Abstract
Carbonic anhydrase isozyme XII is a recently discovered member of the α-carbonic anhydrase gene family with a suggested role in von Hippel-Lindau gene-mediated carcinogenesis. Increased expression of its mRNA has been observed in renal and lung carcinomas. This paper presents the localization of CA XII in the normal human gut and in colorectal tumors. Immunohistochemistry performed using a polyclonal antibody raised against truncated CA XII revealed prominent polarized staining for CA XII in the basolateral plasma membrane of the enterocytes of the normal large intestine, the reaction being most intense in the surface epithelial cuff region. Most colorectal tumors displayed abnormal expression of CA XII; the most dramatic change was observed in the deep parts of the adenomatous mucosa, where the positive immunoreaction clearly increased along with the grade of dysplasia. Adenomas with severe dysplasia and carcinomas showed an equal, diffuse staining pattern. The results indicate region-specific regulation of CA XII expression along the cranial-caudal axis of the human gut, whereas its diffuse expression in the most malignant tumors seems to correlate with their biological behavior.
The growing α-carbonic anhydrase (α-CA) gene family includes 11 enzymatically active members that catalyze the reversible hydration of carbon dioxide, CO2 + H2O ⇔ HCO3− + H+, and participate in various biological processes, including respiration, bone resorption, renal acidification, gluconeogenesis, and formation of cerebrospinal fluid and gastric acid. 1-6 The CA isozymes differ in their kinetic properties, their tissue distribution and subcellular localization, and their susceptibility to various inhibitors. Nine isozymes with CA activity have been identified in humans to date, and all of them are expressed in the alimentary tract. In addition, Mori et al 7 recently isolated and characterized a novel membrane-bound isozyme, CA XIV, from mouse kidney. Its presence in human tissues has not yet been reported. A novel gene for CA XIII has also been identified in mouse, but its human orthologue has not yet been determined (D. Hewett-Emmett and L. C. Shimmin, personal communication).
CA XII is a transmembrane isozyme that has recently been identified in human renal cell carcinoma. 8,9 The cDNA encoding it has been cloned and characterized, and its gene has been mapped to chromosome 15. 8,9 Northern blot analysis of normal human tissues has demonstrated CA XII mRNA in the kidney, colon, prostate, pancreas, ovary, testis, lung, and brain. Türeci et al 8 have shown that in 10% of patients with renal cell carcinoma the CA XII transcript was expressed at higher levels in the tumor than in the surrounding normal kidney tissue. Ivanov et al 9 recently identified CA XII gene as a von Hippel-Lindau target gene. They found that wild-type von Hippel-Lindau protein strongly down-regulated the CA XII mRNA, suggesting a potential role for CA XII in von Hippel-Lindau carcinogenesis. Because it has been shown that acidic pH enhances the invasive behavior of tumor cells in vitro, 10 Ivanov et al 9 proposed that CA XII may acidify the immediate extracellular milieu surrounding the cancer cells and thus create a microenvironment conducive to tumor invasion.
This study was designed to investigate the expression of CA XII in the human intestine and in colorectal tumors. CA XII was absent from the small intestine but was expressed in all segments of the normal large intestine. The positive signal was confined to the basolateral plasma membranes of the epithelial cells of the surface epithelial cuff. The abnormal localization of CA XII in some colorectal tumors suggests a role for the enzyme in carcinogenesis.
Materials and Methods
Antibodies against CA Isozymes
cDNAs expressing truncated human CA XII were ligated into the mammalian expression vector pCXN. 8 To produce a secretory form of CA XII, a stop codon was introduced at Q260X. These gene constructs were used to transfect CHO-K1 cells by electroporation. After selection in 400 μg/ml G418 for 10 days, colonies were isolated and cultured. Clones secreting high levels of human CA XII into the medium were identified by CA activity assay. 11 CA XII secreted into the medium was affinity-purified using a sulfonamide-agarose resin and used to prepare antibody as previously described. 1,12 The polyclonal antibody raised against the Q260X secretory form of human CA XII has been characterized by Karhumaa et al (manuscript submitted for publication). The specificity of the anti-human CA XII antiserum was tested by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. The antiserum identified a strong 45-kd and weaker 40-kd polypeptide bands of CA XII on Western blots of Chinese hamster ovary (CHO) cells transfected with wild-type human CA XII cDNA (data not shown). The anti-CA XII antibody showed no cross-reactivity with other CA isozymes analyzed (CA I, II, IV, VI, and IX).
