Abstract
In the present study we have used a novel, comprehensive mRNA profiling technique (GeneCalling) for determining differential gene expression profiles of human endothelial cells undergoing differentiation into tubelike structures. One hundred fifteen cDNA fragments were identified and shown to represent 90 distinct genes. Although some of the genes identified have previously been implicated in angiogenesis, potential roles for many new genes, including OX-40, white protein homolog, KIAA0188, a homolog of angiopoietin-2, ADAMTS-4 (aggrecanase-1), and stanniocalcin were revealed. Support for the biological significance was confirmed by the abrogation of the changes in the expression of angiogenesis inhibitors and in situ hybridization studies. This study has significantly extends the molecular fingerprint of the changes in gene expression that occur during endothelial differentiation and provides new insights into the potential role of a number of new molecules in angiogenesis.
Angiogenesis, defined as the process whereby new blood vessels are formed from previously existing ones, plays an important role in the development and progression of a number of disease states, including various cancers, diabetic retinopathy, macular degeneration, psoriasis, and rheumatoid arthritis. During the last 10 years there have been many advances in our understanding of the biology of and the molecules that are involved in angiogenesis. A number of different growth factors, including vascular endothelial growth factor (VEGF), fibroblast growth factors, platelet-derived growth factor, hepatocyte growth factor, angiopoietins 1 and 2, as well as various endothelial surface molecules, such as CD31 (PECAM), CD144 (VE-Cadherin), and αvβ3 integrins, have been implicated in various steps of angiogenesis. These advances have enabled the development of new therapeutic strategies for inhibiting angiogenesis (eg, to inhibit tumor growth) or promoting angiogenesis (coronary and peripheral ischemia, wound healing).
Understanding the molecular events that direct angiogenesis and the order in which they occur and identifying new pathways that are required for this process are of fundamental importance for all researchers who study angiogenesis. The present study was undertaken to identify the alterations in gene expression that occur in an in vitro model of angiogenesis. In this model, endothelial cells are suspended in a three-dimensional gel composed of type I collagen and incubated with a mixture of stimuli (phorbol myristate acetate (PMA), basic fibroblast growth factor (bFGF), and vascular endothelial cell growth factor (VEGF)). Previous studies by our laboratory demonstrated that this combination of stimuli resulted in the optimal formation of a three-dimensional tubular network of endothelial cells with interconnecting lumenal structures. 1 In this model, endothelial differentiation into tubelike structures is completely blocked by inhibitors of new mRNA (actinomycin D) or protein synthesis (cycloheximide). Furthermore, the cells progress through this differentiation process in a coordinated and synchronized manner, thus optimizing the profile of gene expression.
The goal of the present study was to identify a molecular fingerprint or transcriptional profile of endothelial differentiation into tubelike structures, using amplification and an imaging approach called GeneCalling. 2 This method was previously shown to provide a comprehensive sampling of cDNA populations in conjunction with the sensitive detection of quantitative differences in mRNA abundance for both known and novel genes. 2 We describe the identification of 115 differentially expressed cDNA fragments, which corresponded to 90 previously identified genes. The identification and differential expression of these genes was confirmed by a second independent method employing real-time quantitative polymerase chain reaction (PCR). Although some of the cDNA fragments identified were genes previously known to play some role in the process of angiogenesis, many other differentially expressed genes were unexpected and suggest possible roles for these additional genes in endothelial differentiation and vessel assembly.
Materials and Methods
Materials
Human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics (San Diego, CA) and maintained in endothelial growth medium (EGM) media supplemented to a final concentration of 10% fetal bovine serum. Type I rat tail collagen was from Upstate Biotechnology (Lake Success, NY), and recombinant bFGF was purchased from Collaborative Biomedical Products (Becton Dickinson Labware, Bedford, MA). Recombinant VEGF was from Genentech (South San Francisco, CA). Medium 199 (10×) (M199, M0650), PMA, ITS (insulin, transferrin, and selenium-A), trypsin, actinomycin D, and cycloheximide were from Gibco-BRL (Gaithersburg, MD).
Formation of Three-Dimensional Collagen Gels
Collagen gels were formed by mixing together ice-cold gelation solution (10× M199, H2O, 0.53 mol/L NaHCO3, 200 mmol/L l-glutamine, type I collagen, 0.1 mol/L NaOH, 100:27.7:50:10:750:62.5 v/v) and cells in 1× basal medium (see below) at a concentration of 3 × 10 6 cells/ml at a ratio of four volumes gelation solution to one volume of cells. The gels were allowed to form by incubation in a CO2-free incubator at 37°C for 30 minutes to 1 hour. The gels were then overlaid with 1× basal medium consisting of M199 supplemented with 1% FBS, 1× ITS, 2 mmol/L l-glutamine, 50 μg/ml ascorbic acid, 26.5 mmol/L NaHCO3, 100 U/ml penicillin, and 100 U/ml streptomycin. In the tube-forming experiments, the culture medium was supplemented with 80 nmol/L PMA, 40-ng/ml bFGF, and 40 ng/ml VEGF.
mRNA Isolation and cDNA Synthesis
Medium was aspirated from the surface of the collagen gels, and the gels were scraped into a 50-ml polypropylene tube containing three volumes of Tri-Reagent-LS (Molecular Research Center, Cincinnati, OH). The tubes were incubated for 10 minutes at 23°C with intermittent gentle agitation. The tubes were stored at −80°C until all experimental samples had been collected. The tubes were then thawed at room temperature, and the RNA was extracted following the manufacturer’s specifications. The RNA pellets were resuspended in diethyl-pyrolidine-carbonate-treated water, and the RNA content was quantified spectroscopically at 260 nm. RNA samples were stored at −20°C. Samples used for GeneCalling analysis were shipped on dry ice to CuraGen (New Haven, CT). Samples from time points of 4, 24, and 48 hours were used for the GeneCalling analysis, and in separate experiments, samples from additional time points of 30 minutes and 2, 4, 8, 16, 24, 38, and 46.5 hours were prepared for TaqMan confirmation. For the quantitative expression analysis, contaminating DNA was removed by treatment of the isolated RNA with DNase I (Promega, Madison, WI). PolyA+ RNA was prepared by fractionation of total RNA with an mRNA purification kit that uses the biotinylated oligo-dT-streptavidin magnetic bead method (MPG, Lincoln Park, NJ), followed by cDNA synthesis by reverse transcription of oligo-dT-primed mRNA (Superscript II; Life Technologies) and second-strand synthesis. Terminal phosphate removal is achieved by treatment with arctic shrimp alkaline phosphatase (Amersham Life Sciences, Piscataway, NJ), followed by purification of cDNA by phenol-chloroform extraction. Yield of cDNA was quantitated by fluorometry using PicoGreen dye (Molecular Probes, Eugene, OR). Double-stranded DNA was digested using pairs of restriction enzymes with 6-bp recognition sites. More than 48 enzyme pairs were used and were chosen such that a representative coverage of most of the possible sequences in a given DNA sample was achieved. 2 PCR amplification using specific linkers was carried out as described previously. 2 The final DNA products were denatured by heating to 96°C and electrophoresed on ultrathin polyacrylamide gels under denaturing conditions in 6 mol/L urea. PCR products were visualized by the presence of 6-carboxy fluorescein (FAM) label on the product, using a multicolor laser excitation (Niagara; CuraGen, New Haven CT) imaging system.
Data Interpretation
The data obtained from Niagara gels were queried (ie, “GeneCalled”) against public and proprietary databases. 2 GeneCalling is the process that takes the restriction enzyme pair recognition site information and the cDNA fragment size determined from the migration of the labeled fragment on Niagara gels and uses that information (the size of the fragment and the relative position of the terminal sequences defined by the restriction enzyme pairs) to search public and proprietary databases for likely gene matches, using statistical and mathematical criteria. A GeneCall is defined as the probability of a cDNA fragment belonging to a known gene. 2 The cDNA fragment data were compiled as a list of likely genes to which that cDNA fragment might belong. If a provisional identification of a cDNA fragment could not be obtained by querying databases, the cDNA fragment was designated as belonging to a putative novel gene.
Confirmation of Gene Calls
GeneCalls were confirmed in a competitive PCR reaction, “GeneCall poisoning,” in which the known sequence of the likely gene of interest is used to design poisoning primers as previously described. 2 Ablation of the cDNA fragment of interest confirmed that the cDNA fragment belonged to the gene for which the specific poisoning primer was designed.
