Abstract
A spontaneous, autosomal, recessive mouse mutation exhibiting mild scaly skin, progressive scarring alopecia, slightly runted growth, and photophobia arose at The Jackson Laboratory in 1993 in the inbred mouse strain DBA/1LacJ. Because this mutant mouse showed genetic, anatomical, and laboratory similarities to the asebia mutation, crosses were done between the new mutant and mice carrying the asebia-J allele. Because the F1 offspring were affected, indicating the two mutants were allelic, the new mutation was named asebia-2J. Careful histological analysis of skin development of mice homozygous and heterozygous for either asebia-J or asebia-2J revealed that both types of mutant mice are very similar regardless of their background. Notable histopathological features of mice homozygous for either allele included extreme sebaceous gland hypoplasia, abnormally long anagen follicles, retained inner root sheath, hair fiber perforation of the anagen follicle base, and progressive follicular replacement by scarring. In this article we present a new pathogenetic hypothesis based on the importance of the sebaceous gland in hair fiber sheath dissociation: in the absence of a functional sebaceous gland the hair follicle is destroyed. The cutaneous pathology of this mutant mouse underscores the importance of the sebaceous gland to follicular biology and presents an animal model for studying the human scarring alopecias, which characteristically begin with sebaceous gland ablation.
The original asebia mouse mutation, an autosomal recessive trait characterized by hypoplastic sebaceous glands, arose spontaneously more than 30 years ago in a colony of BALB/cCrglGa mice. 1 Although in the original study it was thought that these mice lacked sebaceous glands, hence the name asebia (gene symbol ab), later studies showed that sebaceous glands and modified sebaceous glands (meibomian, preputial, clitoral, and ceruminous glands) are present but hypoplastic. 2-4 In 1968, a similar mutation arose spontaneously in the BALB/cJ inbred strain at The Jackson Laboratory; this mutant was found to be allelic to ab (Dr. S. J. Mann) and was named asebia-J (abJ). Mice carrying the abJ mutation were out-crossed to C3H/Di and back-crossed to F8 5 from which time the mutant was maintained by brother-sister matings (P. Lane, personal communication) on the inbred strain ABJ/Le. 6 The abJ remutation was mapped to mouse chromosome 19. 5
Compared to heterozygous or normal littermates, abJ mutants are small and have a hunched back. Adult homozygous asebia mice develop generalized alopecia and scaly skin. Although the hair shafts form normally, 3,7 they are sparse and short. 8 Histological studies have shown that the epidermis is thickened from birth with enlarged intercellular spaces. Hair follicles are excessively long extending at a sharp angle into the deep subcutis. All growth phases of the cycle, anagen, catagen, and telogen, last longer than those of the controls. 2,8 Abnormalities in the inner root sheath (IRS) include the absence of typical transverse corrugations at the level of the sebaceous glands, and an abnormal persistence of the IRS in the upper pilary canal. The IRS remains undegraded, plugging the hair canal and adhering to the emerging hair shafts. It is not unusual to find hair shafts free of sheath lying in the deep dermis of the mutants. 8 The cutaneous changes can be seen histologically late in skin development. By the 20th day after conception the mutants show abnormal sebaceous cell cytodifferentiation. Ultrastructural studies of the glands show that there are fewer lipid droplets, the smooth endoplasmic reticulum is distorted and dilated, and normal mitochondria development is impaired. 4 These abnormalities were interpreted to be because of an abnormal synthetic or degenerative process necessary for completion of normal sebum production. The mutant mice have a thickened dermis characterized by increased vascularity, increased cellularity, and prominent fibroblasts. The dermal cellular infiltrate is rich in mast cells and macrophages. 9,10 The latter cell population contains lipid crystals; such crystals have been thought to form the basis for the dermal inflammation and epidermal hyperplasia. 10 Subcutaneous fat is scant.
Studies of dermal-epidermal recombination grafts (done with 14-day gestational fetuses) suggested that the functional defect was in the epidermis, not the dermis 2 and that normal skin could alleviate the phenotype by means of “some diffusible substance.” 1 Laboratory studies showed that whole skin, skin surface, and epidermal lipids are abnormal in the mutant; they showed deficiency in sterols esterified with long-chain fatty acids, in wax esters, and in wax diesters. 11 These findings suggested a global defect in fatty acid metabolism more than just involving sebum synthesis. Recently we have found that the gene defective in the asebia mouse is stearoyl CoA desaturase-1 (Scd1); the enzyme product of this gene is rate-controlling in the formation of mono-unsaturated fatty acids from saturated fatty acids. 12
In this article we describe a new spontaneous mutation in the DBA/1LacJ mouse that shows clinical, histological, and laboratory features of asebia mutant mice. As crosses with the new homozygous mutant and abJ/abJ mice yielded affected offspring in three matings, we have concluded that the new mutation, which we call asebia-2J (ab2J), is allelic with abJ. Because the mutated gene is now known, the complete gene symbol is Scd1ab2J. Careful histopathological study of these mice demonstrates that the asebia mutant illustrates the importance of the sebaceous gland in shaft-sheath interactions and serves as a model for the pathogenesis of some forms of scarring alopecia.
