Abstract
To assess the participation of the plasminogen activation system in the invasiveness of esophageal squamous cell carcinoma, we performed immunohistochemistry and in situ hybridization to study the distribution of a urokinase-type plasminogen activator (u-PA), u-PA receptor (u-PAR), and plasminogen activator inhibitor-2 (PAI-2). u-PA and PAI-2 were expressed heterogeneously in cancer cells, and restricted expression was found in stromal cells, especially fibroblasts, that were located in the immediate proximity of the cancerous cells. u-PAR was found only in cancer cells located at the periphery of tumors. Compared with patients with u-PA-negative cancer cells, patients with u-PA-positive cancer cells more frequently showed a neoplastic invasion beyond the muscularis propria and lymph node metastases. They also showed a significantly shorter 5-year overall survival. Patients with PAI-2-positive fibroblasts showed significantly lower levels of local invasiveness, represented by a neoplastic invasion beyond the muscularis propria, than those who were PAI-2 negative. Our results suggest that the expression of u-PA in esophageal squamous cell carcinoma is predictive of poor survival, whereas the expression of PAI-2 in the fibroblasts surrounding them is protective. An analysis of u-PA and PAI-2 expression in cancer cells and their surrounding fibroblasts may be useful for predicting the prognosis of patients with esophageal squamous cell carcinoma.
Cancer invasion and metastasis is the result of several interdependent processes. Extracellular proteolytic enzymes (eg, serine proteases and metalloproteases) have been implicated in cancer metastasis. The premise behind this theory is that release of proteolytic enzymes from tumors leads to the breakdown of basement membranes and the extracellular matrix, thereby facilitating cancer cell invasion into the surrounding normal tissue. 1,2,3 The plasminogen activation system includes the serine proteases plasmin and urokinase-type plasminogen activator (u-PA), plasminogen activator inhibitors 1 and 2 (PAI-1 and PAI-2, respectively), and the u-PA receptor (u-PAR). During the past decade, evidence for involvement of the u-PA system in cancer invasion and metastasis has increased substantially, and it now seems beyond reasonable doubt that the u-PA-mediated pathway of plasminogen activation is central to this process. 1-5
u-PA was the first protease shown to be a prognostic marker in human malignancy. Duffy et al showed that patients with breast tumors containing high levels of u-PA enzyme activity had a significantly shorter disease-free interval than did patients with low levels. 6 Later, high u-PA antigen levels were found to correlate with a shortened overall survival in this disease. 7 In addition, u-PA is a prognostic marker in other malignancies, including cancers of the lung, 8 esophagus, 9 stomach, 10 and colorectum. 11 However, the precise localization and expression of u-PA in both cancer and stromal cells are still unknown.
u-PA binds to the cell surface-bound u-PAR, and the bound form induces activation much more rapidly than does the fluid-phase u-PA. 12 In colon adenocarcinoma, u-PA was expressed in stromal cells, and u-PAR was expressed in cancer cells in invasive foci. 13 In this situation, u-PAR expression, but not u-PA, seemed to be specific for the carcinoma. u-PAR appears to be a prognostic marker in other cancers, including colorectal carcinoma, 14 breast cancer, 15 and squamous cell carcinoma (SCC) of the lung. 8
In vitro assays have shown that PAI-2 inhibits u-PA- and u-PAR-dependent invasion of cancer cells into human amniotic membranes 16 and into Matrigel. 17 In some situations, increased levels of PAI-2 are associated with a favorable prognosis. For example, low levels of PAI-2 in breast tumors correlated with a shorter metastasis-free survival in the overall population, as well as in the node-negative subgroup. 18 However, high levels of PAI-2 were associated with aggressive colorectal cancer. 11 These observations show that the biological consequences of PAI-2 expression in malignant tumors remain unclear.
Esophageal SCC has one of the poorest prognoses among the malignancies of the gastrointestinal tract. Biological markers for the malignant potential of this neoplasm are being investigated, and the discovery of such markers is likely to have a considerable effect on diagnosis and therapy. In a previous study, Hewin et al evaluated esophageal carcinoma by using enzyme-linked immunosorbent assays. 19 u-PA levels in the tumor were elevated compared with those of normal tissue, although PAI-1 concentrations did not differ. 19 Compared with nontumor tissue, the level of u-PA antigen was 13-fold higher in the samples of esophageal SCC. 20 Recently, it was reported that u-PA concentration and expression are prognostic factors in esophageal adenocarcinoma 21 and SCC. 9
In the present study, we describe and compare the expression and distribution of u-PA, u-PAR, and PAI-2 in cancer cells and stromal cells of esophageal SCC and evaluate the relationship between the clinicopathological factors, including the prognosis and expression of u-PA and PAI-2.
Materials and Methods
Patients
The study population consisted of 56 patients (54 men, 2 women) with surgically resected esophageal carcinoma. A potentially curative resection (esophagectomy) was defined as the absence of a distant metastasis, the removal of all gross tumor, and the histologically confirmed absence of tumor tissue at the surgical margins. Of the 56 patients, 15 had distant metastases during the period of follow up, 6 had lung metastases, 6 had hepatic metastases, and 4 had bone metastases; one patient had bone, bronchial, renal, and skeletal muscle metastases. Of the 22 patients who have died, death was caused by distant metastasis in 15 patients and by local recurrence in 3. Four patients died from causes other than carcinoma. The patient age at diagnosis ranged from 27 to 78 years (mean 62.2 years). The disease stage was classified based on the tumor-node metastasis (TMN) staging criteria. 22 Accordingly, 12 tumors were classified as pT1 (21.4%), 13 as pT2 (23.2%), 26 as pT3 (46.5%), and 5 as pT4 (8.9%); 21 cases were classified as pN0 (37.5%) and 35 as pN1 (62.5%). Microscopic evaluations were made by guidelines established by the Japanese Research Society for Esophageal Diseases. 23
Tissue Preparation
Samples of surgically resected esophageal cancer tissues from 28 patients were immediately fixed in 4% (w/v) paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4) at 4°C for 4 hours and embedded in paraffin, and 3-μm sections were subjected to in situ hybridization as described below. Paraffin-embedded specimens that had been fixed in 10% formaldehyde were available from all 56 patients and were used for immunohistochemical staining.
