Abstract
Brain abscess represents the infectious disease sequelae associated with the influx of inflammatory cells and activation of resident parenchymal cells in the central nervous system. However, the immune response leading to the establishment of a brain abscess remains poorly defined. In this study, we have characterized cytokine and chemokine expression in an experimental brain abscess model in the rat during the acute stage of abscess development. RNase protection assay revealed the induction of the proinflammatory cytokines interleukin (IL)-1α, IL-1β, IL-6, and tumor necrosis factor-α as early as 1 to 6 hours after Staphylococcus aureus exposure. Evaluation of chemokine expression by reverse transcription-polymerase chain reaction demonstrated enhanced levels of the CXC chemokine KC 24 hours after bacterial exposure, which correlated with the appearance of neutrophils in the abscess. In addition, two CC chemokines, monocyte chemoattractant protein-1 and macrophage inflammatory protein-1α were induced within 24 hours after S. aureus exposure and preceded the influx of macrophages and lymphocytes into the brain. Analysis of abscess lesions by in situ hybridization identified CD11b+ cells as the source of IL-1β in response to S. aureus. Both intercellular adhesion molecule-1 and platelet endothelial cell adhesion molecule expression were enhanced on microvessels in S. aureus but not sterile bead-implanted tissues at 24 and 48 hours after treatment. These results characterize proinflammatory cytokine and chemokine expression during the early response to S. aureus in the brain and provide the foundation to assess the functional significance of these mediators in brain abscess pathogenesis.
Brain abscess is a focal, intracerebral lesion that begins as a localized area of cerebritis and develops into a collection of pus surrounded by a well-vascularized capsule. The end result of a brain abscess includes the replacement of the abscessed area with a fibrotic scar, loss of brain tissue by surgical removal, or abscess rupture and death. Brain abscesses represent a serious medical problem accounting for approximately 1 in 10,000 hospital admissions in the United States. 1 The leading etiological agents of brain abscess are the streptococcal strains and Staphylococcus aureus. 2,3 Our laboratory has developed an experimental brain abscess model in the rat 4 that allows for dissection of the basic cellular and immunological mechanisms involved in the progression and resolution of brain abscesses.
The rat brain abscess model closely mimics human disease in that the abscess progresses through a series of well-defined stages. In the rat, each stage is defined on the basis of predominant cell types and the histological appearance of the abscess. 4 In the human, these stages have been identified using CT scanning techniques. 1 The early stage, or early cerebritis, occurs from days 1 to 3 and is typified by neutrophil accumulation, tissue necrosis, and edema. The intermediate, or late cerebritis stage, occurs from days 4 to 9 and is associated with a predominant macrophage and lymphocyte infiltrate. The final, or capsule, stage occurs from day 10 onward and is associated with the formation of a well-vascularized abscess wall that sequesters the lesion and protects the surrounding normal brain parenchyma. Myofibroblasts are found associated with the developing abscess wall at this stage and plasma cells predominate the lesion. Currently, it is not clear what cells are responsible for mediating the immunopathological response to S. aureus in the brain.
Chemokines represent a family of low molecular weight chemotactic cytokines, classified into groups based on the presence and position of conserved cysteine residues. 5-7 CXC or α chemokines function as neutrophil and lymphocyte chemoattractants and prototypical family members include IL-8, KC (rodent homologue of growth related oncogene-α), interferon-inducible protein-10 kd (IP-10), and monokine induced by interferon γ (MIG). CC or β chemokines are chemotactic for monocytes and T cells and include such members as macrophage inflammatory protein-1α (MIP-1α), monocyte chemoattractant proteins (MCPs) 1–5, and regulated on activation T cell expressed and secreted (RANTES). Chemokines play an important role in recruiting leukocytes into areas of active inflammation. They are synthesized locally at sites of inflammation and establish a concentration gradient to which target cell populations migrate. Several studies have examined the expression of chemokines in response to central nervous system (CNS) insults such as stab wound injury in the brain 8,9 and experimental autoimmune encephalomyelitis (EAE). 10-12 In these models, KC, IP-10, MCP-1, and MIP-1α are up-regulated after injury. However, the signals responsible for the initial recruitment of inflammatory cells into brain abscesses have not yet been defined.
