Abstract
Experimental allergic encephalomyelitis (EAE) is the principal genetically determined animal model for multiple sclerosis (MS), the major inflammatory disease of the central nervous system (CNS). Although genetics clearly play a role in susceptibility to MS, attempts to identify the underlying genes have been disappointing. Considerable variation exists between MS patients with regard to the severity of clinical signs, mechanism of demyelination, and location of CNS lesions, confounding the interpretation of genetic data. A mouse-human synteny mapping approach may allow the identification of candidate susceptibility loci for MS based on the location of EAE susceptibility loci. To date, 16 regions of the mouse genome have been identified that control susceptibility or clinical signs of EAE. In this work, we examined the genetic control of histopathological lesions of EAE in an F2 intercross population generated from the EAE susceptible SJL/J and EAE resistant B10.S/DvTe mouse strains. Composite interval mapping was used to identify 10 quantitative trait loci (QTL), including seven newly identified loci controlling the distribution and severity of CNS lesions associated with murine EAE. QTL on chromosome 10 control lesions in the brain, whereas QTL on chromosomes 3, 7, and 12 control lesions in the spinal cord. Furthermore, sexually dimorphic QTL on chromosomes 2, 9, and 11 control CNS lesions in females, whereas QTL on chromosomes 10, 11, 12, 16, and 19 control lesions in males. Our results suggest that the severity and location of CNS lesions in EAE are genetically controlled, and that the genetic component controlling the character and severity of the lesions can be influenced by sex.
Multiple sclerosis (MS) is the major inflammatory disease of the central nervous system (CNS), affecting 0.1% of the North American population. Susceptibility to MS is controlled by genetic and environmental factors. 1,2 Although a clear genetic link to MS susceptibility has been established, attempts to identify the underlying genes have been disappointing. 3-7 The clinical spectrum of MS is diverse, including relapsing-remitting, primary progressive, secondary progressive, and progressive-relapsing disease types. 8 The clinical heterogeneity characteristic of MS contributes to the difficulty in searching for susceptibility genes because the underlying genetic components of the disease may differ between patients. 9,10
Besides the clinical heterogeneity in MS, considerable variation exists in the type and anatomical location of the lesions. Typical MS lesions consist of inflammation and infiltration of T cells and macrophages accompanied by edema, myelin swelling, and endothelial cell activation. 11-14 Lucchinetti et al 11 described several different patterns of demyelination, including: demyelination with relative preservation of oligodendrocytes; myelin destruction with concomitant, complete destruction of oligodendrocytes; primary destruction or disturbance of myelinating cells with secondary demyelination; demyelination with secondary oligodendrocyte loss; and loss of myelin, oligodendrocytes, axons, and astrocytes. Different immunological pathways including, but not limited to, cytotoxic cytokines, demyelinating antibodies, cell-mediated cytotoxicity, and apoptosis may be responsible for the different patterns of demyelination. 11-13 Although the pattern of demyelination is heterogenous between patients, lesions within the same patient tend to be homogenous, suggesting that different mechanisms of demyelination may operate in different patient subgroups, perhaps reflecting an underlying genetic influence. 11-13
In addition to the differences in lesion type, variation is seen in the location of MS lesions. 14 Patients with primarily progressive MS often exhibit lesions in the spinal cord (SC) without cerebral involvement. 15,16 In the classical form of MS, called Charcot’s variant, lesions appear to be randomly distributed, involving the optic nerves, brain stem, cerebellum, and SC. 14 Neuromyelitis optica or Devic’s disease is an MS variant characterized by lesions in the optic nerves and SC with significantly less involvement at other sites. 14 The fact that Devic’s disease is more prevalent in India and the Far East, particularly Japan, suggests that genetic factors can contribute to the anatomical location of the lesions seen in MS and its variants. 10,17
Experimental allergic encephalomyelitis (EAE) is the principal animal model for MS. Inoculation with crude CNS tissue homogenate, purified myelin proteins, or their encephalitogenic peptides with appropriate adjuvants can elicit EAE in genetically susceptible strains of mice. 18-20 Autoreactive CD4+ T cells infiltrate the CNS and subsequently recruit additional lymphocytes and mononuclear cells, resulting in inflammation and demyelination. 21,22 Clinically, disease is manifest as an ascending paresis followed by paralysis of the tail and hindlimbs, frequently accompanied by fecal and urinary incontinence. Although EAE is typically known as a chronic relapsing disease, 20 inducing antigen, induction protocol, and mouse strain can influence the disease course. 23-25 Histologically, EAE in SJL/J mice is characterized by white matter lesions primarily in the SC, optic nerve, cerebellum, and the medulla/pons whereas the midbrain, basal gray, and cerebrum are infrequently involved. 20,24,25 SC lesions are found in the lumbosacral portion with a more patchy distribution seen rostrally. 24
In contrast to the simple relapsing remitting disease pattern of EAE in purebred SJL/J mice, we recently described four distinct clinical subtypes of EAE in our F2 intercross population between the EAE-susceptible SJL/J and EAE-resistant B10.S/DvTe mouse strains, including: relapsing-remitting; monophasic remitting-nonrelapsing; chronic nonremitting; and acute progressive subtypes. 26 We identified novel EAE-modifying (eae-m) loci and unique loci with gender-specific effects that govern susceptibility to these distinct subtypes of EAE. 26 In this study, we used composite interval mapping (CIM) 27-29 to identify quantitative trait loci (QTL) controlling the distribution and severity of CNS lesions in our F2 population. We report the existence of QTL on chromosome 10 that control lesion severity and mononuclear cell infiltration in the brain. Lesions in the SC are controlled by QTL on chromosomes 3, 7, and 12. Furthermore, analysis of the data following stratification by sex revealed significant gender-specific differences in the genetic control of the distribution and severity of lesions. Female-specific QTL were found on chromosomes 2, 9, and 11, whereas male-specific QTL were found on chromosomes 10, 11, 12, 16, and 19.
Materials and Methods
Animals
Male and female SJL/J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B10.S/DvTe mice were generated from breeding stock originally obtained from Dr. Chella David (Mayo Clinic, Rochester, MN). Six hundred and seventy-three F2 animals (322 male and 351 female) were generated continuously throughout the course of 12 months from the same F1 hybrid (B10.S/DvTe × SJL/J) breeding stock in the animal colony at Brigham Young University (Provo, UT). Animals were fed Purina mouse pellets (Ralston-Purina, St. Louis, MO) and acidified water ad libitum.
