Abstract
Expression of cyclins A and E and cyclin-dependent kinase 2 (cdk2) was examined immunohistochemically in 55 cases of soft tissue smooth muscle tumors, including vascular leiomyoma, and compared to expression of Ki-67 and proliferating cell nuclear antigen. Cyclin A was expressed in 70% of the leiomyoma cases, but with much lower labeling indexes than in leiomyosarcoma. Cyclin E was expressed exclusively in leiomyosarcoma. Although the differences of cyclin A- and cyclin E-labeling indexes between leiomyoma and leiomyosarcoma were statistically significant, no significant differences were found in the other markers. Furthermore, cyclin A- and/or E-positivity predicted a poor prognosis in recurrence- or metastasis-free survivals and overall survival. Immunoblotting revealed that cyclins A and E were expressed, in complex with cdk2, exclusively in tumors. In addition, not only leiomyosarcoma, but also leiomyoma specimens that exhibited negligible levels of complex expression, manifested detectable cdk2 activity. These results suggest 1) up-regulation of active cyclin A/cdk2 expression and associated kinase activity is critical for unrestrained cell proliferation; 2) cyclin E/cdk2 complexes may play a crucial role in leiomyosarcoma; 3) immunohistochemical detection of cyclins can be a more reliable tool for differential diagnosis between leiomyoma versus leiomyosarcoma than that of Ki-67 or proliferating cell nuclear antigen, and be a possible prognostic indicator.
The external soft tissue is one of the locations where leiomyosarcoma (LMS) is occasionally found, with a frequency ranging from 2.3 to 3.8% of all sarcomas in that location. 1,2 Regarding the histopathological diagnostic criteria for differentiation of LMS from leiomyoma (LM), in the present consensus, the most reliable and reproducible measure in predicting malignancy is mitotic activity although several factors, such as size, cellularity, and necrosis correlate with malignancy to some extent. 3-6 Unlike uterine lesions, more than 2 mitoses/10 high-power fields (HPFs) have been thought to warrant a finding of potential malignancy in smooth muscle tumor (SMT) of the external soft tissue, 7 illustrating the strict criteria applied to the diagnosis of LMS in that location. However, even when these stringent criteria are applied, exceptions are not uncommon. In reality, histologically benign-looking SMTs occasionally metastasize after long latent periods, leading to the suspicion that the vast majority of SMTs with high cellularity are potentially malignant. 4,5 As a result of these observations, a more strict criteria has been recently proposed, namely, greater than 1 mitosis per 10 HPFs for cutaneous SMT and greater than zero for that in deep soft tissue should be regarded as LMS. 8-10
In soft tissue LMS, the factors reported to reliably predict a better prognosis are superficial location (cutis rather than subcutis or deeper) and small size (<5 cm in diameter). 4,11 Attempts have been made to correlate the biological behavior of LMS with aberrations of oncogene and tumor suppressor gene products, such as overexpression of mdm2, aberration of p53, Rb, and/or DCC, and inactivation of p16. 12-14 However, the clinicopathological implications of these aberrations are still uncertain, and their ability to distinguish diagnoses of LMS and LM has not been established. In addition, although there has been significant accumulation of morphological observations in human LM and LMS, the pathological mechanisms of cell proliferation and, more particularly, the ultimate participation of various cell cycle regulators have not been analyzed.
It is now widely known that cell proliferation is precisely regulated by cell-cycle control mechanisms that depend on the activities of various cyclins and cyclin-dependent kinase (cdk) complexes. 15-21 These complexes are regulated both positively and negatively. One positive regulator is cdk-activating kinase (CAK), which activates cdks by phosphorylation at specific threonine residues. 22-25 In addition, several negative regulators have been identified, including p21, p27, and p57, which are universal cdk-inhibitors, and p16, p15, p18, and p19, which are INK4-family-inhibitors of cdk4/cdk6. 19-21,26-29 Thus, cell proliferation is regulated by mechanisms that are complex and redundant.
In this study, we examined the expression of cyclin A and cyclin E, as well as their catalytic partner cdk2, by immunohistochemistry and evaluated the utility in providing a differential diagnosis between benign and malignant SMTs of the external soft tissue and further evaluated the applicability for predicting the prognosis. In addition, we performed immunoblotting analysis and in vitro kinase reaction assays in matched sets of normal/tumor tissues to examine whether the expression of these molecules and their associated kinase activities are responsible for the unrestrained cell proliferation of SMTs.
Materials and Methods
Cases and Histological Classification
This study examined 55 cases of primary SMTs of the external soft tissue, including vascular leiomyoma, obtained from surgical material in the Department of Pathology, Kitasato University Hospital, between 1973 and 1999, as well as consultation cases from outside the hospital. The diagnosis of SMT was made based on the histological features and immunohistochemical positivity for α-smooth muscle actin, HHF-35, and desmin. 30,31 In addition to these histopathological criteria for SMT, a diagnosis of LMS was made for the tumors showing more than 1 mitotic figure per 10 HPFs (1 HPF = 0.16 mm2) for cutaneous lesions and more than zero for subcutaneous or deeper lesions. 8-10 In practice, mitoses were estimated by counting 2000 tumor cells in 50 high-power fields in 10 regions each on five sections, and expressed as the number of mitotic figures per 10 HPFs (mitotic index). These diagnostic criteria indicated that 30 cases of LM and 25 cases of LMS were present among the specimens examined. As listed in Table 1 ▶ , size, locations, and major clinical profiles of the LMS cases were as follows: 17 cases were larger than 5 cm in diameter and eight were smaller, eight resided in cutis and 17 in subcutis or deeper, 12 were in males and 13 were in females. Local recurrence was noted in eight patients, distant metastasis in five patients, and five patients died of disease. The clinicopathological profiles and the staining results of those cases were detailed in Table 2 ▶ . In 26 out of 30 LM cases, the absence of recurrence or metastasis was confirmed by patients’ follow-up data.
Table 1.
Clinicopathological Profiles of the Cases
| LM (30 cases) | LMS (25 cases) | |
|---|---|---|
| Age (mean) | 19–77 (39) | 24–73 (48) |
| Sex (male, female) | 11, 19 | 12, 13 |
| Size (<5 cm, >5 cm) | 30, 0 | 8, 17 |
| Location (cutis, deeper) | 28, 2 | 8, 17 |
| Follow-up length | 8–61 months | 6–191 months |
| (Median duration) | (31) | (55) |
| Local recurrence | 0/26* | 8/25 |
| Metastasis | 0/26* | 5/25 |
| Death | 0/26* | 5/25 |
*LM, leiomyoma; LMS, leiomyosarcoma.
*Follow-up data were available in 26 out of 30 cases.
Table 2.
Clinical and Immunohistochemical Profiles of the Cases of Leiomyosarcoma Showing Recurrence, Metastasis, or Death
| Locations | Labeling index on IHC (%) | Clinical course (months, after surgery) | ||||
|---|---|---|---|---|---|---|
| Cyclin A | Cyclin E | cdk2 | Recurrence | Metastasis | Death | |
| CU | 14.1 | 3.6 | 28.2 | 20 | N.S. (26) | N.S. (26) |
| CU | 7.6 | 0 | 20.3 | 36 | N.S. (66) | N.S. (66) |
| SC | 6.7 | 0 | 20.8 | N.S. (60) | 52 | 60 |
| SC | 23.3 | 0 | 25.8 | 15 | N.S. (41) | N.S. (41) |
| SC | 8.5 | 0 | 45.1 | 37 | N.S. (47) | N.S. (47) |
| SC | 7.8 | 0 | 17.4 | 64 | N.S. (114) | N.S. (114) |
| IM | 29.4 | 24 | 27.6 | 10 | 23 | 28 |
| IM | 17.5 | 10.9 | 38.2 | 17 | 24 | 30 |
| IM | 8.5 | 12.0 | 10.3 | N.S. (23) | 16 | 23 |
| IM | 12.9 | 6.7 | 31.0 | 23 | 34 | 41 |
IHC, immunohistochemistry; CU, cutis (intradermal); SC, subcutaneous; IM, intramuscular; N.S., not seen.
