Abstract
The adult rodent liver contains at least two recognized populations of cells with stem-like properties that contribute to liver repair/regeneration under different pathophysiological circumstances: (i) unipotential committed progenitor cells (differentiated hepatocytes and biliary epithelial cells) and (ii) multipotential nonparenchymal progenitor cells (oval cells). In retrorsine-induced hepatocellular injury the capacity of fully differentiated rat hepatocytes to replicate is severely impaired and massive proliferation of oval cells does not occur. Nevertheless, retrorsine-exposed rats can replace their entire liver mass after 2/3 surgical partial hepatectomy through the emergence and expansion of a population of small hepatocyte-like progenitor cells that expresses phenotypic characteristics of fetal hepatoblasts, oval cells, and fully differentiated hepatocytes, but differ distinctly from each type of cell. The activation, proliferation, and complete regeneration of normal liver structure from small hepatocyte-like progenitor cells have not been recognized in other models of liver injury characterized by impaired hepatocyte replication. We suggest that the selective emergence and expansion of small hepatocyte-like progenitor cells observed in the retrorsine model reflect a novel mechanism of complete liver regeneration in the adult rat. Furthermore, we suggest that these cells may represent a novel progenitor cell population that (i) responds to liver deficit when the replication capacity of differentiated hepatocytes is impaired, (ii) expresses an extensive proliferative capacity, (iii) can give rise to large numbers of progeny hepatocytes, and (iv) can restore tissue mass.
The major properties of stem cells have been inferred from investigations of classic stem cell-fed lineage renewal systems, including bone marrow, intestinal epithelium, and skin. Essential properties expected of stem cells include the capacities to proliferate repeatedly, to renew the stem cell population, and to generate sufficient differentiated progeny to maintain or regenerate the functional capacity of a tissue. 1,2 Classic stem cells are thought to be undifferentiated and to express variable differentiation potentials; fully (terminally) differentiated cells are not considered to manifest these properties. 1,2 Although classic stem cell-fed lineage systems have been used to infer the properties of stem cells, evidence now suggests the existence of stem-like cells in many tissues, such as the central nervous system 3-6 and liver, 7,8 that appear to be composed predominately of terminally differentiated parenchymal cells and do not contain active stem cell-fed lineages. The stem-like cells of tissues that lack high cellular turnover appear to exhibit properties that differ from those proposed for classic stem cells.
Adult rodent livers contain at least two well-recognized cell populations with major stem-like properties, unipotential (committed) progenitor cells (fully differentiated hepatocytes and biliary epithelial cells), and multipotential nonparenchymal epithelial (ductular) progenitor cells, both of which can contribute to liver repair under different pathophysiological circumstances, although neither cell type proliferates to any significant extent under normal circumstances. 9 Replacement of hepatocytes (and liver tissue mass) lost to surgical resection (partial hepatectomy) or toxic injury (necrosis) is typically achieved through the proliferation of fully differentiated, normally quiescent hepatocytes and biliary epithelial cells con-tained in the residual (viable) tissue. 10-13 Adult rodent hepatocytes, for example, possess extensive growth potential 14-16 and can proliferate through at least 86 cell doublings in vivo under experimental circumstances. 17 Thus, fully differentiated hepatocytes exhibit essential properties ascribed to the stem cells of classic stem cell-fed lineage systems, 7,9 including the ability to proliferate repeatedly and produce large numbers of differentiated progeny, and can be viewed as a unipotential progenitor cell for the generation of additional hepatocytes. 9 Multipotential epithelial stem-like (oval) cells are not activated during liver repair in rodents if the mature residual hepatocytes and biliary epithelial cells are capable of proliferating to restore the normal liver mass and structure. 18,19 However, after certain forms of toxic hepatocellular injury which impair the replicative capacity of hepatocytes, the liver parenchyma may be replaced from oval cells. 7,8 In the current study, we provide evidence that a third liver progenitor cell with some stem-like properties, small incompletely differentiated hepatocyte-like cells, also may participate in liver tissue repair in rats. We have examined the cellular responses and time course for liver regeneration in rats with retrorsine-induced hepatocellular injury. Retrorsine is a member of the pyrrolizidine alkaloid (PA) family of naturally occurring compounds that are toxic to various mammalian tissues, including liver, lung, kidney, brain, muscle, heart, thymus, lymph nodes, and blood vessels. 20-25 The hepatotoxic effects of PAs are long-lasting 26-28 and include inhibition of hepatocyte cell division coupled with induction of polyploidy and megalocytosis. 27,28 The acute development of megalocytosis in the livers of retrorsine-exposed rats results from the antimitotic action of the PA and its metabolites on hepatocytes that are stimulated to divide, such as is induced by partial hepatectomy (PH) or hepatocellular necrosis. 29 In this model, neither retrorsine-injured, fully differentiated hepatocytes nor oval cells proliferate abundantly to contribute significantly to the restoration of liver mass after PH. Instead, the entire liver mass is reconstituted after PH through a novel cellular response that is mediated by the emergence and rapid expansion of a population of small hepatocyte-like progenitor cells, which share some phenotypic traits with fetal hepatoblasts, oval cells, and fully differentiated hepatocytes, but are morphologically and/or phenotypically distinct from each. Small hepatocyte-like cells emerge early following PH, proliferate rapidly to form expanding cellular aggregates that replace megalocytic hepatocytes, and concurrently acquire the panoply of differentiated features typical of mature hepatocytes. Replacement of lost hepatocytes and complete hepatic regeneration from the progeny of small hepatocyte-like progenitor cells have not been observed in other models of liver injury in which replication of residual mature hepatocytes is impaired, suggesting a novel mechanism for liver regeneration, mediated by the expansion of a previously uncharacterized liver progenitor cell type of the adult rat liver.
Materials and Methods
Animals
Male German-strain Fischer 344 DPPIV-deficient rats were used in these studies. The rats were bred and maintained in a colony at the University of North Carolina at Chapel Hill. The original breeders were obtained from the Department of Medical Oncology, Rhode Island Hospital, Brown University (Providence, RI).
Retrorsine Administration and PH
Male 6-week-old littermate Fischer 344 rats (approximately 100 g body weight) were randomized into retrorsine treatment (n = 72) and vehicle-treated control (n = 39) groups at the outset of the experiment. Rats in these groups received two treatments of retrorsine (30 mg/kg i.p.) or vehicle (equal volume of 150 mmol/L saline solution) 2 weeks apart, at 6 and 8 weeks of age. The retrorsine working solution was prepared as described. 28 Retrorsine (12,18-dihydroxysenecionan-11,16-dione; β-Longilobine, Sigma, St. Louis, MO) was added to distilled water at 10 mg/ml and titrated to pH 2.5 with 1 N HCl to completely dissolve the solid. Subsequently, the solution was neutralized using 1 N NaOH, and NaCl was added for a final concentration of 6 mg/ml retrorsine and 150 mmol/L NaCl, pH 7.0. The working solution was used immediately after preparation. Five weeks after the second retrorsine or vehicle treatment, experimental and control rats were randomized into the following groups: control (n = 5), control/PH (n = 34), retrorsine only (n = 15), and retrorsine/PH (n = 57). The control and retrorsine-only groups were not surgically manipulated. There was no mortality associated with vehicle or retrorsine treatment in the absence of PH. Surgical PH was performed essentially as originally described. 30 Mortality rates after PH were higher in retrorsine-exposed rats (35%) than controls (18%), likely due to effects of retrorsine toxicity. Surviving rats in the retrorsine/PH (n = 37) and control/PH (n = 28) groups were euthanized and livers harvested at 1, 3, 5, 7, 10, 14, 17, 23, and 30 days after PH (n = 3–6 per time point). Likewise, rats in the retrorsine-only treatment group were euthanized and livers harvested at days 0, 7, 14, 21, and 30 after PH (n = 3 per time point) where day 0 represents 5 weeks after the final retrorsine treatment. The livers of unmanipulated control rats were collected concurrent with the 30-day post-PH time point (n = 5). At each endpoint, rat body weight and liver weight were recorded. Liver tissue was fixed in 10% neutral buffered formalin and processed routinely for paraffin-embedded sections. In addition, tissue was frozen and cryosections were prepared. Studies involving animals were carried out in accordance with federal and state guidelines put forth by the National Institutes of Health and the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Histology
Formalin-fixed, paraffin-embedded liver tissue was sectioned at 6 μm and stained with hematoxylin and eosin (H&E) according to standard procedures. Cellular polysaccharide deposits were detected routinely using the periodic acid-Schiff (PAS) reaction. Verification of glycogen as a major component of cellular polysaccharides was accomplished by pretreatment of tissue sections with salivary amylase.
