Abstract
Bcl-2 and p53 gene products have been both linked to cell death by apoptosis. In the present study, we examined the relationship of Bcl-2 and p53 protein expression, p53 mutation and apoptosis in normal human ovaries and different types of human ovarian epithelial tumors by immunohistochemical localization, in situ terminal transferase-mediated dUTP nick end labeling and polymerase chain reaction-single strand conformation polymorphism. It was found that Bcl-2 expressed strongly in the surface epithelium of normal ovaries and benign and borderline ovarian tumors but weakly in the malignant tumors. On the contrary, strong protein expression of p53 was found in 54% (25/46) of the malignant epithelial tumors examined but similar expression of p53 was not observed in borderline and benign tumors and normal ovarian surface epithelium. A significant inverse correlation between Bcl-2 and p53 expression was found in the malignant ovarian tumors examined. p53 gene mutation at exons 5–11 was however not a pre-requisite for p53 expression in both borderline and malignant tumors. Apoptotic activities, as reflected by apoptotic indices, were low in normal ovarian surface epithelium and benign tumors but were increased in borderline and malignant tumors, with the highest average apoptotic index found in grade III malignant tumors. Statistical analyses showed a positive correlation between apoptosis and p53 expression, but similar correlation was not found between apoptosis and Bcl-2 expression. Our results also indicate that although expression of Bcl-2 is important during ovarian carcinogenesis, the Bcl-2 protein may have other roles to play apart from being a modulator of apoptosis in human ovarian epithelial cancers.
Ovarian cancer is the most common cause of death among all gynecologic malignancies. 1 The overall 5-year survival rate of patients with ovarian cancer is only about 30%, partly due to absence of symptoms at early stages and poor prognosis. 2,3 More than 90% of ovarian cancers are of epithelial cell origin and multiple genetic alterations are believed to occur during malignant transformation of ovarian epithelial cells. 4 Several oncogenes and tumor suppressor genes including HER-2/neu, 5,6 K-ras, 7,8 SPARC, 9 BRCA1, 10 and DOC-2 11 have been found to be involved in ovarian carcinogenesis. It has also been demonstrated that deregulation of the genes involved in apoptosis, such as Bcl-2 and p53, plays a crucial role in tumor formation. 12 Bcl-2 has been proposed to be able to inhibit apoptosis. 13 High levels and aberrant patterns of Bcl-2 expression have been found in a wide variety of human cancers, 14-20 and have been shown to alter drug resistance in cancers. 21 On the other hand, p53 functions as a tumor suppressor by arresting cell cycle at G1 phase 22,23 and by triggering apoptosis. 24 Overexpression of p53 induces apoptosis in human colon tumor and leukemic cells. 24,25 Conversely, the absence of functional p53 is associated with the inability of cells to undergo apoptosis leading to the development of a variety of malignancies. 26 Mutation of p53 gene is the most common molecular genetic change associated with many cancers 27 including ovarian cancers. 28-30
Many recent studies have focused on the interaction between these two apoptosis regulatory genes in carcinogenesis. Immunohistochemical studies on human cancer tissues had demonstrated a significant inverse relationship between Bcl-2 and p53 protein expression in non-small-cell lung cancers, 31,32 follicular lymphomas, 33 gastric carcinomas, 34 esophageal squamous cancers, 35 non-melanoma skin cancers, 36 and breast carcinomas. 37,38 In prostatic carcinomas 39 and colorectal adenomas and carcinomas, 40 the expressions of Bcl-2 and p53 proteins in the same tissue section were almost reciprocal. It appeared that alteration of both Bcl-2 and p53 proteins may be involved in a common genetic pathway that is shared by a number of different human cancers. It has also been suggested that in human ovarian cancers, protein expression patterns of Bcl-2 and p53 are inversely related. 41 In the present study, we aimed to examine thoroughly the relationship among Bcl-2 and p53 expression, apoptosis, and p53 gene mutation. The protein expression patterns of Bcl-2 and p53 were firstly examined by immunohistochemical staining in normal human ovaries, benign ovarian tumors, ovarian tumors of low malignancy (borderline tumors), and malignant ovarian epithelial tumors of different histological grades to see whether their expression patterns were related to malignancy of the tumors. Correlation between Bcl-2 and p53 protein expression was also made. Polymerase chain reaction-single strand conformation polymorphism (PCR-SSCP) was used to investigate p53 gene mutation. Apoptotic activities as reflected by the apoptotic index were then analyzed by means of an in situ terminal transferase-mediated dUTP nick end labeling (TUNEL) technique in normal ovarian epithelial and tumor tissues.
Materials and Methods
Patient Samples
A total of 127 fresh human ovarian tissues including 14 normal ovaries, 11 benign ovarian tumors, 37 borderline ovarian tumors, and 65 malignant epithelial ovarian tumors were obtained from the Brigham and Women’s Hospital, Harvard Medical School, with patient consent before the patient received treatments. All tissues were fixed in 10% buffered formalin for paraffin histology. The tumors were histopathologically diagnosed and classified according to International Federation of Gynecology and Obstetrics 42 criteria by two gynecology pathologists (W. R. W. and D. A. B.). Of the samples obtained, all normal ovaries and benign ovarian tumors, 27 borderline, and 46 malignant ovarian tumors, which were all well-preserved histologically, were used for immunohistochemistry, and 19 borderline and 31 malignant ovarian tumors with DNA samples available were used for PCR-SSCP analysis.
Immunohistochemistry
For identification of Bcl-2 or p53 protein expression, the avidin-biotin peroxidase complex (ABC) method with diaminobenzidine as the chromogen was used. Bcl-2 and p53 antigens were retrieved by microwave in 0.01 mol/L citric acid buffer, pH 6.0, for 10 minutes. Two monoclonal antibodies, clone 124 mouse anti-human Bcl-2 primary antibody (DAKO, Glostrup, Denmark, 1:20) and p53 pantropic (Ab-6, Calbiochem, MA; 1:25), both in 1% bovine serum albumin-phosphate buffered saline (BSA-PBS), were used. Negative control for every experiment was done by replacing the primary antibodies with 1% BSA-PBS.
Semiquantitation of Bcl-2 and p53 Immunoreactivities
Five to seven sections were randomly selected from each specimen. The total cell number and the number of positive cells were counted with a Metamorph software under a microscope by two independent observers. Immunoreactivities were quantified with a 12-point weighted score: 11,43,44 First, the percentage of positive cells in each section was scored with a 5-point scale: 0 for <5%, 1 for 5 to 25%, 2 for 25 to 50%, 3 for 50 to 75%, and 4 for over 75%. Second, the intensity of positive signal was scored with a 3-point scale: 1 for weak, 2 for medium, and 3 for intense. Then, the weighted score for each section was obtained by multiplying the percentage score by the intensity score. The bcl-2 staining intensity was compared to the staining intensity of the section taken from a follicular lymphoma, which served as the positive control and was scored as 3.
