Abstract
Collagen XVII/BP180, an epidermal adhesion molecule, exists as a full-length transmembrane protein and as a soluble 120-kd ectodomain that is shed from the keratinocyte surface by furin-mediated proteolysis. Despite a number of studies on autoantibody targets in blistering skin diseases, it has remained unclear whether the physiologically shed ectodomain of collagen XVII plays a role as an autoantigen. Here we isolated the authentic, soluble form of human collagen XVII and showed that it is an autoantigen recognized by IgG and IgA autoantibodies in different blistering skin diseases and is, in some cases, the preferential target. The ectodomain was isolated from the epidermis, keratinocyte media, amniotic fluid, and pemphigoid blister fluid, and autoantibodies affinity-purified with this ectodomain bound to the proximal surface of the epidermis in normal skin but not in collagen XVII-deficient skin. The antibody reactivity was not dependent on the native conformation or the N-glycosylation of the soluble ectodomain, but was abolished by collagenase treatment. Sera of 81 patients with a clinically active blistering skin disease were reacted with full-length collagen XVII, the authentic soluble ectodomain, and recombinant fragments. In bullous and cicatricial pemphigoid, IgG reactive with full-length collagen XVII also recognized the soluble ectodomain. In linear IgA dermatosis and chronic bullous dermatosis of childhood, IgA targeted the soluble ectodomain more efficiently than the full-length protein. The use of recombinant fragments demonstrated that epitopes were present in several noncollagenous and collagenous subdomains of the molecule, and that a significant portion of the sera targeted Col15 domain, a hitherto unrecognized epitope region.
Collagen XVII, also referred to as 180-kd bullous pemphigoid antigen (BP180), is a structural hemidesmosomal transmembrane protein 1,2 with a globular cytoplasmic domain and a large collagenous extracellular domain. 3-7 It maintains the linkage between intracellular and extracellular structures and anchors the keratinocytes to the basement membrane. 8,9 Collagen XVII occurs in two forms, as a full-length transmembrane protein and as a distinct, soluble ectodomain that is shed from the cell surface by furin-mediated proteolytic processing. 1 The 120-kd soluble form is a rod-like, flexible molecule that contains 15 collagenous domains flanked by 16 short noncollagenous sequences. 3,6 However, the overall structure of the shed ectodomain is collagen-like and triple helical, with a significant thermal stability. 1
Bullous pemphigoid and other acquired subepidermal blistering skin diseases, such as gestational and cicatricial pemphigoids, are characterized by IgG and C3 deposits along the cutaneous basement membrane zone. In addition to BP230, the classic bullous pemphigoid antigen-1, 10 the autoantibodies target collagen XVII. As a result of a number of investigations on the autoantigenicity of this molecule, it is well established that the full-length collagen is recognized by patient autoantibodies. Fifty to seventy percent of pemphigoid sera react with collagen XVII in immunoblots, 11-15 and the pathogenicity of the autoantibodies was demonstrated in a passive transfer mouse model. 16 Epitope mapping studies using different prokaryotic and eukaryotic recombinant fragments demonstrated the reactivity of bullous pemphigoid sera predominantly with the NC16a domain, 14,15,17-19 although other epitopes in extreme amino- and carboxy-terminal regions have also been identified. 11,20-22 After the recent demonstration that the shedding of the collagen XVII ectodomain occurs physiologically, 1,9 the ques- tion prevails whether this independent, soluble molecule also acts as an antigen in a etiopathologically relevant manner.
Linear IgA disease (LAD) is a distinct subepidermal blistering disorder, characterized by tissue-bound and circulating IgA autoantibodies targeting the dermo-epidermal junction. 23-27 The linear IgA disease antigen, LAD-1, was characterized with patient sera and with a monoclonal antibody and shown to be a 120-kd secreted keratinocyte protein that is proteolytically processed into a 97-kd form found in epidermis extracts. 26,28 Some autoantisera in linear IgA disease recognized collagen XVII 12,23,27,29 ; however, lack of cross-reactivity of the monoclonal antibody to LAD-1 with collagen XVII impeded the characterization of the autoantigens in question. The report that the 97-kd LAD-1 from epidermal extracts showed partial amino acid sequence identity with the extracellular domain of collagen XVII 30 led to the assumption that LAD-1 is highly homologous with, if not identical to, the ectodomain of collagen XVII. However, this hypothesis has not been proved.
Similarly, autoantigens with a molecular mass of approximately 120 kd have been proposed in bullous pemphigoid. Gao et al 22 reported reactivity of pemphigoid sera with a 125-kd protein that localized to the dermal side of sodium chloride split skin. Pas et al 23,24 found reactivity of some pemphigoid and LAD sera with a 120-kd secreted protein that appeared to have cross-reactivity with collagen XVII. However, in these studies the molecular identity of the involved antigens was not firmly established, rendering the evidence circumstantial.
In the present study, we isolated the authentic, shed form of collagen XVII and used it as an antigen to test patient autoimmune sera. The results verified that the 120-kd soluble ectodomain of collagen XVII is indeed targeted by both IgG and IgA autoantibodies. Moreover, a significant number of the autoantisera reacted with the Col15 domain, a hitherto unrecognized epitope region.
Materials and Methods
Patients and Controls
Eighty-one patients with a clinically active subepidermal blistering skin disease and no immunosuppressive therapy were included in this study. The age range varied from 8 to 99 years, the average being 73 years. Of the 81 patients, 50 had a blistering skin disease with tissue-bound IgG autoantibodies and 31 a bullous disorder with predominantly IgA autoantibodies. Forty-three patients had bullous pemphigoid, six cicatricial pemphigoid, and one gestational pemphigoid. Twenty-four patients had linear IgA disease and seven children chronic bullous disease of childhood (CBDC) with IgA autoantibodies (Table 1) ▶ . The diagnoses were based on the following criteria: 1) typical clinical presentation with blistering of the skin and/or mucosa; 2) characteristic histology with subepidermal blistering and eosinophil- and/or neutrophil-rich inflammatory infiltrates; 3) either linear IgG and C3 (pemphigoid group) or IgA (linear IgA disease of adults and CBDC of children) deposits at the dermal-epidermal junction, revealed by direct immunofluorescence staining of a skin biopsy; and 4) localization of the immunglobulin deposits in the blister roof of sodium chloride-split skin. 25,31 Most patient sera produced a positive indirect immunofluorescence staining with sodium chloride-split human skin. 31 Sera or blister fluid was used as a source of autoantibodies; the use of blister fluid has previously been reported. 27 As controls, sera of 44 patients with pemphigus and 30 individuals with atopic dermatitis, psoriasis, lupus erythematosus, scleroderma, dermatitis herpetiformis, or Behcet’s disease were analyzed. The latter group included 23 females (age range 18–83 years, average 55 years) and seven males (age range 2–78 years, average 50 years).
Table 1.
Reactivity of the Autoimmune and Control Sera with the Two Forms of Collagen XVII and the Recombinant Domains*
| Disorder | No. of sera tested | Immunopositive sera | ||||
|---|---|---|---|---|---|---|
| Full-length collagen XVII | Shed ectodomain | Recombinant domains | ||||
| NC16a | Col15 | Ecto2 | ||||
| Bullous pemphigoid | 43 | 34 | 29 | 21 | 11 | 17 |
| Cicatricial pemphigoid | 6 | 6 | 6 | 1 | 3 | 3 |
| Gestational pemphigoid | 1 | 0 | 0 | 0 | 0 | 0 |
| LAD | 24 | 9 | 12 | 2 | 2 | 1 |
| CBDC | 7 | 3 | 7 | 0 | 1 | 0 |
| Pemphigus group | 44 | 2 | 1 | 0 | 0 | 0 |
| Others† | 30 | 0 | 0 | 0 | 0 | 0 |
* LAD indicates linear IgA dermatosis and CBDC chronic bullous disease of childhood. The pemphigus group contains pemphigus vulgaris, pemphigus foliaceus, and pemphigus erythematosus.
† Others include control sera from eczema, psoriasis, and nonbullous autoimmune skin diseases.
Isolation of Collagen XVII and the Soluble Ectodomain
Collagen XVII was extracted from normal human keratinocytes grown in serum-free medium (SFM) (Gibco, Grand Island, NY) for 48 hours in the presence of 50 μg/ml ascorbic acid to allow for prolyl hydroxylation and correct folding of the collagen. 32 Confluent keratinocyte layers were extracted for 30 minutes on ice with 1 ml/75 cm 2 of a neutral buffer containing 1% NP-40, 0.1 mol/L NaCl, 25 mmol/L Tris-HCl (pH 7.4), 10 mmol/L EDTA, 1 mmol/L Pefabloc (Merck, Darmstadt, Germany), and, when appropriate, 14 μg/ml chymostatin, 7 μg/ml antipain, 7 μg/ml leupeptin, and 14 μg/ml pepstatin as proteinase inhibitors. 1,28 The cell lysate was then scraped with a rubber policeman, and the extract was centrifuged at 15,000 × g at 4°C to remove insoluble material.
