Abstract
Specific nonparenchymal epithelial cell (NPEC) clusters derived from normal adult porcine livers demonstrate a characteristic developmental pattern in the presence of other types of nonparenchymal cells in vitro. This pattern includes scattering, colonial growth, and an emergence of duct-like structures (DLSs) in the colonies. It has been confirmed that 96% of the scattered cell clusters in these cultures develop into colonies containing DLSs. In this study, we examine the differentiation of NPEC clusters using the scattered formation as a marker of the DLS-emerged colonies. We report that the NPECs expressed albumin, α-fetoprotein, transferrin, cytokeratin (CK) 18, CK7, and c-met, but not α-1-antitrypsin (AAT), at the scattering stage. In addition, at the same stage, NPECs expressed oval-cell-related markers such as OV6, but not biliary epithelial cell (BEC) markers such as γ-glutamyltransferase, CK19, and CK14. At the DLS emerging stage, hepatocyte markers, including AAT, were detectable in the cells either at the periphery of colonies or in the cells surrounded by the DLSs. On the other hand, the cells constituting DLSs expressed BEC markers, suggesting a bile duct nature of the DLSs. Furthermore, the cells in the colonies possessed an ultrastructural appearance of differentiated hepatocytes and BECs. These results suggest that certain NPECs are bipotent, and that, in culture, they mimic hepatoblast development in vivo.
Studies of embryonic rodent liver development indicate that the emerging liver contains hepatoblasts, which are bipotential progenitor cells that can differentiate along either the hepatocyte or the biliary epithelial cell (BEC) lineage. 1,2 Marceau et al showed that the number of these progenitor cells decreases rapidly after birth and presented substantial evidence suggesting that such progenitor cells are absent in the adult liver. 2,3 The epithelial compartment of the liver is composed of hepatocytes, BECs, and other nonparenchymal epithelial cells (NPECs). 4 Certain NPECs, isolated from either an adult mouse or rat, are thought to be capable of acting as progenitor cells. 5,6 In addition, others have suggested that NPEC cell lines behave as putative hepatic stem cells. 4,5 These studies show that NPECs display a capacity for hepatocytic differentiation, although their ability to differentiate along the BEC lineage remains to be determined. On the other hand, it is well known that oval cells, which proliferate in the peri-bile duct region when hepatocytes are impaired and unable to mount an appropriate growth response, are capable of differentiation into either mature hepatocytes or BECs in an adult liver. 7-9 Thus, NPECs and oval cells exhibit the phenotypic characteristics of hepatoblasts and may give rise to both hepatocyte and BEC lineages. 10,11 However, the identification of NPECs as hepatoblasts or hepatoblast precursors remains obscure. One reason for this uncertainty is that the NPECs have not been determined to differentiate morphologically into bile ducts under physiological conditions.
Previously, we reported that normal adult porcine liver tissue may contain a population of cells, NPECs, with the capacity to differentiate into either hepatocytes or BECs. 12 However, the nature of NPECs remained to be defined due to the lack of experimental data and insufficient cellular characterization. In the study reported here, we investigated the nature of the NPECs using several experimental approaches. First, we used a nonparenchymal cell fraction, prepared from normal porcine livers, rather than a parenchymal cell fraction, to obtain NPECs. A nonparenchymal cell fraction should contain more NPECs and, most likely, more bipotential progenitor cells than a parenchymal cell fraction. Second, to study the differentiation of NPECs, we examined the relation between the scattered cell clusters and the duct-like structure (DLS)-emerged colonies. Finally, we investigated the expression of several phenotypic markers for epithelial cell lineages in cell culture, and include an ultrastructural analysis. Together, these studies aimed at defining the nature of NPECs, including their cellular origin.
Materials and Methods
Isolation and Culture of NPECs
Liver cell suspensions were prepared from 6- to 6.7-month-old abattoir pigs by a four-step retrograde perfusion method, as described elsewhere. 13 The suspension was centrifuged once at 160 × g for 5 minutes, and the cell pellet fraction was centrifuged once at 60 × g for 2 minutes, and twice at 60 × g for 1 minute. All of the supernatant fractions from each centrifugation were mixed and centrifuged either once or twice at 350 × g for 5 minutes. Finally, the cell pellet, a nonparenchymal cell fraction, was resuspended in National Institute for Advanced Interdisciplinary Research (NAIR)-1 medium, plated on type I collagen-coated culture dishes at a cell density of about 2 × 10 4 cells/60-mm dish, and cultured at 37°C in a humidified incubator with 5% CO2 in air. In some cases, cells were frozen at this time in freezing medium (90% NAIR-1 medium and 10% dimethylsulfoxide) and stored at −190°C. NPEC clusters, the target cell clusters, were marked by an object marker (Nikon Co., Tokyo, Japan) on day 1 in culture, and other cells near the NPEC clusters, within the field of vision using a 10× magnifying object lens, were eliminated with a scraper on days 2–3. The NAIR-1 medium, originally formulated by us, consists of Dulbecco’s modified Eagle’s medium/F12 (1:1) medium supplemented with 10 mmol/L HEPES, 5% fetal bovine serum (v/v), 10 ng/ml glucagon, 10 μg/ml insulin, 60 ng/ml hydrocortisone, 25 ng/ml epidermal growth factor (EGF), 10 μg/ml transferrin (Tf), 50 nmol/L triiodothyronin, 5 ng/ml sodium selenate, 10 mmol/L nicotinamide, 0.1 mmol/L L-ascorbic acid phosphate magnesium salt, 1 μg/ml [α]-tocopherole acetate, 50 ng/ml linoleic acid, and antibiotics.
Phase-Contrast Micrographs of Cells
The same fields of cultured NPECs, identified by the needle marks, were photographed each day on a Nikon phase-contrast microscope.
Scattered NPEC Cluster and Duct-Like Structure-Emerged Colony Counting
Freshly isolated cells of the nonparenchymal fraction were plated on type I collagen-coated 60-mm grid culture dishes in NAIR-1 medium. Frozen cell samples were thawed and used similarly. Scattered NPEC clusters were marked, as described above, on day 1. The marked cell clusters were checked on a phase-contrast microscope on days 3 and 4 and the number of scattered cell clusters were manually counted. On days 8 to 10, the number of duct-like structure-emerged colonies, derived from marked scattered cell clusters, were similarly counted.
