Abstract
The twitcher mouse is a murine model of globoid cell leukodystropy, a genetic demyelinating disease caused by a mutation of the galactosylceramidase gene. Demyelination of the central nervous system commences around 20 postnatal days. Using GFP-transgenic mice as donors, the distribution of hematogenous cells after bone marrow transplantation was investigated in the twitcher mice. Bone marrow transplantation was carried out at 8 postnatal days. In twitcher chimeric mice examined before 30 postnatal days, numerous GFP+ cells were detected in spleen and peripheral nerve but only a few were detected in the liver, lung, and spinal white matter. In contrast, at 35 to 40 postnatal days when demyelination is evident, many GFP+ cells with ameboid form were detected in the white matter of the spinal cord, brainstem, and cerebrum. Approximately half of these GFP+ cells were co-labeled with Mac-1. In twitcher chimeric mice examined after 100 postnatal days, the majority of GFP/Mac-1 double-positive cells displayed the morphological features of ramified microglia with fine delicate processes and was distributed diffusely in both gray and white matter. These results suggest that a significant number of donor hematogenous cells are able to infiltrate into the brain parenchyma, repositioning themselves into areas previously occupied by microglia, and to ameliorate lethality.
Bone marrow transplantation (BMT) has been carried out as a potential treatment for lysosomal storage disorders in humans 1-4 and in animal models. 5-15 In many of these disorders, the effectiveness of the BMT treatment is often limited to the visceral organs, with little improvement in the central nervous system (CNS). 1,13 However, BMT has been reported to ameliorate clinical course and pathological process of the lysosomal storage diseases affecting the white matter. 3,4 In the previous BMT study using the twitcher mouse, a murine model of globoid cell leukodystrophy (GLD), we have detected donor-derived cells in the central nervous system 5 in mice that were significantly improved clinically as well as pathologically. 16,17 An activity of the galactosylceramidase, the enzyme deficient in GLD, 18 was elevated in the brains of the twitcher that received BMT. 5 More recently, we have observed that, after the injection of the fluorescent dye rhodamine isothiocyanate into the peritoneal cavity, massive infiltration of rhodamine-labeled peripheral monocytes/macrophages were detected in the CNS of nonirradiated twitcher mice along with progression of the demyelination (Y-P Wu, J Matsuda, A Kubota, K Suzuki, and K Suzuki, unpublished observation). These findings are consistent with the hypothesis that in lysosomal diseases affecting the white matter, peripheral hematogenous lineage cells infiltrate into the CNS and peripheral nervous system as a natural disease process.
In this report, we describe the time course and distribution of the infiltrated donor hematogenous cells, and their morphological transformation into microglia in the twitcher mice that received BMT from GFP transgenic mice with normal galactosylceramidase activity.
Materials and Methods
Generation of GFP-Transgenic Mice (C57BL/6-H-2KbP-EGFP)
The transgene was engineered by subcloning the entire H-2Kb promoter region (1960 bp) into SalI and BamHI restriction sites found upstream of a variant GFP (EGFP) gene encoded by pEGFP-1 (Clontech, Palo Alto, CA). The EGFP gene encodes a protein with a single red-shifted excitation peak and fluoresces 30- to 40-fold more intensely than wild-type GFP when excited at 488 nm. Expression of the H-2KbP-EGFP recombinant was verified by transfection into EL-4 cells. A SalI-ClaI 3000-bp fragment was isolated and microinjected into C57BL/6 derived embryos. Integration of the transgene was initially determined by Southern blotting of genomic tail DNA with a GFP-specific probe, and subsequently confirmed by flow cytometry analysis of peripheral lymphocytes prepared from founder mice.
Identification of Twitcher Mice
The original breeder pairs were obtained from the Jackson Laboratory (Bar Harbor, ME) and the colony has been maintained in our institution. The genotypes of twitcher (GALCtwi/twi) on the C57BL/6 (B6) background and wild-type littermate (GALC+/+) mice were identified by polymerase chain reaction using genomic DNA from the clipped tail on postnatal days 5 to 6. 19
Bone Marrow Transplantation
Either sex-matched adult GFP-transgenic mice (C57BL/6-H-2KbP-EGFP) or wild-type C57BL/6 mice were used as donors. The BMT was carried out in twitcher and wild-type littermate control mice at 8 postnatal days or in 5-week-old adult wild-type B6 mice as described previously. 5 Briefly, donor bone marrow cells were obtained by flushing the femurs of GFP-transgenic or wild-type mice with Hanks’ balanced solution. Intraperitoneal injection of 0.2 ml of the solution, containing 3 to 5 × 10 7 bone marrow cells, were carried out into the mice that were irradiated 1 day before with a sublethal dosage 8.0 or 9.0 Gy of 60Co γ rays for 7-postnatal-days young and 5-week-old adult mice, respectively.
