Summary
Osteoblasts and adipocytes originate from a common progenitor, which arises from bone marrow mesenchymal stroma/stem cells (mMSC). Aging causes a decrease in the number of bone-forming osteoblasts and an increase in the number of marrow adipocytes. Here, we demonstrate that, during aging, the status of mMSC changes with respect to both their intrinsic differentiation potential and production of signaling molecules, which contributes to the formation of a specific marrow microenvironment necessary for maintenance of bone homeostasis. Aging causes a decrease in the commitment of mMSC to the osteoblast lineage and an increase in the commitment to the adipocyte lineage. This is reflected by changes in the expression of phenotype-specific gene markers. The expression of osteoblast-specific transcription factors, Runx2 and Dlx5, and osteoblast markers, collagen and osteocalcin, is decreased in aged mMSC. Conversely, the expression of adipocyte-specific transcription factor PPAR-γ2, shown previously to regulate osteoblast development and bone formation negatively and to regulate marrow adipocyte differentiation positively, is increased, as is a gene marker of adipocyte phenotype, fatty acid binding protein aP2. Furthermore, production of an endogeneous PPAR-γ activator(s) that stimulates adipocyte differentiation and production of autocrine/paracrine factor(s) that suppresses the osteoblastic phenotype are also increased. In addition, expression of different components of TGF-β and BMP2/4 signaling pathways is altered, suggesting that activities of these two cytokines essential for bone homeostasis change with aging.
Keywords: adipocyte, aging, marrow stem cells, osteoblast, PPAR-γ, TGF-β/BMP
Introduction
Age-related bone loss or type II osteoporosis occurs universally in animals and humans and, in contrast to post-menopausal bone loss or type I osteoporosis, affects individuals regardless of their sex steroid status (Frost, 1973; Manolagas, 1998). Maintenance of bone homeostasis throughout life relies on the bone remodelling process, which continually replaces old and damaged bone with new bone in order to maintain bone strength and elasticity (Parfitt, 1994). Two types of cells are involved in bone remodelling: osteoclasts, originating from haematopoietic cells, are responsible for bone resorption; and osteoblasts, originating from mesenchymal cells, are responsible for formation of new bone. Age-related bone loss results from attenuated and unbalanced bone turnover and occurs only on the surface in contact with bone marrow. Thus, an oversupply of osteoclasts, relative to the need for bone resorption, and/or an undersupply of osteoblasts, relative to the need for cavity repair, are critical pathogenic factors in type I and type II osteoporosis, respectively (Manolagas, 1998).
Although aging has a negative effect on osteoblast production, it has a positive effect on the proportion of fatty marrow (Tavassoli, 1984; Moore & Dawson, 1990; Gimble et al., 1996a; Robey & Bianco, 1999). In neonatal mammals, adipocytes are all but absent in the bone marrow, which is primarily haematopoietic at this stage. However, with advancing age, the number of adipocytes in the bone marrow increases, resulting in the appearance of fatty marrow. In humans, most of the femoral cavity is occupied by fat by the third decade of life.
Osteoblasts and adipocytes are derived from mesenchymal marrow stroma/stem cells (mMSC). mMSC are also progenitors for marrow fibroblasts and cartilage cells, and function as haematopoiesis-supporting stroma (Bianco et al., 2001; Jiang et al., 2002). The milieu of intracellular and extracellular signals controls mMSC differentiation into osteoblast or adipocyte. Activation of phenotype-specific transcription factors, such as osteoblast-specific Runx2/Cbfa1 and adipocyte-specific PPAR-γ2, determines lineage commitment (Tontonoz et al., 1994b; Ducy et al., 1997; Komori et al., 1997; Karsenty, 2001; Rosen & Spiegelman, 2001). We previously demonstrated that the adipocyte-restricted PPAR-γ2 transcription factor is a key regulator of osteoblast and adipocyte differentiation (Lecka-Czernik et al., 1999). In a cellular in vitro model of murine mMSC differentiation, PPAR-γ2 acts as a positive regulator of adipocyte differentiation and a dominant-negative regulator of osteoblast differentiation. Ectopic expression of recombinant PPAR-γ2 in osteoblastic UAMS-33 cells irreversibly suppressed Runx2/Cbfa1 expression and the osteoblast phenotype and simultaneously converted these cells to terminally differentiated adipocytes. In vivo, an essential role of PPAR-γ in maintaining bone homeostasis was demonstrated in two opposing, but complementary, models (Akune et al., 2004; Rzonca et al., 2004). First, in a model of bone loss, a high-affinity ligand and activator for PPAR-γ, rosiglitazone (Werner & Travaglini, 2001), was administered to mice for 7 weeks and resulted in a significant decrease in bone mineral density (BMD), bone volume and changes in bone microarchitecture. Moreover, rosiglitazone treatment decreased the number of osteoblasts while simultaneously increasing the number of marrow adipocytes (Rzonca et al., 2004). Second, in a model of increased bone formation due to PPAR-γ insufficiency, heterozygous PPAR-γ-deficient mice exhibited high bone mass and increased osteoblastogenesis (Akune et al., 2004). In humans, PPAR-γ polymorphism, resulting from a silent C to T transition in exon 6, is associated with reduced BMD (Ogawa et al., 1999).
signaling through TGF-β/BMP cytokines exemplifies extracellular mechanisms that modulate intracellular processes (Miyazono et al., 2001; Derynck & Zhang, 2003). TGF-β regulates osteoblast differentiation in a biphasic manner. It stimulates development and proliferation of early osteoblasts, but it inhibits their maturation and expression of phenotype-specific genes, such as osteocalcin and alkaline phosphatase (Alliston et al., 2001; Nishimura et al., 1999; Lee et al., 2000; Banerjee et al., 2001). In contrast, BMP2 and BMP4 cytokines are essential for osteoblasts to achieve their mature phenotype, which is characterized by the ability to form collagen-based extracellular matrix and mineral deposits (Abe et al., 2000; Canalis et al., 2003; Devlin et al., 2003). BMP2/4 cytokines positively regulate expression of osteoblast-specific genes, such as Runx2/Cbfa1, Dlx5, collagen and alkaline phosphatase. Inhibition of this pathway by noggin, a natural antagonist, leads to suppression of the osteoblast phenotype and lack of mineralization in vitro and in vivo (Abe et al., 2000; Devlin et al., 2003).
TGF-β/BMP cytokines also control adipocyte formation. TGF-β inhibits adipocyte differentiation by inactivating C/EBP transcription factors via physical interaction with Smad3 or an unclear mechanism involving Smad6 and Smad7 proteins (Choy et al., 2000; Choy & Derynck, 2003). In contrast, BMP2/4 cytokines stimulate adipocyte differentiation by either committing multipotential cells to adipocyte lineage and/or augmenting their adipocyte differentiation, through both Smad-dependent and p38 kinase-dependent mechanisms (Sottile & Seuwen, 2000; Hata et al., 2003; Tang et al., 2004).