Enzyme Histochemistry
Bile canalicular ATPase was detected as described. 31 Briefly, 6-μm liver cryosections were incubated for 30 minutes at 37°C in a substrate solution containing 0.5 mg/ml ATP (sodium salt), 10 mmol/L MgSO4, 3.63 mmol/L lead (II) nitrate, and 80 mmol/L Tris-maleate buffer, pH 7.2. ATPase activity was visualized by incubating the sections in a 0.22% solution of ammonium sulfide at room temperature (r.t.) for 3 minutes. Tissue sections were counterstained with methyl green and mounted in glycerol.
Immunohistochemical Detection of Hepatocyte, Bile Duct, and Oval Cell Markers
Hepatocyte markers were identified using mouse monoclonal antibody H.4, 32,33 rabbit anti-rat transferrin antibodies (Cappel, Aurora, OH), and rabbit anti-rat albumin antibodies (Cappel). Bile duct markers were detected using mouse monoclonal antibodies (mAbs) BD.1, 34 BD.2, 35 and GST-π (Dako, Carpinteria, CA). Oval cell markers were detected using mAbs OV6, 36 OC.2, 32,33 OC.4, OC.5, and OC.10 (Hixson DC, unpublished). Immunostaining of transferrin and albumin was performed on 6-μm paraffin sections. Other immunostaining reactions were performed on 6-μm liver cryosections. Indirect immunoperoxidase analysis was performed on paraffin sections that were cleared with xylene and passed through a graded series of alcohols ending with a short incubation (15 minutes) in PBS (136 mmol/L NaCl, 2.7 mmol/L KCl, 10 mmol/L Na2HPO4, and 1.76 mmol/L KH2PO4, pH 7.2) at r.t. to fully rehydrate tissue sections. Detection of primary antibodies was accomplished using the avidin/biotin peroxidase system (Vectastain rabbit kit, Vector, Burlingame, CA). Endogenous peroxidase activity was quenched using 0.3% H2O2 in PBS for 10 minutes at r.t. Blocking of nonspecific activity was accomplished by incubation in buffer containing serum of the secondary antibody species for 30 minutes at r.t. Polyclonal antibodies to transferrin and albumin were diluted 1:200 in PBS, incubated on tissue sections for 30 minutes, and detected using diaminobenzidine (DAB kit, Vector) with Gill’s hematoxylin counterstain. Indirect immunofluorescence analysis was performed on cryosections fixed in cold acetone (−20°C, 10 minutes). Blocking of nonspecific activity was accomplished by incubation in 1% normal goat serum in PBS for 30 minutes at r.t. Primary antibodies were diluted at 1:100 (OV6 at 1:2000, GST-π at 1:25) in PBS/1% normal goat serum and incubated with tissue sections for 30 minutes at r.t. The FITC-conjugated polyvalent secondary antibody (Sigma) was diluted to 1:100 in PBS/1% normal goat serum. Incubation with secondary antibody was done at 4°C for 30 minutes. Sections were counterstained with propidium iodide. Images were captured using a Nikon FXA microscope and color transparency film.
Estimation of the Proliferative Cell Fraction after PH
The nuclear antigen Ki-67 was used as a marker of dividing cells 37 and is present in proliferating cells exclusively. Indirect immunoperoxidase analysis was performed using 6-μm paraffin sections that were cleared with xylene, passed through a graded series of alcohols, and incubated for 15 minutes in TBS (25 mmol/L Tris-Cl, 136 mmol/L NaCl, and 27 mmol/L KCl, pH 7.2) at r.t. to fully rehydrate tissue sections. Antigen retrieval was accomplished by microwaving (∼750 watts) slides in antigen retrieval buffer (1.8 mmol/L citric acid, 8.2 mmol/L sodium citrate in deionized water) for a total of four 5-minute cycles, then cooled in TBS at r.t. Endogenous peroxidase activity was quenched using 0.3% H2O2 in TBS for 10 minutes. Cells in the growth cycle were decorated with a mouse monoclonal antibody to Ki-67 antigen (Immunotech, Marseille, France) at a dilution of 1:50 in TBS. Detection was accomplished using the avidin/biotin peroxidase system (Vectastain kit, Vector), developed in True Blue peroxidase substrate (Kirkegaard & Perry, Gaithersburg, MD), and counterstained with contrast red.
Characterization of Retrorsine-Induced Inflammation after PH
To assess the inflammatory response observed in H&E-stained sections in retrorsine/PH rats, indirect immunoperoxidase analysis was performed on 6-μm paraffin sections from retrorsine-exposed and control rats at 1 and 3 days post-PH using mouse monoclonal antibodies to rat ED1 (monocytes and tissue macrophages) and ED2 (tissue macrophages alone), as described 38 with minor modifications. Monoclonal antibodies to rat ED1 and ED2 were purchased from Serotec (Kidlington, Oxford, UK). Paraffin sections were cleared with xylene and rehydrated through a graded series of alcohols. To inactivate endogenous peroxidase, sections were incubated at r.t. for 15 minutes in a 0.5% solution of H2O2 in PBS. To block nonspecific staining, sections were incubated at r.t. for 10 minutes in 20% normal rabbit serum/PBS. Primary antibodies were diluted in normal rabbit serum/PBS (ED1 1:100, ED2 1:10) and incubated on sections for 45 minutes at 4°C. The secondary antibody, peroxidase conjugated rabbit anti-mouse immunoglobulin (Dako), was diluted 1:20 in normal rabbit serum/PBS and incubated on sections for 30 minutes at r.t. Antibodies were detected using diaminobenzidine (DAB kit, Vector) with Gill’s hematoxylin counterstain.
Morphometric Analysis
Computerized morphometry was used to quantify the area of parenchyma occupied by clusters of small hepatocytes in regenerating liver of retrorsine/PH rats at various times after PH and to estimate the size of hepatocytes, megalocytes, and small hepatocyte-like progenitor cells in control and retrorsine-exposed rat livers. This analysis was performed using a Macintosh G3 computer and NIH Image software (http://rsb.info.nih.gov/nih-image/). For area quantification, two random H&E-stained sections were chosen for analysis from three different rats per time point after PH (3, 7, 14, and 30 days). Small hepatocyte area was expressed as a percentage of the total liver section area averaged over six sections per time point. For cell size estimation, one random H&E-stained section was chosen for analysis from two different rats from each of the unmanipulated control rats, control/PH rats (at 3 days post-PH), and retrorsine/PH rats (at 3 days post-PH). Cell size was expressed (in units of μm2) as the average of 15 different random measurements per group.
Ultrastructure
Formalin-fixed tissue was immersed in 3% glutaraldehyde in a 100 mmol/L sodium cacodylate buffer, pH 7.4, with 0.05% CaCl2 overnight. Thin sections were postfixed in osmium tetroxide, stained with uranyl acetate and lead citrate, and examined using transmission electron microscopy.
Statistical Analysis
A two-tailed unpaired t-test was used to generate P values and determine the significance of all quantified differences in liver weights and liver/body weight ratios between experimental and control groups. GraphPad Prism software (v. 2.01) was used for all calculations.
Results
Cellular Responses after PH in Retrorsine-Injured Rat Liver
At 1 day post-PH, rats in the retrorsine/PH group have pale, friable livers that normalize in gross appearance by 5 days post-PH. Between 1 and 3 days post-PH, there is microscopic evidence of inflammation, mild coagulative necrosis, and hepatocyte apoptosis (data not shown). The inflammatory infiltrate at 1 day post-PH consists mainly of ED1-positive blood monocytes and ED2-positive Kupffer cells that increase in number by 3 days post-PH, possibly via local proliferation and continued recruitment to the liver. H&E-stained liver sections at 5 days post-PH show minimal evidence of continued inflammation. The livers of rats in the control and control/PH groups display normal color and texture at all time points, and demonstrate no indication of inflammation in H&E-stained sections. The livers of rats in the retrorsine-only group exhibit a mild inflammatory response that is confined to infrequent areas of localized necrosis. Verification of the lack of a generalized inflammatory response in these livers is demonstrated by ED1/ED2 immunohistochemistry (data not shown).
