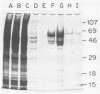
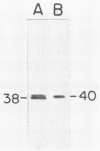
Abstract
A fragment of the nifH gene was amplified from natural populations of Trichodesmium spp. and cloned into a maltose-binding protein (MBP) expression vector. The peptide product of the amplified 359-bp fragment of nifH was cleaved from the fusion protein, purified, and used to generate a specific antibody to the Fe protein of nitrogenase. The antiserum recognized the MBP-nitrogenase fusion protein and the cleaved nif peptide product but not MBP. The antibody cross-reacted with nitrogenase from natural populations of Trichodesmium spp. from the Caribbean Sea and with a cultured isolate from the Kuroshio waters (Trichodesmium sp. strain NIBB1067). The same nifH fragment was amplified, cloned, and sequenced from Trichodesmium sp. strain NIBB1067 and was found to be 98% identical at both the protein and DNA levels to nifH from the Caribbean populations. Three of the six nucleotide differences between the Trichodesmium sp. strain NIBB1067 and the Trichodesmium spp. nifH sequence had also been found in a second sequence from the natural populations, indicating either that there is more than one strain of Trichodesmium sp. in natural assemblages or that there are multiple copies of nifH in the genome. This DNA fragment, which is easily amplified with the polymerase chain reaction, may provide a good indicator of species relatedness without requiring extensive cloning or sequencing. Furthermore, the use of the polymerase chain reaction in combination with a MBP protein fusion vector provides a rapid method for production of highly specific sera, starting with a small amount of DNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capone D. G., O'neil J. M., Zehr J., Carpenter E. J. Basis for Diel Variation in Nitrogenase Activity in the Marine Planktonic Cyanobacterium Trichodesmium thiebautii. Appl Environ Microbiol. 1990 Nov;56(11):3532–3536. doi: 10.1128/aem.56.11.3532-3536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Postgate J. R. Nitrogenase. Nature. 1974 Jun 28;249(460):805–810. doi: 10.1038/249805a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Purification and properties of nitrogenase from Rhodospirillum rubrum, and evidence for phosphate, ribose and an adenine-like unit covalently bound to the iron protein. Biochem J. 1978 Oct 1;175(1):251–259. doi: 10.1042/bj1750251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina C. V., Riggs P. D., Grandea A. G., 3rd, Slatko B. E., Moran L. S., Tagliamonte J. A., McReynolds L. A., Guan C. D. An Escherichia coli vector to express and purify foreign proteins by fusion to and separation from maltose-binding protein. Gene. 1988 Dec 30;74(2):365–373. doi: 10.1016/0378-1119(88)90170-9. [DOI] [PubMed] [Google Scholar]
- Mevarech M., Rice D., Haselkorn R. Nucleotide sequence of a cyanobacterial nifH gene coding for nitrogenase reductase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6476–6480. doi: 10.1073/pnas.77.11.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl H. W., Priscu J. C., Brawner D. L. Immunochemical localization of nitrogenase in marine trichodesmium aggregates: relationship to n(2) fixation potential. Appl Environ Microbiol. 1989 Nov;55(11):2965–2975. doi: 10.1128/aem.55.11.2965-2975.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr J. P., McReynolds L. A. Use of degenerate oligonucleotides for amplification of the nifH gene from the marine cyanobacterium Trichodesmium thiebautii. Appl Environ Microbiol. 1989 Oct;55(10):2522–2526. doi: 10.1128/aem.55.10.2522-2526.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Guan C., Li P., Riggs P. D., Inouye H. Vectors that facilitate the expression and purification of foreign peptides in Escherichia coli by fusion to maltose-binding protein. Gene. 1988 Jul 15;67(1):21–30. doi: 10.1016/0378-1119(88)90004-2. [DOI] [PubMed] [Google Scholar]