Abstract
The role and significance of microsatellite instability (MSI) in gastric carcinogenesis remain unknown. This study determined the chronology of MSI in gastric carcinogenesis by examining intestinal metaplasia (IM) from patients with and without gastric cancer. DNA was obtained from gastric specimens of 75 patients with gastric IM (30 cancer, 26 peptic ulcer, and 19 chronic gastritis patients) and was amplified with a set of eight microsatellite markers. Eight (26.7%) tumors and seven (9.3%) IM samples (three from cancer-free patients) displayed high-level MSI (three or more loci altered). Low-level MSI (one or two loci altered) was detected in 50% of the tumors, in 40% of IM samples coexisting with cancer, and in 38% of IM tissues of cancer-free individuals. Among the 30 cancer patients, microsatellites were more frequently altered in IM coexisting with tumors that showed MSI (P = 0.003). In addition, patients with low-level MSI in the tumor tissues were more likely to have active Helicobacter pylori infection than those with stable tumors (P = 0.02). In conclusion, this study indicates that MSI occurs not only in gastric IM of patients with gastric carcinoma, but also in IM of cancer-free individuals. These data suggest that the progressive accumulation of MSI in areas of IM may contribute to gastric cancer development, representing an important molecular event in the multistep gastric carcinogenesis cascade.
Gastric cancer is the second most frequent malignant tumor in the world 1 and contributes to significant cancer mortality, particularly in Asia. Multiple environmental factors, including Helicobacter pylori infection, 2 high salt intake, 3 N-nitroso compounds, 4 and anti-oxidant deficiency 5 in the gastric mucosa, have been implicated in the initiation of gastric carcinogenesis. These factors potentially trigger genetic changes in the host gastric epithelium, such as oncogene activation, 6 allelic loss of putative tumor suppressor genes, 7 and genetic instability, 8 which ultimately lead to cancer development.
Microsatellite instability (MSI), a form of genomic instability, was first described in hereditary nonpolyposis colorectal cancer. 9 Since then, MSI has been reported in a variety of familial and sporadic human cancers. 10 Microsatellites are ubiquitous, short, repetitive DNA sequences widely and randomly distributed throughout the human genome, with unknown function. 11 Microsatellite instability in tumors is identified when alleles of novel sizes are detected in microsatellite sequences derived from cancer DNA that are not present in normal tissues of the same individual. Gastric cancers, both familial and sporadic, have been shown to exhibit various levels of MSI (17%–59%). 7,8,10
Intestinal metaplasia (IM) is generally believed to be a preneoplastic lesion of the stomach, which is epidemiologically linked to chronic H. pylori infection. 12,13 Identification of genetic changes common to both gastric IM and carcinomas should broaden our understanding of the molecular pathways of gastric carcinogenesis. In contrast to colorectal cancer, current knowledge of these intermediate genetic changes is still superficial and largely limited to reports on mutations of p53 and APC genes in IM and dysplasia. 14,15 Recently, several studies 16-18 reported MSI in areas of IM from gastric cancer patients, suggesting that MSI may be an early event in the multistep progression of gastric carcinogenesis. However, the issue of whether foci of IM in individuals without cancer also harbor MSI was not addressed. Because most gastric cancer patients have evidence of IM in the stomach and not all patients with IM will develop cancer, this question has important biological implications and perhaps clinical applications. We examined IM tissues obtained from patients with gastric cancer and from individuals without gastric malignancy at the time of examination, to elucidate the significance and chronology of MSI in the multistep gastric carcinogenesis pathway.
Materials and Methods
Patients
Gastric tissue samples were selected from archival pathological specimens of patients undergoing upper gastrointestinal endoscopy in Guro Hospital, Seoul, Korea. Endoscopic biopsies were obtained by a standard mapping protocol that included six biopsies from corpus and six biopsies from gastric antrum. Three different groups of patients, including 30 patients with sporadic gastric cancer, 26 patients with peptic ulcer diseases (17 with gastric ulcers and 9 with duodenal ulcers), and 19 patients with chronic gastritis, were studied. All patients showed extensive metaplasia in the stomach. Ulcer patients also had evidence of chronic gastritis on histological examination.
Histological Examination
Gastric carcinomas were classified into diffuse and intestinal types as defined by Lauren. 19 For nontumor tissues (IM and control), the Genta stain 20 was used for grading of H. pylori infection, chronic and active inflammation, and IM according to the updated Sydney classification for gastritis. 21 Samples showing IM were further stained with high iron diamine/Alcian blue, pH 2.5, for subtyping as described by Filipe et al. 22 Type III or incomplete IM was diagnosed only if sulfomucin was detected in the cytoplasm of cells with absorptive cell morphology; otherwise IM was scored as a complete type. Active H. pylori infection was identified by the presence of typical organisms in any of the available gastric biopsies of the same patient by histology.