Immunocytochemistry
At appropriate times during culture, the cells were fixed in 4% neutral buffered paraformaldehyde, for subsequent examination for albumin (Alb), α-fetoprotein (AFP), Tf, α-1-antitrypsin (AAT), c-met, and vimentin, in an absolute ethanol at −30°C for detection of cytokeratin (CK) 18 and CK19, and in absolute methanol at −30°C for detection of CK 7, CK 14, OV6, BD.1, BD.2, H.4, OC.2, OC.5, and OC.10. Rabbit anti-rat Alb (Cappel, Durham, NC), rabbit anti-human AFP (Dako, Copenhagen, Denmark), rabbit anti-human Tf (Dako), rabbit anti-human AAT (Zymed Lab. Inc., San Francisco, CA), rabbit anti-human c-met (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), mouse monoclonal anti-human CKs, 7 (Dako), 14 (Cymbus Biotechnology Ltd., Hants, UK), 18 (Progen, Heidelberg, Germany), and 19 (Progen), mouse monoclonal anti-porcine vimentin (Dako) and mouse monoclonal anti-rat OV6, BD.1, BD.2, H.4, OC.2, OC.5, and OC.10 (gifts from Drs. D. C. Hixson and S. Sell) were used as primary antibodies. Immunostaining was carried out using a LSAB kit containing 3-amino-9-ethylcarbazole as a substrate of horseradish peroxidase (Dako LSAB Kit, Dako-JAPAN Co., Kyoto, Japan), as described previously. 14 Hep.G2 cells (hepatoblastoma), HuH-7 cells (hepatocellular carcinoma), THLE-5b cells (SV40 T antigen-immortalized human liver epithelial cell line), Ac2F cells (rat liver epithelial cell line), OZ cells (human gallbladder cancer cell line), rat hepatocytes, normal human dermal fibroblasts, and Chinese hamster ovary-K1 cells were used as controls. 5,14
Enzyme Cytochemistry
For the histocytochemical demonstration of γ-glutamyltransferase (GGT) in the cultured cells, the method of Rutenberg et al was used. 15 Ac2F cells were used as a positive control.
Transmission Electron Microscopy
Cultured cells, plated on a type I collagen-coated toluen-resistant plastic sheet (Wako, Tokyo, Japan), were fixed with 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer, pH 7.4, at room temperature for 30 minutes, postfixed with 2% osmium tetraoxide in the buffer, and embedded in situ in Epon 812. Semithin and ultrathin sections were cut on a Bromma 2088 ultratome V (LKB, Schweden, Germany). The semithin sections were stained with 1% toluidine blue and examined with a light microscope. The adjacent thin sections were stained with uranyl acetate, followed by lead citrate and examined at 60 KV with a H7000 transmission electron microscope (Hitachi, Tokyo, Japan).
Results
Phase-Contrast Microscopic Observation
A number of the NPEC clusters (target cell clusters) among the several cell clusters that attached to the dishes on day 1 displayed a slower spreading rate (Figure 1A) ▶ . Cultures of the nonparenchymal cell fraction produced many more such NPEC clusters than cultures of the parenchymal cell fraction examined in the previous study (data not shown). 12 NPEC clusters required approximately 48 hours in culture to spread well, whereas the other cell clusters spread within 24 hours (Figure 1B) ▶ . The NPEC clusters began to proliferate and scatter on days 3 and 4, while maintaining their polygonal shapes (Figure 1, C ▶ -F). Thereafter, the cells grew rapidly to form colonies (Figure 1G) ▶ . Although the cells at the periphery of colonies were morphologically similar to those observed on days 3 to 4, the cells in the central regions of the colonies were smaller in size, as previously reported (Figure 1, H ▶ -K). 12 After day 7, several duct-like structures emerged within the colonies (Figure 1H) ▶ . In addition, not only the cells constituting the duct-like structures, but also those surrounded by the structures were morphologically changed after further time in culture. As shown in Figures 1L and 2 ▶ ▶ , the cells surrounded by the duct-like structures appeared cobblestone-like with dark cytoplasms by day 14.
Figure 1.
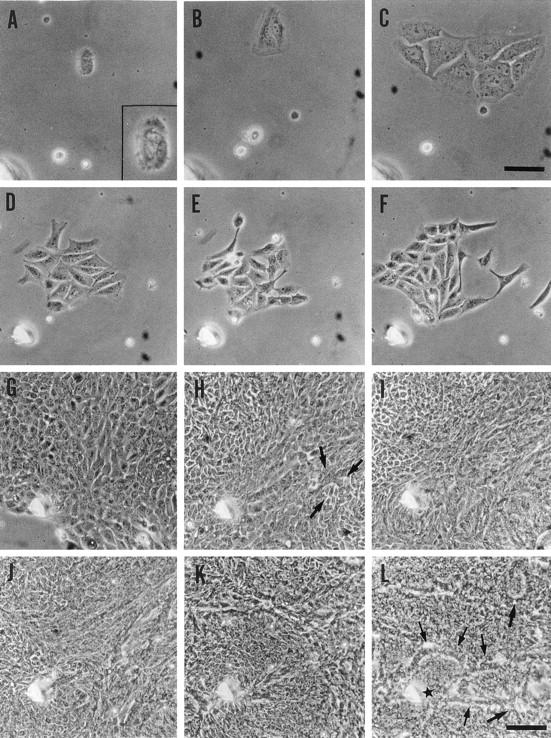
Development of colonies derived from porcine nonparenchymal epithelial cell clusters. The cells within the same field marked by a needle are shown. A: Day 1 (28 hours after plating). The photograph within the square is at a higher magnification. NPECs are observed as clusters. B: Day 2 (50 hours). NPECs spread to about twice in area. C: Day 3 (81 hours). NPEC clusters begin to proliferate. D: Day 4 (101 hours). NPEC clusters begin to scatter. E: Day 4 (130 hours). F: Day 5 (141 hours). G: Day 7 (166 hours). H: Day 8 (197 hours). Arrows indicate a duct-like structure. I: Day 9 (220 hours). J: Day 10 (244 hours). K: Day 12 (292 hours). L: Day 15 (360 hours). Arrows indicate several duct-like structures. The cells, containing dark cytoplasm that surrounded by the duct-like structures, adopt a cobblestone-like morphology. The star indicates the position highly magnified in Figure 2 ▶ . Original magnifications, ×200 (A−C; scale bar, C, 50 μm) and ×100 (D−L; scale bar, L, 100 μm).