Both TGF-β and BMP2/4 cytokines communicate with cells through Smad proteins, which serve as signal mediators between cell surface receptors and the nuclear transcriptional apparatus (Derynck & Zhang, 2003; Shi & Massague, 2003). In general, extracellular binding of TGF-β and BMP2/4 to their receptors recruits pathway-restricted Smads (R-Smads), which are phosphorylated by the serine/threonine kinase activity of the receptors. Smad2 and -3 are activated in response to TGF-β signals, whereas Smad1, -5 and -8 are activated in response to BMP2/4 signals. These receptor-activated R-Smads are released from the receptor complex to form a new complex with Smad4 and translocate into the nucleus, where they serve as transcriptional regulators. The Smad signaling pathway also includes two inhibitory Smads (I-Smads), Smad6 and Smad7, which help regulate the cellular response to TGF-β/BMP cytokines. I-Smads operate through a negative feedback loop mechanism, and their expression is under the positive control of activated R-Smads (Stopa et al., 2000; von Gersdorff et al., 2000; Ishida et al., 2003).
Here, we have examined the effects of aging on the differentiation potential of mMSC and the role of PPAR-γ2 transcription factor in this process. Moreover, we have examined the effects of aging on expression of different components of TGF-β/BMP2/4 signaling pathways. Our results indicate that changes in the differentiation potential of mMSC are accompanied by alterations in the intracellular mechanisms and extracellular signaling that control their fate.
Results
Age-related changes in bone and bone marrow structure
Age-related changes in the rate of bone formation are accompanied by an increase in the proportion of marrow occupied by adipocytes (Moore & Dawson, 1990). Histological examination of the bone marrow in proximal tibiae of old (26-month-old) C57BL/6 mice indicates the presence of significantly more fat cells than in the marrow of adult (8-month-old) mice (Fig. 1). The increase in the number of adipocytes in the marrow of old mice is accompanied by a decrease in the number of bone-forming osteoblasts at bone remodelling sites and reduced BMD, which indicates lower bone mass.
Fig. 1.
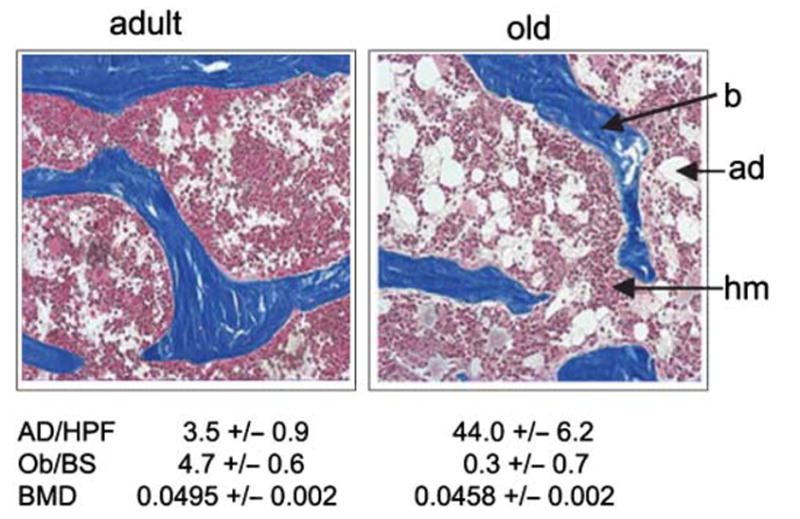
Histological examination of proximal tibiae sections of 8-month- and 26-month-old C57BL/6 mice. Vertical sections of undecalcified tibiae specimens were stained with Masson Trichrome, and images were taken at 4× magnification. The number of osteoblasts at the osteoid sites and the number of adipocytes were determined in six randomly chosen microscopic fields per specimen and calculated as the average of eight animals per group (± SD). b, bone: ad, adipocytes; hm, haematopoietic marrow; AD/HPF, number of adipocytes per field at 20× magnification; Ob/BS, number of osteoblasts per osteoid; BMD, bone mineral density.
Aging alters the differentiation potential of mMSC
Bone marrow consists of a variety of cell types, including cells of haematopoietic and mesenchymal lineage. The conditions of primary bone marrow cultures select for cells that adhere to a plastic surface. This population of cells, historically referred to as marrow stroma cells (MSC), is primarily composed of cells of mesenchymal lineage but also includes a substantial number of macrophages and a relatively small number of myeloid and endothelial cells (Owen et al., 1987; Phinney et al., 1999). A population of adult mesenchymal MSC is heterogeneous and consists of both multipotential primitive stem cells and cells at various stages of differentiation toward specific lineages (Nuttall et al., 1998; Pittenger et al., 1999; Jiang et al., 2002). For clarity, we will refer to these cells as mesenchymal marrow stroma/stem cells or mMSC.
Prompted by the evidence that osteoblasts and adipocytes of the bone marrow are derived from a common set of mesenchymal progenitor cells, we used bone marrow aspirates of adult and old mice to assess the number of mMSC that were able to differentiate into osteoblasts, as judged by the ability to form mineralized colonies, and adipocytes, as judged by the ability to form colonies of fat-laden cells. mMSC were cultured at conditions that allowed a single mesenchymal cell to proliferate and form a separate colony, referred to as a colony forming unit (CFU). For formation of mineralized colonies, mMSC were cultured in the presence of pro-osteoblastic stimuli, whereas the adipocytic phenotype was stimulated by treatment with a pro-adipocytic cocktail, as described in the Experimental procedures. Bone marrow derived from old animals developed relatively fewer osteoblastic colonies (CFU-OB) and more adipocytic colonies (CFU-AD) than marrow derived from adult animals (Fig. 2A,B). A calculated ratio of CFU-OB to CFU-AD formation indicates changes in the differentiation potential of mMSC. In adult animals the prevalence of CFU-OB formation was fourfold greater than CFU-AD, whereas in old animals the frequency of CFU-OB and CFU-AD formation was equal (Fig. 2C). Thus, aging changes the differentiation potential of mMSC for more adipogenic and less osteoblastogenic.
Fig. 2.
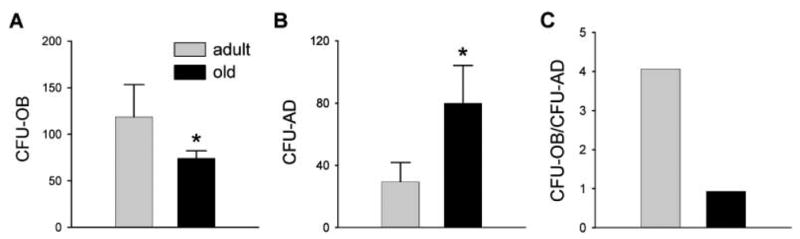
Formation of osteoblastic (CFU-OB) and adipocytic (CFU-AD) colonies in primary bone marrow cultures. Primary bone marrow cell cultures were prepared from 8-month- and 26-month-old C57BL/6 mice. Cells from each animal were cultured separately and examined for the formation of: (A) colonies containing mineralized bone nodules (CFU-OB), which developed in osteoblastic medium; (B) colonies containing fat laden cells (CFU-AD), which developed in adipogenic medium; and (C) ratio of CFU-OB to CFU-AD formation. Each bar represents the average from eight animals; the number of colonies was calculated per 2.5 × 106 plated cells. Error bars indicate SD. Shaded bars, 8-month-old; black bars, 26-month-old animals. *P < 0.05.
