Abstract
The purpose of these studies was to determine the minimal requirements to induce granzyme B, cytotoxic granules and perforin-dependent lytic capacity. To our surprise, both IL-2 and IL-15 induced not only proliferation, but also profound granzyme B and lytic capacity from CD8+ T cells in the absence of antigen or TCR-stimulation. Mouse splenocytes were incubated with mouse r-IL-2 or r-IL-15 for three days, tested by anti-CD3 redirected lysis and examined for intracellular granzyme B and for T cell activation markers. With 10−8 M IL-2 or IL-15, there was excellent lytic activity at 1:1 effector to target ratios mediated by T cells from wild type but not from perforin-gene-ablated mice, consistent with multiclonal activation. Lower interleukin concentrations induced less lytic activity. Granzyme B was undetectable on day 0, and greatly elevated on day 3 in CD44hi CD8+ T cells as detected by flow cytometry. Cytokines alone elevated the granzyme B as much as concanavalin A combined with the cytokines. Some ex vivo CD8+ T cells were CD122+, as were the cultured granzyme B+ cells, thus both populations had low affinity receptors for the interleukins. Only some of the activated cells were proliferating as detected by CFSE labeling. When the cytokines were withdrawn, the cells lost lytic activity within 24 hours and then within the next 24 hours, died. Our results suggest that high concentrations of either IL-2 or IL-15 will activate the lytic capacity and granzyme B expression of many T cells and that antigen recognition is not required.
Keywords: T Cells, Cytotoxic, Cytokines, Cell Activation, Cell proliferation, Cytotoxicity. Granzyme B
1. Introduction
T cells, removed from a naïve mouse, lack the granules necessary to kill target cells. It is an unresolved issue how to activate the cytotoxic mechanisms of T killer lymphocytes. It is generally assumed that activation of cytotoxicity requires two or more signals including one initiated by antigen [1,2] and that bystander activation is rare [3]. Recently, Dr. Ann Kelso and colleagues demonstrated that IL-2 regulates perforin and granzyme message independently of its effects on cell growth [4]. In those studies, the T cells were TCR-stimulated1. In a serendipitous experimental control, we observed that high IL-2 or high IL-15 without antigen induced extremely high cytotoxicity. We monitored cytotoxicity with redirected lysis rather than with antigenic target cells and thus detected multiclonal activation by both cytokines. It is known that IL-2 or IL-15 will activate NK cells without receptor stimulation [5,6]. IL-15 without antigen(s) activates cytotoxic capacity of human T cells with a memory-associated phenotype and may also activate naïve human CD8+ T cells [7,8]. However, both characterization of granzyme B induction and a direct comparison between IL-15 and IL-2 for their ability to induce antigen-independent cytotoxic T cell activation were lacking until our study.
Differences between the effects of IL-2 and IL-15 would be anticipated only at the low concentrations at which these interleukins interact with different receptors. IL-2 and IL-15 are T cell growth factors that support adaptive immune responses [9,10]. Both cytokines share a pair of receptor subunits, the gamma chain common to several cytokine receptors (γc, CD132) combined with the IL-2/15 beta receptor chain (IL-2Rβ, CD122) [11]. Binding of either IL-2 or IL-15, with Kd’s ~10−9 M [12,13,14], will activate this dimeric receptor to transmit intracellular signals via the JAK1/3-STAT3/5 pathways [15,16,17]. There are substantial numbers of these CD122/CD132 receptors on T cells and, at high concentrations (10−8 M) of either IL-2 or IL-15, these receptors will be saturated. IL-2 and IL-15 each have specific high affinity receptors (Kd ~ 10−11 M) that are formed when separate specific alpha receptor chains combine into trimeric receptors with the CD122/CD132 pair [18,19]. The IL-2Rα chain (CD25) in its trimer is thought to mediate subsequent signaling via the CD122/CD132 pair. T cell activation with antigens induces high cell surface expression of CD25. Thus, with moderate levels of IL-2 (10−10 M) and after antigen stimulation, many specific trimeric IL-2 receptors can be activated. The situation is somewhat different for the IL-15α receptors. There are far fewer of these IL-15α’s per T cell [20,21] and the intracellular signaling is less well defined. These differences between high affinity receptors for IL-2 vs. IL-15 contribute to differential T cell growth responses and might be expected to cause differences between the two cytokines for induction of cytotoxicity.
For induction of cytotoxicity, which in our case is without antigen, we evaluated low interleukin concentrations that would saturate only specific trimer receptors as well as higher concentrations that would saturate the dimeric receptors. Engagement of the low affinity dimeric receptors (by either interleukin) induced the most cytotoxic capacity. We use the word ‘capacity’ advisedly because, physiologically, in the absence of antigen, the capacity would normally go unnoticed. We detected this capacity with anti-CD3 redirected lysis of P815 cells and then extended our investigation to characterize intracellular levels of GrzB, the phenotype of the multiclonally activated cells, and the stability of their induced lytic capacity.
The antigen-independent effects of IL-2 and IL-15 that we report here have both theoretical and practical implications. The theoretical implication is that bystander cytotoxicity and granzyme B can be induced in memory phenotype T cells without antigen if there are sufficient cytokines. The practical implications affect IL-2 and IL-15 as therapeutic agents. IL-2 has been used in several clinical trials for conditions ranging from cancer to AIDS, primarily to enhance lymphocyte proliferation and function [22,23,24,25]. These trials may have produced unrecognized and significant side effects on T cell cytotoxic capacity and caused the subsequent death of the activated memory cells upon cytokine withdrawal.
In this report, we have used exclusively murine cytokines with murine lymphocytes and compared IL-2 and IL-15 concurrently. Both cytokines similarly induce cytotoxic capacity and granzyme B in an antigen-independent process when their concentrations are sufficient to be bound by the CD122/CD132 dimeric receptors. The induced cells included CD4+ T cells as well as CD8+ T cells and both had the CD44hi phenotype after induction.
2. Materials and Methods
2.1 Animal Studies
The animal protocols for this study were approved by the University of Nevada Animal Use Review Committee.
2.2 Cell Culture
Spleens were harvested from C57BL/6 mice (Jackson Labs or the NCI) and the cells cultured at 5x105 cells/ml in 24 well plates (CoStar) with RPMI-1640 media (Sigma), 10% FBS (HyClone), and 1% Pen-Strep (Sigma) with or without mouse recombinant (r-) IL-2 or IL-15 (PeProtech or eBiosciences). The specific activity of PeProtech r-IL-2 was 5x106 CTLL-2 growth units per mg protein and 8.6x1013 units/mole. The specific activity of PeProTech r-IL-15 was 2x105 units CTLL-2 growth units per mg protein and 2.66x1012 units/mole. Incubation was at 37°C with 5% CO2. Cytokine stimulated cultures received 10−8 M IL-2 or IL-15 unless otherwise stated. Pfn−/− C57BL/6 mice were obtained from Jackson Labs. In some experiments, the cells also received 2.5 ug/ml concanavalin A (Sigma).
2.3 Flow Cytometric Analysis
Cells were harvested and phenotyped with mAbs to the following mouse antigens: CD3e (hamster IgG, clone 145-2C11), CD4 (rat IgG2b, clone GK1.5), CD8a (rat IgG2a, clone 53–6.7), ICOS (hamster IgG, clone C398.4A)), NKG2D (rat IgG1, clone CX5), CD25 (rat IgG1, clone PC61.5), CD28 (hamster IgG, clone 37.51), CD38 (rat IgG2a, clone 90), CD69 (hamster IgG, clone H1.2F3), PD-1 (hamster IgG, clone J43), CD11a (rat IgG2a, clone M17/4), CD2 (rat IgG2b, clone RM2-5), NK1.1 (rat IgG2a, clone PK136), CD71 (rat IgG2a, clone R17217), and anti-mouse/human CD44 (rat IgG2b, clone IM7). The fluorochromes included fluorescein (FITC), R-fluorsphycoerythrin (PE), PE-cyanine 5 (PC5) and PE-cyanine 7 (PC7) as indicated in the figures. After surface labeling, the cells were washed, fixed, and permeabilized (IntraPrep Kit, Beckman Coulter, Fullerton, CA). Grz B was detected with PE anti-human Grz B mAb (mouse IgG1, clone GB-12) that cross-reacts with mouse Grz B and fails to react with mouse GrzB−/− T cells (data not shown). Samples were measured with a Beckman Coulter XL/MCL flow Cytometer with optical filters to detect 525, 575, 670, and >740nm. The data were analyzed with FlowJo software (Tree Star, Inc., Ashland, OR), including proliferation kinetics. For histograms, the “relative cell number” ordinate value represents a scale normalized to 95% as equal to the number of cells in the histogram channel with the most cells. Recovered cell numbers were normalized to a known bead standard added to each tube (Polymer Laboratories, 9.2um diameter, Polymer Laboratories, Amherst, MA).
