Abstract
The angiopoietins are recently described growth factors for vascular endothelium. The Tie1 and Tie2 receptors are expressed by endothelium. Acquired immune deficiency syndrome (AIDS)-associated Kaposi’s sarcoma (KS) and cutaneous angiosarcoma are malignancies of endothelial origin. KS involves primarily the skin and mucosal surfaces and is common in AIDS patients. In an effort to determine whether the angiopoietins and Tie receptors play a role in the pathobiology of angiosarcoma and KS, we studied the expression of angiopoietin-1, angiopoietin-2, angiopoietin-4, Tie1, and Tie2 mRNAs in biopsies of KS from 12 AIDS patients, in biopsies of cutaneous angiosarcoma from two patients, and in control biopsies of normal skin from three volunteers by in situ hybridization. Strong expression of angiopoietin-2, Tie1, and Tie2 mRNAs was detected in the tumor cells of KS and cutaneous angiosarcomas, in contrast to the focal low-level expression in normal skin biopsies. Focal low-level expression of angiopoietin-1 was seen in KS, cutaneous angiosarcomas, and in normal skin. Focal low-level expression of angiopoietin-4 was identified in a minority of KS lesions. These findings suggest that the angiopoietins and Tie receptors may play an important role in the pathobiology of KS and cutaneous angiosarcoma and identify additional potential targets for therapeutic intervention in these vascular malignancies.
Recently, a new family of growth factors specific for the vascular endothelium has been identified, termed the angiopoietins. 1-4 The specificity of the angiopoietins for the vascular endothelium results from the restricted distribution of the angiopoietin receptor, Tie2, to these cells. 5-7 All known angiopoietins bind to Tie2, but it is still unclear as to whether they use the closely related receptor Tie1. The actions of the angiopoietins seem to be quite different from those of the well-characterized angiogenic factor vascular permeability factor/vascular endothelial growth factor (VPF/VEGF). The angiopoietins seem to act in complementary and coordinated fashion with VPF/VEGF, playing a later role in vascular development. Thus, in mouse embryos lacking either angiopoietin-1 or Tie2, the early stages of VPF/VEGF-dependent vascular development seem to occur rather normally and result in the formation of a primitive vasculature, but remodeling and stabilization of this primitive vasculature is severely perturbed. 3,8,9 Transgenic overexpression of angiopoietin-1 leads to striking hypervascularization. 10 In contrast, transgenic overexpression of angiopoietin-2, a natural competitor of angiopoietin-1 for the Tie2 receptors, seems to severely disturb vessel stabilization and remodeling. 2 Angiopoietin-3 and angiopoietin-4 are more recently described members of this family that seem to represent the mouse and human counterparts of the same genetic locus. 1 Angiopoietin-4 seems to act as an agonist and is expressed at high levels in lung. 1
Angiopoietin-2 is dramatically induced at sites of vascular remodeling in an otherwise stable adult vasculature, 2 and also in tumor vessels, 11 leading to the proposal of a model in which angiopoietin-2 plays a facilitative role at sites of vascular remodeling by blocking a constitutive stabilizing action of angiopoietin-1, with such destabilization facilitating an angiogenic response in the presence of VPF/VEGF, but leading to vessel regression in the absence of VPF/VEGF.
Acquired immune deficiency syndrome (AIDS)-associated Kaposi’s sarcoma (KS) and angiosarcomas are malignancies of endothelial origin. KS involves primarily the skin and mucosal surfaces and is common in AIDS patients. 12 In an effort to determine whether Tie and angiopoietins play a role in the pathobiology of KS and angiosarcoma, we studied the expression of Tie1, Tie2, angiopoietin-1, angiopoietin-2, and angiopoietin-4 in biopsies of KS from 12 AIDS patients, biopsies of angiosarcoma from two patients, and in control biopsies of normal skin in three volunteers.
