Abstract
Alterations of protein tyrosine kinase are often associated with uncontrolled cell growth and tumor progression. Knowledge of the overall expression pattern of tyrosine kinases should prove beneficial in understanding the signaling pathways involved in gastric cancer oncogenesis and in providing possible biomarkers for gastric cancer progression. To establish a general tyrosine-kinase expression profile, degenerated polymerase chain reaction primers designed from the consensus catalytic kinase motifs were used to amplify protein tyrosine kinase molecules from gastric cancer tissues. We observed more than 50 tyrosine and serine/threonine kinases from matching pairs of gastric cancer tissue and normal mucosa. Based on this new kinase profile information, we selected the MKK4 gene for further immunohistochemical studies. Statistical analysis of MKK4 protein expression and clinicopathological features indicated that MKK4 kinase expression could serve as a significant prognostic factor for relapse-free survival and for overall survival. We demonstrated a simple and sensitive method for establishing protein tyrosine-kinase expression profiles of human gastric cancer tissues as well as for discovering novel and useful clinical biomarkers from such kinase expression profiles.
Protein kinases are defined as enzymes that transfer a phosphate group from a phosphate donor, usually ATP, onto an acceptor amino acid in a substrate protein. Protein kinases can be classified into several major subgroups including protein serine/threonine kinases and protein tyrosine kinases (PTKs). 1,2 It has been suggested that PTKs participate in signal transduction and play important roles in the regulation of cell growth, cell cycle, and development. 3 Although their abundance only accounts for <10% of all cellular kinases, many PTKs have been shown to be oncogenic once they lose their biological regulation either by gene amplification, somatic mutation, or viral activation. 4 Among the limited reports regarding PTKs in human gastric cancer progression, over-expression of erbB2/neu and c-met kinases have often been cited. 5,6 In addition to PTKs, protein serine/threonine kinase and dual specificity kinases also play important roles in cellular signaling functions. One example is mitogen-activated protein kinase kinase 4 (MKK4). MKK4 kinase belong to the mitogen-activated protein kinase kinase family, which are dual kinases that act upstream of mitogen-activated protein kinase (MAPK). 7
MAPKs are activated by dual phosphorylation on the threonine and tyrosine residues by mitogen-activated protein kinase kinase . MAPKs are involved in the transduction of extracellular signals for growth factors or environmental stresses, which commonly result in cell growth or differentiation. 8 The MAPK isoforms are distinguished into three subgroups based on the tripeptide dual phosphorylation motif: extracellular signal-regulated protein kinase (erk), c-Jun amino-terminal kinase (JNK), and p38/HOG kinase. Although erk1 and erk2 are involved in growth-factor signaling mechanisms, JNK and p38/HOG play essential roles in proinflammatory cytokines, UV radiation, and stress-related signal pathways. 9 MKK4 is not only the main activator of JNK, but it can also phosphorylate p38. 7 This cross-talk between two MAPK pathways places MKK4 in a central regulatory position in the cell stress response pathway. 7,9 Therefore, MKK4 is also known as SEK1 (SAPK/ERK kinase-1) and JNK kinase. The potential role of MKK4 in tumor progression has attracted the attention of researchers because MAPKs affect cell proliferation, differentiation, and apoptosis. Homologous deletion and mutation of the MKK4 gene have been found in some human cancer tissues and cell lines. 10,11 Thus far, there is no report of MKK4′s potential clinical relevance in human gastric cancers.
Gastric cancer is the second most-common cancer type in the world and the fourth leading cause of cancer death in Taiwan. Gastric cancer also has a high incidence rate around the Far East region, including Japan, India, and Korea. 12 The survival rate for gastric cancer is poor and research efforts need to focus on prognostic and diagnostic advances. Among the limited prognosis factors, two important factors that influence survival in resectable gastric cancer are the depth of invasion through the gastric wall and the presence or absence of regional lymph node involvement. 13 In previous studies, we examined the expression profile of PTKs in a human gastric cancer cell line and one gastric cancer tissue. 14,15 To gain further understanding of PTK expression profiles in human gastric cancers, in the present study we extended the profile analysis using matching normal gastric mucosa tissues from the same patients with degenerated primers from the highly conserved kinase domains using a reverse transcriptase-polymerase chain reaction approach. Because there are several hundred protein kinases that have been identified/cloned, 3 it was important to use such an expression profiling technique in examining overall kinase expressions in the present study. We identified 50 different protein kinase genes expressed in human gastric cancer tissues and adjacent normal gastric mucosa tissues.
Materials and Methods
Surgical Specimens and Protein Tyrosine Kinases Profiling
Gastric cancer tissues and their corresponding normal gastric mucosa tissues were obtained from patients who underwent gastrectomy at Veterans General Hospital–Taipei. Informed consent was obtained from all patients. Specimens were immediately frozen in liquid nitrogen after resection. Total RNA was extracted from gastric cancer and normal mucosa tissues by direct guanidine isothiocyanate lysis and cesium-chloride gradient separation method as described. 14 Reverse transcription was done with 2 μg of total RNA, oligo (dT)15, and Moloney murine leukemia virus (MMLV) reverse transcriptase from Promega (Madison, WI). Degenerated polymerase chain reaction (PCR) primers were derived from the conserved DFG and DVW motifs of the tyrosine-kinase catalytic domain. Three forward primers were designed from two amino acid sequences: 5′-K[V/I][S/C/G]DFG and 5′-K[V/I][A/S/T]DFG. One reverse primer was designed from the amino acid sequence of 5′-DVW[S/A][F/Y]G. 14,15 The PCR reactions were conducted at a 42°C annealing temperature for five cycles and then at 55°C for 25 cycles. The final PCR products were eluted from gels and purified. After ligation with T-vector plasmid from Promega, the resulting recombinants were analyzed by performing sequence identifications on individual clones. Sequence comparison to the GenBank nonredundant database was performed using the BLAST server at the National Center for Biotechnology and Information, National Institutes of Health, Bethesda, MD. We analyzed 50 to 150 clones for each tissue sample. More than 95% of clones analyzed were protein kinases.