2.4 Proliferation Assay
Cells were treated with 2.5μM CSFE (Molecular Probes) for five minutes, washed and cultured for 72 hours with or without cytokines. Harvested cells were labeled with antibodies and assayed as described above.
2.5 Cytotoxicity Assays
T cell lysis was redirected to the FcIgGR-bearing P815 cells with anti-mouse CD3 mAb (hamster IgG, clone 145-2C11). P815 target cells were loaded with Na51CrO4 (Amersham CJS4) and assays run in quadruplicate with 10,000 radio-labeled cells per well for 4 hours. Specific release was calculated by the formula: % Specific Release = [(Experimental CPM) - (Spontaneous Release)] / [(SDS Total Release) - (Spontaneous Release)]. Splenocyte: target ratios were determined by taking aliquots of cells used for the lytic assay and performing hemocytometer counts. CD8+ effector:target ratios were calculated by using the splenocyte population and multiplying it by the fraction of CD3+CD8+ as determined by flow cytometry. Comparison of the activities of different cytotoxic T cells was made by comparing lytic units of activity per 109 effector cells, where 1 lytic unit is defined as the number of cells that will kill half of the 10,000 targets in the 4 hour assay [26].
2.6 Assay for Granule Retention/Cell Survival
After culture for 72 hours as described, the cells were harvested and washed. Experimental groups were held in new media for 2 hours before being recultured in fresh media with 10−8 M IL-15, 10−8 M IL-2 or without cytokine. Flow cytometry was used to detect the CD8+ T cells and their intracellular Grz B content as described above.
2.7 Reproducibility and Statistics
Representative experiments are illustrated from at least three replicate experiments, with the exception of the experiment depicted in Figure 5 which was done twice in its entirety. Standard errors of the mean were calculated for cytotoxic assays and are indicated where appropriate. Student’s t tests were applied to these assays to determine the significance of the results. In cases where flow cytometry was used to determine the frequency of T cell subpopulations, the means were calculated for three or more tubes in the same experiment as indicated. The flow cytometric data were also subjected to Overton subtraction statistical analyses [27] to assess frequency of antibody-positive cells in the populations.
Figure 5. After cytokine withdrawal, cytokine-activated CD8+ T cells survived for 24 hours but lost their cytotoxic capacity and Grz B.
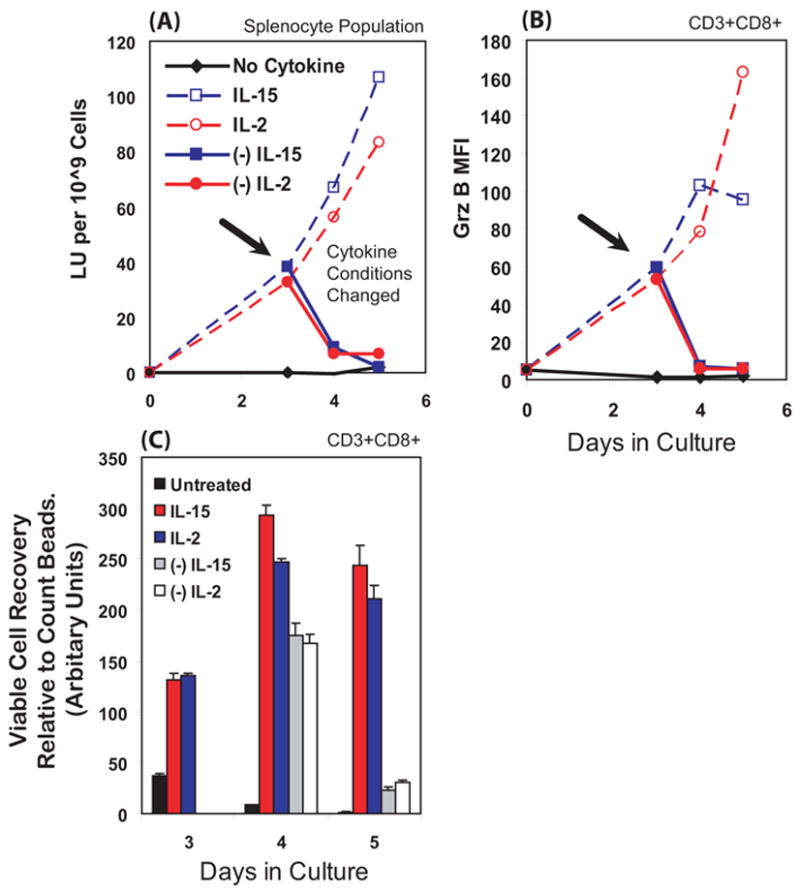
Splenocytes were initially cultured for 3 days with IL-2, IL-15, or no cytokine. On day 3, the cells were washed and re-cultured with renewed cytokine or without cytokine. At day 3, the withdrawal of cytokine from cells is indicated by the arrow and by the closed symbols with solid lines. The dashed lines represent the cells re-cultured with new media and cytokine. Cells were stained and Grz B MFI determined as in figure 2. Cytotoxicity was determined as in Figure 1 and is expressed in lytic unit (LU) per 109 splenocytes. We monitored viable cell recovery (by forward and side scatter, and confirmed by exclusion of propidium iodide) using internal calibration beads added as flow standards to quantify the relative number of viable CD3+CD8+ T cells after cytokine withdrawal. (A) Cells deprived of cytokine lost most of their lytic capability within 24 hours. (B) Grz B, as indicated by MFI values, was also lost within 24 hours after cytokine withdrawal. Replenishing cytokine results in a general increase in Grz B. (C) For 24 hours after cytokine withdrawal, the CD3+CD8+ T cells remained viable and in similar in numbers to before cytokine withdrawal. If given cytokine they continued to grow for 24 hours. After another 24 hours, the cytokine-deprived T cells had died and disintegrated within the cultures. The cells re-cultured with cytokines remained viable. Taken together, these data indicate that the T cells lose cytotoxicity well before they die.
3. Results
3.1 Selection of day 3 and 10−8 M concentrations of IL-15 or IL-2 as the conditions for comparison of antigen-independent activation of T cell cytotoxic capacities
Given the differing proliferative units/ml between IL-15 and IL-2 at 10−8 M (860 u/ml and 27 u/ml respectively), we chose an early time of splenic cell culture, day 3, in order to minimize potential differences induced by selective proliferation of particular T cell subsets. On day 3, proliferation in cytokine-treated populations had just begun and later increased on days 4 and 5 (not illustrated). Anti-CD3 antibody redirected lysis of FcIgG-receptor-bearing P815 cells was performed to monitor the cytotoxic capacity of all the T cells, regardless of antigen specificity. IL-15 induced a strong antigen-independent cytotoxic capability at 10−8 M, as illustrated by a dose dependent response in which 5 x 10−9 M IL-15 was nearly as effective (Fig. 1A). A dose titration using IL-2 yielded a similar result with the highest cytotoxicity at 10−8 M IL-2 (Fig. 1B). To illustrate more effectively that 10−8 M IL-2 and IL-15 induced comparable antigen-independent cytotoxicity, we expanded splenocytes concurrently in either cytokine and found similar lytic activity (Fig. 1C). The high redirected cytotoxic activity at a CD3+CD8+ T cell effector to target (E:T) ratio of 1:1 indicates a high frequency of killer cells in the population and suggests activation of a large number of different T lymphocytes within the original cell population. The activity without anti-CD3 antibody was tenfold less (based on lytic units, not illustrated), indicating that the redirected lysis was T cell-dependent. Even though there were CD3− CD16+ NK cells present in the 3 day cultures, the NK cells lacked activity towards the P815 targets. It is possible that the lysis without anti-CD3 antibody was due to a subset of T cells with allogeneic cross reactivity, arising from the C57BL/6 effectors (H2b) vs. P815 (H2d) targets. The dependence of 90% of the cytotoxicity on anti-CD3 antibody was measured and observed in all the experiments reported in this paper. Furthermore, we detected no anti-CD3 redirected lysis by splenocytes cultured from Pfn−/− mice (not illustrated), indicating that we are monitoring only perforin-dependent cytotoxicity. The concentrations of IL-2 and IL-15 that induced highest observed antigen-independent cytotoxicity were sufficient to saturate the shared IL-2/15 receptor consisting of beta and gamma chains (CD122-CD132).