Archival Tissue Samples and Immunohistochemistry
All archival tissue samples were routinely fixed in formalin and embedded in paraffin. Deparaffinized sections were autoclaved (120°C, 2 atm., 20 minutes) in 20 mmol/L citrate buffer (pH 6.0). 29 Immunostaining was performed with primary antibodies at the following dilutions: anti-cyclin A (monoclonal, clone 6E6, #NCL-CYCLIN A; Novocastra, Newcastle, United Kingdom); 1:500 dilution, anti-cyclin E (monoclonal, clone 13A3, #NCL-CYCLIN E; Novocastra); 1:100, anti-cdk2 (polyclonal, #sc-748; Santa Cruz Biotechnology, Santa Cruz, CA); 1:2500, anti-CAK (monoclonal, clone MO-1.1, #NCL-CAK; Novocastra); 1:200, anti-Ki-67 (monoclonal, clone MIB-1, #0505; Immunotech, Marseille, France); 1:100, anti-PCNA (proliferating cell nuclear antigen, monoclonal, clone PC-10, #MO879; DAKO, Glostrup, Denmark); 1:1000. The specificity of these antibodies was previously confirmed by immunohistochemistry and immunoblotting analysis. 32,33 The conventional streptavidin-biotinylated horseradish peroxidase complex method (LSAB kit; DAKO Japan, Ltd., Kyoto, Japan) was used as directed by the manufacturer’s instruction. Colorization was performed by the peroxidase-diaminobenzidine method.
Scoring Immunoreactivity
The percentages of tumor cells with positive staining were estimated by counting 2000 tumor cells in 50 high-power fields in 10 regions each on five sections, and expressed as a labeling index (LI). 8,9 LIs for all marker proteins were counted in almost identical fields of nearby sections.
Paired Normal/Tumor Tissue Collection
Fresh fragments of paired tumor and adjacent normal tissues, composed of fibroblasts, capillaries, collagenous fibers, inflammatory cells, and/or striated muscle, were obtained from surgically resected specimens. These comprised six cases of LM and five cases of LMS.
Preparation of Tissue Extracts
For protein extraction, fresh tissues were homogenized in high-salt lysis buffer (0.5% Nonidet P-40, 0.1% sodium dodecyl sulfate, 50 mmol/L Tris-HCl, pH 8.0, 0.25 mol/L NaCl, 5 mmol/L ethylenediaminetetraacetic acid, 50 mmol/L NaF, 0.5 mmol/L phenylmethyl sulfonyl fluoride, 5 μg/ml of aprotinin, 5 μg/ml of leupeptin) 34 on ice and the resultant lysates were sonicated on ice four times for 10 seconds each time. 32,35 Lysates were clarified by centrifugation at 10,000 × g for 5 minutes.
Immunoblotting Analysis
Equal amounts (50 μg) of protein were used for immunoblotting analysis. Each protein was detected by the sequential application of the same specific primary antibodies that were used for immunohistochemistry in the following dilutions: cyclin A (1:500), cyclin E (1:100), cdk2 (1:200), and alkaline phosphatase-conjugated secondary antibody (1:6,000; Promega, Madison, WI). Colorization was performed with nitro blue tetrazolium and 5-bromo-4-chloro-3-indol-phosphate (Bio-Rad, Gaithersburg, MD) in 100 mmol/L Tris buffer (pH 9.6). For immunoprecipitation followed by immunoblotting, tissues were homogenized and lysed in NP-40 lysis buffer (50 mmol/L Tris-HCl, pH 7.4, 0.5% Nonidet P-40, 0.15 mol/L NaCl, 50 mmol/L NaF, 1 mmol/L dithiothreitol, 1 mmol/L phenylmethyl sulfonyl fluoride, 5 μg/ml of aprotinin, 5 μg/ml of leupeptin). 36 Lysates (200 μg of protein) were incubated with p13suc1-Sepharose for 4 hours at 4°C. The precipitates were used for further immunoblotting analysis for cyclin A or cyclin E. Alternatively, lysates were incubated with anti-cyclin A (1:500 dilution) or anti-cyclin E (1:100) antibodies for 1 hour at 4°C followed by an additional 1 hour incubation with protein G-Sepharose beads at 4°C. 36 The immunoprecipitates were used for immunoblotting analysis for cdk2.
In Vitro Kinase Reaction
For cdk2 immunocomplex kinase reactions, fresh tissues in which the presence of a cdk2 doublet band had been confirmed by immunoblotting analysis, were homogenized and lysed in solubilizing buffer (50 mmol/L Tris-HCl, pH 7.2, 1% Nonidet P-40, 0.15 mol/L NaCl, 5 mmol/L ethylenediaminetetraacetic acid, 50 mmol/L β-glycerophosphate, 0.05 mmol/L NaF, 5 mmol/L dithiothreitol, 1 mmol/L Na3VO4, 0.1 mmol/L phenylmethyl sulfonyl fluoride, 5 μg/ml aprotinin, 5 μg/ml leupeptin). 34 Lysates (200 μg of protein) were incubated with anti-cdk2 antibody (diluted 1:200) for 1 hour followed by an additional 1 hour incubation with protein A-Sepharose beads at 4°C. For cyclin A- or cyclin E-associated kinase reactions, lysates (200 μg of protein) were incubated with anti-cyclin A (diluted 1:500) or anti-cyclin E antibody (1:100) for 1 hour followed by an additional 1 hour incubation with protein G-Sepharose beads at 4°C. A bacterially-expressed fragment of the retinoblastoma protein (pRB, amino acids 385 to 928) fused to glutathione S-transferase was used as a substrate (0.5 μg protein) in 50 μl of kinase reaction buffer (50 mmol/L Tris-HCl, pH 7.2, 10 mmol/L MgCl2, 1 mmol/L dithiothreitol, 20 μmol/L [γ-32P]ATP (5 μCi; 1 μCi = 37 kBq; ICN, Irvine, CA). 34 After incubation for 10 minutes at room temperature, the sample was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by autoradiography.
Patients’ Follow-up
Patients’ outcome data were collected from hospital charts. Informative patients’ charts were available for 26 cases of LM and 25 cases of LMS. Follow-up period ranged from 6 months to 16 years after the pathological diagnosis (Tables 1 and 2) ▶ ▶ .
Statistical Analysis
The differences in the LIs between LM and LMS specimens obtained by immunohistochemical staining with each antibody were analyzed by the paired comparison t-test. The degree of correlation between the LIs obtained by two particular antibodies was analyzed and calculated by the Spearman’s rank correlation coefficient test. Correlations between immunohistochemical LIs of cyclin A, cyclin E, and clinical outcome were analyzed by the method of Kaplan-Meier analysis and differences between the curves were tested for statistical significance with the log-rank test. Multivariate analysis was performed with the Cox regression model to evaluate the additional prognostic value of the expression of cyclins as well as mitotic index to the other prognostic variables. For these analyses, the cases were divided into two groups: 1) cyclin A-positive group includes cases having LIs of more than 10%, and 2) cyclin E-positive group having LIs of more than zero. The other cases were automatically categorized into their respective negative groups. Dividing by mitotic index, the cut-off value was set at 6 mitoses/10 HPFs.