Figure 2.
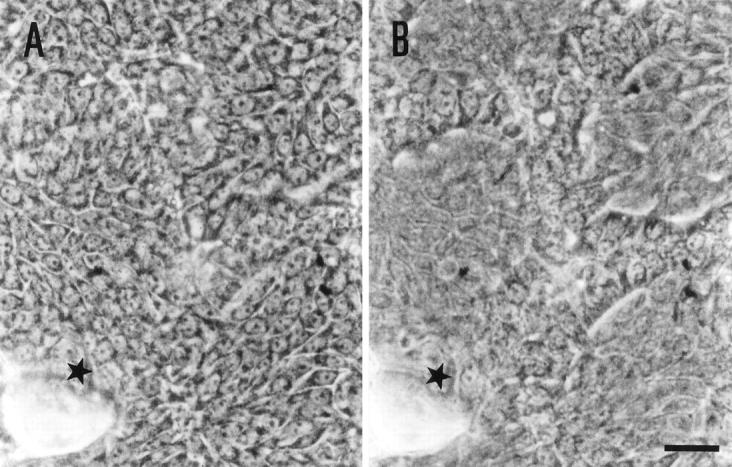
High magnified microphotographs of Figure 1L ▶ . Cells surrounded by the duct-like structures are in focus (A) and those constituting duct-like structures are in focus (B). Original magnifications, ×400. Scale bar, 25 μm.
Counts of Scattered NPEC Clusters and Duct-Like Structure-Emerged Colonies
Previously, and in this study, we observed the scattering of NPEC clusters on day 3 to 4 in culture (Figure 1, D ▶ -F). 12 In the present experiments, 25 (33%) out of 75 clusters examined, displayed the described scattering (Table 1) ▶ . To assess whether the scattered formation could be useful as an early marker of the duct-like structure-emerged colonies, the incidence of colonies with emerging duct-like structures derived from scattered cell clusters was examined. We found that 96% of the scattered NPEC clusters developed into duct-like structure-emerged colonies, as shown in Table 1 ▶ . These data suggest that there is a close relation between the scattered formation and the development of duct-like structure-emerged colonies and that the scattered formation is useful as an early marker of duct-like structure-emerged colonies.
Table 1.
Incidence of Duct-Like Structure-Emerged Colonies Derived from Scattered Epithelial Cell Clusters in Primary Cultures of Adult Porcine Livers
| Primary culture | No. of clusters examined | No. of scattered clusters (A) | No. of duct-like structure-emerged colonies derived from scattered clusters (B) | B/A (%) |
|---|---|---|---|---|
| 1-F* | 116 | 32 | 31 | 97 |
| 2-F* | 64 | 5 | 5 | 100 |
| 3 | 149 | 43 | 42 | 98 |
| 3-F* | 95 | 45 | 41 | 91 |
| 4-F* | 44 | 13 | 13 | 100 |
| 5-F* | 25 | 10 | 9 | 90 |
| 6 | 32 | 14 | 13 | 93 |
| Average | 75 | 23 | 22 | 96 |
*Cultures frozen immediately after cell isolation were thawed in use.
Cytochemical Analysis
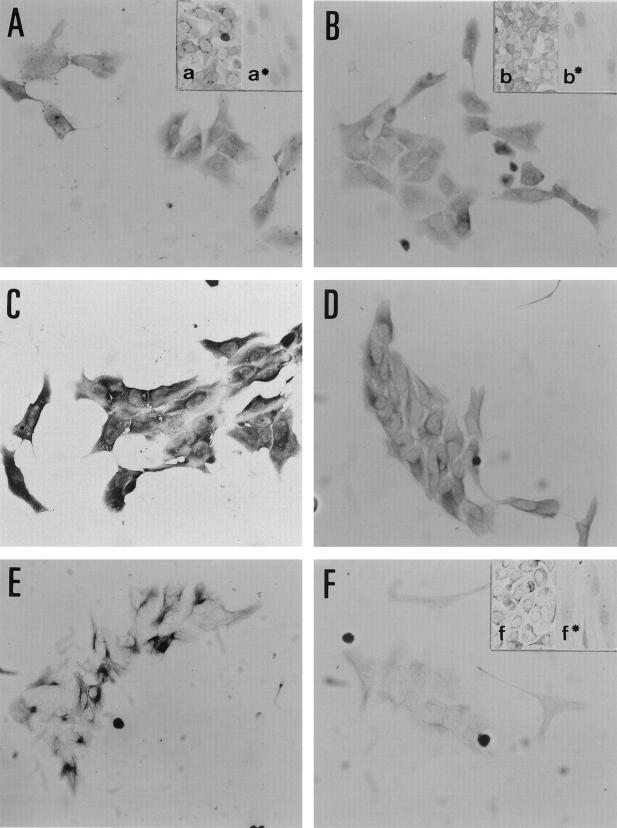
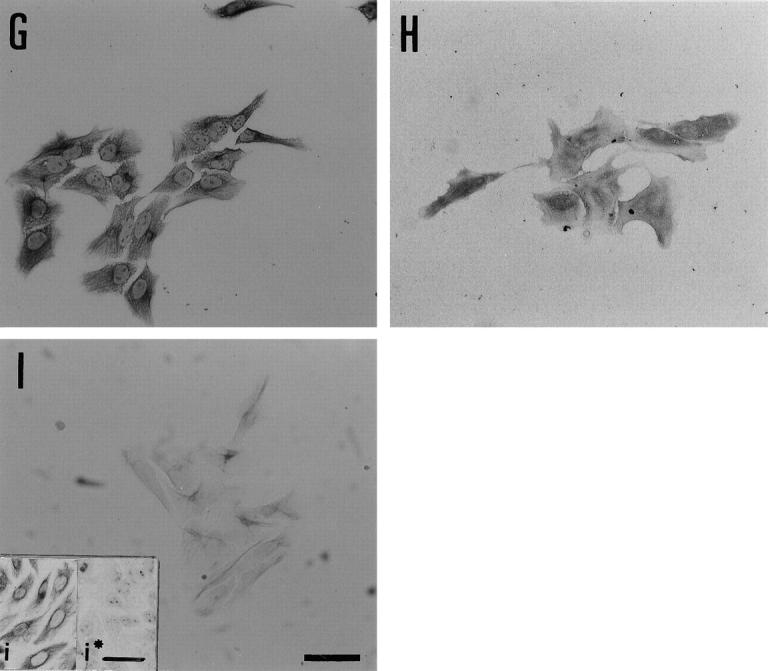
At the scattering stage (day 3–4), the NPEC clusters were recognized by antibodies against Alb, AFP, and Tf (Figure 3, A ▶ -C), but not by antibodies against AAT and H.4 (data not shown). Alb, AFP, and Tf were expressed to a similar extent by all NPECs, suggesting a homogenous expression of the proteins. At the duct-like structure-emerging stage (day 8–10), all of these hepatocytic markers, except H.4, were expressed in a subset of both the cells at the periphery of colonies and those surrounded by the duct-like structures, although the intensity and the frequency of expression was slightly different between each marker. The cells at the periphery of the colonies expressed Alb and AFP more strongly than those surrounded by the