Abstract
The fibrotic response after diverse forms of injury is characterized by the accumulation of extracellular matrix proteins, proliferation of myofibroblast-like cells, and organ contraction. Myofibroblasts are key effector cells in the development of the fibrotic response. They contribute to fibrosis through both increased cell number (proliferation) and enhanced matrix synthesis. Integrins, a class of cell adhesion molecules, are mediators of cell-extracellular matrix protein interactions that are important in the proliferative and migratory response of cells to matrix proteins. We have previously cloned the human integrin subunit α8, documented its high expression in lung tissue, and established it as a receptor for the matrix proteins fibronectin, vitronectin, and tenascin. We now demonstrate that alveolar interstitial cells are the primary cell type expressing α8β1 in the lung parenchyma. Expression of α8β1 is concentrated primarily along the thinned extensions of cells and at the tips of filopodia. Because of its unique distribution in alveolar interstitial cells, we hypothesized that it may play a role in the fibrotic response after injury. In bleomycin-induced pulmonary fibrosis, there is increased expression of α8β1 by interstitial fibroblasts, the majority of which coexpress α smooth muscle actin, a marker of tissue myofibroblasts. To establish a more general role for α8β1 during organ fibrosis, we further examined its expression in two rat models of liver fibrosis. During hepatic injury due to either carbon tetrachloride injury or bile duct ligation, we demonstrate de novo expression of α8β1 in activated hepatic stellate cells, the myofibroblast equivalent in liver. Taken together, the data localize α8β1 to myofibroblast-like cells during wound healing and suggest that signal transduction through the α8β1 integrin may contribute to the fibrotic response of organs to injury.
Myofibroblasts are essential effectors of the fibroproliferative response to injury in lung, liver and other parenchyma. Key features of this response to tissue injury include the proliferation of myofibroblasts, increased extracellular matrix (ECM) production, and remodeling of the extracellular matrix. Increased matrix production in this setting results from both increased myofibroblast cell number (proliferation) and enhanced cellular extracellular matrix synthesis.
In normal lung, the interstitium contains a population of mesenchymal cells called alveolar myofibroblasts or contractile interstitial cells (CICs). 1 CIC-mediated functions are particularly important during the establishment of pulmonary fibrosis. These cells express myogenic intermediate filaments, including desmin and vimentin. CICs are postulated to control the ventilation-perfusion ratio by alveolar wall contraction during breathing. 1-3 In contrast to pericytes (myofibroblasts associated with capillaries) and alveolar ring cells (smooth muscle-like cells found at the corners of alveolar ducts), 4 CICs/myofibroblasts do not express α-smooth muscle actin under normal conditions. 5 However, during pulmonary fibrosis, CICs undergo a phenotypic modulation to a smooth muscle-like phenotype characterized by expression of α-smooth muscle actin 5 and proliferation and increased synthesis of ECM proteins, including collagen and fibronectin. As pulmonary fibrosis progresses, the percentage of CICs in the lung increases from 30% to 80%, accompanied by a 10-fold increase in cell number. 6
In the liver, the cell type analogous to the alveolar contractile interstitial cell is the hepatic stellate cell (formerly known as the lipocyte or Ito cell). 7 In the normal liver, stellate cells manifest a quiescent phenotype and do not express smooth muscle-specific proteins. After liver injury, stellate cells “activate,” whereby they express α-smooth muscle actin, 8 proliferate, and produce abundant extracellular matrix proteins. 9 Activated stellate cells also acquire the ability to contract, a key feature of the cirrhotic liver. 10
Altered extracellular matrix composition in fibrosis leads to cellular responses that are mediated through integrins. Integrins are a family of cell adhesion receptors composed of α and β subunits that influence many cellular activities, including adhesion, migration, proliferation, apoptosis, and cell survival, all of which can modulate the development of pulmonary fibrosis. For example, treatment of mice with antibodies to the leukocyte integrin β2 subunit dramatically decreases collagen deposition after lung injury. 11 Mice lacking the β6 integrin subunit, which is expressed in airway epithelium after injury, 12 are protected from the development of bleomycin-induced pulmonary fibrosis. 13 The prevention of pulmonary fibrosis in β6-deficient mice is due to a defect in the activation of TGF-β1 in the lung. 13
Previous work from our laboratory has identified a potential role for the integrin α8β1 in lung fibrosis. We have cloned the human integrin subunit α8, which is found it to be highly expressed in the normal lung, 14 and showed that α8β1 is a receptor for the matrix proteins fibronectin, tenascin, and vitronectin. 15-17 We now demonstrate that alveolar contractile interstitial cells are the predominant α8-expressing cells in the lung parenchyma. Because interaction of CICs with the ECM may be important for the development of pulmonary fibrosis, we examined the expression pattern of α8 during bleomycin-induced pulmonary fibrosis. We also examined two models of hepatic fibrosis to determine whether this integrin has a generalized role in the response to organ injury. We find the integrin α8β1 is a marker of activated CICs/myofibroblasts and that proliferation of α8-expressing cells appears to be a common feature of fibrosis.