PCR-SSCP
The oligonucleotide primers were synthesized by Genosys Biotechnologies, Inc. (Woodlands, TX). Exons 5–11 of p53 gene were amplified by PCR, and SSCP analysis was performed according to procedures described by Mok et al. 45 DNA with an altered mobility demonstrated by SSCP was reamplified using the same primers and PCR conditions. The PCR product was then purified and sequencing of sense and anti-sense complimentary DNA strands was performed using a commercial PCR gene sequencing kit (U.S. Biochemical Corp., Cleveland, OH).
In Situ Terminal Transferase-Mediated dUTP Nick End Labeling (TUNEL)
Apoptotic activity in the epithelial cells of the ovarian tissue sections was detected with an In situ Apoptosis Detection Kit (Oncor, Gaithersburg, MD) with TUNEL. The peroxidase activity was then visualized by the reaction with the chromogen diaminobenzidine. Negative control was performed using PBS instead of the enzyme TdT. Apoptotic activity was quantified by the apoptotic index which represented the percentage of apoptotic epithelial cells in each tissue sample. A total of 10 fields from each tissue section were randomly chosen, and 100 epithelial cells from each field were counted. Five to seven sections were randomly taken from each specimen for scoring.
Statistics
Bivariate comparisons between the apoptotic indices and weighted scores were made using the Pearson’s product moment correlation coefficient. Significance was defined at P < 0.05.
Results
Expression of Bcl-2 and p53 Proteins in Human Ovarian Tissues
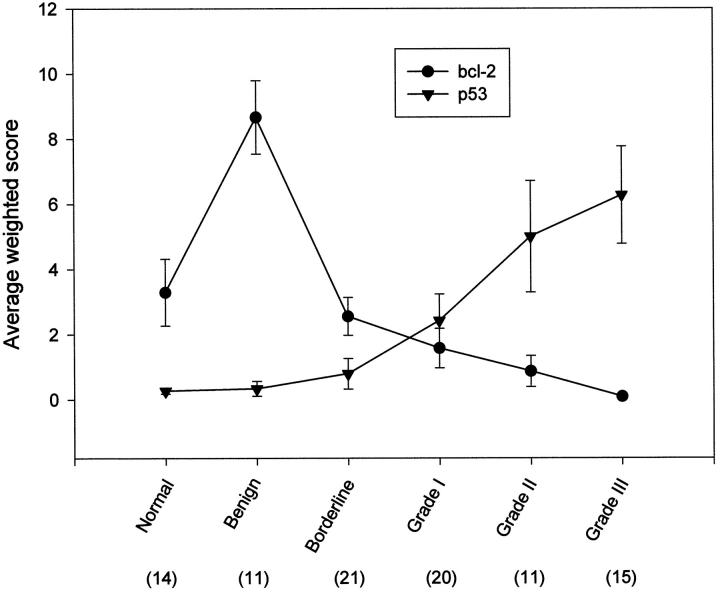
Bcl-2-immunoreactive products appeared as brown granules localized mainly in the perinuclear locations and cytoplasmic regions of epithelial cells (Figure 1, a, b, f, and g) ▶ , which presumably are locations for mitochondria and endoplasmic reticulum. 46 Most of the normal ovaries (79%, 11/14; Figure 1a ▶ ), benign tumors (100%, 11/11), and borderline tumors (78%, 21/27) showed positive Bcl-2 immunoreactivity. Their average weighted scores, which reflected both the staining intensity and the percentage of positive cells, were 4.2 ± 1.2, 8.7 ± 1.1, and 2.6 ± 0.6, respectively (Table 1) ▶ . However, Bcl-2 immunoreactivity was observed in only one-third (33%, 15/46) of the malignant tumors examined and their average weighted score was significantly decreased to 0.9 ± 0.3. Of the 20 grade I tumors examined, only 11 (30%) showed Bcl-2 immunoreactivity (Figure 1f) ▶ , and their average weighted score was 1.6 ± 0.6. The expression of Bcl-2 proteins was detected in only 3 (27%) of the 11 grade II tumors examined, and their weighted score was reduced to 0.9 ± 0.5. Only 1 of 15 grade III tumors (1/15, 7%) examined showed weak Bcl-2 immunoreactivity (weighted score = 1.3), and the remaining grade III tumors (93%, 14/15) did not show any positive Bcl-2 immunoreactivity.
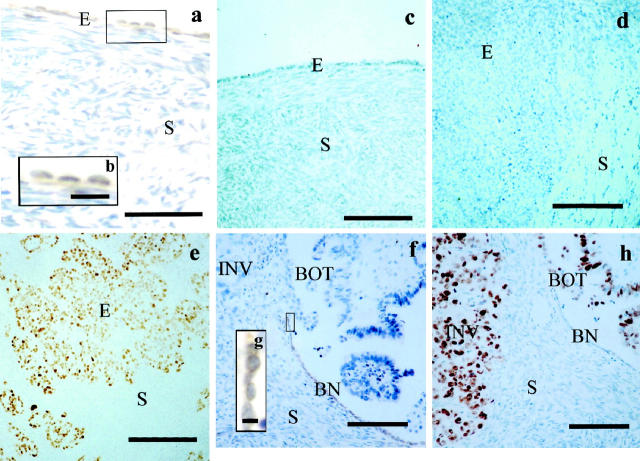
Figure 1.
Photomicrographs of sections taken from normal ovaries (a, c), grade III malignant ovarian tumors (d, e), and a grade I malignant ovarian tumor (f, h), showing reciprocal immunoreactivities of Bcl-2 (a, d, f) and p53 (c, e, h). a: In the normal ovary, Bcl-2 is mainly localized in the surface epithelial cells (E) with occasional staining in the stroma (S). b: Higher magnification of the box in a, showing that Bcl-2-immunoreactive products appear as orange to brown granules in the cytoplasm of the surface epithelial cells. c: p53 immunoreactivity is not observed in both the surface epithelium and stroma of the normal ovary. d: Bcl-2 immunoreactivity is not found in the invading epithelial cells (E) and the stroma (S) of a grade III malignant tumor, but (e) strong p53 immunoreactivity is observed in the epithelial cells (E) of the grade III malignant tumor. f: In a section taken from a serous grade I malignant tumor, Bcl-2-positive cells are located only in the histologically benign appearing epithelium (BN) but not in the epithelial cells of the borderline appearing (BOT) and malignant appearing (INV) components or other epithelial regions of the tumor, whereas in a successive section (h), p53 immunoreactivity is observed in the epithelial cells of the malignant (INV) and borderline (BOT) appearing components but not in the histologically benign epithelium (BN). g: Higher magnification of the box in f showing the Bcl-2 staining is mainly cytoplasmic. Stroma (S) is negative for both Bcl-2 and p53 staining in f and h. Counterstained with Mayer’s hematoxylin. Scale bars, 100 μm (a, c-f, h), 30 μm (b), 12 μm (g).