In this report, we characterize chemokines and cytokines that are expressed immediately after the introduction of S. aureus into the brain. Several proinflammatory cytokines, including IL-1α, IL-1β, IL-6, and tumor necrosis factor-α (TNF-α), were found to be induced. Consistent with the observed neutrophil influx into early abscess lesions, KC expression was enhanced within 24 hours after S. aureus exposure. In addition, IP-10, MCP-1, and MIP-1α expression were enhanced in abscesses, preceding the observed influx of macrophages and lymphocytes. In situ hybridization revealed that CD11b+ cells produce IL-1β after S. aureus exposure. Analysis of adhesion molecule expression revealed enhanced levels of both intercellular adhesion molecule-1 (ICAM-1) and platelet endothelial cell adhesion molecule (PECAM) on vessels associated with S. aureus but not sterile bead-implanted tissues. These findings characterize cytokine and chemokine expression in early brain abscess lesions, and provide a basis for future studies to assess the functional significance of individual mediators in abscess pathogenesis.
Materials and Methods
Preparation of S. aureus-Laden Agarose Beads
Live S. aureus were encapsulated in agarose beads before implantation in the brain as previously described. 4 The use of agarose beads prevents widespread bacterial dissemination or rapid wound sterilization by the host. Briefly, a stock culture of coagulase-positive S. aureus obtained from the microbiology laboratory at Dartmouth Hitchcock Medical Center was grown overnight (16 hours) at 37°C in Luria-Bertani broth (Fisher Biotech, Fair Lawn, NJ). A total of 1 × 10 9 bacteria were added to a solution of 1.4% low melt agarose (type XII, Sigma, St. Louis, MO) at 40°C. The mixture was then added to rapidly swirling heavy mineral oil (Sigma) pre-warmed to 37°C and cooled to 0°C on crushed ice. Beads were washed four times in 1× DPBS (Mediatech Cellgro, Herndon, VA) to remove mineral oil. Beads with dimensions between 50 and 100 μm, as determined by phase contrast microscopy, were used for implantation into the brain. Sterile beads were prepared in the same manner, without bacteria. The bacterial viability or sterility of bead preparations was confirmed by overnight culture in LB medium.
Animals and Generation of Experimental Brain Abscesses
Adult female Lewis rats were obtained from Charles River Laboratories (Wilmington, MA). The animal use protocol has been approved by the Dartmouth College Institutional Animal Care and Use Committee. Anesthesia was provided by an i.p. injection of 60 mg/kg ketamine and 5 mg/kg xylazine on a body weight basis. A 1-cm longitudinal incision was made along the midline between the ear and the eye to expose the frontal sutures. A burr hole was drilled 5 mm caudal and 0.5 mm lateral to the frontal suture. A Hamilton syringe fitted with flexible tubing and a pulled, fine-tipped glass-fired pipet (diameter < 1 mm) was used to deliver beads into the brain parenchyma. A total of 5 μl of beads were placed 5 to 6 mm deep from the external surface of the calvarium to prevent reflux during injection. Using this approach, bacteria were reproducibly deposited into the head of the caudate or frontal lobe white matter. Control animals were implanted with sterile agarose beads. Previous studies have established that implanting sterile beads into the brain induces minimal inflammation and edema 4 (Kielian, unpublished observations). Incisions were closed using sterile wound clips. The mortality rate associated with abscess generation was minimal, with >95% of animals surviving the procedure.
Processing of Tissues for Immunostaining and RNA Analysis
To prepare tissues for immunohistochemistry, animals were perfusion-fixed with 4% paraformaldehyde in 0.1 mol/L phosphate buffer, pH 7.4. The brain was removed, postfixed in paraformaldehyde for 30 minutes, and washed in 0.2 mol/L phosphate buffer, pH 7.4, overnight. Tissues were cryoprotected in 30% sucrose and snap-frozen in OCT for immunohistochemistry.
To prepare tissues for RNA isolation, samples of brain tissue containing S. aureus or sterile beads were snap-frozen in liquid N2 and stored at −70°C until RNA extraction was performed.
In Situ Hybridization (ISH) Probe Labeling
ISH was performed using digoxigenin (DIG)-labeled riboprobes specific for rat IL-1β and MCP-1. IL-1β and MCP-1 cDNA fragments were amplified by reverse transcription-polymerase chain reaction (RT-PCR) from mRNA isolated from 24-hour abscess tissue using the following primers: IL-1β sense (5′-TTCAGGAAGGCAGTGTCA-3′), IL-1β anti-sense (5′-TCTTTGGGTATTGTTTGG-3′), MCP-1 sense (5′-ATGCAGGTCTCTGTCACGCTTC-3′), and MCP-1 anti-sense (5′-AGTTCTCTGTCATACTGGTCAC-3′). The size of products amplified by the IL-1β and MCP-1 primer pairs were 448 and 445 bp, respectively and verified by sequence analysis. DIG-labeled riboprobes were synthesized from cDNA templates subcloned into the PCR vector pGEM T (Promega, Madison, WI) using the DIG RNA labeling kit (Boehringer Mannheim, Indianapolis, IN) according to the manufacturer’s instructions. The efficiency and intensity of riboprobe labeling was compared with a DIG-labeled RNA standard using dot blot analysis.
