Abstract
Papillary thyroid carcinomas in humans are associated with the ret/PTC oncogene and, following loss of p53 function, may progress to anaplastic carcinomas. Mice with thyroid-targeted expression of ret/PTC1 developed papillary thyroid carcinomas that were minimally invasive and did not metastasize. These mice were crossed with p53−/− mice to investigate whether loss of p53 would promote anaplasia and metastasis of ret/PTC1-induced thyroid tumors. The majority of p53−/− mice died or were euthanized by 17 weeks of age due to the development of thymic lymphomas, soft tissue sarcomas, and testicular teratomas. All ret/PTC1 mice developed thyroid carcinomas, but tumors in p53−/− mice were more anaplastic, larger in diameter, more invasive, and had a higher mitotic index than tumors in p53+/+ and p53+/− mice. Thyroid tumors did not metastasize in any of the experimental p53+/+ and p53+/− mice ≤28 weeks of age or p53−/− mice ≤ 17 weeks of age; however, an older (170-day-old) male p53−/− mouse used to maintain the colony developed anaplastic thyroid carcinoma with liver metastases. These findings demonstrate that the lack of functional p53 in ret/PTC1 mice promotes anaplasia and invasiveness of thyroid carcinomas.
The majority of thyroid carcinomas in humans are papillary thyroid carcinomas (PTCs). 1 They occur primarily in iodine-sufficient regions of the world, and external radiation is the only known etiological factor. 2,3 PTCs are histologically characterized by well differentiated papillary and follicular structures, distinctive nuclear features, and infrequent mitotic figures. 4-7 Despite the common occurrence of regional lymph node metastases, 3 PTCs have a relatively benign biological behavior. 8 In contrast, anaplastic thyroid carcinomas (ATCs) have a variety of histological patterns resulting in poorly differentiated cells that usually are negative for thyroglobulin and have numerous, often abnormal, mitotic figures. 4-7 Furthermore, 20 to 50% of patients with ATCs have distant metastases to lung, bone, brain, and liver at the time of initial presentation. 9 PTCs are uniquely associated with the ret/PTC oncogene, 10-12 in which the cytoplasmic tyrosine kinase domain of the ret proto-oncogene is fused to the amino-terminal dimerization domain of a donor gene expressed ubiquitously in the thyroid gland. 13 This results in constitutive tyrosine phosphorylation of ret/PTC independent of ligand binding. Many investigators have found p53 mutations in up to 83% of ATCs by immunohistochemistry and single strand conformation polymorphism. 14-22 These findings suggest that follicular and ret/PTC-negative papillary thyroid carcinomas may progress to ATCs following loss of p53 function. 23,24
We and others have generated transgenic mice expressing ret/PTC1 under the control of the bovine 25 or rat 26 thyroglobulin (Tg) promoters. These mice developed bilateral, thyroid-stimulating hormone (TSH)-responsive PTCs with cystic and solid regions. 25,27 The solid regions were composed primarily of spindle cells, 27 which are a common feature of ATCs in humans. 4-7 However, thyroid tumors in ret/PTC1 transgenic mice were minimally invasive, did not metastasize, and did not overexpress p53 by immunohistochemical staining. 27 In the present study, we investigated whether loss of p53 would promote anaplasia and metastasis of ret/PTC1-induced thyroid tumors in vivo. To generate three groups of animals that differed only in their p53 functional status, ret/PTC1 transgenic mice were crossed with p53−/− mice. Various features of the thyroid tumors that developed in these different genotypic groups were evaluated including tumor morphology, neoplastic thyroid lobe diameter, mitotic index, invasion, and the occurrence of metastasis in extrathyroidal sites.
Materials and Methods
Animals
Female heterozygous FVB/N mice expressing ret/PTC1 under the control of the bovine Tg promoter 25 were crossed with male p53−/− mice of the 129/SV strain (JR 2080: 129/Sv-Trp53tm1Tyj, Jackson Laboratories). In these p53−/− mice, exon 2 through intron 7 of both p53 alleles is replaced by a neomycin resistance cassette, which disrupts 40% of the coding sequence, completely blocking production of p53 protein. 28 The resulting ret/PTC1+ p53+/− and ret/PTC1− p53+/− (F1) mice were bred to generate six genotypic (F2) groups: ret/PTC1+ p53+/+; ret/PTC1− p53+/+; ret/PTC1+ p53+/−; ret/PTC1− p53+/−; ret/PTC1+ p53−/−; and ret/PTC1− p53−/−.
Genotyping
Two polymerase chain reactions (PCR) were performed on genomic DNA from tail clippings. Primers used to amplify a 203-bp DNA fragment from ret/PTC1 transgenic mice were as previously reported (Kd-2: 5′-AGTTCTTCCGAGGGAATTCC-3′ and TPC-4: 5′-GTCGGGGGGCATTGTCATCT-3′). 25 A set of 4 primers and a touchdown protocol were used to detect both normal and mutant p53 alleles in a single reaction. A 280-bp DNA fragment was amplified from the neomycin cassette of the disrupted p53 allele using the primers 5′-CTTGGGTGGAGAGGCTATTC-3′ and 5′-AGGTGAGATGACAGGAGATC-3′. A 600-bp DNA fragment was amplified from the region between exons 6 and 7 of the wild-type p53 allele using the primers 5′-ATAGGTCGGCGGTTCAT-3′ and 5′-CCCGAGTATCTGGAAGACAG-3′.