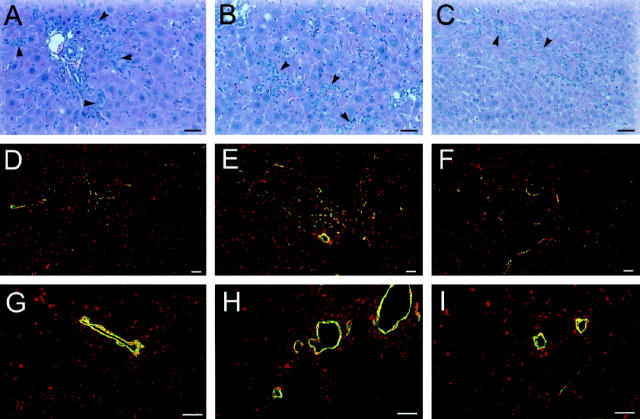
At 1 day post-PH in retrorsine/PH rats, residual hepatocytes already show megalocytosis coupled with DNA synthesis and apoptotic bodies are frequent. Easily identifiable small hepatocytes appear as isolated clusters of 3 to 6 cells at 3 days post-PH (Figure 1A) ▶ and are never colocalized with oval cells. At this time point emerging clusters of small hepatocyte-like progenitor cells are observed in 16% (range, 10–25%) of liver lobules. Examination of H&E-stained liver sections from multiple rats show that emerging clusters of small hepatocyte-like cells are not confined to periportal areas, as are emerging oval cells, and are found in all lobular zones. Assessment of the sublobular localization of expanding clusters of small hepatocyte-like progenitor cells reveals that 31% are found in the periportal region, 43% are mid-lobular, and 26% are pericentrally located (n = 126 clusters counted on ten random H&E-stained liver sections from four rats). Small hepatocyte-like cells proliferate to form lobule-sized aggregates by 7 days post-PH (Figure 1C) ▶ , coalesce into large patches by 10 days post-PH (Figure 1E) ▶ , and occupy nearly 50% of the area of the parenchyma by 14 days post-PH (Figure 1G) ▶ . By 30 days post-PH, normal liver structure is restored by the proliferating small hepatocytes (data not shown). At this time point the structure of the livers of retrorsine/PH rats are nearly indistinguishable from that of control and control/PH rats (data not shown). Newly formed hepatocytes are arranged in regularly spaced one-cell-thick plates with intervening sinusoids, and portal tracts and central veins occur in a regular pattern. Rats in the retrorsine only treatment group have marked hepatocytomegaly (data not shown) and rare regions of localized necrosis. Cells with the characteristics of small hepatocyte-like cells are never observed during liver regeneration in control/PH rats (Figure 1, B, D, F, and H) ▶ . Furthermore, small hepatocyte-like cells are not observed in the livers of control rats or retrorsine-only rats (data not shown).
Figure 1.
Emergence and expansion of small hepatocyte-like cells after PH in retrorsine-exposed rats. A, C, E, and G: Retrorsine-exposed rat livers at 3, 7, 10, and 14 days post-PH, respectively (H&E). B, D, F, and H: Control rat livers at 3, 7, 10, and 14 days post-PH, respectively (H&E). Aggregates of small hepatocyte-like cells are seen in easily identifiable clusters of 3 to 6 cells by 3 days post-PH in retrorsine-exposed rats (A), approach lobule size by 7 days post-PH (C), and coalesce into large patches by 10 days post-PH (E). Small hepatocyte-like cells occupy nearly 50% of the parenchyma by 14 days post-PH (G). No small hepatocyte-like cells are observed in control/PH rats at any time point (B, D, F, H). Bar, 50 μm. Black arrows indicate small hepatocyte-like cells; white arrows indicate megalocytes. Inset in A shows a higher magnification of a different field.
Mature hepatocytes do not contribute to the restoration of tissue mass and cell number after PH in this model, but undergo proliferation-arrest as megalocytes and/or display hallmark characteristics of apoptosis (Figure 2) ▶ . Initiation of DNA synthesis is evident 1 day post-PH by the positive staining for nuclear antigen Ki-67 in a subset of residual hepatocytes and biliary epithelial cells in retrorsine/PH rats. By 3 days post-PH hepatocyte megalocytosis is clearly visible and the majority of hepatocytes and bile duct epithelial cells stain positive for Ki-67 (Figure 2A) ▶ , reflecting an attempt at cell proliferation in response to liver deficit, although hepatocytes in this model are unable to complete the cell cycle and undergo mitosis. 23,28,29 At 5 days post-PH retrorsine-injured hepatocytes exhibit increased megalocytosis and most fail to stain positive for Ki-67, whereas the clusters of small hepatocyte-like cells are Ki-67-positive and contain frequent mitotic figures, indicative of continued proliferation. By 14 days post-PH, only the small hepatocyte-like cells in expanding cellular aggregates are positive for Ki-67 (Figure 2B) ▶ . Thirty days post-PH the liver mass is fully reconstituted and the proliferative activity of the newly formed hepatocytes diminish, with few Ki-67-positive cells (Figure 2C) ▶ .
Figure 2.
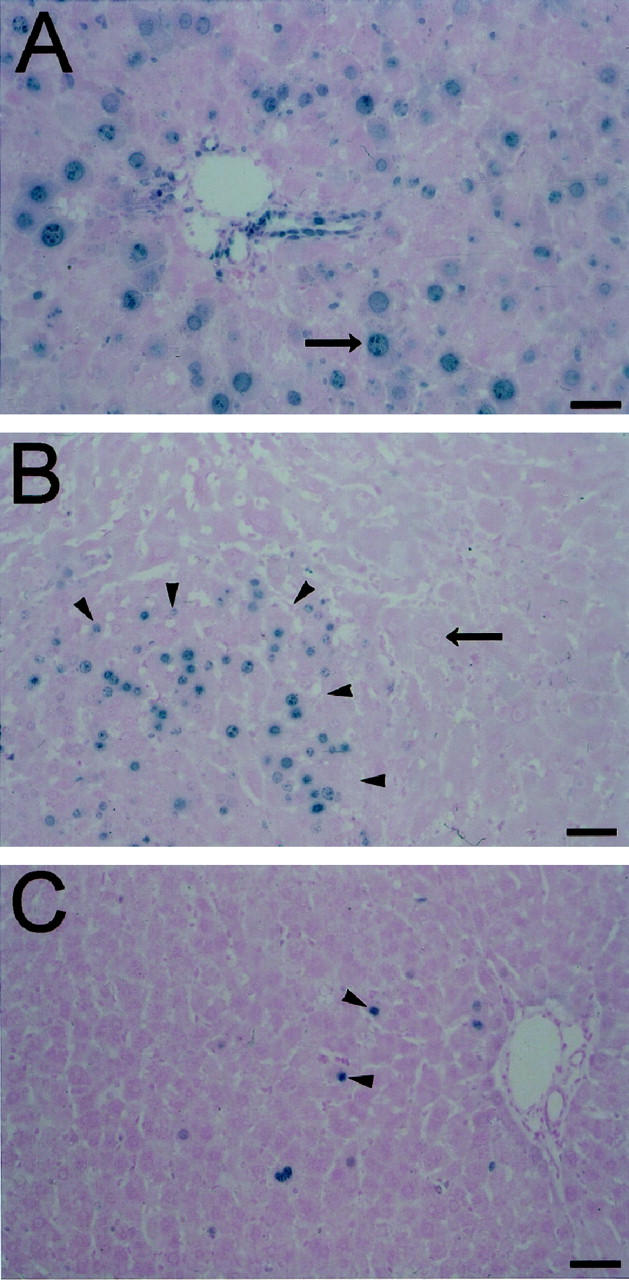
Indirect immunoperoxidase detection of Ki-67 nuclear antigen in retrorsine-exposed rat livers after PH. A: Retrorsine-exposed rat liver section labeled with the nuclear antigen Ki-67 3 days after PH. B: Retrorsine-exposed rat liver section labeled with the nuclear antigen Ki-67 14 days after PH. C: Retrorsine-exposed rat liver section labeled with the nuclear antigen Ki-67 30 days after PH. At 3 days post-PH (A) hepatocyte megalocytosis is clearly visible. The majority of hepatocytes and bile duct epithelial cells stain positive for nuclear antigen Ki-67 (blue chromagen), indicating an attempt at replication, although hepatocytes do not complete the cell cycle. By 14 days post-PH (B), only small hepatocyte-like cells show evidence of proliferation, and only when the liver mass was fully reconstituted at 30 days post-PH (C) did the proliferative activity of the small hepatocyte-like cells diminish. Bar, 50 μm. Long arrows indicate megalocytes and short arrows indicate small hepatocyte-like cells.