The shed ectodomain of collagen XVII was precipitated from keratinocyte culture media. For this purpose, 1 mmol/L Pefabloc (Merck) and 10 mmol/L EDTA were added immediately after the samples were collected. After cellular debris was removed by centrifugation at 1000 rpm for 10 minutes, the proteins from a 10-ml sample were precipitated with ammonium sulfate to 30% saturation for 4 hours at 4°C. After centrifugation at 15,000 × g for 60 minutes at 4°C, the pellets were dissolved in 100 μl of a buffer containing 65 mmol/L NaCl, 25 mmol/L Tris-HCl (pH 7.4), 1 mmol/L Pefabloc, and 1 mmol/L EDTA. The ectodomain was immunoprecipitated from keratinocyte medium, amniotic fluid, or pemphigoid blister fluid with domain-specific antibodies against recombinant collagen XVII fragments, essentially as described. 1 The shed ectodomain was extracted from the epidermis, using a protocol reported previously, 33 with minor modifications. Briefly, the epidermis was separated from the dermis after an incubation in a buffer containing 20 mmol/L EDTA, 50 mmol/L Tris-HCl (pH 7.4), and 1 mmol/L Pefabloc (Merck) and 14 μg/ml chymostatin, 7 μg/ml antipain, 7 μg/ml leupeptin, and 14 μg/ml pepstatin as proteinase inhibitors. The epidermis was then extracted three times with a buffer containing 1 mol/L NaCl, 50 mmol/L Tris-HCl (pH 7.4), 10 mmol/L EDTA, 1 mmol/L Pefabloc, 14 μg/ml chymostatin, 7 μg/ml antipain, 7 μg/ml leupeptin, and 14 μg/ml pepstatin and dialyzed extensively against 1 mmol/L Pefabloc. All incubations were at 0–4°C. The dialyzed protein extract was precipitated with ethanol before use as antigen in immunoblots.
For some experiments, the soluble ectodomain of collagen XVII from the cell culture medium was digested with 40 U/ml bacterial collagenase (Advanced Biofactures, Lynbrook, NY) at 37°C for 2 hours in a buffer consisting of 0.065 mmol/L NaCl, 0.01 mol/L CaCl2, and 0.05 mol/L Tris-HCl (pH 7.4). Alternatively, for deglycosylation, 100 μl of the protein extract was treated with 10% β-mercaptoethanol for 10 minutes at 60°C before digestion with 10 U/ml of N-glycosidase F (Boehringer Mannheim, Mannheim, Germany) overnight at 37°C. All reactions contained 1 mmol/L Pefabloc (Merck), 14 μg/ml chymostatin, 7 μg/ml antipain, 7 μg/ml leupeptin, and 14 μg/ml pepstatin as proteinase inhibitors. 1
Immunoadsorption of Antibodies with the Soluble Ectodomain
Patient autoantibodies were affinity-purified on nitrocellulose stripes containing the 120-kd ectodomain of collagen XVII, which was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 4.5–15% polyacrylamide gradient and electrotransferred to nitrocellulose as described. 34 Briefly, 50 μl of serum diluted 1:10 in blotting buffer were reacted with the nitrocellulose stripe overnight, and the antibodies were eluted in 200 μl of glycine buffer (pH 2.5). After neutralization, the antibodies were used for immunofluorescence staining of intact or sodium chloride-split 29 human skin according to standard techniques. As second antibodies, fluorescein isothiocyanate-labeled anti-human IgG or anti-human IgA (Dako) was used.
Production of Eucaryotic Fragments of Collagen XVII
A cDNA fragment spanning the amino acids 567–807 of human collagen XVII 3 was amplified by reverse transcriptase polymerase chain reaction (Titan RT-PCR; Boehringer Mannheim) of mRNA from normal human keratinocytes according to the manufacturer’s instructions. The sense primer was 5′-GCGCGCTAGCAGGAAGCCCTGGCCCTAAA-3′ and the antisense primer 5′-ATTAGCGGCCGCTCACTTGCCTGGAGCTCC-3′; the underlined sequences representing the NheI and NotI restriction sites, respectively. Another cDNA fragment encoding the amino acids 1175–1497 of human collagen XVII was generated similarly, with the sense primer 5′-GCGCGCTAGCAGGCATCGTTGGACCCCCA-3′ and the antisense primer 5′-ATTAGCGGCCGCTCACGGCTTGACAGC-3′. After NheI and NotI digestions the resulting fragments, ColXVII-Col15 and ColXVII-Ecto2 (Figure 1) ▶ , were cloned into a modified episomal expression vector pCEP-4 (InVitrogen, Leek, the Netherlands) containing the signal peptide sequence of BM-40 and a puromycin resistance gene. 35 The resulting clones, pCEP-ColXVII-Col15 and pCEP-ColXVII-Ecto2, were verified by dideoxynucleotide sequence analysis.
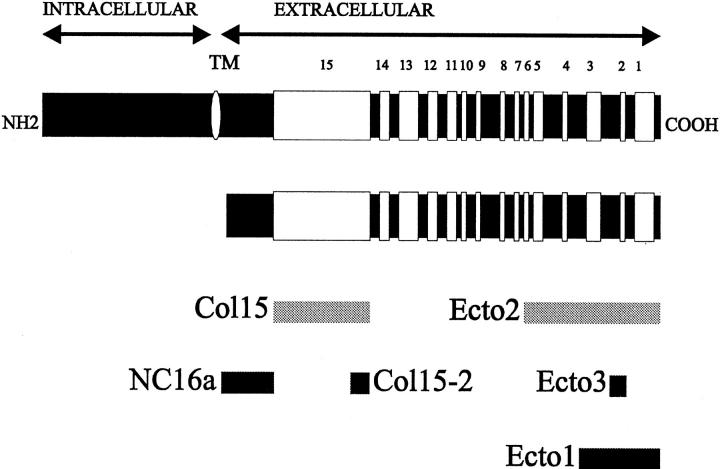
Figure 1.
Schematic representation of collagen XVII, its shed ectodomain, the recombinant fragments, and the specificities of the antibodies used. Collagen XVII is a type II transmembrane protein (upper scheme) with an amino-terminal intracellular domain, a transmembrane domain, and a carboxy-terminal extracellular domain about 1000 amino acids in length. The soluble ectodomain (lower scheme) consists of 15 collagenous and 16 noncollagenous domains. Shedding occurs by proteolytic cleavage carboxy-terminally from the transmembrane domain, presumably within the NC16a region. White bars: Collagenous sequences; black bars: noncollagenous sequences; the white oval indicates the transmembrane domain (TM). The domains covered by the eucaryotic recombinant fragments, ERF-ColXVII-Col15 and ERF-ColXVII-Ecto2 (designated Col15 and Ecto2, respectively), are shown as light gray bars. The dark gray bars indicate the bacterial fusion proteins and the epitopes of the corresponding antibodies Ab-ColXVII-NC16a recognizing the NC16a domain, Ab-ColXVII-Col15-2 for the amino terminus of the Col15 domain (Col15-2), and Ab-ColXVII-Ecto3 (Ecto3) for the Col2 and NC3 domains. Ab-ColXVII-Ecto1 (Ecto1) against the distal carboxy terminus of the molecule has been described previously. 1
The human embryonic kidney 293 EBNA cells were grown in Dulbecco’s modified Eagle’s/Nutrient mix F-12 medium (Gibco BRL, Life Technologies) containing 10% fetal calf serum and 0.35 μg/ml G418 (Invitrogen). One million cells/10-cm culture dish were transfected with 25 μg of pCEP-ColXVII-Col15 or pCEP-ColXVII-Ecto2 DNA by the calcium phosphate method. Transfected cells were selected with 0.5 μg/ml puromycin (Sigma, Deisenhofen, Germany), and serum-free medium containing 50 μg/ml ascorbate (Fluka, Deisenhofen, Germany) added every 24 hours was collected every 48 hours. The recombinant fragments ERF-ColXVII-Col15 (Col15) and ERF-ColXVII-Ecto2 (Ecto2) were dialyzed against 50 mmol/L Tris (pH 8.6) and chromatographed on a DEAE cellulose (Whatman) column in the same buffer. Col15 did not bind to DEAE-cellulose, but a significant amount of contaminating proteins was removed by this step. In contrast, Ecto-2 bound to the DEAE cellulose column and was eluted with a linear 0–0.5 mol/L NaCl gradient. The partially purified recombinant fragments were precipitated with ethanol and dissolved in SDS-PAGE sample buffer before immunoblotting.