Preparation of Genomic DNA
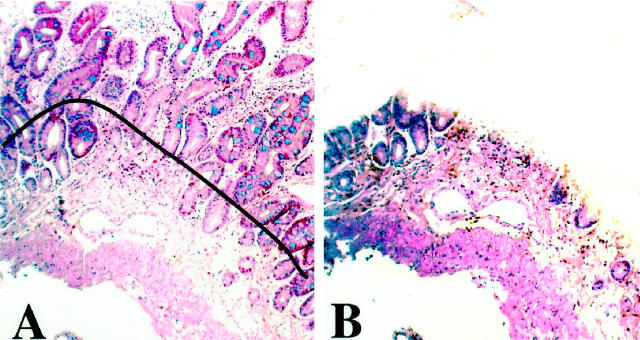
Serial 5-μm-thick sections of selected tissue blocks were obtained on glass slides, and the areas of interest were microdissected after matching with an adjacent section stained with hematoxylin and eosin or Genta stain. Microdissection was performed to enrich the DNA content from the tumor or metaplastic tissues 23 and to minimize DNA contamination by other inflammatory or stromal cell nuclei. For tumor samples, tissues were selected to contain more than 90% of the representative tumor in the section. One or two IM samples were selected from biopsy sites that contained sufficient IM tissue for microdissection (more than 70% of the gastric epithelium replaced by IM on the tissue section), and typically they were associated with only mild chronic inflammation and did not display atypical or dysplastic features (Figure 1, A and B) ▶ . IM tissues from cancer patients were chosen from an area distant from dysplasia or the tumor (embedded in different tissue blocks). Corresponding samples of gastric mucosa that were free of tumor, metaplasia, and dysplasia were obtained from each case as control tissue. Therefore, cancer patients had three sets of samples (IM, tumor, and control) whereas noncancer patients had two sets (IM and control). After tissue deparaffinization, DNA was extracted with the QIAamp Tissue Kit (Qiagen, Chatsworth, CA) following the manufacturer’s instructions.
Figure 1.
Microdissection of IM. A: Tissue section from a typical area of gastric IM stained with the Genta stain. B: The immediately adjacent matched tissue section after microdissection of the mucosa containing IM. Dissection of IM tissue for DNA extraction was performed following the contour of the black line drawn over the matching adjacent tissue section, to exclude most of the lamina propria and the muscularis mucosae.
MSI Analysis
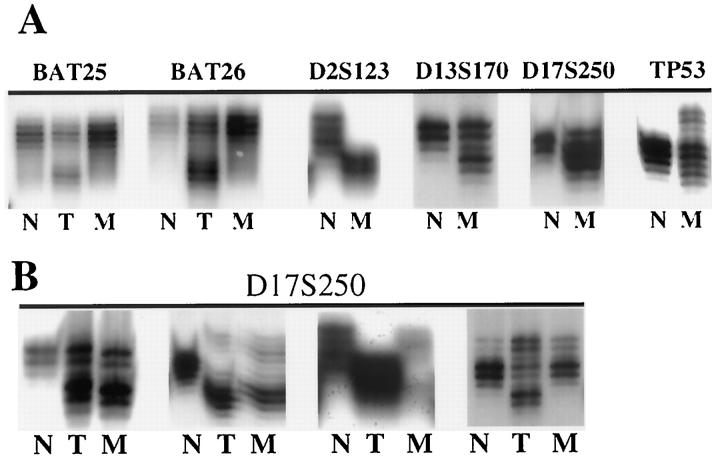
All samples were analyzed by a set of eight microsatellite markers, which included the five reference panel markers recommended by the National Cancer Institute workshop on MSI for cancer detection and familial predisposition. 23 There were three mononucleotide (A)n repeat markers (BAT25, BAT26, and BAT40) and five dinucleotide (CA)n repeat markers (D2S123, D5S346, D13S170, D17S250, and TP53). 24 Oligonucleotides were synthesized by Life Technologies Inc. (Gaitherburg, MD). One of the oligonucleotide primers was end labeled with γ-32P-labeled ATP, using T4 polynucleotide kinase (Amersham, Arlington Heights, IL). Polymerase chain reaction (PCR) amplifications were then carried out in 50-μl reaction volumes, containing 1× PCR reaction buffer (GeneAmp; Perkin-Elmer, Branchburg, NJ), 20 pmol each of deoxynucleotide triphosphate (dATP, dCTP, dTTP, and dGTP; Promega, Madison, WI), 50 pmol of both labeled and unlabeled primer, and 0.25 U of Taq polymerase (AmpliTaq Gold, Perkin Elmer). PCR products were diluted in equal volumes of formamide gel loading buffer (80% formamide, 0.1% xylene cyanol, 0.1% bromophenol blue, 2 mmol/L ethylenediaminetetraacetic acid), denatured at 95°C, and separated through 5.6 mol/L urea, 32% formamide, 7% polyacrylamide gels. 24 MSI-positive samples were confirmed by 7% polyacrylamide and 5.6 mol/L urea sequencing gels. MSI was defined as a band shift in either of the two alleles or the appearance of a band with different size in the tumor or IM sample (Figure 2) ▶ . All autoradiograms were independently interpreted by two investigators (W. K. L. and A. R. S.). In discordant cases, the opinion of a third observer was obtained. PCR was repeated in samples displaying MSI, for confirmation of the results. A specimen was defined as high-level MSI (MSI-H) if more than 30% of the markers examined showed instability, low-level MSI (MSI-L) if less than 30% of the markers displayed instability, and microsatellite stable (MSS) if none of the markers exhibited MSI. 23
Figure 2.