A detailed examination of the adipogenic potential of mMSC revealed that marrow derived from old C57BL/6 mice possessed a greater number of cells able to differentiate spontaneously into adipocytes in the absence of pro-adipocytic stimuli. Bone marrow cultures derived from old mice spontaneously developed approximately three times more CFU-AD than cultures derived from adult animals (Table 1). This, together with our former observation, suggests not only that aging increases the commitment of mMSC to adipocytic lineage but also that a higher proportion of these committed cells are present at the late stages of differentiation.
Table 1.
Potential of adult and old mMSC for spontaneous and rosiglitazone-stimulated CFU-AD development
| Mice age (months) | Basal conditions (CFU-AD)‡ | Rosiglitazone† (CFU-AD)‡ |
|---|---|---|
| 8 | 19.3 ± 13.1 | 485.7 ± 216.0 |
| 26 | 57.5 ± 28.6* | 1131.7 ± 268.8* |
Culture medium supplemented with 1 μM rosiglitazone.
Mean values of six individual cultures are presented per two femora bone marrow aspirates from a single mouse.
P < 0.05 vs. 8-month-old animals.
We used quantitative real-time RT-PCR to examine whether changes in the differentiation potential of mMSC were accompanied by changes in the expression of phenotype-specific gene markers (Fig. 3). In basal, non-differentiating conditions, mMSC derived from old animals express more mRNA encoding fatty acid binding protein aP2, a marker for the adipocyte phenotype, and less mRNAs for osteoblast-specific transcription factors, Runx2/Cbfa1 and Dlx5, and phenotype-specific markers, collagen and osteocalcin, than mMSC isolated from adult animals. These data support our previous observation that aging increases mMSC commitment to adipocyte and decreases commitment to osteoblast lineage.
Fig. 3.
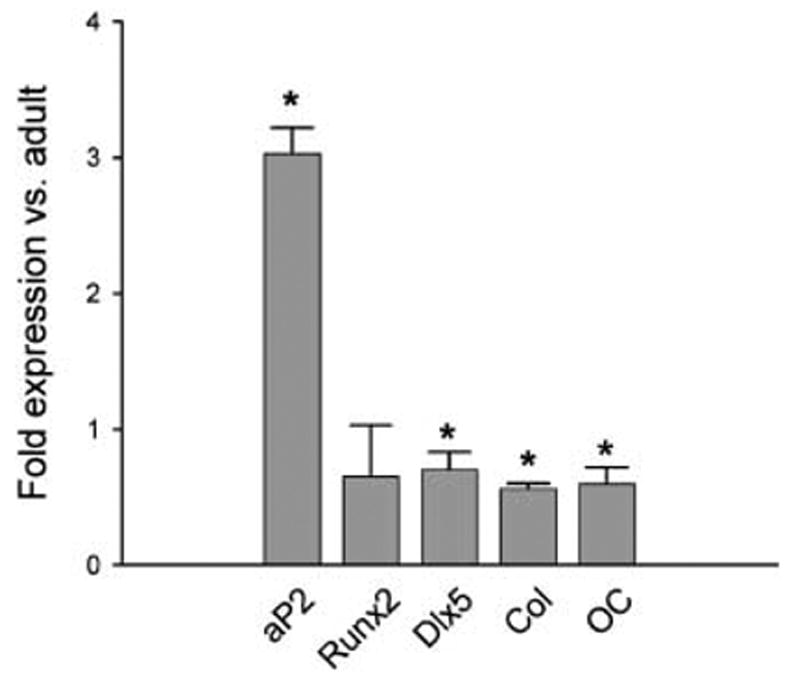
Gene expression of adipocyte and osteoblast markers in mMSC derived from 6-month- and 20-month-old mice. Gene expression was determined using quantitative real-time RT-PCR as described in Experimental procedures. Values were normalized to the expression of GAPDH, and expression in old marrow (20-month-old) is presented as the fold increase or decrease of that in adult marrow (6-month-old). Error bars represent SD. Col, collagen; OC, osteocalcin. *P < 0.05.
PPAR-γ 2 transcription factor accounts for increased adipogenic potential of mMSC with aging
PPAR-γ is a key regulator of adipocyte differentiation and is essential for maintenance of adipocyte phenotype and function (Rosen & Spiegelman, 2001). The thiazolidinedione, rosiglitazone, is an artificial, highly specific PPAR-γ agonist, causing induction of pro-adipocytic and anti-diabetic activities of this transcription factor (Lehmann et al., 1995). Treating bone marrow cultures with rosiglitazone significantly increased the pro-adipocytic response of old marrow as compared with adult marrow (Table 1). In addition to increasing in number, adipocytes developed in cultures derived from old animals accumulated more fat than adipocytes in the cultures derived from adult animals (Fig. 4A). These results indicate that sensitivity to PPAR-γ is increased in old marrow. This increased sensitivity was evidenced by both the large number of cells responding to rosiglitazone and the robust adipocytic response in individual cells.
Fig. 4.
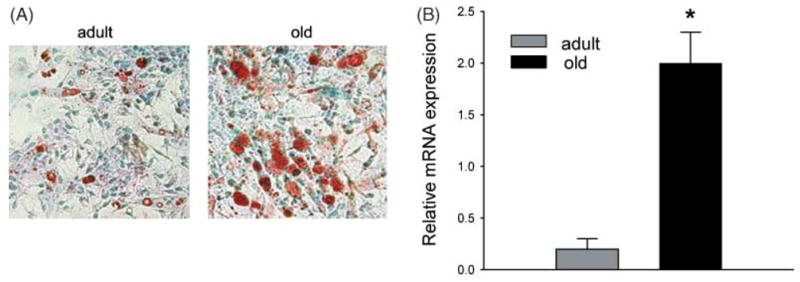
Effects of aging on mMSC sensitivity to rosiglitazone and expression of PPAR-γ2 transcription factor. (A) Photomicrographs (40× magnification) show a response to 5 μM rosiglitazone treatment of mMSC derived from adult (6-month-old) and old (20-month-old) mice. Cells stained with Oil Red O (red) for fat and counterstained with methyl green (blue). (B) Expression of PPAR-γ2 in mMSC derived from 6-month- and 20-month-old mice. mMSC were grown for 10 days in basal medium followed by RNA isolation and analysis of PPAR-γ2 expression using quantitative real-time RT-PCR. Values were normalized to the expression of 18S rRNA, and bars represent average expression from three independent experiments; error bars indicate SD. Shaded bars, 6-month-old; black bars, 20-month-old. *P < 0.001.
This finding suggests that expression of PPAR-γ is increased in old marrow as compared with adult marrow. PPAR-γ nuclear receptor is expressed in two isoforms, PPAR-γ1 and PPAR-γ2, which result from alternative splicing and alternative promoter usage (Zhu et al., 1995). Although both isoforms play important roles in fat tissue homeostasis, recent evidence indicates that PPAR-γ2, but not PPAR-γ1, is essential for adipocyte formation (Ren et al., 2002). Moreover, whereas PPAR-γ2 expression is restricted to adipocytes, PPAR-γ1 is expressed in many cell types, including osteoblasts (Lecka-Czernik et al., 1999). Therefore, we focused our attention on the PPAR-γ2 isoform.