Production of Bacterial Fusion Proteins and Domain-Specific Antibodies
For the production of bacterial fusion proteins with the glutathione S-transferase (GST) Gene Fusion System (Amersham Pharmacia Biotech, Uppsala, Sweden), three different collagen XVII cDNA fragments were amplified from human keratinocyte mRNA by Titan reverse transcriptase PCR (Boehringer Mannheim), using sense primers with BamHI and antisense primers with EcoRI restriction sites (underlined). The fusion protein GST-ColXVII-Col15-2 spanning the amino acids 774–807 was amplified with the sense primer 5′-GCGCGGATCCGACCCAGGAAAGCCAGGT-3′; the antisense primer 5′-GCGCGGAATTCACTTGCCTGGAGCTCC-3. GST-ColXVII-Ecto3, corresponding to amino acids 1365–1413, was amplified with sense primer 5′-GCGGGATCCGCTGACTTTGCTGGAGATCT-3′; and antisense primer 5′-CGCGGAATTCAGCTGATGCCGGGTGGCCC-3′ (modified from Ref. 11 ) and GST-ColXVII-NC16a (NC16a), corresponding to amino acids 490–566, were amplified with the sense primer 5′-GCGCGGATCCGAGGAGGTGAGGAAGCTGAA-3′ and the antisense primer 5′-GCGCGGAATTCAT CCTCGGAGATTTCCATT-3′. The fragments were cloned into the 3′ end of the GST gene in the expression vector pGEX-2T (Amersham Pharmacia Biotech), and dideoxynucleotide sequence analysis was performed. The constructs were expressed in Escherichia coli DH5α, and the recombinant proteins were purified using the GST-glutathione affinity system according to the manufacturer’s instructions (Amersham Pharmacia Biotech).
Rabbits were immunized with the GST-fusion proteins, using standard immunization protocols (Eurogentec, Ougrée, Belgium), and the specificities of high-titer antisera Ab-ColXVII-NC16a, Ab-ColXVII-Col15-2, and Ab-ColXVII-Ecto3 were tested with immunoblotting and immunofluorescence methods. The chicken antibody Ab-ColXVII-Ecto1 has been described previously. 1
Immunoblotting and Autoantibody Detection
For immunoblotting, collagen XVII, the 120-kd shed ectodomain, and the recombinant fragments were concentrated by precipitation with either 70% ethanol or with 10% trichloroacetic acid, neutralized, and dissolved in the SDS-PAGE sample buffer containing 1 mmol/L dithiothreitol. The proteins were separated on SDS-PAGE, using gels with 4.5–15% or with 6–22% polyacrylamide gradients, and transferred onto nitrocellulose. The human autoimmune and control sera were diluted by 1:20, and the rabbit and chicken anti-collagen XVII antibodies 1 were diluted by 1:100–1:000. The incubations with the first antibodies were allowed to proceed overnight, and incubations with the alkaline phosphatase-linked anti-rabbit, -chick, or -human second antibodies (Sigma) lasted for 1.5 h. For detection of IgG and IgA autoantibodies, alkaline phosphatase-labeled, chain-specific goat anti-human IgG and IgA antibodies were used (Sigma).
Results
The Physiologically Shed Ectodomain of Collagen XVII Is an Autoantigen
Collagen XVII and its ectodomain were isolated from keratinocyte cultures as described previously. 1 In addition, we found that under physiological conditions the ectodomain was shed into the epidermal basement membrane zone, because it could be extracted with EDTA and a neutral salt buffer from normal human epidermis (Figure 2) ▶ . Interestingly, amniotic fluid and, under pathological conditions, pemphigoid blister fluid, also contained this form of collagen XVII (Figure 2) ▶ . This authentic ectodomain was recognized by circulating IgG and IgA autoantibodies from patients with blistering skin disorders (Figure 3) ▶ . Several strategies were used to exclude the possibility that the 120-kd band recognized by patient autoantibodies in immunoblots contained other contaminating, antigenic proteins in addition to the ectodomain. First, the shed form of collagen XVII was immunoprecipitated from keratinocyte medium with antibodies raised against the recombinant NC16a domain of collagen XVII (see Figure 1 ▶ ) and then cross-blotted with the patient sera. The immunoprecipitated, 120-kd polypeptide was recognized by IgG and IgA in the sera, indicating that the band targeted by human autoantibodies indeed represented the soluble ectodomain of collagen XVII (Figure 3A) ▶ . Second, as a control, collagen XVII was immunoprecipitated from cultured keratinocytes of a patient with generalized atrophic benign epidermolysis bullosa (GABEB) ( Refs. 6, 36 ) who was a COL17A1 nullizygote. The compound heterozygous mutations R1226X and L855X resulted in premature termination codons and lack of collagen XVII synthesis. 37 In this case, none of the patient sera recognized a 120-kd band (Figure 3A) ▶ , confirming that the immunoreactive 120-kd polypeptide was eliminated by COL17A1 nonsense mutations. Third, treatment of the antigen preparation with a highly purified collagenase abolished the reactivity of human IgG and IgA autoantibodies and of rabbit anti-collagen XVII antibodies with the 120-kd polypeptide, indicating that the antigen had a collagenous structure (Figure 3B) ▶ . Deglycosylation of the ectodomain with N-glycosidase F accelerated its mobility on SDS-PAGE but did not abolish the reactivity with the autoantibodies (Figure 3C) ▶ .
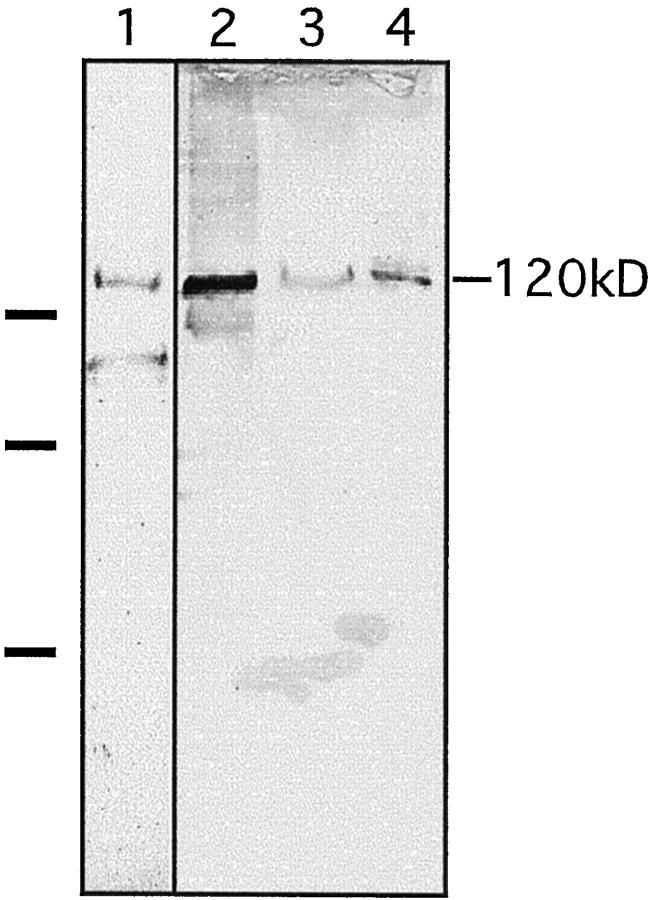
Figure 2.
Identification of the 120-kd soluble ectodomain of collagen XVII in amniotic fluid and pemphigoid blister fluid. Immunoblotting of the shed ectodomain extracted from the epidermis (lane 1) and from keratinocyte culture medium (lane 2). Antibodies used for the blot were anti-NC16a in lane 1 and Ecto1 in lane 2. The soluble ectodomain was immunoprecipitated with the anti-NC16a antibody from amniotic fluid (lane 3) and bullous pemphigoid blister fluid (lane 4) and immunoblotted with the Ecto1 antibody. On the left, the migration positions of molecular mass markers are indicated: from top to bottom 112, 80, and 50 kd. The lower band of approximately 90 kd in lane 1 represents a degradation product of the shed ectodomain. 1
Figure 3.