Immunohistochemical Staining
Monoclonal antibody against u-PA was provided by Y. Sakata (Division of Hemostasis and Thrombosis Research, Jichi Medical School, Tochigi, Japan). This antibody was confirmed by Western blotting to react with both the free and the complex forms of u-PA (Y. Eguchi and Y. Sakata, unpublished data). The monoclonal antibody against u-PAR was purchased from American Diagnostics Co. (Greenwich, CT), and a monoclonal antibody to PAI-2 was obtained from Biopool Co. (Umea, Sweden). Immunohistochemical staining was performed using the Histofine SAB-PO kit (Nichirei, Tokyo, Japan), by the manufacturer’s instructions. To unmask tissue antigens, the sections were digested with 0.3% (w/v) pepsin (Sigma Chemical Co., St. Louis, MO) in 0.01 N HCl for 15 minutes at room temperature. The sections were incubated at 4°C overnight with a 10-μg/ml primary antibody, which was diluted in PBS containing 0.1% rabbit normal serum. Hydrogen peroxide-supplemented diaminobenzidine was used to visualize the bound antibodies. The sections were counterstained with hematoxylin and then mounted. In the control experiments, the sections were incubated with the nonimmune murine immunoglobulin G in place of the primary antibody.
Riboprobe Preparation
Human u-PA complementary DNA (cDNA), 24 subcloned into the vector pBR322, was obtained from the Japanese Cancer Research Resources Bank. The PstI fragment of u-PA cDNA (15.8 kb) was subcloned into the vector pBluescript KS+ (Stratagene, La Jolla, CA).
The Riboprobe preparations were carried out essentially as described. 28,30 Briefly described, total RNA was extracted from an HT 1080 cell line (Japanese Collection of Research Bioresources) and then reverse-transcribed to isolate the coding regions of human u-PAR and PAI-2, . The resulting cDNA was amplified by the polymerase chain reaction, using two synthetic-oligonucleotide primers for each region: 5′-GGGGATTGCCGTGTGGAAGAG-3′ and 5′-GGTGATGGTGAGGCTGAAGTG-3′ for u-PAR, and 5′-CTGGAGTCGGGGAAGGGAGTC-3′ and 5′-GCTTAGTTTTAGGGTGAGGAA-3′ for PAI-2. The conditions for the 35 cycles of amplification were 94°C for 60 seconds, 55°C for 90 seconds, and 72°C for 120 seconds. The amplified 880-bp u-PAR and 406-bp PAI-2 fragments were subcloned into the vector pCRTMII (Invitrogen, San Diego, CA) and then used as the probes for in situ hybridization.
Linearized plasmids were transcribed using T7 polymerase in the presence of digoxigenin (Dig)-labeled UTP to generate sense RNA probes; for antisense probes, T3 polymerase was substituted. 25 The transcription was carried out by using a digoxigenin RNA-labeling kit (Boehringer-Mannheim Biochemicals, Mannheim, Germany) by the manufacturer’s instructions.
In Situ Hybridization
In situ hybridization was performed essentially as described by Springer et al. 26 To summarize, the sections were deparaffinized, incubated with 0.2 N HCl for 10 minutes, treated with proteinase K (Merck, Darmstadt, Germany; 10 μg/ml in 20 mmol/L Tris-HCl, pH 7.5, containing 2 mmol/L CaCl2) for 10 minutes, postfixed with 4% (w/v) paraformaldehyde in PBS for 10 minutes, rinsed with glycine (2 mg/ml in PBS) for 10 minutes, and immersed in 0.25% (w/v) acetic anhydride in 0.1 mol/L PBS for 10 minutes. The sections were prehybridized at 37°C for 1 hour in 50 μl of hybridization buffer (10 mmol/L Tris-HCl, pH 7.5, containing 0.6 mol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 50% (w/v) formamide, 1× Denhardt’s solution, 0.5 mg/ml yeast transfer RNA, and 0.5 mg/ml salmon sperm DNA). Hybridization was performed at 37°C overnight in a reaction mixture (20 μl) consisting of 4% (w/v) paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4), 10% (w/v) dextran sulfate, and the Dig-labeled RNA probe (1 μg/ml). The hybridized sections were washed at 37°C with 2× standard saline citrate (SSC), 50% (w/v) formamide (three times, 20 minutes per wash), 2× SSC (three times, 20 minutes per wash), and 1× SSC (three times, 20 minutes per wash). The following incubations and washes were performed at 25°C unless otherwise specified. The sections were rinsed for 10 minutes with buffer I (0.1 mol/L Tris-HCl, pH 7.5, containing 0.15 mol/L NaCl and 2 mmol/L MgCl2), incubated for 30 minutes in 5% (w/v) bovine serum albumin in buffer I, and then incubated for 10 minutes with anti-Dig alkaline phosphatase-conjugated antibody (Boehringer-Mannheim Biochemicals; diluted 1:500 with buffer I). This incubation was followed by three washes with buffer I (5 minutes per wash) and then a 5-minute wash with buffer II (0.1 mol/L Tris-HCl, pH 9.0, containing 0.15 mol/L NaCl and 50 mmol/L MgCl2). To detect signals, the sections were incubated with a substrate solution containing nitro-blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Life Technologies, Inc., Gaithersburg, MD) for about 60 minutes and then counterstained with methyl green or nuclear fast red.
A431 vulvar epidermoid carcinoma cells (source of u-PA) and HT 1080 fibrosarcoma cells (source of u-PAR and PAI-2) were obtained from the Japanese Collection of Research Bioresources. Cells (1 × 107) were injected under the skin of nude mice to generate control tissue for in situ hybridization. No signals were detected in these tissues hybridized with the u-PA and PAI-2 sense probes (negative control).
Statistical Analysis
The following analyses were performed by using the StatView program. Fisher’s exact probability test and unpaired Student’s t test were used to assess the relationship between the variables. Differences were considered significant when P values were less than 0.05. A life table analysis of overall survival was generated by using the product limit (Kaplan-Meier) method; the log-rank test was used to determine equality over strata; and the stepwise Cox proportional hazards model was used for multivariate analysis of overall survival.