FACS Analysis
To quantify the amount of engraftment, the peripheral blood mononuclear cells from 5-week-old chimeric wild-type B6 mice were analyzed at 3, 4, and 10 weeks after BMT. Briefly, the blood from the ventral tail vein was collected in Gibson’s solution, an anti-coagulant, and the red blood cells were lysed with a Tris-ammonium chloride red blood cell lysis buffer. The peripheral blood mononuclear cells were then analyzed by flow cytometry using a Becton-Dickinson FACScan (San Jose, CA). The leukocytes were gated on and fluorescence measured under the fluorescein isothiocyanate channel. All analysis and quantitation were performed using the Cyclops software from Cytomation, Inc. (Fort Collins, CO).
Tissue Preparation
A total of 15 twitcher mice and nine wild-type littermate control mice transplanted with GFP+ bone marrow cells were examined at 25 to 30 postnatal days (group I), 35 to 40 postnatal days (group II), and 100 to 150 postnatal days (group III). Each group consisted of five chimeric twitcher mice and three chimeric wild-type littermate mice. Three additional adult GFP-transgenic mice were also examined. The mice were anesthetized with ether and perfused transcardially with physiological saline (0.9% NaCl), followed by cold-buffered 4% paraformaldehyde. The entire brain, spinal cord, sciatic nerve, liver, spleen, and kidney were then removed, postfixed in the same fixative for 4 hours, and immersed in the phosphate buffer containing 20% sucrose overnight at 4°C. Serial sections of the cerebrum, cerebellum, brainstem, spinal cord, liver, spleen, and kidney were cut at 40-μm thickness with a vibratome. Sciatic nerves were teased gently with fine forceps. The sections were then coverslipped with Vectashield mounting medium for fluorescence (Vector Laboratories, Burlingame, CA) and examined/photographed under a Nikon microphoto FXA microscope (Nikon, Garden City, NJ) equipped with a fluorescein isothiocyanate filter. A strong GFP fluorescence was observed in the cell bodies and the processes in the sciatic nerve, spleen, liver, and kidney. In the sections from CNS, the fluorescence was somewhat weaker and thus, sections were further immunostained with anti-GFP antibody (Clontech) to enhance the fluorescence.
Immunocytochemistry
The brain and spinal cord sections were pretreated with 10% normal goat serum with 0.2% Triton X-100, and then processed for single- and double-immunostaining. In brief, all brain and spinal cord sections were incubated with polyclonal antibody against GFP (Clontech) at a dilution of 1:200. After washing briefly, the sections were exposed to fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (Sigma Chemical Co., St. Louis, MO). The immunostained sections were then examined/photographed with the microscope. Sections with or without GFP immunostaining were further processed for Mac-1 double-immunostaining. After incubating with monoclonal antibody against mouse Mac-1 (Boehringer-Mannheim, Indianapolis, IN) at a dilution of 1:500, the sections were incubated for 1 hour in biotinylated anti-rat IgG and then for 1 hour in avidin-biotin-peroxidase complex solution (Vector). After rinsing, the sections were finally reacted in a solution of 0.05% 3,3[hyph]diaminobenzidine containing 0.01% H2O2 in Tris-HCl buffer (pH 7.6). The sections were mounted and photographed again.
Results
GFP-Transgenic Mouse
In the adult GFP-transgenic mouse (Figure 1A) ▶ , ∼90% of the peripheral blood mononuclear cells are GFP+ cells. However, in the brain, GFP+ cells were rarely detected even after additional anti-GFP antibody immunostaining. Very rare ramified microglia with a few long, delicate processes and a small cell body in the fimbria hippocampi and Bergmann glial cells in the cerebellum were GFP-positive (data not shown). GFP+ cells were not detected in the spinal cord and brainstem.
Figure 1.