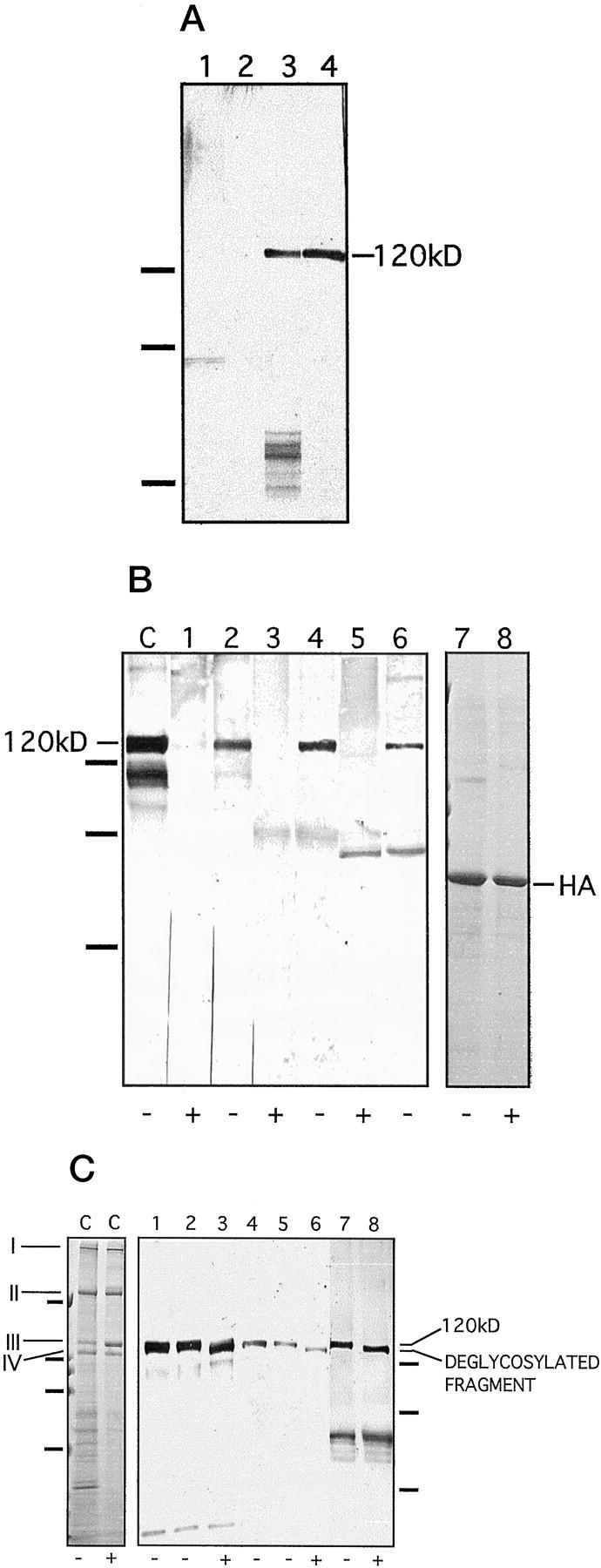
Characterization of the soluble ectodomain as an antigen. A: The soluble ectodomain was immunoprecipitated from keratinocyte medium with the NC16a antibody and cross-blotted with patient sera. Lane 1: Medium of mutant, collagen XVII-deficient keratinocytes, blotted with IgG antibodies from a pemphigoid serum. Lane 2: Medium of mutant, collagen XVII-deficient keratinocytes, blotted with IgA antibodies form an LAD serum. Lane 3: Normal keratinocyte medium, blotted with a LAD serum. Lane 4: Normal keratinocyte medium, blotted with a pemphigoid serum. The absence of the 120-kd band in the samples from collagen XVII-deficient keratinocyte cultures verifies its identity as the soluble ectodomain. On the left, migration positions of molecular weight markers are indicated. From top to bottom: 112, 80, and 50 kd. B: Immunoblotting of the soluble ectodomain treated with (lanes 1, 3, and 5) or without (lanes 2, 4, and 6) highly purified bacterial collagenase under identical conditions. The collagenase digestion abolished the 120-kd band detected with the NC16a antibody (lanes 1 and 2), with pemphigoid serum 1803/95 (lanes 3 and 4), and with a LAD serum 44/98 (lanes 5 and 6). C: an unincubated, concentrated ectodomain control. In this lane, the lower bands of approximately 105 and 90 kd represent degradation products of the shed ectodomain, as described previously. 1 As a control for nonspecific proteolytic activity in the collagenase preparation, human albumin (HA) was incubated without (lane 7) or with (lane 8) collagenase conditions identical to those as above. The enzyme treatment did not affect the protein, proving the specificity of the collagenase and excluding other proteolytic activities. Lanes 7 and 8 were stained with Coomassie blue. +, with enzyme; −, without enzyme. On the left, migration positions of molecular mass markers are indicated. From top to bottom: 112, 80, and 50 kd. C: Deglycosylation with N-glycosidase F. As a control, lane C on the left shows deglycosylation of rat-tail tendon collagen I under conditions identical to those used for collagen XVII. As expected, treatment with N-glycosidase F did not result in a shift in the mobility of the γ (I), β (II), α1 (III), or α2 (IV) chains of collagen I, a protein that does not contain N-linked carbohydrates. This experiment also showed that the enzyme preparation did not have contaminating proteolytic activity. Lane C is stained with Coomassie blue. On the left, the positions of the molecular mass markers are indicated for this gel. From top to bottom: 200, 112, 80, and 50 kd. Lanes 1–8 show an immunoblot of deglycosylated soluble ectodomain of collagen XVII. The ectodomain was incubated without (lanes 2, 5, and 7) and with (lanes 3, 6, and 8) N-glycosidase F. Lanes 1 and 4 show unincubated controls. Lanes 1–3 were detected with the control antibody Ecto1, lanes 4–6 with LAD serum 39/98, and lanes 7 and 8 with the LAD serum 61/98. N-Glycosidase F digestion resulted in a shift in the mobility of the protein but did not eliminate the reactivity with the IgA autoantibodies. We have shown previously 1 that IgG antibodies recognize the ectodomain after deglycosylation. +, with enzyme; −, without enzyme. On the right, migration positions of molecular mass markers are indicated for the immunoblot. From top to bottom: 112, 80, and 50 kd.
Immunoadsorption of IgG and IgA Antibodies with the Soluble Ectodomain
The ectodomain bound to nitrocellulose was used as an affinity matrix to adsorb IgG and IgA autoantibodies from patient sera. After elution from the nitrocellulose matrix, the immunoglobulins were used for indirect immunofluorescence staining of NaCl-split normal human skin or GABEB skin. Bullous pemphigoid sera contained IgG and LAD sera IgA, which stained the blister roof of NaCl-split skin (Figure 4, A and B) ▶ , indicating that the serum autoantibodies that recognized the shed ectodomain in immunoblots also reacted with it in situ. In contrast, they did not react with collagen XVII-deficient GABEB skin (Figure 4C) ▶ . As a further control, a serum from a pemphigus patient was tested in a similar manner (Figure 4D) ▶ . No immunoglobulins reactive with normal skin were adsorbed from this serum, ruling out nonspecific binding to the nitrocellulose-bound antigen. In concert, controls with normal human sera also yielded a negative staining (not shown).
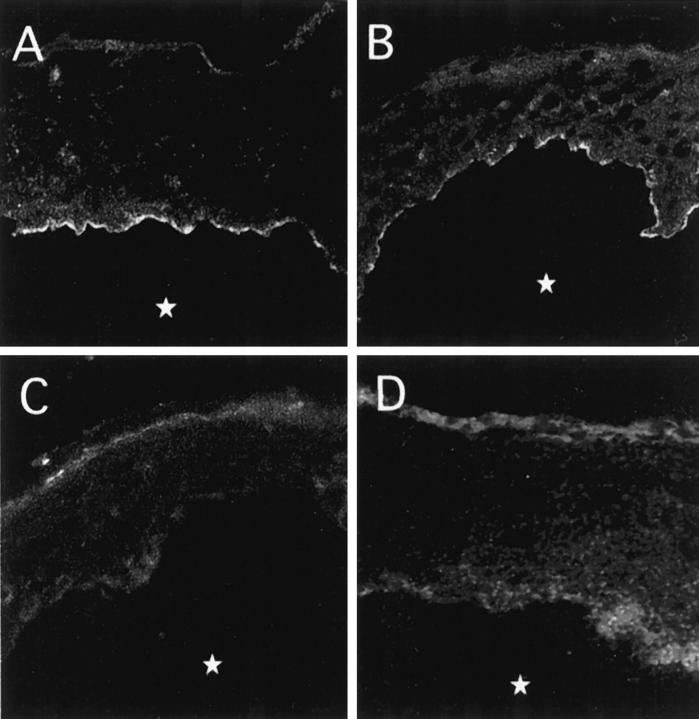
Figure 4.