Figure 1. Culture with IL-15 or IL-2, without antigen, induced cytotoxic capacity in T cells.
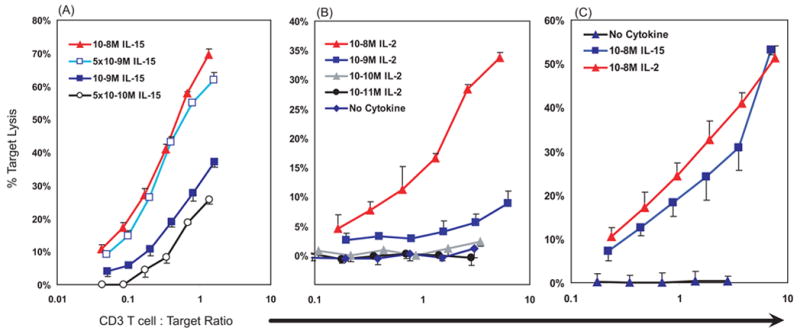
Mouse splenocytes were cultured with IL-15, IL-2 or no cytokine for 3 days. Cytotoxicity was assayed by anti-CD3 antibody-redirected lysis against51Cr-P815 targets. The abscissa indicates the effector CD3+ T cell to target cell ratios (potential E:T) as indicated, on a log10 scale. (A) and (B) Dose titrations of either IL-2 or IL-15. Antigen-independent cytotoxic activity was optimal at 10−8 M concentrations and readily detectable at E:T ratios of 1:1. Additional assays without anti-CD3 antibody (not illustrated) demonstrated ~10 fold less cytotoxic activity by lytic units, indicating that most of the activity illustrated represents TCR-dependent cell responses. (A) and (B) depict independent experiments, while (C) illustrates a single, representative experiment that indicates that IL-2 or IL-15 induce similar cytotoxic potentials.
3.2 Grz B is induced in CD8+ and CD4+ T lymphocytes by both cytokines without antigen
When analyzed directly ex vivo, murine T cells lack detectable Grz B (Fig. 2A & D), but both CD8+ and CD4+ T cells can be induced without antigen to express Grz B (Fig. 2B and E). After 3 days in culture with 10−8 M IL-15 or IL-2, the CD8+ T cell population acquired high levels of Grz B (MFI = ~50) relative to the control series receiving no cytokine (MFI = ~1) and relative to the isotype control for intracellular anti-granzyme labeling (MFI = ~2.5) (Fig. 2A–C). We estimated that 77.5% of the IL-15 treated CD8+ T cells were Grz B+ and 80% of the IL-2 treated cells were Grz B+ using Overton subtraction statistical comparisons with the PE-isotype labeled cells. A similar MFI for Grz B was induced when cells were also stimulated with con A and cytokines, indicating that the antigen-independent Grz B had attained extremely high levels. Con A stimulates granzyme B mRNA even better than allogeneic stimulation [28]. Approximately 30% of the CD8(−) T cells also developed a distinct Grz B+ population when treated with either IL-15 or IL-2 (with MFIs ~90–100) relative to the culture without cytokine (MFI = ~1) and to the isotype controls (MFI = ~1) (Fig. 3D–F). In the experiments illustrated, the fourth antibody was to NK1.1, which we used as a concurrent label to establish that the CD4+ and CD8+ T cells illustrated differed from NKT cells. In other experiments we used direct staining for anti-CD4 and observed that >95% of the CD3+CD8(−) cells were CD3+CD4+ and that ~25–30% of the CD4+ directly stained cells were positive for granzyme B (data not illustrated).
Figure 2. Both IL-2 and IL-15 induced Grz B in T cells.
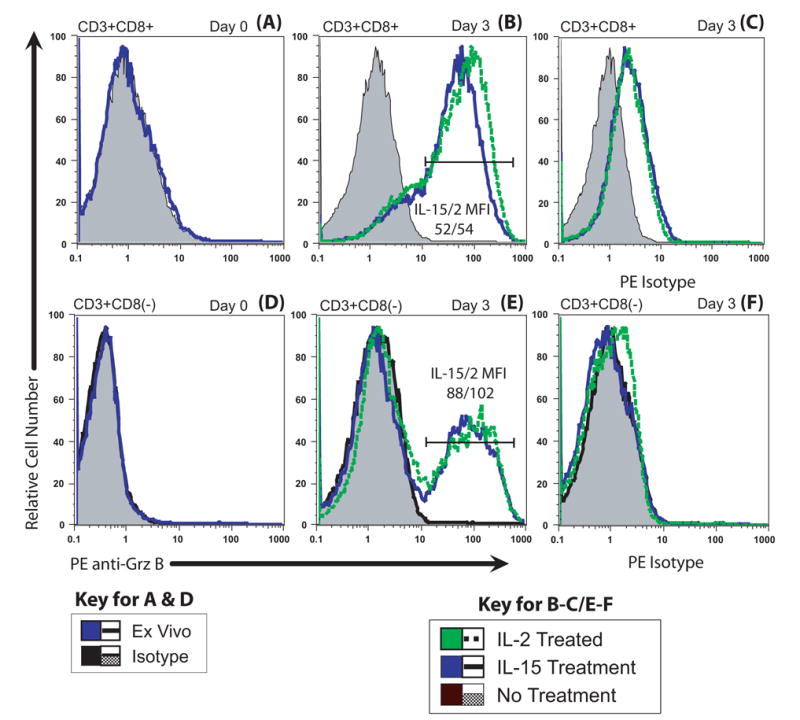
Both CD8+ and CD8(−) T cells (>95% CD4+ cells) were induced to express granzyme B. Splenocytes were cultured alone or with 10−8 M IL-2 or IL-15 for three days. On days 0 and 3, the cells were surface-labeled with FITC-anti NK1.1, PE-Cy5 anti-CD3 and PE-Cy7 anti-CD8, then fixed and permeabilized and reacted with PE anti-Grz B or PE-isotype. The histograms are displayed with relative cell numbers (see Methods). (A) CD8+ T cells possessed little or no Grz B on day 0. (B) Grz B levels of CD8+ T cells increased greatly with cytokine treatment. Both IL-2 and IL-15 induced similar levels of granule formation based on Grz B expression. Both cytokine-treated populations were highly positive, with a day 3 MFI of 52 for IL-15 and 54 for IL-2. Approximately 80% of both the IL-2 treated and IL-15 treated CD8 T cells were positive for Grz B by Overton subtraction. (C) Isotype staining of the CD8+ T cells demonstrated minimal non-specific binding of antibody. (D) The CD3+CD8(−)(CD4) T cells also had no detectable Grz B ex vivo, a condition identical to the CD8+ cells (part A). (E) Approximately 40% of the surviving CD8(−) (CD4) T cells became Grz B+ by day 3 in response to either IL-2 and IL-15, suggesting that a subpopulation of CD4+ T cells responds. MFIs of the Grz B-positive CD4+ T populations were 88 for IL-15 and 102 for IL-2, similar to the MFIs of Grz B-staining of CD8+ T cells in (B). (F) Isotype staining of the CD8(−) population indicates little, if any, nonspecific antibody reaction. Taken together, these data demonstrate that IL-2 and IL-15 induce cytotoxic capability in a large number of T cells in the absence of antigen.
