Abstract
Rhabdomyosarcomas are a heterogeneous group of tumors with respect to their molecular basis, degree of differentiation, histology, and clinical behavior. Because of the wide variation of tumor morphology, it is often difficult to distinguish between the distinct subtypes of rhabdomyosarcomas. By using cryosections of tumor specimens and immunohistochemistry, in the present study we show that strong expression of myogenin in rhabdomyosarcoma is associated with alveolar histology (P = <0.0001, Fisher’s exact test). Although staining for myogenin was observed in 22 of 26 rhabdomyosarcomas, all alveolar rhabdomyosarcomas (nine of nine) showed high levels of staining for myogenin, as defined by the frequency and intensity of staining of the tumor cells. The staining pattern suggests that the tumor cells are clonally derived from myogenin-positive progenitor cells. In contrast, most embryonal rhabdomyosarcomas (13 of 15) were either negative or showed a low level of staining for myogenin. In these tumors a larger proportion of tumor cells were distinctly negative for myogenin. Six of seven alveolar rhabdomyosarcomas that strongly stained for myogenin were also positive for Pax3–7/Forkhead (FKHR) by polymerase chain reaction/reverse transcriptase-polymerase chain reaction. One of two embryonal rhabdomyosarcomas that strongly stained for myogenin was retrospectively found to be positive for Pax3/FKHR transcripts. Quantitative analysis for myogenin by Western blotting using a smaller subset of rhabdomyosarcomas revealed that in general there was a good correlation between immunohistochemical staining and Western blotting (P = 0.01, Pearson Correlation), although the former technique was more sensitive for detecting tumors with low levels of the protein. On average, alveolar rhabdomyosarcomas expressed at least threefold more myogenin than embryonal rhabdomyosarcomas. Our data show that staining for myogenin will be a simple, rapid, and accurate adjunct for distinguishing between alveolar and embryonal rhabdomyosarcomas. We propose that embryonal rhabdomyosarcomas result from an early block in myogenesis, before the expression of myogenin. In contrast, we propose that alveolar rhabdomyosarcomas either originate from a late block in myogenesis (after expression of myogenin) or that the pathological mechanisms involved in these neoplasms also induce strong expression of this protein.
Rhabdomyosarcomas are malignant myogenous tumors that can occur in any part of the body, including both skeletal muscle tissue and sites that are devoid of muscle. 1 These tumors are highly heterogeneous, not only with respect to their clinical behavior, but also with respect to the tumor morphology and spectrum of differentiation. At one end of the spectrum they comprise primitive cells that show little cytoplasmic evidence of lineage-specific differentiation, and, at the other end of the spectrum, they comprise highly differentiated cells with well-defined cross-striations characteristic of mature skeletal muscle.
The conventionally accepted histological classification scheme of Horn and Enterline 2 recognizes embryonal, botryoid, alveolar, and pleomorphic tumors as distinct subtypes of rhabdomyosarcomas. Recent studies have shown that additional subgroups may be identified based on prognostic data. 3 The need to accurately distinguish between the subtypes of rhabdomyosarcomas is becoming increasingly clear as we realize that distinct entities exhibit different clinicopathological behaviors and resulting prognosis. 3-12 Alveolar rhabdomyosarcomas are particularly aggressive because of their capacity for invasive growth and metastasis in the early stages of the disease. 3,6,11,12 However, it is sometimes difficult to distinguish between alveolar and embryonal rhabdomyosarcomas by histology alone. 5
Cytogenetic data may be used to distinguish between the two subtypes, as embryonal rhabdomyosarcomas show deletion of chromosome 11p 13-15 and alveolar rhabdomyosarcomas show reciprocal translocations involving chromosomes 1 16,17 or 2 18-20 with chromosome 13. However, cytogenetic analysis is technically difficult, and fewer than 50% of tumors can successfully be karyotyped. 21 Although polymerase chain reaction (PCR) may be used for diagnosing alveolar rhabdomyosarcomas, using primers that amplify the Pax3 or Pax 7-Forkhead (FKHR) chimeric genes involved in the chromosome translocation, this technique is not widely available in clinical laboratories, and furthermore it requires the destruction of tissue. Apart from histology and cytogenetic characteristics, there are currently no definitive protein markers that can accurately distinguish between the subclasses of rhabdomyosarcomas. Therefore it is desirable to identify new markers that may be used to distinguish between the different subtypes of rhabdomyosarcomas. Such markers could be clinically useful diagnostic tools and make possible accurate comparisons of different therapeutic protocols.
The myogenic determination (MyoD) gene, the human homolog of which is known as myf3, was first identified by virtue of its ability to convert nonmuscle cells into muscle cells. 22 Subsequent to the identification of MyoD, three other myogenic regulatory genes, myf5, myogenin (the human homolog of which is myf4), and MRF4 or herculin (the human homolog of which is myf6), 23-25 were identified. The products encoded by these genes share sequence homology with MyoD, induce myogenic differentiation, and constitute the family of myogenic regulatory factors. 26-28 Antibodies to MyoD can be used to diagnose rhabdomyosarcomas from other pediatric and adult tumors. 29-32 In the present study we provide preliminary evidence showing that the intensity and frequency of immunostaining of tumor cells for myogenin can be used to distinguish alveolar rhabdomyosarcomas from embryonal rhabdomyosarcomas.
Materials and Methods
Tumor Tissue and Clinical Data
A total of 26 rhabdomyosarcomas were obtained from collaborating institutions of the Intergroup Rhabdomyosarcoma Study (IRS) 33 or the tumor bank of St. Jude Children’s Research Hospital. All tissues were snap frozen in liquid nitrogen and stored at −70°C. Clinical data for all tissues were obtained from the respective institutions. For each tumor, the diagnosis of rhabdomyosarcoma was confirmed from available clinical and histopathological data that also included confirmed positive staining for other myogenic markers such as desmin and MyoD. 30 Analysis for PAX 3/7-FKHR was performed by polymerase chain reaction/reverse transcriptase-polymerase chain reaction (PCR/RT-PCR). The histological subclassification of all tumors used in the study was obtained from IRS Pathology review. 33
To confirm that there were differences in expression of myogenin between embryonal and alveolar rhabdomyosarcomas, a subgroup of 12 additional rhabdomyosarcomas obtained from the Cooperative Human Tissue Network (CHTN, Columbus, Ohio) were subsequently analyzed for quantitative differences in expression of myogenin by Western blot analysis. These tumors were also examined by immunohistochemistry to confirm data from Western blotting.
