Abstract
Transforming growth factor-β (TGF-β1, -β2, and -β3) has been implicated in the ontogenetic transition from scarless fetal repair to adult repair with scar. Generally, TGF-β exerts its effects through type I and II receptors; however, TGF-β modulators such as latent TGF-β binding protein-1 (LTBP-1), decorin, biglycan, and fibromodulin can bind and potentially inhibit TGF-β activity. To more fully explore the role of TGF-β ligands, receptors, and potential modulators during skin development and wound healing, we have used a rat model that transitions from scarless fetal-type repair to adult-type repair with scar between days 16 and 18 of gestation. We showed that TGF-β ligand and receptor mRNA levels did not increase during the transition to adult-type repair in fetal skin, whereas LTBP-1 and fibromodulin expression decreased. In addition, TGF-β1 and -β3; type I, II, and III receptors; as well as LTBP-1, decorin, and biglycan were up-regulated during adult wound healing. In marked contrast, fibromodulin expression was initially down-regulated in adult repair. Immunostaining demonstrated significant fibromodulin induction 36 hours after injury in gestation day 16, but not day 19, fetal wounds. This inverse relationship between fibromodulin expression and scarring in both fetal and adult rat wound repair suggests that fibromodulin may be a biologically relevant modulator of TGF-β activity during scar formation.
Fetal repair is fundamentally different from adult repair. Adult skin wounds heal by scar formation, whereas fetal skin wounds heal by regeneration with restoration of normal skin architecture. This transition from scarless fetal repair to adult-type healing with scar occurs at specific times during gestation. 1 The mechanism for scarless fetal repair is unknown, but it does not require systemic factors such as the fetal immune system, fetal serum, or amniotic fluid. 2-4 Isolated human fetal skin transplanted into adult athymic mice can heal without scar. 5 Thus the capability for scarless repair is inherent to fetal skin itself.
Fetal skin contains fetal fibroblasts and fetal extracellular matrix (ECM) that is distinct from adult fibroblasts and ECM. 5 For instance, the fetal ECM has a higher ratio of type III to type I collagen, 6,7 as well as a different profile of proteoglycan and glycosaminoglycan synthesis. 8 It is probable that both the fetal skin cells and the ECM they synthesize are critical in scarless repair. In addition, fetal cells and fetal ECM likely have a dynamic and reciprocal relationship whereby fetal cells secrete a fetal ECM that can, in turn, modulate fetal cell migration, proliferation, and collagen synthesis. 9 These results suggest that cellular gene regulation rather than the external (amniotic) environment is the critical factor in scarless repair.
We hypothesize that fundamental differences in gene expression govern the transition to adult-type repair in fetal skin, whereby profibrotic molecules will be up-regulated and antifibrotic molecules will be down-regulated during the onset of adult-type repair. The transforming growth factor-β (TGF-β1, -β2, and -β3) ligands have been implicated in the ontogenetic transition from scarless fetal repair to adult repair with scar. Specifically, TGF-β1 and TGF-β2 may promote scar formation, whereas TGF-β3 may reduce scarring. 10 The addition of exogenous TGF-β1 to normally scarless fetal wounds results in scar, 11,12 while neutralizing antibodies against TGF-β1 and TGF-β2 in adult wounds, decreases scarring. 10 Although the addition of TGF-β alone is enough to induce scarring in the fetus, TGF-β neutralizing antibodies alone do not entirely prevent scarring in the adult, suggesting that factors other than TGF-β may also be important for scarless fetal repair.
TGF-β ligands have a wide range of effects on cellular motility, proliferation, matrix production, and differentiation. 13 Most cells secrete TGF-β in an inactive latent form that undergoes proteolytic cleavage for activation or associates with one of several latent TGF-β-binding proteins (LTBP) that may function in ECM storage, secretion, and activation of TGF-β. 13,14 Once activated, TGF-β exerts its biological effects through binding to type II and type I receptors. 13 Type III receptor (also known as betaglycan) is a membrane-anchored proteoglycan that facilitates TGF ligand binding to the type II receptor. 14 Besides betaglycan (type III receptor), several small ECM proteoglycans of the decorin family, including biglycan and fibromodulin, can bind activated TGF-β and suppress TGF-β activity. 15 The addition of decorin to a rat model of glomerulonephritis decreased TGF-β-mediated matrix accumulation and glomerular injury. 16 Furthermore, both decorin and fibromodulin, in addition to modulating TGF-β activity, can regulate collagen fibril formation and tensile strength. 17-19
In the present study, we have used a rat model that makes the transition from scarless fetal-type repair to adult-type repair with scar between days 16 (E16) and 18 (E18) of gestation (term = 21 days). 4 To address our hypothesis, we first confirmed the existence of the transition period through histology and confocal microscopy of E16 and E19 fetal wounds. Next, we analyzed the expression of potential profibrotic molecules (eg, TGF-β1 and -β2 and type I, II, and III receptors) and potential antifibrotic molecules (eg, TGF-β3, LTBP-1, decorin, biglycan, and fibromodulin) during rat fetal skin development. Surprisingly, we found that fetal skin levels of TGF-β ligand and receptor levels did not increase during the transition period. Instead, we showed that levels of LTBP-1 and fibromodulin, two possible modulators of TGF-β, decreased with the onset of scar formation in the fetus. Diminished fetal expression of LTBP-1 and fibromodulin during the transition to adult-type repair with scar suggests that LTBP-1 and fibromodulin may have antifibrotic roles in fetal wound repair.
To further clarify the role of LTBP-1 and fibromodulin in scar formation, we examined their expression relative to TGF-β1, -β2, and -β3; TGF-β type I, II, and III receptors; and decorin and biglycan in an adult rat model of wound repair. We demonstrated that TGF-β1 and -β3; type I, II, and III receptors; as well as LTBP-1, decorin, and biglycan, were up-regulated during adult wound healing. The up-regulation of LTBP-1 in an adult model of repair with scar is inconsistent with its role as a possible antifibrotic molecule. Fibromodulin expression, however, in marked contrast to that of the other molecules, was initially down-regulated in adult repair (consistent with its role as a possible antifibrotic molecule).
After this, we examined fibromodulin protein expression in E16 and E19 fetal wounds. We showed significant induction of fibromodulin expression in 16-day fetal wounds and only minimal induction in 19-day wounds. Thus our findings revealed a consistently inverse relationship between fibromodulin expression and scar formation in both fetal and adult rat models of wound repair. This suggests that fibromodulin may be a biologically relevant modulator of TGF-β activity during scar formation. Relative abundance of fibromodulin compared with TGF-β in early gestation wounds may partly effect scarless fetal repair through decreased TGF-β bioavailability. In addition, increased fibromodulin induction in fetal wounds offers a novel explanation of how, mechanistically, fetal wound collagen is deposited in a more organized fashion than collagen in adult wounds. Potential strategies for the manipulation of adult wounds into being more “fetal-like” may include the addition of fibromodulin to modulate both TGF-β activity and ECM assembly.
Materials and Methods
Animals
Sprague-Dawley rats were housed in a controlled animal facility at the University of California, Los Angeles, and fed food and water ad libitum.
