Abstract
Clusterin has been implicated in numerous processes including active cell death, immune regulation, cell adhesion and morphological transformation. The purpose of this study was to examine clusterin expression in a large series of breast carcinomas by immunohistochemistry and in situ hybridization. The study included 40 samples of non-neoplastic glandular epithelia, 42 benign lesions, 15 atypical intraductal hyperplasias, 35 carcinomas in situ, 114 invasive carcinomas, and lymph node metastases from 40 patients. Epithelial normal cells were always negative for clusterin expression and only 19% of the benign lesions presented positive staining. In contrast to the benign lesions, however, the frequency of clusterin positive samples increased in atypical hyperplasias (47%, P = 0.08), intraductal carcinomas (49%, P = 0.01) and invasive carcinomas (53%, P < 0.001). Positive staining presented a cytoplasmic pattern, except in 3 cases of invasive carcinomas which had nuclear staining. Clusterin mRNA by in situ hybridization confirmed the specific cellular pattern of clusterin expression by immunohistochemistry. Clusterin expression was associated with large tumor size (P = 0.04), estrogen and progesterone receptor negative status (P = 0.02 and P = 0.001, respectively) and with the progression from primary carcinoma to metastatic carcinoma in lymph nodes (80% metastatic nodes had positive expression) (P = 0.004). Ten of 15 (67%) primary carcinomas without clusterin expression became positive in lymph node metastases, while most (22 of 25, 88%) of the clusterin-positive primary carcinomas were also immunoreactive in metastases. In survival analysis, clusterin expression did not represent a prognostic indicator by uni- or multivariate analysis. The increased clusterin expression in breast carcinomas tended to correlate inversely with the apoptotic index (P = 0.09) which indicates that clusterin gene expression is not a prerequisite to cellular death by apoptosis. From these results, we suggest that clusterin may have a role in tumorigenesis and progression of human breast carcinomas.
Originally named for its activity in aggregating spermatozoa, 1 clusterin has been implicated in a number of biological processes, including sperm maturation, lipid transport, programmed cell death, regulation of the complement cascade, membrane recycling, cell-adhesion, and src-induced transformation. 2 Because it has been studied independently by a number of laboratories and in different systems, it has been given various names, such as SGP-2, clusterin, apolypoprotein J, SP 40–40, complement lysis inhibitor, gp80, glycoprotein III, and T64. 3 The human homologue of clusterin is comprised of 449 amino acids with two 40-kd subunits (α and β) joined by a unique five disulfide bond motif. 4 The protein precursor is encoded on a single 2 kb mRNA that is transcribed from a single copy gene located on chromosome 8 (8p21). 5 The high degree of sequence conservation across species, the widespread tissue distribution and the high circulating concentration suggest that clusterin plays an important biological role. Whether clusterin is truly a multifunctional protein or whether a common mechanism underlies its various functions is unclear.
Some functions proposed for clusterin may be relevant in the setting of tumorigenesis: these include complement defense, the initiation of apoptosis, membrane protection and the maintenance of cell-cell or cell-substratum contacts. There is no definitive biochemical evidence to support a specific function for clusterin, except for its role in the modulation of the immune system. 6 Clusterin is part of the fluid phase membrane attack complex (SC5b-9) and functions as a complement inhibitor. 6-8 One of the physiological roles of the complement system is the lysis of foreign cells, including virus-infected and tumor cells. 9 Therefore, tumor cells that express surface components that inactivate the complement system would be able to evade destruction by the complement system and thus continue to grow in size as well as progress into more malignant tumors.
On the basis of the high level of expression in apoptotic tissues, it was originally proposed that the protein might be causally involved in apoptosis. 6 Thus, clusterin mRNA has been widely used as a genetic marker of apoptotic cell death. 3 In hormone-dependent tissues, such as the prostate and the mammary gland, clusterin expression is induced after hormone ablation. 10,11 However, these studies did not analyze which cells account for the rise in clusterin gene expression, the cells undergoing apoptosis or those destined to survive. More recently, however, evidence has accumulated suggesting that clusterin expression is not enhanced, but rather down-regulated in the cells undergoing apoptosis, and that its expression in the apoptotic tissue is restricted to the surviving bystander cells. 12-14 Moreover, the expression of the gene is induced in processes other than apoptosis, including mitosis and necrosis, as has been shown in the rat liver. 15 These processes involve substantial membrane remodeling, suggesting that clusterin expression may be involved in maintaining membrane integrity and prevents complement attack during membrane remodeling. 8 If clusterin is involved in preventing immune attack during membrane remodeling, aberrant expression of clusterin may be involved in the pathogenesis of inflammatory and neoplastic diseases. Previous experimental work has suggested a role for clusterin in morphological transformation. 16-18
As clusterin may provide a means of eluding immune surveillance and apoptosis, a better understanding of the biology of its expression might lead to new insights into cancer progression. In this study, we have examined the expression of this molecule in a large cohort of breast tumors. This is the first report showing that clusterin is expressed in the malignant epithelium from early to late stages of carcinogenesis in the breast.