Results
Immunohistochemical Stains and LI Values
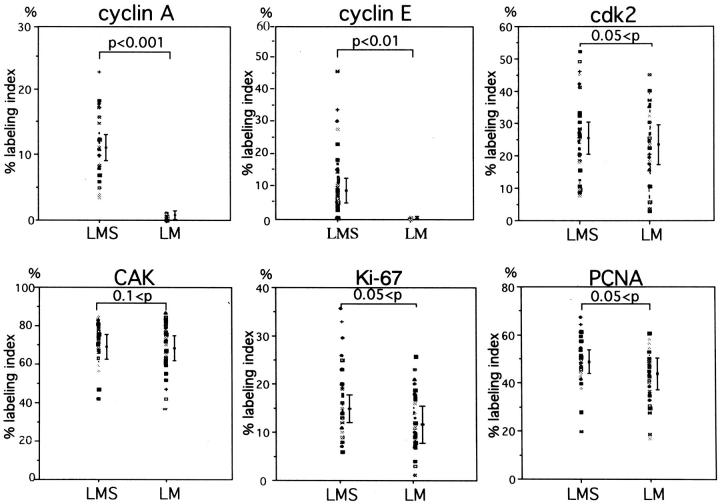
Positive immunohistochemical staining in this study was confined almost exclusively to the nuclei for all of the antibodies used (Figure 1) ▶ . The overall results of the LIs from the various immunohistochemical analyses are shown in Figure 2 ▶ .
Figure 1.
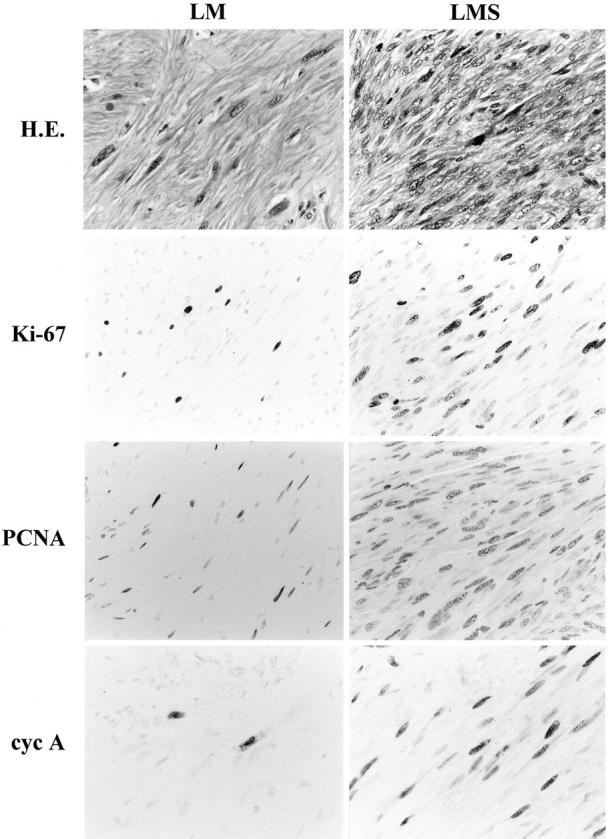
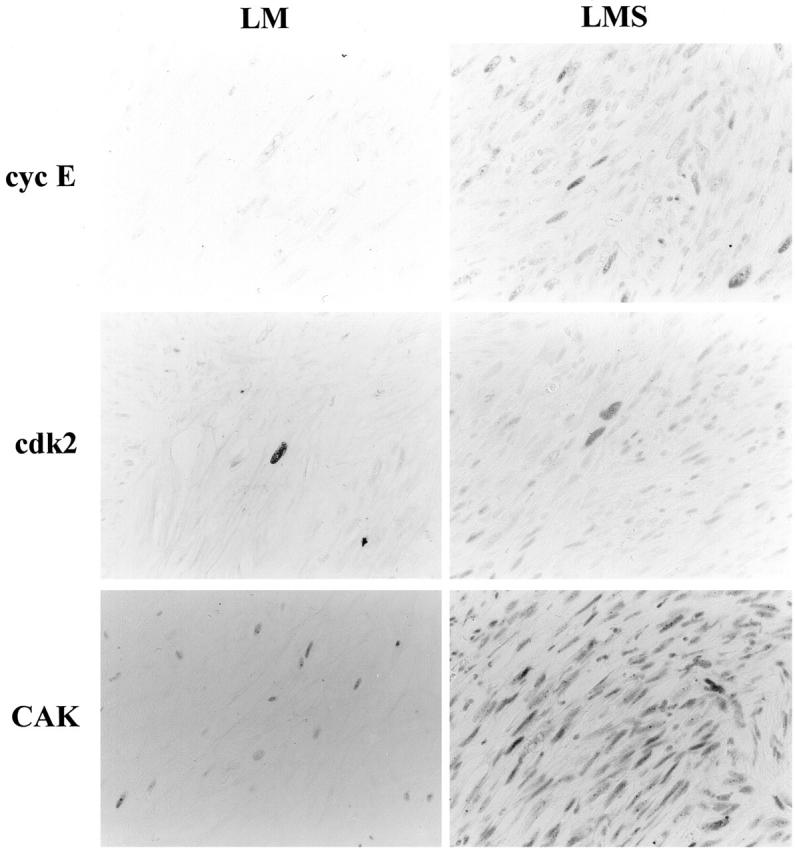
Histological features of LM and LMS as well as immunohistochemical staining for Ki-67, PCNA, and cell-cycle regulator proteins. Streptavidin-biotin peroxidase method with hematoxylin counterstain; original magnification, ×700. PCNA, proliferating cell nuclear antigen; cyc A, cyclin A; cyc E, cyclin E; cdk, cyclin-dependent kinase 2; CAK, cdk-activating kinase.
Figure 2.
LI obtained from immunohistochemical staining. LIs in LM and LMS are expressed as dots and mean values ± 2× standard errors are represented by bars. PCNA, proliferating cell nuclear antigen; cyc A, cyclin A; cyc E, cyclin E; cdk, cyclin-dependent kinase 2; CAK, cdk-activating kinase.
Ki-67
Proliferative activity was evaluated from the Ki-67 LI observed among the tumor cells in LM and LMS specimens and the cells in nonneoplastic tissue. The Ki-67 LI was 10 to 20 times higher than the mitotic counts in LMS specimens, confirming the reliability of the antibody and staining procedure. 37 However, this correlation did not hold in the cases of LM, because Ki-67 positivity was detectable despite the lack of mitotic figures in LM cases. LIs of the tumor cells in LMS ranged from 6.3 to 35.5%, higher than those in LM, which ranged from 1.7 to 26.2%, although this difference was not statistically significant (0.05 < P by t-test; Figure 2 ▶ ). Most of the cells in the nonneoplastic tissue stained negatively except for occasional fibroblasts and vascular endothelial cells.
PCNA
The PCNA LI of the tumor cells in LMS ranged from 19.6 to 68.1%, higher than those in LM, which ranged from 16.5 to 62.0%, although this difference was not statistically significant (0.05 < P; Figure 2 ▶ ). Occasionally, lymphocytes, vascular endothelial cells, or fibroblasts also revealed positive staining.
Cyclin A
The staining profile of cyclin A seemed to parallel that of Ki-67 staining. Cyclin A staining revealed that the vast majority of LMS cases exhibited positive staining, with LIs ranging from 3.3 to 23.5%. In contrast, although positive staining was identified in 70% of the LM cases, the highest LI was 1.8%. Thus, the cyclin A LIs in the tumor cells of LMS were higher than those in LM, and this difference was statistically significant (P < 0.001; Figure 2 ▶ ). Positive staining in nonneoplastic cells was also occasionally observed; for instance, in cells within the germinal center of the lymphoid follicles.