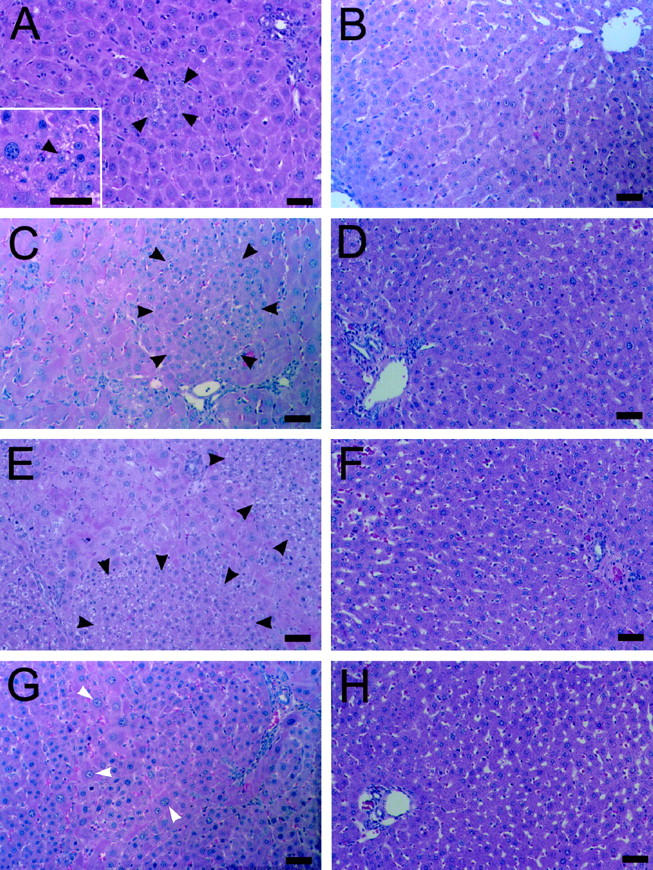
There is a minimal oval cell response during liver regeneration in retrorsine/PH rats (Figure 3) ▶ . At early time points after PH, cells with small ovoid nuclei and scant cytoplasm that morphologically resemble classic oval cells 19,39-41 are located in the vicinity of the portal tracts (Figure 3A) ▶ . These cells are positive for oval cell and bile duct markers, including OV6, OC.2, OC.5, and BD.2, but are negative for OC.4, OC.10, and BD.1. The proliferation of oval cells after PH can be followed using antibodies to the OV6 cell surface marker beginning at day 3 post-PH to observe the relative numbers of positive-staining cells in the parenchyma (Figure 3D) ▶ . Oval cells proliferate only moderately, reaching a peak response by 7 days post-PH (Figure 3E) ▶ , and are never associated with foci of small, basophilic hepatocytes, suggestive of oval cell differentiation. After 7 days, oval cells gradually decrease in number and by 30 days post-PH, the few remaining oval cells colocalize with residual megalocytes at the periphery of parenchymal regions occupied by the progeny of small hepatocytes (Figure 3F) ▶ . Identification and characterization of oval cell proliferation using other positive markers reveal similar frequencies of this cell type in the parenchyma at days 3, 7, and 10 post-PH (data not shown). In control/PH rats, antibodies to OV6 (Figures 3G, 3H, and 3I) ▶ , as well as other oval cell and bile duct markers, decorate only bile ducts at 3, 7, and 30 days post-PH. Similar results are obtained with the livers of unmanipulated control rats (data not shown). Rats in the retrorsine-only group do not exhibit extensive oval cell proliferation (data not shown) although cells morphologically resembling oval cells can infrequently be identified near portal tracts. OV6-positive oval cells in retrorsine-only rats are rare, occasionally occurring at both periportal and pericentral sites. OC.2-positive and OC.5-positive oval cells in livers from retrorsine only rats are more abundant than OV6-positive oval cells and are located predominately near portal tracts with rare instances of mid-lobular localization.
Figure 3.
Oval cell proliferation in retrorsine-exposed rat livers after PH. A−C: H&E-stained liver sections of retrorsine-exposed rats at 3, 7, and 30 days post-PH, respectively. D−F: Indirect immunofluorescence analysis of OV6 in liver cryosections from retrorsine-exposed rats at 3, 7, and 30 days post-PH, respectively. G−I: Indirect immunofluorescence analysis of OV6 in liver cryosections from control rats at 3, 7, and 30 days post-PH, respectively. Oval cell proliferation in this model was minimal. Evidence of oval cell proliferation after PH in retrorsine-exposed rats is observed in the vicinity of the portal tracts at 3 days post-PH (A and D), reaching a peak response by 7 days post-PH (B and E), then slowly diminishing until 30 days post-PH (C and F) when remaining oval cells colocalize with megalocytes. There is no oval cell proliferation in control rats after PH at the same time points (G−I). Only bile ducts are OV6-positive. Bar, 50 μm. Arrows indicate oval cells.
Phenotypic Analysis of Small Hepatocyte-Like Progenitor Cells
Small hepatocyte-like progenitor cells are not readily identified in H&E-stained liver sections of retrorsine/PH rats at 1 day post-PH, but are easily recognized in these liver sections at 3 days post-PH. These cells are of significantly (P < 0.001) smaller size (average = 135.4 ± 9.6 μm2) than hepatocytes from control rats (average = 294.9 ± 15.0 μm2) and regenerating hepatocytes from control/PH (average = 391.5 ± 23.0 μm2) rats. Small hepatocyte-like progenitor cells possess small basophilic nuclei, scant and highly vacuolated cytoplasm, and form clusters that lack sinusoids or well developed hepatic plates. The small size and highly vacuolated appearance of these cells persists in the early phase of liver regeneration from 3 to 10 days post-PH (Figure 1) ▶ . By 14 days post-PH, the clusters of small hepatocyte-like cells in expanding nodular aggregates are organized into one- cell-thick plates with intervening sinusoids, and individual cells lack the vacuolated appearance that characterize the early cells and are morphologically indistinguishable from hepatocytes of control rats (Figure 1) ▶ .
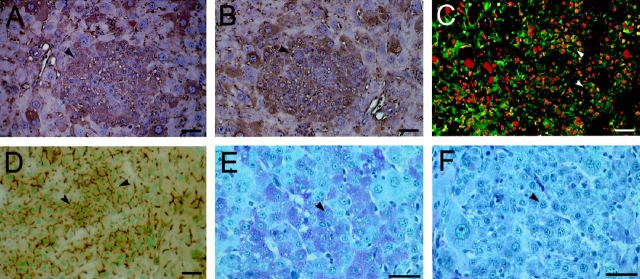
At the time of their emergence, small hepatocyte-like cells express hepatocyte-specific differentiation markers, including albumin and transferrin. At 5 days post-PH, all observed clusters of small hepatocytes clearly express albumin (Figure 4A) ▶ , transferrin (Figure 4B) ▶ , and the hepatocyte-specific antigen recognized by the monoclonal antibody H.4 (Figure 4C) ▶ . In addition, small hepatocyte-like cells possess bile canaliculi (Figure 4D) ▶ and store glycogen (Figure 4, E and F) ▶ . Monoclonal antibodies to bile duct markers BD.1, BD.2, and GST-π do not recognize small hepatocyte-like cells at any point. Ultrastructural observations confirm the hepatocyte-specific phenotype of these cells (data not shown). Transmission electron microscopy shows tight junctions between small hepatocyte-like cells and well-formed bile canaliculi with microvilli in emerging foci. Individual small hepatocyte-like cells have abundant mitochondria, glycogen rosettes, rough endoplasmic reticulum, and peroxisomes (identified based on the presence of single membrane organelles with crystalline inclusions).
Figure 4.
Phenotypic characterization of small hepatocyte-like cells in retrorsine-exposed rats at 5 days post-PH. A-F: Formalin-fixed, paraffin-embedded liver sections and fixed liver cryosections show clusters of small hepatocyte-like cells staining positive for markers of hepatocyte differentiation. A: Indirect immunoperoxidase analysis shows small hepatocyte-like cell clusters are albumin-positive. B: Indirect immunoperoxidase analysis shows small hepatocyte-like cell clusters are transferrin-positive. C: Indirect immunofluorescence analysis shows that small hepatocyte-like cells are positive for the cell surface antigen recognized by the hepatocyte-specific monoclonal antibody H.4. D: Small hepatocyte-like cells possess well-formed bile canaliculi, as evidenced by ATPase histochemical staining on liver cryosections. E: Small hepatocyte-like cells have abundant cellular polysaccharides as evidenced by the PAS reaction. F: Glycogen was confirmed to be the major polysaccharide component by pretreatment with salivary amylase. Bar, 50 μm. Arrows indicate small hepatocyte-like cells.