Preparation of Fetal Tissue
Female Sprague-Dawley (SD) rats (∼300 g) were mated. The time of detection of a vaginal plug as evidence of pregnancy was considered day 0.5 of gestation. To examine cutaneous gene expression during development, pregnant rats were anesthetized on days 14, 16, 17, 18, 19, 20, and 21 of gestation. After this, the animals were shaved and a midline laparotomy was performed to expose the uterine segments. Uterine myotomies were then made, and full-thickness dorsal fetal skin from between the articulation of the hindlimbs and the articulation of the forelimbs was excised (N = 10–15 fetuses/time point). The isolated tissue was immediately frozen in liquid nitrogen and stored at −70°C until RNA extraction.
For fetal wound tissue, pregnant rats were anesthetized on days 16 and 19 of gestation. The laparotomies were performed (as described above). A 7-O nylon pursestring suture was placed before each uterine myotomy. Two-millimeter punch biopsies were then used on the dorsum of each fetus to create full-thickness excisional wounds. Wounds were then stained with blue vital dye for later identification, and the amniotic fluid was replaced with normal saline before uterine closure. Wounds were harvested 12, 36, and 72 hours postoperatively and fixed in 4% paraformaldehyde (N = 4 wounds per time point, two separate pregnancies). Nonwounded skin from E16 and E19 embryos, as well as from each wound harvest time point, was used as controls.
Preparation of Adult Tissue
To assess gene expression during cutaneous wound repair, 20 rats were anesthetized and shaved. To minimize regional cephalocaudal and dorsoventral differences in wound healing, spatial landmarks such as the forelimb and hindlimb articulation sites as well as the spine were used to consistently mark excisional wound locations. 20 Eight full-thickness, excisional skin wounds, including the panniculus carnosus, were made (7 mm2; N = 128 wounds) on the backs of 16 animals. All wounds were separated by at least 2 cm to minimize any effects from neighboring wounds. Two animals at a time were reanesthetized, and all eight wounds on each animal were excised in their entirety (including the base) at 12 hours and 1, 2, 3, 5, 7, 10, and 14 days after injury (N = 16 wounds per time point). Controls consisted of nonwounded skin (0 hour) from identical locations in four animals. To minimize possible wound healing responses to shaving with an electric clipper, nonwounded control animals underwent skin harvest within 1 minute of hair removal. Tissue for RNA isolation was immediately frozen in liquid nitrogen and stored at −70°C until RNA extraction.
RNA Isolation
Total RNA was extracted using a monophasic solution of phenol and guanidine isothiocyanate (TRIzol Reagent; Gibco BRL, Gaithersburg, MD) and treated with at least 1 U of DNase I (Gibco)/1.5 μg RNA to remove chromosomal DNA contamination. 21
Reverse Transcriptase-Polymerase Chain Reaction
To confirm DNA removal, specific primer polymerase chain reaction (PCR) was carried out using total RNA (without reverse transcription) and a control primer set that is known to flank an intron-containing region (see below for PCR methods). Relative RNA integrity and quantitation accuracy were assessed by fractionating 2 μg of total RNA in a glyoxal/dimethyl sulfoxide gel and visualizing more prominent 28S versus 18S ribosomal RNA bands. 22 First-strand cDNA synthesis was accomplished using 1 μg of total RNA with 100 pmol of oligo(dT)15 and 1 μl (200 U) of SUPERSCRIPT II (Gibco) in a 40-μl reaction volume at 42°C for 60 minutes. The SUPERSCRIPT was then inactivated by heating at 72°C for 15 minutes. To relatively quantify differences in gene expression during gestation, reduced cycle-specific primer PCR was carried out. Reduced cycle PCR is based on the principle that, in the linear amplification cycle range, the resultant number of PCR molecules reflects the number of starting mRNA molecules. 23 For each candidate molecule, we determined the cycle number most likely to fall within the linear amplification range by successively reducing the number of cycles (range = 30 to 10 cycles). PCR was carried out using the following: 1.5 μl (1/40) of the first-strand reverse transcriptase (RT) reaction, 2 pmol of each forward and reverse amplification primers, and 0.2 μl (1 U) of Taq DNA polymerase (Gibco) in a 20-μl reaction volume. All PCR reactions were performed using the GeneAmp PCR System 9600 (Perkin Elmer, Branchburg, NJ). PCR temperatures were 95°C for 1 minute (one time only), followed by the annealing temperature (variable among different primer sets) for 1 minute, 72°C for 1 minute, and 95°C for 30 seconds for a variable number of cycles. Concomitant glyceraldehyde-3-phosphate dehydrogenase (GAPDH) PCR was also performed in separate tubes for each RT reaction as a control (Figures 2D and 3D) ▶ ▶ . The PCR products were separated by size on a 1.2% agarose gel and blotted onto a nylon membrane by capillary diffusion for Southern analysis.
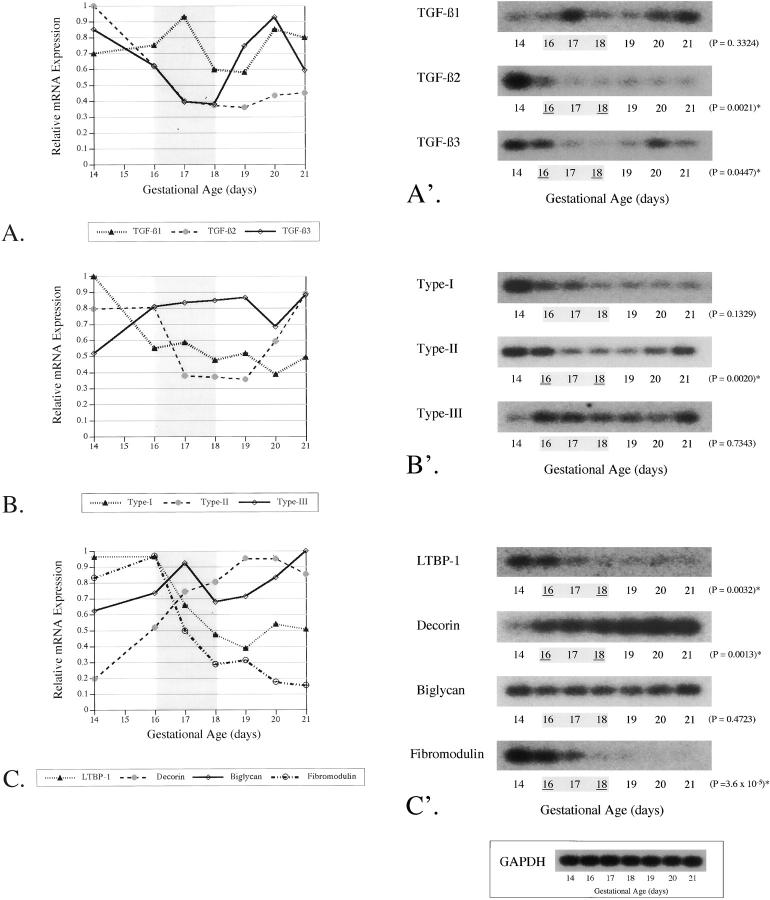
Figure 2.