Novel cDNA Fragments
If no GeneCall was obtained for a cDNA fragment, the cDNA fragment was eluted and subcloned into Escherichia coli with the standard TA-cloning vector (Invitrogen, Palo Alto, CA). The cDNA fragment was then sequenced, and the resulting sequence was used to design poisoning primers for confirmation as described above.
Validation and Confirmation of Gene Expression by Quantitative Reverse Transcriptase-Polymerase Chain Reaction (TaqMan)
To confirm the expression data from GeneCalling by an independent technique, gene-specific PCR oligonucleotide primer pairs and an oligonucleotide probe labeled with a reporter fluorescent dye at the 5′ end and quencher fluorescent dye at the 3′ end were designed using Oligo 4.0 software (National Bioscience, Plymouth, MN). Table 1 ▶ provides the sequences for the primers and probes used in this study. Total RNA (50 ng) was added to a 50 μl reverse transcriptase-polymerase chain reaction (RT-PCR) reaction mixture according to the manufacturer’s protocol (Roche Molecular Systems, Branchburg, NJ). The thermal cycling conditions included one cycle at 48°C for 30 minutes, one cycle at 95°C for 10 minutes, 40 cycles at 95°C for 15 s, annealing at 60°C for 1 minute, and a final hold at 25°C for 2 minutes. Standard curves for the expression of each gene were generated by serial dilution of a standard preparation of total RNA isolated from quiescent HUVECs grown in monolayer culture. Data are expressed as the fold induction normalized to the same gene from quiescent HUVEC RNA.
Table 1.
Taqman Primer and Probes Sets
| Gene name | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| Hormones/growth factors | |||
| Placental growth factor (PLGF) | GACGTTCTCTCAGCACGTTCG | CACCTTTCCGGCTTCATCTTC | CGAATGCCGGCCTCTGCGG |
| Stanniocalcin precursor | CGAGTGGCGGCTCAAAA | CCGCAGCCGACCTGTAGA | TCAGCTGAAGTGGTTCGTTGCCTCAA |
| Fibroblast growth factor 16 (FGF-16) | CCTTAGCTGACTCCCCAGGTT | CTGCAGCTTCCCCTCGATT | CCTGAACGAGCGCCTGGGCC |
| Angiopoeitin-2 Hlog (ai079861) | GGCCTGCAGCGGGTAGTA | GGCAGAAGCTTAAGAAGGGAATC | CGGCCCCGCCAGGTCTTCC |
| Connective tissue growth factor | TGCACCGCCAAAGATGGT | GGACTCTCCGCTGCGGTAC | CTCCCTGCATCTTCGGTGGTACGG |
| Cell cycle/apoptosis | |||
| Human replication factor C | TTGCTTGTAATGCTTCGGATAAGA | TGTGTACCGGAGGACTGCAC | CATCGAGCCCATTCAGTCCCGC |
| ALG-2 interacting protein | TGAGCAATGGATCTGTTAACCAA | TCCCGTGTCAGTACAGCAGTCT | TCGGCTTTCCTCTGATTATAGGCAGCCA |
| p120 | CCTCCGGACCAATACCTTGA | CCCCACGATTGATTAGAGCCT | CCCGACGCCGAGACCTTGCA |
| CDEBP (amyloid precursor-like protein 2) | GATGCCTCGTTGGTACTTCGA | CGCCGCAGCCACCATA | AAGCGCACGCACTTTCCCTTGG |
| BCL-2 related A1 protein | CAGCTCAAGACTTTGCTCTCCA | AGTCCTGAGCCAGCCTGTAAAT | ATCCAAATTCACAGTCTGTCATCTTCTGCCTG |
| CDC28/CDC2 associate protein CLK | TTGTTGCCTGGGCTGGA | TGAAACGAGAGCGCGAAGA | CGGGCGCAGCAGACAAAACCA |
| Human binding protein | GGCTACATCGAGGCTCTTGC | GGCTACATCGAGGCTCTTGC | GGCTACATCGAGGCTCTTGC |
| Polo-like kinase | GGATCACACCAAGCTCATCTTG | CCCGCTTCTCGTCGATGT | CCCACTGATGGCAGCCGTGACC |
| p53 | GAGGTGCGTGTTTGTGCCT | TTCTTGCGGAGATTCTCTTCCT | TGCGCCGGTCTCTCCCAGGA |
| DNA binding proteins/transcription factors/histones/repair | |||
| OS-9 | AAGGCCTCCAAGCAGCATC | GGTCGCACTTGGACCCATT | TCTTAAACGCTACCACAGCCAGACCTATGG |
| Mel-18 homolog | GCGCTTCCTGCGATGC | TGCGGAGAAACTTGGCAAG | CAGCAGCCATGACCGTCATGCA |
| RNA synthesis/ribosomes | |||
| Clone 23689 | CTCAGCCCTCCGAGACCA | CTCCCGGATAATCTTGAGCACT | CGTGGGCAACATGGTGCGGA |
| Ribosomal protein L37 | TGTCCTGGCTGGACGCTACT | TGAGGTGCCATCATCAATGTTC | TCACGATGACAGCTTTGCGTCCG |
| Chemokines | |||
| Interleukin-8 (IL-8) | AAGGAACCATCTCACTGTGTGTAAAC | ATCAGGAAGGCTGCCAAGAG | TGACTTCCAAGCTGGCCGTGGC |
| GRO-1α (MGSA) | TGAGGAGCCTGCAACATGC | CATTGGCCATTTGCTTGGA | CCGCCAGCCTCTATCACAGTGGCT |
| Tyrosine kinase receptors | |||
| axl | GCATGAAGGAATTTGACCATCC | TCTCTCGTTCAGAACCCTGGA | CAGACACCGATGAGCCTCATGACGTT |
| Epithelial cell tyrosine kinase (ECK) | GCCTGTTCACCAAGATTGACAC | GCCTCGAAGTCGCTGCTG | TTGCGCCCGATGAGATCACCG |
| Serine/threonine kinases | |||
| Serum-inducible kinase | GAGGATCGTCCCAGTTTGGA | GAAGACAGTCTGTCCGGAGTGAA | CATCATTCGACATGACTTTTTTTTGCAGGG |
| Branched chain alpha keto acid | CGGTTCCCCTTCATCCCTAT | TGTGGCTCTCATGGCATTCTT | CCACTGGACTACATCCTGCCGGAGCT |
| Dehydrogenase kinase | |||
| Thymidylate kinase | AAGCASTCGAAGCTGTCCATGA | TCTCTGTGGCAGTGCGGAT | TCCGCGTGCTCTCTGAGGACGC |
| Other receptors/integral membrane glycoproteins | |||
| OX40 | CCAACTCTGCACCGTTCTAGG | GGTATGCATGGCATACGTAAGC | CCGATGGCTGCCTCCGGCT |
| CXCR-4 | CGCTACCTGGCCATCGTC | CATAGACCACCTTTTCAGCCAAC | CGCCACCAACAGTCAGAGGCCA |
| Podocalyxin-like protein | GGGCATGGTGAGGTTTCATCT | TTTACGCCCAGAACGATGG | CCATGGCGAAAGTTCAACATTCCACA |
| Alpha-2 integrin | TCTGAGACTGCCAAGGTCTTCA | CAGCTGGTATTTGTCGGACATC | AGGACTAGATCAGAAATGCAAAGTCCATCCTCA |
| JuSo MUC18 glycoprotein | GAACACAGTGGGCGCTATGA | CCTGTGGTTCACTCAGCAGC | CAGGCCTGGAACTTGGACACCATGATAT |
| MHC class 1 antigen | GCGCTCCGCTACTACAACCA | CGTCGCAGCCAAACATCA | AGGCCGGTTCTCACACCCTCCAG |
| gp130 | ATCCGCGCAAGATGTTGAC | ACCTGTAGATTCAGTGGTGAGGAAA | ACAAGGCTTGCACTACCCAAGTCTGCA |
| T-cell receptor Beta 2 | GAGGGTCTCGGCCACCTT | AGAACTGGACTTGACAGCGGAA | TGGCAGAACCCCCGCAACCA |
| Protein zero-related protein | TGTGTCATATCAATTTCTGGATTCATAA | TTGATCCAACTGTGTCCAGAATG | TGACTTCGGCATTTATCCTTTGCTAATCTTGCT |
| Proteases/protease inhibitors | |||
| Tissue factor pathway inhibitor-2 (TFPI-2) | CGATGCTTGCTGGAGGATAGA | ACACTGGTCGTCCACACTCACT | AAAGTTCCCAAAGTTTGCCGGCTGC |
| Aggrecanase (ADAMTS4; KIAA0688) | ACTGGTGGTGGCAGATGACA | TCACTGTTAGCAGGTAGCGCTTT | ATGGCCGCATTCCACGGTGC |
| Matrix metalloproteinase-9 (MMP-9) | CCCGGAGTGAGTTGAACCA | CCTAGTCCTCAGGGCACTGC | TGGACCAAGTGGGCTACGTGACCTATG |
| Matrix metalloproteinase-1 (MMP-1) | CATGAAAGGTGGACCAACAATTT | CCAAGAGAATGGCCGAGTTC | CAGAGTACAACTTACATCGTGTTGCGGCTCA |
Treatment with 15d-PGJ2
mRNA was harvested from endothelial cells incubated 4 and 24 hours in the absence (control) or presence of 10 μmol/L of the PPARγ ligand 15-deoxy-Δ2,14–15-prostaglandin J2 (15d-PGJ2). In both groups the cells were incubated with the mixture of growth stimuli (ie, PMA, VEGF, and bFGF), and the cells were incorporated in the collagen gels as described above.