Figure 3. The Grz B+ T cells were CD44hi with elevated CD122 and some cells were non-proliferating.
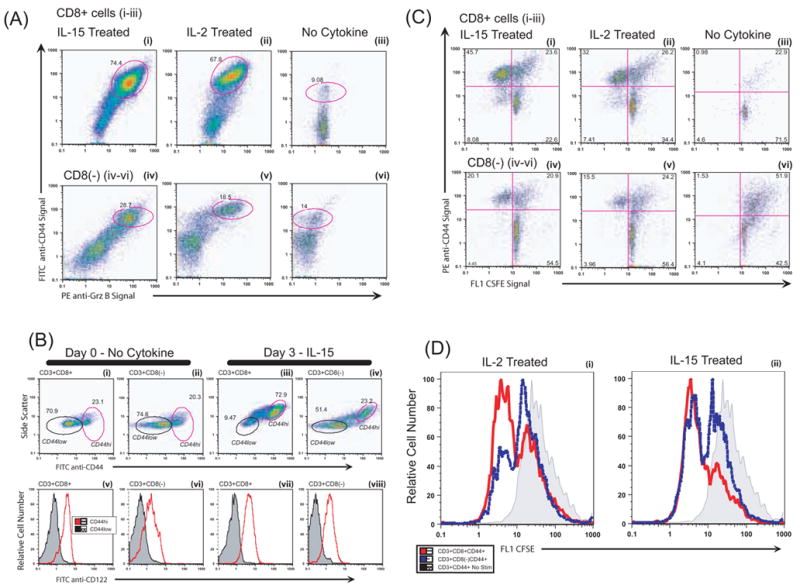
Splenocytes were cultured as in Figure 2. The cells were surface-labeled with FITC-anti CD44, PE-Cy5 anti-CD3 and PE-Cy7 anti-CD8, then fixed and permeabilized and reacted with PE anti-Grz B or PE-isotype. CD3+ gated cells were analyzed as indicated in A-D. (A) Grz B and CD44. (i) Grz B+CD8+ T cells induced with IL-15 possessed elevated levels of CD44, a marker associated with memory cells. On day 3, double positive Grz B+ CD44+ T cells represented 74.4% of the CD8+ T cells, as indicated by the circled population. (ii) A similar fraction of Grz B+CD44+ T cells was observed for cells treated with IL-2. (iii) Cells not cultured in cytokine lacked Grz B and had fewer CD44hi cells. The PE-isotype controls for intracellular staining (not illustrated) were similar to the cells cultured without cytokine. (iv – vi) The CD8(−) T cells demonstrated a pattern similar to the CD8+ population, with cytokine treatment increasing the CD44hi cells and inducing Grz B within the CD44hi population. The responding Grz B+ subset was smaller for the CD8(−) T cells than for the CD8+ T cells. The CD8(−) cells were also NK1.1(−), not illustrated. (B) Activated CD44hi cells and CD122 expression. Splenocytes on day 0 or after three days were analyzed for CD44 and CD122 (the common IL-2/15 beta receptor). (i – ii) On day 0, there are few cells with high side scatter. Most (more than 72.9%) of the IL-15 treated T cells had high side scatter on day 3 (iii – iv). The circled cells within figures B i – iv indicate the gates of the CD44hi or CD44low cells that were analyzed for CD122. The bottom row (v – viii) shows CD122 expression of the circled cells of the plots just above. The MFI of autofluoresce for in the FITC/CD122 channel was ~0.4 for all unstained cells. (v – vi) The CD44hi subset of freshly isolated T cells was CD122hi, while that of the CD44low cells lacked CD122. (vii – viii) The trend continued to day 3 in the cytokine-treated groups, with the CD44hi T cells expressing CD122. The CD122 MFI of the CD44low cells was similar to negative controls, indicating that CD122 was undetectable. (C) Cell division and CD44. Splenocytes were labeled on day 0 with CFSE to track cell division. We used cell surface CD44hi expression as a surrogate marker for Grz B since the intracellular CFSE labeling was greatly diminished when cells were permeabilized for intracellular Grz B staining. On day 3, cells were surface-labeled with PE-Cy5 anti-CD3 and PE-Cy7 anti-CD8, and PE anti-CD44. (C i–iii, CD8+ T cells ) (i) Approximately 66% of the CD8 T cells activated with IL-15 were dividing (calculated from the 45.7% CD44hi CFSElow cells included within the 69.3% of all cells that were CD44hi). Similar calculations indicated that 55% of the CD8 T cells activated with IL-2 (ii) were dividing. (iii) Without cytokine, only 23.9% of the cells on day 3 were CD44hi. Similar cytokine effects were observed in the CD8(−) population (C iv–vi). The proliferating cell populations treated with either IL-15 (iv) or IL-2 (v) were CD44hi, and higher than the unstimulated control cells (vi). IL-2 and IL-15 induced expansion of only the CD44+ T cells. There were more CD44+ CD8− T cells in this experiment than in the experiment illustrated in A. (D) Many but not all memory-phenotype CD44hi CD8+ or CD8− (CD4+) T cells proliferated after 3 days, as detected by CFSE staining. The histograms represent the data from the experiment displayed in (C). (i–ii) The shaded bar illustrates unstimulated cells on day 3 that were not proliferating.
3.3 Grz B+ CD8+ T cells are also CD44hi CD122+ and may acquire granzyme B without cell division
IL-15 is considered a “memory” cytokine, driving homeostatic replacement of memory lymphocytes [29,30,31,32]. It is important to know whether naive as well as memory T cells acquire granzyme B when cytokine-stimulated and also if the cells can respond without dividing. To address this issue, we first monitored whether the responding cells had the CD44hiCD122+ phenotype displayed by many antigen-specific memory cells and by CD8+ T cells undergoing “homeostatic proliferation” in vivo independently of antigen. On day 3, the Grz B+ CD8+ T cells and the Grz B+ CD8− (CD4+) T cells were CD44hi (Fig. 3A). Since the CD44hi cells have the low affinity cytokine receptor and most of the naïve cells lack it (Fig 3B), and the cells are being exposed to 10−8 M concentrations of cytokines that would saturate this receptor, it would be expected that the memory phenotype cells would be the responsive T cells. Our data indicate that the responding cells look like memory phenotype cells though the issue remains equivocal. Others have observed granzyme B induction in responses to IL-15 with human cells but the induction of granzyme B by IL-2 without antigen was unexpected.
A separate issue is whether the cytokines stimulate the Grz B+ cells to divide without antigen. Acquisition of granzyme B without cell division could facilitate rapid activation of bystander T cells in inflammatory situations with high cytokines but without cognate T cell antigens. We used the dye CFSE to monitor the cell division, looking for twofold dilutions of dye inside the cells as an indicator of each cell division. Considerable CFSE signal was lost upon permeabilization of the cells to label for intracellular Grz B, so we relied upon the CD44hi phenotype as a correlative indication for the Grz B+ cytotoxic cell population. Over 50% of the CD44hiCD8+ T cells were dividing (Fig 3C & D). It is difficult to estimate the number of rounds of proliferation of the CD44hiCD8+ T cells because the unstimulated CD44hi T cells had CFSE label that spanned almost a 10 fold range (indicated by the shaded cells in Fig 3D). It is noteworthy that more than a third of the GrzB+CD44hi CD8+ T cells remained noncycling on day 3 (Fig 3D), indicating that CD8+ T cells can be partially activated to express granzyme B without being activated into cell division. Thus the cytokines alone induce responses that can mimic or differ from antigen-driven proliferative responses.
The data also provide the means to compare the responses of CD8+ T cells with CD4+ T cells to see if these 2 major types of T cells differ in their antigen-independent responses to IL-2 and IL-15. Some but not all of the CD8− [CD4+] CD44hi T cells were dividing (Fig 3D dashed lines),. Thus, it would appear that CD4+ T cells can also be activated without antigen to become cytotoxic and can do so without entering cell division. Overall, this series of experiments indicates that T cells respond similarly to IL-2 or IL-15, and that the responding cells are probably recruited from cells with the memory phenotype. The responses are multiclonal expansion as opposed to outgrowth of a small number of lymphocyte clones.