Immunocytochemistry
Samples of human duodenum (n = 1), jejunum (n = 2), ileum (n = 3), cecum (n = 3), ascending colon (n = 3), transverse colon (n = 3), descending colon (n = 3), sigmoid colon (n = 2), and rectum (n = 4) were obtained together with routine histopathological specimens taken during surgical operations for gastric carcinoma (n = 2), rectal carcinoma (n = 1), colon polyposis (n = 2), or colon inertia (n = 1). In addition to the samples of normal human gut, 67 colonic lesions from 59 patients, including eight hyperplastic polyps, one juvenile polyp, 38 adenomas, and 20 adenocarcinomas, were studied. Seven metastases of colorectal adenocarcinomas were also analyzed, including six mesenteric lymph node metastases and one liver metastasis. The adenomatous lesions included 18 tubular, 11 tubulovillous, and four villous tumors. The grade of dysplasia was low in 12 lesions, moderate in 18, and high in eight. Some tumors showed varying dysplasia or adenomatous and invasive histology in the same lesion. There were two patients with familial adenomatous polyposis disease.
The group of 20 malignant colorectal tumors consisted of six well-differentiated, nine moderately differentiated, and five poorly differentiated adenocarcinomas. There were five adenocarcinomas with a mucinous component. Of the carcinomas, two were at stage A in Dukes’ classification, nine at stage B, seven at stage C, and two at stage D. The primary lesions had been isolated from the right colon (n = 13), transverse colon (n = 17), descending colon (n = 6), sigmoid colon (n = 12), and rectum (n = 13).
The procedures had the approval of the ethics committee of Oulu University Hospital, and the research was carried out according to the provisions of the Declaration of Helsinki. Each tissue sample was divided into several small pieces about 5 mm thick. The specimens were fixed either in Carnoy’s fluid (absolute ethanol + chloroform + glacial acetic acid 6:3:1) for 6 hours (samples of the normal human gut) or in 4% neutral-buffered formaldehyde for 24–48 hours (tumor samples). Carnoy’s fluid fixation preserved the antigenicity in the plasma membranes better, whereas formaldehyde fixation produced a more diffuse immunoreaction within the cell (data not shown). The samples were then dehydrated and embedded in paraffin in a vacuum oven at 58°C, and sections of 5 μm were placed on gelatin-coated microscope slides. The CA isozymes were immunostained by the biotin-streptavidin complex method, employing the following steps: 1) pretreatment of the sections with undiluted cow colostral whey (Biotop Oy, Oulu, Finland) for 30 minutes and rinsing in phosphate-buffered saline (PBS), 2) incubation for 1 hour with anti-CA XII serum or normal rabbit serum diluted to 1:100 in 1% bovine serum albumin–phosphate-buffered saline (BSA-PBS), 3) incubation for 1 hour with biotinylated swine anti-rabbit IgG (Dakopatts, Glostrup, Denmark) diluted to 1:300 in 1% BSA-PBS, 4) incubation for 30 minutes with peroxidase-conjugated streptavidin (Dakopatts), and 5) incubation for 2 minutes in DAB solution containing 9 mg 3,3′-diaminobenzidine tetrahydrochloride (Fluka, Buchs, Switzerland) in 15 ml PBS + 5 μl 30% H2O2. The sections were washed three times for 10 minutes in PBS after incubation steps 2 and 3, and four times for 5 minutes in PBS after step 4. All of the incubations and washings were carried out at room temperature. After the immunostaining, the tumor sections were counterstained with hematoxylin. The stained sections were examined and photographed with Leitz Aristoplan (Wetzlar, Germany) or Olympus BH (Tokyo, Japan) microscopes.
The intensity of staining for CA XII was scored by four of the investigators (A. K., T. J. K., S. P., and J. S.) on a scale of 0–4 as follows: 0, no reaction; 1, occasional positive cells; 2, weak reaction; 3, moderate reaction; 4, strong reaction. The distribution of immunoreactivity between the superficial and deep parts of the mucosa was recorded separately.
To examine the subcellular localization of CA XII in the human colonic epithelium, tissue sections were stained by an immunofluorescence method and analyzed by confocal laser scanning microscopy. The steps in the immunofluorescence were as follows: 1) pretreatment of the sections with 1% BSA-PBS for 40 minutes, 2) incubation for 1 hour with 1:100 diluted anti-CA XII serum in 1% BSA-PBS, 3) washing three times for 5 minutes in PBS, 4) incubation for 1 hour with 1:30 diluted fluorescein-conjugated goat anti-rabbit IgG (Dakopatts) in 1% BSA-PBS, and 5) washing three times for 5 minutes in PBS. The immunofluorescent sections were examined with a confocal laser scanning microscope (Leitz CLSM; Leica Microsystems, Heidelberg, Germany).