Results
Localization of u-PA Antigen and mRNA
In normal esophageal tissues, u-PA antigen and mRNA were not detected in the epithelial or stromal cells of normal esophageal tissues (data not shown). In contrast, in the cancer cells, u-PA antigen was detected in the cytoplasm of 13 of the 56 samples (23.2%; Figure 1A ▶ ). The in situ hybridization analysis detected u-PA mRNA expression in the cytoplasm around the nuclei of these cells (Figure 1B) ▶ . The intensity of positive immunostaining was heterogeneous among the tumors. In the stroma, u-PA antigen and mRNA signals were observed in some fibroblasts, lymphocytes, and macrophage-like cells (inset in Figure 1A ▶ ). In the fibroblasts, u-PA antigen was detectable in 12 samples from 56 patients (21.4%). The positive fibroblasts were located in the immediate proximity of the cancerous cells, and no signal was observed in distant fibroblasts.
Figure 1.
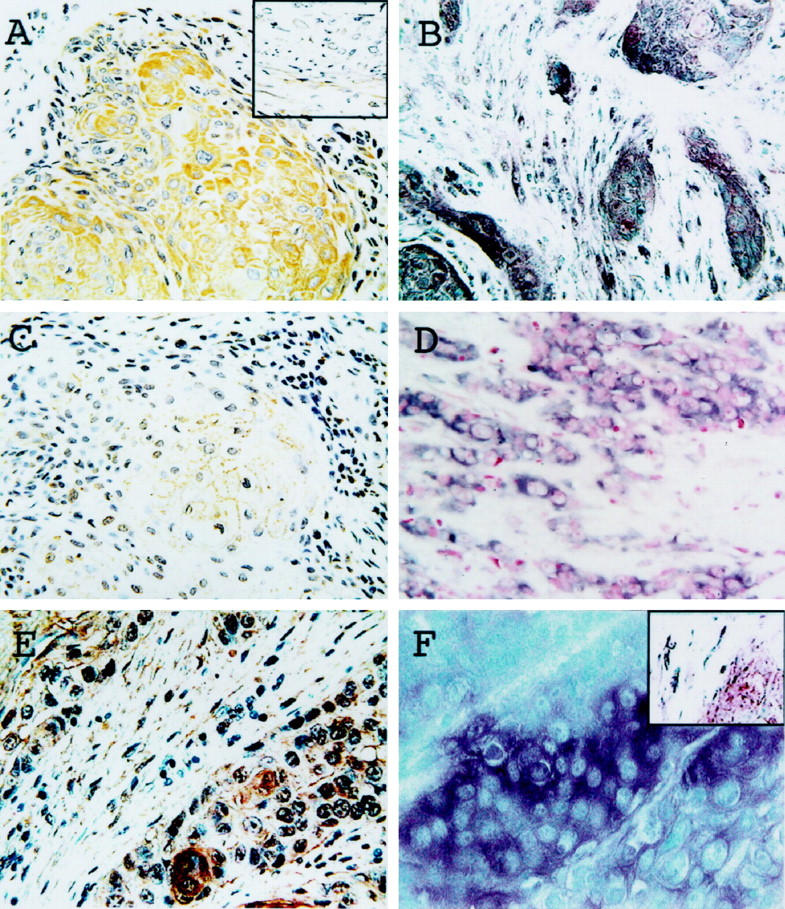
Expression of u-PA, u-PAR, and PAI-2 in esophageal SCC. Immunostaining with antibodies to u-PA (A), u-PAR (C), and PAI-2 (E). Representative data of in situ hybridization using antisense probes for u-PA (B), u-PAR (D), and PAI-2 (F). Positive staining was observed in cancer cells (A–F) and the fibroblasts surrounding them (B, E, insets in A and F).
Localization of u-PAR Antigen and mRNA
The epithelial and stromal cells of normal esophageal tissues and cancerous stromal fibroblasts were negative for both u-PAR antigen and mRNA. Of the 56 samples, 14 (25.0%) had u-PAR antigen at the surface of the neoplastic cells (Figure 1C) ▶ . The positive signal was primarily localized at the periphery of the tumors. u-PAR mRNA signals were found in the cytoplasm of the protein-positive cells (Figure 1D) ▶ .
Localization of PAI-2 Antigen and mRNA
Neither the epithelial nor stromal cells of normal esophageal tissue expressed PAI-2 protein or mRNA. In contrast, PAI-2 antigen was detected in the cytoplasm of cancer cells in 10 of the 56 patients (17.9%; Figure 1E ▶ ), and, in these positive cells, PAI-2 mRNA was found in the perinuclear cytoplasm (Figure 1F) ▶ . The positive immunostaining signals were distributed heterogeneously in the tumors. In the cancerous stroma, PAI-2 protein and mRNA signals were observed in various fibroblasts, lymphocytes, and macrophage-like cells (inset in Figure 1F ▶ ). PAI-2 antigen was found in the fibroblasts in 25 of the 56 cases (44.6%). These PAI-2-expressing fibroblasts were scattered adjacent to the cancer cells.
Correlation of u-PA and u-PAR Expression with Various Clinicopathological Factors
Compared with patients with u-PA-negative tumors, patients with u-PA-positive cancer cells were more frequently pT3 and pT4 at operation (P < 0.05), had more frequent lymph node metastasis at operation (P < 0.05), and developed hepatic metastasis (P < 0.05) and distant metastasis (P < 0.001) more frequently during the follow-up period (Table 1) ▶ .
Table 1.