Antibodies
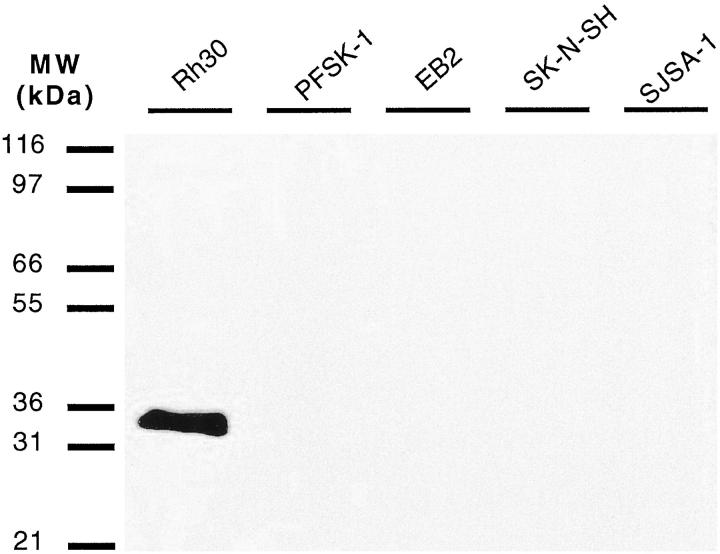
The monoclonal antibody to myogenin (Imgenex, San Diego, CA) was raised against glutathione S-transferase (GST)-myogenin fusion protein. The antibody was found to react specifically with a peptide sequence that corresponds to amino acid residues 144–158 of rat myogenin protein. 34 This region is specific to myogenin and falls distal to the second helix of the HLH domain. Although the monoclonal antibody to myogenin was raised against rat myogenin fusion protein, it cross-reacts with human (myf4) and mouse myogenin, and its specificity for myogenin was demonstrated using a panel of small round cell tumor cell lines, including an alveolar rhabdomyosarcoma cell line (Figure 1) ▶ . A monoclonal antibody to desmin (clone D33; Dako, Carpenteria, CA) was used as an isotype-matched positive control antibody, and a monoclonal antibody to leukocyte common antigen (CD45) (clone 2B11; Dako) was used as an isotype-matched negative control antibody.
Figure 1.
Western blot analysis of myogenin expression in various small round cell tumor lines using the anti-myogenin monoclonal antibody. The antibody only reacts with a protein band corresponding to the molecular mass of myogenin (approximately 34 kd) in the Rh30 rhabdomyosarcoma cell lysate. All other small round cell tumor lysates were negative. Rh30, alveolar rhabdomyosarcoma cell line; PFSK-1A, primitive neuroectodermal tumor cell line; EB2, lymphoma cell line; SKNSH, neuroblastoma cell line; SJSA-1, Ewing’s sarcoma cell line.
Western Blot Analysis
Cell Lines
To confirm the specificity of the monoclonal antibody for myogenin, cell lysates were prepared from a panel of small round tumor cell lines and tested by Western blotting. The cell lines included Rh30 (alveolar rhabdomyosarcoma), PFSK-1A (primitive neuroectodermal tumor), EB2 (lymphoma), SKNSH (neuroblastoma), and SJSA-1 (Ewing’s sarcoma). All cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD). For each cell line, cell lysates were prepared by homogenizing in a lysis buffer (10 mmol/L Tris (pH 7.5), 130 mmol/L NaCl, 1% Triton X-100, 10 mmol/L NaF, 10 mmol/L NaPi (pH 7.5), 10 mmol/L NaPPi pH 7.5). The protein concentration of each lysate was determined and used to load an equal amount of protein per well (10 μg/well) of a sodium dodecyl sulfate/polyacrylamide gel. The proteins were resolved through the gel, transferred to a nylon membrane, and tested for immunoreactivity with the antimyogenin antibody as initially described by Towbin et al. 35 An isotype-matched IgG1 antibody was used as a negative control. For Western blotting, purified antibodies were diluted to 2 μg/ml in 5% nonfat powdered milk in Tris-buffered saline Tween (TBST) blocking buffer, whereas antibody-containing hybridoma culture supernatants were used at 1:3 dilution in the same blocking buffer. Excess unbound primary antibody was washed off, and the membrane was incubated with horseradish peroxidase-conjugated anti-mouse Ig (Jackson Immuno Research Laboratories, West Grove, PA). Antibody binding was detected using the Super Signal chemiluminescent substrate (Pierce, Rockford, IL) and photographic films (Eastman Kodak Company, Rochester, NY).
Tumor Tissue
Tumor lysates were obtained from an additional 12 rhabdomyosarcoma specimens, using lysis buffer as described above. For each tumor lysate, an equal amount of protein was loaded per well (10 μg/well) and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The resolved proteins were then transferred to a nylon membrane and tested for immunoreactivity with 2 μg/ml anti-myogenin antibody as described above. Antibody reactivity with each tumor lysate was analyzed by densitometry, using an Alpha Innotech image analysis system (Alpha Innotech Corporation Corporation, San Leandro, CA).
Immunohistochemical Staining
Immunohistochemical staining for myogenin, desmin, and CD45 was performed by the avidin-biotin-complex (ABC) peroxidase method 36 with a Vectastain kit (Vector Laboratories, Burlingame, CA). Briefly, 5-μm cryosections of tumor tissue on slides were fixed in 1% paraformaldehyde in phosphate-buffered saline (PBS) for 20 minutes, permeabilized in 0.25% Triton X-100 in PBS for 20 minutes, and incubated with 1% bovine serum albumin in PBS for 30 minutes to block for nonspecific staining. The rest of the staining method was performed as previously described. 30 All tissues were incubated with the primary antibody overnight at 4°C. For each tumor, serial sections were used for staining with myogenin, desmin, and CD45. The antibody to myogenin was used at an approximate antibody concentration of 5 μg/ml as a culture supernatant. The antibodies to desmin and CD45 were used in respective PBS dilutions of 1:400 and 1:100 according to the manufacturer’s instructions.
Scoring and Analysis of the Staining Data
For each tumor specimen, the antibody-stained sections were examined independently by P. D., D. P., B. D., H. H., S. Q., and J. T. Positive staining was scored when there was discrete staining of cell nuclei that were not in close proximity to the edge of the section, tissue folds, or areas of necrosis. Staining was scored as follows: −, all tumor cells negative; +, approximately 1–10% tumor cells moderately positive (see Figure 2 ▶ , top panels); ++, approximately 10–50% tumor cells moderately positive; +++, >50–100% tumor cells strongly positive (see Figure 2 ▶ , bottom panels).
Figure 2.
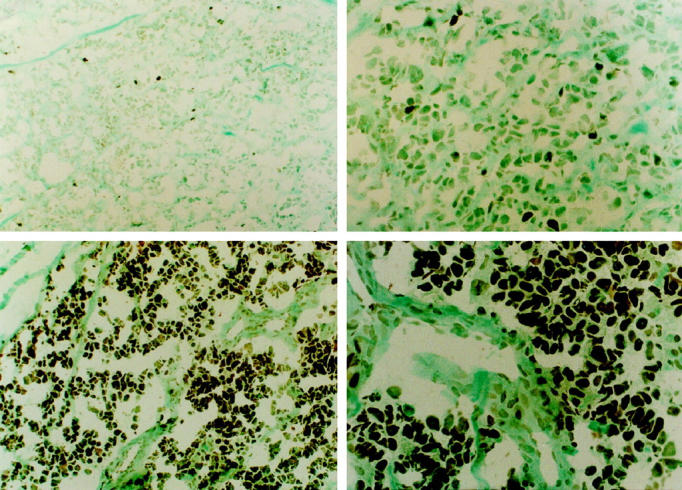
Representative staining for myogenin in embryonal and alveolar rhabdomyosarcoma. The embryonal rhabdomyosarcoma (top panels) in this figure was less than 10% positive for myogenin and was scored as (+), whereas most tumor cells (85–95%) in the alveolar rhabdomyosarcoma tumor (bottom panels) were homogeneously strongly positive for myogenin. This tumor was scored as (+++). Left: Lower magnification (original magnification, ×200). Right: Higher magnification (original magnification, ×400). ABC staining, methyl green counterstain.