MSI in gastric carcinoma (T) and IM (M). A: Representative samples from different patients displaying MSI in tumor (BAT25 and BAT26) and in IM (D2S123, D13S170, D17S250, and TP53). N, Normal control. B: Samples demonstrate MSI in both tumor and IM. The two panels on the left showed similar banding patterns in tumor and IM, whereas the third panel showed a different banding pattern between tumor and IM. The panel on the right showed MSI in tumor only.
Statistical Analysis
The χ 2 or Fisher-exact test and unpaired Student’s t-test were used for analysis of most of the categorical or numerical data, respectively. In addition, the Mann-Whitney U test was used in the comparison of nonparametric differences between two groups, whereas the Kruskal-Wallis test was applied in the analysis of differences between three groups. A P value of less than 0.05 was considered statistically significant.
Results
MSI in Gastric Carcinomas
Thirty gastric cancer patients (21 males and 9 females; mean age 60.1 years, range 38–79 years) were studied. None of them had a family history of gastric cancer or hereditary nonpolyposis colorectal cancer syndrome. 25 Of all carcinomas, 21 (70%) were of the intestinal type, and 9 (30%) were of the diffuse type. Tumors were located in the distal stomach or antrum in 16 cases (53%), and active H. pylori infection was detected in 27 cases (90%).
Among the 30 tumors examined, MSI-H was detected in 8 (26.7%) and MSI-L in 15 tumor samples (50%). All MSI-H tumors were of intestinal type and had evidence of an active H. pylori infection in the stomach. Of eight tumors displaying high-level MSI, seven were located in the distal stomach (Table 1) ▶ . H. pylori infection was more frequent in patients with MSI-L than in those with MSS tumors (57% versus 100%; P = 0.02). The mean ages of patients with MSI-H, MSI-L, or MSS tumors were similar (Table 1) ▶ .
Table 1.
Characteristics of 30 Gastric Cancer Patients
| Characteristic | MSI in gastric cancer | ||
|---|---|---|---|
| MSS (n = 7) | MSI-L (n = 15) | MSI-H (n = 8) | |
| Mean age (SD) | 58.6 (13.4) | 60.2 (8.0) | 62.6 (7.3) |
| Male:female | 4:3 | 11:4 | 6:2 |
| Histological type | |||
| Intestinal | 4 | 9 | 8 |
| Diffuse | 3 | 6 | 0 |
| Tumor location | |||
| Distal | 3 | 6 | 7 |
| Proximal | 4 | 9 | 1 |
| Active H. pylori infection | 4 (57.1%)* | 15 (100%)* | 8 (100%) |
SD, standard deviation.
*P = 0.02.
MSI in IM
A total of 75 IM samples (30 from patients with gastric cancer and 45 from noncancer patients) were studied. Type III IM was found in 40% of the patients with gastric carcinoma and in 24% of the cancer-free patients, a difference that did not reach statistical significance (Table 3) ▶ . Among noncancer patients, type III IM was detected in 35% of patients with gastric ulcer, 22% with duodenal ulcer, and 16% with simple chronic gastritis.
Table 3.
Characteristics of All Patients with Intestinal Metaplasia
| Characteristic | MSS (n = 39) | MSI-L (n = 29) | MSI-H (n = 7) |
|---|---|---|---|
| Mean age (SD) | 53.9 (12.7) | 56.2 (13.3) | 57.4 (7.3) |
| Male:female | 31:11 | 24:8 | 8:3 |
| H. pylori infection | 35 (89.7%) | 28 (96.6%) | 7 (100%) |
| IM subtypes | |||
| Incomplete | 34.2% | 29.6% | 28.6% |
| Complete | 65.8% | 29.6% | 28.6% |
SD, standard deviation.