Induction and Evaluation of EAE
Induction of EAE was carried out as previously described. 30 Briefly, 1.0 mg of SJL/J SC homogenate, diluted in 0.15 ml of phosphate-buffered saline, was emulsified with an equal volume of complete Freund’s adjuvant and injected subcutaneously at two sites on the posterior flank (0.15 ml/injection site). A booster injection of SJL/J SC homogenate plus complete Freund’s adjuvant, prepared in the same manner as the primary inoculum, was given 7 days after the primary injection. Starting on day 10, mice were monitored for clinical signs and graded from 0 to 4 as follows: 0, no clinical expression of disease; 1, floppy tail without hind limb weakness; 2, hind limb weakness with or without flaccid tail; 3, hind leg paralysis and floppy tail; and 4, hind leg paralysis accompanied by a floppy tail and urinary or fecal incontinence. 31 Animals with a score of 4 were euthanized. Mice that had no symptoms by day 30 were euthanized. Animals exhibiting symptoms any time between days 10 and 30 were monitored for an additional 30 days and euthanized on day 60.
Histopathological Evaluation
Brain and SC were dissected from the calvaria and vertebral columns of all animals and fixed by immersion in 10% phosphate-buffered formalin (pH 7.2) at 4°C. After adequate fixation, brain and SC were trimmed and representative transverse sections embedded in paraffin, sectioned at 5 μm, and mounted on glass slides. Sections were stained with hematoxylin and eosin (H&E) for routine evaluation and luxol fast blue-periodic acid Schiff reagent for evaluation of myelin. Representative areas of the brain and SC, including brain stem, cerebrum, cerebellum, and the cervical, thoracic, and lumbar segments of the SC, were selected for histopathological evaluation based on previous studies. 20,24,25 The following components of the lesions were assessed: 1) severity of the lesion as represented by each component of the histopathological assessment; 2) extent and degree of myelin loss and tissue injury (swollen axon sheaths, swollen axons, and reactive gliosis); 3) severity of the acute inflammatory response (predominantly neutrophils); and 4) severity of the chronic inflammatory response (lymphocytes/macrophages). A score was assigned separately to the entire brain and SC for each lesion characteristic based on a subjective scale ranging from 0 to 5. A score of 0 indicates no lesions; 1 indicates minimal; 2, mild; 3, moderate; 4, marked; and 5, severe lesions. Occasional mice had eosinophils admixed with the dominant neutrophilic inflammatory response.
Genotyping and Linkage Analysis
Genomic DNA was isolated from liver, and polymerase chain reaction parameters for microsatellite typing were as previously described. 32 Microsatellite size variants were resolved by autoradiography on Kodak film (Eastman Kodak, Rochester, NY). A linkage map was generated with 173 informative markers on the 19 autosomes using the Kosambi mapping function in the MAPMAKER/EXP computer package. 33,34 CIM was used for localization of QTL affecting EAE lesions, because this method allows for more precise definition of intervals containing QTL than classical interval mapping. 27-29 In addition, CIM avoids the identification of ghost loci. 29 CIM combines classical interval mapping with multiple regression. Markers flanking the test interval are added to the regression model to control for the presence of linked QTL. Additional markers, unlinked to the test interval, but with significant effects on the trait are added to the model to control for the genetic background. The most significant markers unlinked to the test interval are chosen using a linear regression model with a forward/backward selection procedure in the SRmapqtl program of QTL Cartographer v1.13 (http://statgen.ncsu.edu/qtlcart/cartographer.html). 35 CIM was performed using model 6 of the Zmapqtl program in QTL Cartographer, with a window size of 10 cM and the 20 most significant background markers selected from the output of SRmapqtl. Tests of significant linkage for a QTL are reported in the form of a likelihood ratio test (LRT) statistic. CIM was performed for the combined population (males and females) as well as for males and females separately. Analysis for the combined population was performed with an additional covariate in the regression model to control for differences in lesions between the sexes in our population.
Significance of the QTL identified by CIM was evaluated by permutation theory. 36 Critical values for the declaration of significance were determined by the distribution of the maximum LRT statistic from 1,000 permutations of our data under the null hypothesis of no linkage. Each permutation was done by randomly shuffling the trait values while maintaining genotype data, and re-analyzing the data. Significant linkage was declared when the observed LRT statistic equaled or exceeded 95% of the permuted values generated under random conditions (α = 0.05).
Results
Lesions
Representative areas of the CNS including brain stem, cerebrum, cerebellum, and the cervical, thoracic, and lumbar segments of the SC were evaluated for lesion severity, and the degree of mononuclear cell infiltration, acute inflammation, and demyelination. The character and distribution of CNS lesions observed in this study were consistent with those reported in previous studies. 20,24,25 Inflammatory responses in susceptible animals ranged from those with a predominantly neutrophilic response admixed with a smaller monocytic/lymphocytic component, to those with a predominantly monocytic/lymphocytic response. In a few animals, occasional eosinophils were admixed with the neurophilic exudate. CNS tissue responses ranged from mice with no lesions to those with loss of myelin, reactive gliosis, swollen axon sheaths, and swollen axons. In all mice with lesions, the inflammatory response had a perivascular distribution that was predominantly observed in the meninges and in the white matter. In the SC, predilection for the nerve root entry zone was observed, as previously reported. 25
CNS lesions were found in 488 of the 673 (B10.S/DvTe × SJL/J) F2 mice (Table 1) ▶ . Of the histologically affected animals, 282 also exhibited clinical signs of EAE. Whereas 85% of females had CNS lesions, only 58% of males had CNS lesions (chi-square = 61.8, P = 3.8 × 10−15). Additionally, lesions in the brain and SC were more severe in females than males (P < 0.0001 for each variable: lesion severity, mononuclear cell infiltration, acute inflammation, and demyelination, determined by a Mann-Whitney signed rank test). Within the group of mice with CNS lesions (188 males and 300 females), similar numbers of males and females had clinical signs of EAE (102 males and 180 females, chi-square = 1.56, P = 0.21).
Table 1.