ISH
Frozen sections of fixed tissue were mounted onto RNase-free polyL-lysine-treated glass slides and air-dried for 30 minutes at room temperature before use. Tissues were rehydrated in three changes of Tris-buffered saline (TBS), pH 7.4, before proteinase K treatment. Sections were permeabilized with proteinase K (10 μg/ml) in TBS with 2 mmol/L CaCl2 for 15 minutes at 37°C. Slides were rinsed with TBS at 4°C and transferred to a solution of 0.1 mol/L triethanolamine, pH 8.0, for 3 minutes, followed by incubation in 0.5% acetic anhydride for 10 minutes on a stir plate. Following two washes in TBS, tissues were dehydrated through a series of graded alcohols and allowed to air-dry. Before use, DIG-labeled riboprobes (100 ng/ml) were denatured for 5 minutes at 65°C in hybridization buffer (50% deionized formamide, 10% dextran sulfate, 100 μg/ml salmon sperm DNA, 2× standard saline citrate, 0.02% sodium dodecyl sulfate). Riboprobes were added to tissue sections, coverslipped, and incubated overnight (12–16 hours) at 55°C in a humidified chamber. The following day, slides were washed with increasing stringency using standard saline citrate. Next, tissues were treated with blocking reagent (Boehringer Mannheim) for 15 minutes at room temperature before incubation with alkaline phosphatase-conjugated anti-digoxigenin Fab fragments (1:500 dilution) for 1 hour at room temperature. Following three washes in TBS, slides were developed using a NBT/BCIP substrate (Boehringer Mannheim) at 4°C in the dark. The color reaction was terminated by extensive washes in ddH2O. Tissues were dehydrated through a series of graded alcohols and xylene before coverslipping.
Immunohistochemistry
Frozen sections of fixed tissue were processed for immunohistochemistry using the avidin-peroxidase method as previously described. 13 The following antibodies were used for adhesion molecule analysis: ICAM-1 (TLD-4C9) and PECAM (TLD-3A12; both from Serotec, Raleigh, NC), L-selectin, P-selectin, and vascular cell adhesion molecule-1 (VCAM-1; PharMingen, San Diego, CA), and the isotype control Abs MOPC-21 and UPC10 (mouse IgG1 and IgG2a, respectively, Sigma), and rabbit IgG (Vector Laboratories, Burlingame, CA). Astrocytes and monocytes/microglia were identified on the basis of GFAP (Dako, Carpenteria, CA) and CD11b (OX-42, Serotec) staining, respectively. Biotinylated secondary antibodies included anti-mouse IgG, anti-rabbit IgG (both from Vector Laboratories), or anti-hamster IgG (Caltag, South San Francisco, CA). Slides were developed using the substrate 3,3′-diaminobenzidene (DAB).
Cytokine Immunohistochemistry
Cells producing the proinflammatory cytokine IL-1β were identified by immunohistochemical staining as previously described. 14 Primary mouse anti-rat IL-1β (Serotec) and mouse IgG1 isotype control antibodies (MOPC-21, Sigma) were used to evaluate IL-1β protein expression in S. aureus- and sterile bead-implanted tissues. Biotinylated horse anti-mouse IgG (Vector Labs) was used with the ABC peroxidase reagent (Vector Elite ABC kit, Vector Labs) to detect IL-1β protein. Reaction products were developed using a DAB substrate (Vector Labs).
RNA Isolation and RT-PCR
Total RNA was isolated from brain tissue using the TriZol reagent (Gibco BRL, Grand Island, NY) according to the manufacturer’s instructions. Before use in RT-PCR, RNA samples were treated with DNase I (Gibco BRL). RT-PCR was performed using a GeneAmp RNA PCR kit (Perkin Elmer, Foster City, CA) with 100 ng of total input RNA. After reverse transcription, cDNA templates were denatured for 105 seconds at 95°C, then amplified in 35 cycles of annealing (60°C, 30 seconds), extension (60°C, 30 seconds), and denaturing (95°C, 15 seconds). The final step was incubation for 7 minutes at 42°C. Each experimental sample was amplified in parallel using primers specific for β-actin to assess uniformity in the amount of input RNA and gel loading. Primers used for PCR amplification are listed in Table 1 ▶ .