Table 1.
Correlation of Bcl-2 Expression, p53 Expression, and Apoptosis in Various Types of Human Ovarian Tissue
| Ovarian tissues | Sample size | Bcl-2 average weighted score ± SEM | p53 average weighted score ± SEM | Average apoptotic index ± SEM | Pearson correlation coefficient r and P values | ||
|---|---|---|---|---|---|---|---|
| p53 vs. bcl-2 | p53 vs. apoptosis | bcl-2 vs. apoptosis | |||||
| Normal | 14 | 4.2 ± 1.2 | 0.3 ± 0.1 | 0.2 ± 0.1 | r = −0.026 | r = 0.441 | r = 0.191 |
| P = 0.940 | P = 0.174 | P = 0.573 | |||||
| Benign | 11 | 8.7 ± 1.1 | 0.3 ± 0.2 | 0.1 ± 0.0 | r = 0.044 | r = 0.413 | r = 0.358 |
| P = 0.897 | P = 0.207 | P = 0.280 | |||||
| Borderline | 21 | 2.6 ± 0.6 | 0.8 ± 0.5 | 0.5 ± 0.1 | r = −0.104 | r = 0.130 | r = 0.013 |
| P = 0.655 | P = 0.574 | P = 0.957 | |||||
| Malignant | 46 | 0.9 ± 0.3 | 4.3 ± 0.8 | 0.6 ± 0.1 | r = −0.320 | r = 0.511 | r = −0.227 |
| *P = 0.030 | †P = 0.0003 | P = 0.129 | |||||
| Grade I | 20 | 1.6 ± 0.6 | 2.4 ± 0.8 | 0.3 ± 0.1 | r = −0.343 | r = 0.400 | r = −0.159 |
| P = 0.139 | P = 0.080 | P = 0.504 | |||||
| Grade II | 11 | 0.9 ± 0.5 | 5.0 ± 1.7 | 0.6 ± 0.2 | r = −0.479 | r = 0.533 | r = −0.068 |
| P = 0.136 | P = 0.092 | P = 0.842 | |||||
| Grade III | 15 | 0.1 ± 0.1 | 6.3 ± 1.5 | 0.9 ± 0.2 | r = 0.274 | r = 0.529 | r = 0.0006 |
| P = 0.323 | *P = 0.043 | P = 0.998 |
All ovarian tissues were collected before the patients received treatments. The age of patients at diagnosis of malignant tumors ranged from 22 to 79 years (average, 52.84), and their average survival period was 20.98 months.
*Significant correlation, P < 0.05.
†Significant correlation, P < 0.01.
In contrast, fewer than half of the normal ovaries (43%, 6/14), benign tumors (18%, 2/11), and borderline tumors (19%, 5/27) examined showed positive p53 immunoreactivity. Their staining was weak and their average weighted scores were 0.3 ± 0.1, 0.3 ± 0.2, and 0.8 ± 0.5, respectively (Table 1) ▶ . However, strong expression of p53 proteins, which was found exclusively in the nucleus of the epithelial cells (Figure 1, g and h) ▶ , was found frequently in 54% (25/46) of the malignant ovarian tumors. More high grade tumors, eg, grade III (60%, 9/15) and grade II (64%, 7/11) tumors, exhibited positively (Figure 2d) ▶ than low grade tumors (grade I: 45%, 9/20) tumors. Grade III tumors had the highest average weighted score, ie, 6.3 ± 1.5, followed by grade II tumors (5.0 ± 01.7), whereas grade I tumors had the lowest weighted score (2.4 ± 0.8) among the malignant tumors.
Figure 2.
Line diagram plotted against the average weighted score for the normal ovary and different types of ovarian tumors. The number in parentheses represents the sample number and the error bar represents the SEM.
Correlation between Bcl-2 and p53 Expression in Ovarian Tissues
The average weighted scores for Bcl-2 immunoreactivity in normal ovaries and different types of ovarian tumors were decreasing in the following order: benign > normal > borderline > grade I malignant > grade II malignant > grade III malignant, while p53 nuclear staining was increasing in almost the same order, ie, normal < benign < borderline < grade I malignant < grade II malignant < grade III malignant, except that p53 expression in normal ovaries is weaker than that in benign tumors (Figure 2) ▶ . Statistical analyses did not reveal any correlation between Bcl-2 and p53 expression in normal ovaries and in benign and borderline tumors (Table 1) ▶ . However, a significant inverse correlation (Table 1 ▶ : two-tailed, r = −0.32, P = 0.03, n = 46) between bcl-2 and p53 expression in malignant ovarian tumors was found, indicating that high grade malignant ovarian tumors were always associated with a weak bcl-2 and a strong p53 expression but in low grade tumors the reverse was observed. Interestingly, of the 46 malignant tumors examined, only 6 (13%) exhibited both bcl-2 and p53 expression. In the tumors which showed both bcl-2 and p53 expression, the surface epithelial cells where bcl-2 expression was found did not have p53 expression, whereas the invading epithelial cells expressing p53 did not show bcl-2 expression (Figure 2) ▶ , demonstrating the reciprocity of these two types of protein expression in malignant ovarian tumors.
PCR-SSCP Analysis
A total of 19 borderline ovarian tumors and 31 malignant ovarian tumors (5 grade I, 8 grade II, and 18 grade III) were used to analyze p53 mutation at exons 5–11 using PCR-SSCP (Table 2) ▶ . Only one (5%, 1/19) of the borderline ovarian tumors examined showed p53 mutation, whereas 17 of 31 malignant ovarian tumors (55%) showed p53 gene mutation. The mutations were found in 2 of 5 (40%) grade I tumors, 5 of 8 (63%) grade II tumors, and 10 of 18 (56%) grade III tumors examined (Table 2) ▶ . Of the 17 mutations detected in the malignant tumors, ten mutations (59%) were missense, two (12%) were deletion, two (12%) were insertion, two (12%) were silent, and one (6%) was nonsense. However, 6 (37%, 7/19) tumor samples which expressed p53 proteins did not show p53 mutation and of the 17 samples which showed p53 mutation, 5 (29%) did not exhibit p53 expression (Table 2) ▶ . In other words, p53 expression was not necessarily linked to p53 mutation.