Experimental Groups
The conduct of this study was approved by the Institutional Laboratory Animal Care and Use Committee at The Ohio State University. Mice were separated by sex at the time of weaning (between 3 and 4 weeks of age) and fed iodine-replete normal rodent chow (LM-485; Harlan Teklad, Madison, WI). Experimental groups consisted of 16–19 mice per sex for each of the six genotypes, such that 10 or 11 mice per group were sacrificed at 15 to 17 weeks of age, and 6 to 8 mice per group were sacrificed at 28 weeks of age. During the course of the study, additional ret/PTC1 transgenic mice used to maintain the colony were also evaluated. These included nine F1 p53+/− mice ranging in age from 197 to 407 days, as well as two F3 p53−/− mice, 126 and 170 days old.
Histopathology
Complete postmortem evaluations were performed on all mice. Representative tissues were fixed overnight in 10% neutral buffered formalin, processed by routine methods, and embedded in paraffin wax. Sections (5 μm) were stained with hematoxylin and eosin (HE). Blinded (ie, without knowledge of genotype) histological evaluation of the thyroid glands included: measurement of lobe diameter (mm), classification of tumor morphology (papillary or anaplastic carcinoma), mitotic index (mean of three 40× fields), and assessment of invasion (0–3+). A grade of 1+ was characterized by thyroid capsular fibrosis. In 2+ invasion, small nests of neoplastic cells had invaded through the existing thyroid capsule but were surrounded by fibrosis. Complete penetration through the thyroid capsule with invasion of neoplastic cells into perithyroidal connective tissue, the trachea, or surrounding salivary glands and skeletal muscle constituted 3+ invasion. Metastasis was evaluated by examining all lung lobes in all mice, as well as cervical, axillary, and tracheobronchial lymph nodes (obtained in 47%) in mice with thyroid tumors.
Immunohistochemistry
Immunohistochemical staining was performed on 5-μm paraffin sections cut onto poly-L-lysine slides. Endogenous peroxidase was inhibited by 3% hydrogen peroxide in methanol. For antigen retrieval, slides ware incubated at 90°C with Retrieve-All (Signet, Inc., Dedham, MA). Polyclonal rabbit anti-human primary antibodies were against thyroglobulin (1:500, DAKO Corporation, Carpinteria, CA) and ret (C-19, 1:100, Santa Cruz Biotechnology, Santa Cruz, CA). The secondary antibody for both primary antibodies was biotinylated goat anti-rabbit antibody (Santa Cruz Biotechnology). Specific binding was amplified using the streptavidin-biotin immunoperoxidase technique (DAKO). Chromogen reaction was developed with 3–3′ diaminobenzidine (DAB) solution (DAKO), and nuclei were counterstained with Mayer’s hematoxylin. For negative controls, slides were incubated with nonspecific rabbit IgG at similar concentrations, as a source of irrelevant primary antibody.
Statistical Analysis
Data for the three p53 genotypes of ret/PTC1 transgenic mice were analyzed only to determine whether differences were statistically significant at 14 to 17 weeks of age. Pearson’s χ 2 test was performed on data expressed as proportions (anaplastic tumors, tumor invasion grades, and tumors with mitotic figures) using S-Plus version 4.5 (MathSoft Inc., Cambridge, MA). Kruskal-Wallis rank test was performed on numerical data expressed as mean ± SEM (neoplastic thyroid lobe diameters) using GraphPad InStat version 3.0 (GraphPad Software, San Diego CA). Statistical significance was indicated by P < 0.05.
Results
Tumor Burden and Age of Mice at Death
p53−/− mice and, infrequently, p53+/− mice developed extrathyroidal tumors, consistent with previous reports, 28-34 necessitating euthanasia of most mice before 15–17 or 28 weeks of age (Table 1) ▶ . These tumors included thymic lymphomas, subcutaneous hemangiosarcomas, testicular teratomas, gliomas, and miscellaneous tumors such as undifferentiated soft tissue sarcomas. Additionally, paraphimosis (penile prolapse), which had been previously observed in our ret/PTC1 transgenic mice, 25 occurred in 10% of ret/PTC1+ p53+/− mice and 20% of ret/PTC1+ p53−/− mice, necessitating their early removal from the study. An additional cause for early removal was dyspnea resulting from tumor (thyroid and thymus)-induced tracheal compression. As a result of these premature deaths, data for ret/PTC1+ p53−/− mice were separated according to age at death: 7–11 weeks (n = 8), 11–14 weeks (n = 10), and 14–17 weeks (n = 18).
Table 1.
Incidence of Mice Euthanized Prematurely and Age at Death
| 15–17 Weeks of Age Group | ||||||
|---|---|---|---|---|---|---|
| ret/PTC1+ p53+/+ | ret/PTC1− p53+/+ | ret/PTC1+ p53+/− | ret/PTC1− p53+/− | ret/PTC1+ p53−/− | ret/PTC1− p53−/− | |
| Early removal | 0% | 0% | 0% | 5% | 55% | 29% |
| (0/22) | (0/22) | (0/22) | (1/22) | (12/22) | (6/21) | |
| Age range | 103–120 | 103–111 | 104–109 | 91–115 | 62–120 | 76–117 |
| Mean age | 108 | 106 | 106 | 106 | 93 | 102 |
| 28 Weeks of Age Group | ||||||
|---|---|---|---|---|---|---|
| ret/PTC1+ p53+/+ | ret/PTC1− p53+/+ | ret/PTC1+ p53+/− | ret/PTC1− p53+/− | ret/PTC1+ p53−/− | ret/PTC1− p53−/− | |
| Early removal | 15% | 0% | 17% | 0% | 100% | 92% |
| (2/13) | (0/12) | (2/12) | (0/12) | (14/14) | (11/12) | |
| Age range | 49–204 | 190–197 | 67–194 | 190–195 | 49–117 | 82–190 |
| Mean age | 174 | 192 | 175 | 190 | 92 | 125 |
Age data are stated in days.