RT-PCR of TGF-β ligands, receptors, and modulators during fetal skin development. RT-PCR was performed on RNA isolated from day 14, 16, 17, 18, 19, 20, and 21 fetal dorsal skin (N = 10–15 fetuses per time point). To determine relative changes in mRNA levels during development, densitometry values for each blot were corrected to GAPDH expression at each time point and normalized by setting the highest value to 1. The results are depicted graphically as the mean ± SD (A–C). The transition period is highlighted in gray. An unpaired two-tailed Student’s t-test was performed to detect statistically significant differences in gene expression between E16 (beginning transition) and E18 (end transition) fetal skin. A representative blot is shown for each TGF-β ligand (A′), receptor (B′), or modulator (C′), with the corresponding P value on the right and the transition period highlighted in gray. Statistically significant differences in gene expression between day 16 (beginning transition) and day 18 (end transition) fetal skin are underlined (A′–C′). Statistically significant P values (< 0.05) are marked with an asterisk. A representative fetal GAPDH PCR reaction is shown (inset).
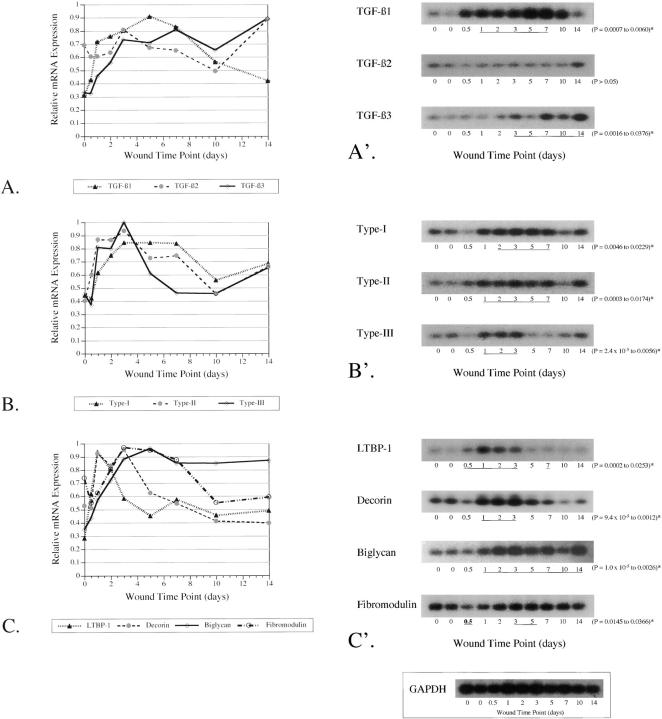
Figure 3.
RT-PCR of TGF-β ligands, receptors, and modulators during adult cutaneous wound repair. RT-PCR was performed on RNA isolated from 12-hour to 14-day wounds and nonwounded controls (N = 16 wounds per time point). To determine relative changes in mRNA levels after wounding, densitometry values for each blot were corrected to GAPDH expression at each time point and normalized by setting the highest value to 1. The results are depicted graphically as the mean ± SD (A–C). An unpaired two-tailed Student’s t-test was performed to detect statistically significant differences in gene expression between nonwounded controls and adult wounds at different postinjury time points, which are underlined (A′–C′). A representative blot is shown for each TGF-β ligand (A′), receptor (B′), or modulator (C′), with the corresponding range of P values on the right. All of the underlined time points represent statistically significant increases in gene expression relative to nonwounded controls, except for the 12-hour fibromodulin time point (in bold), which represents decreased expression levels relative to the control (C′). The range of P values is shown and is marked with an asterisk if P < 0.05. A representative adult GAPDH PCR reaction is shown (inset).
At least two or more RT reactions from which at least four separate sets of cycle-optimized PCR reactions were performed for each PCR primer set. Primer sequences, PCR reaction temperatures, and PCR cycle number are listed in Table 1 ▶ . Primers were designed using the Prime program (version 8.0) from the Wisconsin Sequence Analysis Package (Genetics Computer Group, Madison, WI).
Table 1.
PCR Primer and Probe Sequences and Reaction Conditions
| Gene (Genbank no.) | Forward primers (5′ to 3′) | Reverse primers (5′ to 3′) | ||||
|---|---|---|---|---|---|---|
| TGF-β1 (X52498) | TATCCCGGTGGCATACTGAG | CCCAAGGAAAGGTAGGTGATAGTC | ||||
| TGF-β2 (X71904) | TCCTGCTAATGTTGTTGCCC | AGCAATTATGCTGCACATTCC | ||||
| TGF-β3 (U03491) | CAAAGCAACAGACCTCACCC | ATCTCTTCTCTACCCCACCACC | ||||
| Type I receptor (L26110) | CAAATGGCGGGGAGAAGAAG | TGATAATCCGACACCAACCACAG | ||||
| Type II receptor (L09653) | GGAGGAAGAACGACAAGAAC | TGGATAATGACCAGCAACAG | ||||
| Type III receptor (M77809) | ACACCAACTCCAAAAGTGCC | ATCACCCTGCTAATCCCCTC | ||||
| LTBP-1 (M55431) | TCCCCAGCCACCATTTTTTC | TCCTCCATTTCCACCCAAGTAAG | ||||
| Decorin (X59859) | CACCAACATAACTGCTATTCCTC | AGACTGCCATTTTCCACAAC | ||||
| Biglycan (U17834) | AGTTCACTACCTGTCCATCC | AGAGAGAGAGAGAGAGAGAGAGAG | ||||
| Fibromodulin (X82152) | AACCATCACACACACACACAC | AGCATTTGACCCTTCTCTCC | ||||
| GAPDH (AF106860) | CACCACCATGGAGAAGGC | CCATCCACAGTCTTCTGA |
Probes, Hybridization Conditions, and Autoradiography
Correct PCR product amplification was confirmed by probing the nylon membrane with a 32P-labeled oligonucleotide probe specific for a given gene. The sequences of the probes are described in Table 1 ▶ . Oligonucleotides were end-labeled with T4 kinase (Gibco). Each membrane was placed in 10 ml prehybridization solution (hybridization buffer tablets; Amersham Life Science) and shaken at 42°C in a hybridization oven (Amersham Life Science) for 2 hours. After this, individual membranes were hybridized overnight at 42°C with specific probes. Membranes were then washed in 5× standard saline citrate, 0.1% sodium dodecyl sulfate for 20 minutes, followed by two 20-minute rinses in 0.1× standard saline citrate, 0.1% sodium dodecyl sulfate at 42°C. Autoradiograms were then generated from the radioactive membranes.
To compare general trends in mRNA expression for TGF-β ligands, receptors and modulators, autoradiograms were scanned and analyzed on a Macintosh computer using the public domain NIH Image program. Densitometry values for each TGF-β ligand, TGF-β receptor, and TGF-β modulator were corrected to GAPDH expression at each time point, normalized by setting the highest value to 1, and graphically depicted as the mean ± the SD.
Statistical Analysis
An unpaired two-tailed Student’s t-test was performed to detect statistically significant differences in gene expression between E16 (beginning transition) and E18 (end transition) fetal skin, as well as statistically significant differences in gene expression between controls and adult wounds at different postinjury time points. A P value of <0.05 was considered significant.