Table 2.
PCR-SSCP and p53 Expression in Human Borderline and Malignant Ovarian Epithelial Cancers
| Block no. | Tissues | Age at diagnosis (years) | Survival period (months) | Subtype* | SSCP | p53 weighted score |
|---|---|---|---|---|---|---|
| B16 | Borderline | 34 | 2† | S | None | 0 |
| B42 | Borderline | NA | NA | S | None | 0 |
| B48 | Borderline | NA | NA | S | None | 0 |
| B54 | Borderline | NA | NA | S | None | 0 |
| B58 | Borderline | NA | NA | S | None | 0 |
| 354A | Borderline | 31 | 95† | S | None | 0 |
| 373 | Borderline | 49 | 53 | S | None | 0 |
| 405 | Borderline | 65 | 87† | S | None | 0 |
| 427 | Borderline | 64 | 5 | S | None | 0 |
| 454 | Borderline | 28 | 77† | S | None | 0 |
| 474 | Borderline | 34 | 71† | S | None | 0 |
| B26 | Borderline | 23 | 18† | M | None | 1 |
| B47 | Borderline | NA | NA | M | None | 0 |
| B59 | Borderline | NA | NA | M | Intron 6 G>A | 0 |
| 407 | Borderline | 79 | 62 | M | None | 0 |
| 416 | Borderline | 40 | 27 | M | None | 0 |
| 439 | Borderline | 51 | NA | M | None | 0 |
| 471 | Borderline | 43 | 65† | M | None | 0 |
| 481 | Borderline | 35 | NA | M | None | 0 |
| 315A | Malignant | M | 8(266) GGA>AGA | 0 | ||
| Grade I | 63 | 10 | Gly>Arg | |||
| 473A | Grade I | 34 | 39 | M | None | 7.5 |
| 479B | Grade I | 68 | NA | M | None | 0 |
| 524 | Grade I | 68 | 53 | S | None | 0 |
| 558 | Grade I | 67 | 25 | S | 11(878) Insertion of AC | 2 |
| 308 | Grade II | 44 | 122† | S | 8(273) CGT>CAT | 12 |
| Arg>His | ||||||
| 336 | Grade II | 61 | 12† | S | None | 0 |
| 357 | Grade II | 53 | 21 | S | 7(229) TGT>TAC | 0 |
| GT deletion | ||||||
| 377 | Grade II | 75 | 12 | S | 7(248) CGG>GGG | 12 |
| Arg>Gly | ||||||
| 440 | Grade II | 56 | NA | M | None | 0 |
| 478A | Grade II | 50 | 53 | S | 10(790) G>A silent | 4 |
| 490B | Grade II | 62 | 6 | S | None | 12 |
| 504A | Grade II | NA | NA | S | 11(860) GAA>AAA | 12 |
| Glu>Iys | ||||||
| 6(285) T>C | ||||||
| Ile>The | ||||||
| 312 | Grade III | 50 | 48 | S | None | 12 |
| 316 | Grade III | 54 | 36† | E | 7(245) GGC>AGC | 12 |
| Gly>Ser | ||||||
| 317 | Grade III | 42 | 107† | S | None | 12 |
| 319 | Grade III | 41 | 33 | S | 6(279) A>G | 12 |
| His>Arg | ||||||
| 321A | Grade III | 66 | 44 | S | 8(525) G>A | 12 |
| Cys>Arg | ||||||
| 324 | Grade III | 51 | 55 | S | 10(710) C>T | 12 |
| Arg>Cys | ||||||
| 330 | Grade III | 58 | 10 | S | 6(195) ATC>ATT | 0 |
| Nonsense | ||||||
| 334 | Grade III | 35 | 8 | S | 5(179) CAT>CGT | 12 |
| His>Arg | ||||||
| 341 | Grade III | 48 | 24 | S | None | 8 |
| 344 | Grade III | 56 | 17 | S | 9(645-645) CT del. | 8 |
| (truncation at 700-702 in exon 10) | ||||||
| 345 | Grade III | 59 | 17 | S | None | 0 |
| 349 | Grade III | 41 | 65 | S | None | 0 |
| 351 | Grade III | 48 | 90 | S | 7(435) G>T | 12 |
| Gly>Val | ||||||
| 358 | Grade III | 50 | 21 | S | 7(466) insertion of A | 0 |
| 420 | Grade III | 79 | 15 | S | None | 12 |
| 473 | Grade III | 34 | 39 | M | None | 2 |
| 506A | Grade III | 55 | 18 | S | None | 0 |
| 516 | Grade III | 48 | 14 | S | 10(790) G>A | 0 |
| silent (Arg) |
NA, Data not available.
*S, serous; M, mucinous; E, endometrioid.
†Patient still alive.
Apoptosis in Human Ovarian Tissues
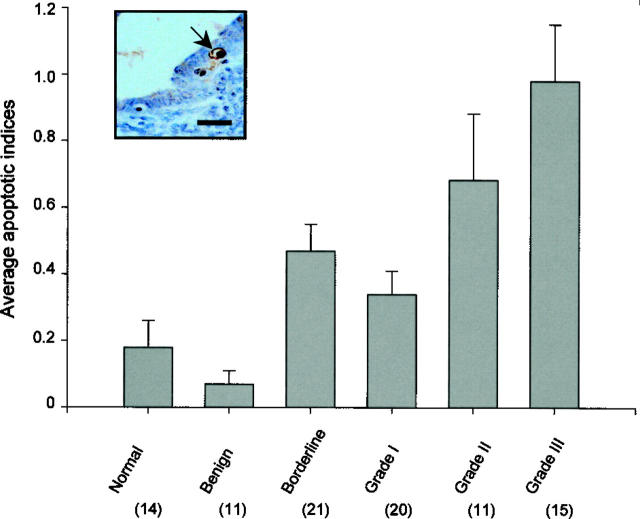
Positive cells for TUNEL were assessed according to their staining and their cellular morphology characteristic of apoptosis. 43,47 The morphological features which were considered to be typical to apoptosis were chromatin condensation on the periphery of the nucleus, and a heavily stained nucleus (Figure 3) ▶ . The apoptotic indices, which were defined as the mean percentage of apoptotic cells, were low for the epithelial cells of normal ovaries (0.2 ± 0.1) and benign ovarian tumors (0.1 ± 0.0; Table 1 ▶ ). Many of normal ovaries (57%, 8/14) and benign ovarian tumors (73%, 8/11) did not even show any apoptotic epithelial cells.