Materials and Methods
Patient Series
One hundred and fourteen invasive breast carcinomas were resected at the Costa del Sol Hospital in Marbella (Spain), between 1994 and 1997. The age range was from 26 to 81 with an average of 54 years. Tissues were collected for evaluation from each case, fixed in formalin and embedded in paraffin. Clinical staging was performed according to the postsurgical International Union Against Cancer Tumor-Node-Metastasis Classification. 19 The histological typing and grading of the tumors was performed according to the World Health Organization Classification. 20 Ninety nine cases were ductal adenocarcinomas and 15 were lobular carcinomas. Local treatment consisted of either (modified) radical mastectomy or tumorectomy plus radiation therapy, in combination with axillary clearance. No patients had previously received endocrine or cytotoxic therapy.
In addition to primary carcinomas, lymph node metastases (1 to 5 per case) from 40 breast carcinomas, 35 carcinomas in situ (DCIS), 20 atypical ductal hyperplasias, 42 benign lesions (18 fibroadenomas, 6 breast fibrocystic diseases, 4 nodular adenosis, 2 papillomas and 12 ductal hyperplasias lacking atypia) and 40 normal tissues (30 of them obtained distant from the lesion and considered normal by the pathologist, and 10 obtained from breast biopsies without microscopic alterations) were analyzed. In this study DCIS was divided into high-grade and low-grade lesions. High-grade lesions were defined as having predominantly high nuclear grade usually associated with central necrosis. Low grade lesions encompassed those with low or intermediate nuclear grade and minimal or absent central necrosis.
Immunohistochemical Staining
A section 5 μm thick from each tumor was mounted on adhesive-coated slides, deparaffinized, and rehydrated through xylene and alcohol. After cooling for 15 minutes in jars containing 10 mmol/L citrate buffer, endogenous peroxidase was blocked with 3% H2O2 in 0.1% sodium azide for 15 minutes. The clusterin E5 monoclonal antibody was kindly provided by Dr. B. Murphy and was used at 1:1000 dilution for 1 hour at room temperature. The specificity of E5 was demonstrated in the original publication by immunoprecipitation and Western blot analysis using human serum. 21 After PBS washes, the sections were incubated with biotinylated link antibody and then with peroxidase-labeled streptavidin. The staining was complete after a 10 minutes of incubation with a freshly prepared substrate-chromogen solution. The reaction products were again washed in PBS, then developed using diaminobenzidine tetrahydrochloride as chromogen. Sections were washed in running tap water and lightly counterstained with hematoxylin, followed by dehydration and coverslip mounting. Negative controls were obtained by omitting the primary antibody.
Clusterin expression was scored as follows: negative if no staining was seen or if immunoreactivity was observed in less than 10% of tumor cells; and positive if more than 10% of tumor cells showed staining. All slides were blind evaluated for immunostaining without any knowledge of the clinical outcome or of other clinical or pathological data. We also studied tumor proliferation and expression of hormone receptors, detecting the expression of Ki67, ERs and PRs (Dako, Copenhagen, Denmark). Tumors with less than 10% of the cells with nuclear staining were considered negative for the expression of hormone receptors or of low proliferation.
In Situ Localization of Apoptotic Cells
To detect apoptotic cells, in situ labeling of the 3′-ends of the DNA fragments generated by apoptosis-associated endonucleases was performed using a commercial apoptosis detection kit (Boehringer Mannheim, Germany). Briefly, deparaffinized sections were incubated with 20 μg/ml of proteinase K (Sigma Chemical Co. St. Louis, MO) for 15 minutes. Following rinsing in PBS, the slides were covered with terminal deoxynucleotidyl transferase plus nucleotide mixture at a 1:35 dilution for 60 minutes at 37°C. Then the slides were covered with an antifluorescein antibody conjugated with alkaline phosphatase. After substrate reaction, the stained cells were analyzed under a light microscope. Pretreatment of sections with DNase served as a positive control for the enzymatic procedures; omission of the enzyme served as a negative control.