Incidence of Clinical EAE and CNS Lesions in F2 Mice by Sex
| Total | Histological EAE | Clinical EAE | |||
|---|---|---|---|---|---|
| No CNS lesions | CNS lesions | Affected | Unaffected | ||
| Male | 322 | 134 | 188 | 102 | 220 |
| Female | 351 | 51 | 300 | 80 | 171 |
| Total | 673 | 185 | 488 | 282 | 391 |
Genetic Control of Lesions in the F2
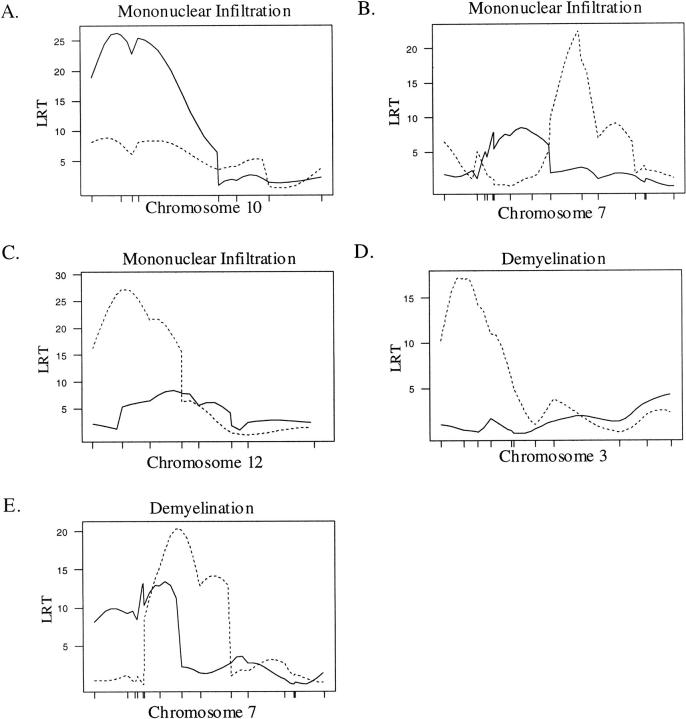
A CIM approach was used to identify QTL for each lesion characteristic (severity, mononuclear cell infiltration, demyelination, and acute inflammation) in the brain and SC. Analyses were carried out for the complete F2 population and on males and females separately. In the combined population, a single QTL affecting lesion severity and mononuclear infiltration in the brain was found on chromosome 10 in an interval from 4 to 19 cM (Table 2 ▶ , Figure 1A ▶ ). We designate this newly found QTL: eae15. Two QTL affected lesion severity and mononuclear cell infiltration in the SC, one on chromosome 7 from 37 to 52 cM, and another on chromosome 12 from 3 to 29 cM (Table 2 ▶ ; Figure 1, B and C ▶ ). Whereas the QTL on chromosome 7 has been previously identified as an eae-m locus, eae4, 26,37 the QTL on chromosome 12 designated eae16 represents a newly identified interval. The SJL/J allele at eae15 and eae16 confers a more severe phenotype and the B10.S/DvTe allele at eae4 confers a more severe phenotype (Table 2) ▶ . QTL on chromosomes 3, 7, and 12 were significantly linked to demyelination in the SC (Table 2 ▶ ; Figure 1, D and E ▶ ). The interval from 4 to 23 cM on chromosome 3 represents a newly identified QTL designated: eae20. The QTL on chromosome 7 resides on an interval from 18 to 50 cM, and overlaps with a previously identified QTL on chromosome 7: eae4. 26,37
Table 2.
Location and Effects of QTL Controlling Lesions in (B10.S/DvTe × SJL/J) F2 Mice
| Trait | Location | Chromosome | Marker* | LRT† | Additive effect‡ | Dominance deviation§ | Percent variance¶ |
|---|---|---|---|---|---|---|---|
| Lesion severity | Brain | 10 | D10Mit2 | 26.26 | −0.239 | −0.080 | 3.5 |
| SC | 7 | D7Mit39 | 21.55 | 0.253 | −0.131 | 3.0 | |
| SC | 12 | D12Mit12 | 19.74 | −0.246 | −0.213 | 2.8 | |
| Demyelination | SC | 3 | D3Mit55 | 17.21 | −0.108 | 0.332 | 2.6 |
| SC | 7 | D7Mit233 | 20.35 | 0.262 | −0.122 | 2.8 | |
| SC | 12 | D12Mit12 | 19.60 | −0.267 | −0.159 | 2.7 | |
| Mononuclear infiltration | Brain | 10 | D10Mit2 | 26.28 | −0.233 | −0.094 | 3.6 |
| SC | 7 | D7Mit39 | 22.41 | 0.244 | −0.106 | 3.0 | |
| SC | 12 | D12Mit12 | 27.27 | −0.243 | −0.194 | 3.5 |
*Marker nearest the peak linkage.
†Likelihood ratio test statistic.
‡Additive effect of the QTL relative to the B10.S/DvTe homozygote. A positive value indicates that the mean trait value for the B10.S/DvTe homozygote was greater than the mean trait value for the SJL/J homozygotes.
§Dominance deviation. Deviation of the trait value for heterozygotes from the midpoint of the SJL/J and B10.S/DvTe homozygotes.
¶Percent variance accounted for by the QTL.
Figure 1.
CIM results for the combined (male + female) population. A solid line represents the plot of the LRT statistic for the brain whereas the broken line represents a plot of the LRT statistic for the SC. Tick marks on the x axis represent the positions of microsatellite markers. Permutation-derived significance cutoffs were based on 1000 permutations at α = 0.05. A–C: Composite interval maps with significant linkage for the trait, mononuclear cell infiltration. Significance cutoffs for this trait are LRT = 16.4 for the brain and 16.8 for the SC. D–E: Composite interval maps with significant linkage for demyelination. Significance cutoffs for this trait are LRT = 17.0 for the brain and 17.6 for the SC.