In Situ Hybridization of Tissue Specimens
Formalin-fixed, paraffin-embedded human tissues were investigated for in situ mRNA expression. Tissues included first-trimester (14–15-week) placenta, adult adrenal cortex, aorta, muscular artery with atherosclerosis, brain, gall bladder, heart, pancreas, prostate, stomach, eye with age-related macular degeneration (AMD), inflamed appendix, pulmonary adenocarcinoma, ductal mammary adenocarcinoma, kidney with renal cell carcinoma, hepatocellular carcinoma, squamous cell carcinoma, osteosarcoma, and chondrosarcoma. In vitro transcription and 33P labeling of sense and antisense riboprobes were performed as described previously. 3 Briefly, stanniocalcin, osteonidogen, podocalyxin, and ADAMTS-4 sequences were PCR-amplified from plasmid DNA, using gene-specific primers that encoded T3 or T7 RNA polymerase initiation sites. Sense and antisense riboprobes were prepared by in vitro transcription from the PCR-amplified templates and diluted in hybridization buffer to a specific activity of 1 × 10 6 cpm/ml. Tissue sections 5 μm thick were deparaffinized, deproteinated in 4 μg/ml of proteinase K for 30 minutes at 37°C, hybridized at 55°C overnight, then washed at high stringency (55°C in 0.1× standard saline citrate for 2 hours). Glass slides were dipped in NBT2 nuclear track emulsion (Eastman Kodak), exposed in sealed plastic slide boxes containing desiccant for 4 weeks at 4°C, developed, and counterstained with hematoxylin and eosin.
Results
cDNA Fragment Selection and Identification
As reported previously, 1 incubation of endothelial cells in 3D gels in the absence of the growth factors resulted in rapid induction of apoptosis. Therefore, no comparison was made of mRNA from cells in 3D gels in the absence of growth factors. Instead we evaluated temporal changes in gene expression in the 3D gel environment in the presence of PMA, VEGF, and bFGF, by comparison of the RNA harvested at 4, 24, and 48 hours. A summary of the differences observed can be found in Table 2 ▶ . The differentially expressed cDNA fragments in the 24-hour versus the 4-hour data set were examined in more detail. As shown in Table 3 ▶ , the identities of 115 cDNA fragments were determined by oligonucleotide poisoning or cloning of the gene fragments, resulting in the identification of 90 distinct genes. In addition (not shown), 80 cDNA fragments were identified as totally novel or as corresponding to expressed sequence tags (ESTs) of unknown function. Full-length cloning of these genes is currently under way.
Table 2.
Summary of Statistics of the Gene Fragments Found to Be Differentially Modulated in GeneCalling at a Given Fold Difference Threshold
| Comparison | Differentially expressed fragments (%)* | |
|---|---|---|
| Difference threshold | ||
| ±2-fold | ±4-fold† | |
| 24 hrs vs. 4 hrs | 1343 (4.8) | 393 (1.4) |
| 48 hrs vs. 24 hrs | 1291 (4.7) | 367 (1.4) |
| 48 hrs vs. 4 hrs | 368 (1.3) | 27 (0.1) |
*Value is the percentage of gene fragments observed to be differentially expressed in the comparison.
†Arbitrary higher cut-off.
Table 3.
Genes Identified by GeneCalling
| Confirmed gene* | Accesion no.† | GeneCalling ratio‡ | TaqMan ratio§ |
|---|---|---|---|
| Hormones/growth factors | |||
| Placental growth factor | X54936 | 6 | 5 |
| Transforming growth factor beta | X02812 | 11 | 2 |
| Stanniocalcin precursor | U25997 | 14 | 8 |
| FGF-16 | AB009391 | 4 | 1 |
| Angiopoetin-2 Hlog | AI079861 | 0.1 | 0.1 |
| Connective tissue growth factor | U14750 | 0.1 | 0.2 |
| Cell cycle/apoptosis | |||
| Human replication factor C | M87338 | 5 | 23 |
| ALG-2 interacting protein | sim to AJ005073 | 5 | 2 |
| p120 proliferation associated antigen | X55504 | 4 | 1 |
| CDEBP (amyloid precursor-like protein 2) | Z22572 | 4 | 1 |
| Bcl-2 related protein A1 | L19597 | 4 | 1 |
| ABC50 ATP binding cassette protein | AF027302 | 2 | ND |
| cdc28/cdc2 associated protein CLK | L29219 | <0.1 | 0.3 |
| Polo-like kinase | U01038 | <0.1 | <0.1 |
| p53 | AA143745 | 0.1 | 1 |
| CDK4 inhibitor (p16-INK4) | L27211 | 5 | ND |
| DNA binding protein transcription factors histones/repair | |||
| KIAA0192/TRAP230 | AF117755 | 3 | 1 |
| Mel-18 hlog | AA477595 | 4 | ND |
| OS-9 | AB002806 | 2 | 1 |
| DNA nucleotide exotransferase | AA744855 | 2 | ND |
| Histone HUMGS00579 | M37583 | 0.2 | 0.3 |
| oriP binding protein (OBP-2) | L29606 | <0.1 | ND |
| RNA synthesis/ribosomes | |||
| Clone 23689 | AF035280 | 8 | 8 |
| Ribosomal protein L37a | L22154 | 4 | ND |
| Chemokines | |||
| Interleukin-8 | M28130 | 0.3 | <0.1 |
| GRO1α (melanoma growth-stimulating activity) | X57019 | <0.1 | <0.1 |
| Tyrosine kinase receptors | |||
| eck | NM_004431 | 3 | 3 |
| axl | P30530 | 0.2 | <0.1 |
| Serine/threonine kinases | |||
| Serum inducible kinase | O60679 | 7 | 2 |
| Branched chain alpha keto acid dehydrogenase kinase | AF026548 | 4 | 1 |
| Thymidylate kinase | L16991 | 0.5 | 0.3 |
| Other receptors/integral membrane glycoproteins | |||
| OX40 | S76792 | 18 | 18 |
| CXCR4 | AF052572 | 13 | 6 |
| Alpha 2 integrin | X17033 | 13 | 2 |
| Podocalyxin-like protein | U97519 | 12 | 2 |
| CD82 | D28137 | 12 | 4 |
| MUC18 | M29277 | 5 | 1 |
| MHC class I | M83191 | 3 | 3 |
| D14343 | |||
| M20022 | |||
| gp130 | M57230 | 3 | 1 |
| T-cell receptor beta 2 | X01411 | 3 | 2 |
| MHC class II | L18885 | 2 | ND |
| Protein zero related protein | R60084 | 6 | 6 |
| Proteases/protease inhibitors | |||
| Tissue factor pathway inhibitor-2 | L27624 | 9 | 7 |
| Aggrecanase (ADAMTS4) | NM_005099 | 18 | 2 |
| Type IV collagenase (MMP9) | J05070 | 6 | 177 |
*Gene identified and confirmed by GeneCalling.
†GeneBank accession number of gene from which cDNA fragment was identified.
‡Refers to the median value of ratios for 24 hours/4 hours for the different cDNA fragments identified for a specific gene.
§TaqMan Ratio refers to the ratio of mRNA at 24 hours versus 4 hours as determined by Taqman (see Materials and Methods).
ND, not determined.