duct-like structures. The expression of these markers in the cells surrounded by duct-like structures decreased with time in culture (Figure 4, A and B) ▶ . In contrast, Tf was expressed homogeneously and strongly in both the cells at the periphery of the colonies and those surrounded by the duct-like structures (Figure 4C) ▶ . AAT expression was observed in a pattern consisting of discrete clusters of cells at the periphery of colonies (Figure 4D) ▶ . In addition, all of the proteins, such as GGT, CK19, and CK14, which are thought to be markers of BECs, were not expressed at detectable levels by the scattered NPECs (data not shown), with the exception of CK7 (Figure 3D) ▶ . However, the cells constituting duct-like structures expressed these proteins (Figure 4, E ▶ -H). Both the scattered NPECs and the cells covering the duct-like structure-emerged colonies expressed CK7 and CK18 at similar levels (Figures 3, D and E, and 4 ▶ ▶ , H and I).
Figure 3.
Immunocytochemistry of scattered NPEC clusters on day 3 to 4. Cells show homogenous expression of each marker protein. Albumin (A) and transferrin (C) are expressed strongly in the cytoplasm and faintly in the nucleus. AFP expression (B) is detected in a granular pattern in the cytoplasm. CK7 (D), CK18 (E), OV6 (F), OC.10 (G), and vimentin (I) are present in a fibrous pattern in the cytoplasm. OV6 and vimentin are faintly detectable as compared to others. C-met (H) is detectable both in the nucleus and in the perinuclear region of the cytoplasm. All photographs are the same magnification. Scale bar (I), 50 μm. Insets show positive and negative (*) controls. a: HepG2. a*: NHDF. b: HepG2. b*: NHDF. f: Ac2F. f*: NHDF. i: CHO-K1. i*: HepG2. Original magnifications, ×200 (except insets, ×160). Scale bar, i*, 50 μm.
Figure 4.
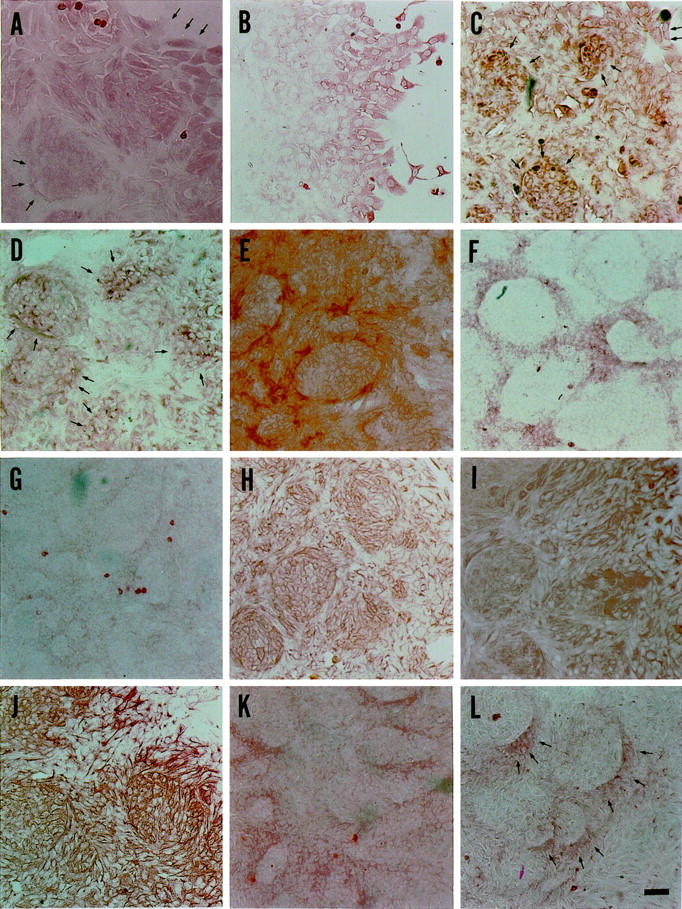
Immunocytochemical analysis of duct-like structure-emerged colonies on Day 8 to 10. Albumin (A), transferrin (C), and AAT (D) are present in the cells at the periphery of colonies and in those surrounded by duct-like structures. AAT is detectable in the cells at the periphery of colonies as colonial spots. AFP (B) is expressed only in the cells at the periphery of colonies after Day 9. GGT (E), CK19 (F), CK14 (G), and OC.10 (L) are present in the cells constituting duct-like structures, although CK14 expression is faint. CK7 (H), CK18 (I), OV6 (J), and BD.2 (K) are expressed in all cells covering the duct-like structure-emerged colonies. Cells at the periphery of colonies express OV6 more strongly than those in the central parts of colonies. Original magnifications, ×100. Arrows indicate positive cells. Scale bar, L, 50 μm.