Figure 3.
Histogram showing average apoptotic indices for different types of ovarian tissues. The number in parentheses represents the sample number and the error bar represents the SEM. The inset figure shows a typical apoptotic cell (arrow) in the surface epithelium of a borderline ovarian tumor. Counterstained with Mayer’s hematoxylin. Scale bar, 30 μm.
Specimens with apoptotic indices >1.0 were found only in borderline and malignant tumors. The average apoptotic indices for borderline and malignant tumors increased to 0.5 ± 0.1 and 0.6 ± 0.1, respectively (Table 1) ▶ . For all of the malignant ovarian tumors examined, high grade tumors were associated with high average apoptotic indices (Figure 3) ▶ . The average apoptotic index for grade III tumors (0.9 ± 0.2) was the highest, whereas the apoptotic index was lowest for grade I tumors (0.3 ± 0.1), with that for grade II tumors having the intermediate index value (0.6 ± 0.2) among the malignant ovarian tumors examined. Such a trend seemed to be similar to the protein expression pattern of p53 found in malignant ovarian tumors. Statistical analyses showed a significant positive correlation between p53 expression and apoptosis (Table 1 ▶ : two-tailed, r = 0.511, P = 0.0003, n = 46), suggesting that p53 expression may be important to apoptosis during ovarian carcinogenesis. However, no correlation was found between the apoptotic index and Bcl-2 protein expression in any of the malignant ovarian tumors examined.
Discussion
Immunohistochemical results of the present study showed that an accumulation of p53 proteins was exclusively found in malignant ovarian tumors, rare in borderline ovarian tumors and absent in normal ovaries and benign tumors. This finding was in line with previous studies that p53 expression was common in malignant ovarian tumors but rare in normal ovaries and benign and borderline ovarian tumors. 48,49 Among malignant ovarian tumors, p53 expression was more prevalent in high grade tumors than in low grade tumors, implicating a possible association of p53 expression with malignancy of ovarian cancers. Interestingly, the expression pattern of bcl-2 appeared to change in a direction opposite to that of p53 expression. Bcl-2 immunoreactivity was much stronger in the epithelial cells of normal ovaries and benign and borderline epithelial tumors than malignant ovarian epithelial tumors. Among the malignant tumors, strongest Bcl-2 immunoreactivity was detected in grade I tumors, whereas grade II and grade III malignant tumors showed moderate immunoreactivities. An inverse correlation was found between the expression of Bcl-2 and p53 proteins in malignant ovarian tumors. Similar bidirectional changes of protein expression patterns of bcl-2 and p53 were also observed by Henriksen et al 41 and Diebold et al, 49 although Diebold et al failed to reveal any correlation between the expression of these two proteins. In addition, our results also showed that within the same single tumor, p53 and bcl-2 were expressed in an opposite direction in 54% (25/46) of the malignant tumors examined. For instance, when p53 expression was detected, bcl-2 expression was either absent or much reduced in the same tumor, while in other cases when bcl-2 was highly expressed, p53 expression was greatly reduced. In cases where both bcl-2 and p53 expressions were found in the same tumor, p53 expression was exclusively found in the invading epithelial cells where Bcl-2 immunoreactivities were negative or much reduced, while Bcl-2-positive cells were found only in the surface or cystic ovarian epithelial cells which were p53-negative. These observations clearly demonstrated that the protein expression patterns of p53 and bcl-2 were negatively correlated during ovarian carcinogenesis. Miyashita et al 50 identified a p53-regulating domain present in the 5′ untranslated region of bcl-2 gene which was able to inhibit bcl-2 expression. Moreover, Haldar et al 51 showed that overexpression of mutant p53 in breast cancer cell line (MCF-7) could induce down-regulation of bcl-2 both at protein and mRNA levels. Together with the findings obtained in the present study, it is speculated that down-regulation of bcl-2 expression may be a result of the inhibitory effect of p53 expression on bcl-2 during ovarian carcinogenesis.
Mutation of p53 during carcinogenesis may lead to an increased stability of the originally unstable p53 proteins, and p53 protein accumulation has been interpreted as a result of p53 gene mutation in some studies. 52-54 However, when Waggoner et al 55 examined p53 expression by immunohistochemistry and gene mutation by PCR-SSCP in clear cell adenocarcinomas of the cervix and vagina, they found that no tumors with positive p53 immunoreactivities had p53 gene mutation, indicating that p53 mutation may not necessarily be causing accumulation of p53 proteins. This finding also demonstrated the presence of wild-type p53 protein accumulation in tumor tissues. In the present study, both p53 expression and mutation are rarely seen in borderline tumors, supporting our previous observations 56 that p53 gene is not important in the pathogenesis of borderline ovarian tumors. However, more than half of the malignant ovarian tumors examined showed either expression of p53 proteins or p53 mutation as detected by PCR-SSCP. Among those malignant tumors that showed expression of p53 proteins, 37% did not have p53 mutation, whereas 29% of the malignant tumors without p53 expression however did show p53 mutation. In other words, although p53 expression and mutation are both commonly found in malignant ovarian tumors, the two events do not have a good correlation. Because the antibody used in the present study detected both the wild-type and mutant form of p53 proteins, strong immunoreactivities in tumors without SSCP abnormalities may indicate an accumulation of wild-type p53 proteins in the tumor, although mutations occurring in other coding regions of the p53 protein can still be possible.