Recently, attention has been drawn to differences between mitogenic potential and regulation of growth by these two interleukins [33]. We, like the D. Cantrell laboratory, observed similar mitogenesis at the start of culture. Our experimental design, with mouse cytokines (rather than human) with mouse cells, without antigen, and at 3 days of tissue culture (Figure 3D) or 5 days upon re-culture with fresh cytokines (Figure 5), rather than at days 8–10 without cytokine replenishment, limits further comparisons of the long term effects of the two cytokines on CD8+ T cell growth.
Taken together, these data support partial or full activation of T cells with the phenotype consistent with memory cells to acquire massive quantities of cytotoxic granules in response to either IL-2 or IL-15. Proliferation without addition of specific antigens is consistent with previous reports for IL-15 and memory CD8+ T cells [34,35], but it is remarkable that proliferation also occurred with IL-2 without specific antigen.
3.4 Antigen-independent culture with IL-15 or IL-2 induces phenotypic changes associated with T cell activation
We assayed an array of activation and functional cell surface markers to create a phenotypic picture of the cellular responses to IL-2 or IL-15. We monitored three molecules involved in cytotoxic function, NKG2D, CD2, CD11a; three molecules involved in the initiation and termination of immune responses, CD28, ICOS, PD-1; two molecules involved in T cell proliferation, CD25, CD71; and two additional molecules, the activation molecule CD69 and the ectoenzyme CD38. Table 1 provides a brief description and the function of each cell surface protein. The antibodies to these proteins were labeled with PE. For reference, the nonspecific binding of antibodies to antigen-negative cells had MFIs of ~ 1 (not illustrated).
Table 1.
Cell surface proteins tracked in figure 4 and their functions.
| Marker | Function |
|---|---|
| Molecules involved in cytotoxicity: | |
| NKG2D | Lectin-like cytotoxicity-activating receptor of activated CD8+ T cells and NK cells [40,41]. |
| CD2 | Adhesion molecule involved in T cell immune synapse formation [42,43]. |
| CD11a | Adhesion molecule involved in T and NK cell immune synapses, also known as LFA-1 [44,45]. |
| Molecules involved in the initiation and termination of immune responses: | |
| CD28 | Costimulatory receptor for B7-1 and B7-2 involved in many T cell- dependent processes [46,47]. |
| ICOS | Costimulatory molecule of the CD28 family [48,49]. |
| PD-1 (CD279) | Molecule of the CD28 family that binds B7-H1 (CD274) and is involved in negative regulation of lymphocytes [50,51]. |
| Molecules involved in cell proliferation: | |
| CD25 | Inducible IL-2 receptor alpha chain [52]. |
| CD71 | Transferrin receptor that mediates cellular mechanisms for iron uptake, indicator of active metabolism [53]. |
| Additional molecules: | |
| CD69 | Activation marker, involved in immunoregulation and mediates some cytotoxic functions [54]. |
| CD38 | Ectoenzyme with ADP ribosyl cyclase activity, adhesion molecule, activation marker [55], may be involved in NK-mediated cytotoxicity. |
We first focus attention on the CD8+ T cells which were greater than 70% granzyme B positive in Figs. 2 & 3. Three molecules involved in cytotoxic function, NKG2D, CD2, CD11a, were markedly up regulated and the effects were indistinguishable between IL-15 and IL-2 (Fig. 4A–C). Thus molecules directly involved in cytotoxicity were simultaneously up regulated together with Grz B. Molecules involved in the initiation and termination of immune responses, CD28, ICOS, PD-1 had a variable pattern, with CD28 largely unchanged (Fig. 4D), and ICOS, another co-stimulatory molecule, up regulated (Fig. 4E), while the programmed death receptor-1 (PD-1, Fig. 4F) was largely unchanged, indicating that at day 3 these cells are responsive to co-stimulation and also likely to survive. The IL-2 alpha receptor, CD25, was elevated by both IL-15 and IL-2, but to a greater extent by IL-15 (Fig. 4G). This observation means that there really was less CD25 on the surface of the IL-2 cultured cells because this particular anti-CD25 monoclonal antibody binds to IL-2R alpha even when IL-2 is already bound to the receptor [36]. In the presence of IL-2, the CD25 IL-2R alpha receptor may be cycling, removing the CD25 protein from the surface of the cell [37]. As expected, the transferrin receptor CD71 was up regulated (Fig. 4H). Most of the cells had substantial increases in CD69 (Fig. 4I). Changes in CD38 expression (Fig. 4J) indicate further changes in the population responding to cytokines. CD122 levels were elevated by both IL-15 and IL-2 above ex vivo and untreated levels (Fig. 4K), though IL-15 induced higher levels than IL-2. Thus the cytokines induced changes in ten different cell surface markers. For perspective, antigen stimulation induced greater expression of CD25 [38], PD-1 [39] and CD38 [author D.R., unpublished data].
Figure 4. Most of the cytokine-treated CD8+ T cells acquired an activated phenotype and up regulated several cell surface proteins that promote cytotoxicity.
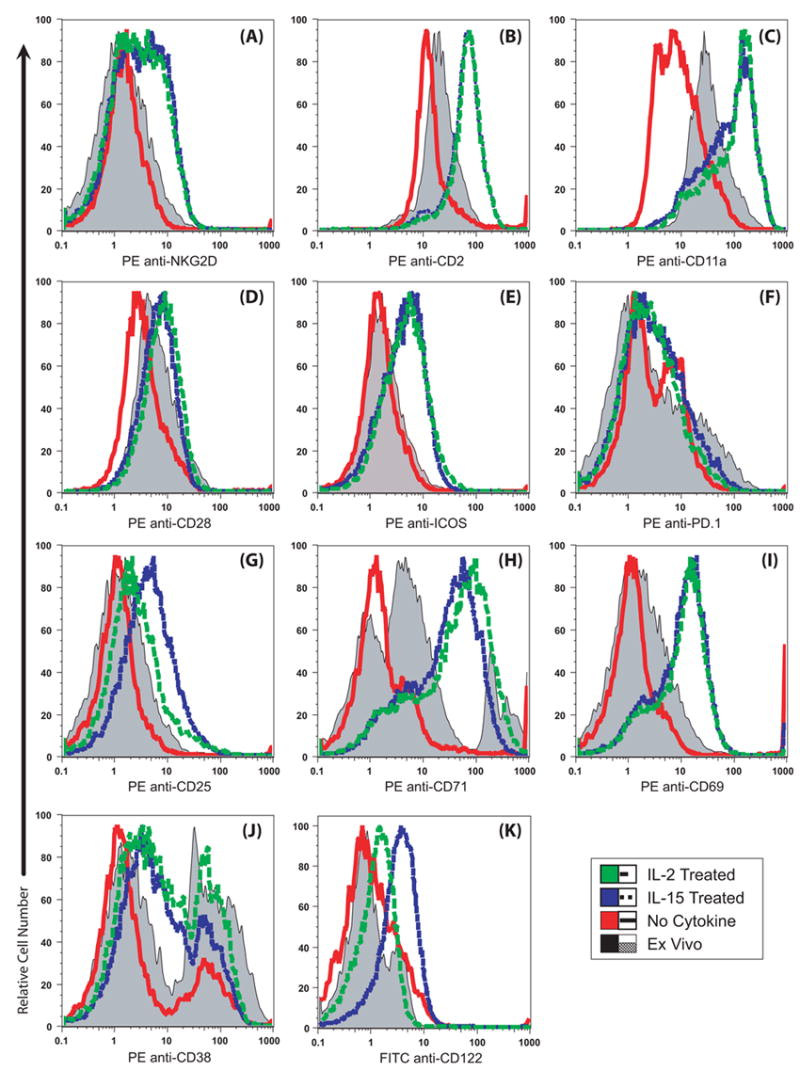
Splenocytes were cultured with no cytokine or 10−8 M IL-2 or IL-15. After three days, the cells were labeled with FITC-anti-CD8, PE-Cy5 anti-CD3 and PE-antibodies to the antigens indicated. The CD3+CD8+ T cells were gated and the PE-signals are illustrated. Three color (−1) PE channel controls had an MFI of 0.6 and PE isotype controls had an MFI of ~ 1 (not illustrated). The ex vivo levels are shaded and the day 3 values are indicated in color. Most of the CD8+ cells also acquired high forward and side scatter after culture (not illustrated). The data are representative of two or more experiments. (A–C) Cell surface proteins NKG2D, CD2 and CD11a were increased equally by either cytokine. (D) CD28 expression was maintained at ex vivo levels by cytokine treatment. (E) ICOS expression increased after culture with cytokines. (F) PD-1 levels were essentially unchanged. (G) IL-15 cultured cells expressed more cell surface CD25 than cells cultured with IL-2. (H) CD71 expression was elevated by IL-2 and IL-15 in the majority of cells. A CD71ultra-bright subset present ex vivo was lost after culture without cytokine stimulation. (I) IL-2 and IL-15 induced increased CD69 expression. (J) CD38 expression is maintained by IL-2 and by IL-15 compared to the cells cultured without cytokines. (K) CD122 increased in response to IL-2 and IL-15, though IL-15 was the more potent stimulator.