Confirmation of cDNA Fragment Identification and Expression
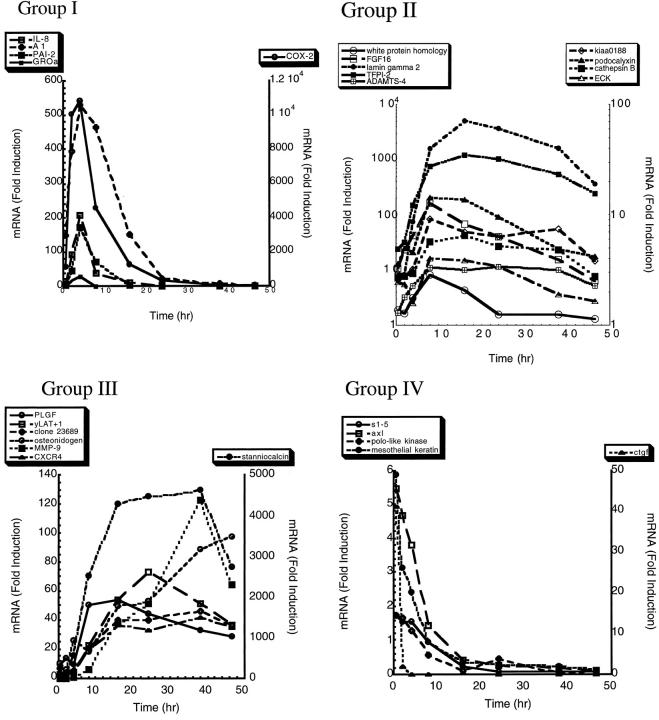
TaqMan probes were prepared to confirm by an independent method the identification of 67 of the genes identified by GeneCalling. As shown in Table 3 ▶ , there was a reasonable agreement in the direction of the fold induction as predicted by GeneCalling and as analyzed by TaqMan. Disagreement between the GeneCalling and TaqMan results are likely due to small differences in the temporal sequence of events in the two separate experiments (ie, the RNA harvested for the GeneCalling experiment versus the RNA harvested for later TaqMan analysis), which could readily account for the discrepancies in the fold induction. A more complete time course analysis of 26 of the genes is depicted in Figure 1, A–D ▶ .
Figure 1.
TaqMan analysis of the changes in gene expression of 26 genes identified by the GeneCalling analysis over the time period of 30 minutes to 46.5 hours. The changes in gene expression were grouped into four patterns, shown as Group 1 (rapid elevation in mRNA, peaking at 2–4 hours, then declining to baseline levels by 24 hours), Group II (more delayed elevation in mRNA, peaking at 8–12 hours, then declining to near-baseline levels by 46.5 hours), Group III (mRNA levels rising somewhat later than Group I or II, peaking at 12–46.5 hours, and remaining markedly above baseline levels at 46.5 hours), and Group IV (mRNA levels declining from the initial value observed at 30 minutes, and by 16–24 hours mRNA levels were below those obtained from quiescent HUVECs (see Materials and Methods)).
Genomic Response of Endothelial Cells in a Tube-Forming Environment
According to the classification schema used, the genes identified fell into most of the major role categories, including cell division, cell signaling, cell adhesion, hormone/growth factors, receptors, cytoskeleton, extracellular matrix, protein turnover, protein modification, and metabolism (Table 3) ▶ . There was no apparent bias in the identification of any given class of gene.
The mRNA changes were clustered based on four basic patterns of expression. Group I, Early Transient (Figure 1A) ▶ , which included interleukin-8 (IL-8), binding protein A1, plasminogen activator inhibitor-2 (PAI-2), growth-related oncogene α (GRO-α), and cyclooxygenase-2 (COX-2), was characterized by mRNA levels that were rapidly and highly induced then declined to the initial levels within 24 hours. Peak mRNA levels were observed at 2–4 hours. Not shown in Figure 1A ▶ because of the small magnitude of fold induction, is an EST with homology to the fibrinogen domain of angiopoeitin-2 (AI79861). The message levels for this EST increased by twofold by 4 hours then returned to baseline levels between 8 and 12 hours. Group II, Delayed Transient, which included the genes white protein homolog, fibroblast growth factor-16 (FGF16), KIAA0188, ADAMTS-4 (aggrecanase-1), tissue factor pathway inhibitor-2 (TFPI-2), podocalyxin-like protein, cathepsin B, and epithelial tyrosine kinase (ECK), was characterized by mRNA levels that peaked somewhat later than those of Group I (8–12 hours), then fell back to near-baseline levels by 46.5 hours. Group III, Stable Induction (Figure 1C) ▶ , was characterized by genes whose mRNA levels rise somewhat later than those of Group I or Group II, peaked at 12–46.5 hours, and remained markedly above baseline levels, even at 46.5 hours. This group included placental growth factor, yLAT1, clone 23689, osteonidogen, matrix metalloproteinase 9 (MMP-9), CXC chemokine receptor 4 (CXCR4), and stanniocalcin precursor (STC). Group IV, Rapid Repression (Figure 1D) ▶ , was quite different from Groups I–III. mRNA levels for genes in Group IV declined from the initial value observed at 30 minutes and, by 16–24 hours, were below the mRNA levels observed in the mRNA controls obtained from quiescent HUVEcs (see Materials and Methods). Genes in Group IV included extracellular protein S1–5 (S1–5), axl, polo-like kinase, and mesothelial keratin.
PPARγ Modulation of Endothelial Gene Expression
Treatment of endothelial cells with 10 μmol/L 15-d-PGJ2 completely blocks endothelial tube formation in response to bFGF, VEGF, and PMA. 4 As shown in Table 4 ▶ , 15d-PGJ2 treatment reduced or, in some cases, abrogated the fold increase (24 versus 4 hours) of PLGF, clone 23689, STC, OX-40, TFPI-2, MMP-9, KIAA0188, +yLAT1, laminin γ2, and PLA2γ. In addition, 15d-PGJ2 reduced the fold decrease (24 versus 4 hours) of IL-8, axl, PAI-2, white protein homolog, and keratin K-7 observed during tube formation. The ratios of other mRNAs (A1, FGF16, eck, podocalyxin, and osteonidogen, for example) were not markedly affected by 15d-PGJ2.
Table 4.
Response of Modulated Genes to PPARγ Ligand 15d-PDJ2
| Confirmed gene* | Accesion no.† | TaqMan ratio‡ | TaqMan ratio in 15d-PDJ2-treated cells§ |
|---|---|---|---|
| Hormones/growth factors | |||
| Placental growth factor | X54936 | 5 | 0.4 |
| FGF-16 | AB009391 | 1 | 1 |
| Stanniocalcin precursor | U25997 | 8 | 3 |
| Cell cycle/apoptosis | |||
| Bcl-2 related protein A1 | L19597 | 1 | 1 |
| Polo-like kinase | U01038 | <0.1 | 15.5 |
| RNA synthesis/ribosomes | |||
| Clone 23689 | AF035280 | 8 | 0.3 |
| Chemokines | |||
| Interleukin-8 | M28130 | <0.1 | 14.5 |
| Tyrosine kinase receptors | |||
| eck | NM_004431.1 | 3 | 2.3 |
| axl | P30530 | <0.1 | 15 |
| Other receptors/integral membrane glycoproteins | |||
| OX40 | S76792 | 18 | 0.7 |
| Podocalyxin-like protein | U97519 | 2 | 1 |
| Proteases/protease inhibitors | |||
| Tissue factor pathway inhibitor-2 | L27624 | 7 | 1.3 |
| Type IV collagenase (MMP9) | J05070 | 177 | <0.1 |
| Plasminogen activator inhibitor-2 | M31551 | <0.1 | 10.2 |
| KIAA0188 | d80010 | 2 | 0.5 |
| Transporters/channels | |||
| Glycoprotein-associated amino acid transporter LAT1 | AJ130718 | 20 | 0.2 |
| White protein Hlog | AF038175 | 0.5 | 2 |
| Intermediate filaments | |||
| Keratin K7 | X03212 | 0.1 | 0.3 |
| Extracellular matrix | |||
| Laminin gamma 2 chain | U31201 | 49 | 4.9 |
| Nidogen-2 (Osteonidogen) | D86425 | 2 | 1 |
| Lipids and lipid turnover | |||
| Phospholipase A2 gamma | AF058921 | 21 | 0.8 |
A subset of genes identified as modulated in experiment were tested for response to PPARγ ligand 15d-PDJ2. Data are expressed as the ratio of expression observed at 24 hours/4 hours in collagen gel.
*Gene identified and confirmed by GeneCalling.
†GeneBank accession number of gene from which cDNA fragment was identified.
‡TaqMan ratio refers to the ratio of mRNA at 24 hours versus 4 hours.
§The ratio of mRNA at 24 hours versus 4 hours in the 15d-PDJ2-treated groups.