Materials and Methods
Reagents and Antibodies
Rabbit polyclonal antibodies to the human α8 cytoplasmic domain were previously characterized. 14,15 The immortalized rat hepatic stellate cell line HSC-T6 was a generous gift from Dr. Scott Friedman (Mount Sinai School of Medicine, New York). The HSC-T6 cell line was generated by transfection of primary hepatic stellate cells with an expression plasmid encoding the SV40 large T antigen as previously described. 18 HSC-T6 cells retain all features of activated stellate cells, including expression of desmin, smooth muscle actin, and glial acidic fibrillary protein, and it can esterify retinol into retinyl esters. 18
Immunoelectron Microscopy
Lungs from adult Sprague-Dawley rats were sequentially perfused through the thoracic aorta with 1) normal saline (0.9%) containing 1000 U/ml of heparin, 2) 50 ml of 3.75% acrolein and 2% paraformaldehyde in phosphate buffer (pH 7.4) (PB), and 3) 200 ml of 2% paraformaldehyde in PB. The lungs were removed and stored in the last fixative for an additional 30 minutes. Sections (50 μm thick) were cut on a vibratome, collected in PB, and treated with 1% sodium borohydride in PB for 30 minutes before immunocytochemical labeling. Rabbit polyclonal antibody to human α8 subunit 14 (1:1000, with 0.035% Triton in the diluent) was localized immunocytochemically, using the avidin-biotin complex (ABC) peroxidase method, and processed for electron microscopy as previously described. 19
Isolation of Rat Lung Fibroblasts
Lungs were initially perfused with phosphate-buffered saline (PBS)/10 mmol/L EDTA via the right ventricle, during simultaneous inflation and deflation through cannulated trachea. The lungs were removed en bloc and lavaged with PBS/10 mmol/L EDTA, followed by instillation of prewarmed collagenase (250 mg/ml)/trypsin (2.5 μg/ml) in PBS with penicillin/streptomycin (P/S)/gentamycin for 30 minutes at 37°C and then instillation with cold trypsin inhibitor (type II-S (Sigma T9128)/DNase (Sigma D-5025) (250 mg TI, 1 mg DNase, 10 ml fetal bovine serum, 90 ml PBS). Trachea, bronchi, major structures, and other extraneous tissues were excised, and the remainder of th tissue was minced into 2–4-mm pieces. Tissue was suspended in PBS/0.5% trypsin, stirred at 37°C for 30 minutes, and then filtered through nylon mesh. Cells were washed with PBS and resuspended in Dulbecco’s modified Eagle’s medium/10% fetal bovine serum/P/S/fungizone. Macrophages were depleted by repeated adherence to IgG-coated plates. The remaining cells were plated and incubated overnight.
Immunofluorescence
Freshly isolated fibroblasts were grown on fibronectin-coated chamber slides for 1 day and fixed and permeabilized with 2% paraformaldehyde/0.1% Triton X-100 for 10 minutes. Slides were blocked with 3% bovine serum albumin and then incubated with anti-α8 antibody (10 μg/ml) for 1 hour at room temperature, washed with PBS, and incubated with biotin-conjugated donkey anti-rabbit IgG (1:50) (Amersham, Arlington Heights, IL) for 1 hour at room temperature, followed by PBS wash. Coverslips were then incubated with fluorescein-conjugated streptavidin (1:100) (Amersham) for 15 minutes at room temperature, washed with PBS, and mounted with Vectashield (Vector Laboratories, Burlingame, CA).
Western Blot
Freshly isolated fibroblasts were grown for 3–5 days, and cells were lysed with 200 mmol/L octyl-α-d-glucopyranoside/100 mmol/L Tris-HCl/1 mmol/L phenylmethylsulfonyl fluoride for 1 hour at 4°C and centrifuged twice at 2000 rpm for 10 minutes each. The protein concentrations were determined by the Bradford Assay. Samples (30 μg/well) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions on 7.5% acrylamide gel, and electrophoretically transferred to a nylon membrane (Hybond-N; Amersham). Filters were blocked with 1% dry milk/1% bovine serum albumin/0.02% Tween-20/PBS for 2 hours, probed with the anti-α8 antibody for 1 hour, rinsed in PBS, and then probed with an alkaline phosphatase-conjugated goat anti-rabbit antibody at a dilution of 1:3000 for 30 minutes. After rinsing in PBS, color was developed with the Protoblot alkaline phosphatase detection system (Promega).
Bleomycin-Induced Pulmonary Fibrosis
C57Bl/6 mice were maintained in a pathogen-free environment. Bleomycin (Mead-Johnson) was dissolved in sterile saline, and 0.075 U was administered intratracheally by direct visualization after cutdown. As a control, saline alone was administered intratracheally. Mice were sacrificed at 2 days, 4 days, 6 days, 2 weeks, 4 weeks, and 9 weeks after initial bleomycin installation. Lungs were perfused with PBS/heparin and then immersed in 10% sucrose solution overnight at 4°C. Tissues were embedded in OCT and then frozen in nitrogen-chilled butane. Five-micron sections were processed as previously described. 14 Sections were incubated in 5% bovine serum albumin for 1 hour at room temperature and then incubated with the primary antibody overnight at 4°C. After washing in PBS, sections were incubated with peroxidase-conjugated goat anti-rabbit secondary antibody (Vector Laboratories) for 1 hour at room temperature. The reaction product was visualized after incubation of the sections with diamino benzidine (DAB Plus kit; Zymed Laboratories). Sections were counterstained with hematoxylin, sequentially dehydrated in ethanol solutions, transferred to xylene, and mounted with Permount (Fisher).
For colocalization immunofluorescence studies, lung sections were incubated with α8 antibody (1:1000) and Cy3-conjugated α-smooth muscle actin antibody (Sigma), followed by biotin-conjugated donkey anti-rabbit IgG (1:100) (Amersham), then fluorescein-conjugated streptavidin (1:100) (Amersham) for 15 minutes at room temperature, washed with PBS, and mounted with Vectashield (Vector Laboratories). Slides were examined on a Zeiss Axioskop (Thornwood, NY) equipped with filter sets for fluorescein isothiocyanate (FITC) and rhodamine/Cy3 (Chroma Technology, Brattleboro, VT). Images were collected using a Spot 2 cCCD camera (Diagnostic Instruments, Sterling Heights, MI). Black-and-white images (8-bit) were collected for each channel (ie, FITC and Cy3) and then merged into the respective RGB channels in Adobe Photoshop. Colocalization of α8 expression (green) and α-smooth muscle actin expression (red) was determined by overlap of the two fluorochromes (yellow).