Established morphological features used to identify apoptosis on H&E were also required in TUNEL-stained slides. Cells were defined as apoptotic if the whole nuclear area of the cell labeled positively. Apoptotic bodies were defined as small positively labeled globular bodies in the cytoplasm of the tumor cells which could be found either singly or in groups.
One thousand cells were counted for each specimen. The number of positively stained cells was then divided by 1000 to estimate the percentage of apoptotic cells in each specimen. As a cut off value for survival studies we used the median of the apoptosis in our series (0.76%) (range, 0.01–2.8%).
In Situ Hybridization
Human clusterin cDNA was kindly provided by Dr. B. Murphy. A digoxygenin-labeled antisense RNA probe was obtained using a HindIII-cut template and T7 RNA polymerase with a DIG RNA labeling kit (Boehringer Mannheim, Germany). Similarly, a sense probe was prepared for negative control experiments by using a NaeI-cut template and SP6RNA polymerase with the same kit. RNA stability was confirmed by hybridization using digoxygenin-labeled oligo-polyT as a probe. Deparaffinized sections were rehydrated and digested with proteinase K (15 μg/ml in 50 mmol/L Tris 20 mmol/L MgCl2 buffer) for 10 minutes. at 37°C, quenched with hydrogen peroxide and postfixed in 4% paraformaldehyde in phosphate buffered saline for 5 minutes. As an ionic block, 0.25% acetic anhydride in 0.1 mol/L triethanolamine pH 8.0 was used. Sections were then dehydrated in gradient ethanol and dilapidated in chloroform for 5 minutes, slightly rehydrated in 100 and 95% ethanol and dried at 37°C for 2 hours. The sections were washed, incubated in prehybridization solution (20 mmol/L Tris-HCl pH 7.4, 1 nmol/L EDTA, 300 mmol/L sodium chloride, 50% formamide, 10% dextran sulfate and 1X Denhardt’s solution) at 42°C for 1 hour, drained, and incubated at 42°C for 16 hours in the hybridization solution. Every milliliter of hybridization solution contained 830 μl of prehybridization solution and 20 ng of cRNA Dig-labeled probe diluted in 45 μl of Ribomix (100 μg/ml salmon sperm DNA, 250 μg/ml yeast total RNA, and 250 μg/ml yeast total tRNA), 50 μl 2 mol/L DTT, 10 μl of 10% sodium thiophosphate, and 10 μl of 10% SDS. The coverslips were removed and sections were washed in decreasing concentrations of SSC (4X to 0.1X) containing 1 mmol/L DTT, equilibrated in 100 mmol/L Tris-HCl pH 7.5, 150 mmol/L NaCl for 5 minutes., and blocked with the same buffer, now containing 2% sheep whole serum and 0.3% triton X-100, for 1 hour at room temperature. Hybridized Dig-labeled cRNA probe was visualized by reaction with anti-Dig-POD conjugated polyclonal antibody diluted 1:100 in the same Tris buffer but containing 1% sheep whole serum and 0.1% triton X-100 for 1 hour and developed with chromogen 3,3′-diaminobenzidine (DAB) and hydrogen peroxide for 1 hour. Tissues were counterstained with hematoxylin, dehydrated and coverslipped.
Follow-Up
One hundred three cases were available for follow-up. They were followed postoperatively at least once every 6 months. Relapsed-free survival (RFS) was calculated as the period from surgery until the date of the first recurrence (RFS). Mean follow-up was 39 months (range, 12 to 68 months).
Statistical Analysis
Statistical analysis was performed using the SPSS statistical software program (SPSS Inc, Chicago, IL). The analysis of the association between gene expression and prognostic factors for breast carcinoma was performed by the χ 2 test for categorical variables and by the analysis of variance test for continuous variables (natural log transformed when necessary). Relapse-free survival was estimated by the Kaplan and Meier method, and survival curves were compared with the log rank test. Cox’s proportional hazards survival analysis was used to determine the relative risk in multivariate analysis.