In Situ Hybridization Analyses
As an additional test of the biological relevance of the genes identified, we evaluated the in situ expression of four of the genes identified in this study, ie, STC, podocalyxin, osteonidogen, and ADAMTS-4, by examining their expression in a number of different tumors as well as sections prepared from a variety of human organs (see Materials and Methods). All four genes were detected at sites of endothelial activation or new blood vessel formation. For example, STC demonstrated strong but variable expression in the vasculature in and around mammary adenocarcinoma and squamous cell carcinoma (Figure 2) ▶ and, to a lesser extent, in chondrosarcoma and renal cell carcinoma (not shown), but there was no significant expression seen in normal vessels (not shown). Detectable expression of podocalyxin in normal adult tissue was limited to glomerular urinary epithelial cells (podocytes) and some endothelial cells in the adventitia around large vessels. Podocalyxin expression was expressed in the endothelium of small vessels associated with chondrosarcoma, squamous and renal cell carcinomas, and ductal mammary adenocarcinoma. (not shown), as well as in arteriolar endothelium in inflamed appendix (Figure 2) ▶ . Osteonidogen expression was absent in normal adult vessels but was observed in endothelial cells of inflamed appendix (not shown) and in peritumor stromal (Figure 2) ▶ endothelium and nonendothelial cell types, as well as in osteosarcoma, chondrosarcoma, and squamous cell CA tumors. ADAMTS-4 expression in adult tissue was intensely expressed in vascular endothelium and smooth muscle in areas of inflammation (appendices, around tumors, in inflamed lung) (Figure 2) ▶ , as well as in scleral and corneal limbic endothelium in an age-related macular degeneration eye (not shown). No detectable expression of ADAMTS-4 was observed in blood vessels of normal adult tissues.
Figure 2.
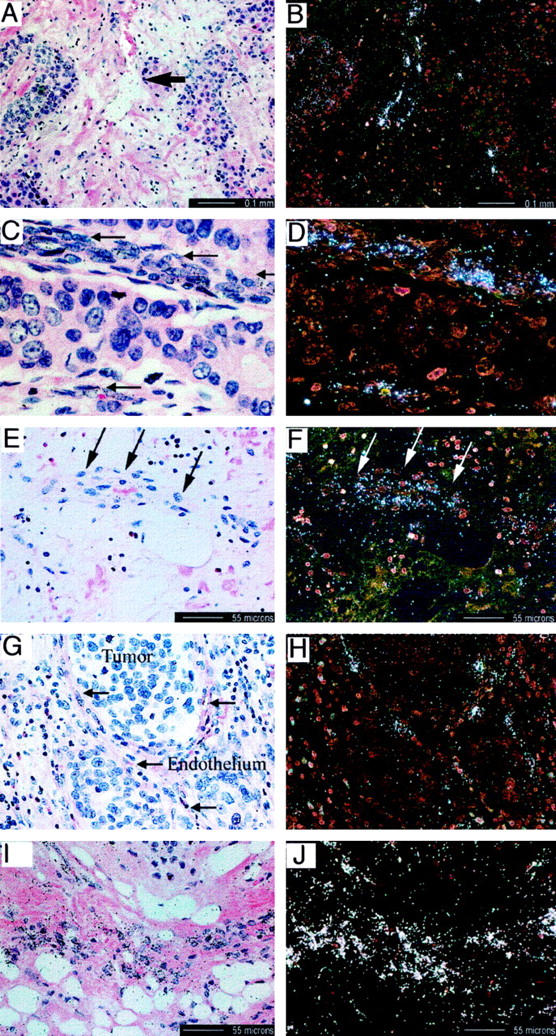
In situ hybridization demonstrating expression of genes identified from the differential expression analysis in the vasculature associated with tumors and with inflammatory disease. A–D: Hematoxylin-eosin (A and C) and in situ (B and D) hybridization demonstrating vascular expression (arrows) of stanniocalcin precursor mRNA in squamous cell carcinoma (A and B) and ductal mammary adenocarcinoma (C and D). E and F: Hematoxylin and eosin stain (E) and in situ (F) hybridization of osteonidogen mRNA in an arteriole (arrows) of inflamed appendix. G and H: Hematoxylin and eosin stain (G) and in situ (H) hybridization of podocalyxin expression in vessels surrounding lung squamous cell carcinoma (arrows). I and J: Hematoxylin and eosin stain (I) and in situ (J) hybridization of ADAMTS-4 expression adjacent to chondrosarcoma.
Discussion
Many of the genes identified in this experiment have previously been implicated in angiogenesis. For example, the mRNA for PLGF, a member of the VEGF family of growth factors, increased during the initial 8 hours of incubation in the gel environment (Figure 1C) ▶ . The EST AI79861 identified from the GeneCalling analysis is 78% identical to the COOH-terminal region of human angiopoietin-2, a naturally occurring antagonist for the tie-2 receptor kinase, 5 suggesting that yet another member of the angiopoietin family may play some role in the regulation of new vessel formation.
TFPI-2, which was a highly up-regulated gene in this study (Figure 1B) ▶ , is a 32-kd serine protease and is associated with the extracellular matrix that inhibits the activation of matrix metalloproteinase zymogens, pro-MMP-1 and MMP-3. 6,7 TFPI has also been reported to be a smooth muscle mitogen. 8 Thus the up-regulation of TFPI-2 by the differentiation of endothelial cells might have a dual role of limiting the extent of matrix degradation and recruiting or promoting the proliferation of mural cells, leading to the assembly of the new vessel wall.
The mRNA levels for a number of proteases increased substantially during the initial 8–10 hours of incubation in the gel, notably the matrix metalloproteinase, MMP-9 (Figure 1C) ▶ , and cathepsin B (Figure 1B) ▶ . MMP-9 is an established participant in angiogenesis, playing important roles in the degradation of basement membrane/matrix in both in vitro and in vivo models of angiogenesis. 9,10 MMP inhibitors reduce the elongation of endothelial cells into tubelike structures in vitro 11 and inhibit tumor angiogenesis in in vivo animal models 12 and in cancer patients. 13 Cathepsin B, a lysosomal cysteine protease, has been observed in many different tumor types and is highly expressed in tumor blood vessels as compared to normal vasculature. 14 One of the newest members of “disintegrin and metalloproteinase family members with thrombospondin motifs,“ ADAMTS-4 was identified as a differentially expressed gene in this study. Although relatively little information is available concerning ADAMTS-4, Tortorella et al 15 recently reported that this protein has aggrecanase activity and suggested that ADAMTS-4 played an important role in the turnover of the proteoglycan aggrecan in diseases such as osteoarthritis. Aggrecan has not previously been reported in vascular tissues, and we were unable to detect the expression of aggrecan mRNA in our endothelial cells under a variety of experimental conditions (data not shown). Intriguingly, however, two other members of the ADAM-TS family, METH-1 (human ADAMTS-1) and METH-2 (ADAMTS-8), were recently reported to be potent antiangiogenic agents. 16 The up-regulation of ADAMTS-4 during endothelial tube formation suggests a potential role of this enzyme in the metabolism of vascular proteoglycans, such as versican or other components of the basement membrane.
The mRNAs for the chemokines IL-8 and Gro-α are up-regulated early in the time course of endothelial differentiation into tubelike structures in the 3D gel environment (Figure 1A) ▶ . These chemokines have previously been reported to have angiogenic activity. 17 mRNA levels for CXCR4, the chemokine receptor for SDF-1α, 18 increased by nearly 40-fold over a time period between 30 minutes and 16 hours, then remained elevated for the duration of the experiment (Figure 1C) ▶ . Deletion studies have shown that both CXCR4 and SDF-1 null mice have defective formation of large blood vessels supplying the gastrointestinal tract. 19
The mRNA level for the receptor “ECK” or epithelial tyrosine kinase (EphA2), a Eph receptor kinase family, increased modestly between 4 and 8 hours, and then slowly declined toward the levels observed at 30 minutes (Figure 1B) ▶ . The ligand for ECK is a gene known as B61 (ephrin A1), initially identified as a tumor necrosis factor-α (TNFα)-induced gene in endothelial cells. 20 Antibodies to B61 block TNFα-induced angiogenesis in a corneal neovascularization assay. 21
The mRNA for the laminin γ2 increased by almost 1000-fold between 0.5 and 24 hours (Figure 1B) ▶ . Laminins are known to play key roles in angiogenesis 22 and are an important component of the basement membrane. Although the precise role of laminin in the differentiation process is unclear, laminin might be important for the establishment of the endothelial apical:basal polarity that may be a prerequisite for the formation of a vascular lumen. The message levels for another basement membrane protein, osteonidogen (also known as Nidogen-2), increased throughout the duration of the HUVEC incubation in the 3D gels (Figure 1C) ▶ . Nidogens can interact with laminin, collagen, and proteoglycans, and there are suggestions that this family of molecules may play some role in angiogenesis. 23
In contrast to the genes discussed above, which have previously been implicated in various steps of angiogenesis, the study also identified a number of genes not previously associated with endothelial cell proliferation, differentiation, or angiogenesis.