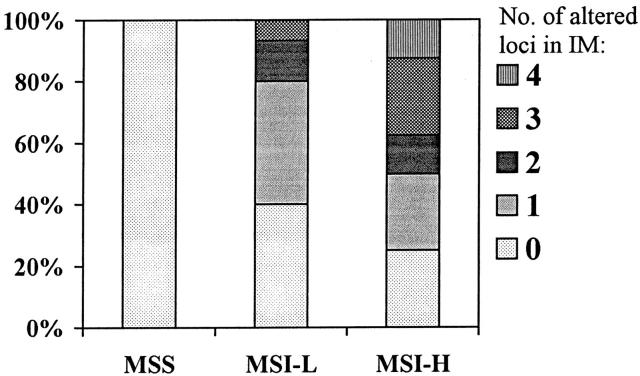
The overall frequency of IM samples that exhibited MSI-H was 9.3%, whereas MSI-L was detected in 38.7% of IM samples (Table 2) ▶ . Notably, compared with tumor samples, IM specimens were more often MSS (52% versus 23.3%; P = 0.009) and less frequently showed high-level instability (26.7% versus 9.3%; P = 0.03; Table 2 ▶ ). Moreover, IM samples from cancer patients had a higher frequency of MSI-H when compared with IM tissues from cancer-free patients, but the difference was not statistically significant (13.3% versus 6.7%; P = 0.4; Table 2 ▶ ). Among the 30 cancer patients, microsatellites were more frequently altered in IM coexisting with tumors that showed instability (Figure 3) ▶ . IM with instability at one or more loci was found in 75% of the cases with MSI-H tumor and 60% of the cases with MSI-L, but none was found in the patients with MSS tumors. In addition, IM samples from patients with MSI-H tumors had significantly more altered loci than IM samples from patients with MSI-L or MSS tumors (P = 0.003).
Table 2.
Frequency of Altered Microsatellites in Different Patient Groups
| Patient group | MSI subtypes | ||
|---|---|---|---|
| MSS | MSI-L | MSI-H | |
| Tumor tissue (n = 30) | 7 (23.3%)* | 15 (50%) | 8 (26.7%)** |
| IM tissue (n = 75) | 39 (52%)* | 29 (38.7%) | 7 (9.3%)** |
| Cancer patients (N = 30) | 14 (46.7%) | 12 (40%) | 4 (13.3%) |
| Noncancer patients (N = 45) | 25 (55.6%) | 17 (37.8%) | 3 (6.7%) |
*P = 0.009; **P = 0.03.
Figure 3.
Frequency of altered microsatellites in IM of patients with coexisting MSI-H, MSI-L, and MSS tumors. The number of altered microsatellite markers in IM (0, 1, 2, 3, and 4) of different tumor groups is indicated by different filling patterns, which are defined on the right side of the Figure ▶ . IM samples from patients with MSI-H tumors had significantly more loci altered than those from MSI-L or MSS (P = 0.003).
The overall rate of active H. pylori infection among the 75 patients with IM was 93.3%, and there was no significant difference in prevalence of H. pylori between the three groups of patients (Table 3) ▶ . Other gastric histological features, such as degree of acute inflammation, chronic inflammation, severity, and subtypes of IM, were not significantly different in patients with MSI-H, MSI-L, or MSS IM. The mean ages of the three groups of patients were similar. It is important that, when microsatellite loci were altered in both tumor and IM, the patterns of affected markers were usually similar; however, discrepancies were observed in three cases (Table 4 ▶ and Figure 2 ▶ ).
Table 4.
Patterns of Altered Microsatellites in All 16 Gastric Cancer Patients with Instability in IM
| Case no. | Age | Sex | Site | BAT25 | BAT26 | BAT40 | D2S123 | D5S346 | D13S170 | D17S250 | TP53 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 17 | 63 | F | T | N | N | N | N | N | N | MSI | N |
| IM | N | N | N | N | N | MSI | MSI | N | |||
| 18 | 68 | M | T | N | N | N | N | MSI | MSI | N | MSI |
| IM | N | N | N | N | N | MSI | N | N | |||
| 19 | 61 | M | T | N | N | N | MSI | N | MSI | MSI | N |
| IM | N | N | N | MSI | N | MSI | MSI | N | |||
| 54 | 55 | M | T | N | MSI | N | MSI | N | MSI | N | N |
| IM | N | N | N | MSI | N | MSI | N | N | |||
| 81 | 57 | M | T | N | N | N | MSI | N | MSI | N | MSI |
| IM | N | N | N | N | N | N | N | MSI | |||
| 87 | 59 | M | T | N | N | N | N | MSI | MSI | MSI | MSI |
| IM | N | N | N | N | N | MSI | MSI | MSI | |||
| 94 | 79 | F | T | N | N | N | N | N | MSI | N | N |
| IM | N | N | N | N | N | MSI | MSI | MSI | |||
| 97 | 59 | M | T | N | N | MSI | N | N | N | N | N |
| IM | N | N | MSI | N | N | N | N | N | |||
| 99 | 49 | M | T | N | MSI | N | MSI | N | N | N | N |
| IM | N | N | N | MSI | N | N | N | N | |||
| 100 | 77 | F | T | MSI | MSI | N | N | N | MSI | N | N |
| IM | N | N | N | MSI | N | MSI | N | N | |||
| 102 | 55 | M | T | N | MSI | N | N | N | N | N | N |
| IM | N | MSI | N | N | N | N | N | N | |||
| 118 | 61 | F | T | N | N | N | N | N | MSI | N | MSI |
| IM | N | N | MSI | MSI | N | MSI | N | N | |||
| 150 | 56 | M | T | N | N | N | MSI | N | N | MSI | N |
| IM | N | N | N | MSI | N | N | MSI | N | |||
| KO57 | 66 | F | T | MSI | MSI | MSI | MSI | N | MSI | MSI | N |
| IM | N | N | MSI | MSI | N | MSI | MSI | N | |||
| KO61 | 66 | M | T | N | N | N | N | N | N | N | MSI |
| IM | N | N | N | N | N | N | N | MSI | |||
| KO100 | 53 | M | T | N | N | N | N | N | MSI | N | N |
| IM | N | N | N | N | N | MSI | N | N |
T, tumor; IM, intestinal metaplasia; N, normal.