SDS-PAGE and Western Blotting
Samples containing 25 μg of protein from homogenized human transverse colon were analyzed by SDS-PAGE under reducing conditions according to the method of Laemmli. 13 All of the reagents for SDS-PAGE were from Bio-Rad Laboratories (Richmond, CA) or Sigma (St. Louis, MO). Electrophoresis was performed in a MiniProtean electrophoresis unit (Bio-Rad Laboratories) with a 10% acrylamide separating gel and a 4% acrylamide stacking gel. The proteins were transferred electrophoretically from the gel to a polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA) in a Novex Blot Module (Novex, San Diego, CA). After the transblotting, the sample lanes were first incubated for 30 minutes with TBST buffer (10 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 0.05% Tween-20) containing 10% cow colostral whey (Biotop Oy) and then for 1 hour with the first antibody diluted 1:2000 in TBST buffer. The membranes were washed five times for 5 minutes with TBST buffer and incubated for 30 minutes with alkaline phosphatase-conjugated goat anti-rabbit IgG (Bio-Rad Laboratories) diluted to 1:3000 in TBST buffer. After they were washed four times for 5 minutes in TBST buffer, the polypeptides were visualized by means of a chemiluminescence substrate (Bio-Rad Laboratories). All of the steps were carried out at room temperature.
Results
Distribution of CA XII in the Normal Human Gut
CA XII showed a characteristic staining pattern in human gut. First, it was highly expressed in the cecum, ascending colon, transverse colon, descending colon, sigmoid colon, and rectum, but was absent from the small intestine. Second, the staining for CA XII was most intense in the surface epithelial cuff region of the large intestine. Third, the positive signal for CA XII was clearly confined to the basolateral plasma membranes in the enterocytes. In the formaldehyde-fixed samples, however, the staining signal was more diffusely distributed within the cells (data not shown).
The immunohistochemical distribution of CA XII in the segments of the human gut is shown in Figure 1 ▶ . Figure 1, A ▶ (duodenum), B (jejunum), and C (ileum), shows that the enzyme was not expressed in the small intestine, whereas in the large intestine, prominent reactions were observed in the epithelial cells of the surface epithelial cuff, although the staining decreased toward the base of the glandular crypts (Figure 1, D–F) ▶ . All segments of the large intestine gave a strong positive signal. The confocal laser scanning microscopy image clearly shows that the signal was confined to the basolateral plasma membrane (Figure 1G) ▶ . Control staining using 1:100 diluted nonimmune rabbit serum showed no immunoreaction (data not shown).
Figure 1.
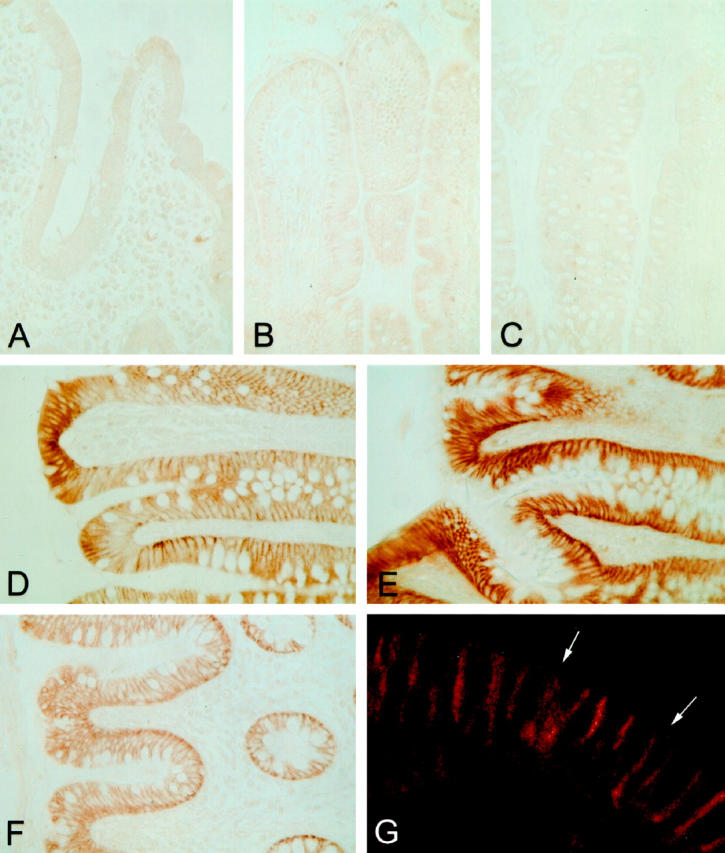
Immunohistochemical staining of CA XII in the segments of the human gut. The epithelia of the duodenum (A), jejunum (B), and ileum (C) give no signal for CA XII, whereas all segments of the large intestine, ie, the cecum (D), ascending colon (E), and rectum (F), demonstrate a strong positive reaction. The intensity of staining for CA XII was highest in the epithelial cells of the surface epithelial cuff and decreased toward the base of the glandular crypts. The subcellular distribution of CA XII in the epithelial cells is confined to the basolateral plasma membrane, as seen in the confocal laser scanning microscopy image (G). The arrows mark the apical brush border. Specimens were fixed with Carnoy’s fluid. Original magnifications: A–F, ×400; G, ×630.