Histology
Wounded fetal skin and nonwounded control skin were fixed overnight in 4% paraformaldehyde. The tissues were then embedded in paraffin and cut into 5-μm sections for hematoxylin and eosin (H&E) staining or immunohistochemistry.
Immunohistochemistry
To determine fibromodulin expression in fetal wounds, immunohistochemistry was performed on E16 and E19 wounds 36 hours after injury, as well as on nonwounded E17.5 (E16 + 36 hours) and E20.5 (E19 + 36 hours) fetal skin. After deparaffinization and rehydration of the tissue sections, endogenous peroxidase activity was quenched with 1% hydrogen peroxide (H2O2) for 30 minutes, followed by 2% H2O2 for 15 minutes, and then the sections were rinsed with phosphate-buffered saline. To reduce nonspecific binding, sections were incubated overnight at 4°C in a humidified chamber with a 1:50 dilution of goat blocking serum (Vector Laboratories, Burlingame, CA). Subsequently, sections were incubated with anti-fibromodulin antibody (from Anna Plaas) 24 for 1 hour at room temperature, rinsed three times with phophate-buffered saline, and then incubated with a 1:50 dilution of goat anti-rabbit IgG (Vector Laboratories) for 1 hour. After this, the sections were rinsed, and Vectastain ABC reagent (Vector Laboratories) was applied for 30 minutes before diaminobenzidine tetrahydrochloride was applied for 5 minutes. Last, the sections were washed with distilled water and counterstained with hematoxylin. Negative controls were incubated with 1:1000 dilution preimmune serum instead of anti-fibromodulin antibodies.
Confocal Microscopy
Confocal microscopy was performed to determine the collagen deposition pattern in E16 versus E19 wounds, 72 hours after injury. First, slightly thicker 7-μm tissue sections were obtained. Next, the sections were deparaffinized, rehydrated, and treated with 0.2% aqueous phosphomolybdic acid for 1 minute and then stained with 0.1% Sirius red F3BA (Pfaltz and Bauer, Stamford, CT) in saturated aqueous picric acid solution for 90–120 minutes. Subsequently, sections were washed in 0.01 N HCl for 2 minutes and then dehydrated, cleaned, and mounted. All sections were analyzed on a Carl Zeiss LSM 310 laser scanning confocal microscope equipped with both an argon ion blue laser (488/514 nm) and a helium-neon green laser (543 nm). After identification of the wound sites by conventional microscopy, confocal pictures were obtained in the frame mode, using a Plan-Neofluar 63×/1.25 NA oil immersion objective with the 543-nm line of the helium-neon laser (attenuation = 1). The emission light beam was recorded with a photomultiplier after passing through a pinhole aperture and emission filter. The depth of the optical section (confocal z resolution) was maintained by keeping the size of the aperture pinhole in the emission pathway constant. The scan time for image collection was 8 seconds. Sections were scanned and collected in 0.5-μm increments for the entire thickness of 7 μm.
Results
Fetal Wounds at Gestation Day 16 Heal without Scar, and Gestation Day 19 Wounds Heal with Scar
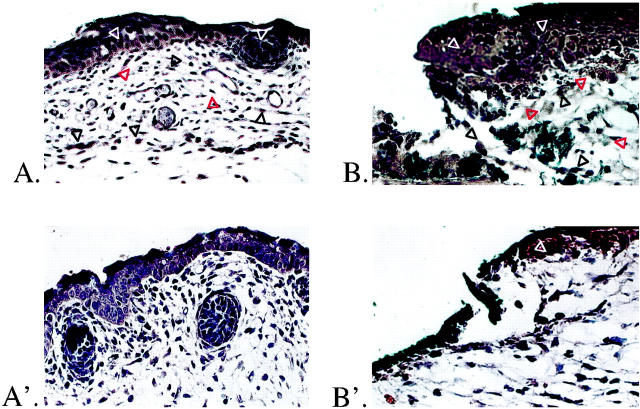
To confirm scarless healing in our model, we compared the effects of full-thickness excisional wounds on E16 and E19 fetal rats, 12 and 72 hours after injury (Figure 1 ▶ , A–C and A′–C′). Minimal inflammatory infiltrate was present at 12 hours postwounding in E16 rats (Figure 1A) ▶ . By 72 hours there was complete healing, with restoration of normal dermal architecture and hair follicle development (Figure 1B) ▶ . The original excision site was identified by the presence of blue vital dye within the dermis. Laser scanning confocal microscopy of day 16 fetal wounds 72 hours after injury revealed restoration of normal skin collagen architecture with no difference between E16 wounds (E16 + 72 hours postwounding = E19) and E19 control skin (Figure 1, C and D) ▶ . To minimize possible overlap with the end of the transition period (gestation day 18), excisional wounds were made on E19 fetal rats. There was increased inflammatory infiltrate relative to the gestation day 16 wounds after 12 hours (Figure 1A ▶ ′). In addition, E19 fetal wounds healed with disorganized dermal collagen deposition and lack of hair follicle regeneration in the wound site at 72 hours (Figure 1B ▶ ′). Confocal microscopy of day 19 wounds at 72 hours postinjury revealed increased density and disorganization of collagen fibers within the scar when compared to control skin of the same age (ie, neonatal day 1; E19 + 72 hours postwounding = N1) (Figure 1, C ▶ ′ and D″).
Figure 1.
H&E staining and confocal scanning laser microscopy of gestation day 16 and 19 fetal rat skin wounds. A: Gestation day 16 excisional skin wound, 12 hours after injury. Original magnification: ×200. The wound edge (solid black triangles) and wound base (open red triangles) are indicated. There is minimal inflammatory infiltrate. Note the presence of blue vital dye used to mark the location of the fetal wound (open black triangles). B: Gestation day 16 excisional skin wound, 72 hours after injury. Original magnification: ×100. There is complete healing, with restoration of normal dermal architecture and regeneration of hair follicles. Small areas containing blue vital dye are visible in the dermis (open black triangles). A′: Gestation day 19 excisional skin wound, 12 hours after injury. Original magnification: ×200. The wound edge (solid black triangles) and wound base (open red triangles) are indicated. There is moderate inflammatory infiltrate. Note again the presence of blue vital dye in the wound (open black triangles). B′: Gestation day 19 excisional skin wound, 72 hours after injury. Original magnification: ×100. To the right of the wound is a small area of nonwounded skin with intact hair follicles. There is complete epithelialization over the wound, but the normal dermal architecture has been disrupted by unorganized collagen deposition with no regeneration of hair follicles. Small areas of blue vital dye are also visible in the dermis (open black triangles). C: Confocal microscopy of nonwounded E19 fetal skin. Original magnification: ×630. The epidermis (E), dermis (D), and a portion of a hair follicle (open white triangles) are indicated. D: Confocal microscopy of an E16 embryo excisional wound, 72 hours after injury. Original magnification: ×630. In addition to hair follicle regeneration (open white triangles), collagen architecture is indistinguishable from nonwounded gestation day 19 skin (E16 + 72 hours postinjury = E19). C′: Confocal microscopy of nonwounded N1 fetal skin. Original magnification: ×630. The epidermis (E), dermis (D), and a portion of a hair follicle (white triangles) are again indicated. D′: Confocal microscopy of an E19 embryo excisional wound, 72 hours after injury. Original magnification: approximately ×630. There is no hair follicle regeneration, and the collagen architecture is disorganized and denser when compared to nonwounded neonatal day 1 controls (E19 + 72 hours postwounding = N1).