Relationship between the Expression of u-PA, u-PAR, and PAI-2 Protein and Various Clinicopathological Factors
| Characteristics | u-PA | u-PAR | PAI-2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer cells | Fibroblasts | Cancer cells | Cancer cells | Fibroblasts | |||||||||||
| Posi- tive | Nega- tive | P value | Posi- tive | Nega- tive | P value | Posi- tive | Nega- tive | P value | Posi- tive | Nega- tive | P value | Posi- tive | Nega- tive | P value | |
| No. of patients | 13 | 43 | 12 | 44 | 14 | 42 | 10 | 46 | 25 | 31 | |||||
| Age | |||||||||||||||
| Mean | 58.8 | 63.3 | N.S.‡ | 62.4 | 62.2 | N.S. | 63.2 | 59.1 | N.S. | 62.3 | 62.2 | N.S. | 59.5 | 64.4 | N.S. |
| Range | 46–73 | 27–78 | 50–72 | 27–78 | 44–78 | 27–72 | 50–69 | 27–78 | 27–74 | 46–78 | |||||
| Sex | |||||||||||||||
| Male | 13 | 41 | N.S. | 11 | 43 | N.S. | 41 | 13 | N.S. | 9 | 45 | N.S. | 24 | 30 | N.S. |
| Female | 0 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Histological type* | |||||||||||||||
| Well | 6 | 14 | N.S. | 5 | 15 | N.S. | 4 | 16 | N.S. | 4 | 16 | N.S. | 11 | 9 | N.S. |
| Moderately | 5 | 21 | 4 | 22 | 8 | 18 | 4 | 22 | 12 | 14 | |||||
| Poorly | 2 | 7 | 3 | 6 | 1 | 8 | 1 | 8 | 2 | 7 | |||||
| Basal cell carcinoma | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | |||||
| T category† | |||||||||||||||
| pT1, pT2 | 2 | 22 | P < 0.05 | 4 | 20 | N.S. | 6 | 18 | N.S. | 5 | 19 | N.S. | 15 | 9 | P < 0.05 |
| pT3, pT4 | 11 | 21 | 8 | 24 | 8 | 24 | 5 | 27 | 10 | 22 | |||||
| N category† | |||||||||||||||
| pN0 | 1 | 20 | P < 0.05 | 4 | 17 | N.S. | 4 | 17 | N.S. | 4 | 17 | N.S. | 12 | 9 | N.S. |
| pN1 | 12 | 23 | 8 | 27 | 10 | 25 | 6 | 29 | 13 | 22 | |||||
| Lymphatic invasion† | |||||||||||||||
| Negative | 1 | 10 | N.S. | 2 | 9 | N.S. | 1 | 10 | N.S. | 0 | 11 | N.S. | 6 | 5 | N.S. |
| Positive | 12 | 33 | 10 | 35 | 13 | 32 | 10 | 35 | 19 | 26 | |||||
| Vascular invasion† | |||||||||||||||
| Negative | 6 | 23 | N.S. | 4 | 25 | N.S. | 8 | 21 | N.S. | 3 | 26 | N.S. | 12 | 17 | N.S. |
| Positive | 7 | 20 | 8 | 19 | 6 | 21 | 7 | 20 | 13 | 14 | |||||
| Prospective hepatic‡ metastases | |||||||||||||||
| Negative | 9 | 41 | P < 0.05 | 11 | 39 | N.S. | 12 | 38 | N.S. | 9 | 41 | N.S. | 24 | 26 | N.S. |
| Positive | 4 | 2 | 1 | 5 | 2 | 4 | 1 | 5 | 1 | 5 | |||||
| Prospective distant‡ metastases | |||||||||||||||
| Negative | 0 | 41 | P < 0.001 | 10 | 31 | N.S. | 11 | 30 | N.S. | 9 | 32 | N.S. | 19 | 22 | N.S. |
| Positive | 13 | 2 | 2 | 13 | 3 | 12 | 1 | 14 | 6 | 9 |
*Well, well-differentiated squamous carcinoma; moderately, moderately differentiated squamous carcinoma; poorly, poorly differentiated squamous carcinoma.
†At operation.
‡During the period of follow up.
N.S., not significant (Fisher’s exact probability test).
We then evaluated the association between various clinicopathological factors and the combined expression of u-PA and u-PAR proteins in cancer cells and fibroblasts (Table 2) ▶ . Group A patients (n = 11) had neoplastic cells that stained for u-PA but not for u-PAR, and their fibroblasts were negative for u-PA. Group B comprised patients whose cancer cells were positive for u-PAR only and whose fibroblasts were positive for u-PA (n = 12). Group C included patients whose cancer cells were negative for both u-PA and u-PAR and whose fibroblasts were negative for u-PA (n = 23). Patients with cancer cells that were positive for both u-PA and u-PAR (n = 2) or who were positive for u-PA in both cancer cells and fibroblasts (n = 5) were excluded from the analysis because of the small number of cases. Compared with group C patients, group A patients more often had pT3 and pT4 (P < 0.05) or pN1 (P < 0.05) at the time of operation, as well as prospective distant metastasis during the follow-up period (P < 0.001). The prospective distant metastasis was more frequently found in group A patients than in group B or group C patients (P < 0.001 and P < 0.05, respectively).
Table 2.
Relationship between Various Clinicopathological Factors and the Combined Expression of u-PA and u-PAR Protein in Cancer Cells and Fibroblasts
| Characteristics | Group A | Group B | Group C |
|---|---|---|---|
| Neoplastic cells | |||
| u-PA staining | + | − | − |
| u-PAR staining | − | + | − |
| Fibroblasts | |||
| u-PA staining | − | + | − |
| u-PAR staining | − | − | − |
| No. of patients | 11 | 12 | 23 |
| T category* | |||
| pT1, pT2 | 2‡ | 6 | 13 |
| pT3, pT4 | 9 | 6 | 10 |
| N category* | |||
| pN0 | 1§ | 4 | 12 |
| pN1 | 10 | 8 | 11 |
| Lymphatic invasion* | |||
| Negative | 1 | 1 | 7 |
| Positive | 10 | 11 | 16 |
| Vascular invasion* | |||
| Negative | 4 | 6 | 14 |
| Positive | 7 | 6 | 9 |
| Prospective hepatic metastases† | |||
| Negative | 8 | 11 | 22 |
| Positive | 3 | 1 | 1 |
| Prospective distant metastasis† | |||
| Negative | 0¶∥ | 11 | 22 |
| Positive | 11 | 1 | 1 |
*At operation.
†During the period of follow-up.
‡P < 0.05, Group A versus C.
§P < 0.05, Group A versus C.
¶P < 0.001, Group A versus B.
∥P < 0.001, Group A versus C.