Similar changes in the ten cell surface proteins illustrated in Fig. 4 occurred for the cytokine-activated subset within the CD8(−) (CD4) T cells (not illustrated). Thus, CD8+ or CD4+ T cells that were activated with either IL-2 or IL-15 without antigen undergo many changes in gene expression consistent with the cytotoxic cell phenotype.
3.5 After cytokine removal, the cytokine-activated T cells lose their Grz B and cytotoxic activity within 24 hours
Given the remarkable multiclonal activation of cytotoxicity, the memory phenotype of the activated cells, and the documented need for IL-15 to maintain memory T cells in vivo, we considered if IL-15-treated cells would selectively maintain cytotoxicity and/or survive after cytokine withdrawal. To address this issue, we withdrew the cytokines from the cells and monitored maintenance of cytotoxicity, retention of Grz B and cell survival. Cells were initially cultured for 3 days with either 10−8 M of IL-15 or IL-2, harvested, washed, and briefly cultured without cytokines and then re-cultured with or without additional cytokine. To assess the cytotoxic capability of the T cells, we once again performed51Cr redirected release assays. Cytotoxic activity was reduced within 24 hours after withdrawal of cytokine (day 4), precipitously declining to the nominal levels of unstimulated cells (Fig. 5A). If either IL-2 or IL-15 were replenished, cytotoxicity continued to increase above day 3 levels in both cultures on days 4–5 (Fig. 5A).
Given that Grz B is a key protein in the cytotoxic process, we expected to see a Grz B expression pattern similar to the observed cytotoxic profile. The Grz B MFI declined within 24 hours post-withdrawal (day 4) to levels similar to the unstimulated control population (Fig. 5B) that lacked granzyme B. The cells replenished with fresh IL-2 or IL-15 increased GrzB expression.
When treated with continual IL-2 or IL-15 stimulation, CD8+ T cell proliferation continued (Fig. 5C). If either IL-15 or IL-2 was withdrawn, the cells survived for the 24 hours between days 3–4, and then died by day 5. Activation by IL-15 failed to protect the activated cells from death, indicating that exposure to high levels of this memory cytokine followed by withdrawal can be toxic.
4. Discussion
In this paper, we documented and compared the antigen-independent induction of T cell cytotoxicity by interleukins 2 and 15. Our novel discovery is that IL-2 as well as IL-15, when delivered at receptor-saturating concentrations, induces antigen independent multiclonal expansion and increases cytotoxic potential of splenic lymphocytes. While others have reported IL-15-induced antigen-independent activation [56], we have advanced the study by directly comparing granzyme B, cytotoxicity and a variety of indicators induced by IL-15 or IL-2 during antigen-independent stimulation. Almost all splenic T cells that expressed CD122 at the time of isolation, whether they were CD4+ or CD8+, appeared to respond in a polyclonal manner to either cytokine and to acquire cytotoxic capacity. The induced cytotoxic capacity would probably have gone unnoticed in assays using target cells with unique cognate antigens because of the low frequency of T cells specific for individual antigens. However, the massive activation of cytotoxicity was detectable by redirected lysis and paralleled by intracellular Grz B expression. The polyclonal nature of the response was indicated by cytotoxicity at low (1:1) effector to target ratios and by the high frequency of Grz B+ cells. The responses to the two cytokines were similar in terms of molar dose-responses, frequencies of responding cells, and the extent of the responses. Even the transient nature of the cytotoxic capacity, and the subsequent death of cytotoxic cells, was similar for both cytokines. This comparison of the antigen-independent effects of IL-2 and IL-15 has implications for T cell biology, immunophysiology, immunopathology and immunotherapy.
The implications for T cell biology demonstrate that cytotoxicity can be induced without antigen engagement of the TCR. Cytokine levels sufficient to stimulate via the CD122/CD132 dimeric receptor induced cytotoxic granules. The marginal effects at concentrations in which the cytokines would have to bind to specific alpha-receptors to be effective, and the upper range of the dose-response, strongly suggest that it is primarily the CD122/CD132 dimers that transduce the signals for granule formation as opposed to the TCR. Further, we found that CD122 was expressed by the responding lymphocyte population. This CD122 expression was observed for both the CD8+ and CD8(−) T responding lymphocyte populations. The signals that induced Grz B usually also induced T cell division, though some cytokine-induced cytotoxic cells failed to divide. For historical perspective, it has been noted in a different context that high IL-2 induced multiclonal activation. When high IL-2 was used for tissue culture induction of antigen-specific responses, “non-specific T killer cell activity” appeared and the non-specific cells were maintained in the cultures [57]. Here we clarify the issue and indicate that these non-specific killers probably resulted from antigen-independent multiclonal activation of lymphocytes.
The immunophysiology of antigen-independent cytokine-induced cytotoxicity is difficult to assess. It is uncertain how often large amounts of IL-2 or IL-15 are readily available in vivo. IL-2 is vectorially secreted by CD4+ T helper cells [58,59], suggesting that exposure is normally carefully regulated. However, because IL-2 is a secreted protein, localized excesses are possible. Excess IL-15 in the periphery is very unlikely since little IL-15 is made and what is produced is bound and trans-presented by the dendritic cells and monocytes that synthesize it [60]. Trans-presentation of IL-15 involves focusing small amounts of IL-15 on the membrane of the dendritic cells and allowing low numbers of receptors per IL-15-receiving T cell to respond. The functional relationship between low amounts of IL-15 presented on dendritic cells and the high concentrations of soluble IL-15 we used is unclear. Thus, if antigen-independent induction of cytotoxicity occurs physiologically for bystander cells, it is much more likely to be in response to IL-2 than IL-15 simply because the bystander cells are more likely to find IL-2 in the periphery than IL-15.
The antigen-independent activation of cytotoxic capacity may contribute to immunopathology. Indeed, IL-2 can be found in circulating blood during diseases [61,62,63,64]. Furthermore, if T cells were trapped in an environment of high local cytokines, these T cells could acquire cytotoxic capacity. It would still require ligation of their T cell receptors for antigen before these T cells mediated damage. In theory, a T cell could be cytokine-induced in one location and then mediate cytotoxicity in another location. Our study suggests that the time frame for movement from the site of induction to the site of antigen must be less than 24 hours. Additionally, high IL-15 might cause pathology similar to the damage observed when dysregulated IL-15 in the gut exacerbates celiac disease by upregulation of human NKG2D (as we report here for mice) to cause TCR-independent cytotoxicity [65].
IL-2 has been used for immunotherapy with some success but, for multiple reasons, clinicians might hope for improved responses with IL-15 [66]. Unfortunately for immunotherapy, our results suggest that high IL-15 may cause some of the same problems that IL-2 does. In vivo murine studies have shown that IL-2 and IL-15 both have similar anti-tumor effects, even tumor free survival was not achieved [67], which supports efficacy. IL-2 affects many lymphocytes including B cells and causes significant systemic toxicity at high concentrations. In vitro stimulation with antigen and IL-2 of cytotoxic T cells destined for adoptive transfer results in subsequent death when IL-2 is withdrawn in vivo [68]. One might have hoped that this death upon cytokine withdrawal would have been less with IL-15, but in our in vitro experiments, death appeared just as widespread upon IL-15 cytokine withdrawal. In vivo administration of rIL-15 to mice has already been demonstrated to induce Grz B in memory phenotype T cells without the re-introduction of antigen [69], which suggests IL-15 efficacy. However, the antigen-independent death may occur in vivo upon cytokine withdrawal and present a new threat in that high concentrations of either cytokine could first activate and then kill memory T cells. We recommend that the survival of antigen-specific CD8 memory cells be monitored in vivo in rodents after high IL-2 or IL-15 treatment to assess potential ablation of memory cytotoxic T cells.