The Transition from Scarless Fetal Repair to Adult-Type Healing with Scar Correlates with Decreased LTBP-1 and Fibromodulin Levels, but Not Increased TGF-β1 and -β2, or Type I, II, and III Receptor Levels in Fetal Skin
To determine whether significant differences in gene expression are present before, during, and after the transition period, RT-PCR was performed on gestation day 14, 16, 17, 18, 19, and 21 (term) fetal RNA, using primer sets for TGF-β1, -β2, and -β3; type I, II, and III receptors; LTBP-1; and decorin, biglycan, and fibromodulin. We hypothesized that molecules promoting scar will be up-regulated and that molecules reducing scar will be down-regulated during the onset of adult-type repair. Student’s t-test was performed to detect statistically significant differences in steady-state mRNA levels between E16 (beginning transition) and E18 (end transition) fetal skin. A P value of <0.05 was considered significant.
Our results show that TGF-β1 levels did not change significantly between gestation days 16 and 18 (P = 0.3324), whereas TGF-β2 and -β3 levels were approximately twofold higher at day 16 relative to day 18 (P = 0.0021 and 0.0447, respectively) (Figure 2, A and A ▶ ′). Type I and III receptor expression, on the other hand, did not change significantly during the transition period (P = 0.1329 and 0.7343, respectively), although type II receptor was elevated by 2.2-fold at day 16 as compared to day 18 (P = 0.0020) (Figure 2, B and B ▶ ′). In terms of TGF-β modulator expression, LTBP-1 mRNA was 2.0-fold (P = 0.0032) and fibromodulin was 3.4-fold (P = 3.6 × 10−5) higher at day 16 versus day 18 (Figure 2, C and C ▶ ′). Decorin levels, however, were increased by 1.5-fold in E18 relative to E16 embryos (Figure 2, C and C ▶ ′). Finally, biglycan expression was not significantly changed during the transition (P = 0.4723) (Figure 2, C and C ▶ ′). Overall, we found that potential profibrotic molecules TGF-β1 and -β2 and type I, II, and III receptors were not up-regulated during the transition period, although three potential antifibrotic molecules, TGF-β3, LTBP-1, and fibromodulin, decreased with the onset of adult-type repair in the fetus.
LTBP-1, Decorin, and Biglycan Are Up-Regulated, and Fibromodulin Is Initially Down-Regulated during Adult Wound Repair
To further clarify the role of LTBP-1 and fibromodulin in scar formation, we examined their expression relative to TGF-β1, -β2, and -β3; type I, II, and III receptors; and decorin and biglycan during adult rat wound repair. RT-PCR was performed on RNA isolated from 0-, 0.5-, 1-, 2-, 3-, 5-, 7-, 10-, and 14-day wounds. We hypothesized that potential antifibrotic molecules would be minimally expressed or down-regulated during actual adult repair. Student’s t-test was performed to detect statistically significant differences in steady-state mRNA levels between nonwounded controls and adult wounds at different postinjury time points. A P value of <0.05 was considered significant.
As expected from previous work, 25 TGF-β1 and -β3 as well as type I and II receptors were up-regulated up to threefold in adult repair, although we did not demonstrate a significant change in TGF-β2 expression (Figure 3, A, A ▶ ′, B, and B′). In addition, we also showed up-regulation of the type III receptor (Figure 3, B and B ▶ ′). Surprisingly, all of the potential TGF-β modulators, LTBP-1, decorin, and biglycan were up-regulated in adult repair, except for fibromodulin, which was initially down-regulated 12 hours after injury (Figure 3, C and C ▶ ′) (P = 0.0366). Fibromodulin levels then returned to baseline by 24 hours with a slight 1.3-fold elevation above control at days 3 and 5 (P = 0.0145 and 0.0318, respectively) (Figure 3, C and C ▶ ′).
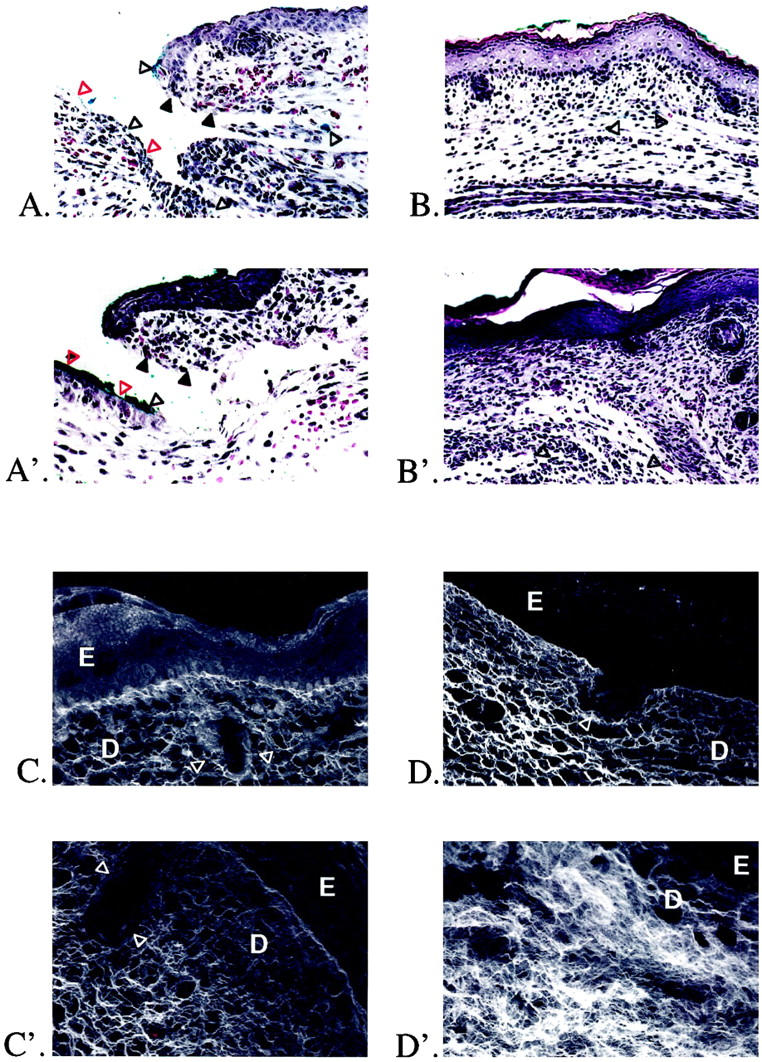
Fibromodulin Is Significantly Up-Regulated in Gestation Day 16 Fetal Wounds, but Not in Gestation Day 19 Wounds
Because fibromodulin is down-regulated during both fetal skin transition to adult-type repair and in actual adult repair, we hypothesized that the absence of fibromodulin, a potential antifibrotic molecule, may promote scar formation. To further test this hypothesis, we immunolocalized fibromodulin expression in E16 and E19 fetal wounds, 36 hours after injury. Our data revealed higher basal fibromodulin localization to the epidermis and dermis in control, nonwounded, gestation day 17.5 (E16 + 36 hour) relative to day 20.5 (E19 + 36 hours) fetal skin (Figure 4, A and A ▶ ′). Fibromodulin expression, however, significantly increased 36 hours after injury in E16 skin, especially in the dermis surrounding the wound (Figure 4B) ▶ . In marked contrast, wounded E19 skin had minimal fibromodulin localized to the dermal wound site 36 hours after injury (Figure 4B ▶ ′).