Survival Rates Analyzed by u-PA and u-PAR Expression
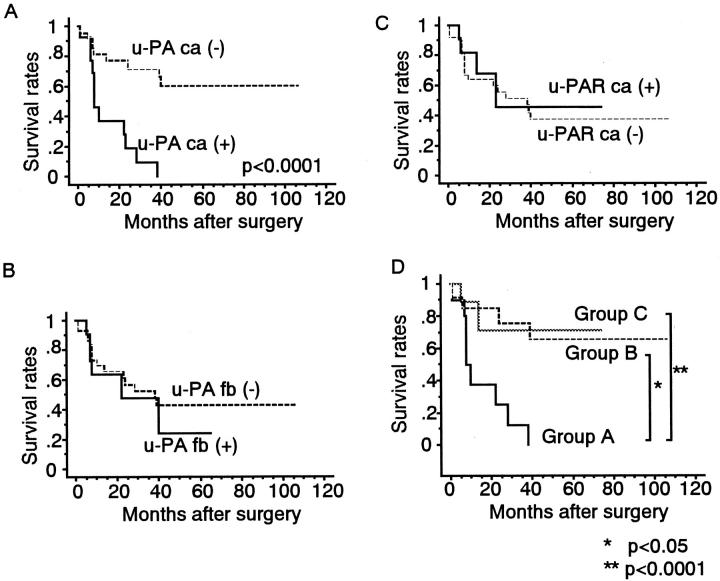
The 5-year overall survival rate of patients with u-PA-positive cancer cells was 0%, compared with 60% for those whose cancer cells were u-PA-negative (Figure 2A) ▶ . The log-rank test showed that these rates differed significantly (P < 0.0001). In contrast, the 5-year overall survival rate did not differ significantly between patients with u-PA-positive or u-PA-negative fibroblasts (23.9% versus 42.7%; Figure 2B ▶ ). Likewise, patients with u-PAR-positive or u-PAR-negative cancer cells had similar 5-year rates of overall survival (45.5% versus 37.7%; Figure 2C ▶ ).
Figure 2.
Overall survival of patients with esophageal SCC as indicated by staining for u-PA in cancer cells (A), u-PA in fibroblasts (B), and u-PAR in cancer cells (C). D: Group A (n = 11) had neoplastic cells that stained for only u-PA and had u-PA-negative fibroblasts. Group B (n = 12) comprises patients whose cancer cells were positive for only u-PAR and whose fibroblasts were positive for u-PA. Patients in Group C (n = 23) had cancer cells negative for both u-PA and u-PAR and fibroblasts negative for u-PA. As shown in A, the prognosis of patients with u-PA-positive cancer cells is significantly poorer than that of patients whose cancer cells are u-PA-negative. D illustrates that group A has a significantly worse prognosis than do groups B and C.
The 5-year overall survival rates were 0%, 65.6%, and 73% for patients in groups A, B and C, respectively (Figure 2D) ▶ . A log-rank test analysis showed that group A had a significantly shorter overall survival than groups B and C (P < 0.05 and P < 0.0001, respectively).
Correlation of PAI-2 Expression with Various Clinicopathological Factors
There was no statistically significant correlation between PAI-2 expression in cancer cells and clinicopathological factors (Table 1) ▶ . However, the incidence of pT3 and pT4 was significantly higher (P < 0.05) in patients whose fibroblasts were negative for PAI-2 protein (Table 1) ▶ .
u-PA and PAI-2 Expression and Survival Rate
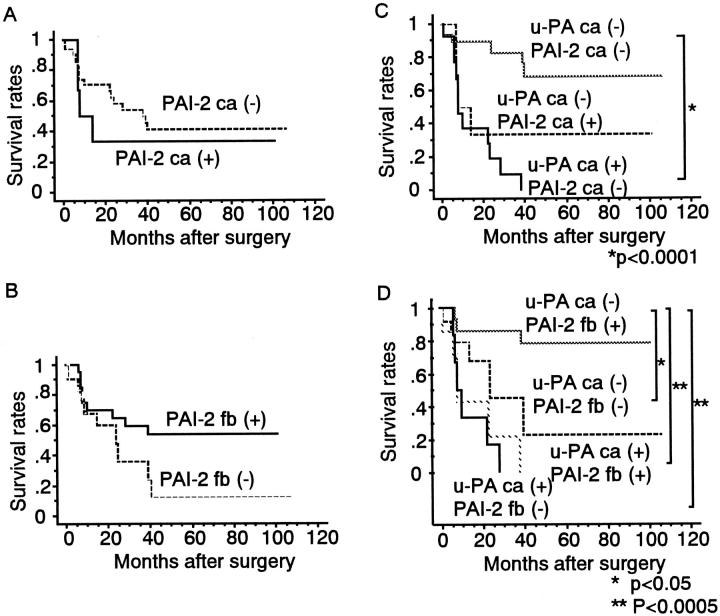
The 5-year overall survival rates of 33.3% for patients with PAI-2-positive cancer cells and 41.4% for those with PAI-2-negative cancer cells did not differ significantly (Figure 3A) ▶ . The estimated rates of overall survival at 5 years were 53.8% for patients whose fibroblasts were positive for PAI-2 and 12.0% for those with PAI-2-negative fibroblasts; again, these values were not significantly different (Figure 3B) ▶ .
Figure 3.
Overall survival of patients with esophageal SCC as indicated by staining for PAI-2 in cancer cells (A), PAI-2 in fibroblasts (B), u-PA in cancer cells and PAI-2 in cancer cells (C), and u-PA in cancer cells and PAI-2 in fibroblasts (D).
When the combined expression of u-PA and PAI-2 was analyzed in cancer cells, there was no case that was positive for both markers, and PAI-2 expression in cancer cells had no effect on the 5-year overall survival rate (Figure 3C) ▶ . In contrast, patients with u-PA-negative cancer cells and PAI-2-positive fibroblasts had a significantly better outcome (P < 0.05) than the group that had u-PA-negative cancer cells and PAI-2-negative fibroblasts. However, among patients with u-PA-positive cancer cells, survival was similar between those with PAI-2-positive and PAI-2-negative fibroblasts (Figure 3D) ▶ .
Multivariate Analysis
The prognostic importance of u-PA, u-PAR, and PAI-2 in cancer cells and fibroblasts and of other variables (pT and pN) was investigated in a multivariate Cox analysis. The factors associated with a poor prognosis were u-PA and PAI-2 in cancer cells (Table 3) ▶ .
Table 3.
