts confocal microscopic sections of the luminal surface of an uninjured rat carotid artery. The characteristic morphology and alignment of endothelial cell nuclei can be appreciated, particularly as the optical plane was lowered 1.2 μm below the cell surface (more sharply focused nuclei in the middle panel in Figure 6A ▶ ), confirming the artery to be intact and uninjured. There was no evidence of de novo fibronectin fiber formation associated with these cells. In contrast, optical sections through the neointima of an injured carotid artery showed an extensive network of polymerized fibronectin (Figure 6B) ▶ . This was evident at the apical surface of the innermost layer of neointimal cells, and it could also be appreciated up to 5 μm into the tissue.
Figure 6.
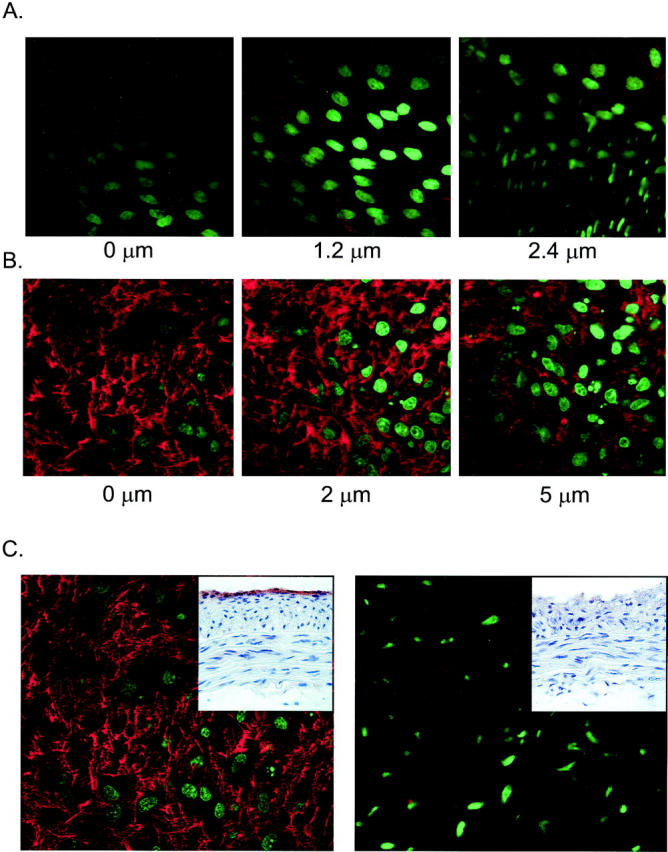
Fluorescence micrographs illustrating in situ assembly of fibronectin fibers by rat carotid artery. Artery fragments were incubated for 25 hours with FITC-labeled fibronectin protomers as described in Materials and Methods and imaged en face by a confocal laser scanning microscope, equipped with a krypton/argon laser for the excitation of fluoroscein dyes and an argon ion laser for the excitation of Hoechst 33258. Nuclear fluorescence (Hoechst 33258) has been pseudocolored green, and fibronectin fiber fluorescence has been pseudocolored red. A: Optical sections 0.6-μm-thick of an uninjured rat carotid artery through the endothelial cell layer (left and middle panels) and the superficial media (elongated and well-aligned nuclei of the right panel). The depths relative to the apical surface are noted. There is no evidence for fibronectin fiber assembly. B: One-micron-thick optical sections of a carotid artery 12 days after injury. Fibronectin fibers have been assembled on the apical surface of the neointimal cells. Nuclei are of variable morphology and display karyorrhexis. C: One-micron-thick optical sections of the rat carotid artery neointimal surface, 12 days after injury, evaluated for fibronectin assembly. The inserts depict frozen sections from an adjacent region of the respective artery studied for fibronectin assembly, immunostained to verify the presence or absence of α5β1 integrin at the luminal edge. The left panel depicts an intact injured artery. The right panel depicts an artery in which the luminal SMCs were gently scraped away before assaying for in situ fibronectin assembly. Fibronectin fibers did not form on this latter surface, which was devoid of α5β1 integrin-expressing SMCs.