Figure 4.
Immunolocalization of fibromodulin in gestation day 16 and 19 fetal wounds. Fibromodulin immunostaining was performed on wounded E16 and E19 fetal skin, 36 hours after injury (N = 4 fetuses per time point, two separate pregnancies). A: Nonwounded gestation day 17.5 (E16 + 36 hours) fetal skin. Original magnification: ×200. There is moderate fibromodulin localization (dark brown staining) to the epidermis (open white triangles) as well as to the extracellular (open red triangles) and intracellular (open black triangles) components of the dermis. B: Gestation day 16 excisional skin wound, 36 hours after injury. Original magnification: ×200. The blue vital dye used to mark the location of the fetal wound is visible at the wound edge and base. There is significantly increased dermal extracellular fibromodulin localization (open red triangles, dark brown staining) relative to nonwounded day 17.5 (E16 + 36 hours) skin, as well as increased dermal intracellular fibromodulin staining (open black triangles) within spindle-shaped, fibroblast-like cells. There is continued fibromodulin staining (dark brown) in the epidermis (open white triangles). A′: Nonwounded gestation day 20.5 (E19 + 36 hours) fetal skin. Original magnification: ×200. There is minimal fibromodulin localization to the epidermis and dermis. B′: Gestation day 19 excisional skin wound, 36 hours after injury. Original magnification: ×200. There is increased epidermal staining (open white triangle, dark brown staining), but still minimal fibromodulin localization to the surrounding dermal wound site.
Discussion
TGF-βs are multifunctional cytokines with diverse effects on cell motility, proliferation, differentiation, and ECM protein expression. 26 In terms of wound repair, TGF-β1 and -β2 are known to promote scarring. 5,10,11 Conversely, TGF-β3 has been shown to decrease scarring in a rat incisional wound model by Shah et al. 10 Our results demonstrated that profibrotic molecules such as TGF-β1 and -β2 were expressed in skin from E16 animals, and that their levels did not increase significantly during the transition from fetal (E16) to adult-like (E18) repair. TGF-β2 levels in fact were significantly lower in E18 animals relative to E16 animals. Thus we did not find a direct correlation between TGF-β1 and-β2 expression and the onset of adult-type repair in the fetus.
Similarly, we found no correlation between TGF-β3 expression and scarless fetal-type repair. Although we showed significant TGF-β3 mRNA down-regulation in E17 and E18 transition period embryos (in keeping with its role as a possible antifibrotic peptide), this down-regulation was not maintained, and TGF-β3 was up-regulated from E19 until term. TGF-β3’s role in epidermal-mesenchymal transformation and epithelial differentiation may explain its increased expression in late gestation 26 ; however, this up-regulation is inconsistent with the role of TGF-β3 as a possible antifibrotic molecule. Wu et al also did not observe TGF-β3 to have any antifibrotic effects when applied to a rabbit dermal ulcer model. 27 This concurs with our adult rat data revealing increased TGF-β3 expression after injury and with other reports of TGF-β3 up-regulation in adult mouse 25 and porcine 28 wound models that repair with scar. Overall, TGF-β3 up-regulation in adult wounds argues against a generalized antifibrotic role for TGF-β3.
Because TGF-β ligand levels did not increase during the fetal skin transition to adult-type repair with scar, we hypothesized that the degree of TGF-β induction after injury may be more relevant than basal TGF-β levels. Earlier immunohistochemistry studies had reported a lack of TGF-β induction in fetal mice, 29 and in human 12,30 and rabbit wound models. 31 However, Martin et al had demonstrated rapid TGF-β1 mRNA induction within 1–3 hours after wounding and rapid protein clearance to near-background levels by 18 hours in a mouse model of embryonic limb repair. 32 We have shown by immunostaining that TGF-β1 and -β2 are present in rat embryo skin wounds and that the degree of expression after injury directly correlated with gestational age. Specifically, E16 (fetal-type repair) fetuses displayed decreased TGF-β1 and -β2 induction, whereas E19 (adult-type repair) animals exhibited increased TGF-β1 and -β2 induction (data in submission). Although decreased induction and increased clearance of TGF-β may be one mechanism regulating scarless fetal repair, TGF-β is nonetheless still present in fetal wounds.
To determine whether TGF-β receptor down-regulation in E16 relative to E18 embryos may further account for scarless fetal repair in the presence of TGF-β, we examined the expression of TGF-β type I, II, and III receptors during the transition period. Type I and II receptors are transmembrane serine/threonine kinases that are essential for TGF-β activity (reviewed in Massague 14 ). In contrast, the type III receptor (betaglycan) is a membrane-bound proteoglycan that has no intrinsic signaling function but it may modulate TGF-β access to the signaling receptor. 14 Our data revealed that type II receptor levels significantly decreased during the fetal transition to adult-type repair, whereas type I and III receptor levels did not change significantly. Thus we demonstrated that neither basal TGF-β ligand nor receptor levels positively correlated with scar formation during the transition from scarless fetal repair (E16) to adult-type repair with scar (E18).
Because profibrotic molecules were not up-regulated in the transition to adult-type repair, we then determined whether potential antifibrotic molecules such as LTBP-1, decorin, biglycan, and fibromodulin would be down-regulated. LTBPs are a relatively new class of molecules that may function to sequester and regulate extracellular TGF-β activity through their dual ability to bind both ECM components and TGF-β. 33 We observed a significant decline in LTBP-1 steady-state mRNA in E18 as opposed to E16 animals. This substantial drop in LTBP-1 expression in late gestation may ultimately increase TGF-β bioavailability and potentially account for scar formation in late-term fetuses. To determine whether LTBP-1 will be correspondingly down-regulated in a model that repairs through scar, we examined LTBP-1 expression in adult rat excisional wounds. Surprisingly, we found up-regulation of LTBP-1 mRNA up to 3.5-fold between 12 hours and 3 days after injury. The up-regulation of LTBP-1 suggests that TGF-β expression may be coordinately linked with LTBP-1 in adult repair. Dallas et al have shown concomitant TGF-β1 and LTBP mRNA expression in human foreskin fibroblasts in vitro. 34 Overall, despite decreased LTBP-1 levels in E18 embryos exhibiting adult-type repair, markedly increased LTBP-1 levels in adult wounds argue against a fundamental antifibrotic role for LTBP-1.
Our results thus far demonstrate that the onset of adult-type repair in the fetus does not directly correspond to increased TGF-β ligand, receptor, or LTBP-1 expression. Although the difference in TGF-β induction in E16 versus E19 embryos may be a major determinant of scar formation, it does not entirely explain how fetal wounds heal without scar despite the presence of TGF-β. Concurrently, the addition of anti-TGF-β antibodies against all three isoforms fails to completely eliminate scar in adult animals. 10 This suggests that other molecules besides TGF-β ligands, receptors, and binding proteins may regulate fetal scarless repair.