Multivariate Analysis
| Parameter | P value | Relative risk | 95% Confidence interval | |
|---|---|---|---|---|
| pT | pT3, pT4 | 0.5412 | 1.449 | 0.441–4.762 |
| pN | pN1 | 0.1216 | 2.551 | 0.779–8.333 |
| u-PA cancer cells | Positive | 0.0012 | 11.569 | 2.633–50.835 |
| u-PA fibroblasts | Positive | 0.3477 | 1.668 | 0.573–4.850 |
| u-PAR cancer cells | Positive | 0.8814 | 1.098 | 0.323–3.731 |
| PAI-2 cancer cells | Positive | 0.0315 | 4.621 | 1.145–18.642 |
| PAI-2 fibroblasts | Negative | 0.1637 | 2.012 | 0.752–5.376 |
Discussion
We used immunohistochemistry and in situ hybridization to investigate the expression of u-PA, u-PAR, and PAI-2 in cancer cells and stromal cells of esophageal SCC. u-PA and PAI-2 were expressed in cancer cells as well as in adjacent fibroblasts. In contrast, u-PAR was expressed only in cancer cells located at the periphery of the tumors.
Localization of the protein and mRNA of u-PA, u-PAR, and the PAIs varies among tumor types. In colon carcinoma, u-PA is expressed in cancer cells, u-PAR occurs in cancer cells and macrophages surrounding cancer cells, and PAI-1 and -2 are found in endothelial cells. 27,28 In breast cancer, u-PA, u-PAR, and PAI-2 are all expressed in cancer cells and the surrounding stromal cells. 29,30 In cutaneous SCC, u-PA and PAI-1 are expressed in cancer cells. 31 Together with our findings, these data suggest that the differential expression of u-PA, u-PAR, and PAIs may contribute to the organ and cellular specificity of various malignant characteristics.
u-PA is a prognostic marker in many malignancies, including cancers of the lung and stomach, 10 esophageal adenocarcinoma, 21 and colorectal cancers. 11 By immunohistochemical analysis in esophageal SCC, the intensity of u-PA staining provided significant prognostic information, in that patients with strongly u-PA-positive tumors had a poorer outcome than patients with weakly u-PA-positive or u-PA-negative tumors, 9 which is almost identical to our result. In addition, u-PAR is a prognostic factor for colorectal carcinoma 14 and SCC of the lung. 8 To evaluate the relationship between u-PA and u-PAR expression and the invasion of cancer cells and survival in esophageal SCC, we divided the patients into three groups based on the combined expression of u-PA in cancer cells and fibroblasts and u-PAR in cancer cells. Metastasis to distant organs during the follow-up period occurred more frequently (P < 0.001) in group A, whose patients had u-PA-positive, but u-PAR-negative cancer cells and u-PA-negative fibroblasts, compared with group B, who had u-PA-negative, but u-PAR-positive cancer cells and u-PA-positive fibroblasts. Furthermore, survival was significantly shorter (P < 0.05) for group A than for group B. These data suggest that u-PA expression by cancer cells may be indicative of poor prognosis in esophageal SCC.
In vitro, u-PAR-bound pro-u-PA is activated much more quickly than is the fluid-phase protein, 12 and u-PAR is considered to enhance the ability of u-PA to mediate invasiveness by localizing u-PA to the surface of the tumor cell. Indeed, recent evidence from Morrissey et al 33 shows that coexpression of u-PA and u-PAR is required for maximal invasiveness of esophageal carcinoma cell lines in vitro. Antisense to either molecule greatly diminished the invasiveness of u-PA–u-PAR coexpressing esophageal cell lines. 32 In our study, two patients with cancer cells that were positive for both u-PA and u-PAR showed shorter overall survival for 6 and 33 months, respectively, indicating that the coexpression of u-PA and u-PAR in cancer cells enhances the invasiveness. We observed the expression of u-PAR only in the cancer cells; therefore, u-PAR may be a cancer-specific, rather than prognostic factor, because it does reflect invasiveness. In melanoma cells, u-PA is able to bind with a low affinity to an unidentified membrane-associated protein. 33 Further, the urine of u-PAR−/− mice contains active u-PA, 34 demonstrating that activation, at least to some extent, can proceed in the absence of u-PAR binding. These observations suggest that u-PA in cancer cells could act in vivo without the intermediacy of u-PAR, and could thus be associated with invasiveness.
The role of PAI-2 expression and its effect on the prognosis in cancer patients have been unclear. In our investigation, patients with PAI-2-positive fibroblasts were pT1 and pT2 significantly more frequently than patients with PAI-2-negative fibroblasts (P < 0.05), which were more frequently pT3 and pT4. Patients with PAI-2-positive fibroblasts also showed a more favorable prognosis when their cancer cells expressed too little u-PA to be detected by immunohistochemistry (P < 0.05). This suggests that PAI-2 expression by fibroblasts may be sufficient to inhibit u-PA activity when a low level of u-PA synthesis occurs in cancer cells. On the other hand, multivariate analysis revealed that PAI-2 expression in cancer cells was significantly associated with poor prognosis (P < 0.05). The genes for PAI-2 and SCC antigen are located within a 300-kb cluster on the long arm of chromosome 18 (18q21.3). 35 In a previous study, 25% of patients with an esophageal SCC had an elevated level of SCC antigen. 36 Therefore, it is possible that PAI-2 might be coexpressed with the SCC antigen in esophageal squamous cell carcinoma.
Although the expression of the SCC antigen does not correlate with malignant potential, 37 transduction of tumor cells with SCC antigen-1 results in inhibition of apoptosis of tumor cells induced by anticancer drugs, tumor necrosis factor α, or natural killer cells. 38 PAI-2 also has been reported to inhibit apoptosis induced by tumor necrosis factor α in HeLa cells. 39 Therefore, PAI-2 expression in cancer cells may have an effect on apoptosis, resulting in a poor prognosis. In summary, PAI-2 may play diverse roles in tumor biology, promoting carcinogenesis via an effect on apoptosis as well as inhibiting tumor invasiveness via the plasminogen activation system. A PAI-2 effect in any one tumor may depend on its cellular distribution.