Because the number of tumors in each group was too low to allow statistical analysis between the individual groups, closely related staining groups were merged such that the group A tumors comprised (−), (+), and (++) tumors and the group B tumors comprised (+++) tumors (Table 1 ▶ and Figure 2 ▶ ; compare upper (+) panels with lower (+++) panels). The two myogenin-stained tumor groups (groups A and B) were then compared with histological classification, using Fisher’s exact test.
Table 1.
Immunohistochemical Staining for Myogenin in Embryonal and Alveolar Rhabdomyosarcomas
| Group A (−, +, ++) | Group B (+++) | ||||||
|---|---|---|---|---|---|---|---|
| Case no. | Subtype | Pax3/7-FKHR RT-PCR | Anti-myogenin staining | Case no. | Subtype | Pax3/7-FKHR RT-PCR | Anti-myogenin staining |
| 1 | Emb | − | − | 16 | Emb | ND | +++ |
| 2 | Emb | − | − | 17 | Emb | + | +++ |
| 3 | Emb | − | − | 18 | Alv | + | +++ |
| 4 | Emb | ND | − | 19 | Alv | + | +++ |
| 5 | Emb | − | + | 20 | Alv | + | +++ |
| 6 | Emb | − | + | 21 | Alv | + | +++ |
| 7 | Emb | − | + | 22 | Alv | + | +++ |
| 8 | Emb | ND | + | 23 | Alv | + | +++ |
| 9 | Emb | − | + | 24 | Alv | ND | +++ |
| 10 | Emb | ND | + | 25 | Alv | ND | +++ |
| 11 | Emb | − | ++ | 26 | Alv | − | +++ |
| 12 | Emb | − | ++ | ||||
| 13 | Emb | − | ++ | ||||
| 14 | Emb | − | ++ | ||||
| 15 | Emb | − | ++ |
Most embryonal rhabdomyosarcomas showed low levels of staining for myogenin, whereas most alveolar tumors showed high levels of staining for myogenin.
Anti-myogenein antibody staining: −, negative; +, < 10% cells positive; ++, approx. 10–50% cells positive; +++, approx. 50–100% cells strongly positive. Most tumors in this group had 70–100% positive cells. ND, not determined.
Pax3-7/FKHR RT-PCT: −, negative; +, positive.
Histology: Emb, embryonal; Alv, alveolar.
RNA Extraction and PAX3/7-FKHR Amplification by RT-PCR
Analysis for the PAX 3/7-FKHR fusion RT-PCR or PCR product in rhabdomyosarcoma specimens was performed on both frozen and formalin-fixed (cases 11, 18, 19, and 22) or alcohol-fixed (case 17) paraffin-embedded tissue. Paraffin-embedded tissue was first cut into 10-μm sections (10–20 sections each, depending on tissue volume) and deparaffinized with 10 changes of xylene for 30 minutes each at room temperature. The tissue was then rehydrated in a decreasing concentration of ethanol, one change each in 100%, 80%, 50%, and finally RNase-free water. Total RNA was extracted from frozen or deparaffinized tissue, using the Purescript RNA Extraction Kit according to the manufacturer’s instructions (Gentra Systems, Minneapolis, MN). For detection of the Pax3-FKHR or Pax7-FKHR fusion mRNA transcripts, the first strand of cDNA was synthesized by reverse transcription (AMV reverse transcriptase; Promega, Madison, WI) of RNA from the tumor tissue in the presence of deoxyribonucleotide triphosphates (dATP, dCTP, dGTP, and dTTP). PCR was performed as described previously, 37 with the same FKHR reverse primer as used in the reverse transcription and a consensus Pax3/7 primer that recognizes both Pax3 and Pax7. This primer pair amplifies a 219-bp fragment for Pax3-FKHR and a 206-bp fragment for Pax7-FKHR.
A control amplification reaction was performed for each RNA sample, using the FKHR reverse primer and the FKHR forward primer to amplify the normal FKHR transcript. The cycling parameters for both PCR reactions were as previously described. 37 The PCR product was resolved through a 10% native polyacrylamide gel and visualized with ethidium bromide under UV light. To confirm the nature of the fusion genes, the samples that were positive for translocations were then amplified separately, using the same FKHR reverse primer and primers specific for Pax3 or for Pax7. For the four paraffin-embedded tissues, a second-round PCR was performed using a set of seminested primers, including the FKHR forward, Pax3, and Pax7 specific primers together with a nested primer, FKHR C (5′ TCT GCA CAC GAA TGA ACT 3′). The amplification and gel analysis procedures were as described above.
Results
Determining the Specificity of the Monoclonal Antibody for Myogenin by Western Blotting
The anti-myogenin monoclonal antibody reacted with a single band with an approximate size of 34 kd, which corresponds to the molecular size of myogenin in the Rh30 alveolar rhabdomyosarcoma cell lysate (Figure 1) ▶ . All other small round cell tumor cell lines, including primitive neuroectodermal tumor (PFSK-1A), lymphoma (EB2), neuroblastoma (SKNSH), and Ewing’s sarcoma (SJSA-1), were negative with the antibody. The Rh30 alveolar rhabdomyosarcoma cell line had previously been shown to express myogenin transcripts by Northern blotting. 30
Immunohistochemical Staining of Tumor Specimens
Twenty-two of the 26 rhabdomyosarcomas were positive for myogenin. Various patterns of staining for myogenin were observed (Table 1) ▶ . Four of the 17 embryonal rhabdomyosarcomas were negative for myogenin. Six of the 17 tumors belonging to this subclass had occasional weakly positive tumor cells, and five of the 17 embryonal rhabdomyosarcomas had approximately 10–50% myogenin-positive tumor cells. The top panels of Figure 2 ▶ show representative staining of most embryonal rhabdomyosarcoma. Only two of the 17 embryonal rhabdomyosarcomas (cases 16 and 17) showed strong staining for myogenin in most of the tumor cells. One of these two embryonal tumors (case 17) was subsequently found to be positive for Pax3/FKHR (Table 1) ▶ , whereas RT-PCR was not performed on the second case, as a tissue specimen was not available. In contrast, all alveolar rhabdomyosarcomas showed strong staining in most of the tumor cells. Almost every alveolar rhabdomyosarcoma had in excess of 70% strongly positive cells. The bottom panels of Figure 2 ▶ show representative staining of alveolar rhabdomyosarcomas. In general, it appeared as if all tumor cells in alveolar rhabdomyosarcomas were clonally derived from a myogenin-positive progenitor cell. In contrast, a smaller proportion of cells in embryonal rhabdomyosarcomas were positive for myogenin against a background of distinctly negative cells. In all myogenin-positive tumors distinct staining of cell nuclei was observed. There appeared to be no strict correlation with staining for myogenin and the morphological differentiation of the tumor cells. Thus strong staining of a high proportion of tumor cells was observed both in cases with unequivocal alveolar histology and in solid variant alveolar rhabdomyosarcomas (tumors with dense sheets of undifferentiated monomorphic tumor cells). However, weak to strong nuclear staining of tumor cells that had acquired a higher status of differentiation was also observed in embryonal rhabdomyosarcomas. Interestingly, one of the two embryonal rhabdomyosarcomas that strongly stained for myogenin appeared monomorphous, with large rounded nuclei and scanty cytoplasm resembling the solid variant forms of alveolar rhabdomyosarcoma.