Materials and Methods
Longitudinal Pathological Characterization
Groups of two male and female mice homozygous for the ab2J mutation (Scd1ab2J/Scd1ab2J), hereafter abbreviated ab2J/ab2J were sacrificed at 3-day intervals from 0 to 26 days and at 8 weeks and 8 months of age. Additional female mutant and control mice were collected daily from 20 to 40 days of age. Sex- and aged-matched DBA/1LacJ wild-type (+/+) or heterozygous (+/ab2J) mice, hereafter +/?, were used as controls. The morphological study used a total of 204 mice (controls: 33 males, 48 females; mutants: 27 males, 56 females; undetermined phenotype 15 days of age and under: 21 males, 19 females). The abJ mice were obtained from The Jackson Laboratory (Bar Harbor, ME) on the ABJ/Le inbred background. ABJ/Le abJ/abJ mice were compared to ab2J mutant and control mice collected at selected ages. A total of 65 mice were studied (+/abJ: 20 females, 21 males; abJ/abJ: 10 females, 14 males).
Tissue collection, husbandry conditions, and disease monitoring were done routinely, as described in detail previously. 13 After fixation and routine processing, sections were stained with hematoxylin and eosin (H&E), Masson’s trichrome, or SACPIC 14 for histopathological examination. Complete sets of organs were collected from mice at birth, 3 weeks, 8 weeks, and 8 months of age. 15
Allelism Breedings
Standard methods were used to establish a stable breeding colony and determine whether the new mutation was dominant or recessive. 16 Matings were set up between this new mutant mouse line and a number of other known mutant mice with a scaly skin phenotype including flaky skin, fsn; 17 harlequin ichthyosis, ichq; 13 matted/flaky tail, ma/ft; 18 chronic proliferative dermatitis, cpdm; 19 lanceolate hair-J, lahJ; 20 and abJ. 18 Homozygous new mutations were crossed with either known heterozygous or homozygous mutant mice depending on fecundity. If the mutations were allelic, then homozygous crosses would yield all F1 offspring with a mutant phenotype. If homozygous new mutant mice were crossed with heterozygous known mutants, then only 50% of the F1 offspring would be affected if the two mutations were allelic.
Transepidermal Water Loss (TEWL)
To investigate differences in skin barrier function between normal and mutant mice, TEWL was determined. All mice used were 6 weeks of age. One group of six (five males and one female) DBA/1LacJ +/?, one group of five (four males, one female) DBA/1LacJ ab2J/ab2J, and one group of six (four males, two female) ABJ/Le abJ mice were sedated with 100 mg/kg ketamine HCl, intraperitoneally (Fort Dodge Laboratories, Fort Dodge, IA) plus 0.5 mg/kg xylazine (Rompun; Miles Laboratories, Shawnee Mission, KS). Dorsal hair was removed with electric clippers and then depilated for 5 minutes with Neet (Reckitt and Coleman, Wayne, NJ). TEWL was measured 24 hours later by placing a Servo Med Evaporimeter EPI probe (Servomed AB, Stockholm, Sweden) on the depilated area. 21
Water consumption was measured in two groups consisting of four DBA/1LacJ +/? and four DBA/1LacJ ab2J/ab2J male mice, 8 weeks of age. A bottle was filled with 450 ml of water and the volume measured daily to determine the 24-hour consumption rate for the four mice in the box. This was done on 4 consecutive days.
Urinary output was determined for each of 10 mice, 8 weeks of age. Five DBA/1LacJ ab2J/ab2J (three females and two males) and five DBA/1LacJ +/? (five males) mice were used. Each mouse was placed in a separate box with an underpad (Safety Assay Mats, Plain; Isolab, Inc., Akron, OH). Water was provided in small bottles and monitored throughout the study period for leaks. The underpads were weighed at zero time and again 1 hour after returning the mice to the box with the weighed under pad.
Morphometric Studies of Skin
Using an image analyzer (Quantimet 600HR Image Analysis System; Leica, Inc., Deerfield, IL), the mean hair follicle length as well as dermal, epidermal, hypodermal, and skin thickness measurements of dorsal skin were determined. Skin was measured from dorsal truncal regions of all mice used in the longitudinal study. Data were pooled per group between 6 and 9 days of age and analyzed using a Student’s t-test. 22 Data were analyzed using a spreadsheet (Excel; Microsoft Corp., Redmond, WA). In addition, hair cycle stage was recorded as slides were reviewed and the stage plotted by age to determine the cycle length as previously described. 22
Scanning Electron Microscopy of Hair Fibers
Dorsal truncal hair of a mutant and a control mouse, both 21-day-old females, were prepared for scanning electron microscopy and screened for abnormalities. Representative hairs from the four major hair types (zigzag, guard, auchene, and awl), plucked at the time of necropsy were mounted onto aluminum stubs with double-stick tape (3M, St. Paul, MN). Samples were sputter-coated with 15 ηm of gold and examined under a JOEL 35C scanning electron microscope operated at 10 kV as previously described. 13
Lipid Analyses
Surface lipids were collected by dipping each sacrificed mouse totally in 40 ml of acetone 10 times and drying the acetone down under argon gas. The plates were analyzed by thin layer chromatography by an established procedure. 23 Briefly, the dried residue was dissolved in toluene and plated in separate lanes on silica gel G chromatographic plates (Merck, Rahway, NJ). The plates were developed to 19 cm in hexane-ether-acetic acid (80:20:1). After drying, the plate was sprayed with 50% sulfuric acid.
Results
Breeding History
A trio consisting of one affected male and two clinically normal females were obtained from The Jackson Laboratory DBA/1LacJ colony in October of 1993. These mice were used to establish the colony used in these studies. Thirteen crosses between known heterozygous mice carrying this new mutation resulted in 136 females (46 mutant, 33%; 90 normal mice) and 107 males (27 mutant, 25%; 80 normal mice) indicating that this was an autosomal recessive mutation.