Thyroid Lesions, Neoplastic Thyroid Lobe Diameter, and Tumor Mitotic Index
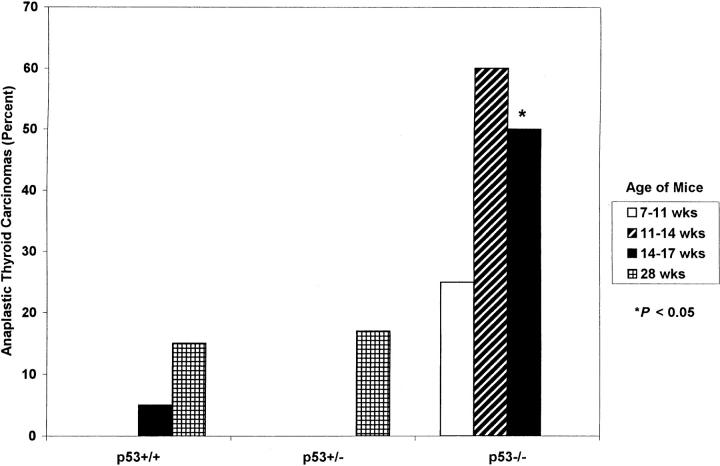
All ret/PTC1 transgenic mice developed bilateral thyroid carcinomas. Papillary thyroid carcinomas were composed of heterogeneous areas of follicular architecture with papillary infoldings and uniform vesicular nuclei (Figure 1A) ▶ . 4,5,7,25,27 Carcinomas were classified as anaplastic if one or both lobes contained solid areas composed of pleomorphic spindle, polyhedral, or multinucleated giant cells (Figure 1B) ▶ . 4-7 The incidence of ATCs in all mice increased with age (Figure 2) ▶ . However, the percentage of ATCs in all ages of p53−/− mice was greater than both age groups of p53+/+ and p53+/− mice, and was significantly greater (P < 0.05) at 14 to 17 weeks of age. Anaplastic thyroid tumors also had larger lobe diameters and a higher mitotic index. The mean diameter of neoplastic thyroid lobes was greater in 11- to 17-week-old p53−/− mice than in p53+/+ or p53+/− mice, but it was significant (P < 0.05) only when 14- to 17-week-old p53−/− mice were compared to the corresponding age groups of p53+/+ and p53+/− mice (Table 2) ▶ . Mitotic figures are not typical histological features of papillary thyroid carcinomas. 4,5,7 None of the thyroid tumors in p53+/+ mice had mitotic figures. In contrast, 30% of all p53−/− tumors and 17% of p53+/− tumors at 28 weeks of age had mitotic figures (Table 2) ▶ . In these tumors, the mitotic index ranged from 1 to 12 per high power field (hpf; mean, 3.9/hpf) in all p53−/− tumors and 1 to 2/hpf (mean, 1.5/hpf) in p53+/− tumors at 28 weeks of age. Furthermore, the mitotic index increased with age in p53−/− tumors (2.5/hpf at 7–11 weeks of age vs. 5.8/hpf at 14–17 weeks of age). Mitotic figures were often atypical (Figure 1D) ▶ .
Figure 1.
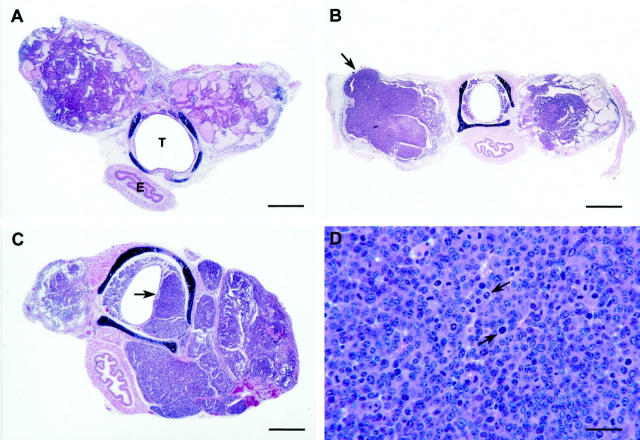
Thyroid tumors in ret/PTC1+ p53−/− mice. A: Minimally invasive (1+) bilateral PTC (79 days old) consisting of a mixture of cystic and follicular areas with papillary infoldings. T, trachea; E, esophagus. HE staining. Bar, 600 μm. B: Bilateral ATC (112 days old) with widespread regions of solid neoplastic polygonal cells, and thyroid capsular (2+) invasion (arrow). HE staining. Bar, 1.1 mm. C: Bilateral ATC (91 days old) with asymmetrical enlargement of the right lobe and extensive (3+) invasion into the tracheal submucosa (arrow). HE staining. Bar, 600 μm. D: ATC (103 days old) with regions of solid neoplastic polygonal cells containing numerous atypical mitotic figures (arrows). HE staining. Bar, 170 μm.
