Abstract
Ocular perfusion studies from all non-human species performed to date consistently demonstrate a perfusion-volume-dependent increase in aqueous outflow facility known as the “washout” effect. However, this “washout” effect does not occur in human eyes. We have recently documented that, in bovine eyes, the washout associated increase in facility correlates with the extent of physical separation between the juxtacanalicular connective tissue (JCT) and the inner wall endothelium lining the aqueous plexus (the bovine equivalent of Schlemm’s canal). We hypothesize that if washout truly correlates with inner wall/JCT separation then this separation should not occur in human eyes that do not exhibit the washout effect, even after prolonged perfusion. Eight enucleated human and eight bovine eyes were used in this study. Aqueous outflow facility was measured at 15 mmHg for long-duration (3 hr) or short-duration (30 min – 1 hr) perfusion (n=4 for each group). All eyes were perfusion-fixed at 15 mmHg, and examined morphologically with both light and electron microscopy. In bovine eyes, outflow facility increased 81% (p=0.049) from 1.06 ± 0.06 μl/min/mmHg at baseline to 1.92 ± 0.30 μl/min/mmHg after 3 hrs due to washout. The pre-fixation outflow facility in long-duration eyes (1.92 ± 0.30 μl/min/mmHg) (mean ± SEM) was 2-fold greater than pre-fixation facility in short-duration eyes (0.92 ± 0.11 μl/min/mmHg; p = 0.0387). In human eyes, washout was not observed; baseline outflow facility was similar between both groups (0.18 ± 0.02 vs. 0.25 ± 0.08 μl/min/mmHg; p=0.518); however, pre-fixation outflow facility in long-duration eyes showed a 40% decrease compared to baseline outflow facility in those same eyes (p = 0.017, paired Student’s t-test). In bovine eyes, significant expansion and rarefaction of the JCT and inner wall/JCT separation was much more prevalent in long-duration eyes, and data from all bovine eyes revealed a correlation between the extent of inner wall/JCT separation and the absolute value of outflow facility measured immediately prior to fixation (p = 0.0024) as well as the washout-induced increase in outflow facility (p = 0.0006). In human eyes, no significant morphologic differences were observed between long- and short-duration perfusion, with no observed change in inner wall/JCT separation or expansion between the two groups. Morphologic analysis revealed that the previously described “cribriform plexus” of elastic-like fibers was far more extensive in the JCT of human eyes, appearing to form numerous connections to the inner wall endothelium. The cribriform plexus appears to function as a mechanical tether that maintains inner wall/JCT connectivity in human eyes by opposing hydrodynamic forces generated during perfusion, potentially explaining the lack of washout in humans.
INTRODUCTION
The “washout effect” is a phenomenon in which perfusion of an eye at physiological pressure results in a volume-dependent increase in the measured facility of aqueous humor outflow. Washout was originally believed to result from a “washing out” of extracellular glycosaminoglyans (Barany and Scotchbrook, 1954; Barany and Woodin, 1955; Barany, 1962, 1964) from the outflow pathway, but biochemical studies have failed to detect an appreciable change in either sulfated proteoglycans (Johnson et al., 1993) or hyaluronic acid (Knepper et al., 1984) from the outflow pathway following prolonged perfusion. While the rate of facility increase during washout can be slowed by perfusion with either serum (Kee et al., 1996; Johnson et al., 1993) or serum proteins (Epstein et al., 1978; Sit et al., 1997) or by perfusion with either pooled homologous aqueous humor (Barany and Woodin, 1955; Gaasterland et al., 1978) or mock aqueous humor with similar biochemical constituents (Erickson and Kaufman, 1981; Gaasterland et al., 1979), the washout effect cannot entirely be eliminated (Barany and Scotchbrook, 1954; Van Buskirk and Brett, 1978).
Possibly the most intriguing aspect of the washout effect, however, is that it does not occur in the human eye (Erickson-Lamy et al., 1990). This suggests that there is some unique aspect of outflow anatomy or physiology that distinguishes human eyes from most other species, including from non-human primate eyes that exhibit washout during perfusion both in vivo (Barany, 1962, 1964; Erickson and Kaufman, 1981; Gaasterland et al., 1978, 1979; Kaufman et al., 1988) and in vitro (Epstein, 1982; Hashimoto and Epstein, 1980; Peterson et al., 1974), despite their anatomical similarity to humans. A thorough understanding of the mechanism of washout, and the reason for its absence in humans would likely provide important insight into the fundamental mechanisms that generate outflow resistance. Such understanding might also permit us to artificially induce a washout-like response in human eyes as a means of reducing intraocular pressure in glaucoma.
Two recent studies (Overby et al; 2002; Sabanay et al., 2004) have documented that washout is a reversible process in both bovine and monkey eyes. A structural correlate to the facility increase during washout in bovine eyes appeared to be the degree of separation of the JCT from the inner wall of the aqueous plexus –the bovine equivalent to Schlemm’s canal (Tripathi, 1971). This separation was proposed to increase outflow facility by disrupting a hydrodynamic interaction between the inner wall and JCT known as “funneling” (Johnson et al., 1992). The funneling theory states that the patterns of outflow through the JCT are confined to those regions nearest the pores in the inner wall, and this flow confinement reduces the filtration area through the JCT, thereby increasing its effective hydrodynamic resistance. Based upon our prior work in bovine eyes, we hypothesized that washout resulted from a disruption in the connectivity between inner wall and JCT that decreased outflow resistance by eliminating the funneling effect (Overby et al., 2002). The washout hypotheses and funneling theory emphasize the role of cellular and extracellular matrix adhesions that maintain the connectivity between the inner wall and JCT in the face of an opposing pressure gradient and thereby influence outflow resistance by controlling the local hydrodynamic patterns of outflow.
In this study, we hypothesize that if the structural correlate for washout is separation between the inner wall and JCT, then these morphological changes should not be found in human eyes subject to prolonged perfusion. Light and electron microscopy were used to compare the baseline morphological differences in the outflow pathway and morphological changes following prolonged perfusion in bovine eyes that exhibit washout and human eyes that do not exhibit washout. Our goal was to determine whether the absence of washout in human eyes relates to morphological differences unique to the human outflow pathway. A corollary of our hypothesis is that the absence of washout in the human eye may result from an enhanced connectivity between the inner wall and JCT, which would have important consequences for the regulation of outflow resistance in this species.
