Abstract
Transthyretin (TTR) is a tetrameric protein synthesized mainly by the liver and the choroid plexus, from where it is secreted into the plasma and the cerebrospinal fluid, respectively. Some forms of polyneuropathy, vitreopathy, and cardiomyopathy are caused by the deposition of normal and/or mutant TTR molecules in the form of amyloid fibrils. Familial amyloidotic polyneuropathy is the most common form of TTR amyloidosis related to the V30M variant. It is still unclear the process by which soluble proteins deposit as amyloid. The treatment of amyloid-related disorders might attempt the stabilization of the soluble protein precursor to retard or inhibit its deposition as amyloid; or aim at the resorption of the deposited amyloid. The anthracycline 4′-iodo-4′-deoxydoxorubicin (I-DOX) has been shown to reduce the amyloid load in immunoglobulin light-chain amyloidosis. We investigated 1) whether I-DOX has affinity for TTR amyloid in tissues, 2) determined the I-DOX binding constants to TTR synthetic fibrils, and 3) determined the nature of the effect of I-DOX on TTR fibrils. We report that 1) I-DOX co-localizes with amyloid deposits in tissue sections of patients with familial amyloidotic polyneuropathy; 2) I-DOX strongly interacts with TTR amyloid fibrils and presents two binding sites with kd of 1.5 × 10−11 mol/L and 5.6 × 10−10 mol/L, respectively; and 3) I-DOX disrupts the fibrillar structure of TTR amyloid into amorphous material, as assessed by electron microscopy but does not solubilize the fibrils as confirmed by filter assays. These data support the hypothesis that I-DOX and less toxic derivatives can prove efficient in the treatment of TTR-related amyloidosis.
Transthyretin (TTR), a tetrameric protein that binds thyroxine and retinol, is synthesized mainly by the liver and the choroid plexus, from where it is secreted into the plasma and the cerebrospinal fluid, respectively. 1 Normal and mutated TTR variants are associated with the deposition of amyloid fibrils in several tissues, causing predominantly polyneuropathy and/or cardiomyopathy. The process that leads to the deposition of soluble TTR into an amyloid fibril is still unknown. It is hypothesized that an amyloidogenic intermediate should be initially formed and prompt the protein to deposit. It is also possible that some tissue-specific factor enables a particular protein to deposit and this may vary in the different types of amyloidosis. 2 In familial amyloidotic polyneuropathy (FAP) associated with a V30M mutation, the amyloid fibrils deposit primarily in the peripheral nerve. During the course of the disease, FAP patients may suffer from vitreous opacities caused by the presence of TTR fibrils, which can be easily removed and represent an excellent native fibrillar material for analysis. The only therapy available for FAP is liver transplant, where the amyloidogenic precursor protein is eliminated from the blood. Some patients improve in autonomic function. Serum amyloid P component scintigraphy analysis has shown regression of visceral amyloid after liver transplantation. 3 One patient has also been described where regeneration of the peripheral nerve was observed without a decrease in the amyloid load. 4 Although it is general agreement that liver transplantation arrests the progression of the disease to some extent, it is a drastic therapy and the clinical improvement is not observed in all instances. In addition, the choroid plexus continues to synthesize the mutated protein, and it is unknown whether the cerebrospinal fluid constitutes a route for TTR access to the nerve. Therefore, potential alternative therapies could aim either at the inhibition of amyloid deposition and/or at the resorption of already formed amyloid. Several compounds are being tested with such purposes in FAP and in other amyloidoses. 5,6
The anthracycline 4′-iodo-4′-deoxydoxorubicin (I-DOX) may be such a drug. I-DOX strongly binds in vitro to various natural amyloid fibrils composed of immunoglobulin light chains, amyloid A, TTR (V30M), β-protein, and β2-microglobulin 7,8 and leads to amyloid resorption in patients with light-chain amyloidosis. 9 In addition, I-DOX delays the appearance of experimental prion disease in hamsters prolonging the survival time. 10 The metabolic active I-DOX metabolite, 13-dihydro-I-DOX (I-DOXOL), can reach the cerebrospinal fluid. 9 This feature may be relevant for diseases where cerebrospinal fluid bathes the site of amyloid deposition.