Cyclin E
Cyclin E staining was found in 68.0% (17 out of 25) of the LMS cases, with LIs ranging up to 45.9% (Figure 2) ▶ . In contrast, none of the LM cases showed positive staining for cyclin E. This difference in LIs was statistically significant (P < 0.01; Figure 2 ▶ ).
Cdk2
Cdk2-positivity was also found restricted to the tumor cells (Figure 1) ▶ . Although, in general, the LIs of the tumor cells were slightly higher in LMS (7.5 to 52.8%) compared to LM (2.3 to 45.1%), this difference was not statistically significant (0.05 < P; Figure 2 ▶ ).
CAK
CAK positivity was ubiquitously demonstrated in both groups, not only in the tumor cells, but also in the cells of nonneoplastic tissue, including infiltrating lymphocytes, vascular endothelial cells, and fibroblasts, with the LI values ranging from 37 to 88% in tumor cells of both groups (Figure 2) ▶ . Staining intensity was also similar among the areas and among various kinds of cells (Figure 1) ▶ .
Specific Correlations in LIs and Staining Patterns
The interactions among cell-cycle regulators and the signal cascades in which they function have been gradually elucidated throughout the past several years. Based on the known relationships among some of these molecules, we statistically evaluated the immunohistochemical results (LIs) obtained in the tumor cells (Table 3) ▶ .
Table 3.
Correlation Coefficients (ρ) between Expression Frequencies of the Related Proteins in Tumor Cells
| Ki-67 | PCNA | cyclin A | cyclin E | cdk2 | CAK | |
|---|---|---|---|---|---|---|
| Ki-67 | — | 0.28 | 0.53† | 0.43* | 0.45* | 0.05 |
| PCNA | 0.28 | — | 0.31 | 0.12 | 0.14 | 0.02 |
| Cyclin A | 0.53† | 0.31 | — | 0.52‡ | 0.58† | 0.03 |
| Cyclin E | 0.43* | 0.12 | 0.52‡ | — | 0.35 | 0.07 |
| cdk2 | 0.45* | 0.14 | 0.58† | 0.35 | — | 0.02 |
| CAK | 0.05 | 0.02 | 0.03 | 0.07 | 0.02 | — |
PCNA, proliferating cell nuclear antigen; cdk, cyclin-dependent kinase; CAK, cdk-activating kinase.
*p < 0.05.
†p < 0.01.
‡p < 0.001.
ρ, Positive ratios of the tumor cells on immunohistochemical staining (labeling indexes) against respective proteins counted in each case were analysed by Spearman’s rank test.
Ki-67 and Cyclin A
The LIs of Ki-67 and cyclin A showed a statistically significant correlation with a coefficient of 0.534 (P < 0.01) as determined by Spearman’s test.
Ki-67 and Cyclin E
Although cases with higher cyclin E LIs tended to also express higher Ki-67 LIs, this correlation was weak (coefficient: ρ = 0.434; P < 0.05), probably because cyclin E-positive cases comprised only 17 of 25 LMS cases.
Cyclin A and Cdk2
The correlation between the cyclin A and cdk2 LIs was positive at a statistically significant level with a coefficient of 0.582 (P < 0.01).
CAK and Cyclin A-cdk2
Although cyclin A-cdk2 positivity was observed almost exclusively in the tumor cells, positive CAK staining was observed ubiquitously, ie, in both the tumor and the nonneoplastic cells (Figure 1) ▶ . The correlation coefficient between CAK and cyclin A LIs was 0.03, and that between CAK and cdk2 was 0.017, neither of which indicated significant correlation.
CAK and Cyclin E-cdk2
The correlation between CAK and cyclin E LIs was very low and the coefficient was 0.07.
Immunoblotting Analysis
To further confirm the results observed by immunohistochemical staining, immunoblotting analysis was performed using lysates obtained from available paired normal/tumor tissue samples. The clinical profiles and the staining results of those cases are summarized in Table 4 ▶ .
Table 4.
Histological and Immunohistochemical Profiles of the Cases Utilized for Immunoblotting, Immunoprecipitation, and for in Vitro Kinase Reaction
| Case No. | Pathological diagnosis | Labeling index on IHC (%) | Clinical course (months after surgery) | ||||
|---|---|---|---|---|---|---|---|
| Cyclin A | Cyclin E | cdk2 | Recurrence | Metastasis | Death | ||
| 1 | Leiomyoma | 0.6 | 0 | 27.5 | FOD (11) | ||
| 2 | Leiomyoma | 0.9 | 0 | 15.1 | FOD (13) | ||
| 3 | Leiomyoma | 0.2 | 0 | 17.4 | FOD (18) | ||
| 4 | Leiomyoma | 1.0 | 0 | 9.3 | FOD (23) | ||
| 5 | Leiomyoma | 0.6 | 0 | 10.5 | FOD (33) | ||
| 6 | Leiomyoma | 1.5 | 0 | 39.8 | FOD (35) | ||
| 1 | Leiomyosarcoma, SC | 8.5 | 0 | 45.1 | 37 | N.S. (47) | N.S. (47) |
| 2 | Leiomyosarcoma, IM | 12.9 | 6.7 | 31.0 | 23 | 34 | 41 |
| 3 | Leiomyosarcoma, CU | 15.1 | 4.5 | 34.7 | FOD (11) | ||
| 4 | Leiomyosarcoma, IM | 17.5 | 10.9 | 38.2 | 17 | 24 | 30 |
| 5 | Leiomyosarcoma, CU | 14.7 | 0 | 46.6 | FOD (14) |
IHC, immunohistochemistry; FOD, free of disease; N.S., not seen; SC, subcutaneous; IM, intramuscular; CU, cutis (intradermal).
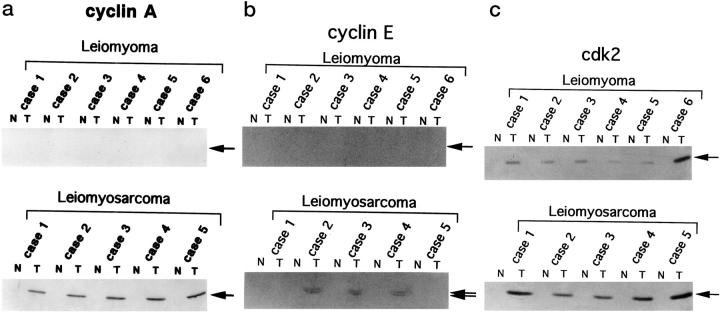
Cyclin A blotting revealed one major band of 58 kd, detectable only in tumor tissues under these experimental conditions (Figure 3a) ▶ . This band was observed in all five cases of LMS, but in none of the LM cases. The level of cyclin A protein expression correlated well with the LIs determined by immunohistochemistry.
Figure 3.
Immunoblotting analysis of cyclin A (a), E (b), and cdk2 (c) proteins expressed in surgically resected tissues. Lysates (50 μg of protein) prepared from paired normal/tumor tissues were subjected to immunoblotting analysis. N, normal tissue; T, tumor tissue.
Cyclin E blotting revealed two major bands of 49 and 43 kd, corresponding to the two alternatively-spliced forms of human cyclin E (Figure 3b) ▶ . 38 Although expression of cyclin E was elevated in three out of five cases of LMS (cases 2, 3, and 4), no elevation was observed in the cases of LM, consistent with the absence of cyclin E positivity on immunohistochemistry.
Cdk2 blotting revealed two major bands of 33 and 34 kd, corresponding to the hyper- and unphosphorylated forms of human cdk2, respectively (Figure 3c) ▶ . 34 In contrast to cyclins A and E, cdk2 expression was observed in both LMS and LM specimens.