MATERIALS AND METHODS
Enucleated eyes from both bovine and human donors were used in this study. Eight bovine eyes were obtained from a local abattoir (Arena and Sons, Hopkinton, MA) and delivered on ice within 6 hours post-mortem. Eyes with any discernible damage or accumulated blood in the angle of the anterior chamber were excluded. Eight human eyes from anonymous donors with no known history of eye disease (ranging from 33 to 89 years of age) were obtained from National Disease Research Interchange (Philadelphia, PA) within 24 hours postmortem. Each eye was confirmed to be grossly normal by examination under a dissecting microscope.
Experimental Ocular Perfusion
All eyes were perfused with Dulbecco’s phosphate-buffered saline containing 5.5 mM glucose (collectively referred to as DPBS) using a previously described constant-pressure ocular perfusion system (Erickson-Lamy et al., 1990). Briefly, this system maintains a constant IOP by a fixed height perfusion reservoir placed at 20.4 cm (15 mmHg) above the eye, while the changing weight of the reservoir is recorded as a measure of the perfusion flow rate. Outflow facility is calculated as a function of time as the ratio of the time-varying flow rate to IOP.
Experimental Perfusion of Bovine Eyes
Bovine eyes were perfused at 15 mmHg for either a short-duration (30 min) or a long-duration (3 hrs) prior to fixation. Previous studies have shown that 3 hrs of perfusion at 15 mmHg is sufficient to induce appreciable washout in bovine eyes (Barany and Woodin, 1955; Erickson-Lamy et al., 1988, 1990; Overby et al., 2002). For the short-duration perfusion, eyes were perfused for 30 min to allow for stabilization of outflow facility and to achieve a baseline facility measurement. For the long-duration perfusion, eyes were perfused for 30 min to achieve a stable baseline facility measurement, followed by 3 hrs of prolonged perfusion at the same pressure. All eyes were perfusion-fixed with 2% paraformaldehyde and 2.5% glutaraldehyde in PBS (pH 7.3) at 15 mmHg for 30 minutes.
Experimental Perfusion of Human Eyes
For the short-duration perfusion, eyes were perfused for 1 hr to allow for stabilization of outflow facility and to achieve a baseline measurement. For the long-duration perfusion, eyes were again perfused for 1 hr to allow for a stable baseline facility measurement, and the perfusion was continued for an additional 4 hours to measure outflow facility during prolonged perfusion. Afterwards, eyes were perfusion-fixed with 2% paraformaldehyde and 2.5% glutaraldehyde in PBS (pH 7.3) at 15 mmHg for 1 hour.
Tissue Processing for Microscopy
Anterior segments of each eye were divided into 4 quadrants (designated superior, inferior, nasal, and temporal) and cut into meridional sections (1–2 mm) that were post-fixed in 2% osmium tetroxide and 1.5% potassium ferrocyanide in dH2O for 2 hours. The sections were then dehydrated in a graded series of ethanols and embedded in Epon-Araldite. Sections from 4 quadrants of each eye were prepared for examination using both light and transmission electron microscopy. For light microscopy, semi-thin radial sections (3 μm) were cut from all quadrants (two blocks per quadrant were examined) and stained with 0.1% Toluidine Blue to localize the inner wall of the aqueous plexus. Light micrographs were taken at an original magnification of 40X and analyzed for the degree of separation between the inner wall and JCT (described below). For transmission electron microscopy, ultra-thin sections (90 nm), from each quadrant, were cut with an ultramicrotome, counterstained with uranyl acetate and lead citrate, and examined with an electron microscope (Model 300: Philips, Eindhoven, The Netherlands). Micrographs from all four quadrants of both human and bovine eyes were taken along the inner wall of SC (or AP) at an original magnification of 3300X.
Morphologic Evaluation of Inner Wall/JCT Separation
A trained masked observer (HG) was presented with light micrographs of the inner wall and JCT from both human and bovine eyes and was instructed to identify regions along the inner wall that exhibited separation from the underlying JCT (Fig. 1). The degree of separation was assigned a qualitative score representing the fraction of inner wall length that appeared separated. Scores ranged from 0-plus to 3-plus:
Figure 1. Morphologic Assessment of Separation between the Inner Wall/JCT in the Bovine Eye.
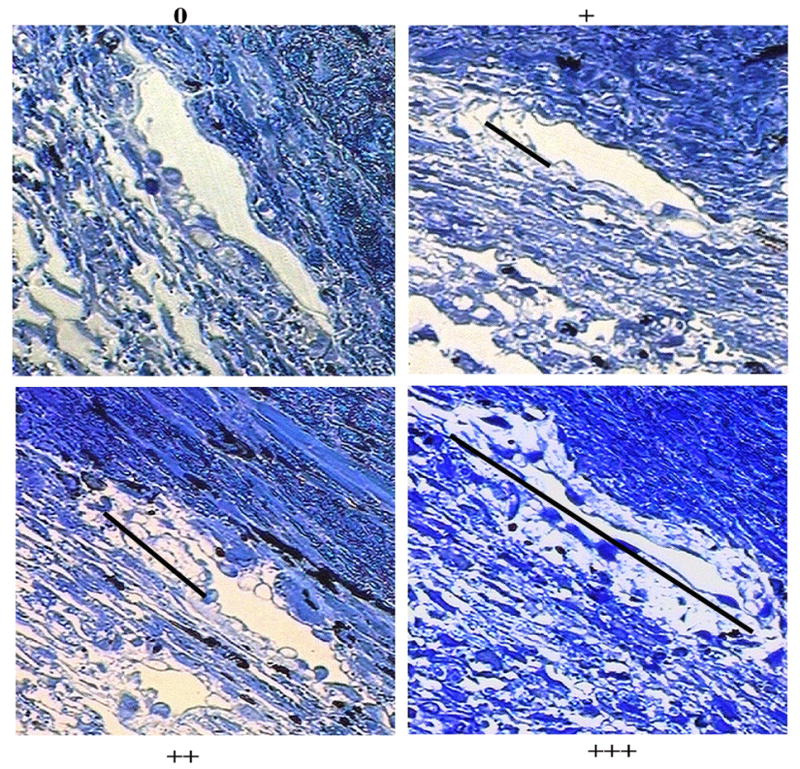
The degree of inner wall/JCT separation observed in each section was assessed based on a scale of 0 (no separation), + (less than approximately 1/3 of total length of the inner wall), ++ (more than approximately 1/3 but less than approximately 2/3 of the total length of inner wall), and +++ (more than approximately 2/3 of the total length of the inner wall). The length of the black lines corresponded to the estimated length of inner wall/JCT separation in each case.