Materials and Methods
In Situ Hybridization
Four-millimeter punch biopsies were obtained with informed consent from seven human immunodeficiency virus (HIV)-positive patients with cutaneous KS lesions and from normal skin of three volunteers following human experimental guidelines of the United States Department of Health and Human Services and of Beth Israel Deaconess Medical Center. Fresh tissue was also obtained in one of the two cases of angiosarcoma studied. Fresh tissue was fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4, for 2 to 4 hours at 4°C and was then transferred to 30% sucrose in PBS overnight at 4°C, frozen in optimum cutting temperature compound (Miles Diagnostics, Elkhart, IN) and stored at −70°C. Archival paraffin sections were studied in an additional five cases of KS as well as in both cases of angiosarcoma. In situ hybridization studies were performed on 4-μm thick frozen sections with antisense single-stranded 35S-labeled RNA probes for angiopoietin-1 (570 bp, including 70 bp of the 5′ untranslated region and 500 bp of the region up to amino acid 166), angiopoietin-2 (640 bp, including 360 bp of the 5′ untranslated region and 280 bp of the region up to amino acid 99), angiopoietin-4 (640 bp), Tie1 (562 bp from the ectodomain amino acid region 162–349), and Tie 2 (508 bp from the ectodomain amino acid region 70–239). Corresponding sense probes were synthesized as controls. Plasmids were provided by Regeneron Pharmaceuticals, Inc., Tarrytown, NY.
Details of in situ hybridization have been published previously. 13 Briefly, frozen section slides were passed through 0.2 mol/L HCl, Tris/ethylenediaminetetraacetic acid (EDTA) with 1 μg/ml proteinase K, 0.2% glycine, 4% paraformaldehyde in PBS (pH 7.4), 0.1 mol/L of triethanolamine containing 1/200 (v/v) acetic anhydride, and 2× standard saline citrate (SSC). Slides were hybridized overnight at 50°C with 35S-labeled riboprobes in the following mixture: 0.3 mol/L NaCl, 0.01 mol/L Tris (pH 7.6), 5 mmol/L EDTA, 50% formamide, 10% dextran sulfate, 0.1 mg/ml yeast tRNA, and 0.01 mol/L dithiothreitol. Posthybridization washes included 2× SSC/50% formamide/10 mmol/L dithiothreitol at 50°C; 4× SSC/10 mmol/L Tris/1 mmol/L EDTA with 20 μg/ml ribonuclease at 37°C; and 2× SSC/50% formamide/10 mmol/L dithiothreitol at 65°C and 2× SSC. Slides were then dehydrated through graded alcohols containing 0.3 mol/L ammonium acetate, dried, coated with Kodak NTB 2 emulsion and stored in the dark at 4°C for 2 weeks. The emulsion was developed with Kodak D19 developer and the slides were counterstained with hematoxylin. Paraffin sections were treated in a similar manner except slides were initially deparaffinized in xylene and passed through graded alcohols and a larger concentration of proteinase K (3 μg/ml) was used.
Results
Lesional biopsies were studied from 12 patients with AIDS-associated KS and two patients with angiosarcoma (one case arose in breast skin after radiation and chemotherapy for breast cancer and the other case arose spontaneously). Punch biopsies of normal skin were studied from three volunteers without AIDS. In our experience, the use of fixed frozen sections results in somewhat stronger and more consistent mRNA labeling compared to sections cut from archival paraffin blocks. Expression of mRNA was graded as strong (more than 10 grains/cell), low level (between 5 and 10 grains per cell), or equivocal (less than 5 grains/cell).