Immunohistochemical Staining of MKK4 Kinase
Human gastric adenocarcinoma tissues were obtained from 96 patients who underwent gastrectomy at the Department of Surgery, Veterans General Hospital–Taipei. Informed consent was obtained from all patients. The pathological features of patients studied are listed in Table 1 ▶ . None of the patients had undergone chemotherapy or radiotherapy before surgery. The maximum time between stomach removal and quenching of tissue was 1 hour. Tissue blocks were fixed overnight at 4°C with 4% neutral-buffered paraformaldehyde solution, dehydrated, cleared with Hemo-De solution (Fisher, Pittsburgh, PA; ingredients: d-limonene, butylated hydroxanisole), then embedded in wax. Five-μm thick sections were used for staining. Gastric adenocarcinomas were histologically divided into intestinal and diffuse by Lauren’s 16 criteria, and they existed either alone or in combination. MKK4 in gastric cancer tissues was localized using the avidin-biotin-peroxidase complex technique as described. 17 Antibodies and blocking peptides were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). All other histochemistry reagents were obtained from Vector Laboratories (Burlingame, CA).
Table 1.
Pathological Features of 96 Patients with Gastric Cancer
| n = 96 | |
|---|---|
| MKK4 immunoreactivity | |
| Negative | 53 |
| Positive | 43 |
| Age | |
| <65 | 35 |
| ≥65 | 61 |
| Sex | |
| Male | 81 |
| Female | 15 |
| Size | |
| <4 cm | 13 |
| 4–8 cm | 61 |
| >8 cm | 22 |
| Location of tumor | |
| Upper | 5 |
| Middle | 28 |
| Lower | 63 |
| Gross appearance | |
| Superficial | 6 |
| Localized | 18 |
| Infiltrative | 72 |
| Stromal reaction pattern | |
| Medullary | 15 |
| Intermediate | 43 |
| Scirrhous | 38 |
| Lauren histology | |
| Intestinal type | 46 |
| Diffuse type | 41 |
| Mixed type | 9 |
| Depth of invasion | |
| Mucosa and submucosa | 8 |
| Propia muscle and subserosa | 41 |
| serosa-exposed and serosa-infiltrated | 63 |
| Lymph node metastasis | |
| Negative | 30 |
| Positive | 66 |
| Venous invasion | |
| Negative | 86 |
| Positive | 10 |
| Liver metastasis | |
| Negative | 87 |
| Positive | 9 |
| Peritoneal dissemination | |
| Negative | 79 |
| Positive | 17 |
| Lymphatic duct invasion | |
| Negative | 10 |
| Positive | 86 |
| Stage | |
| I | 18 |
| II | 19 |
| III | 22 |
| IV | 37 |
MKK4 (C-20) is an affinity-purified rabbit polyclonal antibody raised against a peptide corresponding to amino acids 372 to 391 mapped at the carboxyl terminus of MKK4 of mouse origin. Briefly, the rehydrated tissue sections were first treated with microwave in a sodium citrate buffer (0.06 mol/L, pH = 6), then with normal rabbit serum to remove endogenous peroxidase activity and to reduce nonspecific background staining. The tissue sections were then incubated with rabbit anti-human MKK4 antiserum at 1:100 dilution at room temperature overnight in a moist chamber. The tissue sections were subsequently treated with biotin-labeled goat anti-rabbit immunoglobulin G antibodies (50 μl) in 10 ml of phosphate-buffered saline (PBS), followed by treatment with avidin-biotin-peroxidase complex. Fresh avidin-biotin-peroxidase complex was made by incubating 10 μl of avidin and 10 μl of biotin-peroxidase in PBS. Avidin-biotin-peroxidase complex staining for negative controls was carried out by omission of primary anti-serum or replacement of primary antiserum by nonimmune rabbit normal serum. The section adjacent to that for avidin-biotin-peroxidase complex staining was stained with hematoxylin and eosin for comparison. Several tumor sections were further incubated with MKK4 blocking peptide to demonstrate its specificity.
Assessment of MKK4 Immunoreactivity
All staining procedures were standardized and controlled with an autoimmunostaining workstation machine (Leica ST5050; Leica, Deerfield, IL). Stained tissue sections were independently viewed and recorded by two researchers, and reconfirmed if there was a discrepancy on the reading of a particular slide. For assessing MKK4 immunoreactivity, we used the ratio of positively stained epithelial cells to total epithelial cells. A minus symbol (−) was given if there were no positively stained cells. One plus sign (+) indicated that >1% but <25% of cells were positively stained. Two plus signs (++) indicated that there were 25 to 75% of cells stained for MKK4 immunoreactivity. Three plus signs (+++) were used if >75% of cells were positively stained. We did not consider the use of color intensity of immunohistochemical reactions in stained cells as an evaluating parameter because it is difficult to provide objective and quantitative measurement of color intensity. However, only weakly stained cells were observed occasionally in the matching normal (adjacent nontumor tissue) gastric epithelium.
Statistical Analysis
All data are expressed as means ± SD, and the correlation between various disease parameters were analyzed by Student’s t-test and chi-square test. The relapse-free survival and overall survival curves were generated using the Kaplan-Meier method. Significance of survival differences analyses using Cox’s proportional hazard regression model were carried out to assess the independent contribution of each variable to relapse-free and overall survival. The difference was considered to be significant when the P value was less than 0.05.