Potential Lineage Relationships between Small Hepatocytes and Oval Cells
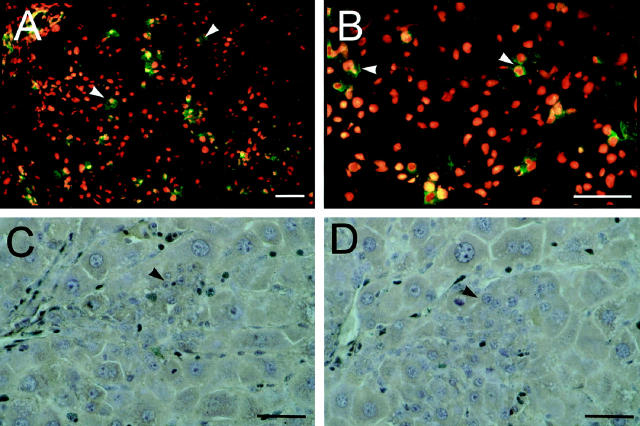
Small hepatocyte-like cells express markers of hepatocyte differentiation as soon as 3 days post-PH in retrorsine/PH rats (Figure 5, C and D) ▶ . It is not possible to identify definitively oval cell marker-positive small hepatocytes at 3 days post-PH due to the difficulty with identifying small numbers of these cells in cryosections at that time. However, at 5 days post-PH when small hepatocytes are easily identified in frozen sections, a subset of these cells are decorated with antibodies to oval cell markers OC.2 (Figure 5A) ▶ and, in lesser numbers, OC.5 (Figure 5B) ▶ . At this time point approximately 30% of all small hepatocyte clusters have OC.2-positive cells and 15% have OC.5-positive cells. Within each positive cluster, roughly 20% of small hepatocyte-like cells are positive for OC.2 or OC.5. Examination of serial sections suggests that these markers are coexpressed on the same subsets of small hepatocytes and that expression of OC.5 is lost before OC.2. By comparison, all OC.2-positive and OC.5-positive cells in retrorsine-only rats express morphological characteristics of oval cells (data not shown). At no point were small hepatocyte-like cells positive for OV6. Expression of these oval cell markers in small hepatocyte-like cells is lost by 7 days post-PH.
Figure 5.
Potential lineage relationships involving small hepatocyte-like cells. A and B: Indirect immunofluorescence analysis of oval cell markers OC.2 and OC.5, respectively, in liver cryosections from retrorsine-exposed rats 5 days post-PH. C and D: Indirect immunoperoxidase analysis of albumin and transferrin, respectively, on liver sections from retrorsine-exposed rats 3 days post-PH. Small hepatocyte-like cells are transiently positive at 5 days post-PH in retrorsine-exposed rats for OC.2 (A) and OC.5 (B) oval cell markers, but are not positive for OV6. Oval cell markers are subsequently lost by 7 days post-PH. Small hepatocyte-like cells express markers of hepatocyte differentiation as soon as they can be definitively identified at 3 days post-PH, staining positive for albumin (C) and transferrin (D). Bar, 50 μm. Arrows indicate small hepatocyte-like cells.
Small Hepatocytes Completely Restore Liver Mass after PH
Retrorsine-exposed rats are able to completely regenerate their liver mass after PH as evidenced by liver weights (Figure 6A) ▶ and liver/body weight ratios (Figure 6B) ▶ . Regeneration is facilitated by proliferation of small hepatocyte-like cells that occupy an increasing percentage of the liver parenchymal area between 5 and 30 days post-PH (Figure 6C) ▶ . The liver weights of retrorsine/PH rats remain around 3 to 4 g through 14 days post-PH. At 14 days post-PH, small hepatocyte-like cells proliferate to encompass nearly 50% of the liver parenchyma by area, yet the liver weight at that time point (average = 3.16 g) is not significantly different from liver weights (average = 3.02 g) at 1 day post-PH (P = 0.495). The lack of increasing liver weights during the early phase of liver regeneration (0–14 days post-PH) in retrorsine/PH rats may reflect the continued loss of megalocytic hepatocytes from the parenchyma through apoptosis. Apoptotic bodies are present in the livers of all retrorsine/PH rats during this time period, are infrequently observed in the livers of retrorsine only rats, and are never observed in the livers of control and control/PH rats. From 17 to 30 days post-PH, retrorsine/PH livers gradually increase in size, eventually approximating liver weights of control/PH rats. At 30 days post-PH, liver weights and liver/body weight ratios are not significantly different between retrorsine/PH and control/PH groups (P = 0.982 and P = 0.294, respectively). By this time, the progeny of small hepatocyte-like cells occupy virtually the entire parenchyma in retrorsine/PH rats (87% by area). Control/PH rats nearly double their liver weight after 3 days and essentially regenerate their liver mass by 10 days, three times as quickly as retrorsine/PH rats (Figure 6A) ▶ .
Figure 6.
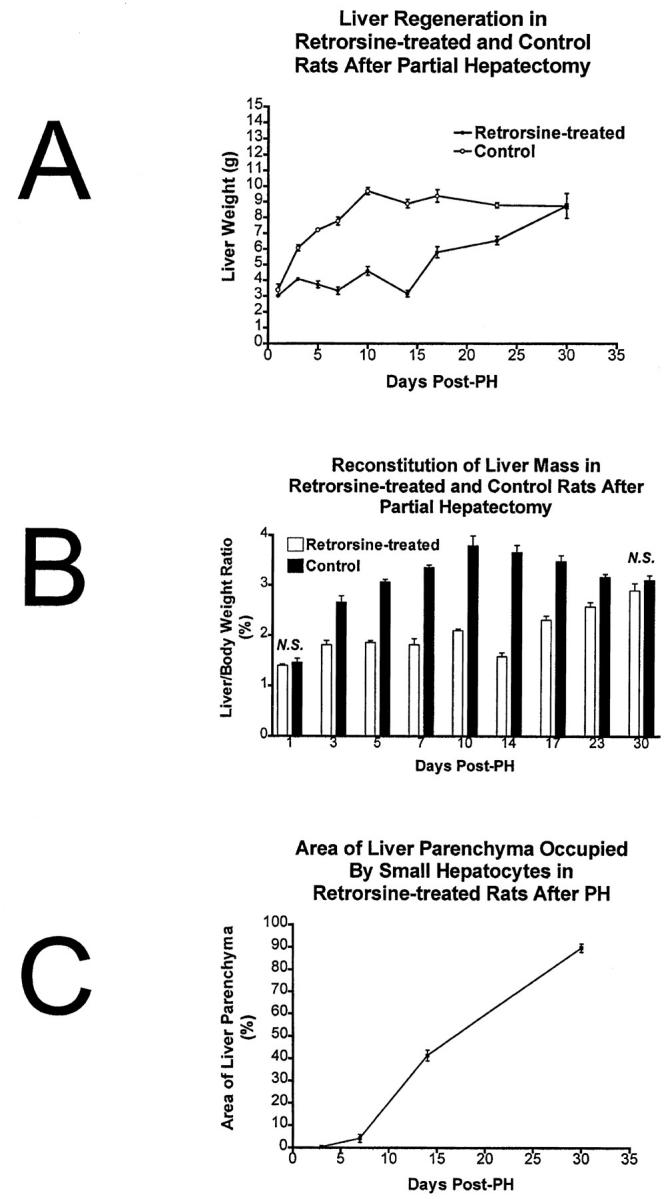
Small hepatocyte-like cells reconstitute liver mass after PH. A: Progeny of small hepatocyte-like cells reconstitute liver mass after PH as evidenced by liver weight. The timeline for complete reconstitution is delayed compared to control rats but is essentially complete by 30 days post-PH. B: Liver mass reconstitution as evidenced by liver/body weight ratios. C: Small hepatocyte-like cells occupy an increasing percentage of the liver parenchyma by area. n = 3–6 per time point. Error bars indicate SEM.
Discussion
Retrorsine-induced hepatocellular injury severely impairs the capacity of fully differentiated rat hepatocytes to replicate and participate in the restoration of liver mass following surgical PH, and significant proliferation of oval cells does not occur. Nevertheless, retrorsine-exposed rats can replace their entire liver mass after PH through the emergence and expansion of a (novel?) population of small hepatocyte-like progenitor cells which express some of the phenotypic characteristics of fetal hepatoblasts, oval cells, and fully differentiated hepatocytes, but differ distinctly from each. The activation, proliferation, and complete regeneration of normal liver structure from small hepatocyte-like progenitor cells has not been recognized in other models of liver injury characterized by impaired hepatocyte replication. In the present study, we describe the time course for liver regeneration in retrorsine-exposed rats and partially characterize the cell types involved. We provide evidence for a novel mechanism of liver regeneration after PH, mediated by the selective expansion of a small hepatocyte-like progenitor cell that participates in liver tissue repair processes under certain pathophysiological circumstances in the adult rat.