The application of I-DOX in the treatment of TTR-related amyloidosis needs investigation at the tissue and molecular levels. We investigated binding of I-DOX in situ on FAP tissues and in vitro on native and synthetic TTR fibrils by morphological and biochemical analysis, to determine the nature of the interaction and the effect on TTR fibrils on binding.
Materials and Methods
Anthracyclines
I-DOX, I-DOXOL, and DOX (doxorubicin) were kindly provided by Pharmacia and Upjohn, Nerviano, Italy.
TTR Fibrils
Native TTR amyloid vitreous fibrils were obtained from a FAP patient with V30M TTR subjected to vitreoctomy, whereas kidney fibrils were extracted, postmortem, from another FAP patient using water extractions as previously described. 11 Both the kidney and vitreous fibrils showed the characteristic apple birefringence under polarized light after Congo red binding. Recombinant TTR proteins were isolated from the periplasmic space of Escherichia coli after osmotic shock, and purified by ion-exchange chromatography and preparative electrophoresis. 12 Wild-type or V30M-TTR synthetic amyloid fibrils were prepared from acidification of 2-mg/ml recombinant TTR solutions and assessed by thioflavin T fluorescence assay. 13,14 Shortly, excitation spectra were recorded on a Jasco FP-770 spectrofluorometer (Jasco International, Tokyo, Japan) at 25°C with 30 μmol/L thioflavin T in 50 mmol/L of glycine-NaOH buffer, pH 9.0, in 1-ml assay volume. Preparations showing the characteristic novel excitation maxima at 450 nm on thioflavin T binding were used in the experiments.
Tissue Sections
Deparaffinized tissue sections of nerve and skin biopsies from FAP patients were incubated for 20 minutes with 80% ethanol saturated with NaCl followed by 0.5% Congo red in 80% alkaline ethanol saturated with NaCl, and analyzed under polarized light. 15,16 Amyloid was identified by the characteristic green birefringence. Immunohistochemistry using polyclonal anti-TTR antibodies (DAKO, Glostrup, Denmark) following standard procedures, and developed with 3,3′-diaminobenzidine confirmed the TTR nature of the amyloid deposits.
Anthracycline Binding
Fluorescence and Confocal Microscopy
Deparaffinized tissue sections were incubated for 20 minutes with 10−5 mol/L I-DOX or I-DOXOL in 80% ethanol saturated with NaCl. Control samples were incubated with 80% ethanol saturated with NaCl. Anthracycline fluorescence was determined with a Leica DMLB microscope (Wetzlar, Germany; excitation filter 585 nm, emission filter 615 nm). Kidney-extracted TTR fibrils were treated in the same way but observed under a Bio-Rad MRC 600 confocal microscope (Bio-Rad, Hercules, CA) using a 568 nm excitation filter.
Quantitative Methods
For the Scatchard analysis a fixed amount of fibrils (TTR or insulin) was incubated for 2 hours at 20°C with solutions of I-DOX in water ranging from 10−7 mol/L to 7.5 × 10−5 mol/L. The samples were centrifuged at 15,000 × g for 10 minutes at 4°C and fibril pellets were washed three times with 1 ml of water. Bound compounds were extracted by adding 200 μl of HCl 0.6 N/EtOH (1:1) and vigorously shaken for 20 minutes. Then the samples were spun at 15,000 × g and supernatants were injected into high performance liquid chromatography. RP-high performance liquid chromatography analysis was performed using the following instrumental conditions: Waters (Milford, MA) equipment comprising 717 Autosampler, 474 spectrofluorometer, and 510 pump. Column was C8 (Waters WAT 054270 4.6 × 250 mm) with precolumn C8 WATO 54250 Sentry. Samples were run at 0.8 ml/min and detected at 479 nm excitation/593 nm emission wavelengths. The mobile phase was 0.05 mol/L KH2PO4/CH3CN (70:30), pH 3.0, with H3PO4. To compare the binding to amyloid fibrils of I-DOX, I-DOXOL, and DOX, synthetic fibrils (100 μg) were incubated for 2 hours at room temperature with these anthracyclines (5 × 10−5 mol/L) in water. The bound compound was determined as described above.