The specificity of the CA XII immunostaining was confirmed by Western blotting of human transverse colon. Anti-CA XII serum recognized a major 46-kd polypeptide of CA XII (Figure 2) ▶ , whereas no signal was observed in the blots immunostained with the normal rabbit serum.
Figure 2.
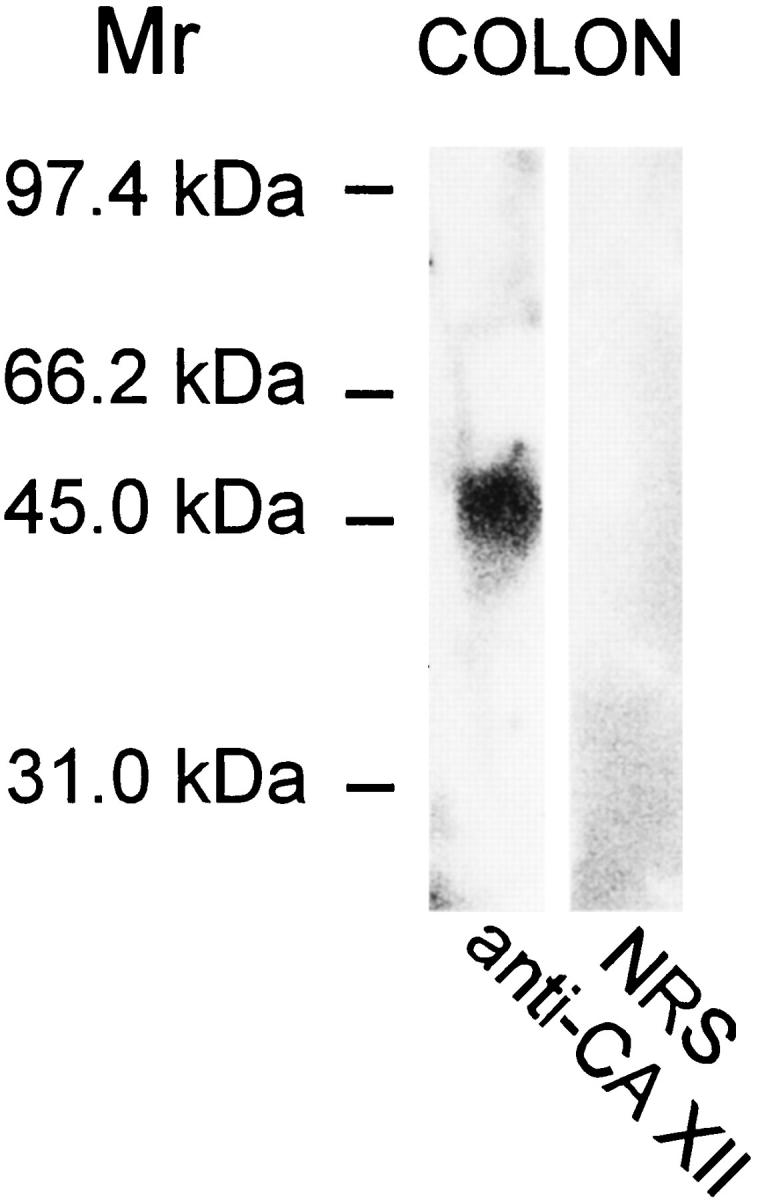
Western blot of CA XII in the human transverse colon. The anti-CA XII serum recognized a major 46-kd polypeptide, whereas normal rabbit serum produced no signal.
Nonneoplastic Polyps of the Large Intestine
The 67 colorectal lesions subjected to immunohistochemical analysis of CA XII included one juvenile polyp and eight hyperplastic polyps. In the juvenile polyp the weak positive immunostaining was restricted to a few occasional epithelial cells, the hyperplastic polyps generally showed a moderate reaction in the enterocytes of the surface epithelial cuff region, whereas the signal was barely apparent in the deep parts of the cryptal epithelium (Figures 3A and 4A) ▶ ▶ . There were no differences in staining intensity between the proximal and distal polyps.
Figure 3.