To determine the proportion of α8-expressing cells that did not express α-smooth muscle actin in fibrotic lesions, we counted the number of α8-positive/α-smooth muscle-negative and α8-positive/α-smooth muscle-positive cells in four independent, ×40 fields containing areas of fibrosis from three different mice, 4–6 days after bleomycin administration.
Models of Hepatic Fibrosis
Hepatic fibrosis was induced in adult Sprague-Dawley rats by carbon tetrachloride (CCl4) administration or bile duct ligation as previously described. 8 To induce biliary obstruction, the common bile duct was ligated and sectioned. Periductal fibrosis occurred by 5 days, and biliary cirrhosis occurred by 14 days. CCl4 was administered at a dose of 1.0 ml CCl4/kg body weight at weekly intervals via gavage. Early bridging fibrosis was apparent by 3–4 weeks. Tissues were processed for immunohistochemistry as above.
Results
α8β1 Is Expressed in Alveolar Interstitial Cells
In the normal lung parenchyma, immunoreactivity for α8β1 was seen within the alveoli septa by light microscopy. 14 To determine the cell type expressing α8β1 in the lung parenchyma, we performed immunoelectron microscopy of normal rat lung. Immunoreactivity for α8 was localized to the interstitial cells (Figure 1, A–D) ▶ . In contrast, epithelial cells and endothelial cells lacked immunoreactivity for α8. Many of the interstitial cells expressing α8 labeling contained contractile filaments (Figure 1A ▶ , asterisk), identifying them as CICs. 2 In these cells, α8 immunoreactivity was found at discrete patches along the cell membrane and was often concentrated at the tips of the filopodia cells as they protruded into the fused basement membrane of the endothelial and epithelial cells (Figure 1C ▶ , arrow). This pattern is reminiscent of the localization of α8β1 at synaptic densities in the hippocampus. 19 α8-labeling was frequently concentrated at areas along the thin extensions of interstitial cells. It is of interest that a lipid droplet was identified within an α8-immunoreactive cell (Figure 1D ▶ , arrowhead), consistent with the notion that these cells are similar to the hepatic stellate cells that contain cytoplasmic droplets of retinyl esters.
Figure 1.
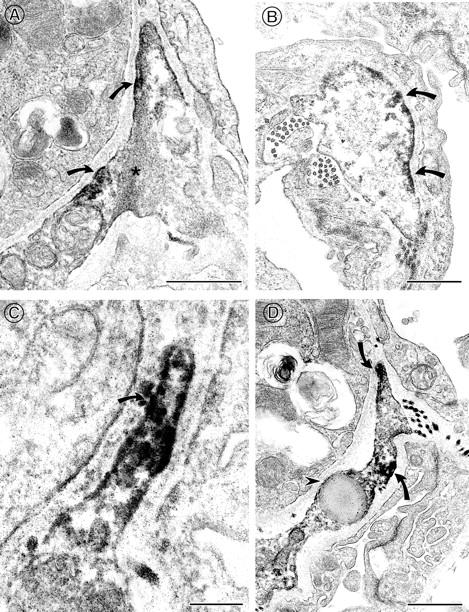
Electron microscopy of normal rat lung demonstrating α8-immunoreactivity in contractile interstitial cells. A–D: α8 immunoreactivity is visualized at the tips and along the membrane (arrows) of contractile interstitial cells (CIC). A: Actin filaments (*) are noted in an α8-expressing CIC. C: Higher magnification of CIC illustrating α8-reactivity (arrow) along the filopodia as it protrudes into the basement membrane. D: Lipid droplet (arrowhead) within an α8-expressing CIC. Immunoperoxidase staining. Scale bars, 0.5 μm in A, B, and D; 0.2 μm in C.
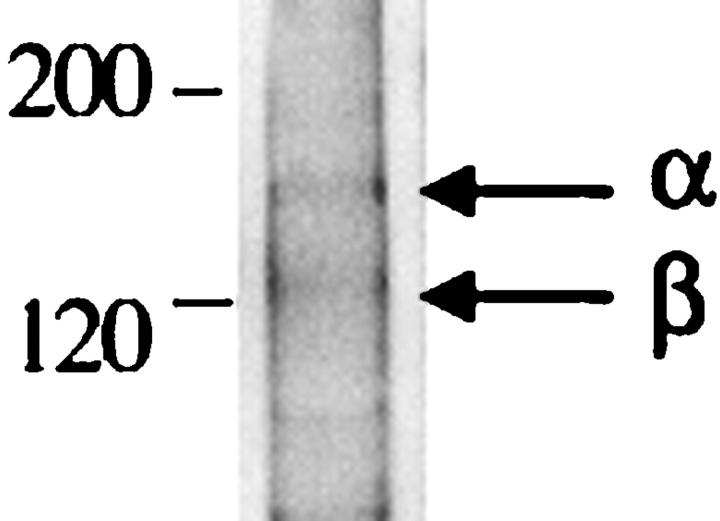
To confirm expression of α8β1 in lung interstitial fibroblasts, we analyzed isolated primary rat lung fibroblasts by Western blot and immunofluorescence. Fibroblasts isolated from normal rat lung express α8β1 by Western blot (Figure 2A) ▶ . Immunofluorescence of isolated fibroblasts identified a subpopulation of α8β1-expressing cells. When pulmonary fibroblasts were grown on fibronectin-coated plates, α8β1 was concentrated at focal contacts, consistent with its role as a receptor for fibronectin (Figure 2B) ▶ .
Figure 2.