The staining results for the group A and B tumors are summarized on Table 2 ▶ . Nine of the 11 tumors that strongly stained for myogenin were alveolar rhabdomyosarcomas and only two were embryonal rhabdomyosarcomas. One of the two embryonal rhabdomyosarcomas (case 17) was characterized as positive for Pax3-FKHR, indicating retrospectively that it was an alveolar rhabdomyosarcoma. When the tumors were examined for correlation of staining (group A versus group B) with tumor subclassification (embryonal versus alveolar) by Fisher’s exact test, there was a highly significant difference (P = <0.0001) in the staining for myogenin between embryonal and alveolar rhabdomyosarcomas.
Table 2.
Summary of Anti-Myogenin Staining in Embryonal and Alveolar Rhabdomyosarcomas
| Subtype | Group A (−, +, ++) | Group B (+++) | Total |
|---|---|---|---|
| Embryonal | 15 | 2 | 17 |
| Alveolar | 0 | 9 | 9 |
| Total 26 |
P < 0.0001, Fishers Exact test.
Anti-myogenin antibody staining: −, negative; +, <10% cells positive; ++, approx. 10–50% cells positive; +++, approx. 50–100% cells strongly positive.
RT-PCR for Pax3/7-FKHR Chimeric Transcripts in Tumor Specimens
RNA samples were available from 20 of 26 tumors to examine for the Pax3–7/FKHR chimeric transcripts characteristic of alveolar rhabdomyosarcomas. Only one of the 13 embryonal rhabdomyosarcomas (case 17) examined was positive for Pax-3/FKHR transcripts. In contrast, six of seven alveolar rhabdomyosarcomas were positive for the Pax3–7/FKHR transcripts. Of these six alveolar tumors, five were positive for Pax3/FKHR and one (case 23) was positive for Pax7/FKHR transcripts. The one tumor that was negative for the Pax3–7/FKHR chimeric transcripts (case 26) appeared to be alveolar rhabdomyosarcoma by histology. This tumor was also strongly positive for myogenin.
Analysis of an Additional Subset of Rhabdomyosarcomas for Quantitative Differences in Expression of Myogenin by Western Blot Analysis and Correlation with Immunohistochemical Staining Data
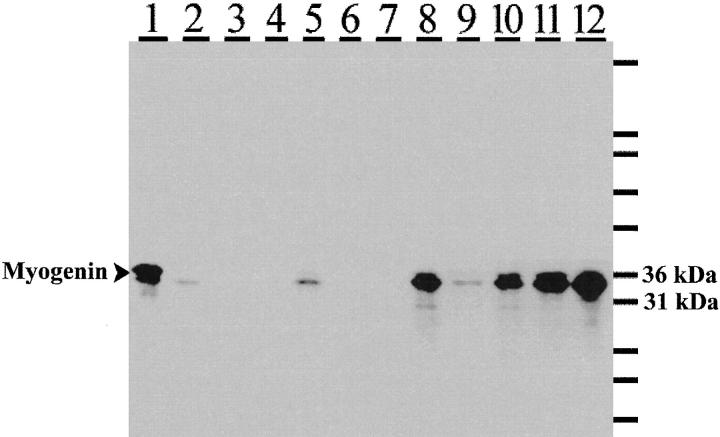
Western blot analysis was carried out on 12 additional rhabdomyosarcomas to test whether the difference in expression of myogenin between embryonal and alveolar rhabdomyosarcoma observed by immunohistochemistry could be shown quantitatively. Molecular/cytogenetic data were unavailable for these 12 tumor cases. There was a good correlation (P = 0.01, Pearson Correlation) between densitometric readings for the Western blot and the levels of staining for myogenin by immunohistochemistry (Figure 3 ▶ and Table 3 ▶ ), although the latter technique was more sensitive. Thus some tumors that were weakly positive by immunohistochemistry were completely negative by Western blotting (see Figure 3 ▶ , lanes 3, 4, and 6, and Table 3 ▶ , cases 3, 4, and 6). High levels of myogenin were detected in four of six alveolar rhabdomyosarcomas (Figure 3 ▶ , lanes 8, 10, 11, and 12, and Table 3 ▶ , cases 8, 10, 11, and 12). Low levels of myogenin were detected in one of the remaining two tumors (Figure 3 ▶ , lane 9), whereas none was detected in the other (Figure 3 ▶ , lane 7). By immunohistochemistry, the myogenin staining pattern of the weakly positive tumor (Figure 3 ▶ , lane 9, corresponding to Table 3 ▶ , case 9) resembled that typically observed for embryonal rhabdomyosarcomas (figure not shown). Interestingly, the alveolar rhabdomyosarcoma that was negative for myogenin by both immunohistochemistry and Western blotting (Figure 3 ▶ , lane 7, and Table 3 ▶ , case 7) was also found to be negative for desmin by immunohistochemistry. Desmin is an intermediate filament protein that is often expressed in rhabdomyosarcomas.
Figure 3.
Western blot analysis for myogenin expression in a smaller subset of rhabdomyosarcoma specimens. Four of six alveolar rhabdomyosarcomas (lanes 8, 10, 11, and 12) were strongly positive for myogenin. One alveolar rhabdomyosarcoma (lane 7) was negative, and one alveolar tumor (lane 9) was weakly positive for myogenin. Three of six embryonal rhabdomyosarcomas (lanes 3, 4, and 6) were negative for myogenin, whereas two of six (lanes 2 and 5) were weakly positive. One embryonal rhabdomyosarcoma (lane 1), a posttreatment tumor, was strongly positive for myogenin. The blot was incubated with anti-myogenin monoclonal antibody. Reactivity was detected using a horseradish peroxidase-conjugated secondary antibody and visualized using a chemiluminescent substrate and exposure to photographic films.
Table 3.
Analysis of an Additional Subset of Rhabdomyosarcomas for Quantitative Differences in Expression of Myogenin by Western Blot Analysis and Correlation with Immunohistochemical Staining Data
| Case no. | Subtype by histology | Densitometric analysis of Western blot (arbitrary units) | Anti-myogenin staining | Anti-desmin Staining |
|---|---|---|---|---|
| 1 | Emb | 77290 | ++ | ++ |
| 2 | Emb | 9136 | + | +++ |
| 3 | Emb | 3472 | ND | ND |
| 4 | Emb | 2969 | + | +++ |
| 5 | Emb | 22601 | ++ | +++ |
| 6 | Emb | 8528 | ++ | +++ |
| 7 | Alv | 9206 | − | − |
| 8 | Alv | 71706 | +++ | +++ |
| 9 | Alv | 21448 | ++ | +++ |
| 10 | Alv | 64627 | +++ | +++ |
| 11 | Alv | 91434 | ND | ND |
| 12 | Alv | 106664 | +++ | +++ |
The table shows immunohistochemical staining for myogenin, desmin, and a summary of the analysis of the Western blot from Figure 3 ▶ .
−, negative; +, weakly positive; ++, moderately positive; +++, strongly positive; ND, not determined.
Histology: Emb, embryonal; Alv, alveolar.
With the exception of the posttreatment embryonal rhabdomyosarcoma (Figure 3 ▶ , lane 1, and Table 3 ▶ , case 1), which showed high levels of myogenin, all other embryonal rhabdomyosarcomas either were negative (Figure 3 ▶ , lanes 3, 4, and 6) or showed low levels of myogenin (Figure 3 ▶ , lanes 2 and 5). This was also reflected in the immunohistochemical staining data, although, as mentioned above, tumors with a low proportion and intensity of myogenin-positive cells were found to be negative by Western blotting. Although the posttreatment embryonal tumor was strongly positive for myogenin by Western blotting, the staining pattern of this tumor was similar to that observed for embryonal rhabdomyosarcomas in that, even though there was a higher proportion of strongly myogenin-positive cells, there was also a significant proportion of cells that were distinctly negative for myogenin.