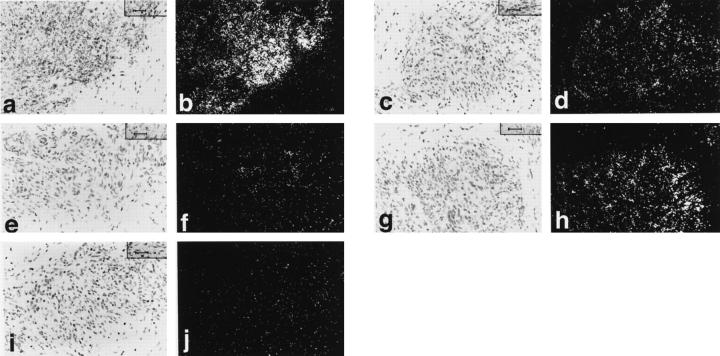
Expression patterns in KS tumor cells were similar in the cases studied (Table 1) ▶ . Strong expression of Tie1 mRNA was seen in eight of the 11 cases studied with low-level labeling in the other three cases (Figure 1, a and b) ▶ . Strong but less intense expression of Tie2 mRNA was seen in nine of the 12 cases and low-level expression in the other three cases (Figure 1, c and d) ▶ . Angiopoietin-2 mRNA was strongly expressed in 11 of the 12 cases studied (Figure 1, g and h) ▶ , and low-level expression was seen in the other case. Expression of angiopoietin-1 mRNA was low level in all 12 cases studied (Figure 1, e and f) ▶ . Angiopoietin-4 mRNA expression was low level in five cases and equivocal in seven (Figure 1, i and j) ▶ . No specific labeling was seen with control sense probe. Expression was also noted in small vessels admixed with the KS including arterioles, venules, and capillaries. With the most strongly expressed mRNAs, ie, Tie1, Tie2, and angiopoietin-2, distinct expression was noted in endothelial cells lining these vessels and expression levels were much stronger than those seen in the control normal skin biopsies. Expression patterns were similar in the three normal skin biopsies studied and showed focal low-level expression of angiopoietin-1, angiopoietin-2, Tie1, and Tie2 associated with the vasculature. Expression of angiopoietin-1 was the most focal and the lowest of the four. Unequivocal expression of angiopoietin-4 could not be detected in normal skin.
Table 1.
mRNA Expression in KS and Angiosarcoma Cells
| No. cases | Strong | Low level | Equivocal | |
|---|---|---|---|---|
| KS tumor cells | ||||
| Ang-1 | 12 | 0 | 12 | 0 |
| Ang-2 | 12 | 11 | 1 | 0 |
| Ang-4 | 12 | 0 | 5 | 7 |
| Tie1 | 11 | 8 | 3 | 0 |
| Tie2 | 12 | 9 | 3 | 0 |
| Angiosarcoma tumor cells | ||||
| Ang-1 | 2 | 0 | 2 | 0 |
| Ang-2 | 2 | 2 | 0 | 0 |
| Ang-4 | 2 | 0 | 0 | 2 |
| Tie1 | 2 | 2 | 0 | 0 |
| Tie2 | 2 | 2 | 0 | 0 |
KS, Kaposi’s sarcoma; ANG, angiopoietin.
Figure 1.
In situ hybridization studies of KS. Bright-field and corresponding dark-field photomicrographs of KS lesions showing strong expression of Tie1 (a and b), Tie2 (c and d) and angiopoietin-2 (g and h) mRNAs. Strong expression of angiopoietin-1 (e and f) and angiopoietin-4 (i and j) mRNAs was not detected. Magnification, ×400.