Figure 2.
Percentage of anaplastic thyroid carcinomas in ret/PTC1+ mice with variable p53 functional status.
Table 2.
Neoplastic Thyroid Lobe Diameter and Incidence of Thyroid Tumors with Mitotic Figures
| 7–11 weeks | 11–14 weeks | 14–17 weeks | 28 weeks | ||||
|---|---|---|---|---|---|---|---|
| p53−/− | p53−/− | p53+/+ | p53+/− | p53−/− | p53+/+ | p53+/− | |
| Mean lobe diameter (mm)* | 1.93 ± 0.20 | 2.60 ± 2.25 | 1.86 ± 0.09 | 1.98 ± 0.08 | 3.36 ± 1.09† | 2.11 ± 0.15 | 2.04 ± 0.13 |
| Tumors with mitotic figures | 29% | 30% | 0% | 0% | 29% | 0% | 17% |
| (2/7) | (3/10) | (0/22) | (0/22) | (5/17)† | (0/13) | (2/12) | |
*Mean ± SEM.
†P < 0.05.
Tumor Invasion
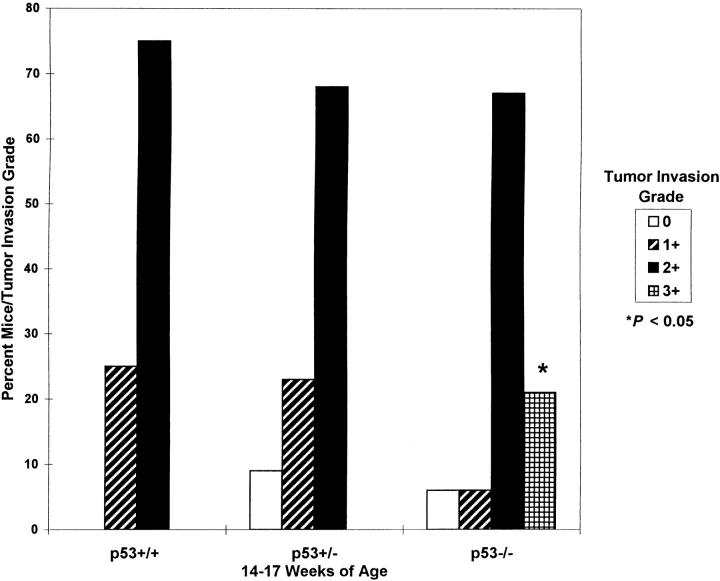
Less than 15% and 30% of thyroid tumors in all mice had invasion grades of 0 or 1+ (Figure 1A) ▶ , respectively. The majority (range, 40–86%; mean, 65%) of thyroid tumors in all groups were characterized by 2+ invasion grades (Figure 2B) ▶ . The most significant finding was that 3+ invasion was seen only in p53−/− mice (Figure 3) ▶ . Thyroid tumors completely penetrated the thyroid capsule and invaded surrounding tissues in 40% and 21% of p53−/− mice aged 11–14 and 14–17 weeks, respectively (Figures 1C and 4B) ▶ ▶ . ATCs with 3+ invasion were further characterized by asymmetrical enlargement of one lobe (up to 25 mm in diameter) in 5/9 older p53+/− mice (F1, 197 to 314 days old), 1/36 experimental p53−/− mice (F2, 102-day-old female), and 1/2 older p53−/− mice (F3, 170-day-old male; Figure 4, A and B ▶ ).
Figure 3.
Incidence of thyroid tumor invasion by grade in 14- to 17-week-old ret/PTC1+ mice with variable p53 functional status.
Figure 4.
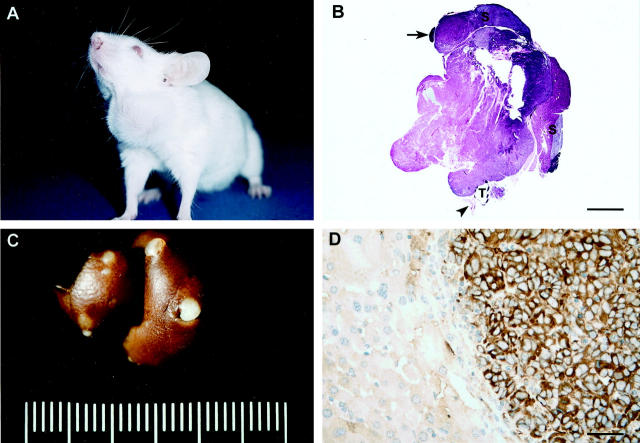
Metastatic ATC in a ret/PTC1+ p53−/−mouse (170 days old). A: Note the asymmetrical swelling in the ventral cervical region corresponding to the thyroid tumor. B: Bilateral ATC with complete penetration through the thyroid capsule (3+ invasion) and marked asymmetrical enlargement on the left. Adjacent salivary glands (S) and lymph node (arrow) are not affected. T, trachea; arrowhead, esophagus. HE staining. Bar, 4.8 mm C: Multiple metastases of ATC in the liver. D: Immunohistochemical stain for thyroglobulin in the liver. The metastatic thyroid tumor cells are labeled positively for thyroglobulin, but the adjacent hepatocytes are negative. Results of immunohistochemical staining for ret were similar (not shown). Hematoxylin counterstain. Bar, 80 μm.