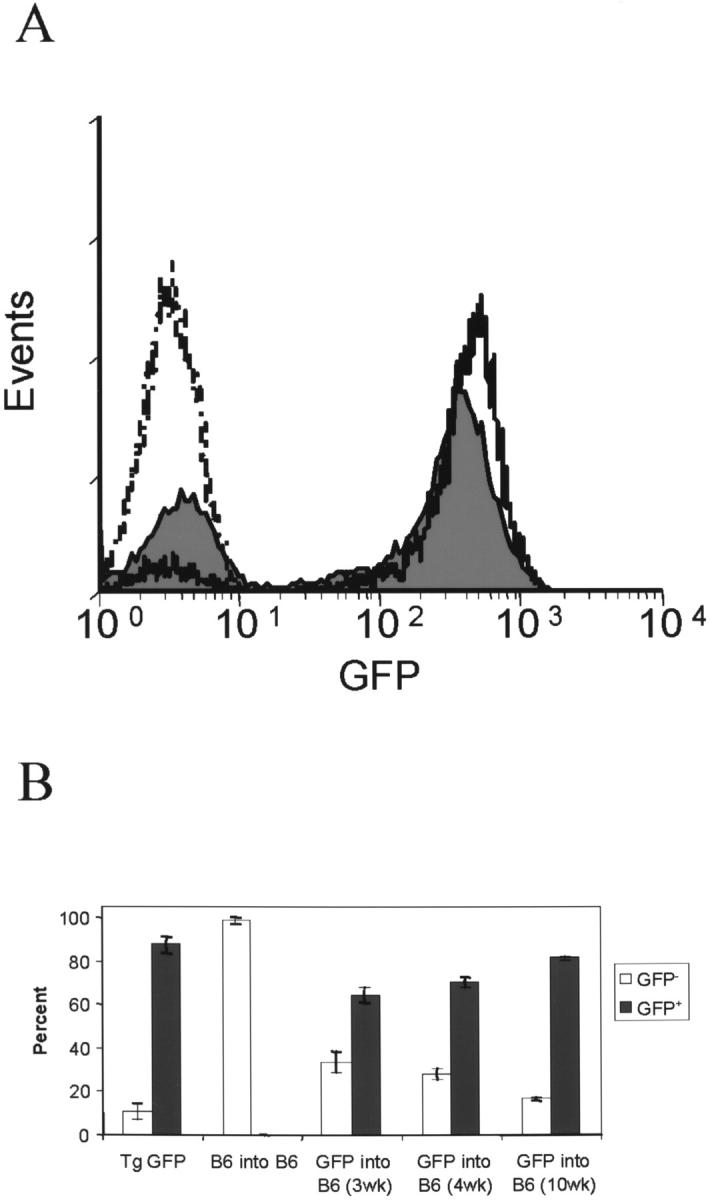
Engraftment of peripheral blood mononuclear cells by GFP+ cells. Peripheral blood mononuclear cells from 5-week-old wild-type B6 mice, which received bone marrow cells from wild-type B6 or GFP-transgenic mice were analyzed by flow cytometry at 3, 4 (A), and 10 weeks after BMT. Histograms: dashed, B6 into B6; solid black, GFP-transgenic; filled gray, B6/GFP chimera 4 weeks after MBT. Results are summarized in B.
Bone Marrow Transplanted Wild-Type and Twitcher Mice
In wild-type adult B6 mice transplanted with GFP-transgenic bone marrow at 3, 4, and 10 weeks after BMT, 65%, 70%, and 82% GFP+ peripheral blood mononuclear cells were detected, respectively, as mean of 5 mice (Figure 1B) ▶ . This demonstrates that cells from GFP-transgenic mice can be detected in the peripheral nervous system and provides a mean to identify and track bone marrow derived cells in the CNS.
Clinical Course of Twitcher Mice with BMT
The untreated twitcher mice could not survive beyond 37 to 45 days of age. However, after BMT, the chimeric twitcher mice were able to survive three to five times longer than nonchimeric twitcher mice with reduced neurological symptoms as reported previously. 5 The body weight of these chimeric twitcher mice remained less than that of chimeric wild-type littermate mice, however.
Distribution of GFP+ Cells
Visceral Organs
Distribution of GFP+ cells in visceral organs was similar in both chimeric twitcher and wild-type mice. In groups I and II, almost all hematopoietic cells in the spleen expressed GFP fluorescence (Figure 2A) ▶ . In contrast to the rapid engraftment in the spleen, only rare GFP+ cells were observed in the liver, kidney, and lung (data not shown). In group III mice, an increased number of GFP+ cells was seen in the liver (Figure 2B) ▶ , kidney, and lung (data not shown).