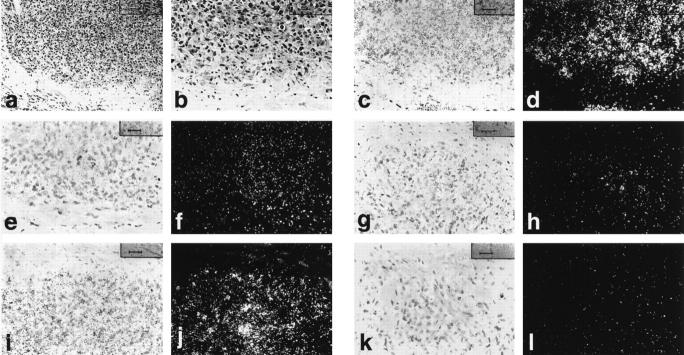
Expression patterns in angiosarcomas (low- and high-power photomicrographs, routine staining; Figure 2, a and b ▶ ) were similar to KS (Table 1) ▶ . Tie1 (Figure 2, c and d) ▶ and angiopoietin-2 (Figure 2, i and j) ▶ were the most strongly expressed mRNAs in malignant endothelial cells with strong but less intense expression of Tie2 (Figure 2, e and f) ▶ , focal low-level expression of angiopoietin-1 (Figure 2, g and h) ▶ , and equivocal expression of angiopoietin-4 (Figure 2, k and l) ▶ .
Figure 2.
Angiosarcoma, hematoxylin and eosin stain at low and high power (a and b). Bright-field and corresponding dark-field photomicrographs of in situ hybridization studies of angiosarcoma showing strong expression of Tie1 (c and d), Tie2 (e and f) and angiopoietin-2 (i and j) mRNAs. Strong expression of angiopoietin-1 (g and h) and angiopoietin-4 (k and l) mRNAs was not detected. Magnification, ×200 (a), ×400 (b–l).
Discussion
In this study, we have shown that there is strong expression of angiopoietin-2, Tie1, and Tie2 in the spindle cells of Kaposi’s sarcoma and the malignant endothelial cells of angiosarcoma. Furthermore, expression is markedly up-regulated compared to the low-level expression detected in biopsies of normal skin. Focal low-level expression of angiopoietin-1 was seen in KS, cutaneous angiosarcoma, and in normal skin biopsies. Focal low-level expression of angiopoietin-4 was detected in a minority of KS biopsies whereas expression in cutaneous angiosarcoma and normal skin was equivocal. These findings suggest that angiopoietins and the Tie receptors may play an important role in the pathobiology of Kaposi’s sarcoma.
Tie1 and Tie2 are receptor tyrosine kinases expressed by endothelial cells. 5-7 Tie1 and Tie2 have been reported to play important roles in embryonic blood vessel development. 3,8,14 Angiopoietin-1, the ligand for Tie2 has also been reported to be important in the development of the embryonic vasculature. 3 Tie has been reported to be up-regulated in arteriovenous malformations in the brain. 15 Tie expression has been reported to be up-regulated in endothelial cells in the angiogenesis associated with wound healing 16 and breast cancer. 17,18 These studies all point to an important role for Tie receptors and angiopoietins in vascular biology and pathology. The results of our study suggest that Tie receptors and angiopoietins may also be playing an important role in the pathobiology of at least two types of malignant vascular tumor, Kaposi’s sarcoma and angiosarcoma.
Angiopoietin-2 and Tie 1 were the most intensely expressed mRNAs studied. The findings that angiopoietin-2 is markedly induced in Kaposi’s sarcoma is particularly interesting in light of recent observations that angiopoietin-2 may be one of the earliest markers induced in the vasculature of other tumors, 11 although the precise signal which leads to this up-regulation remains a mystery. The role of angiopoietin-2 in tumor angiogenesis remains unclear, although it has been proposed that angiopoietin-2 serves to block a stabilizing action of the Tie2 receptor, thus either making the destabilized vessels more susceptible to the angiogenic effects of other agents such as VPF/VEGF, or more vulnerable to regression in the absence of other angiogenic support. 11 This is of particular interest in KS because the expression of VPF/VEGF and its receptor KDR 19-23 have also been reported in KS. Therefore, angiopoietin-2 may be actually playing a stimulatory role in these tumors.
No ligand for Tie1 has been identified, but the intensity of the expression of Tie1 mRNA in these vascular tumors suggests that it could be playing an important role in their pathobiology.