Microsatellite Marker Alterations
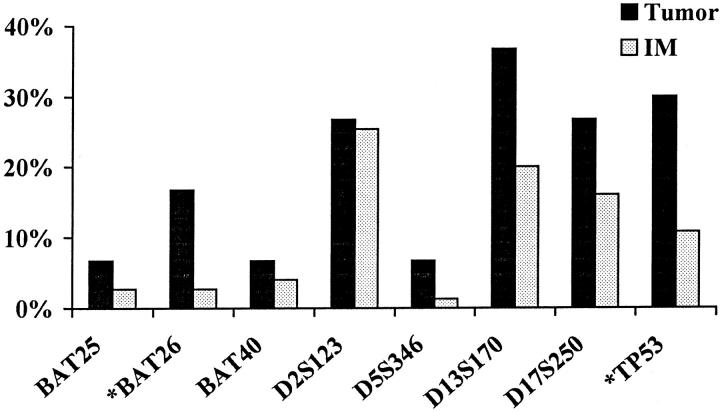
For each marker analyzed, MSI was more frequently detected in tumor tissues than in IM (Figure 4) ▶ . In general, dinucleotide repeat markers were more frequently altered than mononucleotide repeat markers, and alterations in the BAT26 and TP53 markers were significantly more frequent in tumor than in IM (P = 0.02). The results of our expanded panel of eight microsatellite markers and the NCI-recommended panel are listed in Table 5 ▶ . The numbers of patients classified into MSI-H were similar in both panels, but the number of cases with low-level instability increased when the expanded panel was used. Interestingly, when instability in the BAT markers was compared with all other markers, slightly over one third of the MSI-H tumors and IM tissues had BAT instability, and a minority of the MSI-L tumors and IM tissues displayed BAT alterations (Table 6) ▶ .
Figure 4.
Patterns of microsatellite marker alterations in tumor and IM. *, Markers BAT26 and TP53 were more frequently altered in tumor than in IM (P = 0.02).
Table 5.
Comparison of Microsatellite Alterations by Using NCI-Recommended Panel (5 Markers) and the Expanded Panel (8 Markers) in Tumor Metaplasia and IM
| Tissue | Alteration | Expanded panel | |||
|---|---|---|---|---|---|
| MSS | MSI-L | MSI-H | Total | ||
| Tumor | |||||
| MSS | 7 | 7 | 0 | 14 | |
| NCI Panel | MSI-L | 0 | 6 | 3 | 9 |
| MSI-H | 0 | 2 | 5 | 7 | |
| Total | 7 | 15 | 8 | 30 | |
| IM | |||||
| MSS | 39 | 8 | 0 | 47 | |
| NCI Panel | MSI-L | 0 | 17 | 3 | 20 |
| MSI-H | 0 | 4 | 4 | 8 | |
| Total | 39 | 29 | 7 | 75 |
Table 6.
Comparison of Alterations in Mononucleotide Microsatellite Markers and All Markers
| MSS | MSI-L | MSI-H | |
|---|---|---|---|
| Tumor | 0 /7 | 3 /15 | 3 /8 |
| IM | 0 /39 | 4 /29 | 3 /7 |
To test whether MSI occurred in the histologically normal gastric mucosa from individuals without gastric disease, we compared the allelic patterns of DNA extracted from endoscopic biopsies obtained from different parts of the stomach (corpus and antrum) of seven individuals. MSI, defined by the appearance of a nonidentical allele(s) in the two biopsies, was not detected in any individuals with normal gastric mucosa (data not shown).
Discussion
This is the first study to examine the frequency and patterns of microsatellite alterations in IM, a premalignant gastric mucosal lesion, in individuals without gastric cancer and its association with MSI in gastric cancer. If MSI plays an early and significant role in gastric carcinogenesis, one might expect to find MSI in metaplastic tissues before the development of cancer. In this study, a high level of MSI was detected in 26.7% of gastric cancers and in 9.3% of the IM tissues examined. In addition, MSI-stable tumors were associated with MSI-stable IM. These findings suggest a sequential accumulation of instability in the histological progression from IM to cancer. Supporting this hypothesis, we also noted that MSI in IM was more readily detected in individuals with MSI-H tumors than in those with MSS or MSI-L tumors (Figure 4) ▶ . Together, these findings suggest an early and important role of MSI in the gastric carcinogenesis pathway, and they may define a subset of individuals particularly susceptible to gastric cancer.