Electron Microscopy
An equal amount of I-DOX, dissolved in saline, was added freshly everyday, for 7 days, to the same solution containing ∼50 μg of vitreous V30M TTR native amyloid fibrils to reach a final concentration of 7 × 10−6 mol/L. As control, in a similar vitreous amyloid preparation, the same procedure was performed with additions of saline alone. At the end of the 7 days, the samples were analyzed by electron microscopy after negative staining with 2% uranyl acetate. Briefly, 30 μl of fibril suspension were deposited on the grid and let dry. Then the grid was incubated with 2% uranyl acetate solution for 5 minutes and the solution in excess was gently removed from one side of the grid by filter paper. The grid was let to dry at room temperature for 10 minutes before examination by electron microscopy.
Filter Assays
To investigate whether I-DOX was able to dissolve amyloid fibrils, 100 μg of V30M TTR synthetic fibrils (obtained by acidification) or soluble 100 μg V30M TTR were incubated with 1 × 10−6 or 1 × 10−5 mol/L in 0.9% NaCl for 0, 3, 6, 12, and 24 hours, or with 1 × 10−6 or 1 × 10−5 mol/L I-DOX added freshly everyday for 7 days, at room temperature in the dark. The samples were applied to SPINE-X (Costar, Cambridge, MS) centrifuge filter units, centrifuged, and washed with 0.9% NaCl. The protein concentration of the filtered material was determined using the Bio-Rad Protein Assay (Bio-Rad) and albumin as standard.
Results
I-DOX Binding in Situ
As seen in Figure 1 ▶ , the intense fluorescence of I-DOX (C and G) co-localizes with Congophilic fibrils (A and E) immunoreactive for TTR (B and F), both in skin and nerve from FAP patients. Because I-DOX is dissolved in 80% ethanol, the negative control for each section corresponds to the use of 80% ethanol alone (D and H), which represents the endogenous fluorescence of the tissue. I-DOX does not bind nerve from normal individuals (I) that, as expected, shows no Congo red birefringence nor TTR immunoreactivity (not shown), and presents similar endogenous fluorescence (J) as the FAP nerve. The same holds true for normal skin (data not shown).
Figure 1.
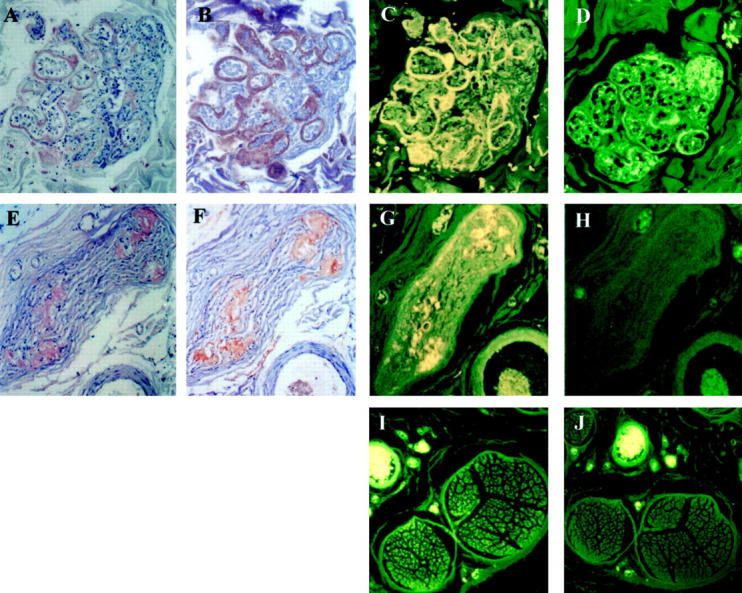
Congo red staining of FAP skin (A), FAP nerve (E); TTR immunoreactivity of FAP skin (B), FAP nerve (F); I-DOX binding to FAP skin (C), FAP nerve (G) and normal nerve (I); ethanol binding to FAP skin (D), FAP nerve (H) and normal nerve (J). Magnification: ×30 (A–H); ×10 (I and J).