In addition to the above markers, the scattered NPECs and the cells of the duct-like structure-emerged colonies were examined for the expression of OV6, BD.1, BD.2, OC.2, OC.5, and OC.10. Hixson et al have reported that both oval cells and BECs express OV6, BD.2, OC.2, OC.5, and OC.10, whereas only BECs express BD.1 (personal communication). 9,16-19 In the present study, the scattered NPECs were stained positively for OV6, OC.5, and OC.10, although the staining intensity for OV6 was weak, while the cells stained negatively for BD.1, BD.2, and OC.2 (Figure 3, F and G ▶ ; data for BD.1, BD.2, OC.2, and OC.5 not shown). On the other hand, the cells constituting duct-like structures were stained positively for all of these six markers. All cells covering the duct-like structure-emerged colonies were OV6-positive and the cells at the periphery of colonies were stained more strongly than cells in the other parts of the colonies (Figure 4J) ▶ . BD.1 and OC.2 were expressed in all cells of the colonies. However, the staining intensity was weak, especially in cells that constituted the duct-like structures, as compared with control cells (data not shown). Only the cells constituting duct-like structures exhibited strong fibrous cytoplasmic staining with antibodies to OC.10 (Figure 4L) ▶ . BD.2 was found to be expressed in a similar pattern as CK18 (Figure 4K) ▶ , and all of the cells of the colonies exhibited a granular cytoplasmic staining with antibodies to OC.5 on day 3 to 4 (data not shown). The NPECs expressed c-met at early culture (Figure 3H) ▶ , regardless of their scattered formation. In the duct-like structure-emerged colonies, only the cells at the periphery of colonies expressed c-met (data not shown). In addition, the scattered NPECs were stained positively for vimentin (Figure 3I) ▶ and most of the cells constituting duct-like structure-emerged colonies, with an exception of the cells surrounded by duct-like structures, were vimentin-positive (data not shown). All of the results of immunocytochemical staining in both the scattered NPECs and the duct-like structure-emerged colonies are summarized in Table 2 ▶ .
Table 2.
Comparison of the Phenotypic Expression of Scattered Nonparenchymal Epithelial Cell Clusters with Duct-Like Structure-Emerged Colonies
| Markers | Scattered cell cluster | Duct-like structure-emerged colony | ||
|---|---|---|---|---|
| D | I | P | ||
| albumin | ++ | − | + | ++ |
| α-fetoprotein | ++ | − | − | ++ |
| transferrin | ++ | − | ++ | ++ |
| α-1-antitrypsin | − | − | ++ | +/− |
| γ-glutamyltransferase | − | ++ | ± | − |
| cytokeratin 19 | − | + | − | − |
| cytokeratin 14 | − | + | − | − |
| cytokeratin 7 | + | ++ | ++ | ++ |
| cytokeratin 18 | ++ | ++ | ++ | ++ |
| OV6 | + | + | + | ++ |
| BD.1 | − | ++ | + | + |
| BD.2 | − | + | + | + |
| OC.2 | − | + | ± | ± |
| OC.5 | + | +/− | +/− | +/− |
| OC.10 | ++ | + | ± | ± |
| H.4 | − | − | − | − |
| c-met | ++ | − | − | + |
| vimentin | + | ++ | − | + |
D, cells constituting duct-like structures; I, cells surrounded by the duct-like structures; P, cells at the periphery of a colony.
−, negative; ±, faintly positive; +/−, partially positive; +, positive; ++, strongly positive.
Ultrastructure of the Cells Constituting Duct-Like Structure-Emerged Colonies
Some of the cells found inside the duct-like structures possessed tight junctions with desmosomes and bile canaliculi-like structures with microvilli, which are thought to be the characteristics of mature hepatocytes (Figure 5A) ▶ . On the other hand, the cells constituting duct-like structures, themselves, had a relatively large nucleus-cytoplasm ratio, formed clear lumens with short microvilli, and interepithelial junctional complexes. From these characteristics, these cells appeared to be BECs (Figure 5, B and C) ▶ .
Figure 5.
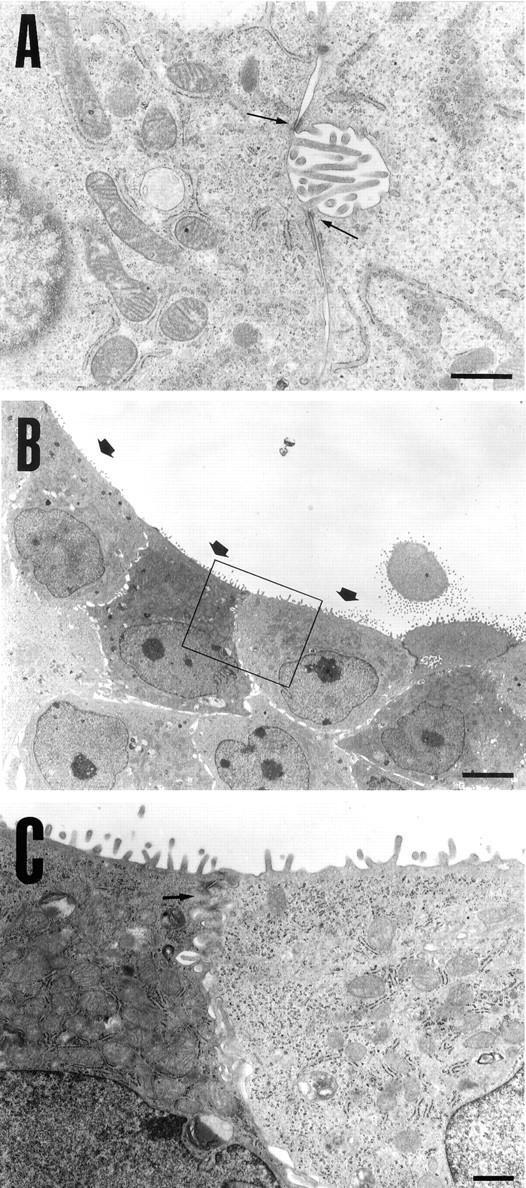
Electron microscopy of cells surrounded by the duct-like structures and those constituting the structures. A: Some of the cells surrounded by the duct-like structures have numerous glycogen rosettes, tight junctions with desmosomes (arrows), and bile canaliculi-like structures with microvilli. B and C: Cells constituting duct-like structures show a large nucleus-cytoplasm ratio, a lumen structure with short microvilli (arrows in B), and juxtaluminal junctional complexes (arrow in C). The nuclei, which are notched in some cells, are located in the basal region of the cells. C: High magnification of the boxed area in B. Original magnifications, ×12,000 (A), ×1500 (B), ×6000 (C). Scale bars, 1 μm (A and C) and 5 μm (B).