Although previous reports 16-18 had addressed the issue of MSI in premalignant gastric lesions, the conclusions were based on relatively small numbers of cancer patients, and analyses of IM in patients without gastric cancer were not performed. Two studies from Japan 16,17 examined IM tissues from patients with intestinal-type gastric cancer and detected MSI in 33% (3 of 9) and 27% (4 of 15) IM tissues, respectively. All cases showed a single altered locus that was identical in IM and tumor, and MSI was limited to incomplete-type IM. In the current study, microsatellites were similarly affected in both complete and incomplete IM subtypes (Table 3) ▶ . Hamamoto et al 17 examined multiple sections from gastrectomy specimens and demonstrated the topographical distribution of microsatellite alterations at a single locus (D1S191) in different IM samples surrounding gastric cancer. In contrast, we identified MSI in IM tissues from gastric endoscopic biopsies that were remote from the primary tumor or in patients without cancer, indicating a potentially significant role of MSI in premalignant metaplastic areas that are not in close proximity to the tumor.
In contrast to gastric tumors without MSI, MSI-H gastric tumors tended to exhibit characteristic features such as predominant distal location and were of intestinal type, similar to a previous report. 8 The pathogenetic mechanisms of MSI-H tumors are better defined than those of MSI-L and MSS tumors. MSI-H tumors may harbor frameshift mutations in coding regions of some cancer-related genes, such as BAX, IGFRII, TGFβRII, hMSH3, and hMSH6, 18,26,27 thereby favoring cancer development. The clinical significance of MSI-L in tumors is less well understood. In the current study, low levels of instability were frequently detected in both tumors and IM samples, which may be attributed to the use of the expanded microsatellite markers panel. BAT or mononucleotide marker alterations are good indicators of MSI-H colorectal cancer with underlying DNA mismatch repair deficiencies, and discordances between mononucleotide simple repeats and dinucleotide repeats appear to be unusual. 23,28,29 Slightly over one third of the MSI-H gastric tumors and IM tissues had alterations in the BAT markers. Therefore, the majority of MSI-H cases were scored on their dinucleotide repeat instability. The reason for the discordance in instability between the BAT loci and the dinucleotide loci is not clear.
Interestingly, the expression of DNA mismatch repair proteins in mismatch-competent cells might be transiently suppressed in the presence of oxidative stress. 30 In the gastric mucosa, reactive oxygen species are commonly released in inflamed gastric mucosa as a result of chronic H. pylori infection, and they could be responsible for the DNA mismatch repair deficiency underlying some MSI-H gastric cancers. 31 All tumor and IM samples with MSI-H in this study had evidence of active H. pylori infection in the stomach. Whether inflammation resulting from chronic H. pylori infection can account for the frequent low-level instability and predominant dinucleotide repeat alterations might deserve further evaluation. Nonetheless, it is tempting to speculate that chronic H. pylori infection, by its effects on cell proliferation and apoptosis, 32,33 may accelerate the accumulation of genetic alterations in the gastric mucosa that would then be carried on to IM and cancer. This was supported by the observation of Wu et al, who reported a significant association between H. pylori infection and gastric cancer with MSI-H. 34 It is interesting that H. pylori was present in the great majority of stomachs with IM (MSI-positive and MSS) and in 100% of patients with MSI-positive tumors, but was absent in about 50% of the stomachs with MSS tumors. This difference can be explained by several possibilities: 1) MSS tumors might develop through molecular pathways that characterize a specific background gastric mucosa that constitutes an unfavorable environment for H. pylori; 2) patients with MSI-positive tumors might be infected with more virulent H. pylori strains; 3) host factor(s) determining susceptibility to MSI development might affect the ability of H. pylori to persist in the gastric environment of patients that ultimately develop MSI-positive tumors. A possibly important role of host susceptibility factors in the development of MSI-positive gastric cancer was suggested by previous studies recognizing a significantly higher frequency of MSI in gastric cancer samples from Korean individuals when compared with tumors from American or Colombian patients. 35
Altered microsatellites have been identified in Barrett’s esophagus 36 and non-neoplastic colonic mucosa of ulcerative colitis patients. 37 Together with the findings of MSI in IM, altered microsatellites have been detected in the most common premalignant gastrointestinal lesions and are likely to play an early role in the carcinogenesis pathway of gastrointestinal malignancies. Although gastric cancer is a common disease, molecular markers for early diagnosis of the disease are lacking. Given the early involvement of MSI in the multistep gastric carcinogenesis model, detection of MSI may serve as a surrogate marker for the risk of gastric cancer development. It might be helpful to identify high-risk patients, by determining MSI of preneoplastic lesions, such that close monitoring or potential intervention can be instituted. Because the majority of patients with IM will not progress to cancer and only a proportion of these patients harbored MSI, it is conceivable that patients with IM displaying MSI are at greater risk of developing gastric cancer than those without instability.