Results
PTK Profiles of Gastric Cancer Tissues
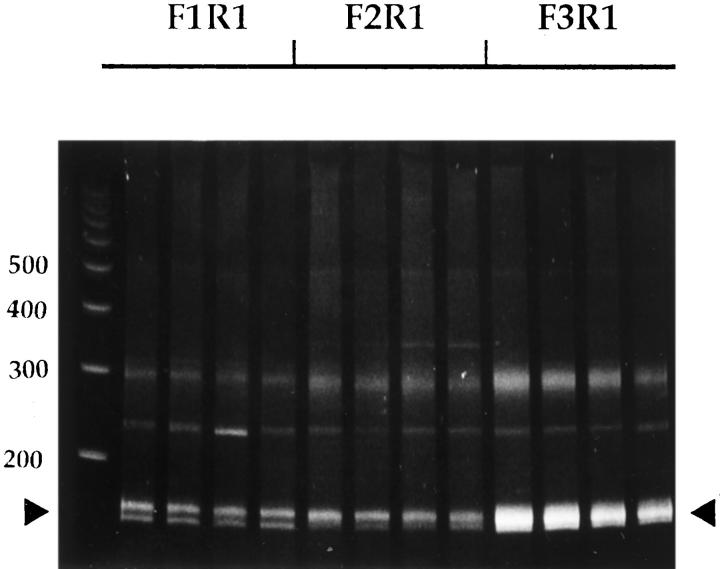
Because in vivo growth conditions and cellular microenvironments are quite different from in vitro conditions, we studied surgical tumor specimens rather than cultured cells to gain insights into the clinical relevance of tyrosine kinase expression. The in vitro cultured cells were maintained in fetal bovine serum and supported on a two-dimensional plastic surface, which may alter the gene expression pattern, including tyrosine kinase. 18 In the present study, we expanded previous kinase profiles of cancer tissues by including matching normal gastric mucosa to obtain a better overview of kinase expression in human gastric cancer progression. As shown in Figure 1 ▶ , 150 to 170 bp of PCR products were generated using a combination of three forward primers and one reverse primer (indicated by the arrowheads). All reactions gave rise to significant amounts of PCR products, with the F3/R1 pair producing the highest yield. Similar results have been observed with RNA isolated from other tissues and cell lines. 14 This result reflected differential codon usages of the three degenerated forward primers. In previous studies, we had analyzed 110 clones from a gastric cancer tissue, 15 and here we further selected 50 clones from the cancer’s matching normal gastric mucosa tissue. For an additional pair of gastric cancers in the present study, 160 clones were analyzed for gastric cancer tissue and 98 clones were analyzed for matching normal mucosa tissue, respectively. More than 95% of clones analyzed originated from protein kinase genes, thereby demonstrating the specificity of the PCR primers used and the effectiveness of the PTK profiling approach used in the present study.
Figure 1.
Gel electrophoresis of amplified 150- to 170-bp PTK reverse transcriptase-polymerase chain reaction products. Total RNA obtained from a gastric cancer tissue was used. Reverse transcriptase-polymerase chain reaction was performed as described in Materials and Methods. Four separate PCR reactions were performed for each set of primers and separated on an 8% polyacrylamide gel.
We performed four independent PCR reactions and pooled the gel-purified amplification products together for subsequent analysis (Figure 1) ▶ . This greatly minimized any inconsistency related to PCR amplification because of its extremely high sensitivity. We hoped that PTK profiles generated from these procedures would be more representative and reproducible by compensating for the most inconstant factor, ie, the PCR reaction step. Although it would be preferable to perform repeated sets of PTK profiling experiments on the same tissue sample to demonstrate the reproducibility of our methods, we currently do not have the resources to sequence thousands of clones.
Because of the limited numbers of clones analyzed (from 50 to 160 clones), it was not feasible to perform further statistical measurements of our profile results. Nonetheless, the number of varieties of PTKs identified in both PTK profiles of the normal tissues is the same. In total, 23 protein kinases were identified in both the normal gastric tissue from patient 1 (with 50 clones sequenced) and the normal tissue from patient 2 (with 98 clones sequenced). We found that 11 kinases overlapped in both normal tissue PTK profiles and that they represented almost all of the abundantly expressed PTKs, with the exception of jak1 and cak in patient 2. Because of the heterogeneous nature of clinical samples, it is intriguing that the representation of PTKs was somewhat compatible in both normal gastric mucosa tissues. This implied that our PTK profiles could still provide a common expression pattern, even with limited numbers of clones analyzed.
However, it was difficult to make similar observations in gastric cancer tissues because of the aberrant gene expression patterns and possible amplification of some PTKs. Nevertheless, a variety of PTKs have been identified in gastric cancer tissues and many of them were not previously implicated in gastric cancers. This PTK profile information provided us an excellent opportunity to be able to choose several important protein kinase genes, which may play critical functions in human gastric cancer progression, out of hundreds of kinases. Subsequent immunohistochemical analysis with archived tissue sections can then be performed with specific PTK antibodies. This approach allowed us to examine large number of samples, to confirm the PTK expression on which particular cell types, and to perform retrospective statistical analysis with clinicopathological features.
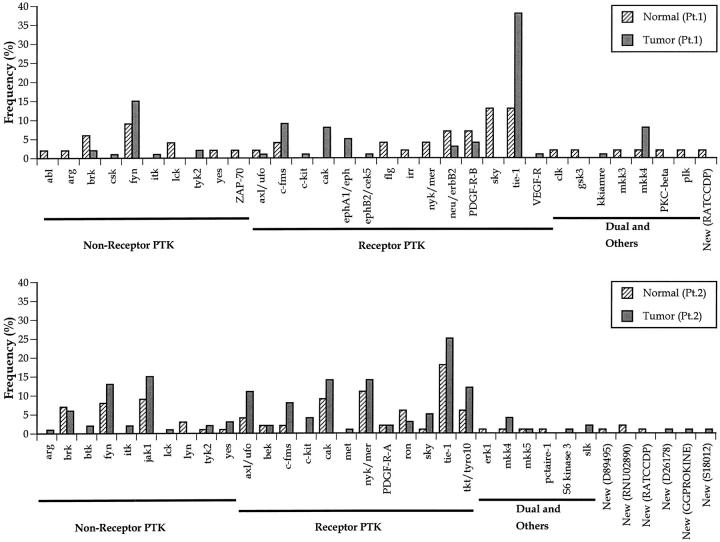
To further assist our identification of important PTKs in human gastric cancer progression, we established and analyzed PTK profiles from two sets of different gastric cancer tissues with matching pairs of normal tissues. The expression of the PTK genes identified is summarized in Figure 2 ▶ . In the first pair of tissues (Figure 2 ▶ , top panel), a total of 32 different PTK genes were identified, including receptor-type PTK genes (14 genes), nonreceptor-type PTK genes (10 genes), as well as dual and other kinases (7 genes). One possible new protein kinase gene was identified. In the second pair of tissues (Figure 2 ▶ , bottom panel), a total of 34 different PTK genes were identified, including receptor-type PTK genes (12 genes), nonreceptor-type PTK genes (10 genes), as well as dual and other kinases (6 genes). Six possible new protein kinase genes were identified. In total, we observed 50 different protein kinases in human gastric tissues and cancers. Differential expression patterns of PTKs in normal and cancer tissues were noted. For example, the tie-1 gene was significantly over-expressed in cancer tissues in patient 1 (Figure 2) ▶ . There were seven nonreceptor-type PTKs, seven receptor-type PTKs, one dual kinase, and one novel kinase presented in both profiles. Among these PTKs, several genes such as fyn, itk, tyk2 (nonreceptor PTK); c-fms, c-kit, cak, tie-1 (receptor PTK); MKK4 (dual kinase) seemed to be expressed more frequently in cancer tissues than in normal tissues. Several of these tyrosine kinases are potentially related to hematopoietic cells, such as fyn, itk, c-fms, and c-kit. It is likely that infiltrating lymphocytes are responsible for expression of these protein tyrosine kinases, not gastric cancer cells. Among the remaining PTKs, we previously examined the expression of tie-1 protein in human gastric cancer tissue sections and found that tie-1 can serve as an independent prognostic biomarker. 15 Therefore, in the present study we examined the expression of the MKK4 gene in gastric cancer tissues by immunohistochemistry to better determine MKK4’s clinical relevance in gastric cancers.