Figure 2.
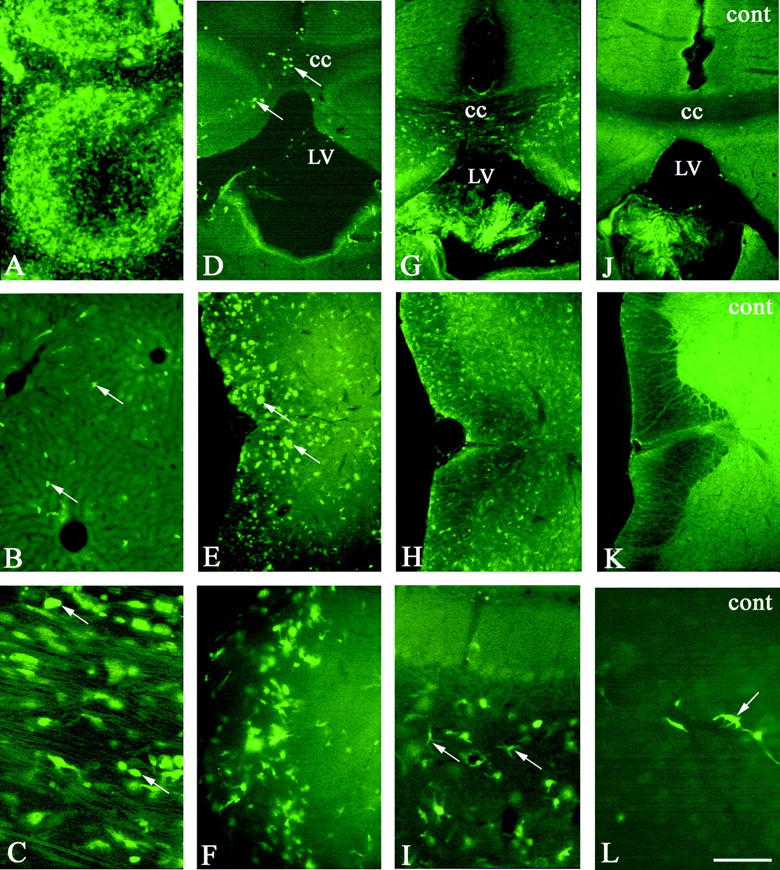
Photomicrographs showing the distribution of GFP+ cell in chimeric twitcher (A–I) and wild-type littermate chimeric mouse (J–L) at 35 postnatal days (A–F) and 150 postnatal days (G–L). Nearly all cells in the spleen (A) showing green fluorescence indicate engraftment with GFP+ donor cells. B: GFP+ cells (arrows) in the liver. In the sciatic nerve (C), many ameboid GFP+ cells (arrows) are observed. Arrows in D and E indicate ameboid cell and in I ramified cell. Arrows in L indicate perivascular cell. CC, corpus callosum; LV, lateral ventricle; cont, wild-type littermate control mice. A–C, without GFP immunostaining. Scale bar, 160 μm in A, B, D. E, G, H, J, K; 80 μm in F, I; and 40 μm in C, L.
Peripheral Nervous System
In wild-type chimeric mice, a few slender spindle-shaped GFP+ cells were detected along the nerve fibers in the sciatic nerve in all three groups (data not shown). Compared with wild-type chimeric mice, numerous GFP+ cells were observed in the sciatic nerve of the chimeric twitcher mice in all three groups (Figure 2C) ▶ . In groups I and II, the majority of the GFP+ cells had a spheroid, round cell body. But in group III, almost all of GFP+ cells were slender and spindle-shaped along the nerve fibers.
Central Nervous System
Control Mice
In wild-type chimeric mice of all groups, although a few GFP+ cells were observed in the CNS (Figure 2, J–L) ▶ , they were far less than those in other organs. They were predominantly seen in the choroid plexus (Figure 2J) ▶ , perivascular region (Figure 2L) ▶ , and leptomeninges. Most GFP+ cells in these areas were spindle-shaped but a few were oval.