To determine whether the α5β1 integrin-negative SMCs deeper within the neointima were capable of mediating fibronectin assembly, arteries were harvested 12 days after injury and longitudinally opened, and the luminal-most neointimal cells were gently scraped away under microscopic guidance, using a plastic coverslip. The artery was then divided into two portions, one of which was frozen, sectioned, and immunostained for the α5β1 integrin. The other portion was subjected to the fibronectin assembly protocol. In this way, the soluble fibronectin protomers could be assured of access to SMCs that were deeper in the neointima. As shown in Figure 6C ▶ , arteries in which the luminal surface was not removed displayed both the expression of the α5β1 integrin by the luminal SMCs and the capacity to assemble a fibronectin matrix on this surface. In contrast, when the α5β1 integrin-expressing lumenal SMCs were removed, in situ fibronectin assembly was not detected. Fibronectin assembly was also not evident on the adventitial side of arterial fragments, studied by pinning arterial segments adventitia side up (data not shown).
To directly ascertain whether the assembly of fibronectin by arterial neointima was integrin-mediated, arterial segments were incubated with fluorescent fibronectin protomers and anti-rat integrin mAbs, anti-integrin peptides, or their respective controls. As shown in Figure 7 ▶ , fibronectin assembly in situ by luminal SMCs was abrogated by the anti-β1 integrin mAb Ha2/5 and by the GRGDNP peptide. The anti-β3 integrin mAb F11 had little to no effect on fibronectin assembly.
Figure 7.
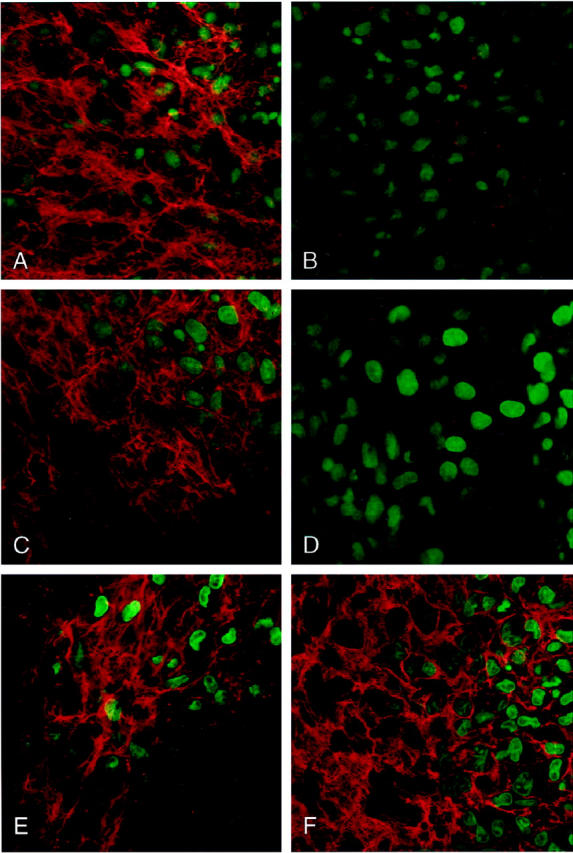
Laser scanning confocal micrographs of 1.0-μm-thick optical sections of the luminal surface of a rat carotid artery, 12 days after balloon injury. Artery fragments were incubated for 25 hours with FITC-labeled fibronectin protomers and imaged en face by confocal laser scanning microscopy. Nuclear fluorescence (Hoechst 33258) is pseudocolored green, and fibronectin fiber fluorescence is pseudocolored red. A: Control IgM (40 μg/ml); B: anti-β1 integrin mAb (40 μg/ml); C: RGESP control peptide (100 μg/ml); D: GRGDNP blocking peptide (100 μg/ml); E: control IgG (40 μg/ml); F: Anti-β3 integrin mAb (40 μg/ml).