Sex-Specific QTL Controlling EAE Lesions
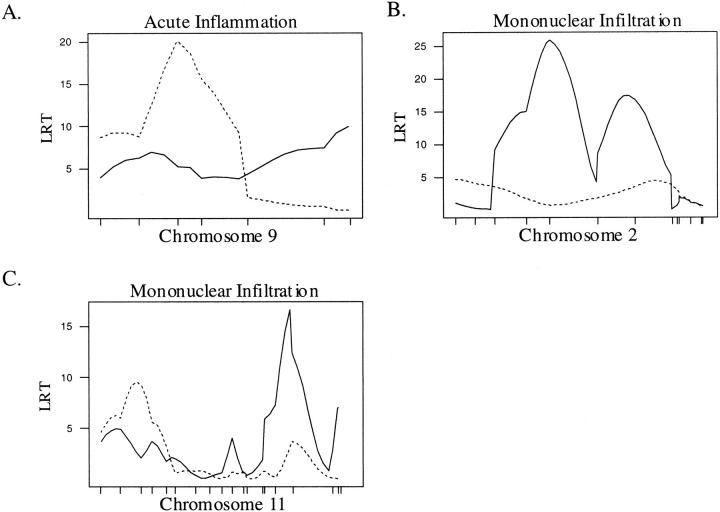
Stratification of our population by sex allowed us to identify QTL with sex-dependent effects. In females, we identified a single QTL on chromosome 9 between 25 and 35 cM affecting acute inflammation in the SC (Table 3 ▶ , Figure 2A ▶ ). This QTL resides in the interval containing eae9, a QTL identified previously controlling duration of disease among affected animals. 37 A B10.S/DvTe allele at this locus increases neutrophilic infiltrates (Table 3) ▶ . Lesion severity and mononuclear infiltration in females were linked to QTL on chromosomes 2 and 11 (Table 3 ▶ ; Figure 2, B and C ▶ ). The QTL on chromosome 2 resides in an interval from 30 to 69 cM, a locus we designate: eae21. Linkage to chromosome 11 is to an interval between 49 and 71 cM, telomeric of eae7, a locus associated with severity of clinical signs. 38 We designate this newly identified QTL: eae22. At both eae21 and eae22, the SJL/J allele increases the severity of the lesions and mononuclear cell infiltration (Table 3) ▶ .
Table 3.
Location and Effects of QTL Controlling Lesions in Female (B10.S/DvTe × SJL/J) F2 Mice
| Trait | Location | Chromosome | Marker* | LRT† | Additive effect‡ | Dominance deviation§ | Percent variance¶ |
|---|---|---|---|---|---|---|---|
| Lesion severity | Brain | 2 | D2Mit9 | 25.71 | −0.312 | 0.050 | 5.7 |
| Brain | 11 | D11Mit330 | 20.21 | −0.343 | −0.312 | 4.7 | |
| Mononuclear infiltration | Brain | 2 | D2Mit9 | 25.90 | −0.307 | 0.064 | 5.8 |
| Brain | 11 | D11Mit330 | 16.64 | −0.300 | −0.148 | 3.9 | |
| Acute inflammation | SC | 9 | D9Mit48 | 20.06 | 0.332 | 0.001 | 4.7 |
*Marker nearest the peak linkage.
†Likelihood ratio test statistic.
‡Additive effect of the QTL relative to the B10.S/DvTe homozygote. A positive value indicates that the mean trait value for the B10.S/DvTe homozygotes was greater than the mean trait value for the SJL/J homozygotes.
§Dominance deviation. Deviation of the trait value for heterozygotes from the midpoint of the SJL/J and B10.S/DvTe homozygotes.
¶Percent variance accounted for by the QTL.
Figure 2.
CIM results for the female population. A solid line represents the plot of the LRT statistic for the brain whereas the broken line represents a plot of the LRT statistic for the SC. Tick marks on the x axis represent the positions of microsatellite markers. Permutation-derived significance cutoffs were based on 1000 permutations at α = 0.05. A: Composite interval map of chromosome 9 with significant linkage for acute inflammation in the SC. Significance cutoffs for this trait are LRT = 16.5 for the brain and 16.8 for the SC. B–C: Composite interval maps with significant linkage for mononuclear cell infiltration. Significance cutoffs for this trait are LRT = 16.4 for the brain and 16.55 for the SC.
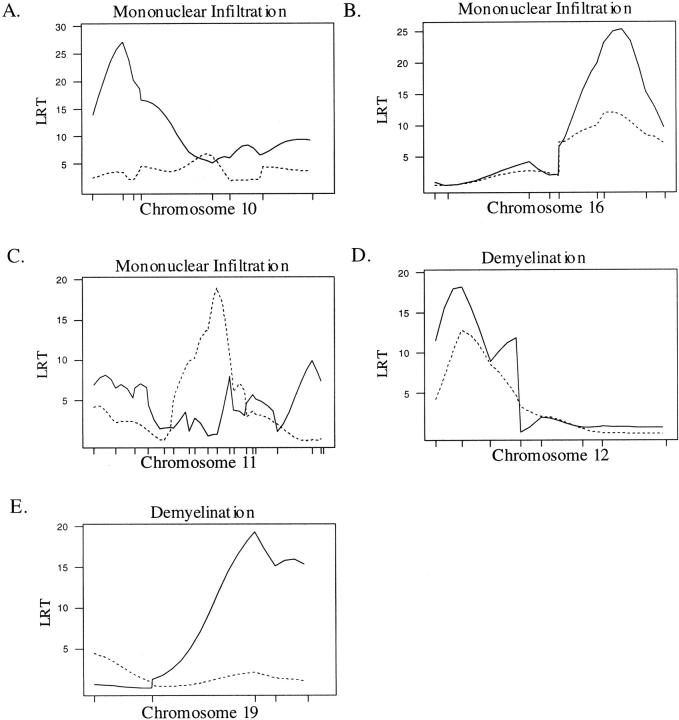
In males, we identified four QTL with effects on lesions in the brain. Eae15, a locus on chromosome 10 affecting mononuclear infiltration and lesion severity in the full F2 population was also significant in the male population (Table 4 ▶ , Figure 3A ▶ ). Significant linkage to mononuclear infiltration, lesion severity, and acute inflammation was found on chromosome 16 in an interval between D16Mit168 and D16Mit140 containing eae11, a previously identified eae-m locus 26 (Table 4 ▶ , Figure 3B ▶ ). A single locus on chromosome 11 had significant effects on lesion severity and mononuclear infiltration in the SC (Table 4 ▶ , Figure 3C ▶ ). This QTL is linked to an interval between markers D11Mit155 and D11Mit194, just centromeric of eae7 and telomeric of eae6b, previously identified QTL controlling severity and duration of disease. 38 The SJL/J allele at this locus, designated eae23, increases lesion severity (Table 4) ▶ . Two QTL were identified affecting demyelination in the brain (Table 4 ▶ ; Figure 3, D and E ▶ ). One, designated eae16, is located on chromosome 12 between 3 and 29 cM, and another on chromosome 19 designated eae19 is located from 26 to 50 cM. At eae16 and eae19, an SJL/J allele increases demyelination (Table 4) ▶ .