Metastasis
Lung and lymph node metastases ware not detected in any of the experimental p53+/+ or p53+/− mice ≤28 weeks of age or p53−/− mice ≤17 weeks of age. A single subpleural aggregate of neoplastic epithelial cells, interpreted to be a micrometastasis of the bilateral anaplastic thyroid carcinoma, was found in one lung lobe of a 111-day-old female experimental p53−/− mouse. However, this aggregation of neoplastic cells was not found in serial sections; therefore, thyroid origin could not be confirmed with thyroglobulin or ret immunohistochemical staining. Evaluation of a 170-day-old male (F3) p53−/− mouse used to maintain the colony, revealed an asymmetrically enlarged thyroid tumor and numerous round, raised, tan nodules (2–3 mm) within the parenchyma and on the capsular surface of the liver (Figure 4) ▶ . Histologically, the neoplastic cells within these metastatic nodules resembled the anaplastic thyroid carcinoma. Results of immunohistochemical staining for thyroglobulin and ret within the anaplastic thyroid carcinoma were variable with distinct but infrequent positive areas interspersed throughout negative areas. Neoplastic cells within the metastatic nodules stained diffusely for thyroglobulin and ret, whereas the adjacent hepatocytes remained negative (Figure 4D) ▶ .
Discussion
The results of this study demonstrated that a lack of functional p53 in ret/PTC1+ p53−/− mice promoted the formation of thyroid tumors that were more anaplastic, larger in diameter, more locally invasive, and contained more mitotic figures than those in ret/PTC1+ p53+/+ and ret/PTC1+ p53+/− mice. All of the experimental p53−/− mice died or were euthanized by 17 weeks of age; however, 25 to 60% of these mice had ATCs at the time of death. Although a low percentage of p53+/+ and p53+/− tumors were also classified as anaplastic by 28 weeks of age, none of the tumors were as large or invasive as the tumors in p53−/− mice. By 28 weeks of age, 17% of tumors in p53+/− mice also had mitotic figures, in contrast to tumors in p53+/+ mice.
In our ret/PTC1 transgenic mouse model, bovine Tg-driven overexpression of the ret receptor tyrosine kinase, led to constitutive activation of the ras/MAPK pathway, inducing the formation of bilateral PTCs. 25,27 Under chronic TSH stimulation, the carcinomas were minimally invasive and did not metastasize. 27 However, the tumors did contain solid regions of spindle cells, 27 a feature of ATCs in humans. 4-7 Mutations of the p53 tumor suppressor gene, primarily located in exons 5–8, are reported frequently in human ATCs. 14-22 However, p53 immunohistochemical analysis of the ret/PTC1 mouse tumors did not reveal overexpression of the p53 protein. 27
There also is substantial evidence documenting the association between thyroid follicular cell differentiation and p53 expression in vitro. 17,35-37 Numerous undifferentiated or anaplastic human thyroid carcinoma cell lines have been established that harbor heterozygous p53 point mutations, fail to concentrate radioiodide, and do not express detectable levels of thyroid-specific genes such as thyroglobulin, TSH receptor, thyroid peroxidase (TPO), paired box-8 (PAX-8), or thyroid transcription factor-1 (TTF-1). However, re-expression of wild-type p53 in these cells restores their ability to respond to TSH and induces the expression of thyroglobulin, TSH receptor, TPO, and PAX-8. 36,37 The lack of functional p53 protein alone is not sufficient to induce the malignant phenotype, since neither ret/PTC1− p53+/− mice nor ret/PTC1− p53−/− mice developed thyroid carcinomas. This finding is supported by the observation that patients with Li-Fraumeni syndrome and germline mutations of p53 develop various types of cancer including breast carcinomas, osteosarcomas, brain tumors, and soft tissue sarcomas, but not thyroid carcinomas. 38,39 In addition, the introduction of mutated p53 in some thyroid cell lines does not induce colony formation in soft agar or tumor formation in nude mice. 35 This suggests that mutated p53 appears to cooperate with other oncogenes to promote, rather than initiate, thyroid tumorigenesis.
ATCs in mice had a variety of histological patterns and high mitotic index, similar to human ATCs. In contrast to human ATCs, the murine ATCs stained variably for thyroglobulin, and none of the mice had microscopic evidence of metastasis by 14–17 weeks of age for p53−/− mice or 28 weeks of age for p53+/+ and p53+/− mice. Many of the ret/PTC1 transgenic p53−/− that were to have been evaluated at 15–17 (55%) and 28 (100%) weeks of age died or had to be euthanized prematurely. The mouse that did develop metastatic ATC survived much longer than the oldest experimental ret/PTC1+ p53−/− mouse (170 vs. 120 days of age). Therefore, it is possible that if ret/PTC1+ p53−/− mice had survived to 28 weeks of age, the incidence of ATCs and metastasis would have been higher. To overcome the premature deaths due to extrathyroidal tumors in p53−/− mice, it will be necessary in future experiments to generate thyroid-restricted p53−/− mice, thereby permitting the mice to live longer and allowing sufficient time for metastases to develop.