0 --- no apparent separation,
+ --- less than approximately 1/3 of total length of the inner wall appears separated
++ --- more than approximately 1/3 but less than approximately 2/3 of the total length of inner wall appears separated,
+++ --- more than approximately 2/3 of the total length of the inner wall appears separated.
This grading system is analogous to the one previously used by Overby et al. (2002) for electron microscopy, but evaluating separation using light microscopy as we do here allows for a much larger field of view to appreciate morphologic changes over tissue-level dimensions that may be more relevant for influencing outflow resistance. We did not quantify other dimensions of JCT/IW separation because any increase in separation hypothetically coincides with increased outflow facility and therefore assumes a secondary importance. Figure 1 shows an example micrograph for each score.
Two types of separation have previously been described to occur between the inner wall and JCT (Overby et al., 2002): (i) separation between the extracellular matrix of the JCT and the basal lamina of the inner wall (matrix-matrix separation) and (ii) separation between the basal lamina of the inner wall and the endothelial cells lining the aqueous plexus (cell-matrix separation). At the level of magnification used for light microscopy (40x), the basal lamina underlying the inner wall could not clearly be identified. Therefore, to more closely examine the inner wall/JCT separation and to distinguish between cell-matrix and matrix-matrix separation, electron micrographs of the inner wall and JCT (3300x) were examined from all 4 quadrants and were compared to determine whether morphological differences were evident between bovine and human eyes and between eyes following short- and long-duration perfusions.
Statistical Methods
Two-tailed Student’s t-test and linear regression analysis were applied with a required significance level of 0.05. For the regressions, the residuals (the difference between the fitted value of the dependent parameter and its measured value) were examined and in all cases appeared random when plotted against the independent variables.
RESULTS
Outflow Facility in Human and Bovine Eyes
A summary of outflow facility data from all human and bovine eyes is presented in Table 1. The average baseline facility for the short- and long-duration perfused bovine eyes was 0.98 ± 0.06 μL/min/mmHg (mean±SEM), which was nearly 5-fold larger than the average human baseline facility 0.21 ± 0.04 μL/min/mmHg. In bovine eyes, long-duration perfusion led to washout with an 81% increase (Table 1; Fig. 2) in outflow facility (p=0.049) from 1.06 ± 0.06 μl/min/mmHg at baseline to 1.92 ± 0.30 μl/min/mmHg after 3 hrs. In human eyes, washout was not observed; baseline outflow facility was similar between both groups (0.18±0.02 vs. 0.25±0.08 μl/min/mmHg; p=0.518); however, pre-fixation outflow facility in long-duration eyes showed a 40% decrease compared to baseline outflow facility in those same eyes (p = 0.017, paired Student’s t-test).
Table 1. Summary of Human and Bovine Outflow Facility.
In long-duration bovine eyes, outflow facility increases compared to the baseline (p = 0.049), while in human eyes outflow facility decreases (p =0.017).
| Outflow Facility (μL/min/mmHg) | |||||||
|---|---|---|---|---|---|---|---|
| Bovine | Human | ||||||
| C0:Baseline | C1:Pre-Fixation | C0:Baseline | C1:Pre-Fixation | ||||
| Short | Long | Short | Long | Short | Long | Short | Long |
| 1.09 | 1.18 | 0.79 | 1.77 | 0.23 | 0.47 | 0.24 | 0.41 |
| 0.82 | 1.16 | 1.22 | 2.72 | 0.21 | 0.27 | 0.16 | 0.19 |
| 0.72 | 0.94 | 0.76 | 1.92 | 0.13 | 0.09 | 0.12 | 0.05 |
| 0.95 | 0.96 | 0.90 | 1.28 | 0.16 | 0.15 | 0.15 | 0.04 |
|
| |||||||
| Mean | |||||||
| 0.89 | 1.06 | 0.92 | 1.92 | 0.18 | 0.25 | 0.17 | 0.17 |
| SD. | |||||||
| 0.16 | 0.13 | 0.21 | 0.60 | 0.05 | 0.17 | 0.05 | 0.17 |
| SE | |||||||
| 0.08 | 0.06 | 0.11 | 0.30 | 0.02 | 0.08 | 0.03 | 0.09 |
Figure 2. Human and Bovine Outflow Facility.
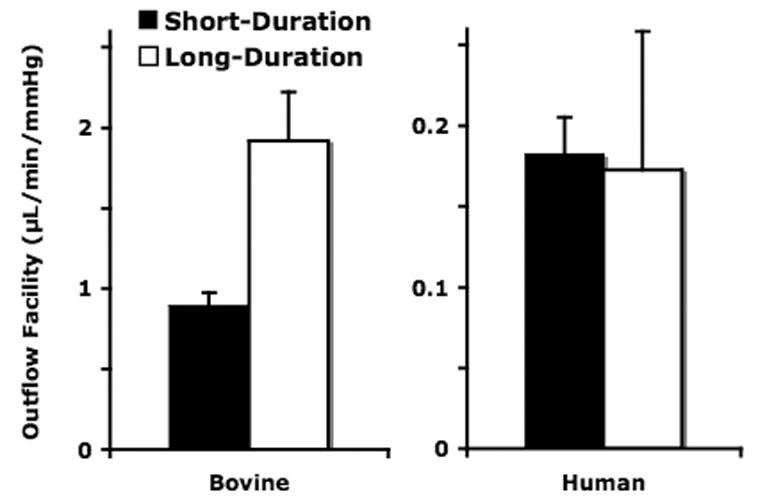
Outflow facility in long-duration bovine eyes increases (81%; p=0.049) vs. short-duration. Human long-duration eyes show no significant change in outflow facility (p>0.3) vs. short-duration.