Table 4.
Location and Effects of QTL Controlling Lesions in Male (B10.S/DvTe × SJL/J) F2 Mice
| Trait | Location | Chromosome | Marker* | LRT† | Additive effect‡ | Dominance deviation§ | Percent variance¶ |
|---|---|---|---|---|---|---|---|
| Lesion severity | Brain | 10 | D10Mit2 | 24.88 | −0.282 | −0.226 | 6.2 |
| Brain | 16 | D16Mit14 | 25.75 | −0.197 | −0.398 | 7.0 | |
| SC | 11 | D11Mit29 | 21.05 | 0.369 | −0.174 | 5.4 | |
| Demyelination | Brain | 12 | D12Mit12 | 17.92 | −0.211 | 0.029 | 5.1 |
| Brain | 19 | D19Mit05 | 19.31 | 0.073 | −0.247 | 4.8 | |
| Mononuclear infiltration | Brain | 10 | D10Mit2 | 27.13 | −0.294 | −0.189 | 6.7 |
| Brain | 16 | D16Mit14 | 25.30 | −0.204 | −0.391 | 7.5 | |
| SC | 11 | D11Mit29 | 18.95 | 0.283 | −0.177 | 4.7 | |
| Acute inflammation | Brain | 16 | D16Mit14 | 32.48 | −0.267 | −0.323 | 10.3 |
| SC | 16 | D16Mit14 | 17.30 | −0.259 | −0.274 | 5.1 |
*Marker nearest the peak linkage.
†Likelihood ratio test statistic.
‡Additive effect of the QTL relative to the B10.S/DvTe homozygote. A positive value indicates that the mean trait value for the B10.S/DvTe homozygotes was greater than the mean trait value for the SJL/J homozygotes.
§Dominance deviation. Deviation of the trait value for heterozygotes from the midpoint of the SJL/J and B10.S/DvTe homozygotes.
¶Percent variance accounted for by the QTL.
Figure 3.
CIM results for the male population. A solid line represents the plot of the LRT statistic for the brain whereas the broken line represents a plot of the LRT statistic for the SC. Tick marks on the x axis represent the positions of microsatellite markers. Permutation-derived significance cutoffs were based on 1000 permutations at α = 0.05. A–C: Composite interval maps with significant linkage for the mononuclear cell infiltration. Significance cutoffs for this trait are LRT = 17.9 for the brain and 16.6 for the SC. D–E: Composite interval maps with significant linkage for demyelination. Significance cutoffs for this trait are LRT = 18.1 for the brain and 18.0 for the SC.
Discussion
Both environmental and genetic factors have been associated with susceptibility to MS. 39 Although significant efforts are underway to identify the genes underlying susceptibility, results have been disappointing. 7,40 Significant heterogeneity in clinical and pathological phenotypes may explain the difficulty in identifying MS susceptibility genes. It has been suggested that different clinical subtypes of both MS and EAE are immunogenetically distinct. 9,26 Differences in pathological manifestation of MS have been observed between Western and Asian populations. 10,17 Asian type MS is characterized by severe inflammation in the optic nerve and SC reminiscent of Devic’s disease. In contrast, MS lesions in Western populations are characterized by widespread demyelination involving the entire CNS. 10,14 Recent findings indicate that MS lesions in Japanese patients rarely affect the cerebellum (6.4%), whereas in Western patients cerebellar involvement is common (50 to 90%). 17 Genetic differences between the Asian and Western populations may account for these differences in the pathological phenotype of MS.
Our findings indicate that lesion characteristics in EAE are under complex genetic control, influencing both the location and severity of lesions. We identified a QTL on chromosome 10 that controlled the severity of lesions and extent of mononuclear/lymphocyte infiltration in the brain. In the SC, lesions were controlled by QTL on chromosomes 3, 7, and 12. Peak linkage for demyelination on chromosome 7 was nearest the marker D7Mit233 at 40 cM, and peak linkage for lesion severity and mononuclear infiltration was nearest the marker D7Mit39 at 50.3 cM. Whereas both QTL reside on an interval contiguous with eae4, these linkages may reflect separate QTL controlling lesion severity/mononuclear infiltration and demyelination in the SC.
Females with MS outnumber males by approximately 2:1. 41 In our mouse population, females were more likely than males to develop the clinical signs and histological lesions of EAE. Among mice with lesions however, males and females progressed in similar proportions to clinical disease. We hypothesize that the differences in EAE between males and females are determined in the early stages of disease, and govern the migration and infiltration of inflammatory cells into the CNS. In this respect, castrated male SJL mice immunized with proteolipid protein residues139 to 151 to induce EAE have widespread perivascular inflammation in the SC at clinical relapse. 42 Intact males fail to relapse, and have no indication of inflammation in the SC, suggesting that susceptibility to CNS lesions in male SJL mice is in part controlled by testosterone. 42,43 Hormone-sensitive genes controlling the ability of inflammatory cells to infiltrate the CNS may therefore regulate the sexual dimorphism in EAE. In this respect, we have identified QTL unique to males and females controlling the expression of EAE lesions in our F2 population. The effect of castration on the individual expression of these QTL is currently being studied in our laboratories.
Overall, the genetic component controlling the lesions in males was stronger (significant QTL on chromosomes 10, 11, 12, 16, and 19) than in females (significant QTL on chromosomes 2, 9, and 11). The presence of unique QTL in males and females regulating the severity and characteristics of lesions in EAE suggests that these QTL are responsive to sex hormones. In this respect, it is known that females have a more robust immune response. 44,45 Androgen treatment has been shown to induce a shift in cytokine production, particularly interleukin-10 toward protective Th2 type cytokines. 46 In an adoptive transfer model using myelin basic protein, draining lymph node cells (LNC) from male SJL mice induced less severe EAE, and produced less interleukin-12 and interferon-γ than LNC from female mice. 47 In a similar experiment, LNC from male SJL mice stimulated with proteolipid protein 139 to 151 were less encephalitogenic than LNC from female mice. 48 Androgen treatment of LNC from SJL females resulted in a decrease in interferon-γ and an increase in interleukin-10 production. 48 Given the protective effect of androgens, susceptibility to EAE in males may require a greater genetic contribution than in females, perhaps explaining the decreased prevalence of MS in males. A similar situation was observed in a backcross using the same strains of mice, where EAE was induced with and without pertussis toxin. 49 In this case, mice induced without pertussis toxin were less susceptible to EAE and had more loci controlling susceptibility than mice induced with PTX.