It was unfortunate that molecular/cytogenetic data, particularly for the two questionable alveolar rhabdomyosarcomas (Figure 3 ▶ , lanes 7 and 9), were not available. Despite the unexpectedly low levels of myogenin in these two alveolar rhabdomyosarcomas, on average, densitometric analysis of the blot revealed that there was at least threefold more myogenin in the alveolar rhabdomyosarcomas than in the embryonal rhabdomyosarcomas. We anticipate that this difference may be even larger and a stronger correlation may be observed if a larger sample size of molecularly characterized tumors is used to validate the present observation.
Discussion
In recent years, multiinstitutional collaborations involving national and international groups such as the Intergroup Rhabdomyosarcoma Study (IRS), the Pediatric Oncology Group (POG), the Children’s Cancer Study Group (CCSG), and the International Society for Pediatric Oncology (SIOP) have allowed controlled studies to be carried out in which a number of selected therapeutic protocols are compared on “pooled” groups of patients with similar cancers from the various institutions. The therapeutic protocol from these cohort studies that gives the most effective response is then subsequently adopted. If the patients are wrongly grouped because of a lack of accurate diagnostic methods or markers, then the findings of these cohort studies will be meaningless and lack therapeutic gain. However, the distinction between subtypes of rhabdomyosarcomas continues to be a problem to pathologists. This is mainly because morphological features that are considered diagnostic are not expressed at the same level among tumors belonging to the same subclass and may even be absent in some tumor specimens. Alveolar foci that are composed of ill-defined pockets of tumor cells separated by hyalinized fibrous septa have traditionally been used to diagnose alveolar rhabdomyosarcomas. However, either a large proportion of the alveolar rhabdomyosarcomas do not exhibit this classical alveolar histological pattern or the foci may be overlooked because of sampling errors. This heterogeneity in tumor histology makes it difficult to apply strict diagnostic criteria for subclassification. As a consequence, alveolar rhabdomyosarcomas are often incorrectly diagnosed as embryonal rhabdomyosarcomas or, conversely, embryonal rhabdomyosarcomas may be incorrectly diagnosed as alveolar rhabdomyosarcomas. Therefore the availability of protein markers that can be used to distinguish between alveolar and embryonal rhabdomyosarcomas by simple histochemical techniques could significantly enhance a diagnosis. In the present study we show that immunohistochemical staining for myogenin can aid in this distinction and note that strong expression of myogenin is associated with tumors of the alveolar histological subtype. Although myogenin is also expressed in embryonal tumors, the level of staining for myogenin was generally distinguishable between the two subtypes of rhabdomyosarcomas. Only two of 17 tumors (from the original tumor series) that had been diagnosed as embryonal rhabdomyosarcomas stained strongly for myogenin. One of these two tumors was retrospectively found to be positive for Pax3/FKHR transcripts, which are found only in alveolar rhabdomyosarcomas, thereby confirming that higher levels of staining for myogenin correlate with alveolar features.
To confirm the observation made by immunohistochemical staining, an additional 12 tumors were quantitatively examined for differences in expression of myogenin by Western blotting. There was a strong correlation in the results from the two techniques, in that higher levels of myogenin were detected in alveolar rhabdomyosarcomas as opposed to embryonal rhabdomyosarcomas. Densitometric analysis of the blot revealed that there was on average at least threefold more myogenin in the alveolar rhabdomyosarcomas than in the embryonal tumors. Although Western blot analysis was the more quantitative of the two techniques, it was less sensitive for detecting weak expression of myogenin in tumors. Furthermore, the immunohistochemical staining patterns for myogenin could clearly be used to distinguish between embryonal and alveolar rhabdomyosarcomas.
From the latter series, there were two alveolar rhabdomyosarcomas with unexpectedly low levels of myogenin; one that was negative and one was weakly positive for myogenin. Unfortunately, there are no cytogenetic or molecular data to confirm the diagnosis of these tumors. One of these two tumors was negative for desmin and myogenin and yet appeared to be an alveolar rhabdomyosarcoma by histology. If tissue preservation was not an issue, then, based on our immunostaining data, it is possible that this tumor is not an alveolar rhabdomyosarcoma. The one embryonal rhabdomyosarcoma that showed strong myogenin expression was a posttreatment tumor. It is not surprising that this tumor was strongly positive for myogenin, as induction of differentiation of tumor cells by therapeutic agents is well documented. A combination of chemotherapy and radiation typically induces a strong cytodifferentiation response in rhabdomyosarcomas. 38-40 It has also been shown that the induction of differentiation by agents such as retinoic acid is associated with activation and increases expression of the myogenin gene. 41 These observations thus suggest that the strong myogenin expression in our single embryonal rhabdomyosarcoma case was a result of the tumor therapy and not an intrinsic component of this tumor. Despite the high levels of myogenin in this tumor, which is a feature we suggest characterizes alveolar rhabdomyosarcomas, by immunohistochemistry this tumor could clearly be distinguished as an embryonal rhabdomyosarcoma. Essentially, the pattern of staining was typical of embryonal tumors in that although there were areas of strong focal staining for myogenin, a fairly large fraction of the tumor cells were negative for myogenin, unlike the staining pattern observed in alveolar rhabdomyosarcomas, where most of the cells were positive. In this smaller subset of tumors, the difference in staining between embryonal and alveolar rhabdomyosarcomas failed to reach statistical significance, although there was a trend toward alveolar rhabdomyosarcomas association with strong myogenin staining (Table 3) ▶ . A study using a larger number of molecularly characterized rhabdomyosarcoma specimens will help to confirm our observations.
Other groups 42-45 have previously examined the expression of myogenin in rhabdomyosarcomas. Tonin et al 42 noted that all eight of their alveolar rhabdomyosarcomas expressed myogenin, whereas only five of eight embryonal rhabdomyosarcomas expressed myogenin transcripts. In the study of Clark et al 43 transcripts of myogenin were detected in 10 of 15 embryonal rhabdomyosarcomas, whereas all three alveolar rhabdomyosarcomas plus one alveolar/embryonal mixed tumor expressed myogenin. Because expression was analyzed by Northern blotting in both studies 42,43 it was not possible to tell the level of expression of myogenin per cell and whether all cells expressed myogenin. Using antibodies to MyoD and myogenin, Wang et al 45 examined expression at the protein level. They noted that 22 of their 25 embryonal rhabdomyosarcomas were positive for myogenin, whereas four of four alveolar rhabdomyosarcomas were positive for myogenin. Unfortunately, these authors did not compare the intensity and frequency of staining between the subgroups of rhabdomyosarcomas. None of the above studies 42,43,45 noted a correlation between the expression of the myogenic regulatory proteins and histological subtypes of rhabdomyosarcomas. Nevertheless it is interesting that in each of these studies some of the embryonal rhabdomyosarcomas were negative for myogenin, whereas all of the alveolar rhabdomyosarcomas were positive for myogenin. In the study of Hosoi et al 44 myogenin expression was examined in two sublines derived from a human rhabdomyosarcoma tumor cell line. Interestingly, stronger expression for myogenin was observed in the subline that appeared to be more differentiated, which is consistent with the role of the protein in myogenic differentiation.