Clinical Features
The original mice were described by the animal care technicians as having “scaly skin.” Hair was sparse and matted over the entire body and was difficult to remove by plucking or shaving at the time of necropsy. The skin had a dry appearance with moderate white flaking evident when the hair was removed. Eyes seemed smaller than normal and eyelids were bound shut (Figure 1, A and C) ▶ . It was also observed that affected mice had a slightly abnormal posture in which their back was arched or hunched. The abJ mice had similar gross features (Figure 1, B and D) ▶ . No other gross lesions were observed.
Figure 1.

Both DBA/1LacJ-ab2J/ab2J (A, C) and ABJ/Le-abJ/abJ (B, D) mutant mice (right in each panel) develop a progressive alopecia with age, have a fine scale on their skin, and seem to be photophobic with squinting of the eyes compared with age and sex-matched littermate controls (left).
Allelism Breedings
Matings were set up between this new mutation and a number of other known mutant mice with clinical features of flaking skin as described in Materials and Methods. Only in the asebia homozygous crosses were the alleles noncomplementary. Histological studies done on representative mutant mice verified the phenotype (data not shown). These results confirm that the new mutation is allelic with abJ; it was therefore called ab2J.
Scanning Electron Microscopy
Examination of plucked hairs or hairs examined in situ on 1 cm 2 of skin did not reveal any abnormalities in 3-week-old mice. Although the data are not shown, these results are similar to those we obtained previously when studying ABJ/Le-abJ/abJ mice. 7
TEWL
These studies were performed controlling for age and sex. TEWL was 60.7 ± 8.0 g/m2/hour for ab2J/ab2J compared to 13.2 ± 7.8 for littermate controls. This was an increase of 4.5-fold. Water consumption was also elevated by 40% in the ab2J/ab2J group (31.3 ml/4 males/24 hours × 4 days) compared with controls (22.5 ml). However, when calculated by milliliters consumption per gram body weight, water consumption was 0.39 ml/g for ab2J/ab2J compared to 0.24 ml/g for +/? mice, an increase of 62%. Urinary output was 321 ± 161 (SE, n = 4) mg/mouse/hour in ab2J/ab2J mice compared to 314 ± 157 (SE, n = 4) in +/? mice, an increase of only 2%. Marked increase in TEWL indicates that the epidermis of the ab2J/ab2J mice does not provide a normal barrier; these mice obviously compensated for the loss by drinking more water. In contrast, in a parallel study, the alellic mutant, abJ/abJ had a normal TEWL (15.5 ± 1.9 g/m2/hour).
Microscopic Description and Comparison between abJ and ab2J
At the time of birth until approximately 6 days of age the ab2J mutant mice were very difficult to distinguish clinically from littermate controls. At 6 days of life, when hair fibers emerge from the follicles, histological examination of skin revealed the presence of very small sebaceous glands in homozygotes compared to controls. Careful and lengthy examination was required to find occasional sebaceous glands. These structures were actually rudimentary outpouchings of the hair follicle into the dermis. There was some early sebaceous differentiation, but unlike normal sebocytes the cells were small and their cytoplasm was brightly eosinophilic (Figure 2) ▶ . Abnormalities were not limited to sebaceous glands of the truncal skin. Most sebaceous and modified sebaceous glands of the body were affected to various degrees including the meibomian glands (Figure 3) ▶ , perianal glands, and ceruminous glands. Male preputial glands and female clitoral glands were not affected. Eccrine glands of the footpads and other types of glands (mammary glands, salivary glands, lingual glands, Harderian glands, and lacrimal glands) were normal.
Figure 2.
Adult DBA/1LacJ-ab2J/ab2J skin has a thickened epidermis, moderate to focally severe orthokeratotic hyperkeratosis, and long hair follicles (A; scale bar, 100 μm). Higher magnification of boxed area illustrates the hypoplastic sebaceous gland (B; scale bar, 100 μm). H&E.
Figure 3.
Eyelid of DBA/1LacJ-+/ab2J control mouse has a prominent and well-differentiated Meibomian gland (A) while the ab2J/ab2J eyelid contains a rudimentary duct and glandular structure (B) or cystic rudiment (C). H&E; scale bar, 100 μm.
The epidermis of young ab2J/ab2J mutant mice, less than 40 days of age, was mildly acanthotic and showed various degrees of orthokeratotic hyperkeratosis and focal areas of parakeratosis (Figures 2 and 4) ▶ ▶ . The dermis was significantly thickened. In the first anagen stage, the skin manifested unusually long hair follicles that extended at a sharp angle into the deep subcutis. As previously described, 1,4 it seemed that the ab2J/ab2J follicles remained in anagen longer than the follicles of their littermate controls (data not shown) but bulbar histology was considerably distorted in the mutant. Eventually, normal telogen follicles developed after a prominent catagen phase (Figures 5 and 6) ▶ ▶ . Although the hair fibers seemed to be normal up to the midfollicle, there was retention of the IRS beyond the midfollicle into the outer piliary canal. The hair shafts showed occasional adherent keratinous debris. Some follicles showed hair shaft penetration of the follicle base (Figure 7) ▶ . The abnormally long hair follicles seemed to be because of the retention of a cornified cell plug (consistent with an IRS fragment) in the pilary canal that blocked, or slowed, the exit of the normally formed hair fiber (Figure 4) ▶ . It was notable that in many late anagen and early catagen follicles the shafts eventually ruptured through the base (bulb) of the hair follicle and induced a foreign body granuloma in the dermal stroma (Figure 7) ▶ . Healing of this inflamed area resulted in dermal scarring and eventual scarring of the hair follicle itself (Figure 8) ▶ . Although the few residual follicles continued to cycle with each cycle there is progressive individual follicle destruction (Figure 9) ▶ . Why some follicles were destroyed and others continued to cycle was not immediately obvious.