Detailed analysis of Figure 1 ▶ indicates that IDOX binds solely to TTR-immunoreactive regions that are also Congophilic.
I-DOX Binding in Vitro
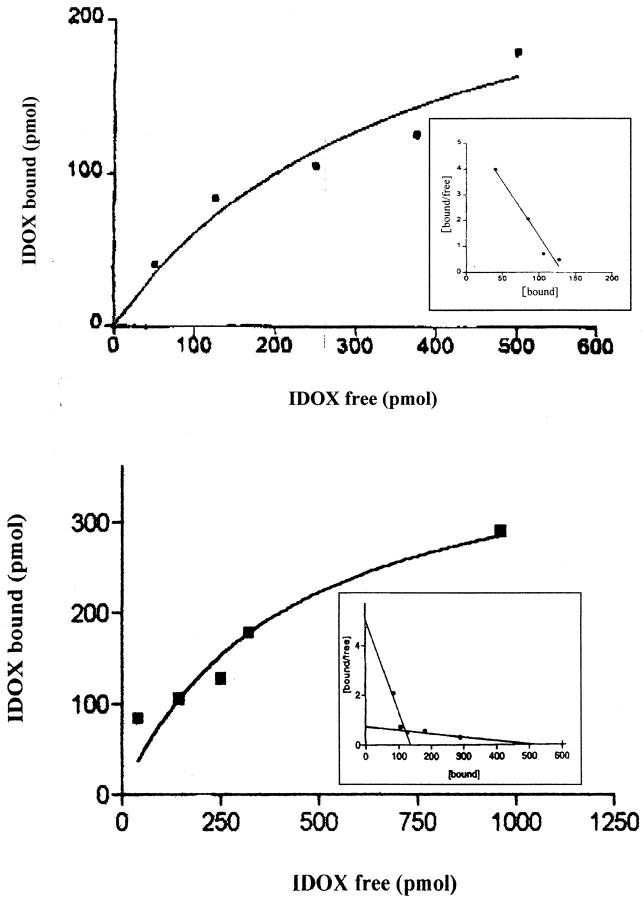
To further demonstrate the specificity of the I-DOX binding to TTR amyloid fibrils observed in FAP tissues, we analyzed the binding of I-DOX to kidney-extracted amyloid fibrils from a patient with FAP. Figure 2 ▶ shows that I-DOX binds strongly to the fibrils (A), above the endogenous fluorescence (B). The amyloidogenic nature of the fibrils was confirmed by the Congo red-green birefringence under polarized light (C). Synthetic fibrils obtained by acidification of recombinant V30M or wild-type TTR also show binding by I-DOX (data not shown). This result prompted us to determine the affinity of I-DOX to the fibrils. To assure that the I-DOX binding was specific to TTR rather than to any other component of the amyloid fibrils, we decided to use for this experiment synthetic fibrils obtained by acidification of TTR solutions. Scatchard analysis reveals the presence of at least two binding sites with kd in the 10−1 nmol/L range of 1.5 × 10−11 mol/L and 5.6 × 10−10 mol/L, respectively (Figure 3) ▶ . I-DOXOL (63 ng/100 μg fibrils) and DOX (44 ng/100 μg fibrils) presented less binding capacities than I-DOX (171 ng/100 μg fibrils) for the synthetic fibrils. Insulin fibrils run in parallel originated identical kd values as previously published, 7 which validated the experiment.