This study used mouse lymphocytes with mouse interleukins 2 and 15. Human cytokines used with mouse cells are considerably less potent [70]. The potency of homologous cytokines raises new caveats for use of high concentrations of either of these cytokines in vivo for human immunotherapy due to (1) polyclonal activation of T lymphocyte cytotoxicity and (2) potential polyclonal deletion of memory T cells upon cytokine withdrawal.
Acknowledgments
We gratefully acknowledge the support of the NIH (USA) through grants R01 CA38942-19 (D.H.), T32 CA09563 (B.N.A. and D.L.T.), and P20 RR016464 (Nevada INBRE), and of the Reno Cancer Center, Inc.
We thank William J. Murphy, Ph.D., School of Medicine, University of Nevada, for helpful editorial suggestions.
Abbreviations used
- Grz
granzyme
- LU
lytic unit
- TCR
T cell receptor for antigen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David L. Tamang, Cell and Molecular Biology Program, University of Nevada, Reno, NV.
Doug Redelman, Cell and Molecular Biology Program, University of Nevada, Reno, NV..
Bryce N. Alves, Cell and Molecular Biology Program, University of Nevada, Reno, NV.
Leanne Vollger, Cell and Molecular Biology Program, University of Nevada, Reno, NV..
Christy Bethley, Southern University, Baton Rouge, LA..
Dorothy Hudig, Cell and Molecular Biology Program, University of Nevada, Reno, NV..
Reference List
- 1.Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 2.Pardigon N, Bercovici N, Calbo S, Santos-Lima EC, Liblau R, Kourilsky P, Abastado JP. Role of co-stimulation in CD8+ T cell activation. Int Immunol. 1998;10:619–630. doi: 10.1093/intimm/10.5.619. [DOI] [PubMed] [Google Scholar]
- 3.Murali-Krishna K, Altman JD, Suresh M, Sourdive D, Zajac A, Ahmed R. In vivo dynamics of anti-viral CD8 T cell responses to different epitopes. An evaluation of bystander activation in primary and secondary responses to viral infection. Adv Exp Med Biol. 1998;452:123–142. doi: 10.1007/978-1-4615-5355-7_14. [DOI] [PubMed] [Google Scholar]
- 4.Janas ML, Groves P, Kienzle N, Kelso A. IL-2 regulates perforin and granzyme gene expression in CD8+ T cells independently of its effects on survival and proliferation. J Immunol. 2005;175:8003–8010. doi: 10.4049/jimmunol.175.12.8003. [DOI] [PubMed] [Google Scholar]
- 5.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuribayashi K, Gillis S, Kern DE, Henney CS. Murine NK cell cultures: effects of interleukin-2 and interferon on cell growth and cytotoxic reactivity. J Immunol. 1981;126:2321–2327. [PubMed] [Google Scholar]
- 7.Liu K, Catalfamo M, Li Y, Henkart PA, Weng NP. IL-15 mimics T cell receptor crosslinking in the induction of cellular proliferation, gene expression, and cytotoxicity in CD8+ memory T cells. Proc Natl Acad Sci U S A. 2002;99:6192–6197. doi: 10.1073/pnas.092675799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alves NL, Hooibrink B, Arosa FA, van Lier RA. IL-15 induces antigen-independent expansion and differentiation of human naive CD8+ T cells in vitro. Blood. 2003;102:2541–2546. doi: 10.1182/blood-2003-01-0183. [DOI] [PubMed] [Google Scholar]
- 9.Michael R Shurin, Irina L Tourkova, Holger Hackstein, Galina V Shurin. Interleukin-15 and 21. In: Angus W Thomson, Michael T Lotze., editors. The Cytokine Handbook. Elsevier Science Ltd; London: 2003. p. 431. [Google Scholar]
- 10.Jian-Xin Lin, Warren J Leonard. Interleukin-15 and 21. In: W Thomson Angus, T Lotze Michael., editors. The Cytokine Handbook. Elsevier Science Ltd; London: 2003. p. 167. [Google Scholar]
- 11.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- 12.Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K, DuBose R, Cosman D, Park LS, Anderson DM. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson DM, Kumaki S, Ahdieh M, Bertles J, Tometsko M, Loomis A, Giri J, Copeland NG, Gilbert DJ, Jenkins NA. Functional characterization of the human interleukin-15 receptor alpha chain and close linkage of IL15RA and IL2RA genes. J Biol Chem. 1995;270:29862–29869. doi: 10.1074/jbc.270.50.29862. [DOI] [PubMed] [Google Scholar]
- 14.Robb RJ, Munck A, Smith KA. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981;154:1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston JA, Bacon CM, Finbloom DS, Rees RC, Kaplan D, Shibuya K, Ortaldo JR, Gupta S, Chen YQ, Giri JD. Tyrosine phosphorylation and activation of STAT5, STAT3, and Janus kinases by interleukins 2 and 15. Proc Natl Acad Sci U S A. 1995;92:8705–8709. doi: 10.1073/pnas.92.19.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston JA, Kawamura M, Kirken RA, Chen YQ, Blake TB, Shibuya K, Ortaldo JR, McVicar DW, O'Shea JJ. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994;370:151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- 17.Witthuhn BA, Silvennoinen O, Miura O, Lai KS, Cwik C, Liu ET, Ihle JN. Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994;370:153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- 18.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- 19.Tagaya Y, Burton JD, Miyamoto Y, Waldmann TA. Identification of a novel receptor/signal transduction pathway for IL-15/T in mast cells. EMBO J. 1996;15:4928–4939. [PMC free article] [PubMed] [Google Scholar]
- 20.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15R[alpha] Recycles and Presents IL-15 In trans to Neighboring Cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 21.Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K, DuBose R, Cosman D, Park LS, Anderson DM. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capuron L, Ravaud A, Miller AH, Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav Immun. 2004;18:205–213. doi: 10.1016/j.bbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Dillman RO. What to do with IL-2? Cancer Biother Radiopharm. 1999;14:423–434. doi: 10.1089/cbr.1999.14.423. [DOI] [PubMed] [Google Scholar]
- 24.Lotem M, Shiloni E, Pappo I, Drize O, Hamburger T, Weitzen R, Isacson R, Kaduri L, Merims S, Frankenburg S, Peretz T. Interleukin-2 improves tumour response to DNP-modified autologous vaccine for the treatment of metastatic malignant melanoma. Br J Cancer. 2004;90:773–780. doi: 10.1038/sj.bjc.6601563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sznol M, Parkinson DR. Clinical applications of IL-2. Oncology (Williston Park) 1994;8:61–67. [PubMed] [Google Scholar]
- 26.Pross HF, Maroun JA. The standardization of NK cell assays for use in studies of biological response modifiers. J Immunol Methods. 1984;68:235–249. doi: 10.1016/0022-1759(84)90154-6. [DOI] [PubMed] [Google Scholar]
- 27.Overton WR. Modified histogram subtraction technique for analysis of flow cytometry data. Cytometry. 1988;9:619–626. doi: 10.1002/cyto.990090617. [DOI] [PubMed] [Google Scholar]
- 28.Babichuk CK, Duggan BL, Bleackley RC. In vivo regulation of murine granzyme B gene transcription in activated primary T cells. J Biol Chem. 1996;271:16485–16493. doi: 10.1074/jbc.271.28.16485. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis a, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu K, Catalfamo M, Li Y, Henkart PA, Weng NP. IL-15 mimics T cell receptor crosslinking in the induction of cellular proliferation, gene expression, and cytotoxicity in CD8+ memory T cells. Proc Natl Acad Sci U S A. 2002;99:6192–6197. doi: 10.1073/pnas.092675799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waldmann T. The contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for the immunotherapy of rheumatological diseases. Arthritis Res. 2002;4(Suppl 3):S161–S167. doi: 10.1186/ar584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weng NP, Liu K, Catalfamo M, Li Y, Henkart PA. IL-15 is a growth factor and an activator of CD8 memory T cells. Ann N Y Acad Sci. 2002;975:46–56. doi: 10.1111/j.1749-6632.2002.tb05940.x. [DOI] [PubMed] [Google Scholar]