Figure 4.

Normal hair fibers exit the follicular os of control mice with retention of few, if any, cornified cells (A). Notice the transverse corrugations (arrow). In contrast, hair fiber exit from follicles is delayed by retention of a cornified cell plug (arrow) in the follicular os of DBA/1LacJ-ab2J/ab2J mice (B). Masson’s trichrome; scale bar, 50 μm.
Figure 5.
Telogen (T) stage hair follicles of DBA/1LacJ-ab2J/ab2J mice extend deep into the hypodermal fat layer near the panniculus muscle and are of equal length to anagen (A) follicles (A, B). H&E; scale bar, 50 μm.
Figure 6.
Normal telogen follicles with scattered follicular dystrophy with pigment accumulation in DBA/1LacJ +/? mice is a strain background phenotype unrelated to the asebia mutation. H&E; scale bar, 100 μm (A) and 50 μm (B).
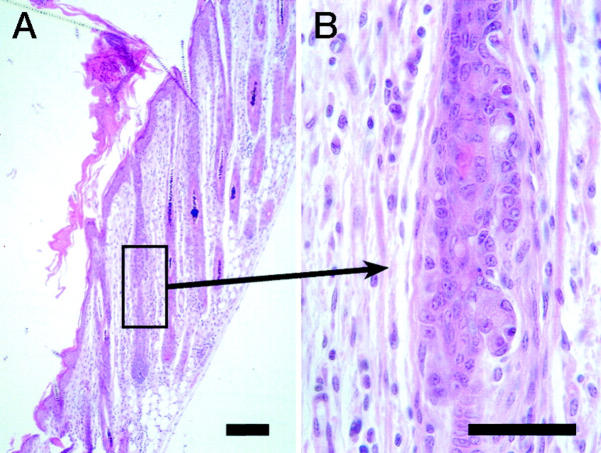
Figure 7.
Retention of hair fibers within follicles of DBA/1LacJ-ab2J/ab2J mice results in elongation of follicles and eventual rupture of the club hair through the base of the telogen follicle (A: Masson’s trichrome; scale bar, 10 μm). The sequella is a foreign body granuloma (B: H&E; scale bar, 50 μm).
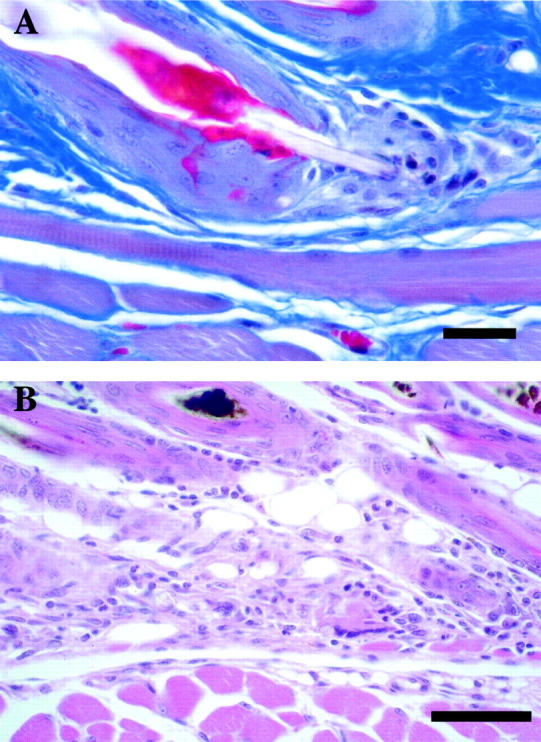
Figure 8.
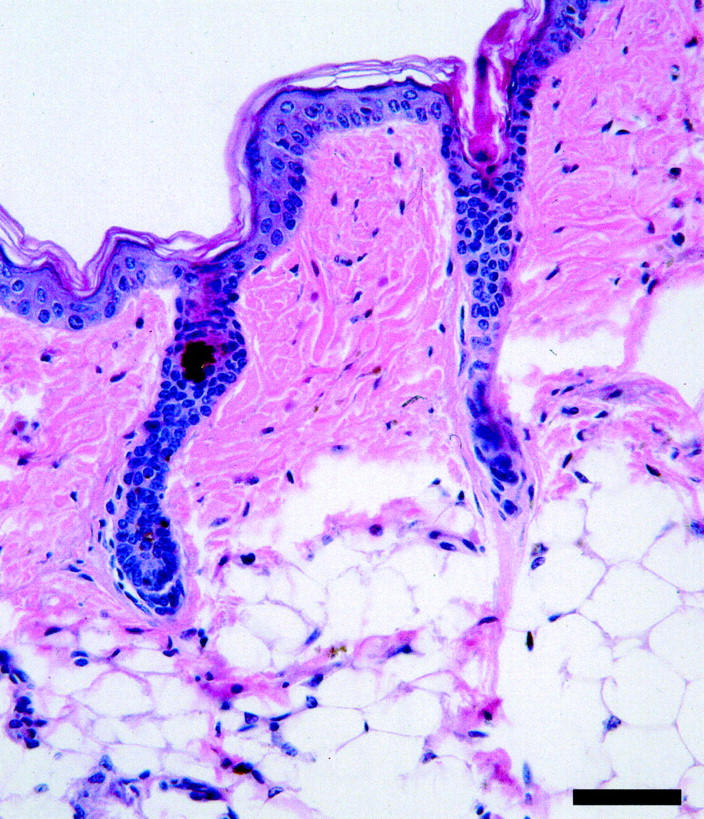
Follicular scarring, in which fibrous connective tissue strands run perpendicular to the epidermis from remnants of dystrophic hair follicles, was found in ABJ/Le-abJ/abJ mice >1 year of age (H&E; scale bar, 50 μm).