Morphological Evaluation of Bovine Eyes
Morphological evaluation by light microscopy of the bovine inner wall and JCT following short-duration and long-duration perfusion is shown in Figures 3A and B. Focal regions of the inner wall appeared distended and displaced into the lumen of the aqueous plexus, with expansion of the underlying JCT. These focal regions were morphologically similar to our previous description of inner wall/JCT separation observed by electron microscopy (Overby et al., 2002), but were much more frequent in eyes following long-duration perfusion (Fig. 3B) compared to eyes after short-duration perfusion (Fig. 3A). Morphometric examination of inner wall/JCT separation in >7 images/eye (N=8 eyes) revealed a 2-fold increase (Table 2) in separation score in long-duration compared to short-duration perfusion (1.31±0.19 vs. 0.65±0.08; mean±SEM; p = 0.032). More importantly, a larger absolute value of outflow facility measured immediately prior to fixation coincided with larger mean inner wall/JCT separation score (Fig. 4), and the correlation between these two parameters was statistically significant (p = 0.0024). A second correlation was observed between the relative increase in outflow facility during perfusion (the ratio of pre-fixation to baseline outflow facility) and mean inner wall/JCT separation score (p = 0.0006).
Figure 3.
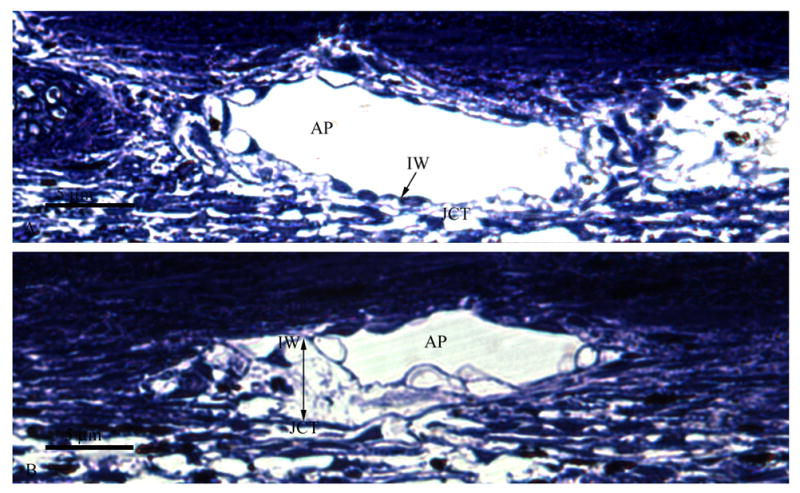
A. Light Micrograph of Bovine Aqueous Plexus after Short-Duration Perfusion. No distention or separation between the JCT and inner wall (IW) of the bovine aqueous plexus (AP) is observed. The JCT/inner wall region appears organized and intact.
B. Light Micrograph of Bovine Aqueous Plexus after Long-Duration Perfusion. A clear separation (Double headed arrow) can be seen between the JCT and the inner wall (IW) of the aqueous plexus (AP). The inner wall distends into the lumen of the AP while the underlying tissue appears disorganized.
Table 2. Inner Wall/JCT Separation Scores in Bovine Eyes.
Long-duration bovine eyes demonstrate a 2-fold increase in separation scores compared with short-duration (p = 0.032).
| Inner Wall Separation Scores | |||||
|---|---|---|---|---|---|
| Bovine Short-Duration | Bovine Long-Duration | ||||
| Eye | #Micrographs | Average Score | Eye | #Micrographs | Average Score |
| 3OD | 10 | 0.80 | 2OD | 8 | 1.25 |
| 6OS | 13 | 0.77 | 3OS | 9 | 1.56 |
| 7OD | 7 | 0.57 | 4OS | 8 | 1.63 |
| 8OS | 8 | 0.47 | 7OS | 10 | 0.80 |
|
| |||||
| Mean | 0.65 | 1.31 | |||
| SD. | 0.16 | 0.38 | |||
| SE | 0.08 | 0.19 | |||
Figure 4. Correlation of Absolute Value of Pre-Fixation Facility and JCT/Inner Wall Separation Scores.
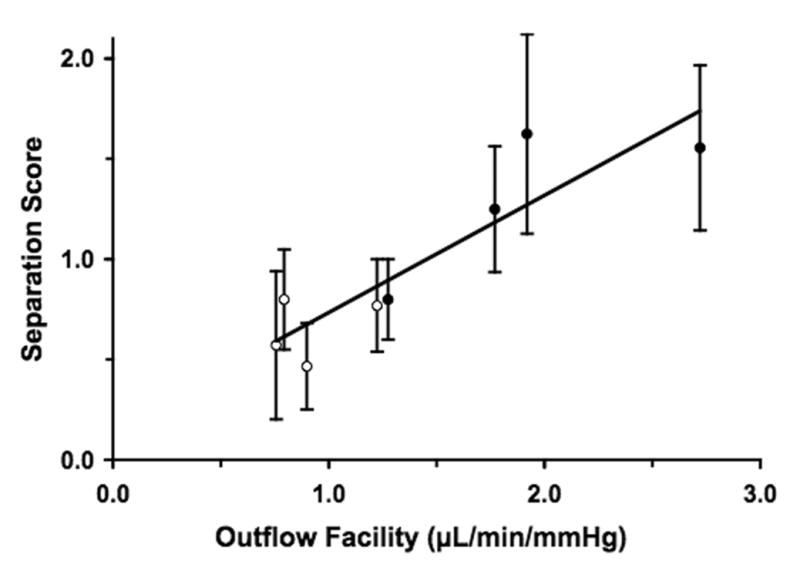
The absolute value of outflow facility measured prior to fixation correlated with the extent of inner wall/JCT separation in all bovine eyes (p=0.0024). Closed circles = long-duration, open circles = short-duration.