Quantitative real-time RT-PCR analysis revealed that the expression of PPAR-γ2 mRNA is 10-fold higher in old than in adult marrow (Fig. 4B). These data, together with the observations on the essential role of PPAR-γ2 in adipocyte development (Ren et al., 2002) (Tontonoz et al., 1994a; Mukherjee et al., 1997), the requirement of this isoform for terminal differentiation of marrow adipocytes and irreversible suppression of the osteoblast phenotype (Gimble et al., 1996b; Lecka-Czernik et al., 1999), indicate that PPAR-γ2 plays a critical role in the decision of mMSC to differentiate into either osteoblasts or adipocytes.
Aging increases production of adipocytic activator(s) and osteoblastic inhibitor(s) in the bone marrow
In vivo, two factors are necessary to achieve increased adipocyte differentiation: increased expression of PPAR-γ2 and increased availability of natural PPAR-γ ligand and/or activator. Therefore, we examined whether bone marrow produces an endogeneous PPAR-γ activator, and whether its level changes with aging. We used conditioned media derived from the cultures of adult and old marrow to assess their pro-adipocytic activities in U-33/γ2 and U-33/c cells (Lecka-Czernik et al., 1999). U-33/γ2 cells represent an in vitro model of mMSC differentiation that is under the control of the PPAR-γ2 transcription factor. U-33/c cells, which lack PPAR-γ2 but naturally express PPAR-γ1, served as a negative control for the processes mediated through the PPAR-γ2 isoform. As a positive control for adipocyte differentiation, U-33/γ2 cells were treated with rosiglitazone. Conditioned media collected from old bone marrow cultures induced fat accumulation in a significantly greater number of U-33/γ2 cells than conditioned medium collected from adult bone marrow cultures (Table 2). No effect on fat accumulation was seen in U-33/c cells (data not shown), indicating that the pro-adipocytic effects of tested conditioned media were mediated through PPAR-γ2.
Table 2.
Effects of conditioned medium derived from bone marrow cultures of adult and old mice on fat accumulation in U-33/γ2 cells
| Medium | ORO/total† | ORO (%) |
|---|---|---|
| Conditioned | ||
| 6-month | 40.7 (10.3)/475.0 (44.6) | 8.6 |
| 20-month | 107.3 (35.5)*/412.7 (30.9) | 26.0 |
| Rosiglitazone (5 μM) | 528.5 (136.5)/689.5 (207.2) | 76.6 |
Average number of cells calculated from five microscopic fields (20× magnification) ± SD (in parentheses).
ORO, cells positively stained for fat with Oil Red O.
P < 0.05 vs. 8-month-old animals.
The same conditioned media were also evaluated for effects on the osteoblastic phenotype of U-33/γ2 and U-33/c cells. Conditioned media from cultures of old marrow cells effectively suppressed the osteoblast phenotype in both U-33/γ2 and U-33/c cells, as measured by formation of mineralized extracellular matrix (Fig. 5). When cells were cultured in the presence of conditioned medium derived from cultures of adult bone marrow, neither cell line demonstrated a significant change in mineralization. These results indicate that old bone marrow not only produces PPAR-γ2 activator(s), but also possesses a PPAR-γ2-independent activity that inhibits osteoblast function. Whether this activity is mediated by the same or different factor(s) remains unclear.
Fig. 5.
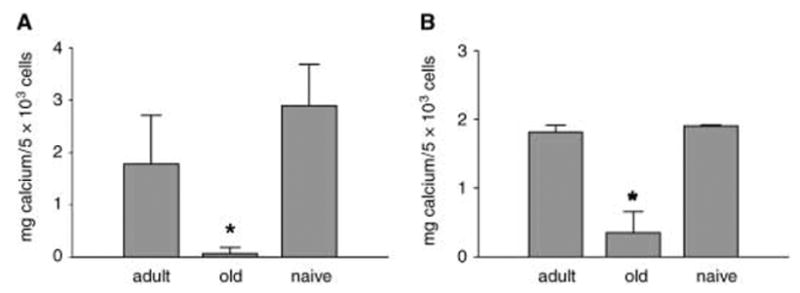
Effects of bone marrow-conditioned media on osteoblast differentiation of U-33/γ2 (A) and U-33/c cells (B). Cell cultures of U-33/γ2 and U-33/c were fed with conditioned media collected from 16th-day primary bone marrow cultures from adult (6-month) and old (20-month) animals, or naïve (non-conditioned medium), freshly supplemented with ascorbic acid and β-glycerophosphate as described in Experimental procedures. After 6 days of treatment extracellular calcium content was measured. *P < 0.05.
Effects of aging on mRNA expression of components of TGF-β/BMP signaling pathways
In bone, both TGF-β and BMP cytokines are produced mainly by cells of the mesenchymal lineage; they control osteoblast and adipocyte differentiation and are essential for bone formation and bone homeostasis. Therefore, changes in the balance between osteoblast and adipocyte differentiation during aging may result from changes in the activities of the TGF-β/BMP signaling pathways. To investigate this possibility, we analysed whether aging changes the expression of different components of these signaling pathways. mRNA expression of two genes encoding I-Smads, Smad6 and Smad7, was decreased in mMSC from old mice (Fig. 6). Because expression of Smad7 is under positive control of TGF-β cytokines, whereas expression of Smad6 is positively regulated by BMP2/4 cytokines, the decrease in expression of these two genes suggests decreased activities of the TGF-β/BMP signaling pathways. These decreased activities may result from changes in the levels of gene expression and activity of components that are limiting factors for TGF-β/BMP signaling. Indeed, whereas mMSC from old mice demonstrated an increase in the mRNA expression of TGF-β1 cytokine, they showed a significant decrease in the mRNA expression of two other cytokines, TGF-β2 and TGF-β3, and the receptor Tβ-R1 (Fig. 6A). Among signaling components of the BMP2/4 pathway, we observed an increase in the mRNA expression of BMP4 cytokine and a 10-fold decrease in the mRNA expression of the receptor BMPR-1B in mMSC derived from old animals (Fig. 6B).
Fig. 6.
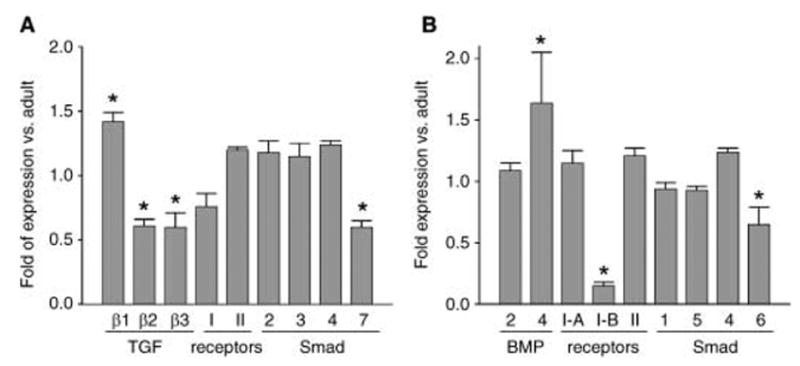
Expression analysis of components of TGF-β (A) and BMP2/4 (B) signaling pathways in mMSCs derived from adult (6-month) and old (20-month) mice. Quantitative real-time RT-PCR was performed as described in Experimental procedures. Values were normalized to the expression of GAPDH, and expression in mMSC from old mice is presented as fold increase or decrease of that in adult animals. Error bars indicate SD. *P < 0.001.