Twitcher Mice
In group I chimeric twitcher mice, the distribution of GFP+ cells was similar to the wild-type chimeric mice, except for a few GFP+ cells with a round cell body which were detected in the white matter of spinal cord (data not shown). In group II chimeric twitcher mice (Figure 2, D–F) ▶ , however, numerous GFP+ cells were detected in the cerebrum especially in the white matter such as the corpus callosum (Figure 2D) ▶ , internal capsule and fimbria hippocampi, spinal white matter (Figure 2E) ▶ , and brainstem (Figure 2F) ▶ . Only a few GFP+ cells were seen in the cerebellum (data not shown). Most of these GFP+ cells in the white matter had a round or spheroid cell body reminiscent of ameboid cells, whereas those in the gray matter displayed a small cell body with two or three fine processes. In group III chimeric twitcher mice (Figure 2, G–I) ▶ , GFP+ cells were still numerous, although more cells were detected in the white matter in the cerebrum (Figure 2G) ▶ . Compared with group I and II chimeric twitcher mice, GFP+ cells were more ubiquitously distributed in both the gray and white matter, in particular, in the spinal cord (Figure 2H) ▶ . The majority of them had two or three fine processes with a small cell body, although few ameboid cells were still observed. At this time point, the increased number of GFP+ cells with both ameboid and ramified form were observed in the cerebellum (Figure 2I) ▶ . The double-immunostaining with Mac-1 antibody showed that more than 50% GFP+ cells in the group II chimeric twitcher mice expressed immunoreactivity with Mac-1 (Figure 3A) ▶ . In group III, almost all of GFP+ cells with fine processes were double-immunostained with Mac-1 (Figure 3, B and C) ▶
Figure 3.
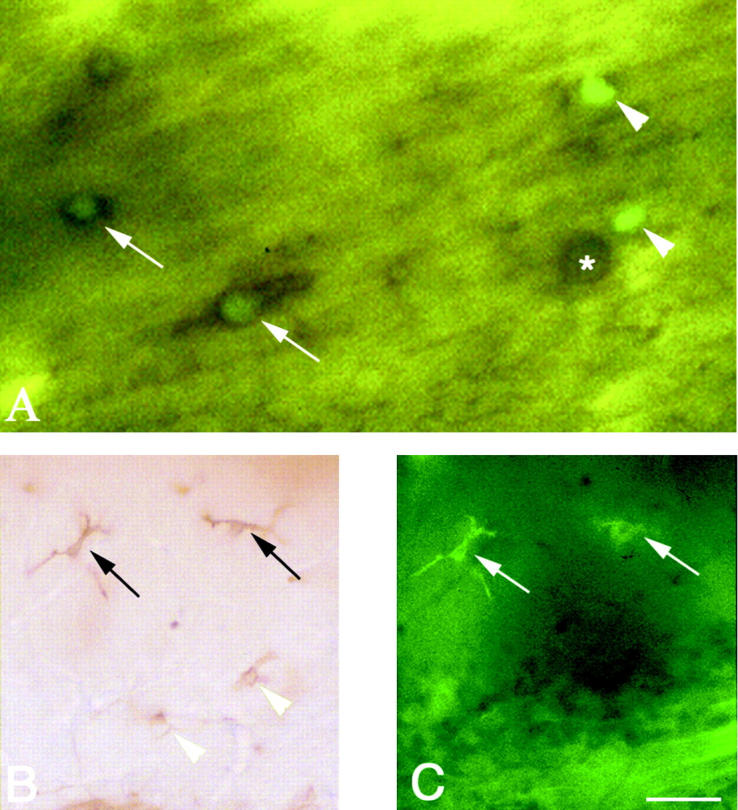
Photomicrographs showing GFP-positive cells double-immunostained with Mac-1 in corpus callosum of 35 postnatal days (A) and cerebellum of 150 postnatal days (B and C) chimeric twitcher. Arrows in A indicate GFP (green) and Mac-1 (brown) double-positive cells and arrowheads indicate cells expressing GFP only (green). Asterisk in A indicates a Mac-1 positive microglia. The black arrows in B and white arrows in C indicate the Mac-1/GFP double-positive ramified microglia. The arrowheads in B indicate cells immunostained with Mac-1 only. A: without GFP immunostaining. Scale bar, 20 μm in A and 40 μm in B and C.