Figure 2.
I-DOX (A), ethanol (B), and Congo red (C) staining of extracted FAP kidney fibrils.
Figure 3.
Titration curves of TTR synthetic amyloid fibrils with I-DOX. Insets: Scatchard plots of the titration data. Determination of high and low affinities are documented in the top and bottom panels, respectively.
I-DOX Effect on TTR Fibrils
Since earlier reports have suggested resorption of amyloid in patients with AL- and AA-amyloidosis after treatment with I-DOX, we investigated the action of I-DOX on TTR fibrils. Because clean fibrils can be obtained directly by vitreoctomy without any further treatment, we incubated with I-DOX vitreous amyloid from a patient with the V30M TTR mutation. Electron microscopy analysis of the fibrils incubated with I-DOX or with saline (control) showed the presence of fibrils in all mesh in the control sample (Figure 4A) ▶ and no fibril in any mesh in the sample incubated with the drug (Figure 4B) ▶ ; I-DOX treatment clearly shows that, in the presence of I-DOX, the fibrillar structure of the material is lost into an amorphous precipitate. In control experiments, when we incubated uranyl acetate and I-DOX under the same conditions but without fibrils no amorphous precipitates were observed.
Figure 4.
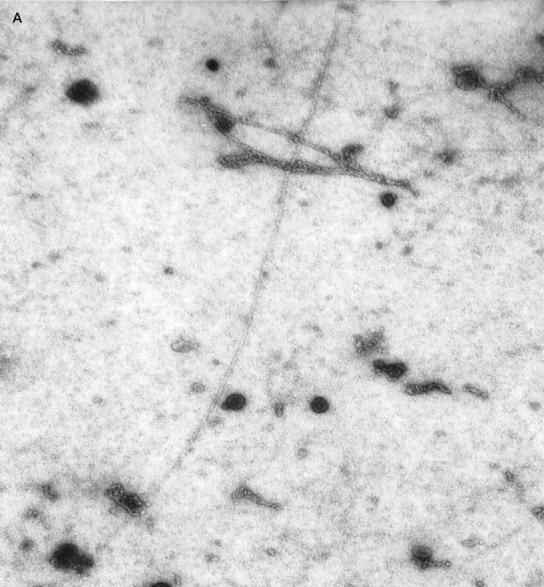
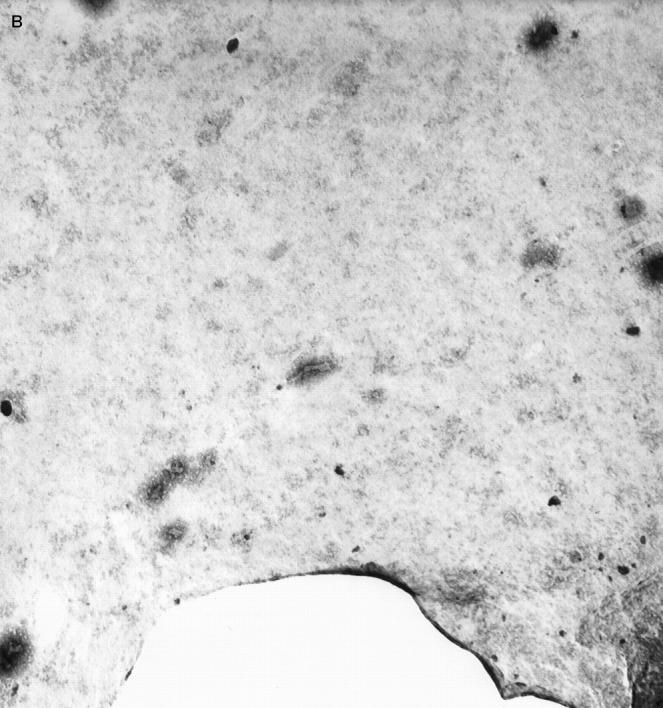
Negative-staining electron microscopy analysis of the FAP vitreous amyloid fibrils without (A) and with (B) I-DOX treatment. Magnification: ×30,000. 1 cm corresponds to 0.15 μm.