With electron microscopy, the inner wall retained many of its characteristic features in both long-duration and short-duration eyes (Fig. 5A and B), including a continuous endothelium containing giant vacuoles residing on a discontinuous basement membrane. In those regions of the inner wall and JCT that were judged to be “separated” by light microscopy, closer examination using electron microscopy revealed that the basal lamina was in close proximity to the inner wall endothelium, despite the apparent displacement of the inner wall from the underlying JCT (Fig. 5A). This morphology fits within the previous definition of “matrix-matrix” separation that occurs between the matrices of the JCT and basal lamina and was previously shown to correlate with the increasing outflow facility during washout (Overby et al., 2002). Morphologic examination of the JCT revealed few elastic-like fibers within the innermost portions of the JCT that occasionally were found immediately underneath the basement membrane of the inner wall (Figs. 5A and B).
Figure 5.
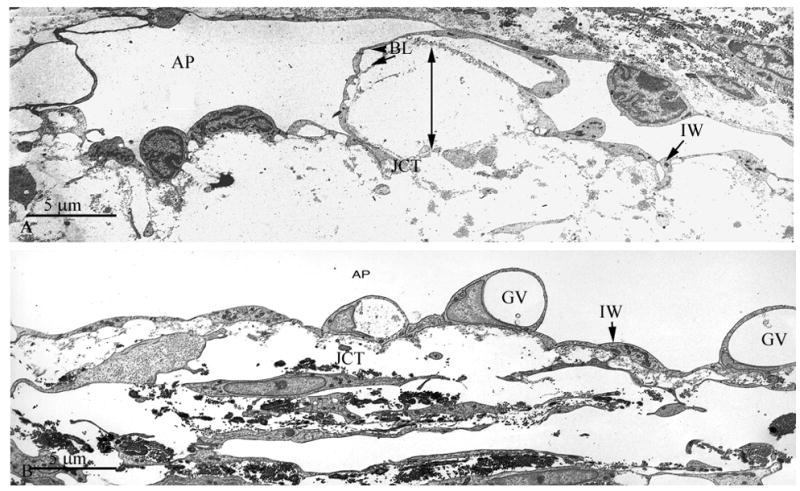
A Transmission Electron Micrograph of Bovine Aqueous Plexus after Long-Duration Perfusion
Significant separation between the basal lamina (BL) of the inner wall (IW) of the bovine aqueous plexus (AP) and underlying extracellular matrix of the JCT region is observed (double headed arrow). Scale bar: 5μm
B Transmission Electron Micrograph of Bovine Aqueous Plexus after Short-Duration Perfusion
No distention of the JCT or separation of the IW lining of the aqueous plexus (AP) was observed. Giant vacuoles (GV) were seen along the inner wall. Scale bar: 5μm
Morphologic Evaluation of Human Eyes
By light microscopy, the inner wall and JCT of human eyes appeared similar between short-duration and long-duration perfusion (Figs. 6A and B). Following long-duration perfusion, there were no observed regions where the inner wall appeared distended and displaced into the lumen of Schlemm’s canal, and the structure of the JCT did not appear to be expanded as observed in bovine eyes (Fig. 3B). The lumen of Schlemm’s canal always appeared patent, with frequent giant vacuoles along the inner wall, with no apparent morphological differences between long and short-duration perfusion (Figs. 6A and B). A morphometric analysis was not performed since no separation between the inner wall and JCT was observed in all micrographs (>24 images/eye; N=8 eyes).
Figure 6.
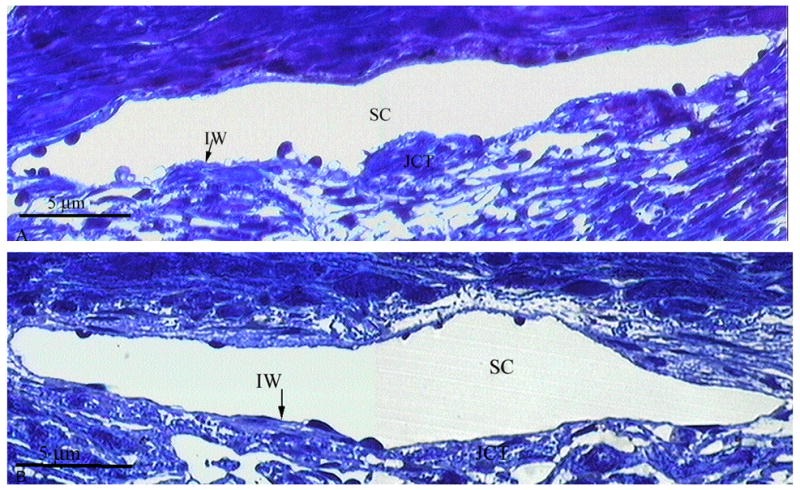
A Light Micrograph of Human JCT/Inner Wall Region after Short-Duration Perfusion. No distention or separation between the JCT and inner wall (IW) of Schlemm’s canal (SC) is observed. The JCT/inner wall region appears organized and intact. Scale bar: 5μm
B Light Micrograph of Human JCT/Inner Wall Region after Long-Duration Perfusion. No distention or separation between the JCT and inner wall (IW) of Schlemm’s canal (SC) is observed. The JCT/inner wall region appears organized and intact. Scale bar: 5μm
With electron microscopy, there was also no apparent morphological difference between short- and long-duration perfusion (Figs. 7A and B). The basal lamina of the inner wall maintained close proximity to both the endothelial cells and extracellular matrix in the JCT, indicating little or no inner wall/JCT separation in either long or short-duration perfusion. Morphologic examination revealed a complex network of elastic-like fibers that was pervasive throughout the JCT of human eyes (Figs. 7A and B). In contrast to the elastic-like fibers observed in bovine eyes (Figs. 5A and B), the elastic-like fibers in human were much more prevalent and were more frequently observed immediately underneath the inner wall endothelium.
Figure 7.