Another confounding factor that may have influenced the development of metastases is the genetic background of the mice. All of the experimental mice were F2 generation from breeding heterozygous ret/PTC1+ p53+/− mice to ret/PTC1− p53+/− mice. All of these mice were from the same generation, and their ret/PTC1 and p53 genotypes were confirmed by PCR. However, the genetic composition of all F2 mice was not identical, in that the exact percentage contributed by the ret/PTC1 FVB/N background versus the p53−/− 129/SV background may vary. This also may explain why only some, not all, of the ret/PTC1+ p53−/− mice had thyroid tumors with anaplastic features. Although it is tempting to compare phenotypes among combined genetically engineered mice to evaluate the roles of various signal transduction pathways in tumor development and progression, it is important to recognize the influence of the genetic background in the resulting phenotype. In this study, the ret/PTC1+ p53−/− mouse that developed metastatic ATC was one generation older (F3) than the experimental mice (F2).
Although tumor development and progression may vary among genetically engineered mice with diverse genetic backgrounds, transgenic mice with thyroid-targeted overexpression of various human oncogenes have provided valuable information on the roles of distinct signal transduction pathways in thyroid tumorigenesis. 25,26,40-48 Alterations of specific signal transduction pathways have been associated with certain neoplastic phenotypes, 25,26,42,45,46,48 and the most malignant and metastatic tumors developed when multiple pathways were altered in thyroid follicular cells. 40,44 This study suggests that loss of p53 alone does not induce thyroid tumor formation. Additional genetic and epigenetic mechanisms may be necessary for the absolute progression and metastasis of thyroid epithelial neoplasms. However, the occurrence of tumors that are anaplastic and locally invasive is promoted by the loss of p53 in ret/PTC1 mice.
Acknowledgments
We thank Ms. Wendy Russo for superior animal husbandry, Dr. Jason Doss for assistance with postmortem evaluations, Mr. Alan Flechtner for slide preparation, Ms. Heather Caprette and Mr. Jerry Harvey for assistance with photography and figure illustrations, Drs. Wayne Buck and Thomas Rosol for statistical support, and Dr. Peter Schweitzer from Jackson Laboratories for assistance with the p53 PCR protocol.
Footnotes
Address reprint requests to Charles C. Capen, D.V.M., Ph.D., The Ohio State University, Department of Veterinary Biosciences, 1925 Coffey Road, Columbus, Ohio 43210. E-mail: Capen.2@osu.edu.
Supported by a fellowship from the Schering-Plough Research Institute, by a T32 Oncology Training Grant from The Ohio State University, Department of Internal Medicine, Division of Hematology and Oncology, and by National Institutes of Health grant R01CA60074.
References
- 1.Mazzaferri EL: Carcinoma of follicular epithelium: radioiodine and other treatments and outcomes. Braverman LE Utiger RD eds. Werner and Ingbar’s The Thyroid: A Fundamental Clinical Text. 1996, :pp 922-945 Lippincott-Raven, Philadelphia [Google Scholar]
- 2.Mack WJ, Preston-Martin S: Epidemiology of thyroid cancer. Fagin JA eds. Thyroid Cancer. 1998, :pp 1-25 Kluwer Academic Publishers, Boston [Google Scholar]
- 3.Schneider AB, Ron E: Carcinoma of follicular epithelium: pathogenesis. Braverman LE Utiger RD eds. Werner and Ingbar’s The Thyroid: A Fundamental Clinical Text. 1996, :pp 902-909 Lippincott-Raven, Philadelphia [Google Scholar]
- 4.Biddinger P, Nikiforov YE: Pathologic features of thyroid tumors. Fagin JA eds. Thyroid Cancer. 1998, :pp 105-137 Kluwer Academic Publishers, Boston [Google Scholar]
- 5.Gleich LL, Biddinger PW: Pathology of thyroid tumours. Johnson JT Gluckman JL eds. Carcinoma of the Thyroid. 1999, :pp 15-31 Isis Medical Media, Oxford [Google Scholar]
- 6.Rosai J, Carcangiu ML, DeLellis RA: Tumors of the thyroid gland: undifferentiated (anaplastic) carcinoma. Rosai J Sobin LH eds. Atlas of Tumor Pathology. 1992, :pp 135-159 DC, Armed Forces Institute of Pathology, Washington [Google Scholar]
- 7.Hedinger C: Histologic typing of thyroid tumours. Hedinger C Williams ED Sobin LH eds. International Histological Classification of Tumours. 1988, :pp 1-67 World Health Organization, Berlin [Google Scholar]
- 8.Mazzaferri EL, Young RL, Oertel JE, Kemmeter WT, Page CP: Papillary thyroid carcinoma: the impact of therapy in 576 patients. Medicine 1977, 56:171-196 [PubMed] [Google Scholar]
- 9.Schlumberger M, Caillou B: Miscellaneous tumors of the thyroid. Braverman LE Utiger RD eds. Werner and Ingbar’s The Thyroid: A Fundamental Clinical Text. 1996, :pp 961-965 Lippincott-Raven, Philadelphia [Google Scholar]