Small Hepatocyte-Like Progenitor Cells Express a Distinct Cell Phenotype
The small hepatocyte-like progenitor cells described in this study share several phenotypic characteristics with fetal hepatoblasts, oval cells, and fully differentiated hepatocytes, but differ from each (Table 1) ▶ . Fully differentiated hepatocytes (and reactive derived megalocytes in retrorsine/PH rats) express albumin, transferrin, and the antigen recognized by monoclonal antibody H.4, store glycogen, and possess bile canaliculi, but do not express oval cell (OV6, OC.2, OC.5, OC.4, OC.10) and bile duct markers (BD.1, BD.2). These cellular characteristics are also expressed by fetal hepatoblasts (fetal hepatocytes) of the E18-E20 rat embryo. 8,33,42,43 At day 7 post-PH in retrorsine/PH rats, small hepatocyte-like progenitor cells in expanding clusters express a cellular phenotype that is indistinguishable from E18-E20 fetal hepatocytes or hepatocytes of the adult liver (Table 1) ▶ . However, before this time point (at 5 days post-PH), a subset of the small hepatocyte-like progenitor cells also expresses oval cell markers OC.2 and OC.5 (Figure 5) ▶ . These markers tend to be transiently expressed in small hepatocyte-like progenitor cells of the smaller clusters, suggesting that the earliest emerging cells exhibit a distinct (more primitive?) phenotype compared to their (more mature?) progeny in the larger cellular aggregates. The expression of OC.2 and OC.5 distinguishes the early small hepatocyte-like progenitor cells from fully differentiated hepatocytes. Bile duct epithelial cells express all of the oval cell and bile duct markers examined, whereas oval cells proliferating in retrorsine-exposed rats express OV6, OC.2, OC.5, and BD.2 (Table 1) ▶ . Thus, the early small hepatocyte-like progenitor cells share the expression of OC.2 and OC.5 with bile duct epithelium and oval cells. However, the expression of multiple hepatocyte-specific markers and the lack of OV6 positivity clearly distinguish the small hepatocyte-like progenitor cells from bile duct epithelium and oval cells. The overall phenotype of the small hepatocyte-like progenitor cells resembles that expected for a transitional cell type that is between the bipotential E14 hepatoblast and the E18-E20 fetal hepatocyte. 43 These results combine to suggest that small hepatocyte-like progenitor cells share several cellular characteristics with other liver epithelial cell populations, but express an overall phenotype that differs from each.
Table 1.
Phenotypic Comparison of the Reactive Cell Types in Regenerating Liver of Retrorsine-Exposed Rats and Hepatocyte Progenitor Cells of the Developing Liver
| Cell lineage marker | Hepatocyte megalocytes* | Oval cells* | BDE* | SHPCs* | Early uncommitted hepatoblast (E10)† | Bipotential hepatoblast (E14)† | Fetal hepatocytes (E18–20)† |
|---|---|---|---|---|---|---|---|
| Albumin | + | +/− | − | + | − | + | + |
| Transferrin | + | − | − | + | − | + | + |
| H.4 | + | − | − | + | − | − | + |
| Glycogen | + | − | − | + | − | − | + |
| Bile canaliculi | + | − | − | + | − | + | + |
| OV6 | − | + | + | − | − | − | − |
| OC.2 | − | + | + | +/− | − | + | − |
| OC.5‡ | − | + | + | +/− | − | − | − |
| BD.1 | − | − | + | − | − | − | − |
| BD.2 | − | + | + | − | − | − | − |
| OC.4‡ | − | − | + | − | − | − | − |
| OC.10‡ | − | − | + | − | − | − | − |
BDE, bile duct epithelium; SHPCs, small hepatocyte-like progenitor cells.
*In retrorsine-treated rats at 5 days post-PH.
†Phenotypic characterization summarized from published literature ( Refs. 8, 33, 42, and 43).
‡DC Hixson, unpublished observations.
Possible Lineage Relationships in Regenerating Liver of Retrorsine-Exposed Rats
As is true for other tissues, putative stem-like progenitor cells of the liver have not been identified microscopically and have not been isolated from the liver in pure form, partially reflecting the fact that stem cells and their phenotypic characteristics are largely intuitive concepts. 8 Likewise, the ultimate progenitor cell that gives rise to expanding clusters of small hepatocyte-like progenitor cells in regenerating livers of retrorsine-exposed rats has not been definitively identified in the current study. However, the phenotype expressed by the small hepatocyte-like progenitor cells may provide clues as to their cells of origin or to lineage relationships with other cells of the adult liver. Some possible candidates for related cells include (i) a pre-existing population of retrorsine-resistant hepatocytes, (ii) proliferative bile duct epithelial cells (oval cells), or (iii) an unknown (novel) stem-like cell compartment.
The small hepatocyte-like progenitor cells resemble mature hepatocytes in cellular morphology, express a number of hepatocyte-specific traits, and emerge from various sites in the lobular hepatic parenchyma. These observations support the possibility that these cells may arise from a pre-existing population of retrorsine-resistant hepatocytes. Retrorsine is metabolized to toxic pyrrolic metabolites through the action of the P450 enzymes. 44,45 Thus, cells that lack expression of the appropriate P450 enzyme(s) or that express some other protective mechanism would not be subject to retrorsine-mediated inhibition of cell replication, and would be available to proliferate in response to the liver deficit generated by PH. Given the extensive proliferative capacity of differentiated hepatocytes, 14-17 the existence of a small population of retrorsine-resistant hepatocytes might account for the regenerative activity observed in retrorsine-exposed rat livers. However, the expression of oval cell markers OC.2 and OC.5 by the small hepatocyte-like progenitor cells early after PH in retrorsine-exposed rats distinguishes these cells from mature hepatocytes. Although it has not been documented, it is possible that oval cell antigens could be expressed by proliferative (and possibly resistant) hepatocytes under the pathophysiological conditions in the retrorsine-exposed rat liver after PH. Alternatively, the expression of these antigens by the small hepatocyte-like progenitor cells might reflect a lineage relationship between these reactive cells and less differentiated stem-like cells. Typical oval cells proliferate modestly in the retrorsine model of liver regeneration, 28,46 suggesting that they (and/or their cells of origin) are resistant to the mito-inhibitory effects of retrorsine. Previous studies have shown that oval cells are resistant to a number of different carcinogens that are mitoinhibitory to mature hepatocytes due to the absence of carcinogen-activated P450 enzymes. 47-50 In liver regeneration models that employ these mitoinhibitory agents, including the modified Solt-Farber hepatocarcinogenic model 40,50-52 and the galactosamine model of necrotic liver injury, 19,39 oval cells proliferate abundantly to regenerate the hepatic parenchyma. Oval cells originate in the periportal regions of the liver and are thought to derive from an undifferentiated stem cell population located in the periportal parenchyma or from hyperplastic bile ductules. 7,8,53 These cells express multipotential differentiation capacity 33 and give rise to hepatocytes under appropriate experimental conditions in vitro 54,55 and in vivo. 50,51 Thus, the expression of shared phenotypic traits between small hepatocyte-like progenitor cells and oval cells may reflect their derivation from a common founder cell (proliferative bile duct epithelial cell or undifferentiated stem-like cell) or a direct precursor-product relationship. However, the overall cellular phenotype expressed by these cell types (Table 1) ▶ and the sublobular site of their emergence argues for different origins for these cell types. The last possibility is that the small hepatocyte-like progenitor cells could represent a novel epithelial progenitor cell population, distinct from both fully differentiated hepatocytes and oval cells of the adult rat liver, that has not been recognized in other models of liver regeneration. The results from the present study strongly suggest that liver regeneration in retrorsine-exposed rats following PH proceeds through a novel cellular response that has not been characterized previously. However, additional studies will be required to discern cell lineage relationships and identify the ultimate cells of origin of the small hepatocyte-like progenitor cells in this model system.