In normal myogenesis, differentiation is an orderly process involving epigenetic regulation of gene expression in the different stages of the myogenic differentiation pathway. Each stage is associated with down-regulation of specific sets of genes and simultaneous transcriptional up-regulation of others. The members of the myogenic bHLH family exhibit different spatiotemporal patterns of expression during embryogenesis. 46-48 Each member may act at a different stage of the myogenic pathway. Through gene inactivation studies in mice it has been shown that whereas either myf 5 or MyoD is necessary for earlier stages of myogenesis, 49 myogenin is obligatory for maturation of muscle. 50 It is anticipated that if a tumor were to occur as a result of a block anywhere in this myogenic differentiation pathway, then the genes expressed by the tumor cells would reflect the stage at which the block in differentiation had occurred. In this regard, we propose that embryonal rhabdomyosarcomas are associated with a block in differentiation before the full expression of myogenin. The relatively small proportion of myogenin-positive cells in these tumors may reflect phenotypic variation due to the environment cues. It is noteworthy that strong staining for myogenin is associated with alveolar rhabdomyosarcomas, because these tumors usually appear to be undifferentiated by morphology. The fact that most of the tumor cells are myogenin positive would seem to suggest that alveolar tumors are clonally derived from myogenin-positive progenitor cells. We propose that either alveolar rhabdomyosarcomas result from a block in differentiation postexpression of myogenin, or that the pathological mechanisms involved in these neoplasms also activate strong myogenin expression. The fact that Pax3/7-FKHR transcripts were detected in six of seven alveolar rhabdomyosarcomas that were strongly positive for myogenin would support the latter notion. However, the one tumor that was negative for Pax3/7-FKHR transcripts indicates that other mechanisms may activate strong expression of myogenin in alveolar rhabdomyosarcoma.
Because there are significant differences in survival between patients with alveolar and embryonal rhabdomyosarcomas, 11,12 our data suggest that strong expression of myogenin in untreated rhabdomyosarcomas is a marker of poor prognosis. It is perplexing that myogenin is strongly expressed in tumors associated with poor prognosis, because, normally, both MyoD 51 and myogenin induce the expression of p21, a tumor suppressor gene, the product of which functions as an inhibitor of cyclin-dependent kinases and causes cells to exit the cell cycle. Because differentiation occurs when the forces that drive proliferation are minimized, it would be anticipated that strong expression of myogenin in untreated rhabdomyosarcomas would result in less aggressive and more differentiated tumors. However, alveolar rhabdomyosarcomas are normally viewed as tumors with an aggressive clinical behavior and poor prognosis.
It is now well established that translocation involving chromosomes 1 or 2 with chromosome 13 is characteristic of alveolar rhabdomyosarcomas. As mentioned above, PCR or RT-PCR for the translocation-associated Pax3/7-FKHR chimeric gene/transcript can be used to diagnose alveolar rhabdomyosarcomas. However, this technique is not widely available in clinical laboratories, whereas immunohistochemistry is widely used for diagnosis. Furthermore, morphological details may be missed by PCR/RT-PCR. From our study we observe that most alveolar rhabdomyosarcomas that strongly stained for myogenin were also positive for Pax3–7/FKHR by PCR/RT-PCR. In fact, one of two embryonal rhabdomyosarcomas that stained strongly for myogenin was also retrospectively found to be positive for Pax3/FKHR transcripts. This indicates that there is a strong association between the translocation-associated Pax3–7/FKHR fusion gene/transcripts and myogenin expression. Therefore we propose that staining for myogenin will be a useful and simple diagnostic test for distinguishing alveolar from embryonal rhabdomyosarcomas. The role of the chimeric gene/transcripts is still not fully understood. The resulting Pax7- or Pax3/FKHR chimeric proteins are potentially oncogenic. 52,53 Epstein et al (1995) 52 showed that the ectopic expression of both Pax3 and Pax3/FKHR was able to inhibit myogenic differentiation of MyoD-expressing 10T1/2 fibroblast cells when these cells were exposed to media with low levels of serum. Interestingly, they observe that Pax-3/FKHR was a more potent inhibitor of myogenesis than Pax-3 alone. In contrast, they showed that under the same conditions, Pax-3 and Pax-3/FKHR were unable to inhibit myogenic differentiation in the myogenin-expressing 10T1/2 cells. The interpretation of this observation in the context of alveolar rhabdomyosarcomas is difficult because we observe that this subtype strongly expresses both MyoD as well as myogenin, and, despite the strong expression of these factors, alveolar rhabdomyosarcomas have a high proliferative potential and are obviously prevented from terminally differentiating. It has also been shown recently that the expression of Pax-3 is sufficient to induce the expression of MyoD 54,55 and myogenin 55 in mice. It is tempting to speculate that the translocation-associated abnormal expression of Pax3/7-FKHR in alveolar rhabdomyosarcomas drives the strong expression of MyoD as well as myogenin in these tumors. In this regard, it would be anticipated that ectopic expression of Pax-3/FKHR would induce strong expression of MyoD and myogenin. However, we do not observe expression of MyoD in 10T1/2 cells transfected with Pax3/FKHR expression vector (P. Houghton, unpublished data).
The expression of MyoD (myf3) has been extensively examined in rhabdomyosarcomas. 29-32 From these studies we observe that antibodies to MyoD can be used to diagnose rhabdomyosarcomas from other pediatric 29,30 and adult 31,32 neoplasms. In these studies we showed that both alveolar and embryonal rhabdomyosarcomas express MyoD. However, we did not observe marked differences in expression of MyoD between embryonal and alveolar rhabdomyosarcomas. There are no reported studies on staining for myf5 and myf6, the two remaining members of the myogenic bHLH family, in rhabdomyosarcomas. It would be interesting to study the expression of these factors in rhabdomyosarcomas, as it may shed some light on the pathological mechanisms involved in these muscle tumors. With this in mind, monoclonal antibody reagents to myf5 and myf6 are currently being developed and characterized.
In summary, we show that there is a strong association in the staining for myogenin in tumors of the alveolar histological subtype. We propose that alveolar rhabdomyosarcomas are clonally derived from myogenin-positive progenitor cells, whereas embryonal rhabdomyosarcomas may be derived from a block in myogenesis before the expression of myogenin. Therefore, the low expression of myogenin in embryonal rhabdomyosarcomas may be associated with phenotypic differentiation of the small proportion of the tumor cells. Thus antibodies to myogenin may not only be useful for diagnosing rhabdomyosarcomas from other small round and spindle cell tumors, but may help in distinguishing between embryonal and alveolar rhabdomyosarcomas by simple immunohistological techniques. Because alveolar rhabdomyosarcomas are associated with poor prognosis, our data suggest that strong expression of myogenin in rhabdomyosarcomas may be used as a marker of poor prognosis. This notion is currently being tested in a study using a large number of tumor specimens for which clinical and cytogenetic data plus patient survival data are available.
Acknowledgments
The authors gratefully acknowledge the excellent technical assistance of Hallie Holt (St. Jude Children’s Research Hospital, Memphis, TN); Lori McLoughlin, Priscilla White, Sandra Brewer-Swartz, and Mary McNulty (Cooperative Human Tissue Network CHTN, Columbus, Ohio) for help with frozen tissue specimens; John Zacker (St. Jude Children’s Research Hospital) for photomicroscopy; and all collaborating investigators of the Intergroup Rhabdomyosarcoma Study for providing freshly frozen tissue. 33
Footnotes
Address reprint requests to Dr. Peter Dias, Imgenex Corporation, 11175 Flintkote Avenue, Suite E, San Diego, CA 92121. E-mail: pbdias@imgenex.com.