- 10.Jhiang SM, Caruso DR, Gilmore E, Ishizaka Y, Tahira T, Nagao M, Chiu I-M, Mazzaferri EL: Detection of the PTC/retTPC oncogene in human thyroid cancers. Oncogene 1992, 7:1331-1337 [PubMed] [Google Scholar]
- 11.Santoro M, Carlomagno F, Hay ID, Herrmann MA, Grieco M, Melillo R, Pierotti MA, Bongarzone I, Della Porta G, Berger N, Peix JL, Paulin C, Fabien N, Vecchio G, Jenkins RB, Fusco A: Ret oncogene activation in human thyroid neoplasms is restricted to the papillary cancer subtype. J Clin Invest 1992, 89:1517–1522 [DOI] [PMC free article] [PubMed]
- 12.Tallini G, Santoro M, Helie M, Carlomagno F, Salvatore G, Chiappetta G, Carcangiu ML, Fusco A: RET/PTC oncogene activation defines a subset of papillary thyroid carcinomas lacking evidence of progression to poorly differentiated or undifferentiated tumor phenotypes. Clin Cancer Res 1998, 4:287-294 [PubMed] [Google Scholar]
- 13.Grieco M, Santoro M, Berlingieri MT, Melillo RM, Donghi R, Bongarzone I, Pierotti MA, Della Porta G, Fusco A, Vecchio G: PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell 1990, 60:557–563 [DOI] [PubMed]
- 14.Dobashi Y, Sakamoto A, Sugimura H, Mernyei M, Mori M, Oyama T, Machinami R: Overexpression of p53 as a possible prognostic factor in human thyroid carcinoma. Am J Surg Pathol 1993, 17:375-381 [DOI] [PubMed] [Google Scholar]
- 15.Dobashi Y, Sugimura H, Sakamoto A, Mernyei M, Mori M, Oyama T, Machinami R: Stepwise participation of p53 gene mutation during dedifferentiation of human thyroid carcinomas. Diagn Mol Pathol 1994, 3:9-14 [DOI] [PubMed] [Google Scholar]
- 16.Donghi R, Longoni A, Pilotti S, Michieli P, Della Porta G, Pierotti M: Gene p53 mutations are restricted to poorly differentiated and undifferentiated carcinomas of the thyroid gland. J Clin Invest 1993, 91:1753–1760 [DOI] [PMC free article] [PubMed]
- 17.Fagin JA, Matsuo K, Karmaker A, Chen DL, Tang S-H, Koeffler HP: High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Invest 1993, 91:179-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho Y-S, Tseng S-C, Chin T-Y, Hsieh L-L, Lin J-D: p53 gene mutation in thyroid carcinoma. Cancer Lett 1996, 103:57-63 [DOI] [PubMed] [Google Scholar]
- 19.Holm R, Nesland JM: Retinoblastoma and p53 tumour suppressor gene protein expression in carcinomas of the thyroid gland. J Pathol 1994, 172:267-272 [DOI] [PubMed] [Google Scholar]
- 20.Ito Y, Seyama T, Mizuno T, Tsuyama N, Hayashi T, Hayashi Y, Dohi K, Nakamura N, Akiyama M: Unique association of p53 mutations with undifferentiated but not with differentiated carcinomas of the thyroid gland. Cancer Res 1992, 52:1369-1371 [PubMed] [Google Scholar]
- 21.Matias-Guiu X, Cuatrecasas M, Musulen E, Prat J: p53 expression in anaplastic carcinomas arising from thyroid papillary carcinomas. J Clin Pathol 1994, 47:337-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura T, Yana I, Kobayashi T, Shin E, Karakawa K, Fujita S, Miya A, Mori T, Nishisho I, Takai S-U: p53 gene mutations associated with anaplastic transformation of human thyroid carcinomas. Jpn J Cancer Res 1992, 83:1293-1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suárez HG: Genetic alterations in human epithelial thyroid tumours. Clin Endocrinol 1998, 48:531-546 [DOI] [PubMed] [Google Scholar]
- 24.Wynford-Thomas D: Origin and progression of thyroid epithelial tumours: cellular and molecular mechanisms. Hormone Res 1997, 47:145-157 [DOI] [PubMed] [Google Scholar]
- 25.Jhiang SM, Sagartz JE, Tong Q, Parker-Thornburg J, Capen CC, Cho J-Y, Xing S, Ledent C: Targeted expression of the ret/PTC1 oncogene induces papillary thyroid carcinomas. Endocrinology 1996, 137:375-378 [DOI] [PubMed] [Google Scholar]
- 26.Santoro M, Chiappetta G, Cerrato A, Salvatore D, Zhang L, Manzo G, Picone A, Portella G, Santelli G, Vecchio G, Fusco A: Development of thyroid papillary carcinomas secondary to tissue-specific expression of the RET/PTC1 oncogene in transgenic mice. Oncogene 1996, 12:1821-1826 [PubMed] [Google Scholar]
- 27.Sagartz JE, Jhiang SM, Tong Q, Capen CC: Thyroid-stimulating hormone promotes growth of thyroid carcinomas in transgenic mice with targeted expression of the ret/PTC1 oncogene. Lab Invest 1997, 76:307-318 [PubMed] [Google Scholar]
- 28.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA: Tumor spectrum analysis in p53-mutant mice. Curr Biol 1994, 4:1-7 [DOI] [PubMed] [Google Scholar]
- 29.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A: Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 1992, 356:215-221 [DOI] [PubMed] [Google Scholar]
- 30.Donehower LA, Harvey M, Vogel H, McArthur MJ, Montgomery CA, Jr, Park SH, Thompson T, Ford RJ, Bradley A: Effects of genetic background on tumorigenesis in p53-deficient mice. Mol Carcinogen 1995, 14:16-22 [DOI] [PubMed] [Google Scholar]