Small Hepatocyte-Like Progenitor Cells May Be Related to Regenerative Transplantable Hepatocytes
It is well known from studies of normal liver regeneration after PH that mature hepatocytes expressing a fully differentiated phenotype can replicate enough times to restore hepatocyte numbers and liver mass. 10,12,56 Nonetheless, the proliferation capacity of fully differentiated adult hepatocytes has been the subject of intensive investigation in recent years. Using transgenic mouse models of hepatocellular injury and hepatocyte transplantation, several investigators have produced evidence that rodent hepatocytes possess an extensive (possibly unlimited) replicative capacity. In transgenic mice that express the urokinase gene in the liver under the direction of the albumin promoter-enhancer, most hepatocytes are killed by the toxic transgene product, but some residual hepatocytes inactivate the toxic transgene and proliferate, undergoing 10 to 12 cycles of cell division to yield discrete nodular aggregates (clones) that repopulate the liver parenchyma. 57 Likewise, when normal (unfractionated) hepatocytes are transplanted into these transgenic mice, nodules of progeny hepatocytes repopulate the damaged liver. 15,58 In a similar experimental system, Grompe and colleagues 16 have examined the ability of transplanted normal (unfractionated) hepatocytes to repopulate the livers of transgenic mice that are deficient for fumarylacetoacetate hydrolase enzyme activity due to the targeted disruption of exon 5 of the Fah gene. 59 These investigators have shown that the transplanted Fah-expressing hepatocytes exhibit a selective growth advantage over host Fah-deficient hepatocytes and effectively repopulate the livers of mutant mice. In these studies, it is estimated that transplanted hepatocytes proliferate through at least 15 cell divisions during repopulation of mutant livers. 16 In more recent studies, these same investigators have examined the repopulating and proliferative potential of normal hepatocytes by serial cycles of transplantation, recovery, and transplantation, each time transplanting 10 3 to 10 6 cells. 17 Full repopulating potential is retained during six consecutive recovery and transplantation cycles, corresponding to at least 86 cell doublings by the transplanted cells since the first transfer, assuming a 15% efficiency of engraftment at each transplant cycle. Based on the results of the serial transplantation experiments, Overturf et al proposed that a subset of transplanted hepatocytes, which they termed the regenerative transplantable hepatocyte (RTH), exhibit high repopulating capacity. 17 It is intriguing to speculate that cells akin to the small hepatocyte-like progenitor cells identified in the present study may be responsible for repopulation of the livers of PA-treated 27 and retrorsine-exposed 28 rats following transplantation of unfractionated hepatocytes, and that these cells may be the RTH proposed by Grompe. 17 The progeny of proliferative small hepatocyte-like progenitor cells in retrorsine/PH rats form expanding nodular aggregates of cells that eventually replace megalocytes (compromised hepatocytes) and remodel to form parenchyma with normal structure in a manner indistinguishable from that observed in these transgenic mouse models. 15,17,57,58
Acknowledgments
We thank Dr. Robert C. Bagnell and Victoria Madden of the Microscopy Services Laboratory of the University of North Carolina at Chapel Hill Department of Pathology and Laboratory Medicine for expert assistance in the preparation of the color photomicrographs and transmission electron microscopy.
Footnotes
Address reprint requests to William B. Coleman, Department of Pathology and Laboratory Medicine, University of North Carolina School of Medicine, CB 7525, Chapel Hill, NC 27599. E-mail: wbcolemn@med.unc.edu.
Supported by grants CA29323 (to J. W. G.) and CA78434 (to W. B. C.) from the National Institutes of Health.
Preliminary findings were presented at the Experimental Biology 1999 Conference and published in abstract form (Gordon GJ, Coleman WB, Hixson DC, Grisham JW: Cellular responses during liver regeneration after partial hepatectomy in rats with retrorsine-induced hepatocellular injury. FASEB J 1999, 13:A189; Gordon GJ, Coleman WB, Grisham JW: Transplantation of WB-F344 stem-like cells into rats following retrorsine-induced hepatocellular injury. FASEB J 1999, 13:A160).
References
- 1.Hall PA, Watt FM: Stem cells: the generation and maintenance of cellular diversity. Development 1989, 106:619-633 [DOI] [PubMed] [Google Scholar]
- 2.Potten CS, Loeffler M: Stem cells: attributes, cycles, spirals, pitfalls, and uncertainties: lessons for and from the crypt. Development 1990, 110:1101-1120 [DOI] [PubMed] [Google Scholar]
- 3.Lois C, Alvarez-Buylla A: Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci USA 1993, 90:2074-2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss S, Reynolds BA, Vescovi AL, Morshead C, Craig CG, van der Kooy D: Is there a neural stem cell in the mammalian forebrain? Trends Neurosci 1996, 19:387-393 [DOI] [PubMed] [Google Scholar]
- 5.Johansson CB, Momma S, Clark DL, Risling M, Lendahl U, Frisen J: Identification of a neural stem cell in the adult mammalian central nervous system. Cell 1999, 96:25-34 [DOI] [PubMed] [Google Scholar]
- 6.Morrison SJ, White PM, Zock C, Anderson DJ: Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell 1999, 96:737-749 [DOI] [PubMed] [Google Scholar]
- 7.Grisham JW, Thorgeirsson SS: Liver stem cells. Potten CS eds. Stem Cells. 1997, :pp 233-282 Academic Press, New York [Google Scholar]
- 8.Coleman WB, Grisham JW: Epithelial stem-like cells of the rodent liver. Strain A Diehl AM eds. Liver Growth and Repair. 1998, :pp 50-99 Chapman & Hall, London [Google Scholar]
- 9.Thorgeirsson SS: Hepatic stem cells in liver regeneration. FASEB J 1996, 10:1249-1256 [PubMed] [Google Scholar]
- 10.Grisham JW: A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver: autoradiography with thymidine-H3. Cancer Res 1962, 26:842-849 [PubMed] [Google Scholar]
- 11.Fabrikant JI: The kinetics of cellular proliferation in regenerating liver. J Cell Biol 1968, 36:551-565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grisham JW: Cellular proliferation in the liver. Recent Results Cancer Res 1969, 17:28-43 [Google Scholar]
- 13.Wright N, Alison M: The Biology of Epithelial Cell Populations. 1984. Clarendon Press, Oxford
- 14.Simpson GEC, Finckh ES: The pattern of regeneration of rat liver after repreated partial hepatectomies. J Pathol Bacteriol 1963, 86:361-370 [DOI] [PubMed] [Google Scholar]
- 15.Rhim JA, Sandgren EP, Degen JL, Palmiter RD, Brinster RL: Replacement of diseased mouse liver by hepatic cell transplantation. Science 1994, 263:1149-1152 [DOI] [PubMed] [Google Scholar]
- 16.Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou C-N, Finegold M, Grompe M: Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat Genet 1996, 12:266-273 [DOI] [PubMed] [Google Scholar]
- 17.Overturf K, Al-Dhalimy M, Ou C-N, Finegold M, Grompe M: Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol 1997, 151:1273-1280 [PMC free article] [PubMed] [Google Scholar]
- 18.Klinman NR, Erslev AJ: Cellular response to partial hepatectomy. Proc Soc Exp Biol Med 1963, 112:338-340 [DOI] [PubMed] [Google Scholar]
- 19.Dabeva MD, Shafritz DA: Activation, proliferation, and differentiation of progenitor cells into hepatocytes in the D-galactosamine model of liver regeneration. Am J Pathol 1993, 143:1606-1620 [PMC free article] [PubMed] [Google Scholar]
- 20.Schoental R, Magee PN: Chronic liver changes in rats after a single dose of lasiocarpine, a pyrrolizidine (senecio) alkaloid. J Pathol Bacteriol 1957, 74:305-319 [DOI] [PubMed] [Google Scholar]
- 21.Bull LB, Dick AT: The chronic pathological effects on the liver of the rat of the pyrrolizidine alkaloids heliotrine, lasiocarpine, and their N-oxides. J Pathol Bacteriol 1959, 78:483-502 [DOI] [PubMed] [Google Scholar]