Supported in part by U.S. Public Health Service grant 7R 44 CA 60198 (to P. D.); the Dean’s CUMG Development Fund, University of Arkansas Medical Sciences (to B. C.); U.S. Public Health Service grant CA 23099; and the American Lebanese and Syrian Associated Charities (to P. H.).
This work was presented at the 87th Annual Meeting of the United States/Canadian Academy of Pathology, Boston MA, March 1998 (Mod Pathol 1998, 11:14A).
References
- 1.Enzinger FM, Weiss SW: Soft Tissue Tumors, 3rd ed. St. Louis, MO, C. V. Mosby, 1995
- 2.Horn RC, Enterline HT: Rhabdomyosarcoma: a clinicopathological study and classification of 39 cases. Cancer 1958, 11:181-200 [DOI] [PubMed] [Google Scholar]
- 3.Gehan EA, Glover FN, Maurer HM, Sutow WW, Hays DM, Lawrence W, Jr, Newton WA, Jr, Soule EH: Prognostic factors in children with rhabdomyosarcoma. Natl Cancer Inst Monogr 1981, 56:83-92 [PubMed] [Google Scholar]
- 4.Harms D, Schmidt D, Treuner J: Soft-tissue sarcomas in childhood. A study of 262 cases including 169 cases of rhabdomyosarcoma. Z Kinderchir 1985, 40:140–145 [DOI] [PubMed]
- 5.Tsokos M, Webber BL, Parham DM, Wesley RA, Miser A, Miser JS, Etcubanas E, Kinsella T, Grayson J, Glatstein E, Pizzo PA, Triche TJ: Rhabdomyosarcoma: a new classification scheme related to prognosis. Arch Pathol Lab Med 1992, 116:847-855 [PubMed] [Google Scholar]
- 6.Raney RB, Teft M, Maurer HM, Ragab AH, Hays DM, Soule EH, Foulkes MA, Gehan EA: Disease patterns and survival rate in children with metastatic soft-tissue sarcoma. A report from the Intergroup Rhabdomyosarcoma Study (IRS)-I. Cancer 1988, 62:1257–1266 [DOI] [PubMed]
- 7.Ghavimi F, Mandell LR, Heller G, Hajdu SI: Prognosis in childhood rhabdomyosarcoma of the extremity. Cancer 1989, 64:2233-2237 [DOI] [PubMed] [Google Scholar]
- 8.La Quaglia MP, Ghavimi F, Penenberg D, Mandell LR, Healey JH, Hadju SI, Exelby PR: Factors predictive of mortality in pediatric extremity rhabdomyosarcoma. J Pediatr Surg 1990, 25:238-244 [DOI] [PubMed] [Google Scholar]
- 9.Leuschner I, Newton WA, Jr, Schmidth D, Sachs N, Asmar L, Hamoudi A, Harms D, Maurer HM: Spindle cell variants of embryonal rhabdomyosarcomas in the paratesticular region. Am J Surg Pathol 1993, 17:221-230 [DOI] [PubMed] [Google Scholar]
- 10.Kodet R, Newton WA, Jr, Hamoudi AB, Asmar L, Jacobs DL, Maurer HM: Childhood rhabdomyosarcoma with anaplastic (pleomorphic) features. Am J Surg Pathol 1993, 17:443-453 [DOI] [PubMed] [Google Scholar]
- 11.Harms D: Alveolar rhabdomyosarcoma: A prognostically unfavorable rhabdomyosarcoma type and its necessary distinction from embryonal rhabdomyosarcoma. Curr Top Pathol 1995, 89:274-296 [DOI] [PubMed] [Google Scholar]
- 12.Newton WA, Jr: Classification of rhabdomyosarcoma. Curr Top Pathol 1995, 89:241-259 [DOI] [PubMed] [Google Scholar]
- 13.Scrable HJ, Witte DP, Lampkin BC, Cavenee WK: Chromosomal localization of the human rhabdomyosarcoma locus by mitotic recombination mapping. Nature 1987, 329:645-647 [DOI] [PubMed] [Google Scholar]
- 14.Scrable HJ, Witte D, Shimada H, Seemayer T, Wang-Wuu S, Soukup S, Koufos A, Houghton P, Lampkin B, Cavenee W: Molecular differential pathology of rhabdomyosarcoma. Genes Chromosom Cancer 1989, 1:23-35 [DOI] [PubMed] [Google Scholar]
- 15.Koufos A, Hansen MF, Copeland NG, Jenkins NA, Lampkin BC, Cavenee WK: Loss of heterozygosity in three embryonal tumors suggests a common pathogenic mechanism. Nature 1985, 316:330-334 [DOI] [PubMed] [Google Scholar]
- 16.Biegel JA, Meek RS, Parmiter AH, Conrad K, Emanuel BS: Chromosomal translocation t(1;13)(p36;q14) in a case of rhabdomyosarcoma. Genes Chromosom Cancer 1991, 3:483-484 [DOI] [PubMed] [Google Scholar]
- 17.Davis RJ, D’Cruz CM, Lovell MA, Biegel JA, Barr FG: Fusion of PAX7 to FKHR by the variant t(1;13)(p36;q14) translocation in alveolar rhabdomyosarcoma. Cancer Res 1994, 54:2869-2872 [PubMed] [Google Scholar]
- 18.Seidal T, Mark J, Haymar B, Angervall L: Alveolar rhabdomyosarcoma: cytogenetic and correlated cytological and histological study. Acta Pathol Microbiol Immunol Scand A 1982, 90:345-354 [DOI] [PubMed] [Google Scholar]
- 19.Douglass EC, Valentine M, Etcubanas E, Parham D, Webber BL, Houghton PJ, Green AA: A specific chromosomal abnormality in rhabdomyosarcoma. Cytogenet Cell Genet 1987, 45:148-155 [DOI] [PubMed] [Google Scholar]
- 20.Shapiro DN, Sublett JE, Li B, Downing JR, Naeve CW: Fusion of PAX3 to a member of the forkhead family of transcription factors in human alveolar rhabdomyosarcoma. Cancer Res 1993, 53:5108-5112 [PubMed] [Google Scholar]
- 21.Fletcher JA: Cytogenetic analysis of soft tissue tumors. Soft Tissue Tumors, 3rd ed. Edited by FM Enzinger, SW Weiss. St. Louis, MO, C. V. Mosby, 1995, pp 105–118
- 22.Davis RL, Weintraub H, Lassar AB: Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 1987, 51:987-1000 [DOI] [PubMed] [Google Scholar]
- 23.Braun E, Buschhausen-Denker G, Bober E, Tannich E, Arnold HH: A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J 1989, 8:701-709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright WE, Sasson DA, Lin VK: Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell 1989, 56:607-617 [DOI] [PubMed] [Google Scholar]
- 25.Rhodes SJ, Konieczny SF: Identification of MRF4: a new member of the muscle regulatory gene family. Genes Dev 1989, 3:2050-2061 [DOI] [PubMed] [Google Scholar]
- 26.Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R, Hollenberg S, Zhuang Y, Lassar A: The MyoD gene family: nodal point during specification of the muscle cell lineage. Science 1991, 251:761-766 [DOI] [PubMed] [Google Scholar]
- 27.Li L, Olson EN: Regulation of muscle cell growth and differentiation by the MyoD family of helix-loop-helix proteins. Adv Cancer Res 1992, 58:95-119 [DOI] [PubMed] [Google Scholar]
- 28.Dias P, Dilling M, Houghton PJ: The molecular basis of skeletal muscle differentiation. Semin Diagn Pathol 1994, 11:3-14 [PubMed] [Google Scholar]