Immunoblotting Analysis of Cdk-Associated Cyclin A and Cyclin E
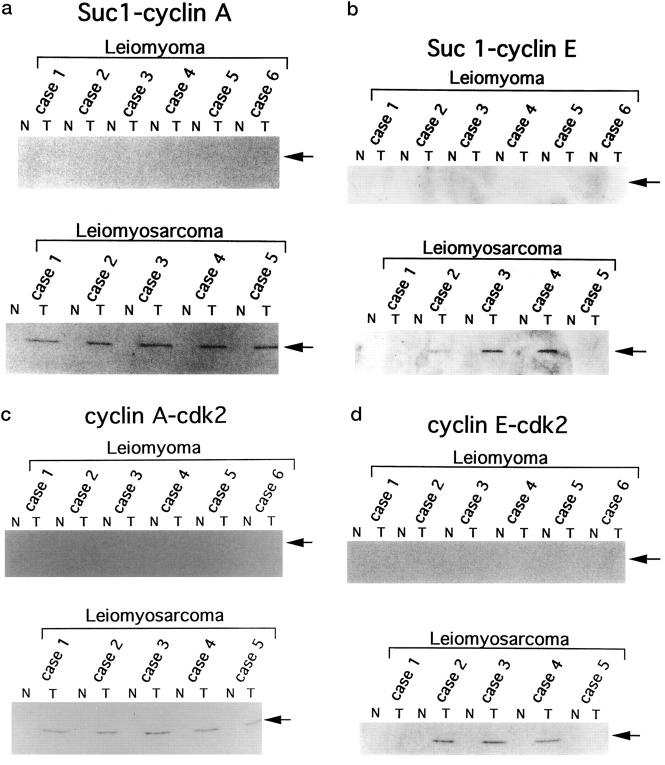
To evaluate the levels of cyclin A and cyclin E associated with cdk, we subjected p13suc1-precipitates prepared from the selected tissue samples to sequential immunoblotting analysis. As shown in Figure 4, a and b ▶ , cyclin A and cyclin E associated with p13suc1-Sepharose-bound cdk were detected only in the tumor samples. To further confirm the association of cdk2 with cyclin A or cyclin E, we subjected anti-cyclin A and anti-cyclin E immunoprecipitates to sequential immunoblotting for cdk2. As shown in Figure 4, c and d ▶ , we observed higher levels of cdk2-cyclin complexes in these tumor tissues. The expression pattern of cyclin A/cdk2 and cyclin E/cdk2 were almost identical to those of cyclin A and cyclin E, respectively. Thus, the expression levels of cyclins/cdk2 complexes seemed to be determined by the expression of the cyclins, not by cdk2.
Figure 4.
Levels of cdk-associated cyclin A and cyclin E and levels of cdk2 associated with cyclin A or cyclin E. Lysates prepared from surgically resected tissues (200 μg) were precipitated with p13suc1-Sepharose (for cyclin A and cyclin E blotting) or immunoprecipitated with anti-cyclin A or anti-cyclin E antibodies (for cdk2 blotting). The precipitates were subjected to further immunoblotting analysis with the respective antibodies. Suc 1-cyclin A (a), Suc 1-cyclin E (b): Precipitation by p13suc1-Sepharose followed by cyclin A or cyclin E blotting, respectively. Cyclin A-cdk2 (c), Cyclin E-cdk2 (d): Immunoprecipitation by anti-cyclin A or anti-cyclin E antibodies followed by cdk2-blotting, respectively. N, normal tissue; T, tumor tissue.
Cdk2- and Cyclin-Associated Kinase Activities
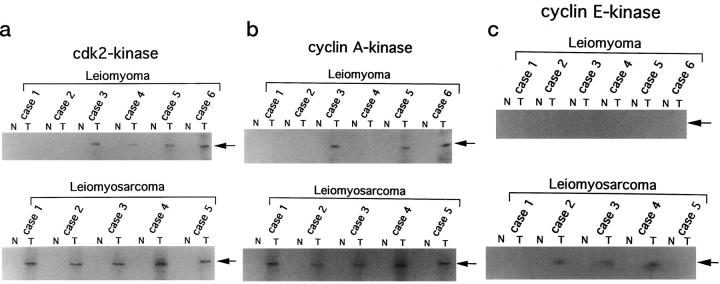
To examine whether the cyclins or cdk2 detected by immunohistochemistry and immunoblotting analysis are functionally active complexes, cyclin A-, cyclin E-, and cdk2-associated kinase activities were examined by in vitro kinase reactions after immunoprecipitation by anti-cyclin A, anti-cyclin E, or anti-cdk2 antibodies. Cdk2- and cyclin A-associated kinase activities were detected in all LMS specimens (Figure 5, a and b) ▶ . However, cyclin E-associated kinase activity was detectable only in three cases in which overexpression of cyclin E had been detected by immunoblotting analysis (cases 2, 3, and 4; refer to Figures 3b and 5c ▶ ▶ ). Cdk2-associated kinase activity was detectable in four cases of LM (cases 3 to 6) in which expression of cdk2 had been detected by immunoblotting analysis (Figures 3c and 5a) ▶ ▶ . Cyclin A-associated kinase activity was also detected in three cases of LM (case 3, 5, and 6) in which expression of cyclin A or cyclin A/cdk2 complexes was not detectable in immunoblotting analysis (Figures 3a and 5b) ▶ ▶ .
Figure 5.
Kinase activities associated with cdk2 (a), cyclin A (b), or cyclin E (c) in surgically resected tissues. Lysates (200 μg) were immunoprecipitated with anti-cdk2, anti-cyclin A, or anti-cyclin E antibodies. The immunocomplexes were assayed for kinase activity using glutathione S-transferase-retinoblastoma fusion protein as a substrate. N, normal tissue; T, tumor tissue.
Correlation of Immunohistochemical Positivity with Patients’ Clinical Course
The clinicopathological factors, in the present consensus, considered to be significant indicators of prognosis in LMS are size (less than or more than 5 cm in diameter) and location (cutis/subcutis and deeper). 1,4,11 Assuming that the proteins examined in this study play critical roles in the kinetics of cell proliferation, we might expect a correlation between our immunohistochemical results and other parameters such as size or location of tumor, local recurrence- or metastasis-free survival, and patients’ overall survival; ie, these molecular markers may have prognostic value. To assess their clinicopathological implications, the results of the cyclin A and cyclin E staining were evaluated for their correlation with the clinicopathological factors mentioned above, because we knew that only cyclin A and cyclin E staining could discrimininate LMS from LM (Figure 2) ▶ . Based on the fact that cutaneous LMS never metastasize, we analyzed the correlation with recurrence in all (superficial and deep-seated) cases of LMS, and with metastasis or overall survival in the cases of deep-seated LMS. As a result, there was no correlation between any LIs and the location (cutis/subcutis or deeper) or the size (less than or more than 5 cm in diameter) of the tumors by paired comparison t-test (0.05 < P; data not shown). The tumor size (larger than 5 cm) was a significant risk factor for metastasis as well as poor overall survival as determined by the Kaplan-Meier analysis in the group consisting of deeper LMS cases (subcutis or deeper soft tissue; Table 5 ▶ ). Mitotic index was a significant risk factor only for local recurrence. The cyclin A and cyclin E LIs were demonstrated to be significant indicators of prognosis: the cyclin A-positive group showed a higher risk of local recurrence, metastasis, and poor survival with statistical significance, compared with the negative group. Cyclin E-positivity was a risk factor for metastasis and poor survival. In multivariate analysis, cyclin A positivity was the most potent independent variable for predicting local recurrence, far exceeding the predictive potential of mitotic index and tumor size (Table 6) ▶ . Cyclin E-positivity turned out to be a significant determinant for overall survival, second to the tumor size (Table 6) ▶ . Accordingly, there was a trend for cyclin E-positive cases to have shorter survival periods (Tables 2 and 4) ▶ ▶ . Tumor size was the strongest variable to predict metastasis and overall survival in multivariate analysis.