We have identified 10 QTL, including seven newly identified loci governing different aspects of the severity and characteristics of the lesions in EAE. Different QTL control EAE lesions depending on location (brain or SC) and sex. Our results reflect the complex nature of genetic control of EAE. EAE is characterized by multiple disease subtypes, sexual dimorphism, and susceptibility that is dependent on multiple minor genes with small individual effects, rather than a few genes of major biological importance. 26,37,38 A summary of EAE modifying loci identified to date is included in Table 5 ▶ . Genetic differences may account for the remarkable heterogeneity observed between MS patients, and may therefore be responsible for confounding the interpretation of human MS linkage data. A mouse-human synteny mapping approach may allow the identification of candidate loci for MS based on the locations of murine eae-m loci. In this regard, Kuokkanen et al 50 identified a MS susceptibility locus on chromosome 5p14-p12 based on the existence of an eae-m locus on mouse chromosome 15 (eae2). Three eae-m loci identified in our population are syntenic with putative MS susceptibility loci. Eae5 on mouse chromosome 17 is syntenic with human 6p21, eae7 on mouse chromosome 11 is syntenic to human 17q22, and eae12 on mouse chromosome 7 is syntenic to human 19q. 3,51,52 Identification of the genes underlying these eae-m QTL will be invaluable in characterizing the molecular basis of the different clinical and pathological subtypes seen in MS. Further genetic studies should account for the possibility that differences between MS patients may represent immunogenetically distinct pathological mechanisms.
Table 5.
Summary of EAE Modifying Loci Identified in Mice to Date
| Locus | Chromosome | Location (cM) | Markers flanking interval | Sex specificity | Traits |
|---|---|---|---|---|---|
| eae1 | 17 | 23 | H-2 | Incidence | |
| eae2 | 15 | 10.6–14.8 | D15Mit51–D15Mit56 | Incidence | |
| eae3 | 3 | 29–52 | D3Mit29–D3Mit105 | Incidence, ACPR subtype | |
| eae4 | 7 | 40–50.3 | D7Mit233–D7Mit39 | Incidence, SC lesions | |
| eae5 | 17 | 24.5–33.3 | D17Mit10–17Mit150 | Incidence | |
| eae6a | 11 | 0.25–13 | D11Mit72–D11Mit294 | Severity | |
| eae6b | 11 | 19–28 | D11Mit307–D11Mit140 | Duration | |
| eae7 | 11 | 44–58 | D11Mit194–D11Mit98 | Severity, M-RNR subtype | |
| eae8 | 2 | 99–107 | D2Mit25–D2Mit200 | Incidence, Severity | |
| eae9 | 9 | 22–42 | D9Mit22–D9Mit8 | Female | Duration, SC acute inflammation |
| eae10 | 3 | 64.1–79.4 | D3Mit14–D3Mit147 | Onset | |
| eae11 | 16 | 21–41 | D16Mit110–D16Mit140 | Male | Incidence, Brain lesions |
| eae12 | 7 | 16 | D7Mit227–D7Mit25 | Female | R/R subtype |
| eae13 | 13 | 37 | D13Mit66 | Male | M-RNR subtype |
| eae14 | 8 | 16–33 | D8Mit3–D8Mit31 | Incidence | |
| eae15 | 10 | 4–19 | D10Mit80–D10Mi214 | Male | Brain lesions |
| eae16 | 12 | 3–19 | D12Mit56–D12Mit2 | SC lesions | |
| eae17 | 10 | 44 | D10Mit42– | Female | Severity index, SC demyelination |
| eae18 | 18 | 41–54 | D18Mit81–D18Mit3 | Male | SC lesions |
| eae19 | 19 | 26–53 | D19Mit19–D19Mit33 | Male | Brain demyelination |
| eae20 | 3 | 5-23 | D3Mit36–D3Mit6 | SC demyelination | |
| eae21 | 2 | 30–69 | D2Mit269–D2Mit17 | Female | Brain lesions |
| eae22 | 11 | 49–71 | D11Mit38–D11Mit168 | Female | Brain lesions |
| eae23 | 11 | 32–44 | D11Mit155–D11Mit194 | Male | SC lesions |
Footnotes
Address reprint requests to Dr. Cory Teuscher, Department of Veterinary Pathobiology, 2001 South Lincoln Ave., University of Illinois at Urbana–Champaign, Urbana, IL 61802. E-mail: cteusche@uiuc.edu.
Supported by National Institutes of Health grants NS36526 (to C. T. and E. P. B) and AI41747 (to C. T.) and National Multiple Sclerosis Society grants RG2650 and RG3129 (to C. T.).