- 29.Dias P, Parham DM, Shapiro DN, Webber BL, Houghton PJ: Myogenic regulatory protein (MyoD1). Expression in childhood solid tumors: diagnostic utility in rhabdomyosarcoma. Am J Pathol 1990, 137:1283–1291 [PMC free article] [PubMed]
- 30.Dias P, Parham DM, Shapiro DN, Tapscott SJ, Houghton PJ: Monoclonal antibodies to the myogenic regulatory protein MyoD1: epitope mapping and diagnostic utility. Cancer Res 1992, 52:6431-6439 [PubMed] [Google Scholar]
- 31.Tallini G, Parham DM, Dias P, Cardon-Cardo C, Houghton PJ, Rosai J: Myogenic regulatory protein (MyoD) expression in adult soft tissue sarcomas. Am J Pathol 1994, 144:693-701 [PMC free article] [PubMed] [Google Scholar]
- 32.Wesche AW, Fletcher CDM, Dias P, Houghton PJ, Parham DM: Immunohistochemistry of MyoD1 in adult pleomorphic soft tissue sarcomas. Am J Surg Pathol 1995, 19:261-269 [DOI] [PubMed] [Google Scholar]
- 33.Parham DM, Webber BL, Holt H, Williams WK, Maurer H: Immunohistochemistry of childhood rhabdomyosarcomas and related neoplasms: results of an Intergroup Rhabdomyosarcoma Study project. Cancer 1990, 67:3072-3080 [DOI] [PubMed] [Google Scholar]
- 34.Wright WE, Binder M, Funk W: Cyclic amplification and selection of targets (CASTing) for the myogenin consensus binding site. Mol Cell Biol 1991, 11:4104-4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Towbin H, Staehelin T, Godron J: Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 1976, 76:4350-4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu S-M, Raine L, Fanger HA: A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol 1981, 75:734-738 [DOI] [PubMed] [Google Scholar]
- 37.Chen B, Dias P, Jenkins JJ, III, Savell VH, Parham DM: Methylation alterations of the MyoD1 upstream region are predictive of subclassification of human rhabdomyosarcomas. Am J Pathol 1998, 152:1071-1079 [PMC free article] [PubMed] [Google Scholar]
- 38.Molenaar WM, Oosterhuis JW, Kamps WA: Cytologic differentiation in childhood rhabdomyosarcomas following polychemotherapy. Hum Pathol 1984, 15:973-979 [DOI] [PubMed] [Google Scholar]
- 39.Lollini PL, De Giovanni C, Del Re B, Landuzzi L, Nicoletti G, Prodi G, Scotlandi K, Nanni P: Myogenic differentiation of human rhabdomyosarcoma cells induced in vivo by antineoplastic drugs. Cancer Res. 1989, 49:3631-3636 [PubMed] [Google Scholar]
- 40.Coffin CM, Rulon J, Smith L, Bruggers C, White FV: Pathologic features of rhabdomyosarcoma before and after treatment: a clinicopathologic and immunohistochemical analysis. Mod Pathol 1997, 10:1175-1187 [PubMed] [Google Scholar]
- 41.Arnold HH, Gerharz CD, Gabbert HE, Salminen A: Retinoic acid induces myogenin synthesis and myogenic differentiation in the rat rhabdomyosarcoma cell line BA-Han-1C. J Cell Biol 1992, 118:877-887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tonin PN, Scrable H, Shimada H, Cavenee WK: Muscle specific gene expression in rhabdomyosarcomas and stages of human fetal skeletal muscle development. Cancer Res 1991, 51:5100-5106 [PubMed] [Google Scholar]
- 43.Clark J, Rocques PJ, Braun T, Bober E, Arnold HH, Fisher C, Fletcher C, Brown K, Gusterson BA, Carter RL, Cooper CS: Expression of members of the myf gene family in human rhabdomyosarcomas. Br J Cancer 1991, 64:1039-1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hosoi H, Sugimoto T, Hayashi Y, Inaba T, Horii Y, Morioka H, Fushiki S, Hamazaki M, Sawada T: Differential expression of myogenic regulatory genes, MyoD1 and myogenin in human rhabdomyosarcoma sublines. Int J Cancer 1992, 50:977-983 [DOI] [PubMed] [Google Scholar]
- 45.Wang NP, Marx J, McNutt MA, Rutledge JC, Gown AM: Expression of myogenic regulatory proteins (myogenin and MyoD1) in small blue round cell tumors of childhood. Am J Pathol 1995, 147:1799-1810 [PMC free article] [PubMed] [Google Scholar]
- 46.Sassoon D, Lyons G, Wright WE, Lin V, Lassar A, Weintraub H, Buckingham M: Expression of two myogenic regulatory factors myogenin and MyoD during mouse embryogenesis. Nature 1989, 341:303-307 [DOI] [PubMed] [Google Scholar]
- 47.Ott M-O, Bober E, Lyons G, Arnold HH, Buckingham M: Early expression of the myogenic regulatory gene, myf-5, in precursor cells of skeletal muscle in the mouse embryo. Development 1991, 111:1097-1107 [DOI] [PubMed] [Google Scholar]
- 48.Montarras D, Chelly J, Bober E, Arnold H, Ott M-O, Gros F, Pinset C: Developmental patterns in the expression of myf5, MyoD, myogenin, and MRF4 during myogenesis. New Biol 1991, 3:592-600 [PubMed] [Google Scholar]
- 49.Rudnicki MA, Schnegelsberg PNJ, Stead RH, Braun T, Arnold H-H, Jaenisch R: MyoD or myf-5 is required for the formation of skeletal muscle. Cell 1993, 75:1351-1359 [DOI] [PubMed] [Google Scholar]
- 50.Hasty P, Bradley A, Morris JH, Edmondson DG, Venuti JM, Olson EN, Klein WH: Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 1993, 364:501-506 [DOI] [PubMed] [Google Scholar]
- 51.Halevy O, Novitch BG, Spicer DB, Skapek SX, Rhee J, Hannon GJ, Beach D, Lassar AB: Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science 1995, 267:1018-1021 [DOI] [PubMed] [Google Scholar]
- 52.Epstein JA, Lam P, Jepeal L, Maas RI, Shapiro DN: Pax3 inhibits myogenic differentiation of cultured myoblast cells. J Biol Chem 1995, 270:11719-11722 [DOI] [PubMed] [Google Scholar]
- 53.Scheidler S, Fredericks WJ, Rauscher FJ, Barr FG, Vogt PK: The hybrid PAX3-FKHR fusion protein of alveolar rhabdomyosarcoma transforms fibroblasts in culture. Proc Natl Acad Sci USA 1996, 93:9805-9809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M: Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and myf-5 act upstream of MyoD. Cell 1997, 89:127-138 [DOI] [PubMed] [Google Scholar]
- 55.Maroto M, Reshef R, Munsterberg AE, Koester S, Goulding M, Lassar AB: Ectopic Pax-3 activates MyoD and myf-5 expression in embryonic mesoderm and neural tissue. Cell 1997, 89:138-148 [DOI] [PubMed] [Google Scholar]