- 22.Schoental R, Magee PN: Further observations on the subacute and chronic liver changes in rats after a single dose of various pyrrolizidine (senecio) alkaloids. J Pathol Bacteriol 1959, 78:471-482 [DOI] [PubMed] [Google Scholar]
- 23.Peterson JE: Effects of the pyrrolizidine alkaloid, lasiocarpine N-oxide, on nuclear and cell division in the liver of rats. J Pathol Bacteriol 1965, 89:153-171 [DOI] [PubMed] [Google Scholar]
- 24.Schoental R: Toxicology and carcinogenic action of pyrrolizidine alkaloids. Cancer Res 1968, 28:2237-2246 [PubMed] [Google Scholar]
- 25.McLean EK: The toxic actions of pyrrolizidine (senecio) alkaloids. Pharmacol Rev 1970, 22:430-463 [PubMed] [Google Scholar]
- 26.Hayes MA, Roberts E, Farber E: Initiation and selection of resistant hepatocyte nodules in rats given the pyrrolizidine alkaloids lasiocarpine and senecionine. Cancer Res 1985, 45:3726-3734 [PubMed] [Google Scholar]
- 27.Laconi E, Sarma DSR, Pani P: Transplantation of normal hepatocytes modulates the development of chronic liver lesions induced by a pyrrolizidine alkaloid, lasiocarpine. Carcinogenesis 1995, 16:139-142 [DOI] [PubMed] [Google Scholar]
- 28.Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, Dabeva MD, Shafritz DA: Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol 1998, 153:319-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jago MV: The development of hepatic megalocytosis of chronic pyrrolizidine alkaloids poisoning. Am J Pathol 1969, 56:405-422 [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins GM, Anderson RM: Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol 1931, 12:186-202 [Google Scholar]
- 31.Wachstein M, Meisel E: Histochemistry of hepatic phosphatases at a physiological pH. Am J Clin Pathol 1955, 27:13-23 [DOI] [PubMed] [Google Scholar]
- 32.Hixson DC, Allison JP: Monoclonal antibodies recognizing oval cells induced in the liver of rats by N-2-fluorenylacetamide or ethionine in a choline-deficient diet. Cancer Res 1985, 45:3750-3760 [PubMed] [Google Scholar]
- 33.Hixson DC, Faris RA, Thompson NL: An antigenic portrait of the liver during carcinogenesis. Pathobiology 1990, 58:65-77 [DOI] [PubMed] [Google Scholar]
- 34.Yang L, Faris RA, Hixson DC: Characterization of a mature bile duct antigen expressed on a subpopulation of biliary ductular cells but absent from oval cells. Hepatology 1993, 18:357-366 [PubMed] [Google Scholar]
- 35.Faris RA, McBride A, Yang L, Affigne S, Walker C, Cha C-J: Isolation, propagation, and characterization of rat liver serosal mesothelial cells. Am J Pathol 1994, 145:1432-1443 [PMC free article] [PubMed] [Google Scholar]
- 36.Dunsford HA, Sell S: Production of monoclonal antibodies to preneoplastic liver cell populations induced by chemical carcinogens in rats and to transplantable Morris hepatomas. Cancer Res 1989, 49:4887-4893 [PubMed] [Google Scholar]
- 37.Gerlach C, Golding M, Larue L, Alison MR, Gerdes J: Ki-67 Immunoexpression is a robust marker of proliferative cells in the rat. Lab Invest 1997, 77:697-698 [PubMed] [Google Scholar]
- 38.Johnson SJ, Hines JE, Burt AD: Macrophage and perisinusoidal cell kinetics in acute liver injury. J Pathol 1992, 166:351-358 [DOI] [PubMed] [Google Scholar]
- 39.Lemire JM, Shiojiri N, Fausto N: Oval cell proliferation and the origin of small hepatocytes in liver injury induced by D-galactosamine. Am J Pathol 1991, 139:535-552 [PMC free article] [PubMed] [Google Scholar]
- 40.Golding M, Sarraf CE, Lalani E-N, Anilkumar TV, Edwards RJ, Nagy P, Thorgeirsson SS, Alison MR: Oval cell differentiation into hepatocytes in the acetylaminofluorene-treated regenerating rat liver. Hepatology 1995, 22:1243-1253 [DOI] [PubMed] [Google Scholar]
- 41.Sarraf C, Lalani EN, Golding M, Anilkumar TV, Poulsom R, Alison M: Cell behavior in the acetylaminofluorene-treated regenerating rat liver: light and electron microscopic observations. Am J Pathol 1994, 145:1114-1126 [PMC free article] [PubMed] [Google Scholar]
- 42.Hixson DC, Faris RA, Novikoff P: Antigenic clues to liver development, renewal, and carcinogenesis: an integrated model. Sirica AE eds. The role of cell types in hepatocarcinogenesis. 1992, :pp 151-182 CRC Press, Boca Raton [Google Scholar]
- 43.Hixson DC, Chapman L, McBride A, Faris R, Yang L: Antigenic phenotypes common to rat oval cells, primary hepatocellular carcinomas and developing bile ducts. Carcinogenesis 1997, 18:1169-1175 [DOI] [PubMed] [Google Scholar]
- 44.Lin G, Cui Y-Y, Hawes EM: Microsomal formation of a pyrrolic alcohol glutathione conjugate of clivorine: firm evidence for the formation of a pyrrolic metabolite of an otonecine-type pyrrolizidine alkaloid. Drug Metab Dispos 1998, 26:181-184 [PubMed] [Google Scholar]
- 45.Mattocks AR: Chemistry and Toxicology of Pyrrolizidine Alkaloids. 1986. Academic Press, London
- 46.Dabeva MD, Laconi E, Oren R, Petkov PM, Hurston E, Shafritz DA: Liver regeneration and α-fetoprotein messenger RNA expression in the retrorsine model for hepatocyte transplantation. Cancer Res 1998, 58:5825-5834 [PubMed] [Google Scholar]
- 47.Ledda GM, Sells MA, Yokoyama S, Lombardi B: Metabolic properties of isolated rat liver cell preparations enriched in epithelial cells other than hepatocytes. Int J Cancer 1983, 31:231-237 [DOI] [PubMed] [Google Scholar]
- 48.Mathis GA, Walls SA, D’Amico P, Gengo TF, Sirica AE: Enzyme profile of rat bile ductular epithelial cells in reference to the resistant phenotype in hepatocarcinogenesis. Hepatology 1989, 9:477-485 [DOI] [PubMed] [Google Scholar]
- 49.Sirica AE, Mathis GA, Sano A, Elmore LW: Isolation, culture, and transplantation of intrahepatic biliary epithelial cells, and oval cells. Pathobiology 1990, 58:44-64 [DOI] [PubMed] [Google Scholar]
- 50.Alison M, Golding M, Lalani E-N, Nagy P, Thorgeirsson S, Sarraf C: Wholesale hepatocytic differentiation in the rat from ductular oval cells, the progeny of biliary stem cells. J Hepatol 1997, 26:343-352 [DOI] [PubMed] [Google Scholar]
- 51.Evarts RP, Nagy P, Nakatsukasa H, Marsden E, Thorgeirsson SS: In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res 1989, 49:1541-1547 [PubMed] [Google Scholar]
- 52.Evarts RP, Hu Z, Omori N, Marsden ER, Thorgeirsson SS: Precursor-product relationship between oval cells and hepatocytes: comparison between tritiated thymidine and bromodeoxyuridine as tracers. Carcinogenesis 1996, 17:2143-2151 [DOI] [PubMed] [Google Scholar]
- 53.Fausto N: Liver stem cells. Arias IM Boyer JL Fausto N Jakoby WB Schachter D Shafritz DA eds. The Liver: Biology and Pathobiology. 1994, :pp 1501-1518 Raven Press, New York [Google Scholar]
- 54.Germain L, Noel M, Gourdeau H, Marceau N: Promotion of growth and differentiation of rat ductulat oval cells in primary culture. Cancer Res 1988, 48:368-378 [PubMed] [Google Scholar]
- 55.Lazaro CA, Rhim JA, Yamada Y, Fausto N: Generation of hepatocytes from oval cell precursors in culture. Cancer Res 1998, 58:5514-5522 [PubMed] [Google Scholar]
- 56.Grisham JW, Tillman RL, Nagel AEH, Compagno J: Ultrastructure of the proliferating hepatocyte: sinusoidal surfaces and endoplasmic reticulum. Lesch R Reutter W eds. Liver Regeneration After Experimental Injury. 1975, :pp 6-23 International Medical Book Corporation, New York [Google Scholar]
- 57.Sandgren EP, Palmiter RD, Heckel JL, Daugherty CC, Brinster RL, Degan JL: Complete hepatic regeneration after somatic deletion of an albumin-plasminogen activator transgene. Cell 1991, 66:245-256 [DOI] [PubMed] [Google Scholar]
- 58.Rhim JA, Sandgren EP, Palmiter RD, Brinster RL: Complete reconstitution of mouse liver with xenogeneic hepatocytes. Proc Natl Acad Sci USA 1995, 92:4942-4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grompe M, al-Dhalimy M, Finegold M, Ou C, Burlingame T, Kennaway NG, Soriano P: Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev 1993, 7:2298–2307 [DOI] [PubMed]