Although the strong expression of angiopoietin-2 and both Tie receptors is intriguing and suggestive of important actions in Kaposi’s sarcoma, understanding the precise roles of these factors depends on further experimentation.
The pathobiology of KS is complex. Several other growth factors and receptors have been reported to be expressed by KS cells in biopsies or in culture including interleukin-1, basic fibroblast growth factor (FGF), granulocyte monocyte colony-stimulating factor (GM-CSF), platelet-derived growth factor (PDGF)-B, and transforming growth factor-β (TGF-β), 24 bFGF, FGF receptor-1, aFGF, FGF-5, and FGF-6 25, PDGFA, PDGFB, and PDGF-A type and PDGF-B type receptors, 26,27 interleukin-6 and interleukin-6 receptors, 28 hepatocyte growth factor and its receptors, 29,30 and thymidine phosphorylase. 31 The human immunodeficiency virus-1 Tat protein has been reported to activate the VPF/VEGF receptor kinase insert domain-containing receptor (KDR). 32 Synergy between bFGF and human immunodeficiency virus-1 Tat protein in the induction of KS in mice has been reported. 33 Clearly much work remains to be done before one can understand the interactions and relative importance of the various growth factors and their receptors in KS.
Manipulation of growth factors and their receptors offers opportunities for therapeutic intervention in KS. Antisense oligonucleotides targeting bFGF have been used to block the growth of KS in nude mice. 34 Inhibition of tumor angiogenesis in certain experimental systems using a soluble Tie 2 receptor has been reported. 35 The manipulation of angiopoietins or their Tie receptors might be of therapeutic benefit to AIDS patients with KS or patients with angiosarcoma.
Acknowledgments
We thank Jo Ann Proper for excellent nursing skills and the patients who participated in this research without whom this work would not be possible.
Footnotes
Address reprint requests to Lawrence F. Brown M.D., Dept. of Pathology, Beth Israel Deaconess Medical Center, 330 Brookine Ave., Boston, MA 02215. E-mail: lbrown@caregroup.harvard.edu.
Supported by United States Public Health Service National Institutes of Health grants CA50453 and HL54465 (to H. F. D.) and grants from the Beth Israel Hospital Pathology Foundation, Inc.
References
- 1.Valenzuela DM, Griffiths J, Rojas J, Aldrich TH, Jones PF, Zhou H, McClain J, Copeland NG, Gilbert DJ, Jenkins NA, Papadopoulos N, Masonpierre PC, Davis S, Yancopoulos GD: Angiopoietins 3 and 4: diverging gene counterparts in mouse and man. PNAS 1999, 96:4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand S, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD: Angiopoietin-2, a natural antagonist for Tie-2 that disrupts in vivo angiogenesis. Science 1997, 277:55-60 [DOI] [PubMed] [Google Scholar]
- 3.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD: Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 1996, 87:1171-1180 [DOI] [PubMed] [Google Scholar]
- 4.Davis S, Aldrich TH, Jones PF, Acheson A, Compton D, Vivek J, Ryan T, Bruno J, Radjiewski C, Masonpierre PC, Yancopoulos GD: Isolation of angiopoietin-1, a ligand for the Tie2 receptor, by secretion-trap expression cloning. Cell 1996, 87:1161-1169 [DOI] [PubMed] [Google Scholar]
- 5.Sato TN, Qin Y, Kozak CA, Audus KL: Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci USA 1993, 90:9355-9358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnurch H, Risau W: Expression of tie-2, a member of a novel family of receptor tyrosine kinases, in the endothelial cell lineage. Development 1993, 119:957-968 [DOI] [PubMed] [Google Scholar]