The mRNA, designated KIAA0188, codes for a novel hypothetical protein. Domain analysis of the predicted amino acid sequence revealed a putative domain, aa 724–732, with homology to the consensus Kunitz-type serine protease inhibitor, and a domain, 455–467, with homology to the subtilase family of serine proteases, suggesting that this gene might code for a proform of a serine protease. KIA0188 mRNA levels increased modestly during the initial 8–10 hours of incubation in the gel, then declined to the initial levels (Figure 1B) ▶ .
STC was first isolated from bony fishes, 24 where this glycoprotein is synthesized and secreted by the corpuscles of Stannius and regulates blood calcium levels through its inhibitory action on calcium ion uptake in the gill, a highly vascularized tissue. 24 Mammalian cDNAs encoding STC have been reported for the human and mouse and are highly homologous to those of the fish, 25 although the role of this protein in mammals is not known. mRNA levels for STC increased over 100-fold between 30 minutes and 16 hours and remained well above baseline levels out to 46.5 hours (Figure 1C) ▶ . What role this gene plays in angiogenesis is unknown, but this observation suggests that further study in the context of angiogenesis is warranted.
The mRNA for FGF-16 demonstrated a biphasic profile, increasing substantially during the initial 8 hours and then declining for the remainder of the experiment (Figure 2B ▶ . This recent member of the FGF family of growth factors was originally cloned from human heart cDNA. 26 FGF-16 weakly stimulates NIH 3T3 fibroblast proliferation and but is a reasonably potent stimulus of primary rat oligodendrocyte proliferation. 27
The mRNA levels of a number of membrane receptors were also increased over the time course of endothelial differentiation into tubelike structures, including the signaling component of the IL-6 receptor gp130, the TNFR-related protein OX40, 28 and the sialomucin, podocalyxin-like protein. There is no known role for any of these receptors in angiogenesis. Indeed, this study documents for the first time the expression of OX40 by endothelial cells. Previously this TNFR family member was thought to be restricted to cells of the lymphocyte lineage. 29 Increased protein expression of OX40 was also confirmed by fluorescence-activated cell sorter analysis (data not shown).
Podocalyxin-like protein, a well-known constituent of the endothelial plasma membrane, 30 was recently shown protein to function as an l-selectin receptor in inflamed lymph nodes, 31 suggesting a role in cell-cell interactions or adhesion.
The mRNA levels for the antiapoptotic bcl-2-related protein A1 were elevated at the early time points and then declined (Figure 1A) ▶ . In the three-dimensional gel environments, HUVECs do not survive well in the absence of growth factors, and they cannot be rescued by supplementation with VEGF or bFGF. 1 However, PMA treatment will induce endothelial survival and tubule formation. The induction of A1 expression may thus be related to inhibition of apoptosis in the 3D gel environment.
The protein designated “white protein homolog” (also known as ATP-binding cassette (ABC) 8) is 84% identical to the Drosophila gene white protein, which codes for a transporter protein whose expression results in white eye color. Many members of the ABC family of proteins function as transporters or channels. The mRNA levels for this gene increased by ∼10-fold between 0.5 and 8 hours (Figure 1B) ▶ . There is little information relating to its expression or function in mammalian cells, although ESTs containing white protein sequence from a variety of tissue sources and tumors can be found in GenBank. The mRNA for y+LAT-1, a new member of a family of polytopic transmembrane proteins, 32 increased by about eightfold between 0.5 and 24 hours (Figure 1C) ▶ . Little is known about the function of this permease, although it undoubtedly plays a role in amino acid transport and protein synthesis, two activities critical to altered endothelial protein expression.
Cyclooxygenase 2 (COX-2), a rate-limiting enzyme in the prostaglandin biosynthesis pathway, was detected in our experiment system. COX2 mRNA levels rose abruptly during the initial few hours of the experiment, then declined (Figure 1A) ▶ . The transient expression we observed for COX2 is consistent with published reports proposing a role for COX2-regulated prostanoid responses after vascular injury. 33
Xin et al 4 recently reported that agonists of PPARγ receptors specifically blocked endothelial tube formation in vitro and VEGF-driven angiogenesis in vivo. Furthermore, Xin et al 4 found that treatment of endothelial cells with the PPARγ ligand 15d-PGJ2 inhibited the induction of kdr, flt-1, and uPA in a three-dimensional collagen gel model identical to the system used in the present investigation. The effects of 15d-PGJ2 on mRNA levels for a number of genes identified in this study were therefore examined for the purpose of identifying genes modulated during tube formation specifically responsive to treatment with the PPARγ ligand. Those genes so identified might represent important targets for therapeutic intervention. As shown in Table 4 ▶ , there were different classes of response to the PPARγ ligand (supermodulation, countermodulation, and no change in modulation), suggesting that the effect of treatment with 15d-PGJ2 was not simply a general phenomenon. The selective and marked effects of 15d-PGJ2 treatment on endothelial gene expression in the three-dimensional collagen system provides further support for the potential roles in angiogenesis of many of the genes identified in this study.
In summary, GeneCalling successfully identified 115 differentially expressed cDNA fragments corresponding to 90 known genes from the study of collagen matrix-driven endothelial cell gene expression. In addition to the known genes identified, 80 fragments considered totally novel, or belonging to ESTs of unknown function, were identified in this study. The identity and expression of 67 of the known genes were confirmed by a second independent method (TaqMan). For the initial confirmations, we focused on membrane proteins and secreted proteins and only confirmed the identity of a few of the other cDNA fragments by this independent technique. However, in every instance the gene identified by the GeneCalling method was confirmed by TaqMan to be expressed by HUVECs in 3D gels, and the magnitude and direction of the changes in expression agreed reasonably well with the GeneCalling estimates. Because the method also identified a number of potentially new genes, we have not, at this time, pursued TaqMan confirmation of the remaining 23 genes, choosing to focus, instead, on the identification of new genes that might play a role in the process of endothelial differentiation into tubelike structures. Most importantly, the biological relevance of many of these newly identified “angiogenesis-associated” genes is strongly supported by the selective abrogation of their differential expression by the PPARγligand, 15d-PGJ2, as well as by the in situ demonstration of selective expression of some of the genes at sites of new blood vessel formation. Although many of the identified genes have previously been associated with angiogenesis or tumor vasculature (eg, cathepsin B, MMP-9, PLGF, IL-8, GRO-α, CXCR4) or have reported roles or expression patterns consistent with a function in the differentiation process (A1, TFPI-2, laminin γ2), a number of genes identified in this study had never previously been associated with angiogenesis (stanniocalcin precursor, OX40, white protein homolog, the angiopoietin-2 homolog, ADAMTS-4, FGF16, KIAA0188), suggesting the need for the further evaluation of the potential biological roles of these genes in the process of new blood vessel formation
Table 1A.