Discussion
In agreement with the previous work, 20,21 our study has shown that in the wild-type chimeric mice, the engraftment of splenic monocytes/macrophages takes place rapidly within a month and slower in the lung, kidney, liver, and brain. The distribution of the donor cells in the CNS was predominantly seen at the perivascular and leptomeningeal, and not parenchymal sites. In the chimeric twitcher mice, distribution of the donor cells in the visceral organs is similar to that of wild-type littermate chimeric mice. In the nervous system, however, significant numbers of the infiltrated donor cells were detected in the chimeric twitcher mice older than 35 postnatal days in groups II and III. The majority of GFP+ ameboid cells with large cell bodies, suggestive of an activated status, noted in the group II chimeric twitcher almost disappeared in the group III chimeric twitcher mice that survived longer than 100 days. In these long-surviving chimeric twitchers, the majority of GFP+ cells resembled ramified microglia with small cell body and fine delicate cellular processes. The morphological difference of GFP+ cells in these two groups suggests that transformation of activated ameboid cells of hematogenous origin to reposition themselves as quiescent ramified microglia. Our preliminary study showed the presence of numerous Ia- and CD8-immunoreactive ameboid cells and increased tumor necrosis factor-α and interleukin-10 mRNA by reverse transcriptase-polymerase chain reaction determination in the group II twitcher mice but such increase in the immunoreactivity or mRNA was not found in the group III twitcher mice. These data are also consistent with the transformation of macrophages/microglia from active to the quiescent state. Although some of the donor cells were not marked by the Mac-1 antibody at the beginning of the infiltration, eventually they became ramified cells and labeled by the Mac-1 antibody. It might suggest, therefore, that some undifferentiated donor cells also infiltrated into the demyelinating CNS of twitcher mice.
The twitcher mouse is a model of GLD in humans that is caused by the mutation of the galactosylceramidase gene. The disease is characterized pathologically by an apoptotic death of oligodendrocytes, 22 diffuse demyelination, and a massive increase of the reactive microglia/macrophages in the CNS and peripheral nervous system. 23,24 The demyelination progressed in orderly fashion and affected the peripheral nervous system earlier than the CNS. 25,26 The reactive microglia/macrophages are detected in the spinal white matter at 20 postnatal days and in the cerebral white matter at 25 postnatal days in twitcher mice. In chimeric twitcher mice, however, GFP+ cells were detected in the CNS only after 35 postnatal days. Although these twitcher mice received irradiation before BMT, this apparently delayed response of hematogenous macrophage infiltration in chimeric twitcher mice is not because of irradiation, because our study with intraperitoneal injection of rhodamine in nonirradiated twitcher mice resulted in delayed cellular infiltration similarly (Y-P Wu, J Matsuda, A Kubota, K Suzuki, and K Suzuki, unpublished observation). We hypothesize that the delayed response may be explained as follows; in the early stage of demyelination, phagocytosis of myelin debris is carried out primarily by local activated microglia. These activated microglia synthesize and secrete a variety of proinflammatory, potentially cytotoxic cytokines, such as tumor necrosis factor-α and interleukin-1. 27-31 Infiltration of the peripheral hematogenous cells may take place in response to these cytokines and/or various chemokines. Thus, the pattern of donor cell infiltration in the twitcher mice in the current study seems to follow this naturally occurring demyelinating process and is totally different from the pattern of donor cell infiltration seen in the murine model of Sandhoff disease, in which the major pathology is a neuronal storage and peripheral hematogenous cells are detected mostly in the gray matter. 15 Thus, natural disease process seems to determine the site and extent of hematogenous cell infiltration and eventual effectiveness of the BMT treatment. Our study also indicates that unlike rapid splenic engraftment, cellular infiltration into the CNS is a slow process because very few GFP+ cells were detected within the CNS of chimeric twitcher mice younger than 35 postnatal days. This slow process may explain the ineffectiveness of BMT on the rapidly progressive infantile form of GLD, although BMT seems effective as the treatment of late onset slowly progressive form of GLD. 3
Acknowledgments
We thank Dr. Robert Bagnell and Ms. Victoria Madden for microscope assistance and Ms. Carol A. Troutner for her editing work.
Footnotes
Address reprint requests to Kinuko Suzuki, MD, Department of Pathology and Laboratory Medicine, 401 Brinkhous-Bullitt Building, CB 7525, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7525. E-mail: kis@med.unc.edu.
Supported by research grants NS-24453, AI-20288, AI-141580 and Mental Retardation Research Center core grant HD-03110 from the United States Public Health Service.