Results
The expression of clusterin in normal epithelial cells was always negative. Benign lesions presented positive staining in only 19% of samples (8 of 30): 2 of 18 fibroadenomas, 1 of 6 fibrocystic diseases, 1 of 4 nodular adenoses, 1 of 2 papillomas, and 3 of 12 ductal hyperplasias lacking atypia showed staining positivity. In contrast with these benign lesions, clusterin expression increased in atypical hyperplasias (7 of 15, 47%, P = 0.08), and ductal carcinomas in situ (17 of 35, 49%, P = 0.01) (Figure 1 ▶ , Table 1 ▶ ). Similar clusterin expression was present in high-grade (7 of 14, 50%) and low-grade (10 of 21, 48%) DCIS. In invasive carcinomas, clusterin expression showed a highly significant increase compared to benign lesions (P < 0.001). Thus, 53% (60 of 114) of primary carcinomas were positive for clusterin expression (Table 1) ▶ . In invasive carcinomas containing areas of in situ disease (31 cases), similar expression was detected in both components. Clusterin expression also showed a significant increase from primary carcinomas to metastatic carcinomas in lymph nodes where only 8 of 40 cases were negative for the expression of clusterin (P = 0.004) (Figure 2 ▶ ; Table 1 ▶ ). Staining intensity and cellular distribution in the lymph nodes was similar to that of the primary tumors. In 10 of 15 cases (67%) in which the primary carcinoma was negative, lymph node metastases became positive, whereas most (22 of 25, 88%) of the primary carcinomas with clusterin expression also showed immunostaining in the lymph node metastases (Figure 2) ▶ .
Figure 1.
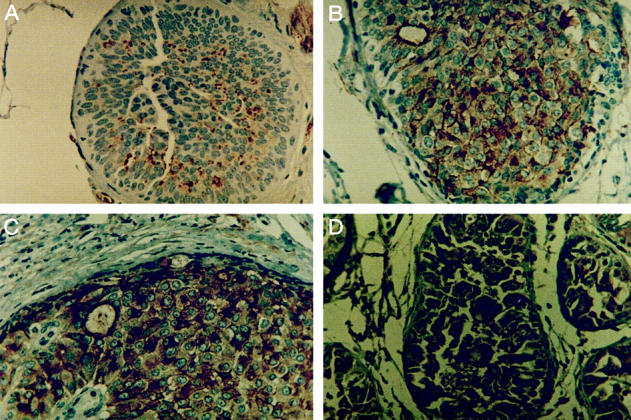
Immunohistochemical analysis of clusterin expression in proliferative lesions and DCIS. A: Clusterin negative ductal hyperplasia without atypias. B: Atypical ductal hyperplasia showing positive staining. C and D: Strong cytoplasmic clusterin staining is seen in a low-grade DCIS with intermediate nuclear grade and absence of necrosis (C) and in a high-grade DCIS (D). All fields are magnified ×400. All sections were counterstained with hematoxylin.
Table 1.
Clusterin Expression in Benign, Premalignant and Malignant Breast Lesions
| Lesions | Clusterin expression | P | |||
|---|---|---|---|---|---|
| Negative | Positive | ||||
| N | % | N | % | ||
| Benign | 34 | 81 | 5 | 19 | |
| Atypical hyperplasias | 8 | 53 | 7 | 47 | 0.08* |
| In situ carcinomas | 18 | 51 | 17 | 49 | 0.01* |
| Invasive carcinomas | 54 | 47 | 60 | 53 | <0.001* |
| Metastatic | 8 | 20 | 32 | 80 | 0.004† |
*Versus benign lesions.
†Metastatic versus primary carcinomas.
Figure 2.