Table 5.
Statistical Analysis (Kaplan-Meier Analysis) of Clinicopathological Parameter and Labeling Indexes of Immunohistochemical Staining in Leiomyosarcoma
| Parameter | Log rank test, P value |
|---|---|
| Size (</=, >5 cm) | |
| Recur* | N.S.¶ |
| Meta† | P < 0.02 |
| Survival‡ | P < 0.05 |
| Location (cutis/deeper) | |
| Recur | N.S.¶ |
| Meta | N.I.∥ |
| Survival | N.I.∥ |
| Mitosis (</=, >6)§ | |
| Recur | P < 0.05 |
| Meta | N.S.¶ |
| Survival | N.S.¶ |
| Cyclin A (</=, >10%) | |
| Recur | P < 0.01 |
| Meta | P < 0.05 |
| Survival | P < 0.05 |
| Cyclin E (0 or >0%) | |
| Recur | N.S.¶ |
| Meta | P < 0.02 |
| Survival | P < 0.05 |
*Recur, local recurrence (all cases analyzed).
†Meta, metastasis-free survival period (only cases of deeper LMS analyzed).
‡Survival; overall survival period (only cases of deeper LMS analyzed).
§Six mitoses/10 high-power-field.
¶N.S.; not significant.
∥N.I.; not included for analysis.
Note: Since cutaneous leiomyosarcoma never metastasizes, we analyzed recurrence in all cases of LMS, and metastasis and overall survival in deep LMS cases. Accordingly, for the analysis of metastasis and overall survival, parameter location was not included.
Table 6.
Statistical Analysis (Multivariate Analysis) of Clinicopathological Parameter and Labeling Indexes of Immunohistochemical Staining in Leiomyosarcoma
| Clinical outcome | Parameter | R.R. | C.I. | P value |
|---|---|---|---|---|
| Recurrence-free survival period | Cyclin A* | 18.4 | 1.2–282.1 | <0.02 |
| Mitosis† | 6.96 | 1.06–45.7 | <0.05 | |
| Size‡ | 3.13 | 0.83–12.0 | N.S.∥ | |
| Location§ | 1.01 | 0.29–3.51 | N.S.∥ | |
| Cyclin E¶ | 0.36 | 0.11–1.18 | N.S.∥ | |
| Metastasis-free survival period | Size | 10.4 | 1.06–102.0 | <0.05 |
| Cyclin E | 1.49 | 0.26–8.54 | N.S.∥ | |
| Cyclin A | 8.90 | 0.22–360.0 | N.S.∥ | |
| Mitosis | 1.26 | 0.31–5.47 | N.S.∥ | |
| Overall survival period | Size | 10.9 | 1.14–104.1 | <0.05 |
| Cyclin E | 6.81 | 1.01–45.9 | <0.05 | |
| Cyclin A | 5.86 | 0.15–230.7 | N.S.∥ | |
| Mitosis | 3.53 | 0.53–23.5 | N.S.∥ |
R.R., relative risk; C.I., 95% confidence interval.
*Cyclin A; < or = >10%.
†Mitosis; < or = > 6 mitoses/10 high-power-field.
‡Size; < or = >5 cm.
§Location; cutis/deeper.
¶Cyclin E; 0 or > 0%.
∥N.S.; not significant.
Note: Since cutaneous leiomyosarcoma never metastasizes, we analyzed recurrence in all cases of LMS and metastasis and overall survival in deep LMS cases. Accordingly, for the analysis of metastasis and overall survival, parameter “location” was not included.
Discussion
A definitive histopathological diagnosis of benign versus malignant SMTs has been a long-standing difficulty, leading to a hypothesis that each of these tumors could be placed at a certain point in a biological sequence. 2,7 Nevertheless, there should be a clear-cut distinction between these two with regards to the patient’s prognosis, because one never recurs nor metastasizes whereas the other may. 2,3,7,11,12 Thus, they should be diagnosed and treated as two related but distinct tumors. Although, in addition to the mitotic index, immunohistochemical analysis by Ki-67 and p53 staining was described to be helpful in differentiation between LM and LMS of the uterus, the borderline (atypical) LM and low-grade LMS revealed LI-values of intermediate level between LM and LMS. 39 This disputable histopathological criteria and the lack of reliable molecular markers of absolute malignancy led us to examine the expression of cell-cycle regulator molecules as potential diagnostic tools.
In this study, we clearly demonstrated the diagnostic and clinical implications of immunohistochemical detection of cyclin A and cyclin E. We also showed that both of these cyclins were present in functionally active complexes with cdk2, more crucially in LMS.
Although positive immunohistochemical staining for cyclin A was detectable in the majority of both LM and LMS cases, the LIs obtained in these two groups were different at a statistically significant level, with the optimal cut-off values at 2 to 3%. Cyclin E immunoreactivity was identified only in LMS, although lack of reactivity does not necessarily indicate a benign tumor. Hence, a cyclin A LI of greater than 2% strongly suggests, and any cyclin E LI greater than zero definitively indicates a diagnosis of LMS. In contrast, Ki-67 and PCNA, both of which are well-known markers of proliferative activity, were not useful in discrimination of LM from LMS. Thus, cyclin A and cyclin E are more reliable histopathological diagnostic markers in these particular tumors. Because LIs of cyclin A correlated well with Ki-67 LIs, it definitely reflects proliferative capability, but seems to more strictly correlate with the capability of rapid and active proliferation. Furthermore, cyclin A- and cyclin E-positive groups revealed higher risk for, at least, one of the three clinical outcomes, such as local recurrence, metastasis, and poor overall survival compared with respective negative groups at statistically significant levels. Thus, immunohistochemical stainings of cyclin A and E are useful not only in pathological diagnosis, but also in predicting prognosis.
Immunoblotting analysis detected cyclins A, E, and cdk2 of an apparent wild-type molecular weight, suggesting that the possibility of rearrangement, truncation, or chimeric protein formation, as previously described, is unlikely. 40-44 The levels of protein expression observed in immunoblotting analysis correlated well with the LIs revealed by immunohistochemistry. Furthermore, it was confirmed that highly expressed cyclins A or E formed complexes with cdk2 and manifested higher kinase activity. Thus, we surmise that the cyclin A and cyclin E detected in immunohistochemical analysis definitely play a role as one of the pathological aspects of LMS. Three cases of LM (cases 3, 5, and 6) also showed cyclin A-associated kinase activity, although those three cases did not reveal any detectable level of cyclin A or cyclin A/cdk2 complexes by immunoblotting analysis. These results indicate that the in vitro kinase reaction is a more sensitive assay than immunohistochemistry or immunoblotting analysis to detect the presence of functional cyclins/cdk complexes. Our results may also prove the idea that up-regulation in kinase activity of cyclin A/cdk2 complexes is a common critical determinant of cell proliferation shared by LM and LMS. Furthermore, cyclin A expression above the threshold level will probably play a role inducing malignant potential and biological aggressiveness of LMS as supported by the results of the statistical analysis revealing the correlation of higher cyclin A LIs and higher risk of local recurrence, metastasis, and poor overall survivals in Kaplan-Meier analysis. Multivariate analysis added further weight to the idea that cyclins could be potential markers predicting prognosis independent of other clinical and pathological parameters: cyclin A for higher risk of recurrence and cyclin E for poor overall survival. This conclusion is partially consistent with a previous study on a large number of soft tissue sarcomas demonstrating that immunohistochemical expression of cyclin A correlated with a poor metastasis-free survival and a poor overall survival although there is the difference of the tumors examined between our series, only LMS of the external soft tissue, and theirs, varieties of sarcomas, including malignant fibrous histiocytoma and liposarcoma. 45 In that study, cyclin A was not a statistically significant prognostic factor in multivariate analysis in which tumor size was the strongest variable. However, using the cyclin A and cyclin E LIs described above, it may be possible to predict a prognosis even from the small specimens obtained by excisional biopsy before radical operation.