Apoptosis was analyzed in the present study by in situ terminal transferase-mediated dUTP nick end labeling (TUNEL) and was semiquantified by the apoptotic index. It was found that in the surface epithelium of normal human ovaries and benign tumors, only a small number of apoptotic cells were found to scatter among surface epithelial cells, reflecting either the rapidity of apoptosis or low apoptotic activity in these two types of ovarian tissues. Borderline tumors and grade I tumors exhibited a slightly greater average apoptotic index, and apoptotic cells were most frequently seen in grade II and grade III malignant tumors, as indicated by the highest apoptotic indices among all of the ovarian samples examined. Diebold et al 49 reported similar observations that apoptosis was particularly prominent in high grade tumors, suggesting that although malignant tumors show high proliferative activity, relatively high apoptotic activity counteracts, leading to high cellular turnover in these tumors. When the proliferative activity of the malignant tumors exceeds apoptotic cell death, an accumulation of tumor cells results. Our present study showed a strong positive correlation between apoptosis and p53 expression, indicating the apoptotic activities that occur during ovarian carcinogenesis are mostly p53-related. Similar p53-dependent apoptotic activities have also been observed in other types of tumors. 57,58 On the contrary, our results showed no correlation between apoptosis and protein expression of bcl-2, despite the fact that the bcl-2 oncogene was the first gene shown to be involved in apoptosis. Many studies, however, have already suggested that bcl-2 may also have other functions in tissue differentiation and development, apart from being a repressor of apoptosis. For instance, the expression of bcl-2 in some neuronal populations beyond the recognized period of cell death 43,59 and its localization to a wide spectrum of early developing tissues 60,61 suggest that bcl-2 may not be simply protecting cells from death, but may also have other roles to play. In the present study, it was found that Bcl-2 immunopositivity was found frequently in the surface epithelial cells of normal human ovaries. Other normal tissues, such as hematopoietic progenitor cells, hormone-responsive organs, 19,60 and several epithelial tissues in which cells are self-renewing or proliferating 62 also show bcl-2 protein expression. The exact function of bcl-2 in normal human ovarian surface epithelial cells has yet to be revealed. It is proposed that bcl-2 may be important in maintaining the normal physiological functioning and integrity of the surface epithelium in the ovulating ovary, where the epithelium undergoes a continuous cycle of rupture and repair. Down-regulation of Bcl-2 protein may thus disrupt the normal physiology of the normal ovarian epithelium, resulting in abnormal or even malignant changes.
To recapitulate, our observations demonstrated a significant inverse correlation between bcl-2 expression and p53 protein accumulation in malignant ovarian tumors. A similar relationship has also been found in various types of human cancer tissues, suggesting that various types of human malignancies may share a common pathway of carcinogenesis in which Bcl-2 and p53 proteins are involved. However, p53 gene mutation was not a prerequisite for the expression of p53 in the malignant ovarian tumor tissues. Our results also showed that despite the fact that apoptosis is regulated by both Bcl-2 and p53 proteins in some neoplastic cells, 63-65 a positive correlation was found only between apoptosis and p53 protein expression, not between apoptosis and bcl-2 expression. The Bcl-2 protein, apart from its inhibiting activity in apoptosis, might also play roles in normal functioning of the normal ovaries’ surface epithelium, which is presumably lost during ovarian carcinogenesis.
Acknowledgments
The authors thank Raymond H. Y. Li for his comments on the statistics.
Footnotes
Address reprint requests to Dr. Samuel C. Mok, 221 Longwood Avenue, BLI 449B, Boston, MA 02115. E-mail: scmok@rics.bwh.harvard.edu.
Supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region (Project No. CUHK4218/97 Mol/L), a RGC direct grant (Medicine Panel, CUHK, 95/96) to W. C. and in part by National Institute of Health Grants CA69453, and CA69291; and U.S. Army Grant DAMD17-99-1-9563 to S. M.
References
- 1.Tortolero-Luna G, Mitchell MF: The epidemiology of ovarian cancer. J Cell Biochem 1995, 233:200-207 [DOI] [PubMed] [Google Scholar]
- 2.Piver MS, Fanning J, Craig KA: Ovarian cancer. Knapp RC Berkowitz RS eds. Gynecologic Oncology. 1993, :pp 250-292 McGraw-Hill, New York [Google Scholar]
- 3.Cannistra SA: Cancer of the ovary. N Engl J Med 1993, 329:1550-1559 [DOI] [PubMed] [Google Scholar]
- 4.Bast RC, Jr, Boyer CM, Xu FJ, Wiener J, Dabel R, Woolas R, Jacobs I, Berchuck A: Molecular approaches to prevention and detection of epithelial ovarian cancer. J Cell Biochem 1995, 23(suppl.):219-222 [DOI] [PubMed] [Google Scholar]
- 5.Medl M, Sevelda P, Czerwenka K, Dobianer K, Hanak H, Hruza C, Klein M, Leodolter S, Mullauer-Ertl S, Rosen A, Saltzer H, Vavra N, Spona J: DNA amplification of HER-2/neu and INT-2 oncogenes in epithelial ovarian cancer. Gynecol Oncol 1995, 59:321-326 [DOI] [PubMed] [Google Scholar]
- 6.Auranen A, Grenman S, Klemi PJ: Immunohistochemically detected p53 and HER-2/neu expression and nuclear DNA content in familial epithelial ovarian carcinomas. Cancer 1997, 79:2147-2153 [DOI] [PubMed] [Google Scholar]
- 7.Mok SC, Bell DA, Knapp RC, Fishbaugh PM, Welch WR, Muto MG, Berkowitz RS, Tsao SW: Mutation of K-ras protooncogene in human ovarian epithelial tumors of borderline malignancy. Cancer Res 1993, 53:1489-1492 [PubMed] [Google Scholar]
- 8.Cuatrecasas M, Villanueva A, Matias-Guiu X, Prat J: K-ras mutations in mucinous ovarian tumors. Cancer 1997, 79:1581-1596 [DOI] [PubMed] [Google Scholar]