Discussion
We have presented evidence that, during aging, the status of mMSC changes with respect to both their intrinsic differentiation potential and production of signaling molecules that contribute to the formation of a specific marrow microenvironment necessary for maintenance of bone homeostasis. With aging, the number of mMSC committed to the adipocytic lineage increases, whereas the number of mMSC committed to the osteoblastic lineage decreases. Increased expression of the adipocyte-specific transcription factor PPAR-γ2 and increased production of its activator might be a driving force for pro-adipocytic and anti-osteoblastic changes in the differentiation potential of mMSC.
As osteoblasts and adipocytes originate from a common progenitor and PPAR-γ2 plays an important, although opposite, role in their differentiation, it is reasonable to hypothesize that with aging, differentiation toward adipocytes occurs at the expense of osteoblast differentiation. Similar age-related changes occur in another type of mesenchymal stem cell, muscle satellite cells (Taylor-Jones et al., 2002). With aging, satellite cells acquired adipocyte-like characteristics in part due to activation of PPAR-γ protein. Thus, it is possible that the common feature of mesenchymal stem cell aging is attaining an adipocytic phenotype. Whether this alteration has physiological reasons or it is just a default and/or by-product of the aging process remains unclear.
Production of an endogeneous PPAR-γ activator by adipocytes was reported previously, but the identity and nature of this activator has not yet been characterized (Kim et al., 1998). Natural ligands for PPAR-γ include polyunsaturated fatty acids and their oxidized forms, certain alkyl phospholipids, and derivatives of prostaglandin J2 (Kliewer et al., 1995, 1997; Davies et al., 2001). We previously reported that oxidized derivatives of linoleic acid, which are found in oxidized LDL and whose levels increase with aging, effectively activate pro-adipocytic and anti-osteoblastic properties of PPAR-γ2 in U-33/γ2 cells (Lecka-Czernik et al., 2002). In vivo, a high-fat atherogenic diet, which increases levels of oxidized LDL, elicited significant bone loss in mice (Parhami et al., 2001). By contrast, mice deficient in 12/15-lipoxygenase production, an enzyme responsible for fatty acid oxidization, exhibit increased bone mass (Klein et al., 2004). These observations suggest that fatty acids and their oxidized derivatives may be good candidates for endogenously produced PPAR-γ2 activators.
Another interesting feature of aging marrow is the production of anti-osteoblastic activity that inhibits the mineralization process in vitro, a hallmark of osteoblast phenotype and function in vivo. This activity is different from the pro-adipocytic activity discussed above because it affects the osteoblastic phenotype regardless of the presence of PPAR-γ2. It is a matter of speculation whether such activity is produced in vivo and whether it affects the function of osteoblasts involved in bone formation at the bone remodelling sites.
Finally, we have demonstrated that aging changes the expression of components of the TGF-β/BMP signaling pathways in mMSC, suggesting changes in the cellular responses to these cytokines. Indeed, the negative effect on Smad7 gene expression, which is positively regulated by TGF-β signaling, suggests that the activity of the TGF-β pathway is decreased with aging. Changes in the expression of TGF-β cytokines and a decrease in the expression of a common type I receptor may contribute to the decrease in the activity of this signaling pathway. It has been demonstrated that TGF-β activity and availability is decreased in murine bone during aging (Gazit et al., 1998). Consistent with TGF-β stimulating osteoblast proliferation and inhibiting adipocyte differentiation, a decrease in TGF-β activity with aging would lead to a decrease in the formation of new osteoblasts and an increase in the formation of new adipocytes. These are the features of mMSC aging that we have reported here.
Similarly, decreased expression of Smad6, which is positively regulated by BMP2/4 cytokines, indicates decreased activity of BMP2/4 signaling. Although BMP2/4 cytokines are necessary for bone formation and osteoblast maturation, they also synergize with signals promoting adipocyte differentiation (Sottile & Seuwen, 2000; Hata et al., 2003; Tang et al., 2004). BMP2/4 cytokines communicate with cells through two type I receptors, BMPR-1A and -1B, that form a complex with a common type II receptor. Studies in pre-osteoblastic 2T3 cells suggest that BMPR-1A controls adipocytic differentiation, whereas BMPR-1B controls osteoblastic differentiation (Chen et al., 1998). According to this model, our findings that aging increases the expression of BMP4 cytokine that commits stem cells to adipocyte lineage (Tang et al., 2004), and decreases the expression of BMPR-1B, a receptor which conveys osteoblast-specific signaling (Chen et al., 1998), are particularly interesting. These results suggest a switch with aging in the activity of the BMP signal transduction pathway from pro-osteoblastic to pro-adipocytic. This possibility needs further investigation.
At the dawn of development of new medical therapies that employ adult stem cell transplants to cure, repair or even grow a new organ, it is necessary to gain a better understanding of their biology and changes that occur in these cells with aging. We have shown that mesenchymal marrow stroma/stem cells undergo age-related changes. Therefore, the therapeutic potential of adult and aged mMSC differs and should be taken into account whenever these cells are considered for therapies against osteoporosis.
Experimental procedures
Animals
Adult (6–8-month-old) and old (20–26-month-old) C57BL/6 mice were obtained from the colony maintained by the National Institute of Aging under contractual agreement with Harlan Sprague Dawley, Inc. (Indianapolis, IN, USA). Animals were housed with free access to water and were maintained at a constant temperature on a 12-h light–dark cycle. The animal treatment and care protocols conformed to National Institute of Health guidelines and were performed using protocols approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee.
BMD measurements and bone histomorphometry
BMD was measured on anaesthetized animals using the small animal dual energy X-ray absorptiometry (DXA) instrument and software V1.46 (GE Lunar, Madison, WI, USA) (Rzonca et al., 2004). Internal variations in repeated measures of total murine body BMD have been determined to be 1.7–2.0%.
For bone histomorphometry measurements, undecalcified tibiae were embedded in methyl methacrylate and sectioned on an automatic, retractable Microtom 355 with a D-profile, tungsten carbide steel knife at 4 μm. Adjacent sections were stained with Masson Trichrome and Von Kossa (Jilka et al., 1996). The histomorphometric examination was performed using an OsteoMeasure system, which includes a Nikon microscope with motorized stage, interfaced with a computer and digitizer tablet (OsteoMetrics Inc., Atlanta, GA, USA). All cancellous measurements were two-dimensional, confined to the secondary spongiosa, and made using a 40× objective lens (numerical aperture 0.75). The terminology and units used were those recommended by the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research (Parfitt et al., 1987). Measurements were performed on six representative fields per bone sample.
Murine primary bone marrow cultures
Bone marrow cultures were established from femur marrow aspirates as previously described (Kajkenova et al., 1997) and maintained in basal medium consisting of α-MEM (Invitrogen, Carlsbad, CA, USA) supplemented with 15% heat-inactivated fetal bovine serum (FBS) (Hyclone, Logan, UT, USA), 100 U mL−1 penicillin, 100 μg mL−1 streptomycin and 0.25 μg mL−1 amphotericin at 37 °C in a humidified atmosphere containing 5% CO2.
Differentiation and assessment of adipocyte and osteoblast cultures
For differentiation assays, bone marrow isolates from individual mice (n = 8) were seeded separately in triplicate at a density of 2.5 × 105 cells cm−2 on six-well plates in basal medium. One-half of the medium was changed every 6 days.