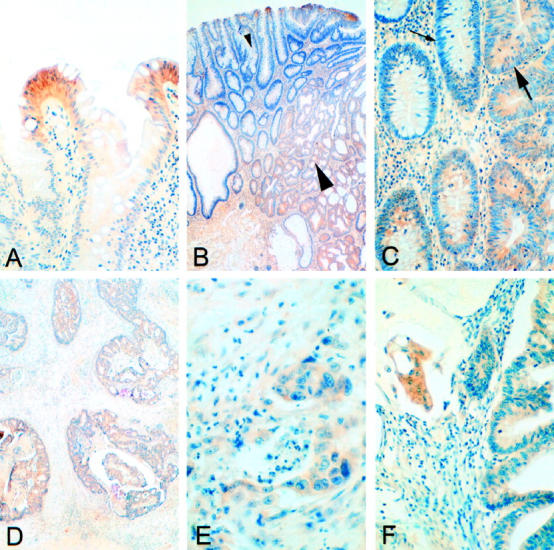
Immunohistochemical staining for CA XII in hyperplastic polyps (A) and adenomas with slight dysplasia (B, small arrowhead, and C, small arrow) is restricted to the surface epithelial cuff region. In adenomas with moderate dysplasia (B, large arrowhead, and C, large arrow), the staining spreads diffusely toward the crypts. B and C represent low and high magnification views of the same section. Adenocarcinomas with (F) or without (D and E) a mucinous component have the staining for CA XII evenly distributed throughout the mucosa. Formaldehyde fixation and hematoxylin counterstaining. Original magnifications: A, C, F: ×200; B, D: ×40; E, ×400.
Figure 4.
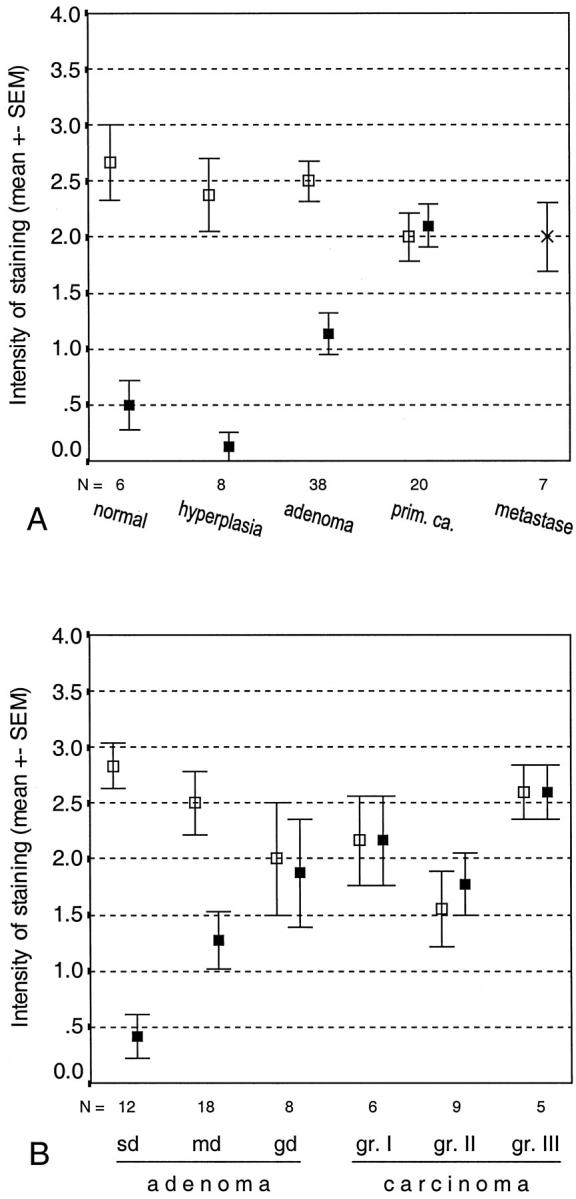
Location and mean intensity of CA XII immunostaining in colorectal tumors and metastases (A) and in colorectal adenomas and carcinomas classified by grade of differentiation (B). sd, slight dysplasia; md, moderate dysplasia; gd, grave dysplasia. □, staining of the surface epithelium; ▪, staining of the cryptal epithelium; ×, staining of the metastases.
Adenomatous Polyps
The 38 adenomatous lesions were obtained from 29 patients, including two with familial adenomatous polyposis disease. The CA XII-positive adenomas were evenly distributed along the cranial-caudal axis of the large intestine. No differences in immunoreactivity were observed between the adenomas obtained from patients with familial polyposis and the sporadic adenomas. No staining for CA XII was found in two lesions (5.3%), which were located in the sigmoid colon and the rectum. The positive immunoreaction was more widely spread toward the crypts in the adenomas than in the normal mucosa or hyperplastic polyps (Figures 3, B and C, and 4A) ▶ ▶ . The intensity of the immunoreaction in the superficial parts of the adenomatous lesions was similar to that in the hyperplastic polyps.