- 9.Mok SC, Chan WY, Wong KK, Muto MG, Berkowitz RS: SPARC, an extracellular matrix protein with tumor-suppressing activity in human ovarian epithelial cells. Oncogene 1996, 12:1895-1901 [PubMed] [Google Scholar]
- 10.Stratton JF, Gayther SA, Russell P, Dearden J, Gore M, Blake P, Easton D, Ponder BAJ: Contribution of BRCA1 mutations to ovarian cancer. N Engl J Med 1997, 336:1125-1130 [DOI] [PubMed] [Google Scholar]
- 11.Mok SC, Chan WY, Wong KK, Cheung KK, Lau CC, Ng SW, Baldini A, Colitti CV, Rock CO, Berkowitz RS: DOC-2, a candidate tumor suppressor gene in human epithelial ovarian cancer. Oncogene 1998, 16:2381-2387 [DOI] [PubMed] [Google Scholar]
- 12.Wyllie AH: The genetic regulation of apoptosis. Curr Opin Genet Dev 1995, 5:97-104 [DOI] [PubMed] [Google Scholar]
- 13.Reed JC: Bcl-2 and the regulation of programmed cell death. J Cell Biol 1994, 124:1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsujimoto Y, Bashir MM, Givol I, Cossman J, Jaffe E, Croce CM: DNA rearrangements in human follicular lymphoma can involved the 5′ or the 3′ region of the bcl-2 gene. Proc Natl Acad Sci USA 1987, 84:1329-1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonnell TJ, Troncoso P, Brisbay SM, Logothetis C, Chung LWK, Hsieh J, Tu S, Campbell ML: Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res 1992, 52:6940-6944 [PubMed] [Google Scholar]
- 16.Pezzella F, Turley H, Kuzu I, Tungekar M, Dunnil M, Pierce CB, Harris A, Gatter KC, Mason DY: Bcl-2 protein in non-small-cell lung carcinoma. N Engl J Med 1993, 329:690-694 [DOI] [PubMed] [Google Scholar]
- 17.Higashiyama M, Doi O, Kodama K, Yogokouchi H, Tateishi R: High prevalence of bcl-2 expression in small cell lung cancer. Anticancer Res 1995, 15:503-505 [PubMed] [Google Scholar]
- 18.Alderson LM, Castleberg RL, Harsh GR, Louis DN, Henson JW: Human gliomas with wild-type p53 express bcl-2. Cancer Res 1995, 55:999-1001 [PubMed] [Google Scholar]
- 19.Lu QL, Elia G, Lucas S, Thomas JA: Bcl-2 protooncogene expression in Epstein-Barr virus associated nasopharyngeal carcinoma. Int J Cancer 1993, 53:29-35 [DOI] [PubMed] [Google Scholar]
- 20.Lauwers GY, Scott GV, Karpeh MS: Immunohistochemical evaluation of bcl-2 protein expression in gastric adenocarcinomas. Cancer 1995, 75:2209-2213 [DOI] [PubMed] [Google Scholar]
- 21.Eliopoulos AG, Kerr DJ, Herod J, Hodgkins L, Krajewski S, Reed JC, Young LS: The control of apoptosis and drug resistance in ovarian cancer: influence of p53 and bcl-2. Oncogene 1995, 11:1217-1228 [PubMed] [Google Scholar]
- 22.Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW: Participation of p53 protein in the cellular response to DNA damage. Cancer Res 1991, 51:6304-6311 [PubMed] [Google Scholar]
- 23.Kastan MB, Zhan Q, El-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJ, Jr: A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia telangiectasia. Cell 1992, 71:587-597 [DOI] [PubMed] [Google Scholar]
- 24.Shaw P, Bovey R, Tardy S, Sahli R, Sordat B, Costa J: Introduction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc Natl Acad Sci USA 1992, 89:4495-4499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M: Wild-type p53 induces apoptosis of myeloid leukemic cells that is inhibited by interleukin 6. Nature 1991, 352:345-347 [DOI] [PubMed] [Google Scholar]
- 26.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Butel JS, Bradley A: Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumors. Nature 1992, 356:215-221 [DOI] [PubMed] [Google Scholar]
- 27.Chang F, Syrjanen S, Syrjanen K: Implications of p53 tumor-suppressor gene in clinical oncology. J Clin Oncol 1995, 13:1009-1022 [DOI] [PubMed] [Google Scholar]
- 28.Okamoto A, Sameshima Y, Yokoyama S, Terashima Y, Surgimura T, Terrada M, Yokota J: Frequent allelic losses and mutations of the p53 gene in human ovarian cancer. Cancer Res 1991, 51:5171-5176 [PubMed] [Google Scholar]
- 29.Kihana T, Tsuda H, Teshima S, Okada S, Matttsura S, Hirohashi S: High incidence of p53 gene mutation in human ovarian cancer and its association with nuclear accumulation of p53 protein and tumor DNA aneuploidy. Jpn J Cancer Res 1992, 83:978-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milner BJ, Allan LA, Eccles DM, Kitchener C, Leonard RCF, Kelly KF, Parkin DE, Haites NE: p53 mutation is a common genetic event in ovarian carcinoma. Cancer Res 1993, 53:2128-2132 [PubMed] [Google Scholar]
- 31.Fontanini G, Vignati S, Bigini D, Mussi A, Lucchi M, Angeletti CA, Basolo F, Bevilacqua G: Bcl-2 protein: a prognostic factor inversely correlated to p53 in non-small-cell lung cancer. Br J Cancer 1995, 71:1003-1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitagawa Y, Wong F, Lo P, Elliott M, Verburgt LM, Hogg JC, Daya M: Overexpression of bcl-2 and mutation in p53 and K-ras in resected human non-small-cell lung cancers. Am J Respir Cell Mol Biol 1996, 15:45-54 [DOI] [PubMed] [Google Scholar]
- 33.Nguyen PL, Zukerberg LR, Benedict WF, Harris NL: Immunohistochemical detection of p53, bcl-2 and retinoblastoma proteins in follicular lymphoma. Am J Clin Pathol 1996, 105:538-543 [DOI] [PubMed] [Google Scholar]
- 34.Saegusa M, Takano Y, Okayasu I: Bcl-2 expression and its association with cell kinetics in human gastric carcinomas and intestinal metaplasia. J Cancer Res Clin Oncol 1995, 121:357-363 [DOI] [PubMed] [Google Scholar]
- 35.Parenti AR, Rugge M, Shiao YH, Ruol A, Ancona E, Bozzola L, Ninfo V: Bcl-2 and p53 immunophenotypes in pre-malignant, early and advanced esophageal squamous cancer. Histopathology 1997, 31:430-435 [DOI] [PubMed] [Google Scholar]
- 36.Wikonkal NM, Berg RJ, van Haselen CW, Horkay I, Remenyik E, Begany A, Hunyadi J, van Vloten WA, de Gruijl FR: bcl-2 versus p53 protein expression and apoptotic rate in human nonmelanoma skin cancers. Arch Dematol 1997, 133:599-602 [PubMed] [Google Scholar]
- 37.Gorczyca W, Markiewski M, Kram A, Tuziak T, Domagala W: Immunohistochemical analysis of bcl-2 and p53 expression in breast carcinomas: their correlation with Ki-67 growth fraction. Virchows Arch 1995, 426:229-233 [DOI] [PubMed] [Google Scholar]