Figure 2.
Profiles of PTK genes expressed in human gastric cancer tissues. Expression of each PTK gene is indicated by the frequency of clones identified. In the top panel (patient 1), 110 clones from a gastric cancer tissue and 50 clones from its matching normal gastric mucosa tissue were sequenced. A total of 32 different PTK genes were identified and grouped by nonreceptor PTK, receptor PTK, dual and other kinases, and new kinases. In the bottom panel (patient 2), 160 clones from gastric tumor tissues and 98 clones from its normal mucosa tissues were sequenced. A total of 34 PTK genes were identified.
MKK4 Protein Expression in Gastric Cancer Tissue Sections and Survival Analysis
As illustrated in Figure 3A ▶ , immunoreactivity of MKK4 in gastric cancer tissue revealed fine reddish-brown particles. Positive MKK4 kinase immunoreactivity existed in the cytoplasm and nucleus. This immunoreactivity was totally blocked by specific MKK4 blocking peptide (Figure 3B) ▶ , attesting to the specificity of the antibody. MKK4 proteins were observed in 44.8% (43 out of 96) of gastric adenocarcinomas and were heterogeneous in type; the intensity varied from weak to strong in different cells in the same cancer cell nests. No MKK4 protein immunoreactivity was observed in muscular and serosal tissues.
Figure 3.
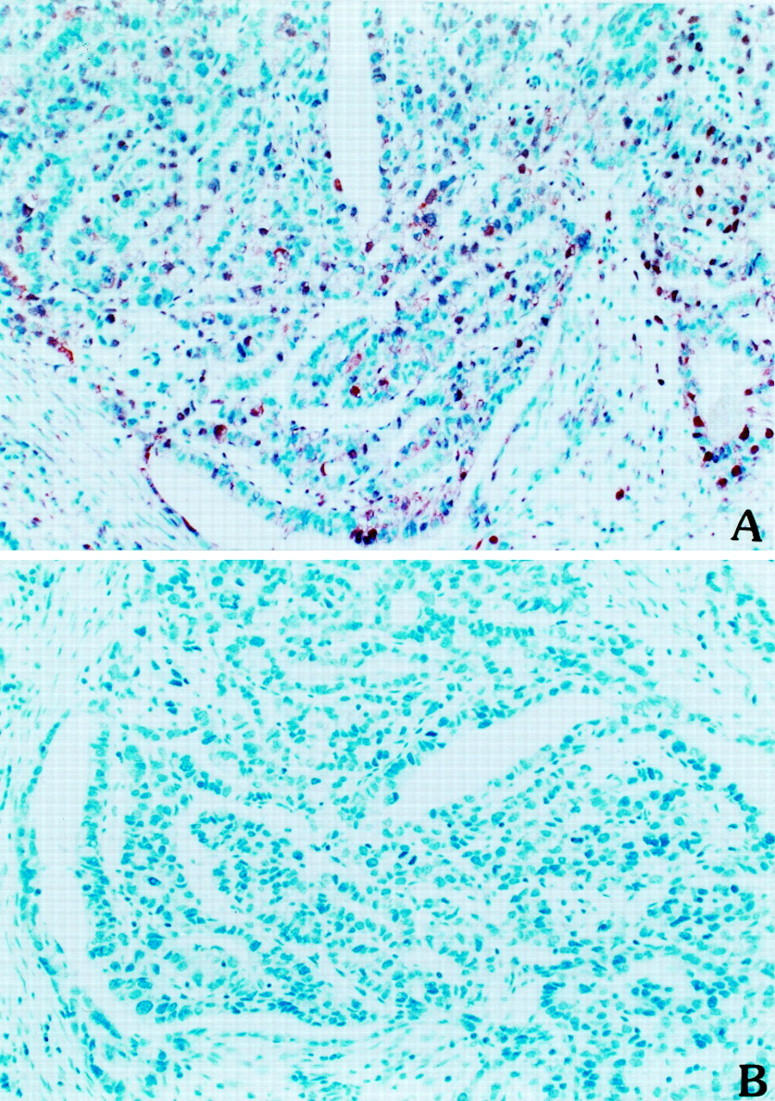
Immunohistochemical staining of MKK4 kinase in human gastric adenocarcinoma sections. Anti-MKK4 protein antibody was used for immunohistochemical staining in human gastric cancer tissues by avidin-biotin-peroxidase complex methods as described in Methods. A: Positive MKK4 kinase immunoreactivity existed in the cytoplasm and nucleus. B: Absence of MKK4 immunoreactivity was observed in the presence of MKK4 blocking peptide in the staining reaction (original magnification, ×400).
When we examined the MKK4 immunoreactivity in matched normal tissue sections, there were 82 normal tissues (85%) positive for MKK4 protein expression. However, only weakly stained cells were occasionally observed in normal gastric epithelium. In addition to the possible general MKK4 expression pattern in normal gastric tissues, it is likely that MKK4 expression in matching normal gastric tissues are a result of stress responses to their adjacent cancer tissues. Although, these normal gastric tissues were taken from tissues at least 2 cm away from apparent cancerous tissues during the surgery, which should be referred as adjacent-tumor tissues. Because MKK4 is mainly involved in stress responses, it is possible that MKK4-JNK or MKK4-p38 pathways are activated in these normal tissues in response to the cancerous microenvironment. This hypothesis is supported in part by the correlation of the size of tumor and lymph node involvement features with MKK4 protein expression in normal gastric epithelium (P = 0.032 and P = 0.024, respectively). Our data also indicated that MKK4 expression might be meaningful in later stages of gastric cancer progression. Patients with the +/− normal versus tumor MKK4 immunoreactivity had significantly better disease-free survival (P = 0.0056) and overall survival (P = 0.0022) than patients with +/+, +/++, and +/+++ MKK4 stainings.