Continued
| Gene name | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| Cathepsin B | GAAGCCATCTCTGACCGGATC | TCCGCCGACACCTCCA | CCACACCAATGCGCACGTCAGC |
| Plasminogen activator inhibitor-2 (PAI-2) | GCAGGCACAAGCTGCAGATA | CCTGTGGATGCATTGATTGC | TCCATTCATCCTTCCGCTCTCTCAGC |
| KIAA0188 | |||
| Transporter/channels | |||
| yLAT1 glycoprotein amino acid | AGGAGGCAATGCCAGGAAG | CTTCATACTCAGTGCTGTCAACCA | TGGTGAAGGGTTTCCTCTCCTCCACC |
| Transporter | |||
| White protein homolog | CCCTTTCAGATCATGTTCCCA | GGACGGCTGCGACGTC | CCAGTACACGATGCTGCAGTAGGCCA |
| Cytoskeleton/motility | |||
| Moesin | ACTGGGCCGAGACAAATACAA | AATGCGCTGCTTGGTGTTG | CCCTGCGCCAGATCCGGC |
| actin bundling protein | CCAGCTGCTACTTTGACATCGA | CCATTGGACGCCCTCAGT | GATGCGCCGGTCACGCCA |
| T-plastin | AATAAAACAGCCATGCTCCCA | CCTTAAGCCATAAGCACTTCACC | TGCATGATTCGCAGGTCAGCTATTTCC |
| Brain ankyrin-2 | AAGCAGCTTCCTGATGCATTC | CGGACACAGCGCCTTACAT | TCGCAGCCAAGAACAGCCACCA |
| Intermediate filaments | |||
| Mesothelial keratin K7 | CCCAGATCTCCGACACATCTG | GCGATGATGCCGTCCAG | CCATGGACAACAGTCGCTCCCTGG |
| Extracellular matrix | |||
| Laminin gamma 2 (nicein B2 chain) | GCTGACAGGCAGGTGTTTGAA | CGAAGTAGCCTGCTTTGCACT | TGTATCCACAACACAGCCGGCATCTACTG |
| Nidogen-2 (osteonidogen) | AAAATCTTAGAACTTTTGTTGGGAAACTA | CCTTGACAGTTGGAGAAGCCA | AAATAATTGGTCCTTTCCCATCAGTTCTGCA |
| Type IV collagen | CCCCTGGAACCTCCTCTGTT | CATTGTGGTGCATCCGTTGT | CACATGGATTTCTTATTACACGCCACAGCC |
| Extracellular protein S1-5 | GGAACCCAGCTGACCCTCA | CTGCTGCACACTGGATACGG | CGCATTCCCTCCAACCCTTCCC |
| p137 | CAGTGCTCCCCGGGATTACT | CCAGAGCCTCGCTTGAAATT | TGCTGATATCCATCCCGTTGATAGCCA |
| Metabolic enzymes | |||
| OXA1 | ACACGGCTCCTATTCCCAGTAG | AAGCCGCAAGGAAGAGGTAGT | CCCGTGCTGCTGTCGCCCA |
| thioredoxin peroxidase AOE 37-2-187F | GAGGCATCCCGGGTATCG | GGCTTGGAAATCTTCGCTTTG | CGCCGACCACTCCCTGCACCTAA |
| Posttranslational protein modification | |||
| Peptidyl-glycine alpha amidating monooxygenase | GAGGGTCTCGGCCACCTT | AGAACTGGACTTGACAGCGGAA | TGGCAGAACCCCCGCAACCA |
| Signal transduction | |||
| TRAF-1 | GGACCCATCTGATGCACCTT | TGTGGTCCTCGGATTGCTTT | TCCCTCACTCGATTCCCCGGG |
| Lipids and lipid turnover | |||
| Phospholipase A2 gamma | GAAGGCGGTGAGCCTGAAC | TTCCAGGGAGGTCCTGGTC | TTCTCGAGCATCTCAGTCAGCCAGGTG |
| Cyclooxygenase-2 | GAATCATTCACCAGGCAAATTG | TCTGTACTGCGGGTGGAACA | TCCTACCACCAGCAACCCTGCCA |
| Coagulation system | |||
| Tissue factor | CACCGACGAGATTGTGAAGGA | CCCTGCCGGGTAGGAGAA | ACCCGTGCCAAGTACGTCTGCTTCA |
| Clathrin components | |||
| Clathrin heavy chain | GGAGAAAATTGTCCTTGATAACTCTGT | TCAGCCTTAATTGCAGTGAGGAT | TTCAGTGAACACAGGAATCTGCAAAACCTCC |
| Unknown | |||
| ALG2 hlog | TGAGCAATGGATCTGTTAACCAA | TCCCGTGTCAGTACAGCAGTCT | TCGGCTTTCCTCTGATTATAGGCAGCCA |
| Sushi-like Repeat Protein | GGGCTTTCGATTGATTGGAAG | GGCAGTTCCAGACCAACGAC | TCGGTGCAATGCCTGCCAAGC |
Table 3A.
Continued
| Confirmed gene* | Accesion no.† | GeneCalling ratio‡ | TaqMan ratio§ |
|---|---|---|---|
| Type I collagenase (MMP1) | X05231 | 10 | 2 |
| Cathepsin B | M14221 | 3 | 2 |
| Plasminogen activator inhibitor-2 | M31551 | <0.1 | <0.1 |
| KIAA0188 | d80010 | 3 | 2 |
| Transporters/channels | |||
| Glycoprotein-associated amino acid transporter | AJ130718 | 6 | 20 |
| LAT1 | |||
| White protein Hlog | AF038175 | 3 | 0.5 |
| Cytoskeleton/motility | |||
| Brain ankyrin 2 | H58696 | 2 | 1 |
| Moesin | M69066 | 2 | 1 |
| Myosin-IC | U14391 | 2 | ND |
| Actin bundling protein | U09873 | 2 | 2 |
| T-plastin | L05491 | 2 | 1 |
| Dynein light chain | U32944 | 3 | ND |
| Intermediate filaments | |||
| Keratin K7 | X03212 | 0.2 | 0.1 |
| Extracellular matrix | |||
| Laminin gamma 2 chain | U31201 | 12 | 49 |
| Nidogen-2 (osteonidogen) | D86425 | 4 | 2 |
| Type IV collagen | Y00706 | 5 | 1 |
| S1-5 (EGF-containing fibulin-like extracellular matrix protein-1) | U03877 | 0.2 | <0.1 |
| p137 | Z48042 | 3 | 1 |
| Signal transduction | |||
| Calmodulin | M27319 | 4 | ND |
| Ras-related protein RAL-A | H94944 | 2 | ND |
| TRAF1 | U59863 | 2 | 1 |
| MT-GRPE precursor | AA989480 | 2 | ND |
| Nonreceptor tyrosine kinase | AF097738 | 4 | 1 |
| Metabolic enzymes | |||
| Z-crystallin/quinone reductase | AA316207 | 4 | ND |
| S-adenosylmethionine synthase Hlog SAMS2 | D11332 | 4 | ND |
| OXA1 subunit of cytochrome oxidase | X80695 | 2 | 1 |
| Antioxidant enzyme A0E37-2 | U25182 | 0.3 | 0.5 |
| Endoplasmic reticulum ATPase | W38423 | 2 | ND |
| Posttranslational protein modification | |||
| Peptidylglycine alpha amidating monooxygenase | AF035320 | 5 | 4 |
| Ubiquitin 52 amino acid fusion protein | D28425 | 0.3 | ND |
| Lipids and lipid turnover | |||
| Phospholipase A2 gamma | AF058921 | 11 | 21 |
| Apolipoprotein E | AA087386 | 0.3 | ND |
| Cyclooxygenase-2 | M90010 | 0.1 | <0.1 |
| Coagulation system | |||
| Tissue factor | J02931 | 0.5 | 0.1 |
| Endosome/lysosome | |||
| Lysosomal membrane sialoglycoprotein(CD36-2L) | D12676 | 2 | ND |
| rab 5 interacting protein | S83365 | 2 | ND |
| Clathrin components | |||
| Clathrin heavy chain | AA100413 | 3 | 1 |
| Clathrin assembly protein | U45976 | 3 | ND |
| Unknown | |||
| PMP41 Hlog (ALG2) | AA226371 | 6 | 2 |
| Insulin-induced protein | W37284 | 5 | ND |
| NK-4 | M59807 | 3 | ND |
| KIAA0726 | AB018269 | 2 | ND |
Acknowledgments
The authors wish to acknowledge the contributions made by the CuraGen Corporation Genomics Facility, the Genentech DNA synthesis facility, and Gretchen Frantz, Department of Pathology, Genentech. The authors gratefully acknowledge the helpful discussions of Dr. David Lowe, Genentech. The authors also acknowledge the University of Michigan (lung adenocarcinoma, breast tumor), National Disease Research Interchange (AMD eyes), and Western Infirmary, Glasgow (inflamed appendices, renal cell carcinoma), for the provision of human tissue samples used for the in situ studies.
Footnotes
Address reprint requests to Dr. Mary E. Gerritsen, Department of Cardiovascular Research, MS 42 Genenetch, 1 DNA Way, South San Francisco, CA 94080. E-mail: meg@gene.com.
Ms. Kahn and Dr. Mehraban contributed equally to this study.