- 31.Harvey M, McArthur MJ, Montgomery CA, Jr, Bradley A, Donehower LA: Genetic background alters the spectrum of tumors that develop in p53-deficient mice. FASEB J 1993, 7:938-943 [DOI] [PubMed] [Google Scholar]
- 32.Harvey M, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A, Donehower LA: Spontaneous and carcinogen-induced tumorigenesis in p53-deficient mice. Nat Genet 1993, 5:225-229 [DOI] [PubMed] [Google Scholar]
- 33.Harvey M, Vogel J, Morris D, Bradley A, Bernstein A, Donehower LA: A mutant p53 transgene accelerates tumour development in heterozygous but not nullizygous p53-deficient mice. Nat Genet 1995, 9:305-311 [DOI] [PubMed] [Google Scholar]
- 34.Purdie CA, Harrison DJ, Peter A, Dobbie L, White S, Howie SEM, Salter DM, Bird CC, Wyllie AH, Hooper ML, Clarke AR: Tumour incidence, spectrum and ploidy in mice with a large deletion in the p53 gene. Oncogene 1994, 9:603-609 [PubMed] [Google Scholar]
- 35.Battista S, Martelli ML, Fedele M, Chiappetta G, Trapasso F, De Vita G, Battaglia C, Santoro M, Viglietto G, Fagin JA, Fusco A: A mutated p53 gene alters thyroid cell differentiation. Oncogene 1995, 11:2029-2037 [PubMed] [Google Scholar]
- 36.Fagin JA, Tang S-H, Zeki K, Di Lauro R, Fusco A, Gonsky R: Reexpression of thyroid peroxidase in a derivative of an undifferentiated thyroid carcinoma cell line by introduction of wild-type p53. Cancer Res 1996, 56:765-771 [PubMed] [Google Scholar]
- 37.Moretti F, Farsetti A, Soddu S, Misiti S, Crescenzi M, Filetti S, Andreoli M, Sacchi A, Pontecorvi A: p53 re-expression inhibits proliferation and restores differentiation of human thyroid anaplastic carcinoma cells. Oncogene 1997, 14:729-740 [DOI] [PubMed] [Google Scholar]
- 38.Li FP, Fraumeni JF, Jr, Mulvihill JJ, Blattner WA, Dreyfus MG, Tucker MA, Miller RW: A cancer family syndrome in twenty-four kindreds. Cancer Res 1988, 48:5358-5362 [PubMed] [Google Scholar]
- 39.Kleihues P, Schäuble B, zur Hausen A, Estève J, Ohgaki H: Tumors associated with p53 germline mutations: a synopsis of 91 families. Am J Pathol 1997, 150:1–13 [PMC free article] [PubMed]
- 40.Coppèe F, Gérard A-C, Denef J-F, Ledent C, Vassart G, Dumont JE, Parmentier M: Early occurrence of metastatic differentiated thyroid carcinomas in transgenic mice expressing the A2a adenosine receptor gene and the human papillomavirus type 16 E7 oncogene. Oncogene 1996, 13:1471-1482 [PubMed] [Google Scholar]
- 41.Ledent C, Dumont J, Vassart G, Parmentier M: Thyroid adenocarcinomas secondary to tissue-specific expression of simian virus-40 large T-antigen in transgenic mice. Endocrinology 1991, 129:1391-1401 [DOI] [PubMed] [Google Scholar]
- 42.Ledent C, Dumont JE, Vassart G, Parmentier M: Thyroid expression of an A<MDSP>2 adenosine receptor transgene induces thyroid hyperplasia and hyperthyroidism. EMBO J 1992, 11:537-542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ledent C, Marcotte A, Dumont JE, Vassart G, Parmentier M: Differentiated carcinomas develop as a consequence of the thyroid specific expression of a thyroglobulin-human papillomavirus type 16 E7 transgene. Oncogene 1995, 10:1789-1797 [PubMed] [Google Scholar]
- 44.Ledent C, Denef J-F, Cottecchia S, Lefkowitz R, Dumont J, Vassart G, Parmentier M: Costimulation of adenylyl cyclase and phospholipase C by a mutant α1b adrenergic receptor transgene promotes malignant transformation of thyroid follicular cells. Endocrinology 1997, 138:369-378 [DOI] [PubMed] [Google Scholar]
- 45.Michiels F-M, Caillou B, Talbot M, Dessarps-Freichey F, Maunoury M-T, Schlumberger M, Mercken L, Monier R, Feunteun J: Oncogenic potential of guanine nucleotide stimulatory factor α subunit in thyroid glands of transgenic mice. Proc Natl Acad Sci USA 1994, 91:10488-10492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powell DJ, Jr, Russell J, Nibu K-I, Li G, Rhee E, Liao M, Goldstein M, Keane WM, Santoro M, Fusco A, Rothstein JL: The RET/PTC3 oncogene: metastatic solid-type papillary carcinomas in murine thyroids. Cancer Res 1998, 58:5523-5528 [PubMed] [Google Scholar]
- 47.Rochefort P, Caillou B, Michiels F-M, Ledent C, Talbot M, Schlumberger M, Lavelle F, Monier R, Feunteun J: Thyroid pathologies in transgenic mice expressing a human activated ras gene driven by a thyroglobulin promoter. Oncogene 1996, 12:111-118 [PubMed] [Google Scholar]
- 48.Santelli G, de Franciscis V, Portella G, Chiappetta G, D’Alessio A, Califano D, Rosati R, Mineo A, Monaco C, Manzo G, Pozzi L, Vecchio G: Production of transgenic mice expressing the Ki-ras oncogene under the control of a thyroglobulin promoter. Cancer Res 1993, 53:5523-5527 [PubMed] [Google Scholar]