Discussion
Neointimal formation after injury entails the generation of new tissue at a location where none previously existed. Using immunohistochemistry and a novel assay for the in situ assessment of fibronectin assembly, these studies demonstrate that a subpopulation of α5β1 integrin-bearing SMCs orchestrates integrin-mediated fibronectin polymerization in the repairing artery wall.
The identification of α5β1 integrin expression after arterial injury augments the repertoire of integrins known to be expressed in the remodeling adult artery wall. Despite the numerous integrins with potential roles in vascular function, 18,19,34 relatively few have been identified in the intima of diseased or injured arteries. The α1β1 integrin is a collagen/laminin receptor identified in the rat carotid artery neointima after balloon injury. 35 The αvβ3 integrin and αvβ5 integrin, which bind several ligands including vitronectin, osteopontin, and fibronectin, have been identified in human atherosclerotic plaque, 36,37 although the αvβ3 integrin was found to be more abundant in the arterial media. 36 Unlike the case for β3 integrins, fibronectin is the only ECM ligand for the α5β1 integrin. Our observation that the α5β1 integrin was expressed in the injured but not normal rat carotid artery suggests that α5β1 integrin plays a role in artery repair, but is not significantly involved in the integrity or function of the normal rat carotid artery. This is consistent with the small amount of fibronectin present in the media of the normal rat carotid artery. 31 Selective use of α5β1 integrin-fibronectin interactions during vascular remodeling has also been suggested during the closure of the ductus arteriosus. The α5β1 integrin is expressed by ductus arteriosus SMCs only after birth, at which time α5β1 integrin-fibronectin interactions are considered important in mediating SMC motility and ductus closure. 9,38,39
It is noteworthy that the expression of the α5β1 integrin after carotid artery injury was not distributed diffusely but was localized. Four to five days after injury, the α5β1 integrin was expressed in SMCs of the medial layers adjacent to the internal elastic lamina and in those of the primordial neointima. Subsequently, the expression localized exclusively to the luminal edge SMCs. This temporal and spatial pattern of expression parallels the well-established pattern for SMC thymidine incorporation after balloon-injury, 25 suggesting a linkage between the α5β1 integrin and SMC replication. An association between the α5β1 integrin and those SMCs actively contributing to lesion formation was further supported in the current study by the inverse relationship between α5β1 integrin expression and smooth muscle α-actin-containing stress fibers. The decline in smooth muscle α-actin in the developing rat carotid neointima is consistent with the previous analyses of tissue lysate, 30 and electron microscopy studies 31 and reflects the presence of less differentiated SMCs. Our findings indicate that the α5β1 integrin expression, and fibronectin polymerization, are specialized features of this population of less differentiated SMCs. Twenty-eight days after injury, the luminal-edge cells expressed immunodetectable smooth muscle α-actin, in keeping with their SMC identity, notwithstanding the lack of smooth muscle α-actin staining 14 days earlier.
Factors that mediate the expression of the α5β1 integrin by SMCs in vivo remain to be elucidated. However, we previously found that FGF2 modestly increased the expression of the α5β1 integrin by human SMCs in culture. 18 In the current study, we observed that PDGF-BB also increased α5β1 integrin expression in cultured SMCs, and appeared to be selective in this response, because there was no detectable increase in the expression of the α2β1, α3β1, or αvβ3 integrin and there was a decline in the expression of the α1β1 integrin. PDGF-BB is an especially noteworthy candidate for mediating the localized expression of the α5β1 integrin within the injured artery, because the tissue concentration of PDGF-BB is likely highest near the luminal surface, by virtue of its release from adherent platelets as well as local expression by SMCs after injury, specifically at this surface. 33
By using fluorescence-labeled soluble fibronectin protomers, we tracked the fibronectin assembly process, independently of fibronectin synthesis by the cell. This enabled us to establish that fibronectin polymerization by SMCs in vitro is a relatively rapid process (evident within 10 minutes in vitro) that progresses. The approach also allowed us to reliably probe for cell surface receptors that mediate assembly, without any confounding effects that the blocking reagents might have on fibronectin production. This established that the α5β1 integrin is essential for fibronectin assembly by human SMCs in culture. The lack of potent blocking antibodies for the rat α5β1 integrin precluded absolute confirmation of the specific integrin heterodimer involved in the injured rat artery wall; however, both an RGD-containing peptide and an anti-β1 integrin mAb blocked in situ fibronectin assembly by luminal neointimal SMCs. Neither the anti-β3 integrin mAb nor a cyclic RGD peptide that preferentially inhibits αvβ3 function had a detectable effect on fibronectin assembly. Furthermore, after removal of the α5β1-integrin expressing cells subjacent to the lumen, in situ fibronectin assembly was no longer detectable. Together, these data strongly suggest that fibronectin assembly in the injured rat carotid artery requires the α5β1 integrin.