In conclusion, we have demonstrated a high frequency of MSI in gastric IM from patients with and without gastric cancer. Taking into consideration the progressive increase in MSI frequency from premalignant to malignant lesions, our results suggest the early involvement and continuous accumulation of MSI in gastric cells that have entered the multistep gastric carcinogenesis pathway. The role of detection of MSI in premalignant gastric lesions as a surrogate marker of risk of gastric cancer development warrants further investigation.
Footnotes
Address reprint requests to Antonia R. Sepulveda, M.D., Ph.D., Digestive Diseases Section (111D), VA Medical Center, 2002 Holcombe Blvd., Houston, TX 77030. E-mail: asepulv@bcm.tmc.edu.
Supported by the Department of Veterans Affairs and by generous support from Hilda Schwartz. W. K. L. is partly supported by the Li Po Chun Charitable Trust Fund Overseas Postgraduate Scholarships of Hong Kong SAR.
References
- 1.Landis SH, Murray T, Bolden S, Wingo PA: Cancer statistics, 1998. CA Cancer J Clin 1998, 48:6-29 [DOI] [PubMed] [Google Scholar]
- 2.Huang JQ, Sridhar S, Chen Y, Hunt RH: Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology 1998, 114:1169-1179 [DOI] [PubMed] [Google Scholar]
- 3.Ramon JM, Serra L, Cerdo C, Oromi J: Dietary factors and gastric cancer risk: a case-control study in Spain. Cancer 1993, 71:1731-1735 [DOI] [PubMed] [Google Scholar]
- 4.Sobala GM, Crabtree JE, Dixon MF, Schorah CJ, Taylor JD, Rathbone BJ, Heatley RV, Axon AT: Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut 1991, 32:1415-1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mannick EE, Bravo LE, Zarama G, Realpe JL, Zhang XJ, Ruiz B, Fontham ET, Mera R, Miller MJ, Correa P: Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res 1996, 56:3238-3243 [PubMed] [Google Scholar]
- 6.Brito MJ: Oncogenes and gastric cancer. Eur J Cancer 1994, 3(Suppl 2):47-49 [DOI] [PubMed] [Google Scholar]
- 7.Strickler JG, Zheng J, Shu Q, Burgart LJ, Alberts SR, Shibata D: p53 mutations and microsatellite instability in sporadic gastric cancer: when guardians fail. Cancer Res 1994, 54:4750-4755 [PubMed] [Google Scholar]
- 8.Hayden JD, Martin IG, Cawkwell L, Quirke P: The role of microsatellite instability in gastric carcinoma. Gut 1998, 42:300-303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aaltonen LA, Peltomaki P, Leach FS, Sistonen P, Pylkkanen L, Mecklin J, Jarvinen H, Powell S, Jen J, Hamilton S: Clues to the pathogenesis of familial colorectal cancer. Science 1993, 260:812-816 [DOI] [PubMed] [Google Scholar]
- 10.Arzimanoglou II, Gilbert F, Barber HR: Microsatellite instability in human solid tumors. Cancer 1998, 82:1808-1820 [DOI] [PubMed] [Google Scholar]
- 11.Weissenbach J, Gyapay G, Dib C, Vignal A, Morissette J, Milasseau P, Vayssaix G, Lathrop M: Linkage map of the human genome. Nature 1992, 359:794-801 [DOI] [PubMed] [Google Scholar]
- 12.Correa P: A human model of gastric carcinogenesis. Cancer Res. 1988, 48:3554-3560 [PubMed] [Google Scholar]
- 13.Correa P: Helicobacter pylori, and gastric carcinogenesis. Am J Surg Pathol 1995, 19:S37-S43 [PubMed] [Google Scholar]
- 14.Correa P, Shiao Y-H: Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res 1994, 54(Suppl):1941-1943 [PubMed] [Google Scholar]
- 15.Nakatsuru S, Yanagisawa A, Furukawa Y, Ichii S, Kato Y, Nakamura Y, Horii A: Somatic mutations of the APC gene in precancerous lesion of the stomach. Hum Mol Genet 1993, 2:1463-1465 [DOI] [PubMed] [Google Scholar]
- 16.Semba S, Yokozaki H, Yamamoto S, Yasui W, Tahara E: Microsatellite instability in precancerous lesions and adenocarcinomas of the stomach. Cancer 1996, 77:1620-1627 [DOI] [PubMed] [Google Scholar]
- 17.Hamamoto T, Yokozaki H, Semba S, Yasui W, Yunotani S, Miyazaki K, Tahara E: Altered microsatellites in incomplete-type intestinal metaplasia adjacent to primary gastric cancers. J Clin Pathol 1997, 50:841-846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ottini L, Palli D, Falchetti M, D’Amico C, Amorosi A, Saieva C, Calzolari A, Cimoli F, Tatarelli C, De Marchis L, Masala G, Mariani-Costantini R, Cama A: Microsatellite instability in gastric cancer is associated with tumor location and family history in a high-risk population from Tuscany. Cancer Res 1997, 57:4523-4529 [PubMed] [Google Scholar]