Figure 9.

Progression of hair cycles results in retention of fibers within follicles because of occlusion of the follicular os, deep extention of catagen, and telogen stage follicles to the depth of anagen follicles, and eventual rupture with formation of a foreign body granuloma. Healing causes follicular scarring with subsequent loss of follicles resulting in alopecia.
The subcutis in the ab2J/ab2J mutant mice was thinned, even in anagen, and showed a paucity of adipose tissue compared to littermate control mice. The histological changes in the whole skin and the hair follicle throughout the cycle were similar in the abJ/abJ mutant mice.
It is of importance that there were occasional dystrophic hair follicles in the wild-type DBA/1LacJ mice. Scattered follicles, in both anagen and telogen, contained structurally abnormal, weak hair fibers that disintegrated leaving debris and accumulations of pigment within the hair follicle (Figure 6) ▶ . Similar dystrophic follicles were found in controls for another spontaneous mutation that occurred in this strain but did not develop any features of asebia. 20
Morphometric Analyses
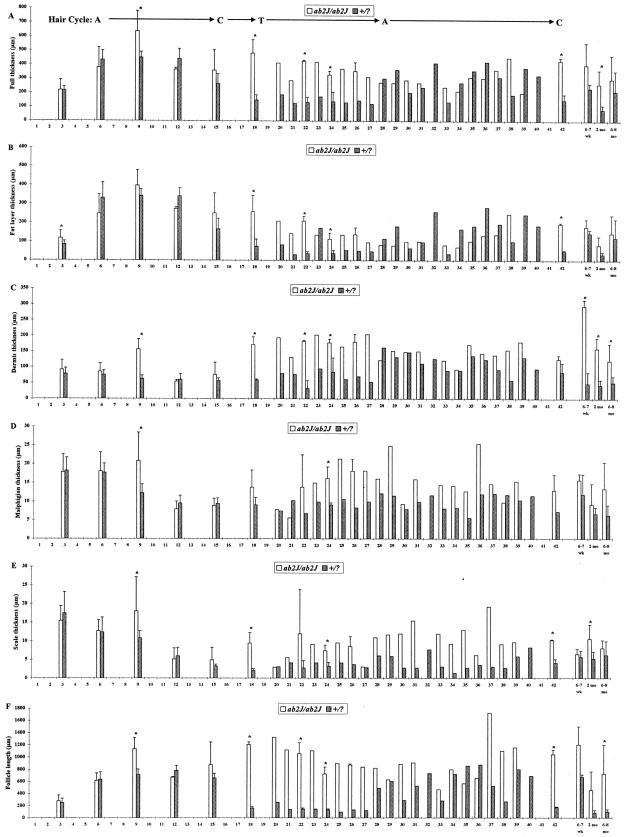
To compare the two extant asebia alleles quantitatively, morphometric studies were executed. Data were similar for both abJ/abJ and ab2J/ab2J mutant mice. Full thickness skin was thicker (epidermal surface to panniculus carnosus) in mutant mice compared to controls except during the onset of the second hair cycle, where control skin became thicker, corresponding to initiation of anagen (Figure 10, A and B) ▶ . 24,25 The prolonged length of the hair follicles (Figure 10F) ▶ coincided with the histological observation of prolonged anagen despite a reduced hypodermal fat layer (Figure 10B) ▶ . The dermis of control mice usually remained relatively similar in thickness throughout life (Figure 10C) ▶ . The epidermis of normal mice was thick at birth and gradually thinned within the first 2 to 3 weeks of life. This was evident for both the thicknesses of the Malphigian layer and stratum corneum (Figure 10, D and E) ▶ . In contrast, these layers were markedly thicker in ab2J/ab2J but only slightly thicker in abJ/abJ mice (data not shown).
Figure 10.
The full thickness of the skin (A) and hypodermal fat layer (B) varied with the hair cycle. The dermis (C), Malphighian layer of the epidermis (D), and stratum corneum (scale, E) remained consistently thicker in mutant mice compared with controls after the second hair cycle. Hair follicle length (F) was dramatically different during the second and subsequent hair cycles. This was because of marked diminution in size of normal mouse follicles in telogen while ab2J telogen follicles remained as long or longer than normal anagen stage follicles. Hair cycle stage is indicated as anagen (A), 224 catagen (C), and 224 telogen (T).