References
- 1.Ebers GC, Sadovnick AD, Risch NJ: A genetic basis for familial aggregation in multiple sclerosis. Canadian Collaborative Study Group [see comments]. Nature 1995, 377:150-151 [DOI] [PubMed] [Google Scholar]
- 2.Ewing C, Bernard CC: Insights into the etiology and pathogenesis of multiple sclerosis. Immunol Cell Biol 1998, 76:47-54 [DOI] [PubMed] [Google Scholar]
- 3.Sawcer S, Jones HB, Feakes R, Gray J, Smaldon N, Chataway J, Robertson N, Clayton D, Goodfellow PN, Compston A: A genome screen in multiple sclerosis reveals susceptibility loci on chromosome 6p21 and 17q22 [see comments]. Nat Genet 1996, 13:464-468 [DOI] [PubMed] [Google Scholar]
- 4.Haines JL, Ter Minassian M, Bazyk A, Gusella JF, Kim DJ, Terwedow H, Pericak-Vance MA, Rimmler JB, Haynes CS, Roses AD, Lee A, Shaner B, Menold M, Seboun E, Fitoussi RP, Gartioux C, Reyes C, Ribierre F, Gyapay G, Weissenbach J, Hauser SL, Goodkin DE, Lincoln R, Usuku K, Oksenberg JR: A complete genomic screen for multiple sclerosis underscores a role for the major histocompatability complex. The Multiple Sclerosis Genetics Group. Nat Genet 1996, 13:469–471 [DOI] [PubMed]
- 5.Ebers GC, Kukay K, Bulman DE, Sadovnick AD, Rice G, Anderson C, Armstrong H, Cousin K, Bell RB, Hader W, Paty DW, Hashimoto S, Oger J, Duquette P, Warren S, Gray T, O’Connor P, Nath A, Auty A, Metz L, Francis G, Paulseth JE, Murray TJ, Pryse-Phillips W, Risch N: A full genome search in multiple sclerosis [see comments]. Nat Genet 1996, 13:472-476 [DOI] [PubMed] [Google Scholar]
- 6.Kuokkanen S, Gschwend M, Rioux JD, Daly MJ, Terwilliger JD, Tienari PJ, Wikstrom J, Palo J, Stein LD, Hudson TJ, Lander ES, Peltonen L: Genomewide scan of multiple sclerosis in Finnish multiplex families. Am J Hum Genet 1997, 61:1379-1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawcer S, Goodfellow PN, Compston A: The genetic analysis of multiple sclerosis. Trends Genet 1997, 13:234-239 [DOI] [PubMed] [Google Scholar]
- 8.Lublin FD, Reingold SC: Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996, 46:907-911 [DOI] [PubMed] [Google Scholar]
- 9.Olerup O, Hillert J, Fredrikson S, Olsson T, Kam-Hansen S, Moller E, Carlsson B, Wallin J: Primarily chronic progressive and relapsing/remitting multiple sclerosis: two immunogenetically distinct disease entities. Proc Natl Acad Sci USA 1989, 86:7113-7117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kira J, Kanai T, Nishimura Y, Yamasaki K, Matsushita S, Kawano Y, Hasuo K, Tobimatsu S, Kobayashi T: Western versus Asian types of multiple sclerosis: immunogenetically and clinically distinct disorders. Ann Neurol 1996, 40:569-574 [DOI] [PubMed] [Google Scholar]
- 11.Lucchinetti CF, Bruck W, Rodriguez M, Lassmann H: Distinct patterns of multiple sclerosis pathology indicates heterogeneity on pathogenesis. Brain Pathol 1996, 6:259-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trapp BD, Bo L, Mork S, Chang A: Pathogenesis of tissue injury in MS lesions. J Neuroimmunol 1999, 98:49-56 [DOI] [PubMed] [Google Scholar]
- 13.Lassmann H: The pathology of multiple sclerosis and its evolution. Philos Trans R Soc Lond B Biol Sci 1999, 354:1635-1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hickey WF: The pathology of multiple sclerosis: a historical perspective. J Neuroimmunol 1999, 98:37-44 [DOI] [PubMed] [Google Scholar]
- 15.Thorpe JW, Kidd D, Moseley IF, Thompson AJ, MacManus DG, Compston DA, McDonald WI, Miller DH: Spinal MRI in patients with suspected multiple sclerosis and negative brain MRI. Brain 1996, 119:709-714 [DOI] [PubMed] [Google Scholar]
- 16.Thompson AJ, Polman CH, Miller DH, McDonald WI, Brochet B, Filippi MMX, De Sa J: Primary progressive multiple sclerosis. Brain 1997, 120:1085-1096 [DOI] [PubMed] [Google Scholar]
- 17.Nakashima I, Fujihara K, Okita N, Takase S, Itoyama Y: Clinical and MRI study of brain stem and cerebellar involvement in Japanese patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 1999, 67:153-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brocke S, Gijbels K, Steinman L: Experimental autoimmune encephalomyelitis in the mouse. Cohen IR Miller A eds. Autoimmune Disease Models, a Guidebook. 1994, :pp 1-14 Academic Press, New York [Google Scholar]
- 19.Encinas JA, Weiner HL, Kuchroo VK: Inheritance of susceptibility to experimental autoimmune encephalomyelitis. J Neurosci Res 1996, 45:655-669 [DOI] [PubMed] [Google Scholar]
- 20.Brown AM, McFarlin DE: Relapsing experimental allergic encephalomyelitis in the SJL/J mouse. Lab Invest 1981, 45:278-284 [PubMed] [Google Scholar]
- 21.Blankenhorn EP, Stranford SA: Genetic factors in demyelinating diseases: genes that control demyelination due to experimental allergic encephalomyelitis (EAE) and Theiler’s murine encephalitis virus. Reg Immunol 1992, 4:331-343 [PubMed] [Google Scholar]
- 22.Wekerle H, Kojima K, Lannes-Vieira J, Lassmann H, Linington C: Animal models. Ann Neurol 1994, 36:S47-S53 [DOI] [PubMed] [Google Scholar]
- 23.Tuohy VK, Sobel RA, Lees MB: Myelin proteolipid protein-induced experimental allergic encephalomyelitis. Variations of disease expression in different strains of mice. J Immunol 1988, 140:1868-1873 [PubMed] [Google Scholar]
- 24.Raine CS, Barnett LB, Brown A, Behar T, McFarlin DE: Neuropathology of experimental allergic encephalomyelitis in inbred strains of mice. Lab Invest 1980, 43:150-157 [PubMed] [Google Scholar]
- 25.Brown A, McFarlin DE, Raine CS: Chronologic neuropathology of relapsing experimental allergic encephalomyelitis in the mouse. Lab Invest 1982, 46:171-185 [PubMed] [Google Scholar]
- 26.Butterfield RJ, Blankenhorn EP, Roper RJ, Zachary JF, Doerge RW, Sudweeks J, Rose J, Teuscher C: Genetic analysis of disease subtypes and sexual dimorphisms in mouse experimental allergic encephalomyelitis (EAE): relapsing/remitting and monophasic remitting/nonrelapsing EAE are immunogenetically distinct. J Immunol 1999, 162:3096-3102 [PubMed] [Google Scholar]