Table 1.
Oligonucleotide Primers
| Primer | Product size (bp) | Sequence (5′ to 3′) |
|---|---|---|
| IP-10 (+) | 286 | GCTGCTGAGTCTGAGTGG |
| IP-10 (−) | CTGTGGCGAGTGGCTTCT | |
| KC (+) | 253 | GCTTGCCTTGACCCTGAA |
| KC (−) | TGAAACGCATCCACATCG | |
| MIP-2 (+) | 286 | GTCCTGCTCCTCCTGCTG |
| MIP-2 (−) | GCCCATGTTCTTCCTTCC | |
| Fractalkine (+) | 394 | CTGCTGGCTGGTTAGAGG |
| Fractalkine (−) | TTCACGGCACATCAAAGT | |
| MIP-1α (+) | 121 | CGCTCTGGAACGAAGTCT |
| MIP-1α (−) | AGGCTGCTGGTCTCAAAA | |
| MCP-1 (+) | 266 | ATGAGTCGGCTGGAGAAC |
| MCP-1 (−) | GTGGAAAAGAGAGTGGAT | |
| RANTES (+) | 127 | CACTCCCTGCTGCTTTGC |
| RANTES (−) | CACTTGGCGGTTCCTTCG | |
| β-actin (+) | 554 | TACAACCTCCTTGCAGCTCC |
| β-actin (−) | GGATCTTCATGAGGTAGTCTGTC |
(+), sense primer; (−), antisense primer; IP-10, gamma interferon inducible protein-10; KC, homolog of growth related oncogene-α; MIP-1α, macrophage inflammatory protein-1α; MCP-1, monocyte chemoattractant protein-1; RANTES, regulated on activation T cell expressed and secreted.
RNase Protection Assay
Cytokine expression in brain tissue was examined by RNase protection assay (RPA) using the RiboQuant RPA kit (PharMingen). The following multiprobe template sets were used, each specific for rat cytokines: CK-1, which detects transcripts for IL-1α and -1β, TNF-β, IL-3, IL-4, IL-5, IL-6, IL-10, TNF-α, IL-2, and interferon (IFN)-γ; and CK-3, which detects IFN-β, TNF-β, granulocyte-macrophage colony stimulating factor (GM-CSF), transforming growth factor (TGF)-β1, -β2, and -β3, lymphotoxin β (Ltβ), TNF-α, macrophage migration inhibitory factor (MIF), and IFN-γ. Both template sets contained probes for the housekeeping genes L32 and GAPDH to serve as internal controls for the assay. Probes were synthesized using [α-33P]UTP (New England Nuclear, Boston, MA) resulting in an average specific activity of 1 × 10 6 cpm/μl. The RNase protection procedure was carried out according to the manufacturer’s instructions using 5 to 10 μg per sample of total RNA. Each assay included normal Lewis rat brain RNA and yeast tRNA as controls. Products were resolved on a 6% acrylamide gel, dried, and exposed to film (Kodak BioMax MR, Rochester, NY).
Results
Chemokine Expression Associated with Brain Abscesses in Vivo
The signals responsible for the initial recruitment of inflammatory cells into brain abscesses have not yet been investigated. The predominant cell type present within early abscess lesions are neutrophils, whereas macrophages begin to accumulate at day 4 after bacterial exposure. 4 We examined the expression of several CXC and CC chemokines to define those that may play a role in the recruitment of neutrophils and macrophages into brain abscess lesions. Consistent with the observed neutrophil influx into early abscess lesions, KC expression was enhanced in animals implanted with S. aureus-containing beads at 24 hours, with levels remaining elevated up to 4 days (Figure 1A) ▶ . This finding suggests that KC may be important for neutrophil recruitment into brain abscesses in vivo. Similarly, the levels of IP-10, a CXC chemoattractant, were up-regulated in the brains of animals receiving S. aureus beads within 24 hours (Figure 1A) ▶ . This increase in IP-10 expression preceded the appearance of macrophages and lymphocytes into the abscess.
Figure 1.