The prognosis of patients with esophageal squamous cell carcinoma is poor, because esophageal carcinomas have frequently metastasized to the lymph nodes and distant organs by the time that the tumor is diagnosed. 40 Therefore, establishing effective treatment for esophageal squamous carcinoma is essential to improve its prognosis. In our opinion, a separate determination of u-PA and PAI-2 in cancer cells and fibroblasts could yield useful prognostic information for patients with esophageal carcinoma. In this regard, recently antisense inhibition of u-PA was shown to significantly reduce invasion and metastasis in a human osteosarcoma cell line. 41 Similar antisense therapy might be effective in patients with u-PA-positive esophageal carcinoma.
Acknowledgments
We are grateful to Dr. Y. Sakata (Division of Hemostasis and Thrombosis Research, Jichi Medical School) for providing the antibodies and for useful advice and discussion; Dr. H. Naitoh and Dr. T. Umeda for preparing the u-PA, u-PAR ,and PAI-2 probes; Dr. A. Kawaguchi for analyzing the overall survival rate; and the Japanese Collection of Research Bioresources for providing the HT 1080 cell line and u-PA cDNA.
Footnotes
Address reprint requests to Yutaka Eguchi, Intensive Care Unit, Shiga University of Medical Science, Otsu, Shiga, 520-2192, Japan. E-mail: eguchi@belle.shiga-med.ac.jp.
Supported by grants-in-aid 04670776 and 07671299 from the Ministry of Education, Science and Culture of Japan.
References
- 1.Danø K, Anderson PA, Grøndahl-Hansen J, Kristensen P, Nielsen LS, Skriver L: Plasminogen activators, tissue degradation and cancer. Adv Cancer Res 1985, 44:139-266 [DOI] [PubMed] [Google Scholar]
- 2.Mignatti P, Rifkin DB: Biology and biochemistry of proteinases on tumor invasion. Physiol Rev 1993, 73:161-195 [DOI] [PubMed] [Google Scholar]
- 3.Andreasen PA, Kjøller L, Christensen L, Duffy MJ: The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer 1997, 72:1-22 [DOI] [PubMed] [Google Scholar]
- 4.Duffy MJ: Proteases as prognostic markers. Clin Cancer Res 1996, 2:613-618 [PubMed] [Google Scholar]
- 5.Schimitt M, Harbeck N, Thomssen C, Wilheim O, Magdolen V, Reuning U, Ulm K, Höfler H, Janiecke F, Graeff H: Clinical impact of the plasminogen activation system in tumor invasion and metastasis: prognostic relevance and target for therapy. Thromb Haemost 1997, 78:285-296 [PubMed] [Google Scholar]
- 6.Duffy MJ, O’Grandy P, Devaney D, O’Siorain L, Fennelly JJ, Luen HJ: Urokinase-plasminogen activator, a marker for aggressive breast cancers. Cancer 1988, 62:531-533 [DOI] [PubMed] [Google Scholar]
- 7.Duffy MJ: Plasminogen activators and cancer. Blood Coagul Fibrinolysis 1990, 1:681-687 [PubMed] [Google Scholar]
- 8.Pedersen H, Brunner N, Francis D, Osterlind K, Ronne E, Hansen HH, Danø K, Grøndahl HJ: Prognostic impact of urokinase, urokinase receptor, and type 1 plasminogen activator inhibitor in squamous and large cell lung cancer tissue. Cancer Res 1994, 54(17):4671-4675 [PubMed] [Google Scholar]
- 9.Torzewski M, Sarbia M, Verreet P, Dutkowski P, Heep H, Willers R, Gabbert HE: Prognostic significance of urokinase-type plasminogen activator expression in squamous cell carcinoma of the esophagus. Clin Cancer Res 1997, 3:2263-2268 [PubMed] [Google Scholar]
- 10.Nekarda H, Siewert J, Schmitt M, Ulm K: Tumor-associated proteolytic factors u-PA and PAI-1 and survival in totally resected gastric cancer. Lancet 1994, 343:117. [DOI] [PubMed] [Google Scholar]
- 11.Ganesh S, Sier CFM, Griffioen G, Vloedgraven H, DeBoer A, Welwaart K, van de Velde CJ, van Krieken JH, Verheijen J, Lamers C, Verspaget HW: Prognostic relevance of plasminogen activators and their inhibitors in colorectal cancer. Cancer Res 1994, 54:4065-4071 [PubMed] [Google Scholar]
- 12.Ellis V, Scully MF, Kakkar VV: Plasminogen activation initiated by single-chain urokinase-type plasminogen activator: potentiation by U937 monocytes. J Biol Chem 1989, 264:2184-2188 [PubMed] [Google Scholar]
- 13.Pyke C, Kristensen P, Ralfkiaer E, Grøndahl-Hansen J, Eriksen J, Blasi FKD: Urokinase-type plasminogen activator is expressed in stromal cells and its receptor in cancer cells at invasive foci in human colon adenocarcinomas. Am J Pathol 1991, 138:1059-1067 [PMC free article] [PubMed] [Google Scholar]
- 14.Ganesh S, Sier CFM, Heerding MH, Griffioen G, Lamers C, Verspaget HW: Urokinase receptor and colorectal cancer survival. Lancet 1994, 344:401-402 [DOI] [PubMed] [Google Scholar]
- 15.Duggan C, Maguire T, McDermott E, O’Higgins N, Fennelly JJ, Duffy MJ: Urokinase plasminogen activator and urokinase plasminogen activator receptor in breast cancer. Int J Cancer 1995, 61:597-600 [DOI] [PubMed] [Google Scholar]
- 16.Brückner A, Filderman AE, Kirchheimer JC, Binder BR, Remold HG: Endogenous receptor-bound urokinase mediates tissue invasion of the human lung carcinoma cell line A549 and Calu-1. Cancer Res 1992, 52:3043-3047 [PubMed] [Google Scholar]
- 17.Stahl A, Mueller BM: Binding of urokinase to its receptor promotes migration and invasion of human melanoma cells in vitro. Cancer Res 1994, 54:3066-3071 [PubMed] [Google Scholar]