Discussion
Bipotential Nature of NPECs
The NPECs, that we describe, expressed not only the hepatocytic markers Alb and Tf, but also the hepatoblast marker AFP at the scattering stage. Others suggest that these cells are relatively immature, possessing characteristics similar to those of hepatoblasts. 1,2,20 However, the NPECs are also thought to be capable of differentiating along the hepatocytic lineage in culture from the expression of AAT, a marker for highly differentiated hepatocytes. 21,22 The differentiation of NPECs into mature hepatocytes is supported by the morphological observations that the cells, when surrounded by duct-like structures, became dark in their cytoplasms, and that tight junctions with desmosomes and bile canaliculi-like structures with microvilli emerged in culture. In contrast to the evidence for hepatocyte differentiation, NPECs expressed both CK7 and CK18 during the whole culture period of this experiment. These two phenotypic markers are reported to be expressed in BECs and oval cells, although hepatocytes are known to express CK18, but rarely CK7. 1 In addition, CK7 has been reported to be expressed in the matured intrahepatic bile duct cells of embryos. 1 Furthermore, in this study it was shown that oval cell markers, such as OV6, OC.5, and OC.10, were expressed in a pattern similar to the cytokeratins mentioned above, and that the other BEC markers were expressed only in duct-like structure-emerged colonies. Moreover, the cells constituting duct-like structures were shown, ultrastructurally, to express some characteristics of BECs, suggesting the morphological differentiation of NPEC clusters into BECs. Together, these results suggest that NPECs are immature cells at the scattering stage, as described above, and that they are capable of differentiation into BECs. In addition, these results suggest that duct-like structure-emerged colonies contain a population of cells that are immature due to the presence of AFP-positive cells among the colonies. A limited number of reports have been presented on the formation of bile ductal structures by either hepatic stem cells or precursor cells in culture. Lazaro et al reported that ductal structures were formed by oval cell lines in the presence of hepatocyte growth factor (HGF) and/or keratinocyte growth factor in a three-dimensional collagen gel matrix system. 23 These results suggest that oval cells are able to differentiate along the bile duct lineage. Block et al reported that mature hepatocytes were induced to dedifferentiate and proliferate in the presence of growth factors such as HGF and/or EGF, followed by the expression of markers of bile ductal epithelium. 24 In the same study, it was shown that nonparenchymal cells and a special microenvironment, such as Matrigel, contributed to the ductal structure formation. Together, these results suggest that immature hepatocytes formed bile duct structures when they were provided the appropriate conditions. Considering all of these results, it appears that hepatocytes and BECs are very similar in nature, even after maturation, and they might be able to alter their phenotype in response to specific conditions. Often, the phenotypic transition between hepatocytes and BECs is observed during normal development and in various pathological conditions of the liver (ductular metaplasia). 25,26 Haque et al describe the possibility that certain ductular hepatocytes, found in liver during regeneration, represent metaplastic bile ductules. 27 The results presented in this study suggest that certain NPECs derived from adult pigs are bipotential and are able to acquire the differentiated properties of both hepatocytes and BECs in culture. The possibility as to whether the formed bile duct-like structures are due to ductular metaplasia remains to be determined.
The Origin of NPECs
As described above, certain NPECs, derived from adult porcine livers, appear to have a functional resemblance to early embryonic liver cells. Maceau et al reported that the progenitor cells of hepatocytes and BECs, embryonic day 12 (E12) cells present in a mouse liver at E10.5–12, already express AFP, Alb, GGT, and BDS7, and are not only morphologically, but also functionally different from the cells found at E15–17. 2,3 Moreover, E12 cells are reported to be capable of differentiation into either hepatocytes or BECs in vitro. 2 In addition, their microenvironments are thought to be important in determining which lineage they will follow. 28,29 These results suggest that embryonic livers possess bipotential stem-like cells as either hepatoblasts or immature hepatocytes. Hepatoblasts express some hepatocytic markers, such as AFP, Alb, CK8, and CK18, as well as BEC markers, such as GGT and CK19. 1,2 The present study shows that NPECs, at early stages in culture, share many phenotypic characteristics with hepatoblasts, although the former does not express the BEC markers examined, except for CK7. These results suggest that certain NPEC cultures mimic hepatoblast development in an embryonic liver.
The data presented here also indicate that the NPECs share some phenotypic characteristics with oval cells. In this study, the NPECs were derived from normal adult livers, whereas oval cells are known to be induced to proliferate from severe damage that renders hepatocytes unrecoverable. It is likely that the destruction of extracellular matrix substances, resulting from dispase and collagenase digestion, induced an activation of the NPECs in this study. Therefore, the NPECs that we observe can be regarded as facultative progenitor cells. Small hepatocytes, assuming the role of committed stem cells, proliferate and become capable of differentiation into mature hepatocytes. 22,29,30 NPECs seem to be distinct from small hepatocytes in that NPECs express AFP in all cells at the scattering stage. AFP expression becomes localized in the cells at the periphery of colonies, whereas small hepatocytes rarely express AFP in culture.