SMCs at the luminal surface of repairing arteries are potentially vulnerable to the mechanical forces imposed by flowing blood, particularly if there is little in the way of a supportive ECM. In the injured rat carotid artery, this vulnerability may be especially relevant for SMCs forming the primordial neointima (eg, 4–5 days after injury), as well as SMCs at the luminal surface of the more developed neointima that have newly arisen by mitosis. For these cells, there must be a mechanism by which an insoluble ECM is rapidly formed. The current findings indicate that this subpopulation of SMCs is selectively equipped to rapidly generate an insoluble fibronectin matrix from soluble precursors. The presence of polymerized fibronectin would not only provide a structure for cell attachment but would facilitate SMC survival by providing ligands for integrin-mediated inhibition of apoptosis. 40 At the same time, the insoluble fibronectin would be conducive to SMC replication, migration, and maintenance of a synthetic phenotype. 6,41 Deposition of fibrin on the injured lumen surface also likely occurs shortly after injury, 42 and we speculate that fibronectin assembly would physically strengthen a provisional matrix initiated by fibrin, a phenomenon suggested in other forms of wound healing. 43 The α5β1 integrin was not expressed in the deeper layers of the neointima, and the SMCs in these layers were not observed to assemble a fibronectin matrix. Presumably, the smooth muscle α-actin-positive SMCs in the deeper layers of the neointima interact with more permanent ECM components such as collagen, which is consistent with a heterogeneity of SMC-ECM interactions within the developing neointima.
We used plasma fibronectin as the source of soluble protomers for integrin-mediated fiber assembly. This may be physiologically relevant because plasma fibronectin is deposited within the vessel wall after injury and is, theoretically, a ready source of substrate for matrix assembly. On the other hand, the expression of alternatively spliced cellular fibronectin is also a response to vessel injury. Interestingly, cellular fibronectin has been localized specifically to the SMCs at the luminal surface 13 and, thus, would be available for assembly by the α5β1 integrin-expressing SMCs. Although there are molecular differences between plasma and cellular fibronectin, both are capable of binding the α5β1 integrin, and it is conceivable that both contribute to the fibronectin assembly process. We do not know if other fibronectin-binding integrins such as the α3β1, α4β1, or α8β1 integrin play a role in fibronectin matrix assembly in repairing arteries; these integrins have not, however, been documented in intimal hyperplasia as yet. The β3 integrin subunit has been identified in neointima after balloon injury; 44 however, the current study suggests that the β3 integrins do not have a dominant role in fibronectin assembly in this context.
In summary, vascular injury induces expression of the α5β1 integrin by a subpopulation of SMCs that can orchestrate rapid, integrin-mediated polymerization of soluble fibronectin. The efficiency of this assembly process may be an important determinant of vascular lesion growth and stability.
Footnotes
Address reprint requests to J. Geoffrey Pickering, M.D., Ph.D., FRCP(C), London Health Science Centre, 339 Windermere Road, London, Ontario N6A 5A5, Canada. E-mail: gpickering@rri.on.ca.