This experiment was not informative of whether I-DOX was able to partially solubilize the amyloid into soluble TTR. Therefore, we next investigated the effect of I-DOX on synthetic V30M fibrils by incubation for periods of time up to 7 days, and checked for the presence of soluble TTR after filtration. Soluble TTR (in the absence or presence of I-DOX) is filtered, whereas TTR fibrils or amorphous precipitate are completely retained (not filtered) when applied on SPIN-X centrifuge filter units. I-DOX does not interfere with soluble TTR on the filtration assay. If I-DOX were able to dissolve TTR fibrils, it would be expected that the solubilized TTR would be filtered and measured by regular protein assays. Because no protein was detected in the filtrate of all incubations, I-DOX does not seem able to dissolve the synthetic fibrils into soluble TTR.
Discussion
Pharmaceuticals able to cure amyloidosis may be thought of on the basis of prevention or treatment. In this way, drugs should either stabilize their soluble precursor to avoid further tissue deposition or destroy preformed fibrils. For the latter situation, drugs must display specific affinity for amyloid. The data presented in this study clearly show that I-DOX binds specifically to TTR amyloid fibrils in tissues from patients with FAP. I-DOX staining does co-localize with TTR amyloid as assessed by the characteristic green birefringence observed under polarized light of Congo red-stained sections and confirmed by immunohistochemistry. Interestingly, I-DOX does not bind to tissue sections that are TTR-positive by immunostaining but that do not stain with Congo red. I-DOX binding was also seen for natural-extracted and synthetic TTR fibrils.
Because normal nonmutated TTR itself is an amyloidogenic protein that deposits in the heart in 80% of the individuals more than 80 years of age, in the so-called senile systemic amyloidosis, 17 we investigated the affinity of I-DOX to synthetic wild-type TTR fibrils. Scatchard analysis indicates the presence of two high-affinity binding sites for I-DOX, with kd in the order of 1.5 × 10−11 mol/L and 5.6 × 10−10 mol/L. The higher affinity binding site is similar to the one observed for synthetic insulin fibrils (kd = 5.9 × 10−11 mol/L) whereas the lower affinity binding site is approximately 10 times higher. 7 In the particular case of TTR, thioflavin T measurements of the fibrils obtained from acidification of the soluble protein (using insulin fibrils as standards), revealed that part of the preparation consisted of protein aggregates rather than pure fibrils. This could account for the unspecific binding of I-DOX which was observed using very high concentration of the drug (in the range of μmol/L; data not shown). Therefore, to diminish the possible interference of amorphous precipitate, we used a narrow range of IDOX concentrations. It should also be noted that the kd of thioflavin binding to different types of amyloid fibrils is diverse. 18