Indirect immunofluorescence staining with autoantibodies immunoadsorbed with the soluble ectodomain. Pemphigoid and LAD sera, and a pemphigus vulgaris serum as a control, were immunoadsorbed with the soluble ectodomain bound to a solid phase and used for indirect immunofluorescence staining of NaCl-split normal human skin. IgG autoantibodies from a pemphigoid serum (A) and IgA autoantibodies from a LAD serum (B) stained the blister roof. C: The IgG autoantibodies from the pemphigoid serum used in A showed a negative reaction on collagen XVII-deficient skin from a GABEB patient. D: From pemphigus vulgaris serum, no antibodies reactive with human skin were immunoadsorbed with the 120-kd ectodomain. In all panels, the asterisk indicates the blister cavity.
IgG and IgA Reactivity with Collagen XVII and the Soluble Ectodomain
Sera of 50 patients with a pemphigoid disease were included in the study (Table 1 ▶ and Figure 5 ▶ ). Specifically, 43 patients had bullous pemphigoid, six cicatricial pemphigoid, and one gestational pemphigoid. Of the bullous pemphigoid patients, 34 (79%) showed IgG reactivity with the full-length 180-kd α1(XVII)-chain on immunoblots. Twenty-nine of the positive sera (85%) also recognized the 120 kd soluble ectodomain. All six patients with cicatricial pemphigoid had IgG autoantibodies to both the full-length collagen XVII molecule and the soluble ectodomain. In contrast, the serum from the individual with gestational pemphigoid showed no reactivity in these assays. Taken together, 35 of 50 patients with a blistering disease of the pemphigoid group (70%) had IgG antibodies targeting the shed ectodomain of collagen XVII. All of these patients showed a positive direct immunofluorescence staining of the skin, and 22 a positive indirect immunofluorescence staining result. Interestingly, bullous pemphigoid blister fluid also contained IgG reactive with both the full-length α1(XVII) chain and the 120-kd ectodomain of collagen XVII (Figure 5, A and B) ▶ .
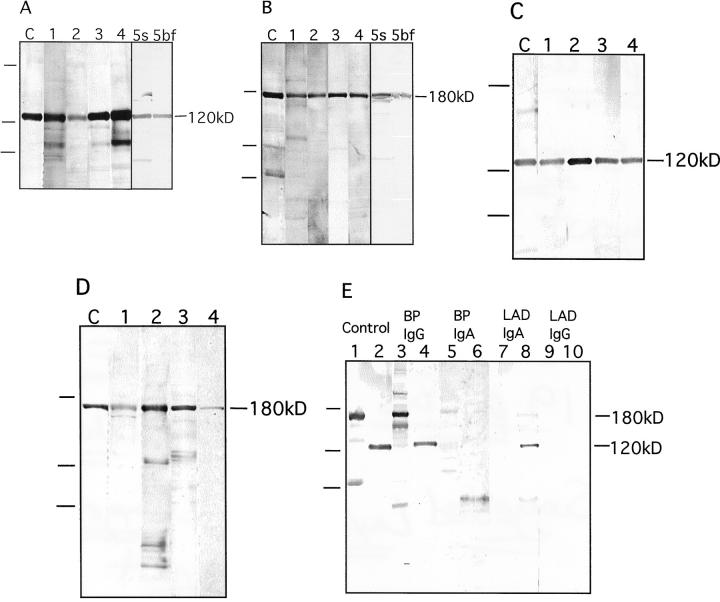
Figure 5.
IgG and IgA autoantibody reactivity with two forms of collagen XVII. A: IgG reactivity with the 120-kd soluble ectodomain of collagen XVII. Lane C: Control antibody Ecto1. Lanes 1–4 show reactivity with four different bullous pemphigoid sera. Serum (lane 5s) and blister fluid (lane 5bf) of yet another bullous pemphigoid patient contained similar amounts of autoantibodies. B: IgG reactivity with 180-kd full-length collagen XVII. Lane C: Control antibody Ecto1. Lanes 1–4 show reactivity with four different bullous pemphigoid sera. Autoantibodies in serum (lane 5s) or in blister fluid (lane 5bf) of yet another bullous pemphigoid patient contained similar amounts of autoantibodies. C: IgA reactivity with the 120-kd soluble ectodomain of collagen XVII. Lane C: Control antibody Ecto1. Lane 1: A CBDC serum. Lanes 2–4: Three different LAD sera. D: IgA reactivity with 180-kd full-length collagen XVII. Lane C: Control antibody Ecto1. Lane 1: A CBDC serum. Lanes 2–4: Three different LAD sera (not the same sera as in C). On the left of the panels, migration positions of molecular weight mass are indicated. From top to bottom: 200, 112, and 80 kd. E: Specificity of the IgG or IgA autoantibody response in the patient serum was tested using chain-specific secondary antibodies, and full-length collagen XVII (lanes 1, 3, 5, 7, and 9) and the soluble ectodomain (lanes 2, 4, 6, 8, and 10) as antigens. The following antibodies were used: control antibody Ecto1 (lanes 1 and 2), bullous pemphigoid serum with anti-human IgG second antibodies (lanes 3 and 4), and anti-human IgA second antibodies (lanes 5 and 6); LAD serum with anti-human IgA second antibodies (lanes 7 and 8) and anti-human IgG second antibodies (lanes 9 and 10). The tests showed that the pemphigoid sera contained practically no IgA reactivity and the LAD sera no IgG reactivity. On the left, migration positions of molecular weight mass are indicated. From top to bottom: 200, 112, and 80 kd.
In addition, sera of 31 patients with linear IgA deposits at the dermal-epidermal junction and subepidermal blistering were studied (Table 1) ▶ . Of the 24 LAD sera, nine (38%) reacted with full-length collagen XVII, and 12 (50%) with the 120-kd soluble ectodomain. Of the 12 patients with antibodies to the shed ectodomain, 10 had a positive indirect immunofluorescence staining result. Interestingly, of the seven CBDC sera, all were positive with indirect immunofluorescence staining and all recognized the soluble form of collagen XVII, but only three recognized the full-length 180-kd α1(XVII) chain (Figure 5, C and D) ▶ , suggesting that shedding had enhanced the immunoreactivity of the molecule.
To exclude false positive results due to cross-reactivity between IgG and IgA autoantibodies present in the same serum sample, separate immunoblots were performed with pemphigoid and LAD sera as first antibodies, and chain-specific anti-human IgG or anti-human IgA immunoglobulins as secondary antibodies. The blots demonstrated that most pemphigoid sera contained only IgG, and not IgA, antibodies to collagen XVII, and, in contrast, most LAD sera contained only IgA, and not IgG antibodies, to both forms of collagen XVII (Figure 5E) ▶ .
As controls, sera from 44 patients with pemphigus and from 30 individuals with dermatitis herpetiformis or random, nonblistering skin diseases were incubated with the collagen XVII-derived antigens. Two of the 44 pemphigus patients had circulating autoantibodies weakly reactive with collagen XVII. Both sera recognized the full-length molecule; one of them also recognized the shed ectodomain. Careful analysis of the clinical history of these patients revealed that the first had pemphigus erythematosus, with IgG deposits both in the intercellular space of the epidermis and at the basement membrane, the latter reflecting autoantibody binding to collagen XVII epitopes. The second patient suffered from a severe pemphigus vulgaris with a recalcitrant, long course of the disease and with numerous extensive relapses. Indirect immunofluorescence staining of this patient’s skin did not reveal immunoglobulin deposits at the basement membrane zone. The positive reaction in the immunoblots may reflect low amounts of autoantibodies to collagen XVII generated after secondary changes, as an epihenomenon, during the tenacious course of the disease. All of the other 42 pemphigus sera and 30 sera from other patients were negative (Table 1) ▶ .
Reactivity with Recombinant Collagen XVII Fragments
For epitope mapping, recombinant fragments spanning the ectodomain of human collagen XVII were used. The eucaryotic fragments ERF-ColXVII-Col15 corresponding to Col15, the largest collagenous domain of 241 amino acids, and ERF-ColXVII-Ecto2, spanning the 324 most carboxy-terminal amino acids of the molecule (Figure 1) ▶ , were produced in human embryonic 293 EBNA cells. The bacterial fusion protein GST-ColXVII-NC16a (Figure 1) ▶ corresponds to the 76-amino acid NC16a domain, which has been reported to contain the immunodominant epitopes. Domain-specific control antibodies were produced by immunizing rabbits with bacterial GST fusion proteins. The antibodies to GST-ColXVII-NC16a, GST-ColXVII-Col15-2, and GST-ColXVII-Ecto3 all showed a highly specific response to the full-length collagen XVII, the ectodomain, and the corresponding recombinant fragments (see Figures 2 and 6 ▶ ▶ ).