There were 12, 18, and 8 lesions showing low, moderate, and severe dysplasia, respectively. The immunostaining in the adenomas is presented according to grade of dysplasia in Figure 4B ▶ . The staining pattern in the specimens with low dysplasia was similar to that in the hyperplastic polyps, whereas the reaction became more diffusely spread in the lower parts of the lesion in the adenomas with a more severe grade of dysplasia.
Malignant Lesions
The material contained 20 malignant colorectal tumors, comprising six well-differentiated, nine moderately differentiated, and five poorly differentiated adenocarcinomas. According to Dukes’ classification these were of stage A in two cases, stage B in nine, stage C in seven, and stage D in two. The malignant tumor specimens also included six mesenteric lymph node metastases of colorectal carcinomas and one liver metastasis.
In the CA XII immunostaining 19 malignant colorectal tumors showed a positive reaction, and only one remained negative. All of the metastases were found to be positive for CA XII (Figure 4A) ▶ . Figure 3, D ▶ —F, illustrates positive immunohistochemical reactions in two adenocarcinoma specimens with (Figure 3F) ▶ or without (Figure 3, D and E) ▶ a mucinous component. In the colorectal carcinomas the immunoreaction was generally diffuse, being present in both the superficial and deep parts of the mucosa (Figure 4, A and B) ▶ . The staining intensity did not correlate with the location of the tumor (data not shown) or with its grade of differentiation (Figure 4B) ▶ . When the tumor samples were grouped according to Duke’s classification, tumors in classes B–D showed significantly more intense staining than those in class A (P < 0.02).
Discussion
CA XII is a novel member of the α-CA gene family, which is overexpressed in a number of carcinomas. 8,9 CA activity measurements have shown that it is enzymatically active 8 and that it binds to the CA inhibitor affinity chromatography matrix (Karhumaa et al, manuscript submitted for publication). The present results demonstrate that this particular transmembrane protein is expressed in the large intestine, a finding that is consistent with previous observations by Türeci et al 8 and Ivanov et al, 9 who showed a positive signal for CA XII mRNA in a Northern blot of the human colon.
Several interesting features of the distribution of CA XII in the gut are demonstrated here: first, its subcellular location is restricted to the basolateral plasma membrane of the epithelial cells; second, its cellular distribution is restricted to the enterocytes of the surface epithelial cuff; and third, its regional expression is distinctive compared with other CAs, as it is present only in the large intestine.
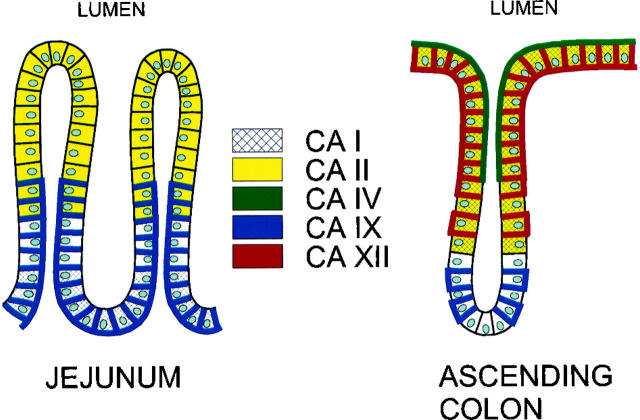
One of the major functions of the large intestine is active Na+ and Cl− transport, which is followed by passive water absorption. Colonic Na+ and Cl− are markedly responsive to alterations in the acid-base balance, 14 which is mainly regulated by Na+-H+ and Cl−-HCO3− exchange processes occurring along the apical border of the colonic epithelial cells. 14-17 These exchangers appear to be regulated by alterations in intracellular pH and HCO3− concentration, which thus control transcellular NaCl absorption as a whole. Based on the present investigations and earlier ones, several CA isozymes can be said to be expressed in the human gut, each of them manifesting a characteristic distribution pattern with respect to segmental, cellular, and subcellular localization. 18 The schematic model for CA isozyme expression in the human jejunum and ascending colon shown in Figure 5 ▶ indicates that the enterocytes of the colonic surface epithelial cuff appear to contain CA I, II, IV, and XII. The existence of at least four CA isozymes in this region (CA I and II in the cytosol, CA IV in the apical brush border, and CA XII in the basolateral plasma membrane) makes it difficult to define the individual role of each, but their cellular distribution suggests an important role in the absorption of water, which is the major physiological function of the colon. Fleming et al 19 proposed that apical CA IV may be functionally coupled with cytosolic CA I and II in the CO2-stimulated uptake of Na+ and Cl− by catalyzing the regeneration of CO2 from excreted H+ and HCO3− at the luminal surface. This would provide additional CO2 to further stimulate the absorption of NaCl and water. The active center of CA XII is located on the cell exterior, beneath the basolateral plasma membrane, 8,9 where it may be functionally involved in transcellular water transport. Through its enzymatic activity, CA XII could convert extracellular water and carbon dioxide to bicarbonate and a proton. This would result in net acidification and a concentration of the extracellular fluid.