Amplification and/or overexpression of cyclins A, D1, and E have been variously described as being positive or negative prognostic indicators of carcinoma of the lungs, breast, ovary, colon, and so forth. 32,46-51 These G1/S cyclins that are genetically altered and overexpressed are, presumably, catalytically active and found predominantly in actively proliferating tumor cells as previously described. 32,33 However, detected cyclins occasionally represent the accumulation of catalytically inactive cyclin-cdk complexes, such as the inactive cyclin E-cdk2 complexes detected in certain kinds of tumors and cultured cell lines. 17,32,34,52 Indeed, in our previous study on human lung carcinomas, positive immunohistochemical staining for cyclin A was demonstrated to be related with shorter survival, whereas that of cyclin E with longer survival, compared with the respective negative groups. 32 In contrast, cyclin E seems to be important for active proliferation of the tumor cells in LMS because cyclin E expression was identified exclusively in LMS. Furthermore, based on our data showing that cases with higher cyclin E-LI and cyclin E protein expression also had higher associated kinase activity, we surmise that cyclin E-cdk2 complexes are expressed in an active form exclusively in the tumor cells of LMS. Consistently, the cyclin E-positive group showed poor metastasis-free or poor overall survival.
Results of immunohistochemical staining for CAK suggest that up-regulation of cdk2 activity is not induced through a CAK-dependent pathway. 25 One alternative explanation is that their activity is up-regulated by the regulation of kinase inhibitors. This possibility needs to be further elucidated by future study.
Altogether, our results show that, for benign and malignant SMTs of the external soft tissues, 1) immunohistochemical LIs of cyclin A and cyclin E are reliable diagnostic markers for LM or LMS; 2) immunohistochemical expression of those proteins represents the presence of active cyclin/cdk2 complexes with high associated kinase activity; 3) immunohistochemical positivity of both cyclin A and cyclin E could be a useful marker predicting clinical outcome; 4) cyclin A/cdk2 complexes may play crucial roles in driving the kinetics of cell proliferation in both LM and LMS; and 5) cyclin E/cdk2 may be one of the possible critical determinants of malignant phenotype.
Footnotes
Address reprint requests to Yoh Dobashi, MD, Department of Pathology, Kitasato University School of Medicine, 1–15-1, Sagamihara, Kanagawa 228-8555, Japan. E-mail: ydobashi@med.kitasato-u.ac.jp.
Supported in part by grant-in-aid for Scientific Research, No. 11670191, from the Ministry of Education, Science and Culture in Japan, Mitsui Life Social Welfare Foundation Research Grant, and by Kitasato University Medical Science Research Project 01A-1999.
References
- 1.Hashimoto H, Daimaru Y, Tsuneyoshi M, Enjoji M: Leiomyosarcoma of the external soft tissues. A clinicopathologic, immunohistochemical, and electron microscopic study. Cancer 1986, 57:2077–2088 [DOI] [PubMed]
- 2.Dahl I, Angervall L: Cutaneous and subcutaneous leiomyosarcoma. A clinicopathologic study of 47 patients. Pathol Eur 1974, 9:307-315 [PubMed] [Google Scholar]
- 3.Ranchod M, Kempson RL: Smooth muscle tumor of the gastrointestinal tract and retroperitoneum. Cancer 1977, 39:255-262 [DOI] [PubMed] [Google Scholar]
- 4.Wile AG, Harry LE, Romsdahl MM: Leiomyosarcoma of soft tissue: a clinicopathologic study. Cancer 1981, 48:1022-1032 [DOI] [PubMed] [Google Scholar]
- 5.Shmookler BM, Lauer DH: Retroperitoneal leiomyosarcoma: a clinicopathologic analysis of 36 cases. Am J Surg Pathol 1983, 7:269-280 [PubMed] [Google Scholar]
- 6.Gustafson P, Willen H, Baldetorp B, Ferno M, Akerman M, Rydholm A: Soft tissue leiomyosarcoma. A population-based epidemiological and prognostic study of 48 patients, including cellular DNA content. Cancer 1992, 70:114-119 [DOI] [PubMed] [Google Scholar]
- 7.Fields JP, Helwig EB: Leiomyosarcoma of the skin and subcutaneous tissue. Cancer 1981, 47:156-161 [DOI] [PubMed] [Google Scholar]
- 8.Kilpatrick SE, Mentzel T, Fletcher CDM: Leiomyoma of deep soft tissue. Clinicopathologic analysis of a series. Am J Surg Pathol 1994, 18:576-582 [DOI] [PubMed] [Google Scholar]
- 9.Fletcher CD, Kilpatrick SE, Mentzel T: The difficulty in predicting behavior of smooth-muscle tumors in deep soft tissue. Am J Surg Pathol 1995, 19:116-117 [DOI] [PubMed] [Google Scholar]
- 10.Raj S, Calonje E, Kraus M, Kavanagh G, Newman PL, Fletcher CDM: Cutaneous pilar leiomyoma: clinicopathologic analysis of 53 lesions in 45 patients. Am J Dermatopathol 1997, 19:2-9 [DOI] [PubMed] [Google Scholar]
- 11.Wascher RA, Lee MYT: Recurrent cutaneous leiomyosarcoma. Cancer 1992, 70:490-492 [DOI] [PubMed] [Google Scholar]
- 12.Patterson H, Gill S, Fisher C, Law MG, Jayatilake H, Fletcher CDM, Thomas M, Grimer R, Gusterson BA, Cooper CS: Abnormality of the p53, MDM2 and DCC genes in human leiomyosarcomas. Br J Cancer 1994, 69:1052-1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dei Tos AP, Maestro R, Doglioni C, Piccinin S, Libera DD, Boiocchi M, Fletcher CDM: Tumor suppressor genes and related molecules in leiomyosarcoma. Am J Pathol 1996, 148:1037–1045 [PMC free article] [PubMed]
- 14.Konomoto T, Fukuda T, Hayashi K, Kumazawa J, Tsuneyoshi M: Leiomyosarcoma in soft tissue: examination of p53 status and cell proliferating factors in different locations. Hum Pathol 1998, 29:74-81 [DOI] [PubMed] [Google Scholar]
- 15.Xiong Y, Connolly T, Futcher B, Beach D: Human D-type cyclin. Cell 1991, 65:691-699 [DOI] [PubMed] [Google Scholar]
- 16.Lees E, Faha B, Dulic V, Reed SI, Harlow E: Cyclin E/cdk2 and cyclin A/cdk2 kinase associated with p107 and E2F in a temporary distinct manner. Genes Dev 1992, 6:1874-1885 [DOI] [PubMed] [Google Scholar]
- 17.Dulic V, Drullinger LF, Lees E, Reed SI, Stein GH: Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive cyclin E - cdk2 and cyclin D1-cdk2 complexes. Proc Natl Acad Sci USA 1993, 90:11034-11038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dulic V, Lees E, Reed SI: Association of human cyclin E with a periodic G1-S phase protein kinase. Science 1992, 257:1958-1961 [DOI] [PubMed] [Google Scholar]
- 19.Sherr CJ: G1 phase progression: cycling on cue. Cell 1994, 79:551-555 [DOI] [PubMed] [Google Scholar]
- 20.Hunter T, Pines J: Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell 1994, 79:573-582 [DOI] [PubMed] [Google Scholar]