The color intensity of immunohistochemical reaction products was also much higher in tumor cells compared with the intensity in normal epithelium, implying a higher expression level of MKK4 in gastric cancer cells. Because of the lack of objective and quantitative measurement means, we did not use this parameter in our analysis. MKK4 expression in tumor tissues actually affected tumor behavior, and its effects on survival were identical to the aforementioned data (normal versus tumor MKK4 immunoreactivity). Therefore, we decided to evaluate the clinicopathological relevance of MKK4 expression in tumor tissue sections. Relations between MKK4 status in gastric cancer tissues and other clinicopathological parameters were determined. There was no significant association between MKK4 status and age (P = 0.70), sex (P = 0.40), tumor size (P = 0.80), tumor location (P = 0.74), gross tumor type (P = 0.27), stromal reaction pattern (P = 0.63), depth of cancer invasion (P = 0.84), lymphatic invasion (P = 0.75), vessel invasions (P = 0.75), lymph node metastasis (P = 0.05), liver metastasis (P = 0.73), peritoneal dissemination (P = 0.46), Lauren’s histological classification (P = 0.37), or TNM stage (P = 0.12). Several investigators previously suggested that tyrosine kinases, such as the c-met receptor PTK, might participate in the formation of lumen-like structures in human mammary ducts, epithelial carcinoma cell lines, 19 and stomach cancer. 20 However, in the present study we did not observe a relationship between MKK4 protein expression and intestinal type gastric cancer (P = 0.366). Moreover, postrecurrence treatments such as chemotherapy or regional radiotherapy did not alter MKK4 biological behavior (data not shown).
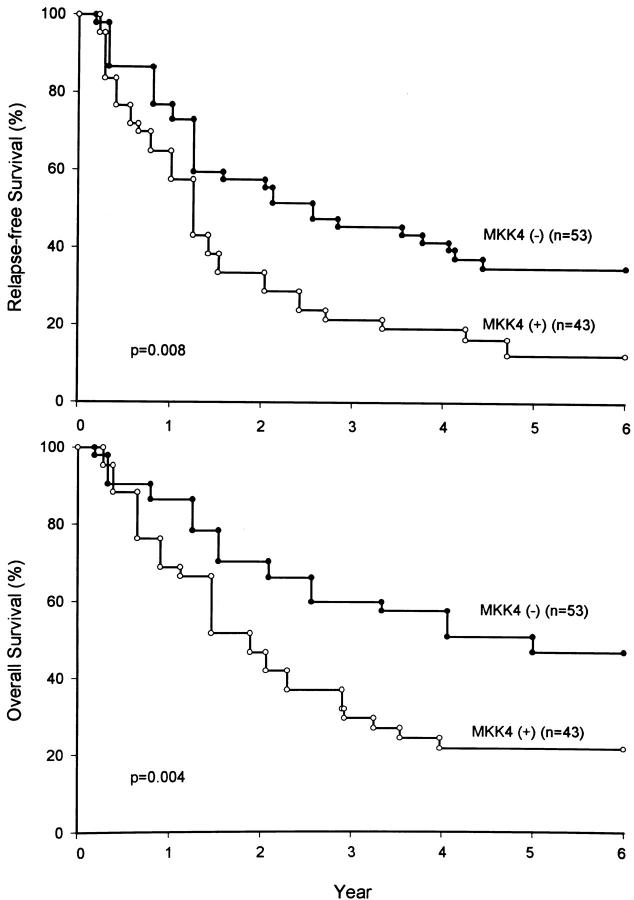
Figure 4 ▶ shows relapse-free and overall survival curves according to MKK4 status. These curves visually represent the increased hazard rates of study subjects who showed MKK4 expression in their gastric cancer tissues. In univariate analysis concerning relapse-free and overall survival rate in gastric cancer patients, significant prognostic factors were tumor size (P < 0.001, P = 0.001), stromal reaction pattern (P = 0.031, P = 0.010), depth of cancer invasion (P = 0.002, P < 0.001), lymph node metastasis (P = 0.002, P < 0.001), liver metastases (P = 0.007, P < 0.001), peritoneal dissemination (P < 0.001, P < 0.001), TNM stage (P < 0.001, P < 0.001), and MKK4 immunoreactivity (P = 0.008, P = 0.004). Lymphatic duct invasion (P = 0.017) and vessel invasion (P = 0.013) were two significant factors affecting overall survival, but not relapse-free survival. Age, gender, location of tumor, and gross tumor appearance were not prognostic factors affecting relapse-free survival or overall survival. After the univariate study, multivariate analysis was conducted to test the independent prognostic role of these variables. When all significant variables were taken into account through a stepwise analysis, the model selected MKK4 expression as the single independent factor regarding relapse-free survival (P = 0.002; hazard ratio = 2.1). Tumor size came next (P < 0.001, hazard ratio = 2.0), then TNM stage (P < 0.001, hazard ratio = 1.9) (Table 2) ▶ . Regarding overall survival, the model selected TNM stage as the most powerful independent factor (P < 0.001, hazard ratio = 3.1). MKK4 status came next (P = 0.007, hazard ratio = 2.1). Stromal reaction pattern, depth of cancer invasion, lymph node metastasis, liver metastases, peritoneal dissemination, lymphatic duct invasion, and vessel invasion were not significant prognostic factors (Table 2) ▶ .
Figure 4.
Relapse-free and overall survival of 96 patients with gastric cancers according to their MKK4 status. Patients with MKK4 protein expressed in gastric cancer tissues had significantly poorer relapse-free and overall survival rates than those without MKK4. Numbers in parentheses represent the total number of patients per group.
Table 2.
Multivariate Cox Analysis of Relapse-Free Survival and Overall Survival of 96 Patients with Gastric Cancer
| Relapse-free survival | Overall survival | |||
|---|---|---|---|---|
| Hazard ratio | P | Hazard ratio | P | |
| MKK4 kinase status | ||||
| Present versus absent | 2.1 | 0.002* | 2.1 | 0.007* |
| Tumor size (mean± sd) | 2.0 | 0.024* | — | 0.276 |
| Stromal reaction | ||||
| Medullary versus intermediate versus scirrhous | — | 0.292 | — | 0.993 |
| Depth of cancer invasion | ||||
| Mucosa and submucosa versus propria muscle and subserosa versus serosa (infiltration) exposed | — | 0.798 | — | 0.859 |
| Lymph node metastasis | ||||
| Negative versus positive | — | 0.296 | — | 0.332 |
| Liver metastasis | ||||
| Negative versus positive | — | 0.360 | — | 0.258 |
| Peritoneal dissemination | ||||
| Negative versus positive | — | 0.745 | — | 0.901 |
| Lymphatic duct invasion | ||||
| Negative versus positive | — | 0.760 | — | 0.648 |
| Vessel invasion | ||||
| Negative versus positive | — | 0.249 | — | 0.351 |
| TNM stage | ||||
| I versus II versus III versus IV | 1.9 | <0.001* | 3.1 | <0.001* |
*Statistically significant.