Figure 5.
Schematic model of the distribution of CA isozymes in the normal human jejunum and ascending colon. The data are extracted from the present paper and previous ones. 18,19,24
The present results show that the expression of CA XII clearly differs from the normal expression in the majority of colorectal tumors. The most dramatic change was observed in the deep parts of the adenomatous mucosa, where the positive immunoreaction clearly increased with the grade of dysplasia. The adenomas with severe dysplasia and the carcinomas showed a diffusely spread staining pattern that seemed to be associated with the malignant behavior of the colorectal tumors. These results suggest that, in addition to CA IX, 20 CA XII is a second transmembrane isozyme that might be useful in the histopathological diagnosis of colorectal tumors. Because diffuse expression was observed in the most malignant tumors, the future investigations are also warranted to assess the prognostic value of the CA XII immunoreactivity.
CA XII has been reported to be related to von Hippel-Lindau-mediated carcinogenesis, in that higher expression levels have been reported in renal cancer, and it has been shown to be down-regulated by expression of the wild-type von Hippel-Lindau tumor suppressor gene. 9 Interestingly, recent studies have indicated that the von Hippel-Lindau gene product is also expressed in the intestinal epithelium, 21 although this has not been considered a major target for von Hippel-Lindau disease. A role for the von Hippel-Lindau gene in the development of colorectal carcinomas was suggested by Zhuang et al, 22 who demonstrated its allelic loss in 64% of patients with primary sporadic colon carcinoma. It would be interesting to compare the expression of CA XII and von Hippel-Lindau protein in colorectal tumor specimens, and it will also be important to investigate the functional role of CA XII in carcinogenesis. It is thought to acidify the immediate extracellular milieu surrounding the cancer cells in malignant tumors, 9 thereby creating a microenvironment conducive to the growth and spread of tumors. 23
Acknowledgments
We thank Ms. Lissu Hukkanen, Mr. Mika Kihlström, and Mr. Eero Oja for their skillful technical assistance.
Footnotes
Address reprint requests to Dr. Antti Kivelä, Department of Anatomy and Cell Biology, Box 5000, FIN-90014 University of Oulu, Finland. E-mail: kivelae@usa.net.
Supported by grants DK40163 and GM34182 from the U.S. Public Health Service (to W. S. S.) and from the Sigrid Juselius Foundation (to S. P.).
References
- 1.Zhu WL, Sly WS: Carbonic anhydrase IV from human lung. Purification, characterization, and comparison with membrane carbonic anhydrase from human kidney. J Biol Chem 1990, 265:8795-8801 [PubMed] [Google Scholar]
- 2.Dodgson SJ: Liver mitochondrial carbonic anhydrase (CA V), gluconeogenesis, and ureagenesis in the hepatocyte. The Carbonic Anhydrases. Cellular Physiology and Molecular Genetics. Edited by SJ Dodgson, RE Tashian, G Gros, ND Carter, New York, Plenum Press, 1991, pp 297–306
- 3.Sly WS, Hu PY: Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem 1995, 64:375-401 [DOI] [PubMed] [Google Scholar]
- 4.Parkkila S, Parkkila A-K: Carbonic anhydrase in the alimentary tract. Roles of the different isozymes and salivary factors in the maintenance of optimal conditions in the gastrointestinal canal. Scand J Gastroenterol 1996, 31:305-317 [DOI] [PubMed] [Google Scholar]
- 5.Pastoreková S, Parkkila S, Parkkila A-K, Opavský R, Zelnik V, Saarnio J, Pastorek J: Carbonic anhydrase IX, MN/CA IX: analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology 1997, 112:398-408 [DOI] [PubMed] [Google Scholar]
- 6.Hewett-Emmett D, Tashian RE: Functional diversity, conversation, and convergence in the evolution of the α-, β-, and γ-carbonic anhydrase gene families. Mol Phylogenet Evol 1996, 5:50-77 [DOI] [PubMed] [Google Scholar]