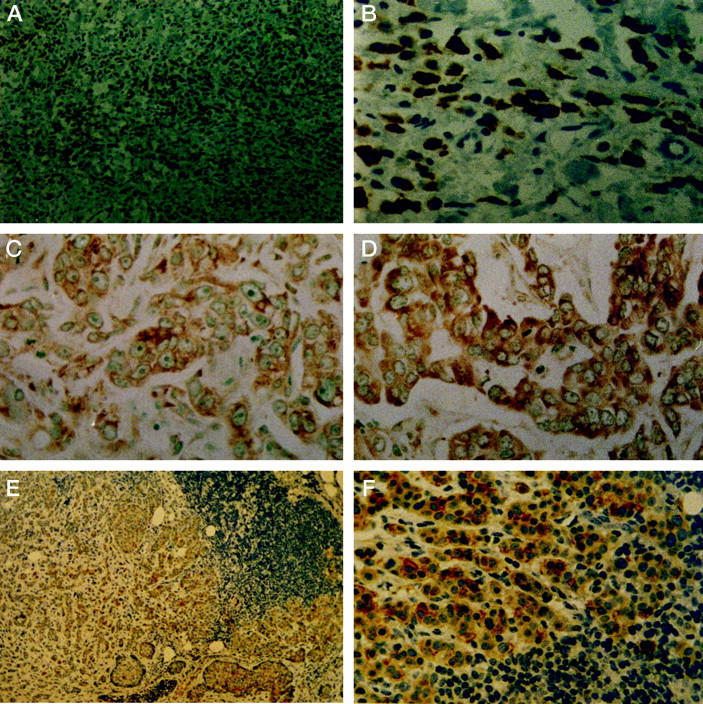
Positive cytoplasmic staining of clusterin in invasive breast carcinomas by immunohistochemistry and in situ hybridization. A and B: Immunoreactivity of clusterin in a lobular carcinoma (original magnifications: ×50 and ×400). C and D: In situ hybridization analysis of clusterin mRNA in poorly (C) and moderately (D) differentiated ductal carcinomas (both ×400). E and F: Lymph node metastasis of an infiltrating ductal carcinoma (×100 and ×400). All sections were counterstained with hematoxylin.
The staining pattern was granular cytoplasmic (Figures 1 and 2) ▶ ▶ , suggesting that clusterin may be contained within secretory vesicles. Nuclear staining was only noted in three cases.
We found a negative correlation between the extent of clusterin immunoreaction and estrogen and progesterone receptor expression (P = 0.02 and P = 0.001, respectively). The mean diameter of clusterin-positive tumors was larger than that of negative ones (3.01 versus 2.28) (P = 0.04). Concerning the degree of differentiation, most poorly differentiated tumors were positive for clusterin expression (9 of 12) although without achieving statistical significance when compared to the expression in well and moderately differentiated tumors. We did not detect any correlation between clusterin immunoreactivity and cancer stage, menopausal status (pre- and postmenopausal), histological type or Ki67 immunostaining (Table 2) ▶ .
Table 2.
Relationship between Clusterin Expression and Pathological Characteristics in Breast Carcinomas
| Variable | Clusterin | P | |
|---|---|---|---|
| Positives | Negatives | ||
| Estrogen receptors | |||
| ER+ | 27 | 36 | 0.02* |
| ER− | 33 | 18 | |
| Progesterone receptors | |||
| PR+ | 9 | 23 | 0.001* |
| PR− | 51 | 31 | |
| Proliferation | |||
| Ki67+ | 30 | 26 | 0.86 |
| Ki67− | 30 | 28 | |
| Differentiation degree | |||
| Grade I | 26 | 28 | 0.15 |
| Grade II | 25 | 23 | |
| Grade III | 9 | 3 | |
| Axillary node status | |||
| No | 32 | 28 | 0.87 |
| N+ | 28 | 26 | |
| Histological types | |||
| Ductal | 51 | 48 | 0.82 |
| Lobular | 9 | 6 | |
| Tumor stage | |||
| Stages I and II | 53 | 46 | 0.60 |
| Stages III and IV | 7 | 8 | |
| Menopause status | |||
| Premenopausal | 19 | 22 | 0.41 |
| Postmenopausal | 41 | 32 | |
| Tumor size | 3.01 ± 0.2† | 2.28 ± 0.18 | 0.04* |
| Apoptotic index | 1.01 ± 0.11 | 0.60 ± 0.18 | 0.09 |
*Statistically significant, P < 0.05.
†Mean ± SEM.
Our findings of increased clusterin expression by immunohistochemistry in breast carcinomas tended to correlate inversely with apoptotic activity in these epithelia. Thus, the mean apoptotic index in clusterin-positive tumors was 0.60 versus 1.1 in clusterin-negative tumors (Table 2) ▶ .
We also examined clusterin mRNA by in situ hybridization in 30 randomly chosen carcinomas to confirm the specific cellular pattern of clusterin expression in breast tumors. The qualitative results of immunohistochemical detection of clusterin in breast carcinoma cells paralleled the changing levels of clusterin mRNA revealed by in situ hybridization (Figure 2) ▶ . In fact, only 2 cases of 30 examined by this method were positive by ISH and negative by immunohistochemistry. In all cases the sense probe gave no hybridization signal, demonstrating the specificity of the labeling observed.