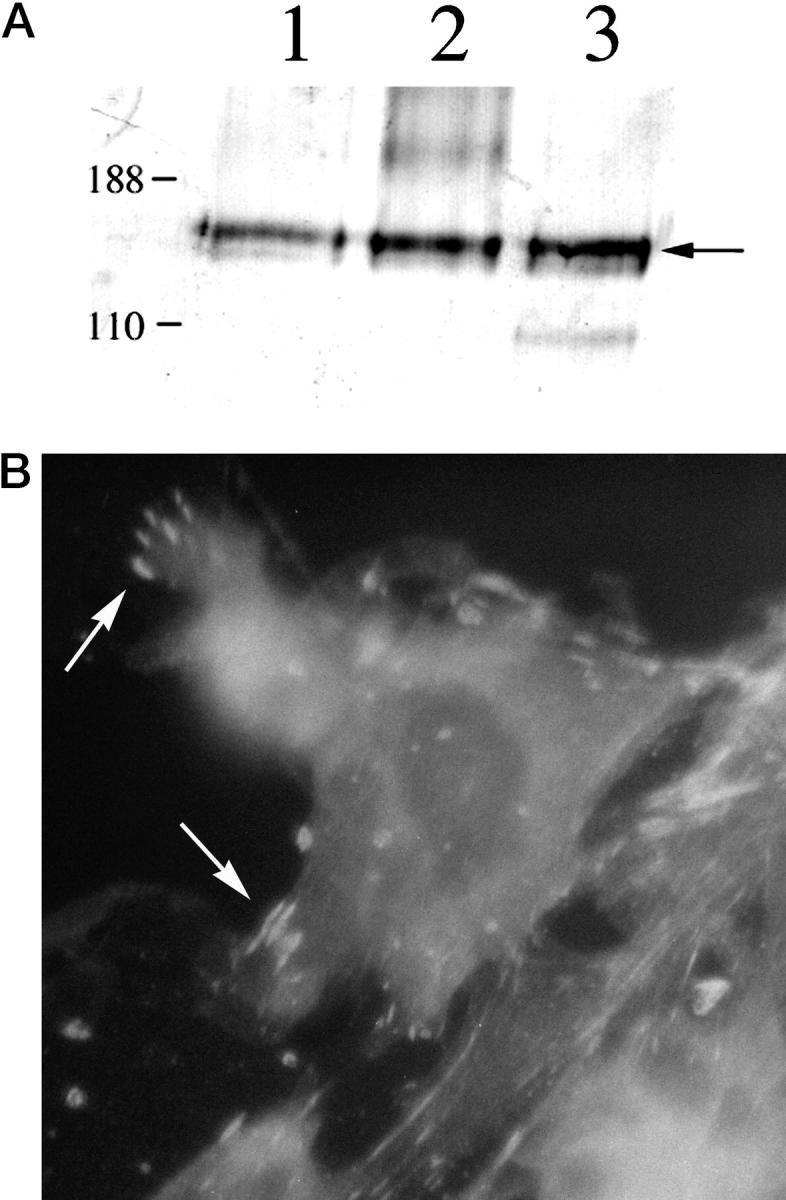
A: Western blot of α8β1 in primary lung fibroblasts. Fibroblasts isolated from rat lung were lysed, and aliquots of lysates were analyzed under nonreducing conditions. The samples were separated on SDS-PAGE gel and immunoblotted with anti-α8 antiserum. Lanes 1–3 represent three different rat fibroblast preparations. The arrow indicates the position of α8. Positions of molecular size markers in kd are shown to the left. B: Immunofluorescence of α8β1 in primary lung fibroblasts. Primary rat lung fibroblasts were grown on fibronectin-coated plates and then fixed, permeabilized, and labeled with anti-α8 antibody. α8-containing focal contacts are indicated by arrows.
Expression of α8β1 during Fibrosis in Vivo
Pulmonary Fibrosis
Because α8β1 is expressed in CICs in the lung, which are important in the development of pulmonary fibrosis, we examined the expression pattern of α8β1 in an experimental model of pulmonary fibrosis. Pulmonary fibrosis in C57/black wild-type mice was produced by intratracheal instillation of bleomycin, a standard technique resulting in reproducible, nonfatal pulmonary inflammation followed by fibrosis in mice (see Materials and Methods). In the saline-treated control mouse lung, there was preservation of normal alveolar architecture with baseline expression of α8β1 in the alveolar interstitial cells (Figure 3A) ▶ . The expression pattern of α8β1 in saline-treated mouse lung was identical to normal mouse lung. Within 2–4 days after bleomycin instillation, alveolar wall thickening was present, with the collapse of some alveoli. The vast majority of cells within areas of alveolar wall thickening were immunoreactive for α8 (Figure 3, B–F) ▶ . Patches of inflammatory cells that were negative for α8β1 were observed within alveolar spaces (Figure 3D) ▶ . Vascular smooth muscle and airway smooth muscle were positive for α8β1 (Figure 3C) ▶ , as observed in normal lung.
Figure 3.
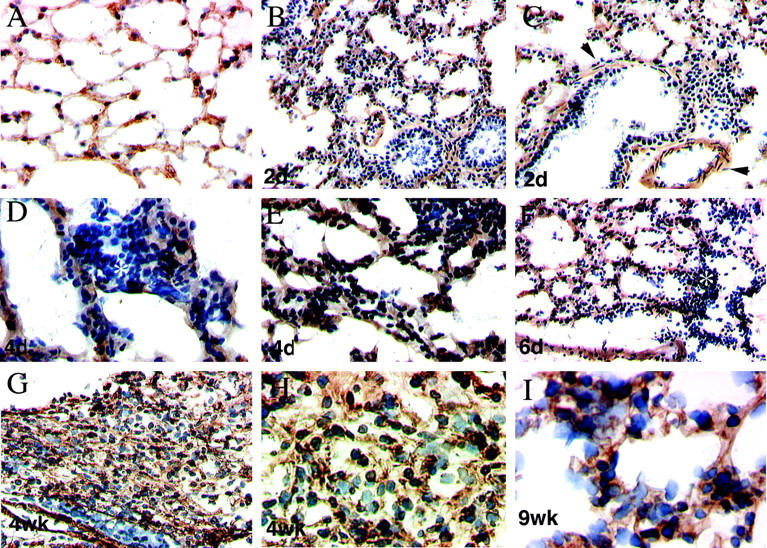
Immunohistochemistry of α8β1 in normal lung and pulmonary fibrosis. Immunohistochemical staining with anti-α8 antibody of mouse lung after saline (A) or bleomycin (B–J) treatment. Frozen sections of lung were stained with hematoxylin and affinity-purified anti-α8 antibodies. In the control (A) 4 weeks after saline treatment, normal lung architecture is preserved, and α8-immunoreactive cells are seen in the interstitium. In the bleomycin-treated animals, alveolar wall thickening, increased cellularity, and obliteration of air spaces are seen at early time points (B–F). The majority of cells within alveolar wall thickening are α8β1-positive. At higher magnification, some areas of α8β1-negative inflammatory cells are observed (asterisk) (D). Vascular smooth muscle and airway smooth muscle are α8β1-positive (arrowheads) (C). By 4 weeks (G and H), areas of dense cellular infiltration with α8β1-positive cells are seen with complete loss of normal alveolar architecture. I: Nine weeks. Higher magnification of a less involved area shows α8β1-positive cells within thickened alveolar septa. Control specimens incubated with preimmune rabbit serum displayed no significant staining (data not shown). Immunoperoxidase staining. Original magnifications: A–C and E–G, ×10; D and H, ×20; I, ×40.