References
- 1.Yang S, Graham J, Kahn J, Schwartz E, Gerritsen M: Differential roles for CD31 and VE-cadherin in formation of vascular tubes and lumens in three dimensional collagen gels. Am J Pathol 1999, 155:887-895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimkets RA, Lowe DG, Tai JT, Sehl P, Jin H, Yang R, Predki PF, Rothberg BE, Murtha MT, Roth ME, Shenoy SG, Windemuth A, Simpson JW, Simons JF, Daley MP, Gold SA, McKenna MP, Hillan K, Went GT, Rothberg JM: Gene expression analysis by transcript profiling coupled to a gene database query. Nat Biotechnol 1999, 17:798-803 [DOI] [PubMed] [Google Scholar]
- 3.Lu L, Gillett N: An optimized protocol for in situ hybridization using PCR generated 33P-labeled riboprobes. Cell Vision 1994, 1:169-176 [Google Scholar]
- 4.Xin X, Yang S, Kowalski J, Gerritsen ME: Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem 1999, 274:9116-9121 [DOI] [PubMed] [Google Scholar]
- 5.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD: Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997, 277:55-60 [DOI] [PubMed] [Google Scholar]
- 6.Iino M, Foster DC, Kisiel W: Quantification and characterization of human endothelial cell-derived tissue factor pathway inhibitor-2. Arterioscler Thromb Vasc Biol 1998, 18:40-46 [DOI] [PubMed] [Google Scholar]
- 7.Petersen LC, Sprecher CA, Foster DC, Blumberg H, Hamamoto T, Kisiel W: Inhibitory properties of a novel human Kunitz-type protease inhibitor homologous to tissue factor pathway inhibitor. Biochemistry 1996, 35:266-272 [DOI] [PubMed] [Google Scholar]
- 8.Shinoda E, Yui Y, Hattori R, Tanaka M, Inoue R, Aoyama T, Takimoto Y, Mitsui Y, Miyahara K, Shizuta Y, Sasayama S: Tissue factor pathway inhibitor-2 is a novel mitogen for vascular smooth muscle cells. J Biol Chem 1999, 274:5379-5384 [DOI] [PubMed] [Google Scholar]
- 9.Rabbani SA: Metalloproteases and urokinase in angiogenesis and tumor progression. In Vivo 1998, 12:135-142 [PubMed] [Google Scholar]
- 10.Werb Z, Vu TH, Rinkenberger JL, Coussens LM: Matrix-degrading proteases and angiogenesis during development and tumor formation. Apmis 1999, 107:11-18 [DOI] [PubMed] [Google Scholar]
- 11.Schnaper HW, Grant DS, Stetler-Stevenson WG, Fridman R, D’Orazi G, Murphy AN, Bird RE, Hoythya M, Fuerst TR, French DL, Quigley JP, Kleinman HK: Type IV collagenase(s) and TIMPs modulate endothelial cell morphogenesis in vitro. J Cell Physiol 1993, 156:235-246 [DOI] [PubMed] [Google Scholar]
- 12.Maekawa R, Maki H, Yoshida H, Hojo K, Tanaka H, Wada T, Uchida N, Takeda Y, Kasai H, Okamoto H, Tsuzuki H, Kambayashi Y, Watanabe F, Kawada K, Toda K, Ohtani M, Sugita K, Yoshioka T: Correlation of antiangiogenic and antitumor efficacy of N-biphenyl sulfonyl-phenylalanine hydroxiamic acid (BPHA), an orally active, selective matrix metalloproteinase inhibitor. Cancer Res 1999, 59:1231-1235 [PubMed] [Google Scholar]
- 13.Gradishar WJ: An overview of clinical trials involving inhibitors of angiogenesis and their mechanism of action. Invest New Drugs 1997, 15:49-59 [DOI] [PubMed] [Google Scholar]
- 14.Keppler D, Sameni M, Moin K, Mikkelsen T, Diglio CA, Sloane BF: Tumor progression and angiogenesis: cathepsin B and co. Biochem Cell Biol 1996, 74:799-810 [DOI] [PubMed] [Google Scholar]
- 15.Tortorella MD, Burn TC, Pratta MA, Abbaszade I, Hollis JM, Liu R, Rosenfeld SA, Copeland RA, Decicco CP, Wynn R, Rockwell A, Yang F, Duke JL, Solomon K, George H, Bruckner R, Nagase H, Itoh Y, Ellis DM, Ross H, Wiswall BH, Murphy K, Hillman MC, Jr, Hollis GF, Newton RC, Magolda RL, Trzaskos JM, Arner EC: Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science 1999, 284:1664-1666 [DOI] [PubMed] [Google Scholar]
- 16.Vazquez F, Hastings G, Ortega MA, Lane TF, Oikemus S, Lombardo M, Iruela-Arispe ML: METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem 1999, 274:23349-23357 [DOI] [PubMed] [Google Scholar]
- 17.Strieter RM, Polverini PJ, Arenberg DA, Kunkel SL: The role of CXC chemokines as regulators of angiogenesis. Shock 1995, 4:155-160 [DOI] [PubMed] [Google Scholar]
- 18.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA: The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 1996, 382:829-833 [DOI] [PubMed] [Google Scholar]
- 19.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T: The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 1998, 393:591-594 [DOI] [PubMed] [Google Scholar]
- 20.Holzman LB, Marks RM, Dixit VM: A novel immediate-early response gene of endothelium is induced by cytokines and encodes a secreted protein. Mol Cell Biol 1990, 10:5830-5838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandey A, Shao H, Marks RM, Polverini PJ, Dixit VM: Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-alpha-induced angiogenesis. Science 1995, 268:567-569 [DOI] [PubMed] [Google Scholar]
- 22.Kubota Y, Kleinman HK, Martin GR, Lawley TJ: Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol 1988, 107:1589-1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicosia RF, Bonanno E, Smith M, Yurchenco P: Modulation of angiogenesis in vitro by laminin-entactin complex. Dev Biol 1994, 164:197-206 [DOI] [PubMed] [Google Scholar]
- 24.Wagner G, Hampong M, Park C, Copp D: Purification, characterization, and bioassay of teleocalcin, a glycoprotein from salmon corpuscles of Stannius. Gen Comp Endocrinol 1986, 63:481-491 [DOI] [PubMed] [Google Scholar]
- 25.Chang AC, Janosi J, Hulsbeek M, de Jong D, Jeffrey KJ, Noble JR, Reddel RR: A novel human cDNA highly homologous to the fish hormone stanniocalcin. Mol Cell Endocrinol 1995, 112:241-247 [DOI] [PubMed] [Google Scholar]
- 26.Miyake A, Konishi M, Martin FH, Hernday NA, Ozaki K, Yamamoto S, Mikami T, Arakawa T, Itoh N: Structure and expression of a novel member, FGF-16, on the fibroblast growth factor family. Biochem Biophys Res Commun 1998, 243:148-152 [DOI] [PubMed] [Google Scholar]
- 27.Danilenko DM, Montestruque S, Philo JS, Li T, Hill D, Speakman J, Bahru M, Zhang M, Konishi M, Itoh N, Chirica M, Delaney J, Hernday N, Martin F, Hara S, Talvenheimo J, Narhi LO, Arakawa T: Recombinant rat fibroblast growth factor-16: structure and biological activity. Arch Biochem Biophys 1999, 361:34-46 [DOI] [PubMed] [Google Scholar]
- 28.Arch RH, Thompson CB: 4–1BB and Ox40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor kappaB. Mol Cell Biol 1998, 18:558-565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imura A, Hori T, Imada K, Ishikawa T, Tanaka Y, Maeda M, Imamura S, Uchiyama T: The human OX40/gp34 system directly mediates adhesion of activated T cells to vascular endothelial cells. J Exp Med 1996, 183:2185-2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stan RV, Roberts WG, Predescu D, Ihida K, Saucan L, Ghitescu L, Palade GE: Immunoisolation and partial characterization of endothelial plasmalemmal vesicles (caveolae). Mol Biol Cell 1997, 8:595-605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sassetti C, Tangemann K, Singer MS, Kershaw DB, Rosen SD: Identification of podocalyxin-like protein as a high endothelial venule ligand for L-selectin: parallels to CD34. J Exp Med 1998, 187:1965-1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torrents D, Estevez R, Pineda M, Fernandez E, Lloberas J, Shi YB, Zorzano A, Palacin M: Identification and characterization of a membrane protein (y + L amino acid transporter-1) that associates with 4F2hc to encode the amino acid transport activity y + L. A candidate gene for lysinuric protein intolerance. J Biol Chem 1998, 273:32437-32445 [DOI] [PubMed] [Google Scholar]
- 33.Pritchard KA, Jr, O’Banion MK, Miano JM, Vlasic N, Bhatia UG, Young DA, Stemerman MB: Induction of cyclooxygenase-2 in rat vascular smooth muscle cells in vitro and in vivo. J Biol Chem 1994, 269:8504-8509 [PubMed] [Google Scholar]