By univariate analysis, the variables associated with short relapse-free survival were high histological grade (P < 0.001), large tumor size (P = 0.01), presence of lymph node metastasis (P < 0.001), estrogen and progesterone receptor negativity (P = 0.02 in both cases) and a high apoptotic index (P = 0.004) (Table 3) ▶ . No differences were observed between clusterin-positive and negative tumors. In a multivariate analysis only axillary nodal status (relative risk, 2.99; 95% confidence interval, 1.41 to 6.35), histological grade (2.33; 1.22 to 4.43) and extent of apoptosis (1.88; 1.11 to 3.18) were of independent prognostic value.
Table 3.
Univariate Analysis of Relapse-Free Survival
| Log rank test | P | |
|---|---|---|
| Differentiation: Grades II+ III vs. I | 11.33 | <0.001* |
| Tumor size: >2 cm vs. ≤2 cm | 6.38 | 0.01* |
| Lymph node metastases: N+ vs. No | 15.21 | <0.001* |
| PgR: negative vs. positive | 5.27 | 0.02* |
| ER: negative vs. positive | 4.77 | 0.02* |
| Apoptosis: ≥0.76% vs. <0.76% | 8.03 | 0.004* |
| Clusterin: positive vs. negative | 0.35 | 0.55 |
| Ki67: positive vs. negative | 0.81 | 0.36 |
| Menopause status: pre- vs. postmenopausal | 2.58 | 0.10 |
*Statistically significant, P < 0.05.
Discussion
Prior studies describe increased expression of clusterin in several types of carcinomas. It increases following malignant transformation of retinal cells by a mutant Rous sarcoma virus. 16 High levels of clusterin expression occur in experimental prostate carcinomas after initiation with N-nitroso-N-methylurea and promotion with testosterone propionate. 17 Up-regulation of clusterin is associated with premalignant prostatic lesions 18 and clusterin expression is higher in prostate carcinomas when compared with normal prostate epithelium or benign prostate lesions. 22 Transcriptional activity of clusterin is three to four fold higher in clear cell carcinomas of the kidney when compared to its mRNA levels in the normal kidney tissue of the same patients. 23
The present study is the first, to our knowledge, to examine the potential role of clusterin in the development of breast carcinoma. Our findings demonstrate that the up-regulation of this gene is closely associated with the different steps of tumor progression from normal tissue to premalignant and malignant breast lesions, with a greater expression in lymph node metastasis. Overexpression of clusterin may thus represent an acquired phenotypic feature which facilitates the local invasion and dissemination of breast tumor cells, in fact, our results also show a positive correlation between clusterin expression and tumor size. Therefore, clusterin could be an important factor in determining the aggressive nature of a given breast tumor.
Clusterin over-expression correlated significantly with negative estrogen and progesterone receptor status. In addition, nuclear clusterin expression was detected in three invasive carcinomas which were also negative for the expression of estrogen and progesterone receptors. In a previous study, Akakura et al 24 reported nuclear clusterin staining in recurrent androgen-independent tumor cells, and these authors suggest that the presence of clusterin in the nucleus may serve to inhibit early events in the apoptotic process.
We also found that clusterin gene expression is not directly associated with programmed cell death, in accordance with other reports. 12,15,25 In fact, we found a lower apoptotic index in clusterin positive tumors than in negative ones. One study has reported a significant increase in cell death following transfection of antisense oligonucleotides to clusterin. 14 This inverse correlation may support the hypothesis that protection from apoptotic death may account, in part, for biologically aggressive tumor behavior. However, the precise biological function of clusterin in mechanisms of cell death/survival remain to be defined. In this sense, a recent study reported different isoforms of clusterin in apoptotic and surviving cells in regressing rat ventral prostate. 26 Apoptosis-associated isoforms were predominantly localized to perinuclear regions while surviving associated isoforms were localized to the cytoplasm as is found in most of the primary and metastatic breast carcinomas included in our study. Further studies are needed to determine the exact function of the different isoforms of clusterin and how changes in their expression may modify properties of tumor cells.