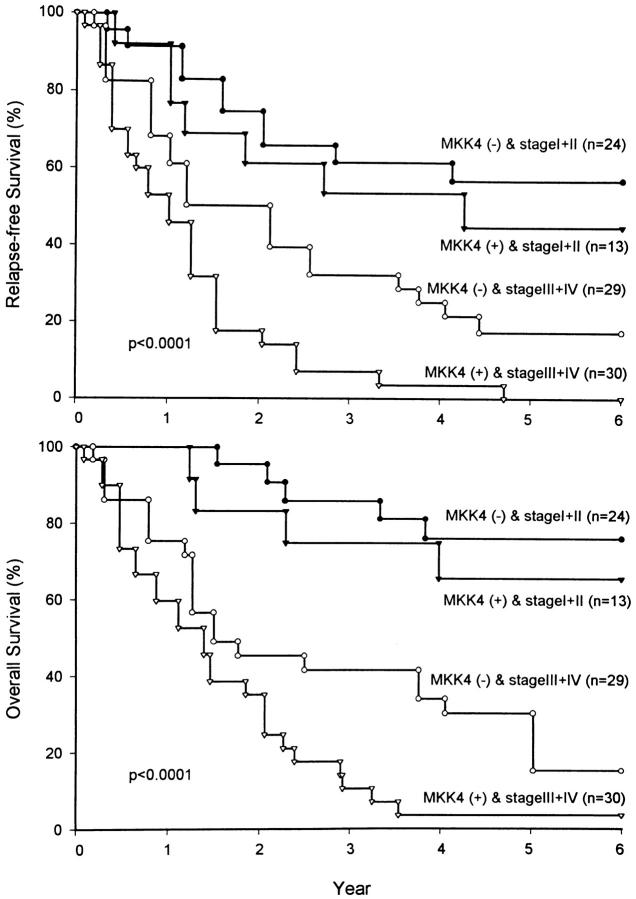
We next combined the two independent prognostic factors, TNM stage and MKK4 status, to define four risk groups. Patients with MKK4 expressed in gastric cancer tissues had a poorer relapse-free survival and overall survival compared to those without (Figure 5) ▶ . The difference in patients with stage III and IV diseases was statistically significant (P = 0.011 and P = 0.018, respectively). However, the difference was not statistically significant in patients with stage I and II diseases (P = 0.522 and P = 0.488, respectively). The MKK4 kinase may play significant roles in later stages of development in gastric cancers.
Figure 5.
Four risk groups were separated according to TNM stage and MKK4 status. The difference of relapse-free and overall survival rates in stage III and IV gastric cancer patients stratified by MKK4 status was statistically significant (P = 0.011 and P = 0.018, respectively). However, the difference of relapse-free and overall survival rates in stage I and II patients was not statistically significant (P = 0.522 and P = 0.488, respectively). Numbers in parentheses represent the number of patients per group.
Discussion
The PTK profiling approach permits a rapid positive identification of tyrosine kinases in a cancer cell and requires only a relatively small amount of specimen. Although some of the kinases identified were expected because of their ubiquitous distribution in epithelial cells, others were previously unknown to be expressed in gastric cancers. These PTK genes could be important for in vivo growth and progression of tumor cells. We observed that PTK profiles generated from gastric cancer in this study were characteristically different from prostate, colon, bladder cancers, and other tissues previously observed, 21-24 even when identical sets of primers were used. This validated the gene family profiling approach with degenerated PCR primers. The simple and rapid PTK profiling technique used here could lead to discoveries on tumor progression-related PTKs. 15,21 Degenerated primers used in PTK profiling allow us to cover almost all known PTKs and some unidentified novel human kinases, although PCR specificity is compromised. However, it is essential to generate an overall PTK profile at first as demonstrated here. Improvement in the quantitation measurements of expressed PTKs is currently being investigated in our laboratory, in collaboration with Dr. Hsing-Jien Kung at the University of California, Davis. Primer degeneracy will be reduced to allow better linear amplification of some overexpressed PTKs and more primer pairs will be required. Gel electrophoresis separation of restriction enzyme-digested PCR fragments with radiolabeled primers will allow us to better identify and measure each PTK expressed. This will also allow us to screen multiple samples in a shorter length of time (unpublished results).
One particular PTK, c-erbB2/neu, has been implicated in gastric cancer progression. 5 Amplification of c-erbB2/neu was observed in ∼18% to 24% of differentiated gastric cancers. 25 Although we did identify c-erbB2/neu or another oncogenic receptor PTK, c-met, in our profiles, they were not over-expressed in tumor tissues analyzed here. This may be attributed to individual patient variations, as a similar PTK profiling procedure readily identified the expression of c-erbB2/neu in prostate cancer tissues. 23
Evidence indicates that lymph node metastasis, depth of cancer invasion, gross appearance of the tumor, 26,27 and stromal reaction 27 are independent factors that affect survival in patients with gastric cancer. The present study showed that MKK4 kinase expression was not associated with these factors. Furthermore, our statistical analyses showed that MKK4 protein expression in primary gastric adenocarcinoma tissue is a useful prognostic marker for identifying high- and low-risk patients. Gastric adenocarcinoma patients with MKK4 present in gastric cancer tissue had a significantly shorter relapse-free survival and overall shorter survival compared to patients without MKK4 expression. In multivariate analysis, MKK4 was found to be an independent and powerful prognostic factor (relative risk = 2.1) in gastric cancer. Because the relative impact of prognostic factors on relapse-free survival and overall survival reflects these prognostic factors’ respective roles in tumor biology, we speculate that MKK4 may play an important role in the progression of human gastric cancer.