Compared to the LTBPs, more is known about the decorin proteoglycan family, which includes biglycan, fibromodulin, and lumican. Proteoglycans have key functions in ECM assembly, cellular interactions, and growth factor storage. 35 Decorin and fibromodulin, and to a lesser extent biglycan, can bind fibrillar collagens (eg, types I, II, III, V, and XI) to delay fibrillogenesis and decrease fibril diameter. 19,36,37 Besides regulating collagen assembly, decorin, biglycan, and fibromodulin can all bind TGF-β1, -β2, and -β3, with fibromodulin exhibiting the greatest affinity for TGF-β1, as well as the latent TGF-β1 precursor. 15 Decorin added to an experimental model of glomerulonephritis has ameliorated glomerular injury associated with TGF-β overexpression. 16 Thus the decorin family is able to regulate both ECM assembly and TGF-β bioactivity, making them possible candidates for the regulation of scarless fetal repair.
Our data showed that decorin mRNA levels increased significantly in E18 relative to E16 embryos, whereas biglycan levels did not change from E14 until term. Fibromodulin mRNA levels, on the other hand, declined dramatically, by ∼3.5-fold in E18 relative to E16 fetuses. Moreover, examination of adult wounds demonstrated increased decorin and biglycan expression by approximately two- to threefold, whereas fibromodulin was initially down-regulated at 12 hours and minimally up-regulated by 1.3-fold between days 3 and 5. Decorin up-regulation in E18 embryos and in adult repair, along with biglycan up-regulation in adult repair, make decorin and biglycan unlikely candidates for mediating scarless fetal repair. In contrast, fibromodulin expression inversely correlated with scar formation during both fetal skin development and adult wound repair. To further elucidate the role of fibromodulin in fetal repair, we immunostained for fibromodulin in E16 and E19 fetal wounds 36 hours after injury. We showed that fibromodulin expression significantly increased 36 hours after injury in E16 but not in E19 wounds. Thus abundant fibromodulin levels are associated with scarless fetal repair, whereas adult-type fetal repair with scar and adult repair are relatively deficient in fibromodulin.
During scarless fetal repair, the combination of minimal induction and rapid clearance of TGF-β, in association with elevated basal and injury-induced fibromodulin levels, may decrease overall TGF-β bioavailability. Diminished TGF-β bioactivity may in turn decrease ECM deposition and scar formation and, more importantly, prevent the activator protein-1 (AP-1)-mediated autoinductive phenomenon that sustains TGF-β1 expression in adult wounds. 38 Concurrently, relative fibromodulin abundance in fetal wounds may affect collagen fibrillogenesis and architecture, as suggested by our confocal data on E16 versus E19 wounds. Consequently, fibromodulin up-regulation in fetal wounds not only elucidates how scarless repair may occur despite the presence of TGF-β; it also explains mechanistically how organized fetal collagen deposition may occur. On the other hand, the lack of early fibromodulin induction in adult wounds may increase overall TGF-β activity and autoinduction, leading ultimately to scar and fibrosis. The small 1.3-fold increase in adult wound fibromodulin expression at days 3 and 5 occurred late in the repair process and is unlikely to contribute a significant antifibrotic effect. Furthermore, the relative lack of fibromodulin in adult wounds also potentially explains why neutralization of all three TGF-β isoforms reduces but does not entirely eliminate scarring in adult animals, inasmuch as adult wounds are deficient in fibromodulin for the regulation of ECM assembly. Future strategies to manipulate adult wounds into being more “fetal-like” may include the addition of fibromodulin protein or virally mediated fibromodulin transfection into wounds for the modulation of both TGF-β activity and ECM assembly.
In summary, our data demonstrate that, just as the macroscopic architecture of fetal skin changes during development from a thickness of few cell layers to a multilayered complex organ with glands and hair follicles, the transcripts for growth factors, receptors, and ECM biological modulators also undergo similar ontogenetic transformation. This suggests that the biology underlying the transition from scarless fetal repair to adult-type repair with scar in late gestation may be governed by dynamic shifts in relative levels of these transcripts at the biomolecular level.
Table 1CONT.
| PCR temp. (°C) | Optimal PCR cycle # | Probes (5′ to 3′) | |
|---|---|---|---|
| Fetal | Adult | ||
| 62 | 15–20 | 18–20 | ATAATTTGAGGTTGAGGGAG |
| 62 | 15–18 | 18–20 | AAAGCAATAGGCGGCATCC |
| 62 | 18–20 | 18–20 | TTCCCCTAACCAACCCACAC |
| 62 | 15–20 | 18–20 | ATTGTCTTTGTTGTCTGCTG |
| 62 | 20–22 | 15–20 | GAAGGAAAAGAAAAGGGCGG |
| 60 | 13–16 | 18–20 | CGTTTTCAGTTTCTGTGTGCC |
| 62 | 10–12 | 12–15 | GTTTAACAGTCACTGCCTGCTCC |
| 57 | 10–13 | 10–12 | ACCCAGCTTAGACAAATTAGAC |
| 57 | 12–15 | 12–15 | CAACTCTGTTATGCTTCCTG |
| 61 | 20–22 | 20–23 | CACCATTATACTGTTCAATACGCTG |
| 61 | 18–20 | 18–20 | CACGATGCCAAAGTTGTC |
Footnotes
Address reprint requests to Dr. Kang Ting, UCLA School of Dentistry, Room 30-113 CHS, 10833 Le Conte Avenue, Los Angeles, CA 90095. E-mail: kting@ucla.edu.
Presented in part at the American College of Surgeons Annual Clinical Congress, Chicago, Illinois, October 1997 and in the International Journal of Oral Biology (1998, 23:201–208).
Supported by the Wunderman Family Foundation, an Oral and Maxillofacial Surgery Foundation grant, the American Association of Orthodontists Foundation, National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR) grant DE10598, Clinical Research Center/NIH grant RR00865, and NIDCR grant K23DE00422.