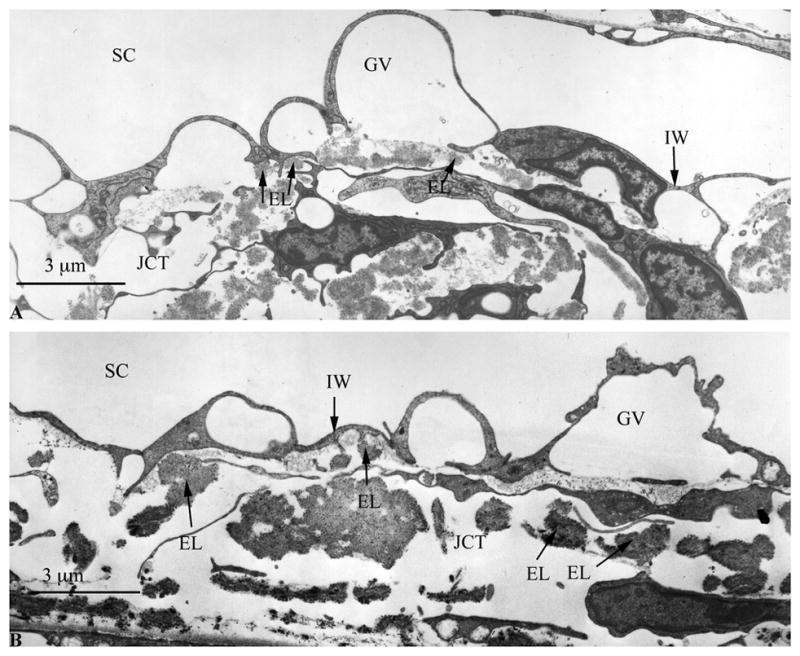
A Transmission Electron Micrograph of Human Schlemm’s Canal after Short-Duration Perfusion
B Transmission Electron Micrograph of Human Schelmm’s Canal after Long-Duration Perfusion.
No separation is observed between the inner wall (IW) of Schlemm’s canal (SC) and the underlying JCT in both short and long-duration perfusion (A and B, respectively). Giant vacuoles (GV) are seen along the IW of SC with a similar distribution in both short and long-duration perfusion. Compared to bovine eyes (Figure 5A & B) there is more complex array of elastic fibers (EL) in the JCT region (single headed arrows), especially in the area immediately underneath the inner wall (IW). Scale bars: 3μm
DISCUSSION
During experimental perfusion of non-human eyes, outflow facility progressively increases due to a phenomenon known as washout. Because washout occurs in many different species (Barany, 1953, 1964; Gaasterland et al., 1978; Kaufman et al., 1988; Melton and Deville, 1960; Pandolfi, 1967; Van Buskirk and Brett, 1978; Yan et al., 1991) including non-human primates (Barany, 1962, 1964; Erickson and Kaufman, 1981, Kaufman et al., 1988) and is observed despite differences in perfusion fluid (Barany and Woodin, 1955; Epstein et al., 1978; Gaasterland et al., 1979; Kee et al., 1996) and enucleated or in vivo state (Barany, 1962, 1964; Erickson and Kaufman, 1981; Epstein, 1982; Gaasterland et al., 1978, 1979; Hashimoto and Epstein, 1980; Kaufman et al., 1988; Peterson et al., 1974), washout likely represents some aspect of normal outflow physiology that is apparently absent from human eyes. Understanding the mechanism of washout would therefore likely provide important insight into the generative mechanism of outflow resistance as well as clues as to how the outflow pathway of human eyes may be functionally different from most other species.
In this study, we investigated the hypothesis that washout is associated with a physical separation between the inner wall endothelium and the underlying JCT. This separation is predicted to increase outflow facility by eliminating the funneling effect (Johnson et al., 1992) that we propose is normally present between the inner wall and JCT and increases the effective resistance generated by extracellular matrix within the JCT. This study focuses on the morphologic aspects of this hypothesis by comparing inner wall/JCT morphology between long- and short-duration and perfusion between species that do and do not exhibit the washout effect.
Following long-duration perfusion of bovine eyes (that exhibit washout) there were two important observations: (i) a 2-fold increase in inner wall/JCT separation score in long- versus short-duration perfusion; and (ii) a statistically significant positive correlation between the inner wall/JCT separation score and the absolute value of outflow facility measured immediately prior to fixation.
These data are consistent with our previous results (Overby et al., 2002) and together strongly suggest that the connectivity between the inner wall and JCT is involved in the regulation of aqueous humor outflow resistance and the increase in outflow facility during washout in bovine eyes. In contrast, after long-duration perfusion of human eyes that do not exhibit washout (Erickson-Lamy et al., 1990), there was no increase in outflow facility and no apparent separation between the inner wall and JCT, as judged either with light or electron microscopy. Interestingly, human eyes subjected to prolonged perfusion showed a time-dependent decrease in outflow facility consistent with previous reports (Erickson et al., 1990; Grant, 1963). Overall, these data support our hypothesis regarding inner wall/JCT separation as a mechanism for washout, but more importantly suggest that the particular cellular and extracellular matrix adhesions that tether the inner wall to the JCT may be involved in maintaining outflow resistance and may contribute to the absence of washout from human eyes.
In our previous study (Overby et al., 2002), we demonstrated that the facility increase during washout in bovine eyes was reversible by reducing IOP to 0 mmHg for one hour. Moreover, the facility change during washout and washout reversal correlated with the extent of “matrix-matrix” separation between the basal lamina of the inner wall and the extracellular matrix of the JCT, as seen by electron microscopy. The previous data suggests that washout reversal coincides with re-establishment of matrix-matrix connectivity. In the current study, light microscopy was used to visualize tissue-level morphologic changes. The extent of inner wall/JCT separation was seen to increase with progressive washout and was associated with increasing outflow facility. Closer examination using electron microscopy revealed that the inner wall/JCT separation seen using light microscopy was consistent with matrix-matrix separation. Thus, our current and previous data (Overby et al., 2002) are complementary, and together suggest that the facility changes during washout and washout reversal in bovine eyes correspond with separation and re-attachment of matrix-matrix connectivity between the inner wall and JCT. Moreover, a recent study (Sabanay et al., 2004) in live non-human primate eyes demonstrated a significant increase in outflow facility as well as expansion and loss of connectivity between the inner wall and JCT following H-7, a serine-threonine kinase inhibitor affecting the cytoskeletal network, and these effects were reversible after withdrawing H-7, supporting the current study and a previous hypothesis (Overby et al., 2002) describing the mechanism of washout.