Because I-DOX has been described to reduce amyloid deposits composed of AA and AL fibrils, 8,9 we analyzed the effect of I-DOX on native vitreous TTR fibrils. We show that I-DOX is able to disrupt the fibrillar structure of the native fibrils into amorphous material. In addition, studies on synthetic fibrils showed that I-DOX does not solubilize the fibrils into soluble TTR or bind to soluble TTR (data not shown). Together, these data indicate the ability of I-DOX to alter the final conformation of the amyloid fibrils. It is interesting to note that I-DOX is also able to intercalate into DNA molecules. 19 We hypothesize that the mechanism by which I-DOX leads to the resorption of amyloid in patients with AA and AL amyloid and eventually in other forms of amyloid, involves the alteration of the fibrillar structure into an intermediate that may make it more readily available for enzymatic degradation. Further studies on the interaction of I-DOX with TTR amyloid should take advantage of the recent finding on the structure of the L55P TTR variant. X-ray data analysis of the L55P TTR has revealed a possible pathway for TTR polymerization into amyloid fibrils. The crystal packing has a tubular structure that has been suggested to be similar to the structure of the TTR amyloid fibril. 20
All forms of amyloidosis are characterized by the extracellular deposition of fibrillar material of protein origin. Despite the fact that in each case the precursor protein is different, the amyloid fibrils share a structural identical β-pleated sheet conformation. 15,16 Because I-DOX is able to bind to all forms of amyloid tested so far it is possible that it has the same effect as observed for the TTR amyloid fibrils. If disruption of the characteristic fibrillar structure by I-DOX proves to be correct, it may be a useful tool for the treatment of all forms of noncerebral amyloid. I-DOXOL, a metabolite of I-DOX formed in vivo also co-localizes with TTR amyloid in tissues from FAP patients (data not shown). In addition, I-DOXOL binds to synthetic fibrils but has lower binding capacity than I-DOX. Because I-DOXOL can cross the blood-brain barrier 9 and reach the brain, it may be useful for diseases where the brain is the predominant site of amyloid deposition.
Acknowledgments
We thank Paul Moreira for excellent technical assistance on the preparation of recombinant TTR and Drs. Eloisa Arbustini, Laura Verga, Monica Concardi, and Lia Asti for the electron microscopy studies.
Footnotes
Address reprint requests to Maria João Saraiva, Amyloid Unit, Instituto de Biologia Molecular e Celular, Rua do Campo Alegre, 823. 4150 Porto, Portugal. E-mail: mjsaraiv@ibmc.up.pt.
Supported by grants SAU/1290/95 from Praxis XXI, Portugal, BMH4-CT98–3689 from the European Community, E.793 from Telethon, Italy and by a grant from the University Hospital IRCCS Policlinico San Matteo, Pavia, Italy.
References
- 1.Dickson PW, Aldred AR, Marley PD, Bannister D, Schreiber G: Rat choroid plexus specializes in the synthesis and secretion of transthyretin (prealbumin). Regulation of transthyretin synthesis in choroid plexus is independent from that in liver. J Biol Chem 1986, 261:3475-3478 [PubMed] [Google Scholar]
- 2.Saraiva MJM: Current trends and perspectives in research on TTR related amyloidosis. Kyle R Gertz M eds. Amyloid and Amyloidosis. 1999, :pp 204-208 Parthenon Publishing, New York [Google Scholar]
- 3.Rydh A, Suhr O, Hietala SO, Ahlstrom KR, Pepys MB, Hawkins PN: Serum amyloid P component scintigraphy in familial amyloid polyneuropathy: regression of visceral amyloid following liver transplantation. Eur J Nucl Med 1998, 25:709-713 [DOI] [PubMed] [Google Scholar]
- 4.Ikeda S, Takei Y, Yanagisawa N, Matsunami H, Hashikura Y, Ikegami T, Kawasaki S: Peripheral nerves regenerated in familial amyloid polyneuropathy after liver transplantation. Ann Intern Med 1997, 8:618-620 [DOI] [PubMed] [Google Scholar]