Within 4–6 weeks, more advanced fibrosis was established. Areas of dense α8-positive cellular infiltration were observed, with an expansion in the number of α8-reactive cells and complete obliteration of normal alveolar architecture (Figure 3, G–I) ▶ . Although the majority of cells within the fibrotic lesions were α8β1-positive, occasional α8β1-negative cells were also identified. In areas of more established fibrosis, there was decreased cellularity.
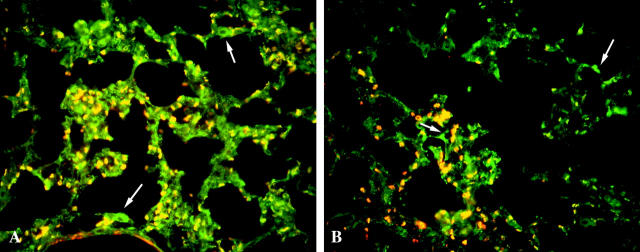
During pulmonary fibrosis, CICs activate and newly express α-smooth muscle actin. To investigate whether the activated CICs coexpressed α8β1 and α-smooth muscle actin, we double-labeled sections for α8β1 and α-smooth muscle actin (Figure 4) ▶ . Colocalization of α8β1 and α-smooth muscle actin occurred in vascular and airway smooth muscle. In the parenchyma, α-smooth muscle actin-positive cells were identified in areas of alveolar wall thickening. The vast majority of these cells were α8β1 immunoreactive. However, a subpopulation of α8β1-positive cells were identified that were α-smooth muscle actin-negative (Figure 4 ▶ , white arrows). In areas of fibrosis, the percentage of α8β1-positive/α-smooth muscle actin-negative cells (compared to α8-positive/α-smooth muscle-positive cells) ranged from 8% to 55%, with an average of 16%. In contrast, normal-appearing areas of lung parenchyma contained >90% cells that were α8-positive/α-smooth muscle-negative.
Figure 4.
α8β1 and α-smooth muscle actin expression during pulmonary fibrosis. Mouse lung sections obtained 4 days after bleomycin treatment were stained for α8β1 (FITC) and α-smooth muscle actin (Cy3). Superimposition of the images reveals that most cells that express α-smooth muscle actin also express α8β1 (yellow). However, some cells express α8β1 but not α-smooth muscle actin (white arrows). Control specimens incubated with preimmune serum displayed no significant staining (data not shown). Original magnification, ×40.
Hepatic Fibrosis
The expression of α8β1 in two mechanistically different models of hepatic fibrosis was examined using carbon tetrachloride-induced hepatic fibrosis and bile duct ligation-induced hepatic fibrosis. In normal liver, α8β1 immunoreactivity was confined to the smooth muscle cells surrounding vessels and bile ducts (Figure 5,A and D) ▶ , with none detected in the parenchyma or hepatocytes. After bile duct ligation (2 weeks), extensive bile duct proliferation occurred, with portal inflammation and fibrosis. The cells surrounding the proliferating bile ductules were immunoreactive for α8β1, and the bile duct epithelium remained negative for α8β1 (Figure 5, B and E) ▶ . The increased α8β1 immunoreactivity was limited to areas of ductal proliferation and perisinusoidal cells; α8β1 was not seen within the parenchyma. The pattern of expression of α8β1 is identical to that observed with stellate cells after bile duct ligation. 8 After CCl4 administration (4 weeks), extensive bridging fibrosis was present between portal areas of the lobule. Cells within the fibrotic bands of tissue were immunoreactive for α8β1 (Figure 5, C and F) ▶ , an expression pattern identical to that of stellate cells after CCl4 administration. 8
Figure 5.
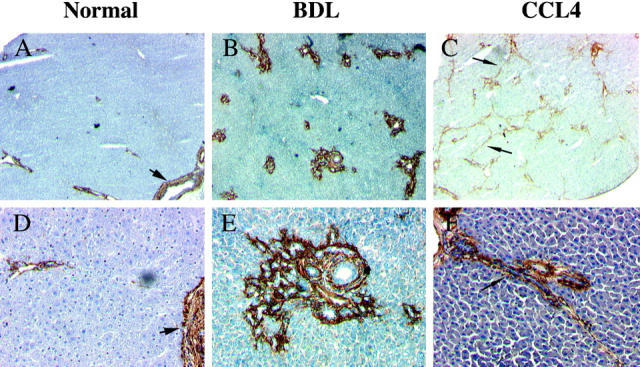
Immunohistochemistry of α8β1 in normal and fibrotic liver. In normal rat liver, α8-positive cells are visualized in smooth muscle surrounding vessels in portal veins and hepatic arteries within portal triads. Parenchyma is negative for α8β1. Vascular smooth muscle cells are α8β1-positive (arrowheads) (A and D). Two weeks after bile duct ligation, α8β1 is detected in cells surrounding proliferating ductules. B and E: After 4 weeks of CCl4 administration, bridging fibrosis is present within lobules (arrow). Cells within fibrotic bands show strong immunoreactivity for α8β1 (arrow) (C and F). Immunoperoxidase staining. Original magnifications: A–C, ×2.5; D–F, ×10.