The Mechanism of NPEC Differentiation
The biological mechanisms responsible for the scattering and differentiation of NPECs remains to be determined. Often, cell-cell or growth factor-cell interactions have been implicated as important for hepatic cell differentiation. 24,25,29,31-33 This study strongly suggests that soluble factors (growth factors), which may be supplied by nonparenchymal nonepithelial cells, such as stellate cells, 34 endothelial cells, and Kupffer cells, are more important than the direct cell-cell contact for the differentiation of NPECs. The nonparenchymal cells, adjacent to the NPEC clusters, were mechanically eliminated, as described in Materials and Methods, at early culture stages so that cell-cell contact was avoided. Scattering, followed by ductal structure formation in MDCK epithelial cells, can be induced by HGF. 35 The exposure of both mouse liver epithelial cell lines 36 and oval cell lines 23 to HGF results in the formation of either hepatic plate-like trabeculae or branching duct-like tubules when grown on collagen gels. Moreover, it is thought that HGF may be closely related to the ontogenesis of the liver from the observation that transgenic mice that have lost HGF gene expression failed in liver organogenesis. 37 In our experiments, NPECs were shown to express c-met at the scattering stage, suggesting that HGF is involved as an inducer in the scattering of these cells and in the subsequent formation of duct-like structures. Several known or unknown growth factors in addition to HGF may be produced by the cells in our cultures. Further work is needed to investigate the cooperative effect of these factors and other components included in the culture medium, NAIR-1, on the growth and/or differentiation of NPECs. 25,35,37
Homogeneity of NPEC Clustered Cells
The determination of whether the cells of an NPEC are of a single cell origin is crucial for the evaluation of the bipotential nature of these cells. NPECs, in the present study, were observed as a cluster consisting of at least three cells on day 1. We have attempted single cell cloning to obtain a pure colony, but technical difficulties have made the methods unsuccessful thus far. We regard the NPEC clustered cells as a homogeneous cell population, although not of a single cell origin, for two reasons: NPEC clusters can be produced at a near constant rate in the same inoculum size of cells derived from different animals, ie, one NPEC cluster per 1–2 × 10 4 cells/60-mm dish; and the NPEC clustered cells homogeneously express either liver-specific or biliary markers at the scattering stage. Further, there are two possible explanations for the presence of cell clusters: i) all of cell clusters were formed by random aggregation of mixed cell populations after cell dispersion, and ii) some of the clusters were derived from enzyme-resistant in vivo cell populations. We hypothesize that enzyme-resistant cell clusters exist in vivo, some of which may retain a stem-like cell nature and are activated by the cell dissociation procedure.
The results presented here suggest that bipotential NPECs, whose culture mimics hepatoblast development, exist in adult porcine livers. NPECs include ductal and ductal epithelial cells, and, most likely, a population of putative progenitor cells. 3,10 We have used the scattered formation as a marker of duct-like structure-emerged colonies to examine the differentiation of NPECs in this study. These methods should provide a powerful means for isolating and studying the biology of liver progenitor cells. These epithelial cells also will provide a valuable system to develop new clinical strategies, such as liver-reconstruction, gene therapy, and cell transplantation, instead of liver transplantation, which faces a shortage of donors in near future.
Acknowledgments
We thank Drs. D. C. Hixson and S. Sell for providing us with antibodies against OV6, BD.1, BD.2, OC.2, OC.5, OC.10, and H.4, and Ms. N. Sugae for transmission electron microscope microphotographs.
Footnotes
Address reprint requests to Dr. Takayoshi Tokiwa, Department of Cell Physiology, Kohno Clinical Medicine Research Institute, 3–4-4 Kita-shinagawa, Shinagawa, Tokyo 140-0001, Japan.
Supported by grant from the Japanese Ministry of Public Welfare (to T. T.).
References
- 1.Shiojiri N, Lemire JM, Fausto N: Cell lineages and oval cell progenitors in rat liver development. Cancer Res 1991, 51:2611-2620 [PubMed] [Google Scholar]
- 2.Germain L, Blouin M-J, Marceau N: Biliary epithelial and hepatocytic cell lineage relationships in embryonic rat liver as determined by the differential expression of cytokeratins, α-fetoprotein, albumin, and cell surface-exposed components. Cancer Res 1988, 48:4909-4918 [PubMed] [Google Scholar]
- 3.Marceau N, Chamberland C, Blouin M-J, Noel M, Loranger A: Progenitor cells in embryonic and post-natal rat livers, their growth and differentiation potential. Bell E eds. Tissue Engineering Current Perspectives. 1993, :pp 58-65 Birkhauser, Berlin [Google Scholar]
- 4.Grisham JW: Cell types in rat liver cultures: their identification and isolation. Mol Cell Biochem 1983, 53/54:23-33 [DOI] [PubMed] [Google Scholar]
- 5.Tokiwa T, Nakabayashi H, Miyazaki M, Sato J: Isolation and characterization of diploid clones from adult and newborn rat liver cell lines. In Vitro 1979, 15:393-400 [DOI] [PubMed] [Google Scholar]
- 6.Sirica AE, Mathis GA, Sano N, Elmore L: Isolation, culture, and transplantation of intrahepatic biliary epithelial cells and oval cells. Pathobiology 1990, 58:44-64 [DOI] [PubMed] [Google Scholar]
- 7.Faust N, Lemire JM, Shiojiri N: Oval cells in liver carcinogenesis: Cell lineages in hepatic development and the identification of stem cells in normal. Sirica AE eds. The Role of Cell Types in Hepatocarcinogenesis. 1992, :pp 89-108 CRC Press, Boca Raton, FL, [Google Scholar]
- 8.Thorgeirsson SS, Evarts RP: Growth and differentiation of stem cells in adult rat liver. Sirica AE eds. The Role of Cell Types in Hepatocarcinogenesis. 1992, :pp 109-120 CRC Press, Boca Raton, FL, [Google Scholar]
- 9.Hixson DC, Faris RA, Yang L, Novikoff P: Antigenic clues to liver development, renewal, and carcinogenesis: an integrated model. Sirica AE eds. The Role of Cell Types in Hepatocarcinogenesis. 1992, :pp 151-182 CRC Press, Boca Raton, FL, [Google Scholar]
