Abstract
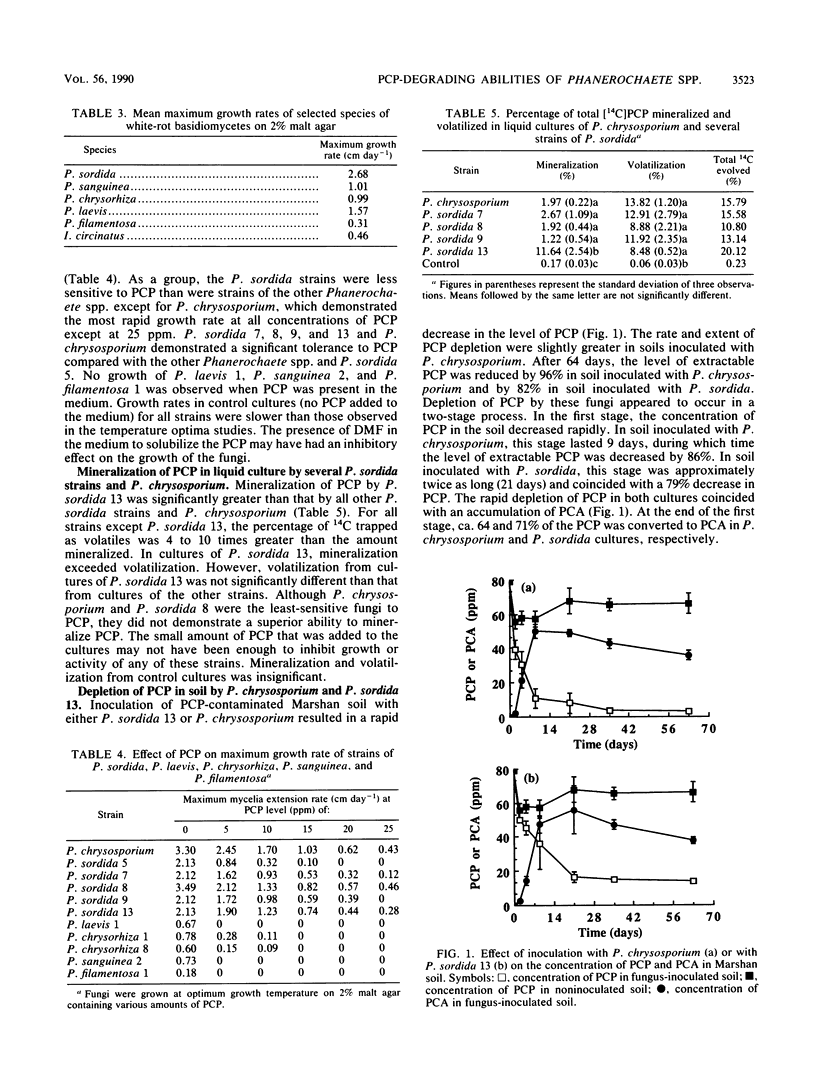
This research measured mycelial extension rates of selected strains of Phanerochaete chrysorhiza, Phanerochaete laevis, Phanerochaete sanguinea, Phanerochaete filamentosa, Phanerochaete sordida, Inonotus circinatus, and Phanerochaete chrysosporium and the ability of these organisms to tolerate and degrade the wood preservative pentachlorophenol (PCP) in an aqueous medium and in soil. Most of the tested species had mycelial extension rates in the range of ≤0.5 to 1.5 cm day−1, but there were large interspecific differences. A notable exception, P. sordida, grew very rapidly, with an average mycelial extension rate of 2.68 cm day−1 at 28°C. Rank of species by growth rate was as follows: P. chrysosporium > P. sordida > P. laevis > P. chrysorhiza = P. sanguinea > I. circinatus = P. filamentosa. There were also significant intraspecific differences in mycelial extension rates. For example, mycelial extension rates among strains of P. sordida ranged from 1.78 to 4.81 cm day−1. Phanerochaete spp. were very sensitive to PCP. Growth of several species was prevented by the presence of 5 ppm (5 μg/g) PCP. However, P. chrysosporium and P. sordida grew at 25 ppm PCP, albeit at greatly decreased mycelial extension rates. In an aqueous medium, mineralization of PCP by P. sordida 13 (ca. 12% after 30 days) was significantly greater than that by all other tested P. sordida strains and P. chrysosporium. After 64 days, the level of PCP had decreased by 96 and 82% in soil inoculated with P. chrysosporium and P. sordida, respectively. Depletion of PCP by these fungi occurred in a two-stage process. The first stage was characterized by a rapid depletion of PCP that coincided with an accumulation of pentachloroanisole (PCA). At the end of the first stage, ca. 64 and 71% of the PCP was converted to PCA in P. chrysosporium and P. sordida cultures, respectively. In the second stage, levels of PCP and PCA were reduced by 9.6 and 18%, respectively, in soil inoculated with P. chrysosporium and by 3 and 23%, respectively, in soil inoculated with P. sordida. PCA was mineralized by both P. chrysosporium and P. sordida in an aqueous medium.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bumpus J. A., Aust S. D. Biodegradation of DDT [1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane] by the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1987 Sep;53(9):2001–2008. doi: 10.1128/aem.53.9.2001-2008.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus J. A. Biodegradation of polycyclic hydrocarbons by Phanerochaete chrysosporium. Appl Environ Microbiol. 1989 Jan;55(1):154–158. doi: 10.1128/aem.55.1.154-158.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus J. A., Tien M., Wright D., Aust S. D. Oxidation of persistent environmental pollutants by a white rot fungus. Science. 1985 Jun 21;228(4706):1434–1436. doi: 10.1126/science.3925550. [DOI] [PubMed] [Google Scholar]
- Cserjesi A. J., Johnson E. L. Methylation of pentachlorophenol by Trichoderma virgatum. Can J Microbiol. 1972 Jan;18(1):45–49. doi: 10.1139/m72-007. [DOI] [PubMed] [Google Scholar]
- Cserjesi A. J. The adaptation of fungi to pentachlorophenol and its biodegradation. Can J Microbiol. 1967 Sep;13(9):1243–1249. doi: 10.1139/m67-169. [DOI] [PubMed] [Google Scholar]
- Gee J. M., Peel J. L. Metabolism of 2,3,4,6-tetrachlorophenol by micro-organisms from broiler house litter. J Gen Microbiol. 1974 Dec;85(2):237–243. doi: 10.1099/00221287-85-2-237. [DOI] [PubMed] [Google Scholar]
- Lamar R. T., Dietrich D. M. In Situ Depletion of Pentachlorophenol from Contaminated Soil by Phanerochaete spp. Appl Environ Microbiol. 1990 Oct;56(10):3093–3100. doi: 10.1128/aem.56.10.3093-3100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileski G. J., Bumpus J. A., Jurek M. A., Aust S. D. Biodegradation of pentachlorophenol by the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1988 Dec;54(12):2885–2889. doi: 10.1128/aem.54.12.2885-2889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckdeschel G., Renner G. Effects of pentachlorophenol and some of its known and possible metabolites on fungi. Appl Environ Microbiol. 1986 Jun;51(6):1370–1372. doi: 10.1128/aem.51.6.1370-1372.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckdeschel G., Renner G., Schwarz K. Effects of pentachlorophenol and some of its known and possible metabolites on different species of bacteria. Appl Environ Microbiol. 1987 Nov;53(11):2689–2692. doi: 10.1128/aem.53.11.2689-2692.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