- 7.Maisonpierre PC, Goldfarb M, Yancopoulos GD, Gao G: Distinct rat genes with related profiles of expression define a TIE receptor tyrosine kinase family. Oncogene 1993, 8:1631-1637 [PubMed] [Google Scholar]
- 8.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y: Distinct roles or the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 1995, 376:70-74 [DOI] [PubMed] [Google Scholar]
- 9.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gerenstein M, Auerbach A, Breitman ML: Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev 1994, 8:1897-1909 [DOI] [PubMed] [Google Scholar]
- 10.Suri C, McLain J, Thurston G, McDonald D, Oldmixon EH, Sato TN, Yancopoulos GD: Increased vascularization in mice overexpressing angiopoietin-1. Science 1998, 282:468-471 [DOI] [PubMed] [Google Scholar]
- 11.Holash J, Masonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Weigand SJ: Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 1999, 284:1994-1998 [DOI] [PubMed] [Google Scholar]
- 12.Enzinger FM, Weiss SW: Soft Tissue Tumors. St. Louis, Mosby, 1995, pp 658–676
- 13.ffrench-Constant C, Van De Water L, Dvorak HF, Hynes RO: Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol 1989, 109:903–914 [DOI] [PMC free article] [PubMed]
- 14.Korhonen J, Polvi A, Partanen J, Alitalo K: The mouse tie receptor tyrosine kinase gene: expression during embryonic angiogenesis. Oncogene 1994, 12:395-403 [PubMed] [Google Scholar]
- 15.Hatva E, Jaaskelainen J, Hirvonen H, Alitalo K, Haltia M: Tie endothelial cell-specific receptor tyrosine kinase is upregulated in the vasculature of arteriovenous malformations. J Neuropathol Exp Neurol 1996, 55:1124-1133 [DOI] [PubMed] [Google Scholar]
- 16.Korhonen J, Partanen J, Armstrong E, Vaahtokari A, Elenius K, Jalkanen M, Alitalo K: Enhanced expression of the Tie receptor-tyrosine kinase in endothelial cells during neovascularization. Blood 1992, 80:2548-2555 [PubMed] [Google Scholar]
- 17.Salven P, Joensuu H, Heikkila P, Matikainen MT, Wasenius VM, Alanko A, Alitalo K: Endothelial Tie growth factor receptor provides antigenic marker for assessment of breast cancer angiogenesis. Br J Cancer 1996, 74:69-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters KG, Coogan A, Berry D, Marks J, Inglehart JD, Kontos CD, Rao P, Sankar S, Trogan E: Expression of Tie2/Tek in breast tumor vasculature provides a new marker for evaluation of tumor angiogenesis. Br J Cancer 1998, 77:51-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weindel K, Marme D, Weich HA: AIDS-associated Kaposi’s sarcoma cells in culture express vascular endothelial growth factor. Biochem Biophys Res Comm 1992, 183:1167-1174 [DOI] [PubMed] [Google Scholar]
- 20.Brown LF, Tognazzi K, Dvorak HF, Harrist TH: Strong expression of kinase insert domain-containing receptor, a vascular permeability factor/vascular endothelial growth factor receptor in AIDS-associated Kaposi’s sarcoma and cutaneous angiosarcoma. Am J Pathol 1996, 148:1065-1074 [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura S, Murakami-Mori K, Rao N, Weich HA, Rajeev B: Vascular endothelial growth factor is a potent angiogenic factor in AIDS-associated Kaposi’s sarcoma-derived spindle cells. J Immunol 1997, 158:4992-5001 [PubMed] [Google Scholar]