Figure 6.
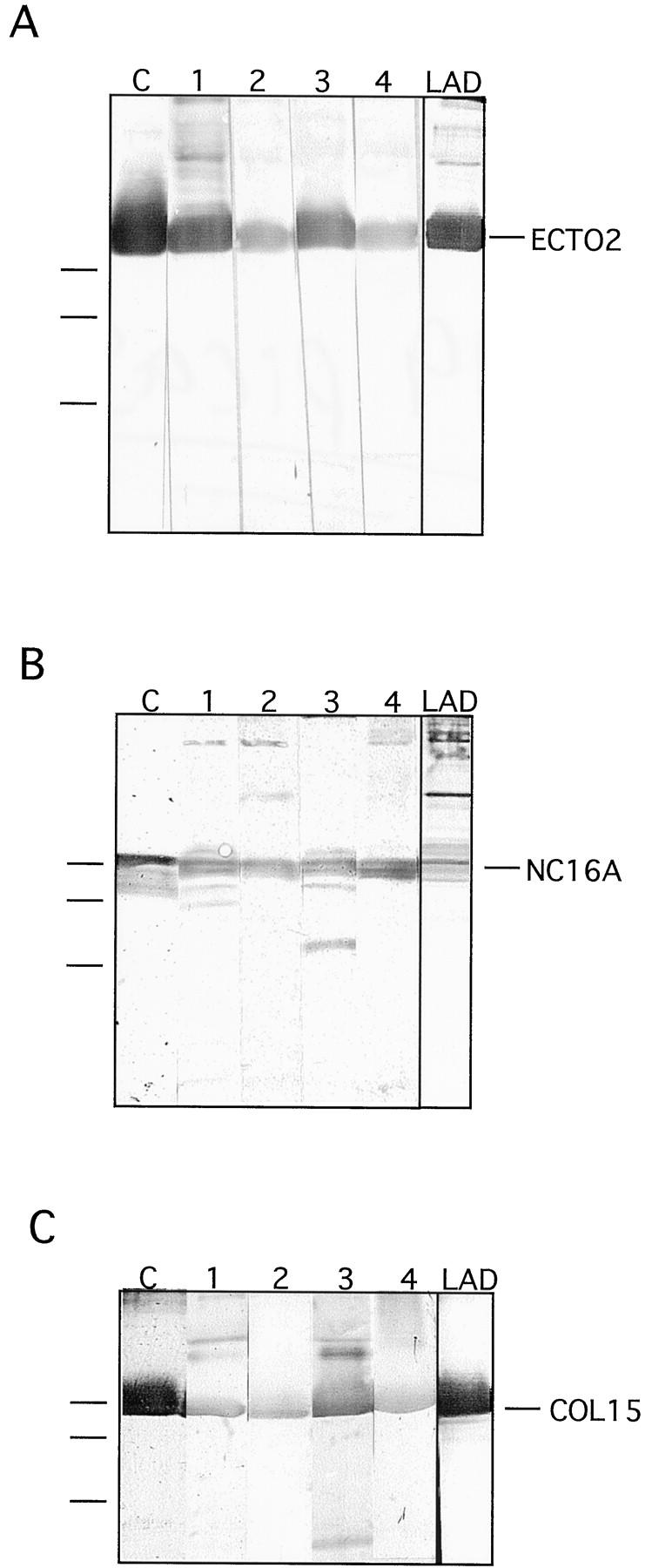
Immunoblotting of IgG and IgA autoantibodies with recombinant collagen XVII fragments. Recombinant fragments spanning the Ecto2 (A), NC16a (B), and Col15 domains (C) were reacted with different bullous pemphigoid sera and with a LAD serum in immunoblots. In each blot, C designates the lane reacted with a control antibody raised against the recombinant fragment. Lanes 1–4 show reactions with different pemphigoid sera, and LAD indicates a reaction with a LAD serum. On the left of the panels, migration positions of molecular mass markers are indicated. From top to bottom: 47, 34, and 28 kd.
To discern the epitopes within the soluble ectodomain of collagen XVII, all patient sera positive with full-length collagen XVII were reacted with the above recombinant fragments (Table 1 ▶ and Figure 6 ▶ ). Twenty-one bullous pemphigoid sera had IgG autoantibodies reactive with the NC16a domain, 11 with the Col15 domain, and 17 with the Ecto2 fragment. Of these, six sera recognized epitopes within all three fragments, 14 in two, and 21 in only one fragment. One pemphigoid serum that recognized both full-length collagen XVII and the soluble ectodomain remained negative with the recombinant fragments. Interestingly, six bullous pemphigoid sera reacted with full-length collagen XVII but not with the soluble ectodomain. Of these, two recognized the NC16a domain but four none of the fragments. Cicatricial pemphigoid sera showed a different reactivity pattern. All six sera recognized both full-length and soluble forms of collagen XVII. However, only one had epitopes in the NC-16a domain, in parallel with the Col15 domain. The other sera had epitopes either in the Col15 domain (two sera) or within the Ecto2 segment (three sera).
The LAD and CBDC sera reactive with the soluble ectodomain were also tested for IgA reactivity to the recombinant collagen XVII fragments (Figure 6) ▶ . Of the LAD sera, two recognized NC16a, two Col15, and one Ecto2 (Table 1) ▶ . One of the sera reacted with two fragments, namely with NC16a and with Col15. Of the seven CBDC sera, only one reacted with the recombinant fragments, specifically with Col15 (Table 1) ▶ . Eleven control sera from individuals with unrelated autoimmune dermatoses or random nonblistering skin diseases such as eczema or psoriasis did not have IgA reactivity with any of the above collagen XVII-related antigens.
Discussion
Here we showed that the 120-kd shed ectodomain of collagen XVII is present in salt extracts 33 of normal epidermis, in amniotic fluid, and in pemphigoid blister fluid, pointing to both physiological and etiopathogenetic relevance of the shedding. However, despite a large body of data generated with help of protein extracts and of different recombinant collagen XVII/BP180 fragments, 11-15,17-22 it has remained unclear whether the native, shed ectodomain is recognized as an autoantigen in human blistering skin diseases. In this study, by using the authentic soluble ectodomain for analysis of sera from 81 patients with an active blistering disease and with IgG or IgA autoantibodies, we unambiguously demonstrated that this is the case and that some autoantibodies preferentially target this form of collagen XVII. The identity of the antigen was established with several strategies: 1) immunoprecipitation with domain-specific antibodies raised against recombinant fragments, 2) treatment with highly purified collagenase, and 3) use of collagen XVII-deficient keratinocytes as controls. Circulating IgG and IgA autoantibodies were immunopurified against the ectodomain and shown to bind to the blister roof of NaCl-split skin, indicating that they also recognized the antigen in situ. The specificity of the immunopurified autoantibodies was demonstrated by the fact that they did not react with collagen XVII-deficient skin.
The keratinocyte-associated target antigens in bullous pemphigoid and in IgA diseases are still incompletely understood. A number of reports have produced circumstantial evidence for the similarity of 97-kd, 120–125-kd, and 180-kd basement membrane proteins 12,19,20,23-27,29,38 , and because Zone et al 30 showed that the epidermal 97-kd LAD antigen exhibited partial amino acid sequence identity with the extracellular domain of collagen XVII, many investigators have assumed that the 120-kd LAD antigen and its 97-kd derivative 28 represent proteolytic fragments of collagen XVII. However, stringent molecular evidence proving this assumption has not been published. In addition, despite similarities of the antigens, the lack of systematic cross-reactivity of LAD patient sera or monoclonal antibodies with the 97-kd/120-kd protein and with collagen XVII remains puzzling. In this study we showed that, at least in some cases, the 120-kd antigen targeted in IgA dermatoses is the soluble ectodomain of collagen XVII. In fact, 50% of our LAD sera and 100% of CBDC sera recognized it. In this context, these data also support the hypothesis that CBDC represents a childhood counterpart of adult LAD, 33 because sera from the two disease groups recognized the same antigens.