MKK4 plays particularly important roles in embryonic development. MKK4 knock-out mice are embryonic lethal; MKK4 (−/−) cells are defective in the downstream JNK and AP-1 activities, indicating the essential roles of MKK4 in the JNK signaling pathway. 28 The most intriguing observation from these knock-out mice was the abnormal hepatogenesis and massive hepatocyte apoptosis that indicate the MKK4-JNK pathway plays an essential role in liver development. 29 These data strongly suggest that MKK4-JNK is essential in protecting hepatocytes from apoptosis in embryonic development; however, contradicting evidence indicates that MKK4 involvement in the apoptosis mechanism is mediated by different stimuli. 30 The MKK4-JNK pathway is a complicated signaling network, and further research needs to be conducted using various cell types and distinct stimuli. JNK and p38 pathways were up-regulated, including MKK4, when cells were exposed to alkylating agents like methyl methanesulfonate. 31 These data indicate that MKK4 could be part of a defense mechanism for cell damaging reagents in cancer cells. It will be important to examine MKK4 kinase activity in tissue sections to understand the activation status of MKK4-JNK pathway. This can be achieved by applying suitable phospho-specific antibodies.
It was previously demonstrated that some human cancer tissues and cell lines have MKK4 genetic changes and lose MKK4 protein expressions or activities. Homozygous deletions were detected in two of 92 pancreatic adenocarcinomas (2%), one of 16 biliary adenocarcinomas (6%), and three of 22 breast carcinomas (15%), and one somatic mis-sense mutation of MKK4 was observed in 45 pancreatic carcinomas. 10 Teng et al 11 reported homozygous deletions in cancer cell lines originating from pancreas and lung cancer lines and four cancer cell lines harboring mutations lost MKK4 kinase activity (in total, six out of 213 cell lines, ∼3%). A major difference between these studies and our report is that in the previous studies a pure cancer cell population was required for DNA preparation and loss of heterozygosity analysis. Therefore, in the previous studies cell lines or xenograft tumor samples were prepared. As mentioned earlier, PTK expression pattern can be altered under different growth environments. In addition, the previous studies did not examine the correlation between the expression of MKK4 protein and clinicopathological features of tumors.
It is possible that multiple genetic alterations are involved in the process of oncogenesis of gastric cancers, and MKK4 might be involved in various signaling pathways. As discussed above, MKK4 is involved in several complicated signaling networks and might play different biological functions in different pathways. We found that only 44.8% of gastric adenocarcinomas examined expressed MKK4 protein, suggesting that genetic alterations of the MKK4 gene may exist in gastric cancer tissues. Loss of MKK4 immunoreactivity in cancer tissues was observed in 46 cases (48% of total samples), while their matching normal tissues were positive for MKK4 expression. Some of these tumors might lose the MKK4 gene loci or otherwise mutate as previously reported, 10,11 hence the exact roles of MKK4 need to be further elucidated. However, there is a previous example of a tumor suppressor gene over-expressed in the late stage of tumors. p53 is a well-known tumor suppressor gene in human cancers including gastric cancers. 25 Mutation of the p53 gene (without loss of heterozygosity phenotype) generated mutant p53 protein with an extended half-life, which resulted in an immunoreactivity against p53 protein. We observed the MKK4 transcript in several gastric cancer tissues examined by reverse transcriptase-polymerase chain reaction (data not shown). Because of the mixtures of cell types in the resected gastric cancer tissues, we were not able to confirm the genetic alterations of the MKK4 gene specifically in cancer cells. Further experiments are required to examine the MKK4 gene transcript in cancer cells only using laser-captured microdissection instruments.
In summary, in the present study we developed a general tyrosine kinase profile for gastric cancer specimens and their normal tissue counterparts, and then examined the in situ expression pattern of a particular PTK (MKK4) identified from the profile by immunohistochemistry. We conclude that MKK4 expression is an independent and powerful prognostic factor in human gastric cancers.
Acknowledgments
The authors would like to thank Yu-Long Chen, Jyh-Shi Lin, and Wenni Su for their excellent technical assistance.
Footnotes
Address reprint requests to Wen-chang Lin, Institute of Biomedical Sciences, Academia Sinica, Taipei 115, Taiwan, Republic of China. E-mail: wenlin@ibms.sinica.edu.tw.
Supported by grants from the National Science Council (NSC 89-2314-B-075-077-M58, and NSC 89-2314-B-001-010-M58).
References
- 1.Hunter A, Cooper JA: Protein-tyrosine kinases. Annu Rev Biochem 1985, 54:897-930 [DOI] [PubMed] [Google Scholar]
- 2.Edelman AM, Blumenthal DK, Krebs EG: Protein serine/threonine kinases. Annu Rev Biochem 1987, 56:567-613 [DOI] [PubMed] [Google Scholar]
- 3.Hunter T: A thousand and one protein kinases. Cell 1987, 50:823-829 [DOI] [PubMed] [Google Scholar]
- 4.Taylor SS, Knighton DR, Zheng J, Ten Eyck LF, Sowadski JM: Structural framework for the protein kinase family. Annu Rev Cell Biol 1992, 8:429-462 [DOI] [PubMed] [Google Scholar]
- 5.Kameda T, Yasui W, Yoshida K, Tsujino T, Nakayama H, Ito M, Ito H, Tahara E: Expression of ERBB2 in human gastric carcinomas: relationship between p185ERBB2 expression and the gene amplification. Cancer Res 1990, 50:8002-8009 [PubMed] [Google Scholar]