- 22.Masood R, Cai J, Zheng T, Smith DL, Naidu Y, Gil PS: Vascular endothelial growth factor/vascular permeability factor is an autocrine growth factor for AIDS-Kaposi’s sarcoma. Proc Natl Acad Sci USA 1997, 94:979-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornali E, Zeitz C, Benelli R, Weniger W, Masiello L, Brier G, Tschachler E, Albini A, Sturzl M: Vascular endothelial growth factor regulates angiogenesis and vascular permeability in Kaposi’s sarcoma. Am J Pathol 1996, 149:1851-1869 [PMC free article] [PubMed] [Google Scholar]
- 24.Ensoli B, Nakamura S, Salahuddin SZ, Biberfeld P, Larsson L, Beaver B, Wong-Staal F, Gallo RC: AIDS-Kaposi’s sarcoma-derived cells express cytokines with autocrine and paracrine growth effects. Science 1989, 243:223-226 [DOI] [PubMed] [Google Scholar]
- 25.Li JJ, Huang YQ, Moscatelli D, Nicolaides A, Zhang WC, Freidman-Klein AE: Expression of fibroblast growth factors and their receptors in acquired immunodeficiency syndrome-associated Kaposi’s sarcoma tissue and derived cells. Cancer 1993, 72:2253-2259 [DOI] [PubMed] [Google Scholar]
- 26.Werner S, Hofschneider PH, Heldin CH, Ostman A, Roth WK: Cultured Kaposi’s sarcoma-derived cells express functional PDGF A-type and B-type receptors. Exp Cell Res 1990, 187:98-103 [DOI] [PubMed] [Google Scholar]
- 27.Sturzl M, Roth WK, Brockmeyer NH, Zeitz C, Speiser B, Hofschneider PH: Expression of platelet-derived growth factor and its receptor in AIDS-related Kaposi’s sarcoma in vivo suggests paracrine and autocrine mechanisms of tumor maintenance. Proc Natl Acad Sci USA 1992, 89:7046-7050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miles SA, Rezai AR, Salazar-Gonzales JF, Vander Meyden M, Stevens RH, Logan DM, Mitsuyasu RT, Taga T, Hirano T, Kishimoto T, Martinez-Maza O: AIDS Kaposi sarcoma-derived cells produce and respond to interleukin-6. Proc Natl Acad Sci USA 1990, 87:4068-4072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maier J, Mariotti M, Comoglio PM, Soria MR: Interleukin 1 induces an autocrine loop hepatocyte growth factor/c-Met in murine Kaposi-like spindle cells. Oncogene 1996, 13:1009-1015 [PubMed] [Google Scholar]
- 30.Maier JA, Mariotti M, Albini A, Comi P, Comogilio PM, Soria MR: Over-expression of hepatocyte growth factor in human Kaposi’s sarcoma. Int J Cancer 1996, 65:168-172 [DOI] [PubMed] [Google Scholar]
- 31.Dada MA, Boshoff CH, Comley MA, Turley H, Schneider JW, Chetty R, Gatter KC: Thymidine phosphorylase expression in Kaposi’s sarcoma. J Clin Pathol 1996, 49:400-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albini A, Soldi R, Giunciuglio D, Giraudo E, Benelli R, Primo L, Noonan D, Salio M, Camussi G, Rockl W, Bussolino F: The angiogenesis induced by HIV-1 Tat protein is mediated by the flk-1/KDR receptor on vascular endothelial cells. Nat Med 1997, 2:1371-1375 [DOI] [PubMed] [Google Scholar]
- 33.Ensoli B, Gendelman R, Markham P, Fiorelli V, Colombini S, Raffeld M, Cafaro A, Chang HK, Brady JN, Gallo RC: Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi’s sarcoma. Nature 1994, 371:674-680 [DOI] [PubMed] [Google Scholar]
- 34.Ensoli B, Markham P, Kao V, Barillari G, Fiorelli V, Gendelman R, Raffeld M, Zon G, Gallo RC: Block of AIDS-associated Kaposi’s sarcoma cell growth, angiogenesis, and lesion formation in nude mice by antisense oligonucleotide targeting basic fibroblast growth factor. A novel strategy for the therapy of KS. J Clin Invest 1994, 94:1736-1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin P, Polverini P, Dewhirst M, Shan S, Rao PS, Peters K: Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie2 in pathologic vascular growth. J Clin Invest 1997, 100:2072-2078 [DOI] [PMC free article] [PubMed] [Google Scholar]