Seventy-nine percent of the bullous pemphigoid sera and all cicatricial pemphigoid sera recognized collagen XVII in this study. These numbers are in general agreement with other immunoblot studies on autoantibody reactivity with collagen XVII. 13,17,20,23 In addition, we showed that 58% of the bullous pemphigoid sera and all cicatricial pemphigoid sera also reacted with the authentic soluble ectodomain. The sera were obtained at early stages of the autoimmune blistering diseases when the patients had clinical manifestations but no immunosuppressive treatment. Not included in the present series, but examined in parallel, were six patients with bullous pemphigoid and three with cicatricial pemphigoid who had undergone long-term (6 weeks to 2 years) immunosuppressive therapy with steroids, azathioprine, and/or endoxan and who were free of clinical manifestations at the time of the test. Despite the fact that eight of the nine patients still had some tissue-bound immunoglobulins demonstrable by direct immunofluorescence staining of the skin, only one showed autoantibody reactivity to both forms of collagen XVII in immunoblots, presumably reflecting the dormant stage of the disease. Four other individuals with an immunosuppressive therapy regimen of less than 2 weeks’ duration and five pemphigoid patients under immunosuppressive therapy but with a current relapse were also tested. All nine had a positive direct immunofluorescence staining of the skin, and six of nine had a positive indirect immunofluorescence staining result. All had circulating autoantibodies reactive with both forms of collagen XVII in immunoblots. These preliminary observations suggest that future studies on the correlation of autoantibodies to both collagen XVII forms with the therapy and the course of the disease may be of interest.
A novel, intriguing aspect of the autoantibody response to collagen XVII revealed here is the fact that some sera react preferentially with the shed ectodomain. In contrast to bullous pemphigoid, all cicatricial pemphigoid sera recognized the soluble ectodomain. Even more explicitly, significantly more IgA sera reacted with the authentic shed ectodomain than with the full-length molecule. Also, a number of sera targeted epitopes in several regions of the molecule, indicating that at least some patients have multiple epitopes spread along the full-length or soluble collagen XVII forms, an aspect that is important for the design of diagnostic tests for these diseases. As shown in Table 1 ▶ , neither full-length collagen XVII nor the recombinant fragments were as sensitive as the authentic shed ectodomain for detection of the autoantibodies, and, therefore, including this form of collagen XVII in the test panels should be considered.
The better reactivity of the cicatricial pemphigoid sera and of certain LAD sera with the physiologically shed ectodomain than with the full-length molecule suggests that the release of collagen XVII from the cell surface exposes novel epitopes. Neoepitopes are known to be generated by proteolytic processing of a wide spectrum of biologically active and disease-relevant proteins, such as plasminogen activator inhibitor type 2, 39 IL-1 receptor, 40 or the extracellular matrix proteins collagen II and aggrecan. Matrix metalloproteinase-1, -8, and -13 cleave collagen II in osteoarthritic cartilage, thereby generating neoepitopes not observed in intact collagen. 41 Cleavage by aggrecanase of the peptide bond Asn341-Phe342 in aggrecan generates a neoepitope VDIPEN341, and monoclonal antibodies to this neoepitope can be used to assess the extent of cartilage destruction during inflammatory arthritis. 42 Therefore, shedding-induced generation of neoepitopes in the collagen XVII ectodomain is conceivable; further studies are ongoing in our laboratories to identify potential neoepitopes.
Finally, we identified the Col15 domain as a hitherto unrecognized epitope region. Previously, the NC16a domain was thought to contain the immunodominant epitopes in bullous pemphigoid, 14,15,17,18 and the carboxy terminus was thought to contain the major epitopes in cicatricial pemphigoid. 11,21,43 Here we detected Col15 domain antibodies in 32% of positive bullous pemphigoid sera, 50% of positive cicatricial pemphigoid sera, 17% of positive LAD sera, and 14% of positive CBDC sera, indicating that this segment may be highly relevant for diagnostic tests with recombinant antigens. In IgA diseases, different epitopes have been reported, 27 and in agreement with these findings, two sera here contained IgA strongly reactive with the recombinant Col15 fragments, and one serum contained IgA strongly reactive with the carboxy-terminal Ecto2. The binding of antibodies to the Col15 domain may be of importance in maintaining blistering, because we have recently observed that Col15 domain of collagen XVII has potential for cell adhesion properties in vitro (K. Tasanen, M. Aumailley, and L. Bruckner-Tuderman, unpublished observation).
Acknowledgments
The authors thank Margit Schubert and Andrea Wissel for expert technical assistance.
Footnotes
Address reprint requests to Dr. Leena Bruckner-Tuderman, Department of Dermatology, University of Münster, Von-Esmarch-Strasse 56, 48149 Münster, Germany. E-mail: tuderma@uni-muenster.de.
Supported by grants from the Alexander von Humboldt Foundation and from the Academy of Finland to K. T., by the Siegfried Roggenbuck-Stiftung to Ha. S., by grants Br 1475/1-2 and SFB 293 Muenster/B3 from the Deutsche Forschungsgemeinschaft and EU contract BMH4-CT97–2062 to L. B.-T., and by the Dunhill Medical Trust to F. W. and by grant 98.073.1 from the Wilhelm-Sander-Foundation to D.Z.
References
- 1.Schäcke H, Schumann H, Hammami-Hauasli N, Raghunath M, Bruckner-Tuderman L: Two forms of collagen XVII in keratinocytes. A full-length transmembrane protein and a soluble ectodomain. J Biol Chem 1998, 273:25937-25943 [DOI] [PubMed] [Google Scholar]
- 2.Nishizawa Y, Uematsu J, Owaribe K: HD4, a 180kD bullous pemphigoid antigen is a major transmembrane glycoprotein of the hemidesmosomes. J Biochem (Tokyo) 1993, 113:493-501 [DOI] [PubMed] [Google Scholar]
- 3.Giudice GJ, Emery D, Diaz LA: Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol 1992, 99:243-250 [DOI] [PubMed] [Google Scholar]
- 4.Li K, Tamai K, Tan EML, Uitto J: Cloning of type XVII collagen. J Biol Chem 1993, 12:8825-8834 [PubMed] [Google Scholar]
- 5.Hirako Y, Usukura J, Nishizawa Y, Owaribe K: Demonstration of the molecular shape of BP180, a 180 kd bullous pemphigoid antigen and its potential for trimer formation. J Biol Chem 1996, 271:13739-13745 [DOI] [PubMed] [Google Scholar]
- 6.Gatalica B, Pulkkinen L, Li K, Kuokkanen K, Ryynänen M, McGrath J, Uitto J: Cloning of the human type XVII collagen gene (COL17A1), and detection of novel mutations in generalized atrophic benign epidermolysis bullosa. Am J Hum Genet 1997, 60:352-365 [PMC free article] [PubMed] [Google Scholar]
- 7.Balding SD, Diaz LA, Giudice GJ: A recombinant form of the human BP180 ectodomain forms a collagen-like homotrimeric complex. Biochemistry 1997, 36:8821-8830 [DOI] [PubMed] [Google Scholar]
- 8.Borradori L, Koch PJ, Niessen CM, Erkeland S, van Leusden MR, Sonnenberg A: The localization of bullous pemphigoid antigen 180 (BP180) in hemidesmosomes is mediated by its cytoplasmic domain and seems to be regulated by the β4 integrin subunit. J Cell Biol 1997, 136:1333-1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirako Y, Usukura J, Uematsu J, Hashimoto T, Kitajima Y, Owaribe K: Cleavage of BP180, a 180-kd bullous pemphigoid antigen, yields a 120-kd collagenous extracellular polypeptide. J Biol Chem 1998, 273:9711-9717 [DOI] [PubMed] [Google Scholar]
- 10.Stanley J: Autoantibodies against adhesion molecules and structures in blistering skin diseases. J Exp Med 1995, 181:1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balding SD, Prost C, Diaz LA, Bernard P, Bedane C, Aberdam D, Giudice GJ: Cicatricial pemphigoid autoantibodies react with multiple sites on the BP180 extracellular domain. J Invest Dermatol 1996, 106:141-146 [DOI] [PubMed] [Google Scholar]