To confirm expression of α8β1 by activated stellate cells, we immunoprecipitated lysates from immortalized activated rat stellate cells (HSC T6 cells) with the anti-α8 antibody. HSC T6 maintains the phenotype of activated stellate cells, including gel contraction, and α-smooth muscle actin expression. Although transfection with SV40 T antigen may alter some patterns of gene expression compared to primary cells, its phenotype is faithful to primary activated stellate cells in all major parameters studied to date. Immunoprecipitation of biotinylated cell lysates revealed two bands corresponding to the α and β subunits (Figure 6) ▶ . In contrast, α8β1 was not detected in endothelial cells, Kupffer cells, or hepatocytes derived from normal or fibrotic livers (data not shown). Thus immunohistochemistry and immunoprecipitation suggest that activated hepatic stellate cells are the primary source of α8β1. Given the increasing recognition that stellate cells represent a heterogeneous population in liver, 20,21 these data do not exclude the possibility that only a subset of this cell type expresses α8β1 in vivo.
Figure 6.
Immunoprecipitation of α8β1 from immortalized rat hepatic stellate cells. HSC T6 cells were surface-labeled with biotin, lysed, and then immunoprecipitated with anti-α8 antibody. Proteins were analyzed by SDS-PAGE under nonreducing conditions. The positions of α8 and β1 subunits are indicated by arrows on the right; positions of molecular mass markers (kd) are indicated on the left.
Discussion
We have demonstrated that in normal lung, the alveolar interstitial cell is the primary parenchymal cell in the lung expressing α8β1 by electron microscopy. The cell surface localization is consistent with its role as a cell adhesion molecule. The ultrastructural localization concentration at tips of cell protrusion is suggestive of a role of α8β1 in the migration or adhesion of CICs and/or matrix remodeling. During pulmonary fibrosis, there is increased immunoreactivity of α8β1. Although we cannot determine whether increased α8β1 expression represents an increased number of α8-positive cells or increased cellular expression per cell, the pattern is consistent with expression by the proliferating population of interstitial cells. It is likely that increased α8β1 expression facilitates interaction of cells with the provisional matrix laid down after injury and mediates cell matrix signal transduction important in the development of fibrosis
By providing an essential link between the cell and the extracellular matrix, integrins play a pivotal role in the fibrotic response to injury. Interaction of integrins with ECM ligands results in intracellular signal transduction and modification of cell behavior. Many of these behaviors, such as migration, contractility, ECM production and degradation, 22 cell survival, and proliferation, 23 contribute to the development of fibrosis. Thus information on the expression of integrins that mediate these behaviors may help us understand the mechanisms involved during the development of fibrosis.
α8β1 is in a unique position to relay specific signals to myofibroblast-like or smooth muscle cells. Much of the focus in the lung has been on the expression of the classic fibronectin receptor α5β1. α5β1 is expressed in a variety of cell types found in the lung after injury, such as macrophages, lymphocytes, neutrophils, endothelial cells, and epithelial cells, in addition to fibroblasts, 24,25 whereas α8β1 expression is limited to interstitial fibroblasts and smooth muscle cells. 14 Therefore, the binding of α8β1 to its ligands, such as fibronectin, vitronectin, or tenascin, may transduce specific signals to activated myofibroblasts that are important in the generation of fibrosis.
Fibronectin, a ligand for α8β1, has been extensively characterized in the context of pulmonary and hepatic fibrosis. Increased fibronectin synthesis and deposition is an important early event following lung injury. 26 In addition, increased capillary permeability is seen with lung injury, which results in the exudation of plasma components such as fibronectin into air spaces. Fibronectin is a proliferative and haptotactic signal for fibroblasts 27,28 and leads to migration of fibroblasts to abnormal locations, such as air spaces, where they deposit ECM proteins and contribute to obliteration of normal lung architecture. Fibronectin staining is concentrated along the slender processes of fibroblasts during pulmonary fibrosis, 29 in a distribution similar to that of α8β1 staining. In the liver, EDA fibronectin is synthesized within 24 hours after injury by endothelial cells. Interaction of EDA fibronectin with stellate cells leads to their synthesis of smooth muscle proteins, extracellular matrix proteins (including fibronectin), and stellate cell activation. 30 Migration of fibroblasts over a provisional matrix in early wound healing is mediated in part by integrins. 31 We have shown that α8β1 mediates the migration of cells on fibronectin and therefore may have a role in the migration of fibroblasts over a fibronectin gradient after injury (unpublished data).
Our data also suggest that α8β1 may contribute to the organ contraction by fibroblasts, through interaction with increased fibronectin deposited in the provisional wound matrix. Contractility of activated myofibroblasts leads to tissue distortion and dysfunction in both lung and liver. Because CICs in lung and stellate cells in liver are both important mediators of contraction during fibrosis, information about the receptors mediating this activity is essential. For example, the collagen-binding integrins α1β1 and α2β1 mediate contraction of collagen gels and play a role in the development of disease in liver. 32 Fibronectin contributes to fibrin clot retraction through the integrin α5β1. 33 Other fibronectin receptors, such as α8β1, may also contribute to the contractile response by interacting with components of the provisional wound matrix during injury and facilitate retraction.