Supported by a grant from the Heart and Stroke Foundation of Canada. J. G. P. was supported by a Career Investigator Award from the Heart and Stroke Foundation of Ontario (HSFO). B. M. C. C. was supported by a Research Scholarship from the Medical Research Council of Canada. E. R. received a studentship from the HSFO.
References
- 1.Ruoslahti E: Fibronectin and its receptors. Annu Rev Biochem 1988, 57:375-413 [DOI] [PubMed] [Google Scholar]
- 2.Glukhova MA, Frid MG, Shekhonin BV, Vasilevskaya TD, Grunwald J, Saginati M, Koteliansky VE: Expression of extracellular domain A fibronectin sequence in vascular smooth muscle cells is phenotype dependent. J Cell Biol 1989, 109:357-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Labat-Robert J, Szendroi M, Godeau G, Robert L: Comparative distribution patterns of type I and III collagens and fibronectin in human arteriosclerotic aorta. Pathol Biol (Paris) 1985, 33:261-265 [PubMed] [Google Scholar]
- 4.Clausell N, de Lima VC, Molossi S, Liu P, Turley E, Gotlieb AI, Adelman AG, Rabinovitch M: Expression of tumor necrosis factor α and accumulation of fibronectin in coronary artery restenotic lesions retrieved by atherectomy. Br Heart J 1995, 73:534-539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clausell N, Molossi S, Rabinovitch M: Increased interleukin-1 β and fibronectin expression are early features of the development of the postcardiac transplant coronary arteriopathy in piglets. Am J Pathol 1993, 142:1772-1786 [PMC free article] [PubMed] [Google Scholar]
- 6.Hedin U, Johan T: Plasma fibronectin promotes modulation of arterial smooth-muscle cells from contractile to synthetic phenotype. Differentiation 1987, 33:239-246 [DOI] [PubMed] [Google Scholar]
- 7.Ingber DE: Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc Natl Acad Sci USA 1990, 87:3579-3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naito M, Hayashi T, Kuzua M, Funaki C, Asai K, Kuzuya F: Effects of fibrinogen and fibrin on the migration of vascular smooth muscle cells in vitro. Atherosclerosis 1990, 83:9-14 [DOI] [PubMed] [Google Scholar]
- 9.Boudreau N, Turley E, Rabinovitch M: Fibronectin, hyaluronan, and a hyaluronan binding protein contribute to increased ductus arteriosus smooth muscle cell migration. Dev Biol 1991, 143:235-247 [DOI] [PubMed] [Google Scholar]
- 10.Molossi S, Elices M, Arrhenius T, Rabinovitch M: Lymphocyte transendothelial migration toward smooth muscle cells in interleukin-1 β-stimulated co-cultures is related to fibronectin interactions with α 4 β 1 and α 5 β 1 integrins. J Cell Physiol 1995, 164:620-633 [DOI] [PubMed] [Google Scholar]
- 11.Lindner V, Reidy MA, Fingerle J: Regrowth of arterial endothelium: denudation with minimal trauma leads to complete endothelial cell regrowth. Lab Invest 1989, 61:556-563 [PubMed] [Google Scholar]
- 12.Kim S, Kawamura M, Wanibuchi H, Ohta K, Hamaguchi A, Omura T, Yukimura T, Miura K, Iwao H: Angiotensin II type I receptor blockade inhibits the expression of immediate-early genes and fibronectin in rat injured artery. Circulation 1995, 92:88-95 [DOI] [PubMed] [Google Scholar]
- 13.Bauters C, Marotte F, Hamon M, Oliviero P, Farhadian F, Robert V, Samuel JL, Rappaport L: Accumulation of fetal fibronectin mRNAs after balloon denudation of rabbit arteries. Circulation 1995, 92:904-911 [DOI] [PubMed] [Google Scholar]