- 12.Ghohestani RF, Nicolas JF, Kanitakis J, Claudy A: Linear IgA bullous dermatosis with IgA autoantibodies exclusively directed against the 180- or 230-kD epidermal antigens. J Invest Dermatol 1997, 108:854-858 [DOI] [PubMed] [Google Scholar]
- 13.Haase C, Büdinger L, Borradori L, Yee C, Merk HF, Yancey K, Hertl M: Detection of IgG autoantibodies in the sera of patients with bullous and gestational pemphigoid: ELISA studies utilizing a baculovirus-encoded form of bullous pemphigoid antigen 2. J Invest Dermatol 1998, 110:282-286 [DOI] [PubMed] [Google Scholar]
- 14.Zillikens D, Pose PA, Balding SD, Liu Z, Olague-Marchan M, Diaz LA, Giudice GJ: Tight clustering of extracellular BP180 epitopes recognized by bullous pemphigoid autoantibodies. J Invest Dermatol 1997, 109:573-579 [DOI] [PubMed] [Google Scholar]
- 15.Zillikens D, Mascaro JM, Rose PA, Liu Z: A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol 1997, 109:679-683 [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Diaz LA, Troy JL, Taylor A, Emery D, Fairley J, Giudice GJ: A passive transfer model of the organ specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest 1993, 92:2480-2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumara K, Amagai M, Nishikawa T: The majority of bullous pemphigoid and herpes gestationes serum samples react with the NC16A domain of 180 kd bullous pemphigoid antigen. Arch Dermatol Res 1996, 288:507-509 [DOI] [PubMed] [Google Scholar]
- 18.Nakatani C, Muramatsu T, Shirai T: Immunoreactivity of bullous pemphigoid (BP) autoantibodies against the NC16A and C-terminal domains of the 180 kd BP antigen (BP180): immunoblot analysis and enzyme-linked immunosorbent assay using BP180 recombinant proteins. Br J Dermatol 1998, 139:365-370 [DOI] [PubMed] [Google Scholar]
- 19.Egan CA, Taylor TB, Meyer LJ, Peterson MJ, Zone JJ: Bullous pemphigoid sera that contain antibodies to BPAg2 also contain antibodies to LABD97 that recognize epitopes distal to the NC16A domain. J Invest Dermatol 1999, 112:148-152 [DOI] [PubMed] [Google Scholar]
- 20.Perriard J, Jaunin F, Favre B, Büdinger L, Hertl M, Saurat J-H, Borradori L: IgG autoantibodies from bullous pemphigoid (BP) patients bind antigenic sites on both the extracellular and the intracellular domains of the BP antigen 180. J Invest Dermatol 1999, 112:141-147 [DOI] [PubMed] [Google Scholar]
- 21.Nie Z, Hashimoto T: IgA antibodies of cicatricial pemphigoid sera specifically react with C-terminus of BP180. J Invest Dermatol 1999, 112:254-255 [DOI] [PubMed] [Google Scholar]
- 22.Gao SQ, Bystryn DJ: A novel bullous pemphigoid antigen (BP125) located in the deeper layers of the basement membrane zone. Arch Dermatol 1994, 130:873-878 [PubMed] [Google Scholar]
- 23.Pas HH, Kloosterhuis GJ, Heeres K, van der Meer JB, Jonkman MF: Bullous pemphigoid and linear IgA disease sera recognize a similar 120-kd keratinocyte collagenous glycoprotein with antigenic cross-reactivity to BP180. J Invest Dermatol 1997, 108:423-429 [DOI] [PubMed] [Google Scholar]
- 24.Pas HH, Kloosterhuis GJ, Nijenhuis M, De Jong MCJM, van der Meer JB, Jonkman MF: Type XVII collagen (BP180) and LAD-1 are present as separate trimeric complexes. J Invest Dermatol 1999, 112:58-61 [DOI] [PubMed] [Google Scholar]
- 25.Wojnarowska F, Whitehead P, Leigh IM, Bhogal BS, Black M: Identification of the target antigen in chronic bullous disease of the childhood and linear IgA disease of adults. Br J Dermatol 1991, 124:157-162 [DOI] [PubMed] [Google Scholar]
- 26.Zone JJ, Taylor TB, Kandunce DP, Meyer LJ: Identification of the cutaneous basement membrane antigen in linear IgA bullous dermatosis. J Clin Invest 1990, 85:812-820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou S, Ferguson DJP, Allen J, Wojnarowska F: The localization of target antigens and autoantibodies in linear IgA disease is variable: correlation of immunogold electron microscopy and immunoblotting. Br J Dermatol 1998, 139:591-597 [DOI] [PubMed] [Google Scholar]
- 28.Marinkovich MP, Taylor TB, Keene DR, Burgeson RE, Zone JJ: LAD-1, the linear IgA bullous disease autoantigen, is a novel 120-kd anchoring filament protein synthesized by epidermal cells. J Invest Dermatol 1996, 106:734-738 [DOI] [PubMed] [Google Scholar]
- 29.Collier P, Wojnarowska F, Allen J, Kirtschig G: Molecular overlap of the IgA target antigens in the subepidermal blistering diseases. Dermatology 1994, 189(Suppl 1):105-107 [DOI] [PubMed] [Google Scholar]
- 30.Zone JJ, Taylor TB, Meyer LJ, Petersen MJ: The 97 kd linear IgA bullous disease antigen is identical to a portion of the extracellular domain of the 180 kd bullous pemphigoid antigen, BPAg2. J Invest Dermatol 1998, 110:207-210 [DOI] [PubMed] [Google Scholar]
- 31.Gammon WR, Fine J-D, Forbes M, Briggaman A: Immunofluorescence on split skin for the detection and differentation of basement membrane zone autoantibodies. J Am Acad Dermatol 1992, 27:79-87 [DOI] [PubMed] [Google Scholar]
- 32.Prockop DJ, Kivirikko KI: Collagen: molecular biology, diseases and potentials for therapy. Annu Rev Biochem 1995, 64:403-434 [DOI] [PubMed] [Google Scholar]
- 33.Zone JJ, Taylor TB, Kadunce D: IgA autoantibodies in chronic bullous disease of childhood react with a 97 kD basement membrane protein. J Invest Dermatol 1996, 106:1277-1280 [DOI] [PubMed] [Google Scholar]
- 34.Bruckner-Tuderman L, Nilssen Ö, Zimmermann D, Dours-Zimmermann M-T, Kalinke UD, Gedde-Dahl T Jr, Winberg J-O: Immunohistochemical and mutation analysis demonstrate that procollagen VII is processed to collagen VII through removal of the NC-2 domain. J Cell Biol 1995, 131:551–559 [DOI] [PMC free article] [PubMed]
- 35.Kohfeldt E, Göhring W, Mayer U, Zweckstetter M, Holak TA, Chu M-L, Timpl R: Conversion of the Kunitz-type module of collagen VI into a highly active trypsin inhibitor by site-directed mutagenesis. Eur J Biochem 1996, 238:333-340 [DOI] [PubMed] [Google Scholar]
- 36.Schumann H, Hammami-Hauasli N, Pulkkinen L, Mauviel A, Küster W, Lüthi U, Owaribe K, Uitto J, Bruckner-Tuderman L: Three novel homozygous point mutations and a new polymorphism in the COL17A1 gene: relations to biological and clinical phenotypes of junctional epidermolysis bullosa. Am J Hum Genet 1997, 60:1344-1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Floeth M, Bruckner-Tuderman L: Digenic junctional epidermolysis bullosa: mutations in COL17A1 and LAMB3 genes. Am J Hum Genet 1999, 65:1530-1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pothupitiya GM, Wojnarowska F, Bhogal B, Black MM: Distribution of the antigen in adult linear IgA disease and chronic bullous disease of the childhood suggests that it is a single and unique antigen. Br J Dermatol 1988, 118:175-182 [DOI] [PubMed] [Google Scholar]
- 39.Saunders DN, Buttigieg KM, Gould A, McPhun V, Baker MS: Immunological detection of conformational neoepitopes associated with the serpin activity of plasminogen activator inhibitor of type-2. J Biol Chem 1998, 273:10965-10971 [DOI] [PubMed] [Google Scholar]
- 40.van Meurs JB, van Lent PL, Singer II, Bayne EK, van de Loo FA, van den Berg WB: Interleukin-1 receptor antagonist prevents expression of the metalloproteinase-generated neoepitope VDIPEN in antigen-induced arthritis. Arthritis Rheum 1998, 41:647-656 [DOI] [PubMed] [Google Scholar]
- 41.Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H: Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest 1997, 99:1534-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singer II, Kawka DW, Bayne EK, Donatelli SA, Weidner JR, Williams HR, Ayala JM, Mumford RA, Lark MW, Glant TT: VDIPEN, a metalloproteinase-generated epitope, is induced and immunolocalized in articular cartilage during inflammatory arthritis. J Clin Invest 1995, 95:2178-2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schumann H, Amann U, Tasanen K, Müller S, Zillikens D, Metze D, Luger T, Bruckner-Tuderman L, Bonsmann G: A child with localized vulvar pemphigoid and IgG autoantibodies targeting the C-terminus of collagen XVII/BP180. Br J Dermatol 1999, 140:1133-1138 [DOI] [PubMed] [Google Scholar]