- 21.Sherr CJ: Cancer cell cycle. Science 1996, 274:1672-1678 [DOI] [PubMed] [Google Scholar]
- 22.Fisher RP, Morgan DO: A novel cyclin associated with MO15/CDK7 to form the CDK-activating kinase. Cell 1994, 78:713-724 [DOI] [PubMed] [Google Scholar]
- 23.Makela TP, Tassan JP, Nigg EA, Frutiger S, Hughes GJ, Weinberg RA: A cyclin associated with the CDK-activating kinase MO15. Nature 1994, 371:254-257 [DOI] [PubMed] [Google Scholar]
- 24.Tassan JP, Schultz SJ, Bartek J, Nigg EA: Cell cycle analysis of the activity, subcellular localization, and subunit composition of human CAK (CDK-activating kinase). J Cell Biol 1994, 127:467-478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan DO: Principles of CDK regulation. Nature 1995, 374:131-134 [DOI] [PubMed] [Google Scholar]
- 26.El-Deiry W, Harper JW, O’Connor PM, Velculescu VE, Canman CE, Jackman J, Pietenpol JA, Burrell M: WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res 1994, 54:1169-1174 [PubMed] [Google Scholar]
- 27.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D: p21 is a universal inhibitor of cyclin kinases. Nature 1993, 366:701-707 [DOI] [PubMed] [Google Scholar]
- 28.Lee MH, Reynisdottir I, Massague J: Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev 1995, 9:639-649 [DOI] [PubMed] [Google Scholar]
- 29.Matsuoka S, Edwards MC, Bai C, Parker S, Zhang P, Baldini A, Harper JW, Elledge SJ: p57KIP2, a structurally distinct member of the p21 cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev 1995, 9:650-662 [DOI] [PubMed] [Google Scholar]
- 30.Kaddu S, Beham A, Cerroni L, Humer-Fuchs U, Salmhofer W, Kerl H, Soyer HP: Cutaneous leiomyosarcoma. Am J Surg Pathol 1997, 21:979-987 [DOI] [PubMed] [Google Scholar]
- 31.Antonescu CR, Erlandson RA, Huvos AG: Primary leiomyosarcoma of bone: a clinicopathologic, immunohistochemical, and ultrastructural study of 33 patients and a literature review. Am J Surg Pathol 1997, 21:1281-1294 [DOI] [PubMed] [Google Scholar]
- 32.Dobashi Y, Shoji M, Jiang SX, Kobayashi M, Kawakubo Y, Kameya T: Active cyclin A-cdk2 complex, a possible critical factor for cell proliferation in human primary lung carcinomas. Am J Pathol 1998, 153:863-872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shoji M, Dobashi Y, Morinaga S, Jiang SX, Kameya T: Mode of tumor extension and cell proliferation in adenocarcinomas of the lung. Am J Pathol 1999, 154:909-918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobashi Y, Kudoh T, Matsumine A, Toyoshima K, Akiyama T: Constitutive overexpression of cdk2 inhibits neuronal differentiation of rat pheochromocytoma PC12 cells. J Biol Chem 1995, 270:23031-23037 [DOI] [PubMed] [Google Scholar]
- 35.Matsushime H, Quelle DE, Shurtleff SA, Shibuya M, Sherr CJ, Kato J: D-type cyclin dependent kinase activity in mammalian cells. Mol Cell Biol 1994, 14:2066-2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong Y, Zhang H, Beach D: D-type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell 1992, 71:505-514 [DOI] [PubMed] [Google Scholar]
- 37.Weidner N, Moore DH, Vartanian R: Correlation of Ki-67 antigen expression with mitotic figure index and tumor grade in breast carcinoma using the novel “paraffin”-reactive MIB-1 antibody. Hum Pathol 1994, 25:337-342 [DOI] [PubMed] [Google Scholar]
- 38.Sewing A, Roenicke V, Burger C, Funk M, Muller R: Alternative splicing of human cyclin E. J Cell Sci 1994, 107:581-588 [DOI] [PubMed] [Google Scholar]
- 39.Jakobsen SS, Hoelund B: Immunohistochemistry (Ki-67 and p53) as a tool in determining malignancy in smooth muscle neoplasms (exemplified by a myxoid leiomyosarcoma of the uterus). APMIS 1996, 104:705-708 [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Chenivesse X, Henglein B, Brechot C: Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature 1990, 343:555-557 [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Zindy F, Chenivesse X, Lamas E, Henglein B, Brechot C: Modification of cyclin A expression by hepatitis B virus DNA integration in a hepatocellular carcinoma. Oncogene 1992, 7:1653-1656 [PubMed] [Google Scholar]
- 42.Tahara E: Genetic alteration in human gastrointestinal cancers. The application to molecular diagnosis. Cancer 1995, 75:1410-1417 [DOI] [PubMed] [Google Scholar]
- 43.Keyomarsi K, Conte D, Toyofuku W, Fox MP: Deregulation of cyclin E in breast cancer. Oncogene 1995, 11:941-950 [PubMed] [Google Scholar]
- 44.Kotani S, Endo T, Kitagawa M, Higashi H, Onaya T: A variant form of cyclin-dependent kinase 2 (Cdk2) in a malignantly transformed rat thyroid (FRTL-Tc) cell line. Oncogene 1995, 10:663-669 [PubMed] [Google Scholar]
- 45.Huuhtanen RL, Blomqvist CP, Boehling TO, Wiklund TA, Tukiainen EJ, Virolainen M, Tribukait B, Andersson LC: Expression of cyclin A in soft tissue sarcomas correlates with tumor aggressiveness. Cancer Res 1999, 59:2885-2890 [PubMed] [Google Scholar]
- 46.Keyomarsi K, O’Leary N, Molnar G, Lees E, Fingert HJ, Pardee AB: Cyclin E, a potential prognostic marker for breast carcinoma. Cancer Res 1994, 54:380-385 [PubMed] [Google Scholar]
- 47.Callender T, Naggar AK, Lee MS, Frankenthaler R, Luna MA, Batsakis JG: PRAD-1 (CCND1)/cyclin D1 oncogene amplification in primary head and neck squamous cell carcinoma. Cancer 1994, 74:152-158 [DOI] [PubMed] [Google Scholar]
- 48.Naggar AK, Steck K, Batsakis JG: Heterogeneity of the proliferative fraction and cyclin D1/CCND1 gene amplification in head and neck squamous cell carcinoma. Cytometry 1995, 21:47-51 [DOI] [PubMed] [Google Scholar]
- 49.Kitahara K, Yasui W, Kuniyasu H, Yokozaki H, Akama Y, Yunotani S, Hisatsugi T, Tahara E: Concurrent amplification of cyclin E and cdk2 genes in colorectal carcinomas. Int J Cancer 1995, 62:25-28 [DOI] [PubMed] [Google Scholar]
- 50.Masciullo V, Scambia G, Marone M, Giannitelli C, Ferrandina G, Bellacosa A, Benedetti-Panici P, Mancuso S: Analysis of cyclin E and cdk2 in ovarian cancer: gene amplification and RNA overexpression. Int J Cancer 1997, 74:390-395 [DOI] [PubMed] [Google Scholar]
- 51.Marone M, Scambia G, Giannitelli C, Ferrandina G, Masciullo V, Bellacosa A, Benedetti-Panici P, Mancuso S: Altered expression of cyclin D1 and cdk4 genes in ovarian carcinomas. Int J Cancer 1998, 75:34-39 [DOI] [PubMed] [Google Scholar]
- 52.Smith E, Schlegel R, Giordano A, Lian JB, Stein JL, Stein GS: Expression of cell cycle regulatory factors in differentiating osteoblasts: postproliferative up-regulation of cyclin B and E. Cancer Res 1995, 55:5019-5024 [PubMed] [Google Scholar]