- 14.Peters DM, Mosher DF: Localization of cell surface sites involved in fibronectin fibrillogenesis. J Cell Biol 1987, 104:121-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C, Keivens VM, O’Toole TE, McDonald J, Ginsberg MH: Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell 1995, 83:715-724 [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q, Magnusson MK, Mosher DF: Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol Biol Cell 1997, 8:1415-1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu C, Bauer JS, Juliano RL, McDonald JA: The α 5 β 1 integrin fibronectin receptor, but not the α 5 cytoplasmic domain, functions in an early and essential step in fibronectin matrix assembly. J Biol Chem 1993, 268:21883-21888 [PubMed] [Google Scholar]
- 18.Pickering JG, Uniyal S, Ford C, Chau T, Laurin MA, Chow LH, Ellis CG, Fish J, Chan BMC: Fibroblast growth factor-2 potentiates vascular smooth muscle cell migration to platelet-derived growth factor: upregulation of α2β1 integrin and disassembly of actin filaments. Circ Res 1997, 80:627-637 [DOI] [PubMed] [Google Scholar]
- 19.Lee RT, Berditchevski F, Cheng GC, Hemler ME: Integrin-mediated collagen matrix reorganization by cultured human vascular smooth muscle cells. Circ Res 1995, 76:209-214 [DOI] [PubMed] [Google Scholar]
- 20.Schwartz SM: Smooth muscle cell migration in atherosclerosis and restenosis. J Clin Invest 1997, 99:2814-2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemler ME, Sanchez-Madrid F, Flotte TJ, Krensky AM, Burakoff SJ, Bhan AK, Springer TA, Strominger JL: Glycoproteins of 210,000 and 130,000 m.w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J Immunol 1984, 132:3011-3018 [PubMed] [Google Scholar]
- 22.Hangan D, Uniyal S, Morris V, MacDonald I, von Ballestrem C, Chau T, Schmidt E, Chambers A, Groom A, Chan BMC: Integrin VLA-2 (α2β1) function in postextravasation movement of human rhabdomyosarcoma cells in the liver. Cancer Res 1996, 56:3142-3149 [PubMed] [Google Scholar]
- 23.Pickering JG, Weir L, Rosenfield K, Stetz J, Jekanowski J, Isner JM: Smooth muscle cell outgrowth from human atherosclerotic plaque: implications for the assessment of lesion biology. J Am Coll Cardiol 1992, 20:1430-1439 [DOI] [PubMed] [Google Scholar]
- 24.Pickering JG, Bacha P, Weir L, Jekanowski J, Nichols JC, Isner JM: Prevention of smooth muscle cell outgrowth from human atherosclerotic plaque by a recombinant fusion protein specific for the epidermal growth factor receptor. J Clin Invest 1993, 91:724-729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clowes A, Reidy M, Clowes M: Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest 1983, 49:327-333 [PubMed] [Google Scholar]
- 26.Pastore C, Isner JM, Bacha P, Kearny M, Pickering JG: Epidermal growth factor receptor-targeted cytotoxin inhibits neointimal hyperplasia in vivo: results of local versus systemic administration. Circ Res 1995, 77:519-529 [DOI] [PubMed] [Google Scholar]
- 27.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, Lobb RR: VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell 1990, 60:577-584 [DOI] [PubMed] [Google Scholar]
- 28.Ruoslahti E, Hayman EG, Pierschbacher M, Engvall E: Fibronectin: purification, immunohistochemical properties, and biological activities. Methods Enzymol 1982, 82:803-831 [DOI] [PubMed] [Google Scholar]
- 29.Mishell BB, Shiigi SM: Modification and use of antibodies to label cell surface antigens. Mishell BB Shiigi SM eds. Selected Methods in Cellular Immunology. 1980, :pp 293-297 W.H. Freeman, San Fancisco [Google Scholar]
- 30.Kocher O, Gabbiani F, Gabbiani G, Reidy MA, Cokay MS, Peters H, Huttner I: Phenotypic features of smooth muscle cells during the evolution of experimental carotid artery intimal thickening: biochemical and morphological studies. Lab Invest 1991, 65:459-470 [PubMed] [Google Scholar]