Examination of the fibrotic response to pulmonary injury has broad relevance to other tissues, particularly liver. Much like CICs in lung, we show that α8β1 expression by stellate cells is increased during hepatic fibrosis. Nonetheless, there are several differences between the two cell types. Although α8β1 expression increases in both pulmonary and hepatic fibrosis, α8β1 is expressed in resting CICs in the lung but is not expressed in stellate cells in the normal liver. In the normal adult liver, stellate cells contain intracellular droplets of retinoids (once thought to be lipids; hence the former nomenclature “lipocytes”). After injury, retinoid droplets are lost. In the developing lung, a similar population of lipid-containing interstitial cells (LICs) exists. 34 However, with lung maturation, LICs lose the lipid droplets and become CICs. Interestingly, another difference between CICs and stellate cells is that CICs express contractile microfilaments at rest, whereas stellate cells only express contractile microfilaments after injury. 35 The data collectively support the notion that there is significant heterogeneity among connective tissue cells in normal and fibrotic tissues. Heterogeneity of cells of fibroblast lineage in the liver is an important, emerging concept. 20 In addition to desmin-positive and desmin-negative stellate cells, a recent report suggests that a subpopulation of liver myofibroblasts, distinct from retinoid-containing stellate cells, exists in the liver and may have a role in the development of fibrosis. 21 The expression of α8β1 may help define a subpopulation of myofibroblast-like cells in the liver that may be important in the development of fibrosis.
The transition of myofibroblast-like cells to an activated, smooth muscle-like phenotype is a characteristic response to injury of many other organs. After lung injury, activated CICs (ie, α-smooth muscle actin-expressing cells) express α8β1. Likewise, during experimental glomerulonephritis, mesangial cells in the kidney proliferate and express α-smooth muscle actin. 36 It is of interest that mesangial cells express α8β1 in normal kidney. 14 Whether α8β1 is involved in the transition of fibroblasts to an activated phenotype remains to be tested.
In summary, we have shown that the integrin α8β1 is expressed in alveolar CICs by electron microscopy and is predominately expressed along the filopodia and thinned extensions of the interstitial cells. We have shown that fibroblasts isolated from lung express α8β1 by Western blot and that α8β1 localizes to focal contacts by immunofluorescence. Furthermore, expression of α8β1 is increased after the development of pulmonary and hepatic fibrosis and colocalized with α-smooth actin expression. Our data support a potential role for the integrin α8β1 in pulmonary fibroblast response after injury. Moreover, parallel expression patterns of α8β1 after liver injury point to a broader role for this integrin in organizing ECM during wound healing. Expression of α8β1 by interstitial cells may be important for signals generated through interaction with extracellular matrix proteins secreted after injury, such as fibronectin. Further studies examining the mechanisms by which α8β1 contributes to tissue injury and repair are under way.
Acknowledgments
We thank Veronica Guille and Min Lu for technical assistance. We also thank Dr. Scott L. Friedman for helpful discussion and critique of the manuscript.
Footnotes
Address reprint requests to Dr. Lynn M. Schnapp, Pulmonary and Critical Care Medicine, Box 1232, Mount Sinai School of Medicine, One Gustave L. Levy Place, New York, NY 10029. E-mail: lynn.schnapp@mssm.edu.
Supported by AHA-Heritage Affiliate Grant-in-Aid (L. M. S.) and NIH HL18974 (T. A. M.).
References
- 1.Kapanci Y, Assimacopoulos A, Irle C, Zwahlen A, Gabbiani G: “Contractile interstitial cells” in pulmonary alveolar septa: a possible regulator of ventilation-perfusion ratio? Ultrastructural, immunofluorescence, and in vitro studies. J Cell Biol 1974, 60:375-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapanci Y, Costabella PM, Cerutti P, Assimacopoulos A: Distribution and function of cytoskeletal proteins in lung cells with particular reference to “contractile interstitial cells.” Methods Achiev Exp Pathol 1979, 9:147-168 [PubMed] [Google Scholar]
- 3.Fukui M, Yasui H, Watanabe K, Fujimoto T, Kakuma T, Yoshida R, Ohi M, Kuno K: Hypoxic contraction of contractile interstitial cells isolated from bovine lung. Am J Physiol 1996, 270:L962-L972 [DOI] [PubMed] [Google Scholar]
- 4.Weibel ER, Crystal RG: Structural organization of the pulmonary interstitium. Crystal RG West JB eds. The Lung: Scientific Foundations, 1991, vol 1.:pp 369-380 Raven Press, New York [Google Scholar]
- 5.Vyalov SL, Gabbiani G, Kapanci Y: Rat alveolar myofibroblasts acquire alpha-smooth muscle actin expression during bleomycin-induced pulmonary fibrosis. Am J Pathol 1993, 143:1754-1765 [PMC free article] [PubMed] [Google Scholar]
- 6.Adler KB, Callahan LM, Evans JN: Cellular alterations in the alveolar wall in bleomycin-induced pulmonary fibrosis in rats. An ultrastructural morphometric study. Am Rev Respir Dis 1986, 133:1043–1048 [DOI] [PubMed]
- 7.Wake K: Perisinusoidal stellate cells (fat-storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin A-storing cells in extrahepatic organs. Int Rev Cytol 1980, 66:303-353 [DOI] [PubMed] [Google Scholar]
- 8.Rockey DC, Boyles JK, Gabbiani G, Friedman SL: Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol 1992, 24:193-203 [PubMed] [Google Scholar]