References
- 1.Imaizumi M, Gushi K, Kurobane I, Inoue S, Suzuki J, Koizumi Y, Suzuki H, Sato A, Gotoh Y, Haginoya K: Long-term effects of bone marrow transplantation for inborn errors of metabolism: a study of four patients with lysosomal storage diseases. Acta Paediatr Jpn 1994, 36:30-36 [DOI] [PubMed] [Google Scholar]
- 2.Ringden O, Groth CG, Erikson A, Granqvist S, Mansson JE, Sparrelid E: Ten years’ experience of bone marrow transplantation for Gaucher disease. Transplantation 1995, 59:864-870 [PubMed] [Google Scholar]
- 3.Krivit W, Shapiro EG, Peters C, Wagner JE, Cornu G, Kurtzberg J, Wenger DA, Kolodny EH, Vanier MT, Loes DL, Dusenbery K, Lockman LA: Hematopoietic stem cell transplantation in globoid cell leukodystrophy. New Engl J Med 1998, 338:1119-1126 [DOI] [PubMed] [Google Scholar]
- 4.Krivit W, Peters C, Shapiro EG: Bone marrow transplantation as effective treatment of central nervous system disease in globoid cell leukodystrophy, metachromatic leukodystrophy, adrenoleukodystrophy, mannosidosis, fucosidosis, aspartylglucosaminuria, Hurler, Maroteaux-Lamy, and Sly syndromes, and Gaucher disease type III. Curr Opin Neurol 1999, 12:167-176 [DOI] [PubMed] [Google Scholar]
- 5.Hoogerbrugge PM, Suzuki K, Suzuki K, Poorthuis BJHM, Kobayashi T, Wagemaker G, van Bekkum DW: Donor-derived cells in the central nervous system of twitcher mice after bone marrow transplantation. Science 1988, 239:1035-1038 [DOI] [PubMed] [Google Scholar]
- 6.Yeager AM, Shinn C, Shinohara M, Pardoll DM: Hematopoietic cell transplantation in the twitcher mouse: the effects of pretransplant conditioning with graded doses of busulfan. Transplantation 1993, 56:185-190 [DOI] [PubMed] [Google Scholar]
- 7.Walkley SU, Thrall MA, Dobrenis K, Huang M, March PA, Siegel DA, Wurzelmann S: Bone marrow transplantation corrects the enzyme defect in neurons of the central nervous system in a lysosomal storage disease. Proc Natl Acad Sci USA 1994, 91:2970-2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krall WJ, Challita PM, Perimutter LS, Skelton DC, Kohn DB: Cells expressing human glucocerebrosidase from a retroviral vector repopulate macrophages and central nervous system microglia after murine bone marrow transplantation. Blood 1994, 83:2737-2748 [PubMed] [Google Scholar]
- 9.Haskins M: Bone marrow transplantation therapy for metabolic disease: animal models as predictors of success and in utero approaches. Bone Marrow Transplant Suppl 1996, 3:S25-S27 [PubMed] [Google Scholar]
- 10.Miranda SRP, Erlich S, Erlich S, Friedrich VL, Haskins ME, Gatt S, Schuchman EH: Biochemical, pathological, and clinical response to transplantation of normal bone marrow cells into acid sphingomyelinase-deficient mice. Transplantation 1998, 65:884-892 [DOI] [PubMed] [Google Scholar]
- 11.Vogler C, Sands MS, Galvin N, Levy B, Thorpe C, Barker J, Sly WS: Murine mucopolysaccharidosis type VII: the impact of therapies on the clinical course and pathology in a murine model of lysosomal storage disease. J Inherit Metab Dis 1998, 21:575-586 [DOI] [PubMed] [Google Scholar]
- 12.Hahn CN, del Pilar Martin M, Zhou XY, Mann LW, d’Azzo A: Correction of murine galactosialidosis by bone marrow-derived macrophages overexpressing human protective protein/cathepsin A under control of the colony-stimulating factor-1 receptor promotor. Proc Natl Acad Sci USA 1998, 95:14880–14885 [DOI] [PMC free article] [PubMed]
- 13.Laine M, Richter J, Fahlman C, Rapola J, Renlund M, Peltonen L, Karlsson S, Jalanko A: Correction of peripheral lysosomal accumulation in mice with aspartylglucosaminuria by bone marrow transplantation. Exp Hematol 1999, 27:1467-1474 [DOI] [PubMed] [Google Scholar]