- 27.Zeng ZB: Precision mapping of quantitative trait loci. Genetics 1994, 136:1457-1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng ZB: Theoretical basis for separation of multiple linked gene effects in mapping quantitative trait loci. Proc Natl Acad Sci USA 1993, 90:10972-10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doerge RW, Zeng ZB, Weir BS: Statistical issues in the search for genes affecting quantitative traits in experimental populations. Stat Sci 1997, 12:195-219 [Google Scholar]
- 30.Korngold R, Feldman A, Rorke LB, Lublin FD, Doherty PC: Acute experimental allergic encephalomyelitis in radiation bone marrow chimeras between high and low susceptible strains of mice. Immunogenetics 1986, 24:309-315 [DOI] [PubMed] [Google Scholar]
- 31.Teuscher C, Hickey WF, Korngold R: An analysis of the role of tumor necrosis factor in the phenotypic expression of actively induced experimental allergic orchitis and experimental allergic encephalomyelitis. Clin Immunol Immunopathol 1990, 54:442-453 [DOI] [PubMed] [Google Scholar]
- 32.Sudweeks JD, Todd JA, Blankenhorn EP, Wardell BB, Woodward SR, Meeker ND, Estes SS, Teuscher C: Locus controlling Bordetella pertussis-induced histamine sensitization (Bphs), an autoimmune disease-susceptibility gene, maps distal to T-cell receptor beta-chain gene on mouse chromosome 6. Proc Natl Acad Sci USA 1993, 90:3700-3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lander E, Green P, Abrahamson J, Barlow A, Daley M, Lincoln S, Newburg L: MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1987, 1:174-181 [DOI] [PubMed] [Google Scholar]
- 34.Lincoln S, Daly M, Lander E: Constructing Genetic Maps with MAPMAKER/EXP 3.0. Whitehead Institute Technical Report. 3rd ed., Cambridge, MA, 1992
- 35.Basten CJ, Weir BS, Zeng ZB: QTL Cartographer: A Reference Manual and Tutorial for QTL Mapping. 1997. North Carolina State University, Raleigh, NC, Department of Statistics
- 36.Churchill GA, Doerge RW: Empirical threshold values for quantitative trait mapping. Genetics 1994, 138:963-971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butterfield RJ, Sudweeks JD, Blankenhorn EP, Korngold R, Marini JC, Todd JA, Roper RJ, Teuscher C: New genetic loci that control susceptibility and symptoms of experimental allergic encephalomyelitis in inbred mice. J Immunol 1998, 161:1860-1867 [PubMed] [Google Scholar]
- 38.Teuscher C, Butterfield RJ, Ma RZ, Zachary JF, Doerge RW, Blankenhorn EP: Sequence polymorphisms in the chemokines Scya1 (TCA-3), Scya2 (monocyte chemoattractant protein (MCP)-1), and Scya12 (MCP-5) are candidates for eae7, a locus controlling susceptibility to monophasic remitting/nonrelapsing experimental allergic encephalomyelitis. J Immunol 1999, 163:2262-2266 [PubMed] [Google Scholar]
- 39.Hogancamp WE, Rodriguez M, Weinshenker BG: The epidemiology of multiple sclerosis. Mayo Clin Proc 1997, 72:871-878 [DOI] [PubMed] [Google Scholar]
- 40.Compston A: The genetic epidemiology of multiple sclerosis. Philos Trans R Soc Lond B Biol Sci 1999, 354:1623-1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duquette P, Pleines J, Girard M, Charest L, Senecal-Quevillon M, Masse C: The increased susceptibility of women to multiple sclerosis. Can J Neurol Sci 1992, 19:466-471 [PubMed] [Google Scholar]
- 42.Bebo BF, Jr, Zelinka-Vincent E, Adamus G, Amundson D, Vandenbark AA, Offner H: Gonadal hormones influence the immune response to PLP 139–151 and the clinical course of relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol 1998, 84:122-130 [DOI] [PubMed] [Google Scholar]
- 43.Cua DJ, Hinton DR, Kirkman L, Stohlman SA: Macrophages regulate induction of delayed-type hypersensitivity and experimental allergic encephalomyelitis in SJL mice. Eur J Immunol 1995, 25:2318-2324 [DOI] [PubMed] [Google Scholar]
- 44.Whitacre CC, Reingold SC, O’Looney PA: A gender gap in autoimmunity. Science 1999, 283:1277-1278 [DOI] [PubMed] [Google Scholar]
- 45.Homo-Delarche F, Fitzpatrick F, Christeff N, Nunez EA, Bach JF, Dardenne M: Sex steroids, glucocorticoids, stress and autoimmunity. J Steroid Biochem Mol Biol 1991, 40:619-637 [DOI] [PubMed] [Google Scholar]
- 46.Dalal M, Kim S, Voskuhl RR: Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J Immunol 1997, 159:3-6 [PubMed] [Google Scholar]
- 47.Kim S, Voskuhl RR: Decreased IL-12 production underlies the decreased ability of male lymph node cells to induce experimental autoimmune encephalomyelitis. J Immunol 1999, 162:5561-5568 [PubMed] [Google Scholar]
- 48.Bebo BF, Jr, Schuster JC, Vandenbark AA, Offner H: Androgens alter the cytokine profile and reduce encephalitogenicity of myelin-reactive T cells. J Immunol 1999, 162:35-40 [PubMed] [Google Scholar]
- 49.Blankenhorn EP, Butterfield RJ, Rigby R, Cort L, Giambrone D, McDermott R, McEntee K, Solowski N, Meeker ND, Zachary JF, Doerge RW, Teuscher C: Genetic analysis of the influence of pertussis toxin on EAE susceptibility: an environmental agent can override genetic checkpoints. J Immunol 2000, 164:3420-3425 [DOI] [PubMed] [Google Scholar]
- 50.Kuokkanen S, Sundvall M, Terwilliger JD, Tienari PJ, Wikstrom J, Holmdahl R, Pettersson U, Peltonen L: A putative vulnerability locus to multiple sclerosis maps to 5p14–p12 in a region syntenic to the murine locus Eae2. Nat Genet 1996, 13:477-480 [DOI] [PubMed] [Google Scholar]
- 51.Barcellos LF, Thomson G, Carrington M, Schafer J, Begovich AB, Lin P, Xu XH, Min BQ, Marti D, Klitz W: Chromosome 19 single-locus and multilocus haplotype associations with multiple sclerosis. Evidence of a new susceptibility locus in Caucasian and Chinese patients. JAMA 1997, 278:1256-1261 [PubMed] [Google Scholar]
- 52.Kuokkanen S, Gschwend M, Rioux JD, Daly MJ, Terwilliger JD, Tienari PJ, Wikstrom J, Palo J, Stein LD, Hudson TJ, Lander ES, Peltonen L: Genomewide scan of multiple sclerosis in Finnish multiplex families. Am J Hum Genet 1997, 61:1379-1387 [DOI] [PMC free article] [PubMed] [Google Scholar]