- 10.Faust N: Liver stem cells. 3rd ed. Arias IM Boyer JE Fausto N Jakoby WB Schachter DA Shafritz DA eds. The Liver: Biology and Pathobiology, 1994, :pp 1501-1518 Raven Press, New York [Google Scholar]
- 11.Grisham JW, Thorgeirsson SS: Liver stem cells. Potten CS eds. Stem Cells. 1997, :pp 233-282 London, Academic Press, San Diego [Google Scholar]
- 12.Kano J, Tokiwa T, Zhou X-D, Kodama M: Colonial growth and differentiation of epithelial cells derived from abattoir adult porcine livers. J Gastroenterol Hepatol 1998, 13(suppl.):62S-69S [PubMed] [Google Scholar]
- 13.Zhou X-D, Tokiwa T, Kano J, Kodama M: Isolation and primary culture of adult pig hepatocytes. Meth Cell Sci 1998, 19:277-284 [Google Scholar]
- 14.Tokiwa T, Kano J, Noguchi M, Kodama M, Tateishi T: Restoration of differentiated functions in multicellular aggregates of a human liver epithelial cell line. Materials Sci Eng 1998, 6:249-252 [Google Scholar]
- 15.Rutenburg AM, Kim H, Fischbein JW, Hanker JS, Wasserkrug HL, Seligman AM: Histochemical and ultrastructural demonstration of γ-glutamyl transpeptidase activity. J Histochem Cytochem 1969, 17:517-526 [DOI] [PubMed] [Google Scholar]
- 16.Dunsford HA, Sell S: Production of monoclonal antibodies to preneoplastic liver cell populations induced by chemical carcinogens in rats and to transplantable morris hepatomas. Cancer Res 1989, 49:4887-4893 [PubMed] [Google Scholar]
- 17.Dunsford HA, Karnasuta C, Hunt JM, Sell S: Different lineages of chemically induced hepatocellular carcinoma in rats defined by monoclonal antibodies. Cancer Res 1989, 49:4894-4900 [PubMed] [Google Scholar]
- 18.Faris RA, Monfils BA, Dunsford HA, Hixson DC: Antigenic relationship between oval cells and a subpopulation of hepatic foci, nodules, and carcinomas induced by the “resistant hepatocyte” model system. Cancer Res 1991, 51:1308-1317 [PubMed] [Google Scholar]
- 19.Yang L, Faris RA, Hixson DC: Characterization of a mature bile duct antigen expressed on a subpopulation of ductular cells but absent from oval cells. Hepatology 1993, 18:357-366 [PubMed] [Google Scholar]
- 20.Cascio S, Zaret KS: Hepatocyte differentiation initiates during endodermal-mesenchymal interactions prior to liver formation. Development 1991, 113:217-225 [DOI] [PubMed] [Google Scholar]
- 21.Alpini GA, Phillips JO, LaRusso NF: The biology of biliary epithelia. 3rd ed. Arias IM Boyer JL Fausto N Bakoby JW Schachter DA Shafritz DA eds. The Liver: Biology and Pathobiology, 1994, :pp 623-653 Raven Press, New York [Google Scholar]
- 22.Tateno C, Yoshizato K: Growth and differentiation in culture of clonogenic hepatocytes that express both phenotypes of hepatocytes and biliary epithelial cells. Am J Pathol 1996, 149:1593-1605 [PMC free article] [PubMed] [Google Scholar]
- 23.Lazaro CA, Rhim JA, Yamada Y, Fausto N: Generation of hepatocytes from oval cell precursors in culture. Cancer Res 1998, 58:5514-5522 [PubMed] [Google Scholar]
- 24.Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, Riley T, Howard TA, Michalopoulos K: Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF α in a chemically defined (HGM) medium. J Cell Biol 1996, 132:1133-1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eyken PV, Desmet VJ: Development of intrahepatic bile ducts, ductular metaplasia of hepatocytes, and cytokeratin patterns in various types of human hepatic neoplasms. Sirica AE eds. The Role of Cell Types in Hepatocarcinogenesis. 1992, :pp 227-263 FL, CRC Press, Boca Raton [Google Scholar]
- 26.Uchida T, Peters RL: The nature and origin of proliferated bile ductules in alcoholic liver disease. Am J Clin Pathol 1983, 79:326-333 [DOI] [PubMed] [Google Scholar]
- 27.Haque S, Haruna Y, Saito K, Nalesnik NA, Atillasoy E, Thung SN, Gerber MA: Identification of bipotential progenitor cells in human liver regeneration. Lab Invest 1996, 75:699-705 [PubMed] [Google Scholar]
- 28.Coleman WB, Wennerberg AE, Smith GJ, Grisham JW: Regulation of the differentiation of diploid and some aneuploid rat liver epithelial (stemlike) cells by the hepatic microenvironment. Am J Pathol 1993, 142:1373-1392 [PMC free article] [PubMed] [Google Scholar]
- 29.Zvibel I, Fiorino AS, Brill S, Reid LM: Phenotypic characterization of rat hepatoma cell lines and lineage-specific regulation of gene expression by differentiation agents. Differentiation 1998, 63:215-223 [DOI] [PubMed] [Google Scholar]
- 30.Mitaka T, Sato F, Mizuguchi T, Yokono T, Mochizuki Y: Reconstruction of hepatic organoid by rat small hepatocytes and hepatic nonparenchymal cells. Hepatology 1999, 29:111-125 [DOI] [PubMed] [Google Scholar]
- 31.Tokiwa T, Kano J, Meng X-Y, Zhou X-D, Kodama K, Matumura T: Multilayer rat hepatocytes aggregates formed on expanded polytetrafluoloethylene surface. Cytotechnology 1997, 25:137-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kano J, Tokiwa T, Meng X-Y, Kodama M, Matumura T: Immobilization of CHO-K1 cells cultured in serum-free medium using glass fiber fabrics or expanded polytetrafluoloethylene membranes. Seitaizairyou 1997, 15:55–62 (in Japanese with English abstract)
- 33.Tokiwa T, Kano J, Meng X-Y, Bahulekar R, Kodama M: Formation of non-spheroidal aggregates on polyacrylamide surfaces. Biotechnol Techniques 1996, 10:845-848 [Google Scholar]
- 34.Tokiwa T, Yata Y, Takahara T, Watanabe A, Enosawa S, Suzuki S, Kano J, Noguchi M: SV40 large T antigen immortalization of rat hepatic stellate like cells. In Vitro Cell Dev Biol 1999, 35:246-247 [DOI] [PubMed] [Google Scholar]
- 35.Santos OFP, Nigam SK: HGF-induced tubulogenesis and branching of epithelial cells is modulated by extracellular matrix and TGF-β. Dev Biol 1993, 160:293-302 [DOI] [PubMed] [Google Scholar]
- 36.Johnson M, Koukoulis G, Matsumoto K, Nakamura T, Iyer A: Heptocyte growth factor induces proliferation and morphogenesis in nonparenchymal epithelial liver cells. Hepatology 1993, 17:1052-1061 [PubMed] [Google Scholar]
- 37.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeir C: Scatter factor/hepatocyte growth factor is essential for liver development. Nature 1995, 373:699-702 [DOI] [PubMed] [Google Scholar]