- 6.Tsugawa K, Yonemura Y, Hirono Y, Fushida S, Kaji M, Miwa K, Miyazaki I, Yamamoto H: Amplification of the c-met, c-erbB-2 and epidermal growth factor receptor gene in human gastric cancers: correlation to clinical features. Oncology 1998, 55:475-481 [DOI] [PubMed] [Google Scholar]
- 7.Lin A, Minden A, Martinetto H, Claret F, Lange-Carter C, Mercurio F, Johnson GL, Karin M: Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science 1995, 268:286-290 [DOI] [PubMed] [Google Scholar]
- 8.Pelech SL, Sanghera JS: Mitogen-activated protein kinases: versatile transducers for cell signalling. Trends Biochem Sci 1992, 17:233-238 [DOI] [PubMed] [Google Scholar]
- 9.Davis RJ: The mitogen-activated protein kinase signal transduction pathway. J Biol Chem 1993, 268:14553-14556 [PubMed] [Google Scholar]
- 10.Su GH, Hilgers W, Shekher MC, Tang DJ, Yeo CJ, Hruban RH, Kern SE: Alterations in pancreatic, biliary, and breast carcinomas support MKK4 as a genetically targeted tumor suppressor gene. Cancer Res 1998, 58:2339-2342 [PubMed] [Google Scholar]
- 11.Teng DH-F, Perry WLI, Hogan JK, Baumgard M, Bell R, Berry S, Davis TDF, Frye C, Hattier T, Hu R, Jammulapati S, Janecki T, Leavitt A, Mitchell JT, Pero R, Sexton D, Schroeder M, Su P, Swedlund B, Kyriakis JM, Avruch J, Bartel P, Wong AKC, Oliphant A, Thomas A, Skolnick MH, Tavtigian SV: Human mitogen-activated protein kinase kinase 4 as a candidate tumor suppressor. Cancer Res 1997, 57:4177-4182 [PubMed] [Google Scholar]
- 12.Wu CW, Tsay SH, Hsieh MC, Lo SS, Lui WY, P’eng FK: Clinicopathological significance of intestinal and diffuse type of gastric carcinoma in Taiwan Chinese. J Gastroenterol Hepatol 1996, 11:1083-1088 [DOI] [PubMed] [Google Scholar]
- 13.Wu CW, Hsieh MC, Lo SS, Tsay SH, Lui WY, P’eng FK: Relation of number of positive lymph nodes to the prognosis of patients with primary gastric adenocarcinoma. Gut 1996, 38:525-527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin JS, Lu CW, Huang CJ, Wu PF, Robison D, Kung HJ, Chi CW, Wu CW, Yang EK, Whang-Peng JJK, Lin Wc: Protein-tyrosine kinase and protein-serine/threonine kinase expression in human gastric cancer cell lines. J Biomed Sci 1998, 5:101–110 [DOI] [PubMed]
- 15.Lin W-c, Li AF-Y, Chi CW, Chung WW, Huang CL, Lui WY, Kung HJ, Wu CW: Tie-1 protein-tyrosine kinase: a novel independent prognostic marker for gastric cancer. Clin Cancer Res 1999, 5:1745–1751 [PubMed]
- 16.Lauren P: The two histological main types of gastric carcinoma. Diffuse and so-called intestinal type carcinoma: an attempt at a histoclinical classification. Acta Pathol Microbiol Scand 1965, 64:31-49 [DOI] [PubMed] [Google Scholar]
- 17.Wu CW, Chung WW, Chi CW, Kao HL, Lui WY, P’eng FK, Wang SR: Immunohistochemical study of arginase in cancer of stomach. Virchows Arch 1996, 428:325-331 [DOI] [PubMed] [Google Scholar]
- 18.Bissell MJ: Glandular structure and gene expression. Lessons from the mammary gland. Ann N Y Acad Sci 1998, 842:1-6 [DOI] [PubMed] [Google Scholar]
- 19.Tsarfaty L, Resau JH, Rulong S, Keydar I, Faletto D, Vande Woude GF: The met proto-oncogene receptor and lumen formation. Science 1992, 257:1258–1261 [DOI] [PubMed]
- 20.Wu CW, Li AFY, Chi CW, Chung WW, Liu TY, Lui WY, P’Eng FK: Hepatocyte growth factor and Met/HGF receptors in patients with gastric adenocarcinoma. Oncol Rep 1998, 5:817-822 [DOI] [PubMed] [Google Scholar]
- 21.Chen W-S, Kung H-J, Yang W-K, Lin W-c: Comparative tyrosine kinase profiles in colorectal cancers: enhanced arg expression in carcinoma as compared to adenoma and normal mucosa. Int J Cancer 1999, 83:579–584 [DOI] [PubMed]
- 22.Tseng TC, Chen SH, Hsu YP, Tang TK: Protein kinase profile of sperm and eggs: cloning and characterization of two novel testis-specific protein kinases (AIE1, AIE2) related to yeast and fly chromosome segregation regulators. DNA Cell Biol 1998, 17:823-833 [DOI] [PubMed] [Google Scholar]
- 23.Robinson D, He F, Pretlow T, Kung H-J: A tyrosine kinase profile of prostate carcinoma. Proc Natl Acad Sci USA 1996, 93:5958-5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu TJ, Lu TL, Su IJ, Lai MD: Tyrosine kinase expression profile in bladder cancer. Anticancer Res 1997, 17:2635-2637 [PubMed] [Google Scholar]
- 25.Ming S-C: Cellular and molecular pathology of gastric carcinoma and precursor lesions: a critical review. Gastric Cancer 1998, 1:31-50 [DOI] [PubMed] [Google Scholar]
- 26.Maruyama K: The most important prognostic factors for gastric cancer patients: a study using univariate and multivariate analyses. Scand J Gastroenterol 1987, 22(suppl 133):63-68 [Google Scholar]
- 27.Wu CW, Hsieh MC, Lo SS, Tsay SH: Prognostic indicators in patients with gastric cancer after resection. Dig Dis Sci 1997, 42:1265-1269 [DOI] [PubMed] [Google Scholar]
- 28.Yang D, Tournier C, Wysk M, Lu HT, Xu J, Davis RJ, Flavell RA: Targeted disruption of the MKK4 gene causes embryonic death, inhibition of c-Jun NH2-terminal kinase activation, and defects in AP-1 transcriptional activity. Proc Natl Acad Sci USA 1997, 94:3004-3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganiatsas S, Kwee L, Fujiwara Y, Perkins A, Ikeda T, Labow MA, Zon LI: SEK1 deficiency reveals mitogen-activated protein kinase cascade crossregulation and leads to abnormal hepatogenesis. Proc Natl Acad Sci USA 1998, 95:6881-6886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Z, Seimiya H, Naito M, Mashima T, Kizaki A, Dan S, Imaizumi M, Ichijo H, Miyazono K, Tsuruo T: ASK1 mediates apoptotic cell death induced by genotoxic stress. Oncogene 1999, 18:173-180 [DOI] [PubMed] [Google Scholar]
- 31.Wilhelm D, Bender K, Knebel A, Angel P: The level of intracellular glutathione is a key regulator for the induction of stress-activated signal transduction pathways including Jun N-terminal protein kinases and p38 kinase by alkylating agents. Mol Cell Biol 1997, 17:4792-4800 [DOI] [PMC free article] [PubMed] [Google Scholar]