- 19.Lauren P: The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965, 64:31-49 [DOI] [PubMed] [Google Scholar]
- 20.Genta RM, Robason GO, Graham DY: Simultaneous visualization of Helicobacter pylori and gastric morphology: a new stain. Hum Pathol 1994, 25:221-226 [DOI] [PubMed] [Google Scholar]
- 21.Dixon MF, Genta RM, Yardley JH, Correa P: Classification and grading of gastritis: the updated Sydney system. Am J Surg Pathol 1996, 20:1161-1181 [DOI] [PubMed] [Google Scholar]
- 22.Filipe MI, Munoz N, Matko I, Kato I, Pompe-Kirn V, Jutersek A, Teuchmann S, Benz M, Prijon T: Intestinal metaplasia types and the risk of gastric cancer: a cohort study in Slovenia. Int J Cancer 1994, 57:324-329 [DOI] [PubMed] [Google Scholar]
- 23.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S: A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998, 58:5248-5257 [PubMed] [Google Scholar]
- 24.Santos AC, Yamaoka Y, Graham DY, Sepulveda AR: Variability in the interpretation of microsatellite patterns with different electrophoretic conditions. J Clin Pathol Mol Pathol 1999, 52:302-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasen HF, Mecklin JP, Khan PM, Lynch HT: The international collaborative group on HNPCC. Anticancer Res 1994, 14:1661-1664 [PubMed] [Google Scholar]
- 26.Yamamoto H, Sawai H, Perucho M: Frameshift somatic mutations in gastrointestinal cancer of the microsatellite mutator phenotype. Cancer Res 1997, 57:4420-4426 [PubMed] [Google Scholar]
- 27.Shinmura K, Tani M, Isogaki J, Wang Y, Sugimura H, Yokota J: RER phenotype and its associated mutations in familial gastric cancer. Carcinogenesis 1998, 19:247-251 [DOI] [PubMed] [Google Scholar]
- 28.Hoang JM, Cottu PH, Thuille B, Salmon RJ, Thomas G, Hamelin R: BAT-26, an indicator of the replication error phenotype in colorectal cancers and cell lines. Cancer Res 1997, 57:300-303 [PubMed] [Google Scholar]
- 29.Halling KC, Harper J, Moskaluk CA, Thibodeau SN, Petroni GR, Yustein AS, Tosi P, Minacci C, Roviello F, Piva P, Hamilton SR, Jackson CE, Powell SM: Origin of microsatellite instability in gastric cancer. Am J Pathol 1999, 155:205-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang CL, Ha HT, Ricciardiello L, Chang DK, Randolph A, Chauhan DP, Carethers JM, Boland CR: The DNA mismatch repair (MMR) system is inappropriately down-regulated in the presence of oxidative DNA damage. Gastroenterology 1999, 116:G1692(abstr.) [Google Scholar]
- 31.Ernst P: Review article: the role of inflammation in the pathogenesis of gastric cancer. Aliment Pharmacol Ther 1999, 13(Suppl 1):13-18 [DOI] [PubMed] [Google Scholar]
- 32.Wagner S, Beil W, Westermann J, Logan RP, Bock CT, Trautwein C, Bleck JS, Manns MP: Regulation of gastric epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology 1997, 113:1836-1847 [DOI] [PubMed] [Google Scholar]
- 33.Jones NL, Shannon PT, Cutz E, Yeger H, Sherman PM: Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am J Pathol 1997, 151:1695-1703 [PMC free article] [PubMed] [Google Scholar]
- 34.Wu MS, Lee CW, Shun CT, Wang HP, Lee WJ, Sheu JC, Lin JT: Clinicopathological significance of altered loci of replication error and microsatellite instability-associated mutations in gastric cancer. Cancer Res 1998, 58:1494-1497 [PubMed] [Google Scholar]
- 35.Sepulveda A, Santos AC, Yamaoka Y, Wu L, Gutierrez O, Kim JG, Graham DY: Marked differences in the frequency of microsatellite instability in gastric cancer from different countries. Am J Gastroenterol 1999, 94:3034-3038 [DOI] [PubMed] [Google Scholar]
- 36.Meltzer SJ, Yin J, Manin B, Rhyu MG, Cottrell J, Hudson E, Redd JL, Krasna MJ, Abraham JM, Reid BJ: Microsatellite instability occurs frequently and in both diploid and aneuploid cell populations of Barrett’s-associated esophageal adenocarcinomas. Cancer Res 1994, 54:3379-3382 [PubMed] [Google Scholar]
- 37.Brentnall TA, Crispin DA, Bronner MP, Cherian SP, Hueffed M, Rabinovitch PS, Rubin CE, Haggitt RC, Boland CR: Microsatellite instability in nonneoplastic mucosa from patients with chronic ulcerative colitis. Cancer Res 1996, 56:1237-1240 [PubMed] [Google Scholar]