- 9.Friedman SL, Roll FJ, Boyles J, Bissell DM: Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci USA 1985, 82:8681-8685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rockey DC, Housset CN, Friedman SL: Activation-dependent contractility of rat hepatic lipocytes in culture and in vivo. J Clin Invest 1993, 92:1795-1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piguet PF, Rosen H, Vesin C, Grau GE: Effective treatment of the pulmonary fibrosis elicited in mice by bleomycin or silica with anti-CD-11 antibodies. Am Rev Respir Dis 1993, 147:435-441 [DOI] [PubMed] [Google Scholar]
- 12.Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, Nishimura SL, Aldape K, Landers DV, Carpenter W, Gillett N, Sheppard D, Matthay MA, Albelda SM, Kramer RH, Pytela R: Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci 1995, 2241–2251 [DOI] [PubMed]
- 13.Griffiths M, Huang X-Z, Wu JF, Sheppard D: Inactivation of the beta 6 subunit gene protects against bleomycin-induced pulmonary fibrosis. Mol Biol Cell 1996, 7:166a [Google Scholar]
- 14.Schnapp L, Breuss J, Ramos D, Sheppard D, Pytela R: Sequence and tissue distribution of the human integrin alpha 8 subunit: a beta 1-associated a subunit expressed in smooth muscle cells. J Cell Science 1995, 108:537-544 [DOI] [PubMed] [Google Scholar]
- 15.Schnapp LM, Hatch N, Ramos DM, Klimanskaya IV, Sheppard D, Pytela R: The human integrin alpha 8 beta 1 functions as a receptor for tenascin, fibronectin, and vitronectin. J Biol Chem 1995, 270:23196-23202 [DOI] [PubMed] [Google Scholar]
- 16.Varnum-Finney B, Venstrom K, Muller U, Kypta R, Backus C, Chiquet M, Reichardt LF: The integrin receptor alpha 8 beta 1 mediates interactions of embryonic chick motor and sensory neurons with tenascin-C. Neuron 1995, 14:1213-1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller U, Bossy B, Venstron K, Reichardt LF: Integrin alpha 8 beta 1 promotes attachment, cell spreading, and neurite outgrowth on fibronectin. Mol Biol Cell 1995, 6:433-448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y, Ratziu V, Choi SG, Lalazar A, Theiss G, Dang Q, Kim SJ, Friedman SL: Transcriptional activation of transforming growth factor beta1 and its receptors by the Kruppel-like factor Zf9/core promoter-binding protein and Sp1. Potential mechanisms for autocrine fibrogenesis in response to injury. J Biol Chem 1998, 273:33750-33758 [DOI] [PubMed] [Google Scholar]
- 19.Einheber S, Schnapp LM, Salzer JL, Cappiello ZB, Milner TA: Regional and ultrastructural distribution of the alpha 8 integrin subunit in developing and adult rat brain suggests a role in synaptic function. J Comp Neurol 1996, 370:105-134 [DOI] [PubMed] [Google Scholar]
- 20.Friedman SL: The virtuosity of hepatic stellate cells. Gastroenterology 1999, 117:1244-1246 [DOI] [PubMed] [Google Scholar]
- 21.Knittel T, Kobold D, Saile B, Grundmann A, Neubauer K, Piscaglia F, Ramadori G: Rat liver myofibroblasts and hepatic stellate cells: different cell populations of the fibroblast lineage with fibrogenic potential. Gastroenterology 1999, 117:1205-1221 [DOI] [PubMed] [Google Scholar]
- 22.Huhtala P, Humphries MJ, McCarthy JB, Tremble PM, Werb Z, Damsky CH: Cooperative signaling by alpha 5 beta 1 and alpha 4 beta 1 integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J Cell Biol 1995, 129:867-879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pozzi A, Wary KK, Giancotti FG, Gardner HA: Integrin alpha1beta1 mediates a unique collagen-dependent proliferation pathway in vivo. J Cell Biol 1998, 142:587-594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roman J, Jeon YJ, Gal A, Perez RL: Distribution of extracellular matrices, matrix receptors, and transforming growth factor-beta 1 in human and experimental lung granulomatous inflammation. Am J Med Sci 1995, 309:124-133 [DOI] [PubMed] [Google Scholar]
- 25.Pilewski JM, Albelda SM: Adhesion molecules in the lung. An overview. Am Rev Respir Dis 1993, 148:S31-S37 [DOI] [PubMed] [Google Scholar]
- 26.Raghow R, Lurie S, Seyer JM, Kang AH: Profiles of steady state levels of messenger RNAs coding for type I procollagen, elastin, and fibronectin in hamster lungs undergoing bleomycin-induced interstitial pulmonary fibrosis. J Clin Invest 1985, 76:1733-1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bitterman PB, Rennard SI, Adelberg S, Crystal RG: Role of fibronectin as a growth factor for fibroblasts. J Cell Biol 1983, 97:1925-1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rennard SI, Hunninghake GW, Bitterman PB, Crystal RG: Production of fibronectin by the human alveolar macrophage: mechanism for the recruitment of fibroblasts to sites of tissue injury in interstitial lung diseases. Proc Natl Acad Sci USA 1981, 78:7147-7151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazenby AJ, Crouch EC, McDonald JA, Kuhn Cd: Remodeling of the lung in bleomycin-induced pulmonary fibrosis in the rat. An immunohistochemical study of laminin, type IV collagen, and fibronectin. Am Rev Respir Dis 1990, 142:206–214 [DOI] [PubMed]
- 30.Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM: Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol 1994, 127:2037-2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greiling D, Clark RA: Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci 1997, 861–870 [DOI] [PubMed]
- 32.Bissell DM: Hepatic fibrosis as wound repair: a progress report. J Gastroenterol 1998, 33:295-302 [DOI] [PubMed] [Google Scholar]
- 33.Corbett SA, Schwarzbauer JE: Requirements for alpha(5)beta(1) integrin-mediated retraction of fibronectin-fibrin matrices. J Biol Chem 1999, 274:20943-20948 [DOI] [PubMed] [Google Scholar]
- 34.Vaccaro C, Brody JS: Ultrastructure of developing alveoli. I. The role of the interstitial fibroblast. Anat Rec 1978, 192:467-479 [DOI] [PubMed] [Google Scholar]
- 35.McGowan SE, Torday JS: The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol 1997, 59:43-62 [DOI] [PubMed] [Google Scholar]
- 36.Johnson RJ, Iida H, Alpers CE, Majesky MW, Schwartz SM, Pritzi P, Gordon K, Gown AM: Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest 1991, 87:847–858 [DOI] [PMC free article] [PubMed]