Lipid Analysis
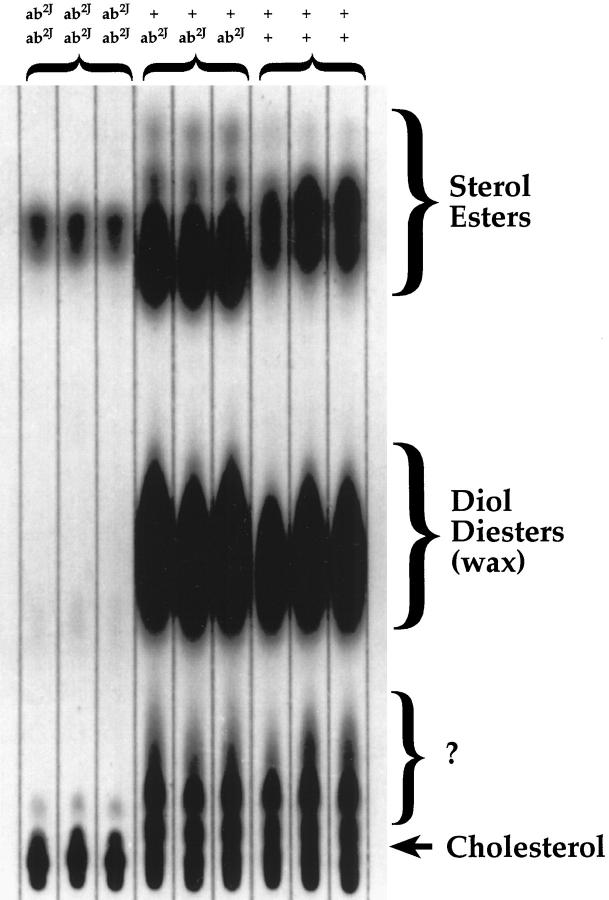
Skin surface lipid analysis of ab2J/ab2J mutant mouse skin revealed a marked reduction in sterol esters and cholesterol with apparently total loss of diol diesters compared with littermate controls (Figure 11) ▶ . These results are similar to those reported for the original asebia (ab) mutant mice. 11 Similar analyses were not performed on the asebia-J mice.
Figure 11.
ab2J mice have marked decreases or absence of specific groups of lipids in the skin.
Discussion
In this paper we report the finding of a new asebia allele, referred to here as ab2J. It is notable that except for the extent of epidermal thickness, scaling, and epidermal permeability properties, the newly found ab2J homozygous mice are similar to homozygous mice for the abJ allele. Both alleles cause progressive scarring alopecia and scaly skin, but normal hair fiber structure. Histologically, their skins have a dermis with progressive scarring, chronic inflammatory infiltrates, thinned subcutis, and hypoplastic sebaceous glands. The meibomian and most of the other modified sebaceous glands are also small.
Recently, using a positional cloning approach, we have mapped 26 and identified the gene mutated in asebia (abJ and ab2J) as stearoyl CoA desaturase-1 (Scd1). 12 The ab2J allele shows a splice junction deletion of exon 2 in Scd1, leading to an in-frame stop codon. A much larger deletion is present in Scd1 gene in the abJ allele that extends over most of the open reading frame. 12
One important difference we found between the two alleles is the thickness of the epidermis and the epidermal permeability barrier as measured by TEWL. Regarding the structure of the epidermis, a TEWL measurement depends directly on the stratum corneum barrier integrity and inversely on the thickness of the stratum corneum. 27 An important caveat in interpreting TEWL results is that TEWL increases under conditions of skin irritation; 28 moreover, TEWL is elevated in dermatitic skin. 29 Although we do not yet understand the basis for the epidermal difference between the alleles, we observed that the epidermal changes of the abJ/abJ mutant mice is less severely involved than that of the epidermis of the ab2J/ab2J mutant mice which are clearly irritated as manifested by the chronic inflammatory infiltrate and the overlying scale. The degree of dermal inflammation in abJ/abJ mutant mice is also reduced compared with ab2J/ab2J mice. Because the same gene is mutated in both mutant mice, we would assume that the Scd1 gene is not central to the epidermal barrier and that the difference in TEWL is because of the extent of the inflammatory reaction in the skin of one allele compared to the other, possibly because of strain background modifying gene effects.
Multiple mechanistic theories have been put forth explaining the asebia phenotype and its genetic basis. Clearly, a satisfactory hypothesis must explain the lipid abnormality, sebaceous gland hypoplasia, adipose tissue atrophy of the subcutis, and epidermal and dermal inflammatory reactions. In all discussions, the primary and secondary events have been difficult to decipher. The study by Wilkinson and Karasek 11 suggested that the primary cutaneous defect was in sebaceous lipid synthesis, although epidermal lipid was assumed to be abnormal as well. That lipid crystals were found within the macrophages in the dermal infiltrate suggested to other workers that macrophage lysis could be the basis for the skin lesions. 10 Data have been presented suggesting that the sebaceous and epidermal changes are because of abnormalities in the dermis and in endocrine glands 4 although the same workers suggest that the defect in asebia was in the epidermis. 2 The epidermal thickening is thought to be because of “dermal inflammation, macrophage lysis, crystals in lysosomes and ultimately to a genetic defect in the metabolism of a branched-chain fatty acid.” 10 It is of interest that by reducing the inflammation and mast cell infiltrate with cyclosporin A, some of the wild-type histological features can be restored. 30