Clusterin mediated inhibition of complement-induced cytolysis probably protects carcinoma cells from complement-mediated lysis and contributes to the highly metastatic phenotype of those cells. Its presence at cell surfaces and its ubiquitous presence in virtually all types of biological fluids may protect cell membranes exposed to deleterious components. This protective effect may favor the metastatic phenotype of tumor cells which may migrate through tissues and fluids without excessive damage. 2 Thus, an attractive hypothesis is that clusterin induction is a reactive response to environmental changes rather than a causative factor in cell death. In this manner, clusterin could be thought of as an extracellular version of a heat shock protein. 26,27
In conclusion, the detection of clusterin in breast carcinoma suggests that this protein may have a role in breast tumorigenesis and progression although it has not been shown to possess clinical utility as a survival prognostic factor. Further studies are needed to distinguish whether clusterin reflects a cause or an effect of increased tumor progression.
Acknowledgments
We thank Dr. Brendan Murphy for the human anti-clusterin antibody and clusterin cDNA clone. We also thank Ana Rabaneda for her excellent technical assistance and Dr. Jose A. Castilla for his helpful discussion. This work was supported by grants from Fondo de Investigaciones Sanitarias (FIS 97/414), Junta de Andalucía (98/154) and Fundación Rey Fahd, Spain.
Footnotes
Address reprint requests to Dr. Maximino Redondo, Departamento de Bioquímica, Hospital Costa del Sol, Carretera de Cádiz Km 187, 29600 Marbella, Málaga, Spain. E-mail: mredondo@hcs.es.
Supported by grants from Fondo de Investigaciones Sanitarias (FIS 97/414), Junta de Andalucía (98/154), and Fundacion Rey Fahd, Spain.
References
- 1.Fritz IB, Burdzy C, Setchell B, Blaschuck O: Ram rete testes fluid contains a protein (clusterin) which influences cell-cell interactions in vitro. Biol Reprod 1993, 28:1173-1188 [DOI] [PubMed] [Google Scholar]
- 2.Jordan-Stark TC, Witte DP, Aronow B, Harmony JAK: Apolipoprotein J: A membrane policeman? Curr Opin Lipidol 1992, 3:75-85 [Google Scholar]
- 3.Tenniswood MP, Guenette RS, Lakins Y, Mooibroek M, Wong P, Welsh JE: Active cell death in hormone-dependent tissue. Cancer Metastasis Rev 1992, 11:197-200 [DOI] [PubMed] [Google Scholar]
- 4.Kirszbaum L, Bozas SE, Walker ID: SP40,40, a protein involved in the control of the complement pathway possesses a unique array of disulphide bridges. FEBS Lett 1992, 297:70-76 [DOI] [PubMed] [Google Scholar]
- 5.Fink TM, Zimmer M, Tschopp J, Etienne J, Jenne DE, Lichter P: Human clusterin (CLI) maps to 8p21 in proximity to the lipoprotein lipase (LPL) gene. Genomics 1993, 16:526-528 [DOI] [PubMed] [Google Scholar]
- 6.Jenne DE, Tschopp J: Clusterin: the intriguing guises of a widely expressed glycoprotein. Trends Biochem Sci 1992, 17:154-159 [DOI] [PubMed] [Google Scholar]
- 7.Choi NH, Mazda T, Tomita M: A serum protein SP40,40 modulates the formation of membrane attack complex of complement on erythrocytes. Mol Immunol 1989, 26:835-840 [DOI] [PubMed] [Google Scholar]
- 8.Kirszbaum L, Sharpe JA, Murphy B, d’Apice AJF, Classon B, Hudson P, Walkeer ID: Molecular cloning and characterization of the novel, human complement-associated protein, SP40,40: a link between the complement and reproductive systems. EMBO J 1989, 8:711-718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakulinen J, Meri S: Complement-mediated killing of microtumors in vitro. Am J Pathol 1998, 153:845-855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rennie PS, Bruchousky N, Buttyan R, Benson M, Cheng H: Gene expression during the early phases of regression of the androgen-dependent Shionogi mammary carcinoma. Cancer Res 1988, 48:6309-6312 [PubMed] [Google Scholar]