These data suggest that the connecting fibrils and adhesion proteins that tether the inner wall to the JCT are important regulators of aqueous humor outflow resistance. In bovine eyes, loss of these adhesions results in inner wall/JCT separation that is shown here to coincide with decreasing outflow resistance (increasing outflow facility), possibly by altering the hydrodynamic patterns of outflow through the JCT or by eliminating the funneling effect. These adhesions are likely under mechanical tension and oppose outward-directed forces generated during perfusion that result from the pressure gradient across the inner wall and JCT and the basal-to-apical driven flow across the inner wall. Therefore, there exists a mechanical equilibrium between hydrodynamic and adhesion forces that likely determines the extent of inner wall/JCT separation and the increase in outflow facility during washout. During experimental perfusion of enucleated or in vivo eyes, the processes driving washout may be accelerated by a greater than normal pressure gradient across the inner wall/JCT region, resulting from a reduction in episcleral venous pressure caused by enucleation or anesthesia (John et al., 1997).
If the connecting fibrils and molecular adhesions between the inner wall and JCT of human eyes function in a manner similar to their bovine counterparts, then these connections should also have an important role in the maintenance of outflow resistance in humans. With this perspective, one likely explanation for the lack of washout from human eyes is an enhanced connectivity between the inner wall and JCT that more strongly opposes hydrodynamic forces, prevents inner wall/JCT separation and maintains outflow resistance. Compared to bovine eyes, we observed a complex array of elastic-like fibers in the JCT that formed frequent contacts with the inner wall endothelium in human eyes, consistent with the previously described “cribriform plexus” that tethers the inner wall via connecting fibrils to the elastic-like fibers and tendons of the ciliary muscle (Rohen, Futa, and Lutjen-Drecoll, 1981). This elastic-like fiber network may act together with other cell-cell (Johnstone, 1979; Grierson et al., 1978) or cell-matrix (Tian et al., 2000) adhesions to anchor the inner wall and transmit hydrodynamic forces exerted during perfusion to the underlying tissue, thereby opposing inner wall/JCT separation and the washout-associated decrease in outflow resistance in human eyes.
In summary, we investigated the mechanism(s) for the increase in outflow facility during washout in the bovine eye and examined the mechanism accounting for the lack of washout in human eyes. Our study suggests that constituents involved in the tethering of the inner wall and JCT are important regulators of outflow resistance and therefore represent possible targets for new pharmacological strategies that disrupt the connectivity between the inner wall and the JCT to reduce aqueous humor outflow resistance in glaucomatous eyes.
Acknowledgments
This work was supported by NIH grant #EY-09699, the Glaucoma Research Foundation, National Glaucoma Research, a program of the American Health Assistance Foundation and The Massachusetts Lions Eye Research Fund, Inc to Boston University. We thank Dr. Kristine Erickson for the generous use of her ocular perfusion system. We are grateful for the technical assistance of Rozanne Richman, MS.
Footnotes
Supported by NIH grants EY007149 and EY-09699, the Glaucoma Research Foundation, National Glaucoma Research, a program of the American Health Assistance Foundation and The Massachusetts Lions Eye Research Fund, Inc to Boston University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bárány EH. In vitro studies of the resistance to flow through the angle of the anterior chamber. Acta Soc Med Uppsala. 1953;59:260–276. [PubMed] [Google Scholar]
- Bárány EH. The mode of action of pilocarpine on outflow resistance in the eye of a primate (Cercopithecus aethiops) Invest Ophthalmol Vis Sci. 1962;1:712–727. [PubMed] [Google Scholar]
- Bárány EH. Simultaneous measurement of changing intraocular pressure and outflow facility in the vervet monkey by constant pressure infusion. Invest Ophthalmol Vis Sci. 1964;3:135–143. [PubMed] [Google Scholar]
- Bárány EH, Scotchbrook S. Influence of testicular hyaluronidase on the resistance to flow through the angle of the anterior chamber. Acta Physiol Scand. 1954;30:240–248. doi: 10.1111/j.1748-1716.1954.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Bárány EH, Woodin AM. Hyaluronic Acid and hyaluronidase in the aqueous humor and the angle of the anterior chamber. Acta Physiol Scand. 1955;33:257–290. doi: 10.1111/j.1748-1716.1955.tb01208.x. [DOI] [PubMed] [Google Scholar]
- Ellingsen B, Grant W. Influence of intraocular pressure and trabeculotomy on aqueous outflow in enucleated monkey eyes. Invest Ophthalmol Vis Sci. 1971;10:705–709. [PubMed] [Google Scholar]
- Epstein DL, Hashimoto JM, Grant WM. Serum obstruction of aqueous outflow in enucleated eyes. Am J Ophthalmol. 1978;86:101–105. doi: 10.1016/0002-9394(78)90023-5. [DOI] [PubMed] [Google Scholar]
- Epstein DL, Patterson MM, Rivers SC, Anderson PJ. N-ethylmaleimide increases the facility of aqueous outflow of excised monkey and calf eyes. Invest Ophthalmol Vis Sci. 1982;22:752–756. [PubMed] [Google Scholar]
- Erickson KA, Kaufman PL. Comparative effects of three perfusates on outflow facility in the cynomolgus monkey. Curr Eye Res. 1981;1:211–216. doi: 10.3109/02713688109001851. [DOI] [PubMed] [Google Scholar]