With the identification of the gene, we now recognize that the primary molecular defect in these mice involves the lack of Scd1 gene function. At what level the synthesis is important, ie, in terms of membrane structure, signal transduction, sebum synthesis, or sebaceous gland morphogenesis is not clear at this stage. Our detailed histological study has given us some mechanistic insight and a new hypothesis. Central to our new hypothesis is the observation that IRS accumulates in the pilary canal and that the shaft does not cleanly shed the sheath in its passage outward. This observation is similar to that of Josefowicz and Hardy 8 who pointed out that the IRS fails to degrade and adheres to the emerging hair fiber. In addition, we know from in vitro studies that the separation of the shaft from the sheath is dependent on an intact midfollicle/sebaceous gland. 31-34 The sebaceous gland is implicated in the degradation of the IRS from the emerging shaft. The adherent sheath seems to restrain the shaft from growing out of the follicle so that the whole follicle is forced to take the reverse direction—toward the subcutis. This action would result in long, deep-lying follicles and short, scale-adherent shafts. Both of these morphological features are observed in our studies and by others. 8 Eventually the shaft penetrates the follicle bulb and destroys the bulb associated with cell necrosis, fragments of shaft in the dermis, and foreign body/chronic inflammatory reaction to the follicular contents (Figure 7) ▶ . Our hypothesis for the pathogenesis of the asebia phenotype is summarized in Figure 9 ▶ . The lack of Scd1 function leads to inadequate sebaceous gland development/function and sebaceous gland function is needed for normal shaft-sheath interactions. This hypothesis begs the question—how does the sebaceous gland normally support shaft-sheath separation. Perhaps a proteolytic enzyme that depends on a unique lipid environment plays a role. Straile 32 observed that the sheath fell from the fiber at the level of the sebaceous duct and postulated not only that removal of the IRS at the follicular neck was required for the orderly emergence of the hair fiber but also that lytic agents released from the sebaceous gland were responsible for the event. Gemmell and Chapman 33 suggested that the source of these lytic agents might be the outer root sheath at the level of the isthmus. Unfortunately, even though we now know the molecular defect in these mutant mice and that the gene is expressed only in the sebaceous glands in mice, 12 how the Scd1 gene product functions is still unknown.
In conclusion, we have described in this paper a new allele at the Scd1 locus, formerly known as the asebia locus. We put forth a new view of the pathogenesis of the alopecia found in this genetic model that is based on the role of the sebaceous gland in degrading the internal root sheath. Unable to easily slip out of the sheath the shaft destroys the follicle and in so doing induces an inflammatory reaction, epidermal hyperplasia, and scarring. How the absence of a gene important to lipid processes induces these dramatic changes is a question needing elucidation and underlies the basic biology of the sebaceous gland.
Acknowledgments
The assistance of Jeff Pote in the TEWL studies, David Yang in the hydration studies, and J. Worcester with the graphics is gratefully appreciated.
Footnotes
Address reprint requests to John P. Sundberg, D.V.M., Ph.D., The Jackson Laboratory, 600 Main Street, Bar Harbor, ME 04609-1500. E-mail: jps@jax.org.
Supported by grants AR43801 and CA34196 from the National Institutes of Health and the Council for Nail Disorders (to J. P. S.).
References
- 1.Gates AH, Karasek M: Hereditary absence of sebaceous glands in the mouse. Science 1965, 148:1471-1473 [DOI] [PubMed] [Google Scholar]
- 2.Pennycuik PR, Raphael KA, Chapman RE, Hardy MH: The site of action of the asebia locus (ab) in the skin of the mouse. Genet Res 1986, 48:179-185 [DOI] [PubMed] [Google Scholar]
- 3.Compton JG, Dunstan RW, Sundberg JP: Asebia, a mutation affecting sebaceous glands, mouse. Monographs on Pathology of Laboratory Animals. Integument and Mammary Glands. Edited by TC Jones, U Mohr, RD Hunt. Heidelberg, Springer-Verlag, 1989, pp 218–222
- 4.Josefowicz WJ, Hardy MH: The expression of the gene asebia in the laboratory mouse. 3. Sebaceous glands. Genet Res 1978, 31:157-166 [DOI] [PubMed] [Google Scholar]
- 5.Sweet HO, Lane PW: Asebia-J is now known to be very close to ru on Chr 19. Mouse News Lett 1977, 57:20 [Google Scholar]
- 6.Festing MFW: Origin and characteristics of inbred strains of mice. 14th listing. Mouse Genome 1992, 90:231–352
- 7.Sundberg JP (Ed): The asebia (ab, abJ) mutations, chromosome 19. Handbook of Mouse Mutations with Skin and Hair Abnormalities. Animal Models and Biomedical Tools. Boca Raton, FL, CRC Press, 1994, pp 171–177
- 8.Josefowicz WJ, Hardy MH: The expression of the gene asebia in the laboratory mouse. 2. Hair follicles. Genet Res 1978, 31:145-155 [DOI] [PubMed] [Google Scholar]
- 9.Brown WR, Hardy MH: Mast cells in asebia mouse skin. J Invest Dermatol 1989, 93:708. [DOI] [PubMed] [Google Scholar]
- 10.Brown WR, Hardy MH: A hypothesis on the cause of chronic epidermal hyperproliferation in asebia mice. Clin Exp Dermatol 1988, 13:74-77 [DOI] [PubMed] [Google Scholar]