To stimulate adipogenesis, after 10 days of growth, cultures were exposed for the next 3 days to IHI medium [0.5 mM iso-butylmethylxanthine (IBMX), 60 μM indomethacin, and 0.5 μM hydrocortisone (Sigma Chemical Co., St. Louis, MO, USA)]. The medium was then changed to basal medium and cells were maintained in culture for a further 3 days (Dorheim et al., 1993). Alternatively, after 10 days of growth in basal medium, cultures were maintained for 3 days in medium supplemented with 1 μM rosiglitazone (Tularik Inc., South San Francisco, CA, USA). Fat-containing cells were visualized with Oil Red O staining and adipogenesis was quantified by enumerating colonies containing at least 10% Oil Red O-positive cells (Lecka-Czernik et al., 2002).
To stimulate osteoblastogenesis, cells were maintained in osteoblastic medium (basal medium supplemented with 50 μg mL−1 ascorbic acid and 10 mM β-glycerophosphate) for 28 days. Mineralization was determined by Von Kossa staining.
Collection and testing activities of conditioned media
Primary bone marrow cultures (three independent bone marrow isolates per age group) were established at a density of 0.5 × 106 cells cm−2 and allowed to grow for 6 days with no media change, and then one-half of the media was replaced with fresh media every 2 days for the next 10 days. At these 2-day intervals, conditioned media were collected and frozen at −70 °C.
Murine marrow-derived UAMS-33 cells stably transfected with PPAR-γ2, referred to as U-33/γ2 cells, and UAMS-33 transfected with an empty vector, referred to as U-33/c cells, have been previously described (Lecka-Czernik et al., 1999). Cultures of U-33/γ2 and U-33/c cells were established in α-MEM supplemented with 10% heat-inactivated FBS and 30 mg mL−1 G418. When cultures were 70% confluent, cells were fed every 2 days for 6 days with conditioned media from 16-day bone marrow cultures that were preselected for their pro-adipocytic activity. At the end of the experiment, U-33/γ2 and U-33/c cells were stained for fat with Oil Red O, and fat cells were counted. As a positive control for adipocyte formation, cells were grown in the presence of 5 μM rosiglitazone for the same period of time as above cultures.
To assess the effects of bone marrow-conditioned media on the osteoblastic phenotype, U-33/γ2 and U-33/c cells were grown in the conditioned media collected from 16th-day primary bone marrow cultures; these conditioned media were freshly supplemented with pro-osteoblastic components (50 μg mL−1 ascorbic acid and 10 mM β-glycerophosphate). As a control for mineralization, U-33/γ2 and U-33/c cells were cultured in non-conditioned media, referred to as naïve, supplemented with 15% FBS, ascorbic acid and β-glycerophosphate. After 6 days of treatment, calcium content was measured using a calcium binding assay (Sigma Chemical Co.) as previously described (Lecka-Czernik et al., 2002).
Gene expression analysis using quantitative real-time RT-PCR
For RNA isolation, primary bone marrow cultures were established from pooled groups of marrow isolates in basal medium. Cells were plated at a density of 2 × 105 cells cm−2 on 100-mm plates. After 10 days of growth, RNA was isolated using RNAeasy (Qiagen, Valencia, CA, USA) and was subjected to DNase I digestion. Gene-specific primer sequences were selected using the Taqman Probe and Primer Design function of the Primer Express v1.5 software (Applied Biosystems, Foster City, CA, USA) and are listed in Table 3. Reverse transcription reactions were carried out using 2 μg RNA and TaqMan Reverse Transcription Reagents (Applied Biosystems), followed by real-time PCR using SYBR Green PCR Master Mix (Applied Biosystems) and ABI Prism 7700 Sequence Detection System (Applied Biosystems). Reactions were performed in the following cycling conditions: 95 °C for 10 min, then 40 cycles of 95 °C for 15 s followed by 60 °C for 1 min, with the exception for PPAR-γ2, which was performed in the following conditions: 95 °C for 10 min, then 40 cycles of 94 °C for 15 s, 53 °C for 15 s, and 72 °C for 20 s. Concentrations of primers and templates used in each reaction were optimized based on the standard curve created prior to the reaction and corresponding to nearly 100% efficiency of the reaction. Results were then normalized to expression of 18S rRNA in the same sample.
Table 3.
Sequences of primers used for quantitative real-time RT-PCR
| Gene | Accession no. | cDNA sequence (5′–3′) F, forward; R, reverse | Amplicon length (bases) | Corresponding cDNA sequence position |
|---|---|---|---|---|
| 18S rRNA | X56974 | F: TTCGAACGTCTGCCCTATCA | 49 | 1535–1555 |
| R: ATGGTAGGCACGGCGACTA | 1584–1566 | |||
| aP2 | NM_024406 | F: GCGTGGAATTCGATGAAATCA | 67 | 246–266 |
| R: CCCGCCATCTAGGGTTATGA | 313–294 | |||
| Osteocalcin | L24430 | F: CGGCCCTGAGTCTGACAAA | 208 | 1023–1041 |
| R: GCCGGAGTCTGTTCACTACCTT | 1231–1210 | |||
| Runx2/Cbfal | NM_009820 | F: GGGCACAAGTTCTATCTGGAAAA | 54 | 169–191 |
| R: CGGTGTCACTGCGCTGAA | 240–223 | |||
| Dlx5 | AF072453 | F: TGACAGGAGTGTTTGACAGAAGAGT | 64 | 184–208 |
| R: CGGGAACGGAGCTTGGA | 248–232 | |||
| α1(I)Collagen | NM_007742 | F: ACTGTCCCAACCCCCAAAG | 59 | 311–329 |
| R: CGTATTCTTCCGGGCAGAAA | 370–351 | |||
| PPAR-γ2 | U09138 | F: GCTGTTATGGGTGAAACTCTG | 351 | 34–54 |
| R: ATAAGGTGGAGATGCAGGTTC | 384–364 | |||
| TGF-β1 | NM_011577 | F: TACAGCAAGGTCCTTGCCCT | 62 | 1873−1892 |
| R: GCAGCACGGTGACGCC | 1935−1920 | |||
| TGF-β2 | BC011055 | F: CAACACCATAAATCCCGAAGC | 66 | 682–702 |
| R: GGTCAGTGGTTCCAGATCCTG | 748–728 | |||
| TGF-β3 | BC014690 | F: GCAACTAGCTATCTCAGGTCCCTT | 79 | 2221–2244 |
| R: CCAGGGAATACATGAGAGAACCA | 2300–2278 | |||
| TβR-1 | NM_009370 | F: AGCAGTGACTGCCATGCG | 67 | 2337–2354 |
| R: CAGGCTAAACGTCTCAACTGCA | 2404–2383 | |||
| TβR-2 | D32072 | F: CATGTGAGAAGAATAAAATACGAGAACA | 93 | 3615–3642 |
| R: AATGTGTAAGGGAAGTTGCCTATGT | 3708–3684 | |||
| BMP2 | AY050249 | F: AACTGGCTAGAATATTAAGCACTGCA | 71 | 6082–6107 |
| R: AGTGATTTCCTAACTGCCCAGG | 6153–6132 | |||
| BMP4 | BC052846 | F: TCAAGGGAGTGGAGATTGGG | 60 | 916–935 |
| R: GCCATCATGGCCAAAAGTG | 976–958 | |||
| BMPR-1 A | BC042611 | F: TGCATCAAGACTCCAATCCTGA | 83 | 2086–2107 |
| R: ACAGAAAGCACCACTTTATGGACA | 2169–2146 | |||
| BMPR-1B | BC065106 | F: GCTGGGCGCAGAATCCT | 73 | 1560–1576 |
| R: GGACTCTGACATTTTGGCAAGG | 1633–1612 | |||
| BMPRII | U78048 | F: TCCACCTGGGTCATCTCCA | 63 | 2975–2993 |