- Erickson-Lamy K, Rohen JW, Grant WM. Outflow facility studies in the perfused bovine aqueous outflow pathways. Eye Research. 1988;7:799–807. doi: 10.3109/02713688809033211. [DOI] [PubMed] [Google Scholar]
- Erickson-Lamy K, Schroeder AM, Bassett-Chu S, Epstein DL. Absence of time-dependent facility increase (“washout”) in the perfused enucleated human eye. Invest Ophthalmol Vis Sci. 1990;31:2384–2388. [PubMed] [Google Scholar]
- Ethier CR, Kamm RD, Palaszewski BA, Johnson M, Richardson TM. Calculations of flow resistance in the juxtacanlicular meshwork. Invest Ophthalmol Vis Sci. 1986;27:1741–1750. [PubMed] [Google Scholar]
- Francois J, Rabaey M, Neetens A. Perfusion studies on the outflow of aqueous humor in human eyes. AMA Arch of Ophthalmol. 1955;54:193–204. doi: 10.1001/archopht.1956.00930030195005. [DOI] [PubMed] [Google Scholar]
- Gaasterland DE, Pederson JE, McLellan HM. Perfusate effects upon resistance to aqueous humor outflow in the rhesus monkey. Invest Ophthalmol Vis Sci. 1978;17:391–397. [PubMed] [Google Scholar]
- Gaasterland DE, Pederson JE, McLellan HM, Reddy VN. Rhesus monkey aqueous humor composition and a primate ocular perfusate. Invest Ophthalmol Vis Sci. 1979;18:1139–1150. [PubMed] [Google Scholar]
- Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783–801. doi: 10.1001/archopht.1963.00960040789022. [DOI] [PubMed] [Google Scholar]
- Grierson I, Lee WR, Abraham S, Howes RC. Associations between the cells of the walls of Schlemm’s canal. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1978;208:33–47. doi: 10.1007/BF00406980. [DOI] [PubMed] [Google Scholar]
- Hashimoto JM, Epstein PL. Influence of intraocular pressure on aqueous outflow facility in enucleated eyes of different mammals. Invest Ophthalmol Vis Sci. 1980;19:1483–1489. [PubMed] [Google Scholar]
- John SWM, Hagaman JR, MacTaggart TE, Peng Li, Smithes O. Intraocular pressure in inbred mouse strains. Invest Ophthalmol Vis Sci. 1997;38:249–253. [PubMed] [Google Scholar]
- Johnson M, Gong H, Freddo TF, Ritter N, Kamm R. Serum proteins and aqueous outflow resistance in bovine eyes. Invest Ophthalmol Visl Sci. 1993;34:3549–3557. [PubMed] [Google Scholar]
- Johnson M, Shapiro A, Ethier CR, Kamm RD. Modulation of outflow resistance by the pores of the inner wall endothelium. Invest Ophthalmol Vis Sci. 1992;33:1670–1675. [PubMed] [Google Scholar]
- Johnstone MA. Pressure-dependent changes in nuclei and the process origins of the endothelial cells lining Schlemm’s canalconfiguration of the endothelial tubules of Schlemm’s canal. Invest Ophthalmol Vis Sci. 1979;18:44–51. [PubMed] [Google Scholar]
- Kaufman PL, True-Gabelt B, Erickson-Lamy KA. Time dependence of perfusion outflow facility in the cynomolgus monkey. Curr Eye Res. 1988;7:721–726. doi: 10.3109/02713688809033201. [DOI] [PubMed] [Google Scholar]
- Kee C, Gabelt BT, Croft MA, Gange SJ, Menage MJ, Kaufman PL. Serum effects on aqueous outflow during anterior chamber perfusion in monkeys. Invest Ophthalmol Vis Sci. 1996;37:1840–1848. [PubMed] [Google Scholar]
- Knepper PA, Farbman AI, Tesler AG. Exogenous hyaluronidases and degradation of hyaluronic acid in the rabbit eye. Invest Ophthalmol Vis Sci. 1984;25:286–293. [PubMed] [Google Scholar]
- Melton CE, Deville WB. Perfusion studies on eyes of four species. Am J Ophthalmol. 1960;50:302–308. [Google Scholar]
- Overby D, Gong H, Qiu G, Freddo TF, Johnson M. The mechanism of increasing outflow facility during washout in the bovine eye. Invest Ophthalmol Vis Sci. 2002;43:3455–3464. [PubMed] [Google Scholar]
- Pandolfi M. Fibrinolysis and outflow resistance in the eye. Am J Ophthalmol. 1967;64:1141–1148. doi: 10.1016/0002-9394(67)93070-x. [DOI] [PubMed] [Google Scholar]
- Peterson WS, Joscon VL. Hyaluronidase effects on aqueous outflow resistance: Quantitative and localizing studies in the rhesus monkey eye. Am J Ophthalmol. 1974;77:573–577. doi: 10.1016/0002-9394(74)90473-5. [DOI] [PubMed] [Google Scholar]
- Rohen JW, Futa R, Lutjen-Drecoll E. The fine structure of the cribriform meshwork in normal and glaucomatous eye as seen in tangential sections. Invest Ophthalmol Vis Sci. 1981;21:574–585. [PubMed] [Google Scholar]
- Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Functional and structural reversibility of H-7 effects on the conventional aqueous outflow pathway in monkeys. Exp Eye Res. 2004;78:137–150. doi: 10.1016/j.exer.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Sit AJ, Gong H, Ritter N, Freddo TF, Kamm R, Johnson M. The role of soluble proteins in generating aqueous outflow resistance in the bovine and human eye. Exp Eye Res. 1997;64:813–821. doi: 10.1006/exer.1997.0276. [DOI] [PubMed] [Google Scholar]
- Tian B, Geiger B, Epstein DL, Kaufman PL. Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci. 2000;41:619–623. [PubMed] [Google Scholar]
- Tripathi RC. Ultrastructure of the exit pathway of the aqueouos in lower mammals (a preliminary report on the “angular aqueous plexus”) Exp Eye Res. 1971;12:311–314. doi: 10.1016/0014-4835(71)90155-2. [DOI] [PubMed] [Google Scholar]
- Van Buskirk MS, Brett J. The canine eye: in vitro dissolution of the barriers to aqueous outflow. Invest Ophthalmol Vis Sci. 1978;17:258–263. [PubMed] [Google Scholar]
- Yan DB, Trope GE, Ethier CR, Menon IA, Wakeham A. Effects of hydrogen peroxide-induced oxidative damage on outflow facility and washout in pig eyes. Invest Ophthalmol Vis Sci. 1991;32:2515–2520. [PubMed] [Google Scholar]