- 11.Wilkinson DI, Karasek MA: Skin lipids of a normal and a mutant (asebic) mouse strain. J Invest Dermatol 1966, 47:449-455 [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y, Eilersten KJ, Ge L, Zhang L, Sundberg JP, Prouty S, Stenn KS, Parimoo S: Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet 1999, 23:268-270 [DOI] [PubMed] [Google Scholar]
- 13.Sundberg JP, Boggess D, Hogan ME, Sundberg BA, Rourk MH, Harris B, Johnson K, Dunstan RW, Davisson MT: Harlequin ichthyosis. A juvenile lethal mouse mutation with ichthyosiform dermatitis. Am J Pathol 1997, 151:293-310 [PMC free article] [PubMed] [Google Scholar]
- 14.Nutbrown M, Randall VA: Recognition of cellular differentiation in the human hair follicle at the light microscope level using SACPIC staining. Van Neste DJJ Randall VA eds. Hair Research for the Next Millenium. 1996, :pp 161-166 Elsevier Science BV, Amsterdam [Google Scholar]
- 15.Sundberg JP, Montagutelli X, Boggess D: Systematic approach to evaluation of mouse mutations with cutaneous appendage defects. Chuong C-M eds. Molecular Basis of Epithelial Appendage Morphogenesis. 1998, :pp 421-435 Landes Bioscience, New York [Google Scholar]
- 16.Boggess D, Cunliffe-Beamer T, Sundberg JP: Colony establishment. Sundberg JP Boggess D eds. Systematic approach to evaluation of mouse mutations. 1999, :pp 1-13 CRC Press Inc., Boca Raton [Google Scholar]
- 17.Sundberg JP, France M, Boggess D, Sundberg BA, Jenson AB, Beamer WG, Shultz LD: Development and progression of psoriasiform dermatitis and systemic lesions in the flaky skin (fsn) mouse mutant. Pathobiology 1997, 65:261-286 [DOI] [PubMed] [Google Scholar]
- 18.Sundberg JP: Handbook of Mouse Mutations with Skin and Hair Abnormalities: Animal Models and Biomedical Tools. Boca Raton, CRC Press, Inc., 1994
- 19.Hogenesch H, Gijbels M, Offerman E, Hooft JV, Bekkum DV, Zurcher C: A spontaneous mutation characterized by chronic proliferative dermatitis in C57BL mice. Am J Pathol 1993, 143:972-982 [PMC free article] [PubMed] [Google Scholar]
- 20.Sundberg J, Boggess D, Bascom C, Limberg BJ, Shultz LD, Sundberg BA, King LE, Montagutelli X: Lanceolate hair-J (lahJ): a mouse model for human hair disorders. Exp Dermatol 2000, 9:201-213 [DOI] [PubMed] [Google Scholar]
- 21.Serup J, Jemec GBE: Handbook on Non-invasive Methods and the Skin. 1995:pp 173-178 CRC Press, Boca Raton
- 22.Sundberg JP, Rourk M, Boggess D, Hogan ME, Sundberg BA, Bertolino A: Angora mouse mutation: altered hair cycle, follicular dystrophy, phenotypic maintenance of skin grafts, and changes in keratin expression. Vet Pathol 1997, 34:171-179 [DOI] [PubMed] [Google Scholar]
- 23.Downing DT, Stranieri AM: Correction for deviation for the Lambert-Beer law in the quantitation of thin-layer chromatograms by photodensitometry. J Chromatogr 1980, 192:208-211 [Google Scholar]
- 24.Hansen LS, Coggle JE, Wells J, Charles MW: The influence of the hair cycle on the thickness of mouse skin. Anat Rec 1984, 210:569-573 [DOI] [PubMed] [Google Scholar]
- 25.Chase HB, Montagna W, Malone JD: Changes in the skin in relation to the hair growth cycle. Anat Rec 1953, 116:75-82 [DOI] [PubMed] [Google Scholar]
- 26.Eilertsen KJ, Tran T, Sundberg JP, Stenn KS, Parimoo S: High resolution genetic and physical mapping of the mouse asebia locus: a key gene locus for sebaceous gland differentiation. J Exp Anim Sci 2000, 40:165-170 [Google Scholar]
- 27.Berardesca E, Distante F: Mechanisms of skin irritations. Curr Probl Dermatol 1995, 23:1-8 [PubMed] [Google Scholar]
- 28.Agner T, Serup J: Sodium lauryl sulphate for irritant patch testing—a dose response study using bioengineering methods for determination of skin irritation. J Invest Dermatol 1990, 95:543-547 [DOI] [PubMed] [Google Scholar]
- 29.Shahidullah M, Ralffle EJ, Rimmer AR, Frain-Bell W: Transepidermal water loss in patients with dermatitis. Br J Dermatol 1969, 81:722-730 [DOI] [PubMed] [Google Scholar]
- 30.Oran A, Marshall JS, Kondo S, Paglia D, McKenzie RC: Cyclosporin inhibits intercellular adhesion molecule-1 expression and reduces mast cell numbers in the asebia mouse model of chronic skin inflammation. Br J Dermatol 1997, 136:519-526 [PubMed] [Google Scholar]
- 31.Williams D, Stenn KS: Transection level dictates the pattern of hair follicle sheath growth in vitro. Dev Biol 1994, 165:469-479 [DOI] [PubMed] [Google Scholar]
- 32.Straile WE: Root sheath-dermal papilla relationships and the control of hair growth. Lyne AG Short BF eds. Biology of Skin and Hair Growth. 1965, :pp 35-37 Angus & Robertson, Sydney [Google Scholar]
- 33.Gemmell RT, Chapman RE: Formation and breakdown of the inner root sheath and features of the pilary-canal epithelium in the wool follicle. J Ultrastruct Res 1971, 36:355-366 [DOI] [PubMed] [Google Scholar]
- 34.Williams D, Siock P, Stenn KS: 13-cis-retinoic acid affects sheath-shaft interactions of equine hair follicles in vitro. J Invest Dermatol 1996, 106:356-361 [DOI] [PubMed] [Google Scholar]