| R: CCCTGTCACTGCCATTGTTG | 3038–3019 | |||
| Smad1 | BC058693 | F: TCCGTCTCTTGCAAACTATCGA | 75 | 1809−1830 |
| R: TTCGTCAGGTCTCCATCCTGT | 1884−1864 | |||
| Smad2 | BC021342 | F: CCCTTCAGTGCGATGCTCA | 74 | 1504–1522 |
| R: GAATACTACGACGGAGGAGCTGTT | 1578–1555 | |||
| Smad3 | NM_016769 | F: CACGCAGAACGTGAACACC | 100 | 485–503 |
| R: GGCAGTAGATAACGTGAGGGA | 585–565 | |||
| Smad4 | BC046584 | F: ACAGAGAACATTGGATGGACGA | 69 | 662–683 |
| R: ACGGGCATAGATCACATGAGG | 731–711 | |||
| Smad5 | BC050001 | F: CAAGGGCCTTGCCTGCT | 76 | 3469–3483 |
| R: GTCCGAGACCTATGACATGAAGACT | 3545–3521 | |||
| Smad6 | BC047280 | F: TGGCTGGAGATCCTACTCAACA | 62 | 1753–1774 |
| R: GGACGCTGCGGCACAG | 1815−1800 | |||
| Smad7 | NM_008543 | F: GCCCCCCCTTCCTGCT | 63 | 3103–3119 |
| R: CCAGCCAAGGGATGGTACC | 3166–3148 |
Statistical analysis
Statistically significant differences between groups were detected using one-way ANOVA followed by post-hoc analysis by Student–Neuman–Keuls within the SigmaStat software (SPSS, Inc., Chicago, IL, USA) after establishing the homogeneity of variances and normal distribution of data. In all cases, P < 0.05 was considered significant.
Acknowledgments
We thank Dr Charlotte Peterson for critical reading and valuable comments regarding this manuscript. We also thank the Office of Grants and Scientific Publications at the University of Arkansas for Medical Sciences for editorial assistance during the preparation of this manuscript. This work was supported by National Institute on Aging grant R01 AG17482 and American Diabetes Association research grant 1-03-RA-46.
References
- Abe E, Yamamoto M, Taguchi Y, Lecka-Czernik B, O’Brian CA, Economides AN, Stahl N, Jilka RL, Manolagas SC. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J Bone Miner Res. 2000;15:663–673. doi: 10.1359/jbmr.2000.15.4.663. [DOI] [PubMed] [Google Scholar]
- Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliston T, Choy L, Ducy P, Karsenty G, Derynck R. TGF-beta-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 2001;20:2254–2272. doi: 10.1093/emboj/20.9.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee C, Javed A, Choi JY, Green J, Rosen V, van Wijnen AJ, Stein JL, Lian JB, Stein GS. Differential regulation of the two principal Runx2/Cbfa1 n-terminal isoforms in response to bone morphogenetic protein-2 during development of the osteoblast phenotype. Endocrinology. 2001;142:4026–4039. doi: 10.1210/endo.142.9.8367. [DOI] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, Celeste AJ, Rosen V, Mundy GR, Harris SE. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol. 1998;142:295–305. doi: 10.1083/jcb.142.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy L, Derynck R. Transforming growth factor-beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J Biol Chem. 2003;278:9609–9619. doi: 10.1074/jbc.M212259200. [DOI] [PubMed] [Google Scholar]
- Choy L, Skillington J, Derynck R. Roles of autocrine TGF-beta receptor and Smad signaling in adipocyte differentiation. J Cell Biol. 2000;149:667–682. doi: 10.1083/jcb.149.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SS, Pontsler AV, Marathe GK, Harrison KA, Murphy RC, Hinshaw JC, Prestwich GD, St Hilaire A, Prescott SM, Zimmerman GA, McIntyre TM. Oxidized phospholipids are specific, high affinity PPAR{gamma} ligands and agonists. J Biol Chem. 2001;26:26. doi: 10.1074/jbc.M100878200. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signaling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Dorheim MA, Sullivan M, Dandapani V, Wu X, Hudson J, Segarini PR, Rosen DM, Aulthouse AL, Gimble JM. Osteoblastic gene expression during adipogenesis in hematopoietic supporting murine bone marrow stromal cells. J Cell Physiol. 1993;154:317–328. doi: 10.1002/jcp.1041540215. [DOI] [PubMed] [Google Scholar]
- Devlin RD, Du Z, Pereira RC, Kimble RB, Economides AN, Jorgetti V, Canalis E. Skeletal overexpression of noggin results in osteopenia and reduced bone formation. Endocrinology. 2003;144:1972–1978. doi: 10.1210/en.2002-220918. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Frost HM. Bone Remodeling and its Relationship to Metabolic Bone Disease. Springfield, MA: Charles C. Thomas; 1973. [Google Scholar]
- Gazit D, Zilberman Y, Ebner R, Kahn A. Bone loss (osteopenia) in old male mice results from diminished activity and availability of TGF-beta. J Cell Biochem. 1998;70:478–488. doi: 10.1002/(sici)1097-4644(19980915)70:4<478::aid-jcb5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- von Gersdorff G, Susztak K, Rezvani F, Bitzer M, Liang D, Bottinger EP. Smad3 and Smad4 mediate transcriptional activation of the human Smad7 promoter by transforming growth factor beta. J Biol Chem. 2000;275:11320–11326. doi: 10.1074/jbc.275.15.11320. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Robinson CE, Wu X, Kelly KA. The function of adipocytes in the bone marrow stroma: an update. Bone. 1996a;19:421–428. doi: 10.1016/s8756-3282(96)00258-x. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Robinson CE, Wu X, Kelly KA, Rodrigue ZBR, Kliewer SA, Lehmann JM, Morris DC. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol Pharm. 1996b;50:1087–1094. [PubMed] [Google Scholar]
- Hata K, Nishimura R, Ikeda F, Yamashita K, Matsubara T, Nokubi T, Yoneda T. Differential roles of Smad1 and p38 kinase in regulation of peroxisome proliferator-activating receptor gamma during bone morphogenetic protein 2-induced adipogenesis. Mol Biol Cell. 2003;14:545–555. doi: 10.1091/mbc.E02-06-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida W, Hamamoto T, Kusanagi K, Yagi K, Kawabata M, Takehara K, Sampath TK, Kato M, Miyazono K. Smad6 is a Smad1/5-induced smad inhibitor. Characterization of bone morphogenetic protein-responsive element in the mouse Smad6 promoter. J Biol Chem. 2003;275:6075–6079. doi: 10.1074/jbc.275.9.6075. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzale ZXR, Reyes M, Lenvik T, Lund T, Du Blackstad MJ, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Takahashi K, Parfitt AM, Manolagas SC. Linkage of decreased bone mass with impaired osteoblastogenesis in a murine model of accelerated senescence. J Clin Invest. 1996;97:1732–1740. doi: 10.1172/JCI118600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajkenova O, Lecka-Czernik B, Gubrij I, Hauser SP, Takahashi K, Parfitt AM, Jilka RL, Manolagas SC, Lipschitz DA. Increased adipogenesis and myelopoiesis in the bone marrow of SAMP6, a murine model of defective osteoblastogenesis and low turnover osteopenia. J Bone Miner Res. 1997;12:1772–1779. doi: 10.1359/jbmr.1997.12.11.1772. [DOI] [PubMed] [Google Scholar]