C. S., F.-Y. H., and X. Z. contributed equally to this work.
References
- 1.Adzick NS, Longaker MT: Characteristics of fetal tissue repair. Adzick NS Longaker MT eds. Fetal Wound Healing. 1992, :pp 53-70 Elsevier, New York [Google Scholar]
- 2.Bleacher JC, Adolph VR, Dillon PW, Krummel TM: Isolated fetal mouse limbs: gestational effects on tissue repair in an unperfused system. J Pediatr Surg 1993, 28:1312-1315 [DOI] [PubMed] [Google Scholar]
- 3.Ihara S, Motobayashi Y: Wound closure in foetal rat skin. Development 1992, 114:573-582 [DOI] [PubMed] [Google Scholar]
- 4.Ihara S, Motobayashi Y, Nagao E, Kistler A: Ontogenetic transition of wound healing pattern in rat skin occurring at the fetal stage. Development 1990, 110:671-680 [DOI] [PubMed] [Google Scholar]
- 5.Adzick NS, Lorenz HP: Cells, matrix, growth factors, and the surgeon. The biology of scarless fetal wound repair. Ann Surg 1994, 220:10-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallock GG, Rice DC, Merkel JR, DiPaolo BR: Analysis of collagen content in the fetal wound. Ann Plast Surg 1988, 21:310-315 [DOI] [PubMed] [Google Scholar]
- 7.Merkel JR, DiPaolo BR, Hallock GG, Rice DC: Type I and type III collagen content of healing wounds in fetal and adult rats. Proc Soc Exp Biol Med 1988, 187:493-497 [DOI] [PubMed] [Google Scholar]
- 8.Mast BA, Flood LC, Haynes JH, DePalma RL, Cohen IK, Diegelmann RF, Krummel TM: Hyaluronic acid is a major component of the matrix of fetal rabbit skin and wounds: implications for healing by regeneration. Matrix 1991, 11:63-68 [DOI] [PubMed] [Google Scholar]
- 9.Raghow R: The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J 1994, 8:823-831 [DOI] [PubMed] [Google Scholar]
- 10.Shah M, Foreman DM, Ferguson MW: Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci 1995, 108:985-1002 [DOI] [PubMed] [Google Scholar]
- 11.Krummel TM, Michna BA, Thomas BL, Sporn MB, Nelson JM, Salzberg AM, Cohen IK, Diegelmann RF: Transforming growth factor beta (TGF-beta) induces fibrosis in a fetal wound model. J Pediatr Surg 1988, 23:647-652 [DOI] [PubMed] [Google Scholar]
- 12.Lin RY, Sullivan KM, Argenta PA, Meuli M, Lorenz HP, Adzick NS: Exogenous transforming growth factor-beta amplifies its own expression and induces scar formation in a model of human fetal skin repair. Ann Surg 1995, 222:146-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonewald LF: Regulation and regulatory activities of transforming growth factor beta. Crit Rev Eukaryot Gene Expr 1999, 9:33-44 [PubMed] [Google Scholar]
- 14.Massague J: TGF-beta signal transduction. Annu Rev Biochem 1998, 67:753-791 [DOI] [PubMed] [Google Scholar]
- 15.Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E: Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J 1994, 302:527-534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E: Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature 1992, 360:361-364 [DOI] [PubMed] [Google Scholar]
- 17.Hedlund H, Mengarelli-Widholm S, Heinegard D, Reinholt FP, Svensson O: Fibromodulin distribution and association with collagen. Matrix Biol 1994, 14:227-232 [DOI] [PubMed] [Google Scholar]
- 18.Hedbom E, Heinegard D: Binding of fibromodulin and decorin to separate sites on fibrillar collagens. J Biol Chem 1993, 268:27307-27312 [PubMed] [Google Scholar]
- 19.Font B, Eichenberger D, Goldschmidt D, Boutillon MM, Hulmes DJ: Structural requirements for fibromodulin binding to collagen and the control of type I collagen fibrillogenesis—critical roles for disulphide bonding and the C-terminal region. Eur J Biochem 1998, 254:580-587 [DOI] [PubMed] [Google Scholar]
- 20.Auerbach R, Auerbach W: Regional differences in the growth of normal and neoplastic cells. Science 1982, 215:127-134 [DOI] [PubMed] [Google Scholar]
- 21.Ausubel FM: Differential display of mRNA by PCR. Current Protocols in Molecular Biology. New York, Greene Publishers Associates and Wiley-Interscience, 1987, Section 15:8.3
- 22.Sambrook J, Maniatis T, Fritsch EF: Electrophoresis of RNA after denaturation with glyoxal and dimethyl sulfoxide. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY, Cold Spring Harbor Laboratory, 1989, Section 7.40–7.42
- 23.Diaco R: Practical considerations for the design of quantitative PCR assays. Innis MA Gelfand DH Sninsky JJ eds. PCR Strategies. 1995, :84-108 Academic Press, San Diego [Google Scholar]
- 24.Plaas AH, Wong-Palms S: Biosynthetic mechanisms for the addition of polylactosamine to chondrocyte fibromodulin. J Biol Chem 1993, 268:26634-26644 [PubMed] [Google Scholar]
- 25.Frank S, Madlener M, Werner S: Transforming growth factors beta1, beta2, and beta3 and their receptors are differentially regulated during normal and impaired wound healing. J Biol Chem 1996, 271:10188-10193 [DOI] [PubMed] [Google Scholar]
- 26.Cox DA: Transforming growth factor-beta 3. Cell Biol Int 1995, 19:357-371 [DOI] [PubMed] [Google Scholar]
- 27.Wu L, Siddiqui A, Morris DE, Cox DA, Roth SI, Mustoe TA: Transforming growth factor beta 3 (TGF beta 3) accelerates wound healing without alteration of scar prominence. Histologic and competitive reverse-transcription-polymerase chain reaction studies. Arch Surg 1997, 132:753–760 [DOI] [PubMed]
- 28.Levine JH, Moses HL, Gold LI, Nanney LB: Spatial and temporal patterns of immunoreactive transforming growth factor beta 1, beta 2, and beta 3 during excisional wound repair. Am J Pathol 1993, 143:368-380 [PMC free article] [PubMed] [Google Scholar]
- 29.Whitby DJ, Ferguson MW: Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol 1991, 147:207-215 [DOI] [PubMed] [Google Scholar]
- 30.Sullivan KM, Lorenz HP, Meuli M, Lin RY, Adzick NS: A model of scarless human fetal wound repair is deficient in transforming growth factor beta. J Pediatr Surg 1995, 30:198–202. [DOI] [PubMed]
- 31.Nath RK, LaRegina M, Markham H, Ksander GA, Weeks PM: The expression of transforming growth factor type beta in fetal and adult rabbit skin wounds. J Pediatr Surg 1994, 29:416-421 [DOI] [PubMed] [Google Scholar]
- 32.Martin P, Dickson MC, Millan FA, Akhurst RJ: Rapid induction and clearance of TGF beta 1 is an early response to wounding in the mouse embryo. Dev Genet 1993, 14:225-238 [DOI] [PubMed] [Google Scholar]
- 33.Mangasser-Stephan K, Gressner AM: Molecular and functional aspects of latent transforming growth factor-beta binding protein: just a masking protein? Cell Tissue Res 1999, 297:363-370 [DOI] [PubMed] [Google Scholar]
- 34.Dallas SL, Park-Snyder S, Miyazono K, Twardzik D, Mundy GR, Bonewald LF: Characterization and autoregulation of latent transforming growth factor beta (TGF beta) complexes in osteoblast-like cell lines. Production of a latent complex lacking the latent TGF beta-binding protein. J Biol Chem 1994, 269:6815–6821 [PubMed]
- 35.Ruoslahti E: Proteoglycans in cell regulation. J Biol Chem 1989, 264:13369-13372 [PubMed] [Google Scholar]
- 36.Vogel KG, Trotter JA: The effect of proteoglycans on the morphology of collagen fibrils formed in vitro. Collagen Related Res 1987, 7:105-114 [DOI] [PubMed] [Google Scholar]
- 37.Hedbom E, Heinegard D: Interaction of a 59-kDa connective tissue matrix protein with collagen I and collagen II. J Biol Chem 1989, 264:6898-6905 [PubMed] [Google Scholar]
- 38.Roberts AB: Molecular and cell biology of TGF-beta. Miner Electrolyte Metab 1998, 24:111-119 [DOI] [PubMed] [Google Scholar]