- 33.Cornish GH, Sinclair LV, Cantrell DA. Differential regulation of T-cell growth by IL-2 and IL-15. Blood. 2006;108:600–608. doi: 10.1182/blood-2005-12-4827. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 35.Kanegane H, Tosato G. Activation of naive and memory T cells by interleukin-15. Blood. 1996;88:230–235. [PubMed] [Google Scholar]
- 36.Lowenthal JW, Corthesy P, Tougne C, Lees R, MacDonald HR, Nabholz M. High and low affinity IL 2 receptors: analysis by IL 2 dissociation rate and reactivity with monoclonal anti-receptor antibody PC61. J Immunol. 1985;135:3988–3994. [PubMed] [Google Scholar]
- 37.Hemar A, Subtil A, Lieb M, Morelon E, Hellio R, utry-Varsat A. Endocytosis of interleukin 2 receptors in human T lymphocytes: distinct intracellular localization and fate of the receptor alpha, beta, and gamma chains. J Cell Biol. 1995;129:55–64. doi: 10.1083/jcb.129.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redelman D, Wormsley S. The induction of the human T-cell growth factor receptor precedes the production of RNA and occurs in the presence of inhibitors of RNA synthesis. Cytometry. 1986;7:453–462. doi: 10.1002/cyto.990070511. [DOI] [PubMed] [Google Scholar]
- 39.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, Collins M, Honjo T, Freeman GJ, Carreno BM. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 40.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol. 2000;1:119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 41.Cerwenka A, Bakker AB, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 42.Ayroldi E, Migliorati G, Cannarile L, Moraca R, Delfino DV, Riccardi C. CD2 rescues T cells from T-cell receptor/CD3 apoptosis: a role for the Fas/Fas-L system. Blood. 1997;89:3717–3726. [PubMed] [Google Scholar]
- 43.Bolhuis RL, Roozemond RC, van de Griend RJ. Induction and blocking of cytolysis in CD2+, CD3- NK and CD2+, CD3+ cytotoxic T lymphocytes via CD2 50 KD sheep erythrocyte receptor. J Immunol. 1986;136:3939–3944. [PubMed] [Google Scholar]
- 44.Shier P, Ngo K, Fung-Leung WP. Defective CD8+ T cell activation and cytolytic function in the absence of LFA-1 cannot be restored by increased TCR signaling. J Immunol. 1999;163:4826–4832. [PubMed] [Google Scholar]
- 45.Cai Z, Kishimoto H, Brunmark A, Jackson MR, Peterson PA, Sprent J. Requirements for peptide-induced T cell receptor downregulation on naive CD8+ T cells. J Exp Med. 1997;185:641–651. doi: 10.1084/jem.185.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harding FA, Allison JP. CD28-B7 interactions allow the induction of CD8+ cytotoxic T lymphocytes in the absence of exogenous help. J Exp Med. 1993;177:1791–1796. doi: 10.1084/jem.177.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 48.McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, Chernova T, Malenkovich N, Jabs C, Kuchroo VK, Ling V, Collins M, Sharpe AH, Freeman GJ. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000;165:5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- 49.Mages HW, Hutloff A, Heuck C, Buchner K, Himmelbauer H, Oliveri F, Kroczek RA. Molecular cloning and characterization of murine ICOS and identification of B7h as ICOS ligand. Eur J Immunol. 2000;30:1040–1047. doi: 10.1002/(SICI)1521-4141(200004)30:4<1040::AID-IMMU1040>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 50.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 51.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 52.Uchiyama T, Nelson DL, Fleisher TA, Waldmann TA. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. II. Expression of Tac antigen on activated cytotoxic killer T cells, suppressor cells, and on one of two types of helper T cells. J Immunol. 1981;126:1398–1403. [PubMed] [Google Scholar]
- 53.Kemp JD, Thorson JA, McAlmont TH, Horowitz M, Cowdery JS, Ballas ZK. Role of the transferrin receptor in lymphocyte growth: a rat IgG monoclonal antibody against the murine transferrin receptor produces highly selective inhibition of T and B cell activation protocols. J Immunol. 1987;138:2422–2426. [PubMed] [Google Scholar]
- 54.Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 55.Deaglio S, Mehta K, Malavasi F. Human CD38: a (r)evolutionary story of enzymes and receptors. Leuk Res. 2001;25:1–12. doi: 10.1016/s0145-2126(00)00093-x. [DOI] [PubMed] [Google Scholar]
- 56.Alves NL, Hooibrink B, Arosa FA, van Lier RA. IL-15 induces antigen-independent expansion and differentiation of human naive CD8+ T cells in vitro. Blood. 2003;102:2541–2546. doi: 10.1182/blood-2003-01-0183. [DOI] [PubMed] [Google Scholar]
- 57.Knuth A, Danowski B, Oettgen HF, Old LJ. T-cell-mediated cytotoxicity against autologous malignant melanoma: analysis with interleukin 2-dependent T-cell cultures. Proc Natl Acad Sci U S A. 1984;81:3511–3515. doi: 10.1073/pnas.81.11.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7:247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 59.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 60.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15R[alpha] Recycles and Presents IL-15 In trans to Neighboring Cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 61.Gallo P, Piccinno MG, Pagni S, Argentiero V, Giometto B, Bozza F, Tavolato B. Immune activation in multiple sclerosis: study of IL-2, sIL-2R, and gamma-IFN levels in serum and cerebrospinal fluid. J Neurol Sci. 1989;92:9–15. doi: 10.1016/0022-510x(89)90171-8. [DOI] [PubMed] [Google Scholar]
- 62.Deckert-Schluter M, Schluter D, Schwendemann G. Evaluation of IL-2, sIL2R, IL-6, TNF-alpha, and IL-1 beta levels in serum and CSF of patients with optic neuritis. J Neurol Sci. 1992;113:50–54. doi: 10.1016/0022-510x(92)90264-l. [DOI] [PubMed] [Google Scholar]
- 63.Ozer G, Teker Z, Cetiner S, Yilmaz M, Topaloglu AK, Onenli-Mungan N, Yuksel B. Serum IL-1, IL-2, TNFalpha and INFgamma levels of patients with type 1 diabetes mellitus and their siblings. J Pediatr Endocrinol Metab. 2003;16:203–210. doi: 10.1515/jpem.2003.16.2.203. [DOI] [PubMed] [Google Scholar]
- 64.Debnath CR, Alam K, Sarker CB, Rahman S, Ahmad N, Rahman S, Khan GK, Sutradhar SR, Miah MT. Serum IL-2 in chronic hepatitis B virus infected patients and its association with disease activity. Mymensingh Med J. 2005;14:125–127. [PubMed] [Google Scholar]
- 65.Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, Raulet DH, Lanier LL, Groh V, Spies T, Ebert EC, Green PH, Jabri B. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–366. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 66.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- 67.Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol. 2004;173:900–909. doi: 10.4049/jimmunol.173.2.900. [DOI] [PubMed] [Google Scholar]
- 68.Yee C, Riddell SR, Greenberg PD. In vivo tracking of tumor-specific T cells. Curr Opin Immunol. 2001;13:141–146. doi: 10.1016/s0952-7915(00)00196-5. [DOI] [PubMed] [Google Scholar]
- 69.Yajima T, Nishimura H, Sad S, Shen H, Kuwano H, Yoshikai Y. A novel role of IL-15 in early activation of memory CD8+ CTL after reinfection. J Immunol. 2005;174:3590–3597. doi: 10.4049/jimmunol.174.6.3590. [DOI] [PubMed] [Google Scholar]
- 70.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proc Natl Acad Sci U S A. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]