- 31.Thyberg J, Blomgren K, Roy J, Tran PK, Hedin U: Phenotypic modulation of smooth muscle cells after arterial injury is associated with changes in the distribution of laminin and fibronectin. J Histochem Cytochem 1997, 45:837-846 [DOI] [PubMed] [Google Scholar]
- 32.Ferns GAA, Raines EW, Sprugel K, Motani AS, Reidy MA, Ross R: Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science 1991, 253:1129-1132 [DOI] [PubMed] [Google Scholar]
- 33.Lindner V, Giachelli CM, Schwartz SM, Reidy MA: A subpopulation of smooth muscle cells in injured rat arteries expresses platelet-derived growth factor-B chain mRNA. Circ Res 1995, 76:951-957 [DOI] [PubMed] [Google Scholar]
- 34.Mogford JE, Davis GE, Platts SH, Meininger GA: Vascular smooth muscle α v β 3 integrin mediates arteriolar vasodilation in response to RGD peptides. Circ Res 1996, 79:821-826 [DOI] [PubMed] [Google Scholar]
- 35.Gotwals PJ, Chi-Rosso G, Lindner V, Yang J, Ling L, Fawell SE, Koteliansky VE: The α1β1 integrin is expressed during neointima formation in rat arteries and mediates collagen matrix reorganization. J Clin Invest 1996, 97:2469-2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoshiga M, Alpers C, Smith LL, Giachelli CM, Schwartz SM: αvβ3 integrin expression in normal and atherosclerotic artery. Circ Res 1995, 77:1129-1135 [DOI] [PubMed] [Google Scholar]
- 37.Dufourcq P, Louis H, Moreau C, Daret D, Boisseau MR, Lamaziere JM, Bonnet J: Vitronectin expression and interaction with receptors in smooth muscle cells from human atheromatous plaque. Arterioscler Thromb Vasc Biol 1998, 18:168-176 [DOI] [PubMed] [Google Scholar]
- 38.Mason CA, Bigras JL, O’Blenes SB, Zhou B, McIntyre B, Nakamura N, Kaneda Y, Rabinovitch M: Gene transfer in utero biologically engineers a patent ductus arteriosus in lambs by arresting fibronectin-dependent neointimal formation. Nat Med 1999, 5:176-182 [DOI] [PubMed] [Google Scholar]
- 39.Clyman RI, Goetzman BW, Chen YQ, Mauray F, Kramer RH, Pytela R, Schnapp LM: Changes in endothelial cell and smooth muscle cell integrin expression during closure of the ductus arteriosus: an immunohistochemical comparison of the fetal, preterm newborn, and full-term newborn rhesus monkey ductus. Pediatr Res 1996, 40:198-208 [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z, Vuori K, Reed JC, Ruoslahti E: The α 5 β 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci USA 1995, 92:6161-6165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mercurius KO, Morla AO: Inhibition of vascular smooth muscle cell growth by inhibition of fibronectin matrix assembly. Circ Res 1998, 82:548-556 [DOI] [PubMed] [Google Scholar]
- 42.Bosmans JM, Kockx MM, Vrints CJ, Bult H, De Meyer GR, Herman AG: Fibrin(ogen) and von Willebrand factor deposition are associated with intimal thickening after balloon angioplasty of the rabbit carotid artery. Arterioscler Thromb Vasc Biol 1997, 17:634-645 [DOI] [PubMed] [Google Scholar]
- 43.Magnusson MK, Mosher DF: Fibronectin: structure, assembly, and cardiovascular implications. Arterioscler Thromb Vasc Biol 1998, 18:1363-1370 [DOI] [PubMed] [Google Scholar]
- 44.Stouffer GA, Hu Z, Sajid M, Li H, Jin G, Nakada MT, Hanson SR, Runge MS: Beta 3 integrins are upregulated after vascular injury and modulate thrombospondin- and thrombin-induced proliferation of cultured smooth muscle cells. Circulation 1998, 97:907-915 [DOI] [PubMed] [Google Scholar]