- Karsenty G. Minireview: transcriptional control of osteoblast differentiation. Endocrinology. 2001;142:2731–2733. doi: 10.1210/endo.142.7.8306. [DOI] [PubMed] [Google Scholar]
- Kim JB, Wright HM, Wright M, Spiegelman BM. ADD1/SREBP1 activates PPARgamma through the production of endogenous ligand. Proc Natl Acad Sci USA. 1998;95:4333–4337. doi: 10.1073/pnas.95.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RF, Allard J, Avnur Z, Nikolcheva T, Rotstein D, Carlos AS, Shea M, Waters RV, Belknap JK, Peltz G, Orwoll ES. Regulation of bone mass in mice by the lipoxygenase gene Alox15. Science. 2004;303:229–232. doi: 10.1126/science.1090985. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Lecka-Czernik B, Gubrij I, Moerman EA, Kajkenova O, Lipschitz DA, Manolagas SC, Jilka RL. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPAR-gamma 2. J Cell Biochem. 1999;74:357–371. [PubMed] [Google Scholar]
- Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143:2376–2384. doi: 10.1210/endo.143.6.8834. [DOI] [PubMed] [Google Scholar]
- Lee KS, Kim HJ, Li QL, Chi XZ, Ueta C, Komori T, Wozney JM, Kim EG, Choi JY, Ryoo HM, Bae SC. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol. 2000;20:8783–8792. doi: 10.1128/mcb.20.23.8783-8792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Manolagas SC. Cellular and molecular mechanisms of osteoporosis. Aging. 1998;10:182–190. doi: 10.1007/BF03339652. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol. 2001;187:265–276. doi: 10.1002/jcp.1080. [DOI] [PubMed] [Google Scholar]
- Moore SG, Dawson KL. Red and yellow marrow in the femur: age-related changes in appearance at MR imaging. Radiology. 1990;175:219–223. doi: 10.1148/radiology.175.1.2315484. [DOI] [PubMed] [Google Scholar]
- Mukherjee R, Jow L, Croston GE, Paterniti JR., Jr Identification, characterization, and tissue distribution of human peroxisome proliferator-activated receptor (PPAR) isoforms PPARgamma2 versus PPARgamma1 and activation with retinoid X receptor agonists and antagonists. J Biol Chem. 1997;272:8071–8076. doi: 10.1074/jbc.272.12.8071. [DOI] [PubMed] [Google Scholar]
- Nishimura R, Harris SE, Hata K, Rubenstein J, Yoneda T. Essential role of the transcription factors Dlx5 and Cbfa1 during osteoblastic differentiation. J Bone Miner Res. 1999;14:S144. [Google Scholar]
- Nuttall ME, Patton AJ, Olivera DL, Nadeau DP, Gowen M. Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: implications for osteopenic disorders. J Bone Miner Res. 1998;13:371–382. doi: 10.1359/jbmr.1998.13.3.371. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Urano T, Hosoi T, Miyao M, Hoshino S, Fujita M, Shiraki M, Orimo H, Ouchi Y, Inoue S. Association of bone mineral density with a polymorphism of the peroxisome proliferator-activated receptor gamma gene: PPARgamma expression in osteoblasts. Biochem Biophys Res Commun. 1999;260:122–126. doi: 10.1006/bbrc.1999.0896. [DOI] [PubMed] [Google Scholar]
- Owen ME, Cave J, Joyner CJ. Clonal analysis in vitro of osteogenic differentiation of marrow CFU-F. J Cell Sci. 1987;87:731–738. doi: 10.1242/jcs.87.5.731. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem. 1994;55:273–286. doi: 10.1002/jcb.240550303. [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Parhami F, Tintut Y, Beamer WG, Gharavi N, Goodman W, Demer LL. Atherogenic high-fat diet reduces bone mineralization in mice. J Bone Miner Res. 2001;16:182–188. doi: 10.1359/jbmr.2001.16.1.182. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999;72:570–585. [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Ren D, Collingwood TN, Rebar EJ, Wolffe AP, Camp HS. PPAR-gamma knockdown by engineered transcription factors: exogenous PPARgamma2 but not PPARgamma1 reactivates adipogenesis. Genes Dev. 2002;16:27–32. doi: 10.1101/gad.953802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey PG, Bianco P. Cellular mechanisms of age-related bone loss. In: Rosen C, Bilezikian JP, editors. The Aging Skeleton. Academic Press; San Diego, CA: 1999. pp. 145–157. [Google Scholar]
- Rosen ED, Spiegelman BM. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004;145:401–406. doi: 10.1210/en.2003-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Sottile V, Seuwen K. Bone morphogenetic protein-2 stimulates adipogenic differentiation of mesenchymal precursor cells in synergy with BRL 49653 (rosiglitazone) FEBS Lett. 2000;475:201–204. doi: 10.1016/s0014-5793(00)01655-0. [DOI] [PubMed] [Google Scholar]
- Stopa M, Anhuf D, Terstegen L, Gatsios P, Gressner AM, Dooley S. Participation of Smad2, Smad3, and Smad4 in transforming growth factor beta (TGF-beta)-induced activation of Smad7. THE TGF-beta response element of the promoter requires functional Smad binding element and E-box sequences for transcriptional regulation. J Biol Chem. 2000;275:29308–29317. doi: 10.1074/jbc.M003282200. [DOI] [PubMed] [Google Scholar]
- Tang QQ, Otto TC, Lane MD. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci USA. 2004;101:9607–9611. doi: 10.1073/pnas.0403100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli M. Marrow adipose cells and hemopoiesis: an interpretative review. Exp Hematol. 1984;12:139–146. [PubMed] [Google Scholar]
- Taylor-Jones JM, McGehee RE, Rando TA, Lecka-Czernik B, Lipschitz DA, Peterson CA. Activation of an adipogenic program in adult myoblasts with age. Mech Ageing Dev. 2002;123:649–661. doi: 10.1016/s0047-6374(01)00411-0. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994a;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994b;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Werner AL, Travaglini MT. A review of rosiglitazone in type 2 diabetes mellitus. Pharmacotherapy. 2001;21:1082–1099. doi: 10.1592/phco.21.13.1082.34615. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Qi C, Korenberg JR, Chen XN, Noya D, Rao MS, Reddy JK. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: alternative promoter use and different splicing yield two mPPAR gamma isoforms. Proc Natl Acad Sci USA. 1995;92:7921–7925. doi: 10.1073/pnas.92.17.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]