- 5.Oza VB, Petrassi HM, Purkey HE, Kelly JW: Synthesis and evaluation of anthranilic acid-based transthyretin amyloid fibril inhibitors. Bioorg Med Chem Lett 1999, 9:1-6 [DOI] [PubMed] [Google Scholar]
- 6.Kisilevsky R: Anti-amyloid drugs: potential in the treatment of diseases associated with aging. Drugs Aging 1996, 8:75-83 [DOI] [PubMed] [Google Scholar]
- 7.Merlini G, Ascari E, Amboli N, Bellotti V, Arbustini E, Perfetti V, Ferrari M, Zorzoli I, Marinone MG, Garini P, Diegoli M, Trizio D, Ballinari D: Interaction of the anthracycline 4′-iodo-4′-deoxydoxorubicin with amyloid fibrils: inhibition of amyloidogenesis. Proc Natl Acad Sci USA 1995, 92:2959-2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez Equiza E, Arguiñano JM, Gastearena J: Successful treatment of AA amyloidosis secondary to Hodgkin’s disease with 4′-iodo-4′-deoxydoxorubicin. Haematologica 1999, 84:93–94 [PubMed]
- 9.Gianni L, Bellotti V, Gianni AM, Merlini G: New drug therapy of amyloidosis: resorption of AL-type deposits with 4′-iodo-4′-deoxydoxorubicin. Blood 1995, 86:855-861 [PubMed] [Google Scholar]
- 10.Tagliavini F, McArthur RA, Canciani B, Giaccone G, Porro M, Bugiani M, Lievens PMJ, Bugiani O, Peri E, Dall’Ara P, Rocchi M, Poli G, Forloni G, Bandiera T, Varasi M, Suarato A, Cassutti P, Cervini MA, Lansen J, Salmona M, Post C: Effectiveness of anthracycline against experimental prion disease in Syrian hamsters. Science 1997, 276:1119-1122 [DOI] [PubMed] [Google Scholar]
- 11.Pras M, Schubert M, Zucker-Franklin D, Rimon A, Franklin EC: The characterization of soluble amyloid prepared in water. J Clin Invest 1968, 47:924-933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuya H, Saraiva MJM, Gawinowicz MA, Alves IL, Costa PP, Sasaki H, Goto I, Sakaki Y: Production of recombinant human transthyretin with biological activities toward the understanding of the molecular basis of familial amyloidotic polyneuropathy (FAP). Biochemistry 1991, 30:2415-2421 [DOI] [PubMed] [Google Scholar]
- 13.Bonifácio MJ, Sakaki Y, Saraiva MJ: ’In vitro ’ amyloid fibril formation from transthyretin: the influence of ions and the amyloidogenicity of TTR variants. Biochim Biophys Acta 1996, 1316:35-42 [DOI] [PubMed] [Google Scholar]
- 14.Naiki H, Higuchi K, Hosokawa M, Takeda T: Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal Biochem 1989, 177:244-249 [DOI] [PubMed] [Google Scholar]
- 15.Glenner GG: Amyloid deposits and amyloidosis: the beta-fibrilloses (part 1). N Engl J Med 1980, 302:1283-1292 [DOI] [PubMed] [Google Scholar]
- 16.Glenner GG: Amyloid deposits and amyloidosis: the beta-fibrilloses (part 2). N Engl J Med 1980, 302:1333-1343 [DOI] [PubMed] [Google Scholar]
- 17.Cornwell GG 3d, Sletten K, Johansson B, Westermark P: Evidence that the amyloid fibril protein in senile systemic amyloidosis is derived from normal prealbumin. Biochem Biophys Res Commun 1988, 154:648–653 [DOI] [PubMed]
- 18.LeVine H: Thioflavin T interaction with amyloid beta-sheet structures. Amyloid. Int J Exp Clin Invest 1995, 2:1–6
- 19.Berger I, Su L, Spitzner JR, Kang C, Burke TG, Rich A: Molecular structure of the halogenated anti-cancer drug iododoxorubicin complexed with d(TGTACA) and d(CGATCG). Nucleic Acids Res 1995, 23:4488-4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sebastião MP, Saraiva MJ, Damas AM: The crystal structure of amyloidogenic Leu55–>Pro transthyretin variant reveals a possible pathway for transthyretin polymerization into amyloid fibrils. J Biol Chem 1998, 271:24715-24722 [DOI] [PubMed] [Google Scholar]