- 11.Kyprianou N, English HF, Davidson NE, Isaacs JT: Programmed cell death during regression of the MCF-7 human breast cancer following estrogen ablation. Cancer Res 1991, 51:162-166 [PubMed] [Google Scholar]
- 12.French LE, Wohlwend A, Sappino A-P, Tschopp J, Schifferli JA: Human clusterin gene expression is confined to surviving cells during in vitro programmed cell death. J Clin Invest 1994, 93:877-884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French LE, Sappino A-P, Tschopp J, Schifferli JA: Clusterin gene expression in the rat thymus is not modulated by dexamethasone treatment. Immunology 1994, 82:328-331 [PMC free article] [PubMed] [Google Scholar]
- 14.Sensibar JA, Sutkowski DM, Raffo A, Buttyan R, Griswold MD, Sylvester SR, Kozlowski JM, Lee C: Prevention of cell-death induced by tumor necrosis factor alpha in LNCaP cells by overexpression of sulfated glycoprotein-2 (clusterin). Cancer Res 1995, 55:2431-2437 [PubMed] [Google Scholar]
- 15.Bursch W, Gleeson T, Kleine L, Teniswood M: Expression of clusterin (testosterone-repressed prostate message-2) mRNA during growth and regeneration of rat liver. Arch Toxicol 1995, 69:253-258 [DOI] [PubMed] [Google Scholar]
- 16.Michel D, Gillet G, Volovitch M, Pessac B, Calothy G, Brun G: Expression of a novel gene encoding a 51.5 kD precursor protein is induced by different retroviral oncogenes in quail neuroretinal cells. Oncogene Res 1989, 4:127-136 [PubMed] [Google Scholar]
- 17.Kadomatsu K, Anzano MA, Slayter MV, Winokur TS, Smith JM, Sporn MB: Expression of sulfated glycoprotein 2 is associated with carcinogenesis induced by N-nitroso-N-methylurea in rat prostate and seminal vesicle. Cancer Res 1993, 53:1480-1483 [PubMed] [Google Scholar]
- 18.Ho SM, Leav I, Ghatak S, Merk F, Jagannathan VS, Mallery K: Lack of association between enhanced TRPM-2/clusterin expression and increased apoptotic activity in sex-hormone-induced prostatic dysplasia of the noble rat. Am J Pathol 1998, 153:131-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Union Against Cancer: TNM Classification of Malignant Tumours, 4th ed, 2nd revision. Edited by P Hermanek P, LH Sobin. Berlin, Springer-Verlag, 1992
- 20.World Health Organization: Histological Typing of Breast Tumors. International Histological Classification of Tumors No.2. Geneva, WHO, 1981
- 21.Murphy BF, Kirszbaum L, Walker ID, DÁpice AJF: SP-40,40, a newly identified normal human serum protein found in the SC5b-9 complex of complement and in the immune deposits in glomerulonephritis. J Clin Invest 1988, 81:1858-1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinberg J, Oyasu R, Lang S, Sintich S, Rademaker A, Lee C, Kozlowski JM, Sensibar JA: Intracellular levels of SGP-2 (clusterin) correlate with tumor grade in prostate cancer. Clin Cancer Res 1997, 3:1707-1711 [PubMed] [Google Scholar]
- 23.Parczyk K, Pilarsky C, Rachel U, Koch-Brandt C: Gp80 (Clusterin; TRPM2) mRNA level is enhanced in human renal clear cell carcinomas. J Cancer Res Clin Oncol 1994, 120:186-188 [DOI] [PubMed] [Google Scholar]
- 24.Akakura K, Bruchousky N, Rennie PS, Coldman AJ, Goldenberg SL, Tenniswood M, Fox K: Effects of intermittent androgen suppression on the stem cell composition and the expression of the TRPM-2 (clusterin) gene in the Shionogi carcinoma. J Steroid Biochem Mol Biol 1996, 59:501-511 [DOI] [PubMed] [Google Scholar]
- 25.Fratelli M, Galli G, Minto M, Pasinetti GM: Role of clusterin in cell adhesion during early phases of programmed cell death in P19 embryonic carcinoma cells. Biochim Biophys Acta 1996, 1311:71-76 [DOI] [PubMed] [Google Scholar]
- 26.Aronow BJ, Lund DS, Brown TL, Harmony JAK, Witte DP: Apolipoprotein J expression at fluid-tissue interfaces: potential role in barrier cytoprotection. Proc Natl Acad Sci USA 1993, 90:725-729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphreys DT, Carver JA, Easterbroock-Smith SB, Wilson MR: Clusterin has chaperone-like activity similar to that of small heat shock protein. J Biol Chem 1999, 274:6875-6881 [DOI] [PubMed] [Google Scholar]