- 18.Bouchet C, Spyratos F, Martin PM, Hacene K, Gentile A, Oglobine J: Prognostic value of urokinase-type plasminogen activator (u-PA) and plasminogen activator inhibitors PAI-1 and PAI-2 in breast carcinomas. Br J Cancer 1994, 69:398-405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hewin DF, Savage PB, Alderson D, Vipond MN: Plasminogen activators in oesophageal carcinoma. Br J Surg 1996, 83:1152-1155 [DOI] [PubMed] [Google Scholar]
- 20.Sier CF, Quax PH, Vloedgraven HJ, Verheijen JH, Griffioen G, Ganesh S, Lamers CB, Verspaget HW: Increased urokinase receptor levels in human gastrointestinal neoplasia and related liver metastases. Invasion Metastasis 1993, 13:277-288 [PubMed] [Google Scholar]
- 21.Nekarda H, Schlegel P, Schmitt M, Stark M, Mueller JD, Fink U, Siewert JR: Strong prognostic impact of tumor-associated urokinase-type plasminogen activator in completely resected adenocarcinoma of the esophagus. Clin Cancer Res 1998, 4:1755-1763 [PubMed] [Google Scholar]
- 22.Union Internationale Contre Cancer: TMN Classification of Malignant Tumors. New York, Wiley-Liss, 1997
- 23.Japanese Research Society for Esophageal Diseases: Guidelines for the Clinical and Pathologic Studies on Carcinoma of the Esophagus. Tokyo, Kanehara & Co., Ltd, 1992
- 24.Ricco A, Grimaldi G, Verde P, Sebastio G, Boast S, Blasi F: The human urokinase-plasminogen activator gene and its promoter. Nucleic Acids Res 1985, 13:2759-2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hölyke HJ, Kessler C: Non-radioactive labeling of RNA transcripts in vitro with the heparin digoxigenin (DIG); hybridization and ELISA-based detection. Nucleic Acids Res 1990, 18:5843-5851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Springer J, Robbins E, Gwag B, Lewis M, Baldino F: Non-radioactive detection of nerve growth factor receptor (NGFR) mRNA in rat brain using in situ hybridization histochemistry. J Histochem Cytochem 1991, 39:231-234 [DOI] [PubMed] [Google Scholar]
- 27.Pyke C, Ralfkiaer E, Ronne E, Hoyer Hansen G, Kirkeby L, Danø K: Immunohistochemical detection of the receptor for urokinase plasminogen activator in human colon cancer. Histopathology 1994, 24:131–138 [DOI] [PubMed]
- 28.Naitoh H, Eguchi Y, Ueyama H, Kodama M, Hattori T: Localization of urokinase-type plasminogen activator, plasminogen activator inhibitor-1,2 and plasminogen in colon cancer. Jpn J Cancer Res 1995, 86:48-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pyke C, Graem N, Ralfkiaer E, Ronne E, Hoyer HG, Brunner N, Danø K: Receptor for urokinase is present in tumor-associated macrophages in ductal breast carcinoma. Cancer Res 1993, 1915, 53:1911-1915 [PubMed] [Google Scholar]
- 30.Umeda T, Eguchi Y, Okino K, Kodama M, Hattori T: Cellular localization of urokinase-type plasminogen activator, its inhibitors and their mRNAs in breast cancer tissues. J Pathol 1997, 183:388-397 [DOI] [PubMed] [Google Scholar]
- 31.Sappino A-P, Belin D, Huarte J, Hirschel-Scholz S, Saurat J-H, Vassalli J-D: Differential protease expression by cutaneous squamous and basal cell carcinomas. J Clin Invest 1991, 88:1073-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrissey D, O’Connell J, Lynch D, O’Sullivan GC, Shanahan F, Collins JK: Invasion by esophageal cancer cells: functional contribution of the urokinase plasminogen activation system by antisense oligonucleotides to urokinase or urokinase receptor. Clin Exp Metastasis 1999, 17:77-85 [DOI] [PubMed] [Google Scholar]
- 33.Koopman JL, Slomp J, de Bart ACW, Quax PHA, Verheijen JH: Mitogenic effects of urokinase on melanoma cells are independent of high affinity binding to the urokinase receptor. J Biol Chem 1998, 273:33267-33272 [DOI] [PubMed] [Google Scholar]
- 34.Bugge TH, Suh TT, Flick MJ, Daugherty CC, Rømer J, Solberg H, Ellis V, Danø K, Degen JL: The receptor for urokinase-type plasminogen activator is not essential for mouse development or fertility. J Biol Chem 1995, 270:16886-16894 [DOI] [PubMed] [Google Scholar]
- 35.Schneider SS, Schick C, Fish KE, Miller E, Pena JC, Treter SD, Hui SM, Silverman GA: A serine proteinase inhibitor locus at 18q21.3 contains a tandem duplication of the human squamous cell carcinoma antigen gene. Proc Natl Acad Sci USA 1995, 92:3147-3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto K, Oka M, Hayashi H, Tangoku A, Gondo T, Suzuki T: CYFRA 21–1 is a useful marker for esophageal squamous cell carcinoma. Cancer 1997, 79:1647-1655 [PubMed] [Google Scholar]
- 37.Matsuda H, Mori M, Tsujitani S, Ohno S, Kuwano H, Sugimachi K: Immunohistochemical evaluation of squamous cell carcinoma antigen and S-100 protein-positive cells in human malignant esophageal tissues. Cancer 1990, 65:2261-2265 [DOI] [PubMed] [Google Scholar]
- 38.Suminami Y, Nawata S, Kato H: Biological role of SCC antigen. Tumour Biol 1998, 19:488-493 [DOI] [PubMed] [Google Scholar]
- 39.Dickinson JL, Bates EJ, Ferrante A, Antalis TM: Plasminogen activator inhibitor type 2 inhibits tumor necrosis factor α-induced apoptosis: evidence for an alternate biological function. J Biol Chem 1995, 270:27894-27904 [DOI] [PubMed] [Google Scholar]
- 40.Katlic MR, Wilkins EW, Grillo HC: Three decades of treatment of esophageal squamous carcinoma at the Massachusetts General Hospital. J Thorac Surg 1990, 99:929-938 [PubMed] [Google Scholar]
- 41.Haeckel C, Krueger S, Roessner A: Antisense inhibition of urokinase: effect on malignancy in a human osteosarcoma cell line. Int J Cancer 1998, 77:153-160 [DOI] [PubMed] [Google Scholar]