- 7.Mori K, Ogawa Y, Ebihara K, Tamura N, Tashiro K, Kuwahara T, Mukoyama M, Sugawara A, Ozaki S, Tanaka I, Nakao K: Isolation and characterization of CA XIV, a novel membrane-bound carbonic anhydrase from mouse kidney. J Biol Chem 1999, 274:15701-15705 [DOI] [PubMed] [Google Scholar]
- 8.Türeci Ö, Sahin U, Vollmar E, Siemer S, Göttert E, Seitz G, Parkkila A-K, Shah GN, Grubb JH, Pfreundschuh M, Sly WS: Human carbonic anhydrase XII, cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc Natl Acad Sci USA 1998, 95:7608–7613 [DOI] [PMC free article] [PubMed]
- 9.Ivanov SV, Kuzmin I, Wei M-H, Pack S, Geil L, Johnson BE, Stanbridge EJ, Lerman MI: Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes. Proc Natl Acad Sci USA 1998, 95:12596-12601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Zaguilan R, Seftor EA, Seftor RE, Chu YW, Gillies RJ, Hendrix MJ: Acidic pH enhances the invasive behavior of human melanoma cells. Clin Exp Metastasis 1996, 14:176-186 [DOI] [PubMed] [Google Scholar]
- 11.Sundaram V, Rumbolo P, Grubb J, Strisciuglio P, Sly WS: Carbonic anhydrase II deficiency: diagnosis and carrier detection using differential enzyme inhibition and inactivation. Am J Hum Genet 1986, 38:125-136 [PMC free article] [PubMed] [Google Scholar]
- 12.Waheed A, Okuyama T, Heyduk T, Sly WS: Carbonic anhydrase IV: purification of a secretory form of the recombinant human enzyme and identification of the positions and importance of its disulfide bonds. Arch Biochem Biophys 1996, 333:432-438 [DOI] [PubMed] [Google Scholar]
- 13.Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227:680-685 [DOI] [PubMed] [Google Scholar]
- 14.Goldfarb DS, Egnor RW, Charney AN: Effects of acid-base variables on ion transport in rat colon. J Clin Invest 1988, 81:1903-1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurtin P, Charney AN: Effect of arterial carbon dioxide tension on amiloride-sensitive sodium absorption in the colon. Am J Physiol 1984, 247:G537-G541 [DOI] [PubMed] [Google Scholar]
- 16.Kurtin P, Charney AN: Intestinal ion transport and intracellular pH during acute respiratory alkalosis and acidosis. Am J Physiol 1984, 247:G24-G31 [DOI] [PubMed] [Google Scholar]
- 17.Wagner JD, Kurtin P, Charney AN: Effect of systemic acid-base disorders on colonic intracellular pH and ion transport. Am J Physiol 1985, 249:G39-G47 [DOI] [PubMed] [Google Scholar]
- 18.Saarnio J, Parkkila S, Parkkila A-K, Waheed A, Casey MC, Zhou XY, Pastoreková S, Pastorek J, Karttunen T, Haukipuro K, Kairaluoma MI, Sly WS: Immunohistochemistry of carbonic anhydrase isozyme IX (MN/CA IX) in human gut reveals polarized expression in the epithelial cells with the highest proliferative capacity. J Histochem Cytochem 1998, 46:497-504 [DOI] [PubMed] [Google Scholar]
- 19.Fleming RE, Parkkila S, Parkkila A-K, Rajaniemi H, Waheed A, Sly WS: Carbonic anhydrase IV expression in rat and human gastrointestinal tract: regional, cellular, and subcellular localization. J Clin Invest 1995, 96:2907-2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saarnio J, Parkkila S, Parkkila A-K, Haukipuro K, Pastoreková S, Pastorek J, Kairaluoma MI, Karttunen T: Immunohistochemical study of colorectal tumors for expression of a novel transmembrane carbonic anhydrase, MN/CA IX, with potential value as a marker of cell proliferation. Am J Pathol 1998, 153:279-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Los M, Jansen GH, Kaelin WG, Lips CJ, Blijham GH, Voest EE: Expression pattern of the von Hippel-Lindau protein in human tissues. Lab Invest 1996, 75:231-238 [PubMed] [Google Scholar]
- 22.Zhuang Z, Emmert-Buck MR, Roth MJ, Gnarra J, Linehan WM, Liotta LA, Lubensky IA: von Hippel-Lindau disease gene deletion detected in microdissected sporadic human colon carcinoma specimens. Hum Pathol 1996, 27:152-156 [DOI] [PubMed] [Google Scholar]
- 23.Warburg O: Über den Stoffwechsel der Tumoren. 1926. Cambridge University Press, Cambridge
- 24.Parkkila S, Parkkila A-K, Juvonen T, Rajaniemi H: Distribution of the carbonic anhydrase isoenzymes I, II, and VI in the human alimentary tract. Gut 1994, 35:646-650 [DOI] [PMC free article] [PubMed] [Google Scholar]