- 38.Hori M, Nogami T, Itabashi M, Yoshimi F, Ono H, Koizumi S: Expression of bcl-2 in human breast cancer: correlation between hormone receptor status, p53 protein accumulation and DNA strand breaks associated with apoptosis. Pathol Int 1997, 47:757-762 [DOI] [PubMed] [Google Scholar]
- 39.Matsushima H, Kitamura T, Goto T, Hosaka Y, Kawabe K: Combined analysis with bcl-2 and p53 immunostaining predicts poorer prognosis in prostatic carcinoma. J Urol 1997, 158:2278-2283 [DOI] [PubMed] [Google Scholar]
- 40.Watson AJ, Merritt AJ, Jones LS, Askew JN, Anderson E, Becciolini A, Balzi M, Potten CS, Hickman JA: Evidence of reciprocity of bcl-2 and p53 expression in human colorectal adenomas and carcinomas. Br J Cancer 1996, 73:889-895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henriksen R, Wilander E, Öberg K: Expression and prognostic significance of Bcl-2 in ovarian tumors. Br J Cancer 1995, 72:1324–1329 [DOI] [PMC free article] [PubMed]
- 42.International Federation of Gynecology, and Obstetrics: Changes in definitions of clinical staging for carcinoma of the cervix and ovary. Am J Obstet Gynecol 1987, 156:246 [PubMed]
- 43.Chan WY, Yew DT: Apoptosis and bcl-2 oncoprotein expression in the human fetal central nervous system. Anat Rec 1998, 252:165-175 [DOI] [PubMed] [Google Scholar]
- 44.Sinicrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B: bcl-2 and p53 oncoprotein expression during colorectal tumorgenesis. Cancer Res 1995, 555:237-241 [PubMed] [Google Scholar]
- 45.Mok CH, Tsao SW, Knapp RC, Fishbaugh PM, Lau CC: Unifocal origin of advanced human epithelial ovarian cancer. Cancer Res 1992, 52:1119-1122 [PubMed] [Google Scholar]
- 46.Krajewski S, Tanaka S, Takayanma S, Schibler MJ, Fenton W, Reed JC: Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res 1993, 53:4701-4714 [PubMed] [Google Scholar]
- 47.Gold R, Schmied M, Giegerich G, Breitschopf H, Hartung HP, Tokyka KV, Lassmann H: Differentiation between cellular apoptosis and necrosis by combined use of in situ tailing and nick translation techniques. Lab Invest 1994, 71:219-225 [PubMed] [Google Scholar]
- 48.Marks JR, Davidoff AM, Kerns BJ, Humphrey PA, Pence JC, Dodge RK, Clarke-Pearson DL, Iglehart JD, Bast RC, Jr, Berchuck A: Over-expression and mutation of p53 in epithelial ovarian cancer. Cancer Res 1991, 51:2979-2984 [PubMed] [Google Scholar]
- 49.Diebold J, Baretton G, Felchner M, Meier W, Dopper K, Schmidt M, Lohrs U: Bcl-2 expression, p53 accumulation, and apoptosis in ovarian carcinomas. Am J Clin Pathol 1996, 105:341-349 [DOI] [PubMed] [Google Scholar]
- 50.Miyashita T, Harigai M, Handa M, Reed JC: Identification of a p53-dependent negative response element in the bcl-2 gene. Cancer Res 1994, 54:3131-3135 [PubMed] [Google Scholar]
- 51.Haldar S, Negrini M, Monne M, Saggioni S, Croce CM: Down-regulation of bcl-2 in human breast cancer cells. Cancer Res 1994, 54:2095-2097 [PubMed] [Google Scholar]
- 52.Aggelopoulou E, Troungos C, Goutas N, Skarlos D, Papadimitriou C, Kittas C: Immunohistochemical detection of p53 protein in HPV positive oral lesions. Anticancer Res 1998, 18:4511-4515 [PubMed] [Google Scholar]
- 53.Kiss A, Wang NJ, Xie JP, Thorgeirsson SS: Analysis of transforming growth factor (TGF)-α/epidermal growth factor receptor, hepatocyte growth Factor/c-met, TGF-β receptor type II, and p53 expression in human hepatocellular carcinomas. Clin Cancer Res 1997, 3:1059-1066 [PubMed] [Google Scholar]
- 54.Lashner BA, Shapiro BD, Husain A, Goldblum JR: Evaluation of the usefulness of testing for p53 mutations in colorectal cancer surveillance for ulcerative colitis. Am J Gastroenterol 1999, 94:456-462 [DOI] [PubMed] [Google Scholar]
- 55.Waggoner SE, Anderson SA, Luce MC, Takahashi H, Boyd J: p53 protein expression and gene analysis in clear cell adenocarcinoma of the vagina and cervix. Gynecol Oncol 1996, 60:339-344 [DOI] [PubMed] [Google Scholar]
- 56.Wertheim I, Muto MG, Welch WR, Bell DA, Berkowitz RS, Mok SC: p53 gene mutation in human borderline epithelial ovarian tumors. J Natl Cancer Inst 1994, 86:1549-1551 [DOI] [PubMed] [Google Scholar]
- 57.Lipponen PK, Aaltomaa S: Apoptosis in bladder cancer as related to standard prognostic factors and prognosis. J Pathol 1994, 173:333-339 [DOI] [PubMed] [Google Scholar]
- 58.Jasty R, Lu J, Irwin T, Suchard S, Clarke MF, Castle VP: Role of p53 in the regulation of irradiation-induced apoptosis in neuroblastoma cells. Mol Genet Metab 1998, 65:155-164 [DOI] [PubMed] [Google Scholar]
- 59.Merry DE, Veis DJ, Hickey WF, Korsmeyer SJ: Bcl-2 expression is widespread in the developing nervous and retained in the adult PNS. Development 1994, 120:301-311 [DOI] [PubMed] [Google Scholar]
- 60.LeBrun DP, Warnke RA, Cleary ML: Expression of bcl-2 in fetal tissues suggests a role in morphogenesis. Am J Pathol 1993, 142:743-753 [PMC free article] [PubMed] [Google Scholar]
- 61.Novak VD, Korsmeyer SJ: Protein expression during murine development. Am J Pathol 1994, 145:61-73 [PMC free article] [PubMed] [Google Scholar]
- 62.Hockenbery DM, Zutter M, Hickey W, Nahm M, Korsmeyer SJ: Bcl-2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci USA 1991, 88:6961-6965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y, Szekely L, Okan I, Klein G, Wiman KG: Wild-type p53-triggered apoptosis is inhibited by bcl-2 in a v-myc-induced T-cell lymphoma line. Oncogene 1993, 8:3427-3431 [PubMed] [Google Scholar]
- 64.Marin MC, Hsu B, Meyn RE, Donehower LA, El-Naggar AK, McDonnell TJ: Evidence that p53 and bcl-2 are regulators of a common cell death pathway important for in vivo lymphomagenesis. Oncogene 1994, 9:3107-3112 [PubMed] [Google Scholar]
- 65.Zhaung SH, Shvarts A, Jochemsen AG, van Oorschot AAAM, van der Eb AJ, Noteborn MHM: Differential sensitivity to Ad5 E1B–21kD and bcl-2 proteins of apoptin-induced apoptosis. Carcinogenesis 1995, 16:2939-2944 [DOI] [PubMed] [Google Scholar]