- 14.Norflus F, Tifft CJ, McDonald MP, Goldstein G, Crawley JN, Hoffmann A, Sandhoff K, Suzuki K, Proia RL: Bone marrow transplantation prolongs life span and ameliorates neurologic manifestations in Sandhoff disease mice. J Clin Invest 1998, 101:1881-1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oya Y, Proia RL, Norflus F, Tifft CJ, Langaman C, Suzuki K: Distribution of enzyme bearing cells in GM2 gangliosidoses mice: regionally specific pattern of cellular infiltration following bone marrow transplantation. Acta Neuropathol 2000, 99:161-168 [DOI] [PubMed] [Google Scholar]
- 16.Suzuki K, Hoogerbrugge PM, Suzuki K, Poorthuis BJHM, Van Bekkum DW, Suzuki K: The twitcher mouse: central nervous system pathology after bone marrow transplantation. Lab Invest 1988, 58:302-309 [PubMed] [Google Scholar]
- 17.Kondo A, Hoogerbrugge PM, Suzuki K, Poorthuis BJHM, van Bekkum DW, Suzuki K: Pathology of the peripheral nerve in the twitcher mouse following bone marrow transplantation. Brain Res 1988, 460:178-183 [DOI] [PubMed] [Google Scholar]
- 18.Suzuki K, Suzuki Y, Suzuki K: Galactosylceramide lipidosis; globoid cell leukodystrophy (Krabbe disease). Scriver CP Beaudet AL Sly WS Valle D eds. The Metabolic and Molecular Basis of Inherited Disease. 1995, :pp 2671-2692 McGraw-Hill, New York [Google Scholar]
- 19.Ezoe T, Vanier MT, Oya Y, Popko B, Tohyama J, Matsuda J, Suzuki K, Suzuki K: Biochemistry and neuropathology of mice doubly deficient in synthesis and degradation of galactosylceramide. J Neurosci Res 2000, 59:170-178 [DOI] [PubMed] [Google Scholar]
- 20.Kennedy DW, Abkowitz JL: Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood 1997, 90:968-993 [PubMed] [Google Scholar]
- 21.Ono K, Takii T, Onozaki K, Ikawa M, Okabe M, Sawada M: Migration of exogenous immature hematopoietic cells into adult mouse brain parenchyma under GFP-expressing bone marrow chimera. BBRC 1999, 262:610-614 [DOI] [PubMed] [Google Scholar]
- 22.Taniike M, Mohri I, Eguchi N, Irikura D, Urade Y, Okada S, Suzuki K: An apoptotic depletion of oligodendrocytes in the twitcher, a murine model of globoid cell leukodystrophy. J Neuropathol Exp Neurol 1999, 58:644-653 [DOI] [PubMed] [Google Scholar]
- 23.Higashi Y, Komiyama A, Suzuki K: The twitcher mouse: immunocytochemical study of Ia expression in macrophages. J Neuropathol Exp Neurol 1992, 5:47-57 [DOI] [PubMed] [Google Scholar]
- 24.Ohno M, Komiyama A, Martin PM, Suzuki K: MHC class II (Ia) antigen expression and T-cell infiltration in the demyelinating CNS and PNS of the twitcher mouse. Brain Res 1993, 625:186-196 [DOI] [PubMed] [Google Scholar]
- 25.Taniike M, Suzuki K: Spacio-temporal progression of demyelination in twitcher mouse with clinico-pathological correlation. Acta Neuropathol 1994, 88:228-236 [DOI] [PubMed] [Google Scholar]
- 26.Suzuki K, Taniike M: Murine model of genetic demyelinating diseases: the twitcher mouse. Microsc Res Tech 1995, 32:204-214 [DOI] [PubMed] [Google Scholar]
- 27.Banati RB, Gehrmann J, Schubert P, Kreutzberg GW: Cytotoxicity of microglia. Glia 1993, 7:111-118 [DOI] [PubMed] [Google Scholar]
- 28.Renno T, Krakowski M, Piccirillo C, Lin JY, Owens T: TNF-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J Immunol 1995, 154:944-953 [PubMed] [Google Scholar]
- 29.Kreutzberg GW: Microglia: a sensor for pathological events in the CNS. Trends Neurosci 1996, 19:312-318 [DOI] [PubMed] [Google Scholar]
- 30.Le Vine SM, Brown DC: IL-6 and TNFα expression in brains of twitcher, quaking and normal mice. J Neuroimmunol 1997, 73:47-56 [DOI] [PubMed] [Google Scholar]
- 31.Ransohoff RM: Chemokines in neurological disease models: correlation between chemokine expression patterns and inflammatory pathology. J Leukocyte Biol 1997, 62:645-652 [DOI] [PubMed] [Google Scholar]