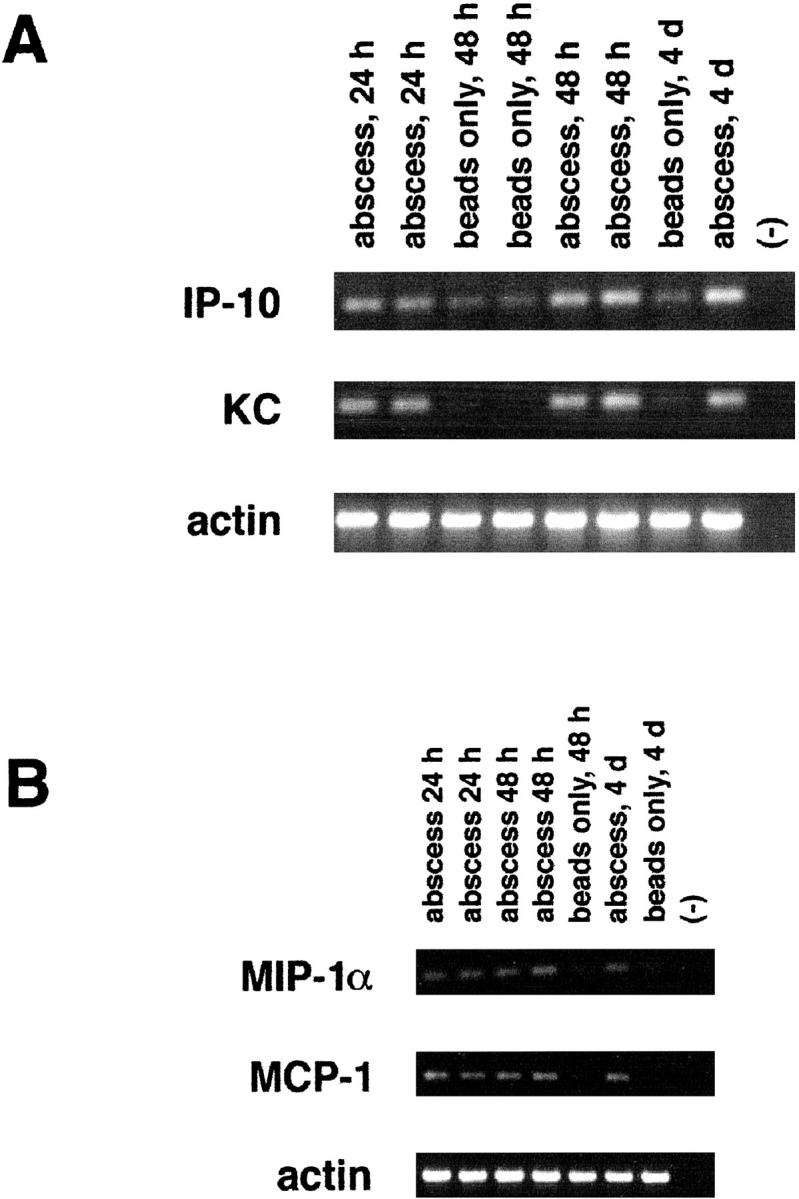
CXC and CC chemokine expression are up-regulated in acute experimental brain abscess. Adult Lewis rats were implanted with S. aureus-containing or sterile beads. Tissues were collected from the lesion site of individual animals at the indicated time points for RNA extraction and RT-PCR. A total of 100 ng of DNase I-treated RNA was added to each RT reaction. A total of 35 cycles of PCR were conducted using primers specific for either the CXC chemokines KC and IP-10 (A), or the CC chemokines MIP-1α and MCP-1 (B). Amplification using primers specific for β-actin was included to account for uniformity in the amount of input RNA and gel loading. Primer controls are denoted by (−). “Abscess” refers to animals receiving S. aureus-containing agarose beads compared to those implanted with sterile beads.
In addition, we have examined the expression of three monocyte-attracting CC chemokines, including MIP-1α, MCP-1, and RANTES, by RT-PCR. As shown in Figure 1B ▶ , MCP-1 and MIP-1α were elevated within 24 hours after S. aureus exposure. This increase in CC chemokine expression precedes the first detectable accumulation of macrophages in the abscess, which occurs at days 4 and 5. 4 At time points before 24 hours, we found no significant differences in the levels of chemokine expression between S. aureus- and sterile bead-implanted tissues (data not shown). However, chemokine induction in response to sterile beads was transient, with levels beginning to decline by 24 hours compared to the continued expression of MCP-1, MIP-1α, KC, and IP-10 in S. aureus-implanted tissues. There was no significant difference in the amount of MIP-2, RANTES, or fractalkine expression between S. aureus- and sterile bead-implanted tissues (data not shown). Importantly, we were not able to detect any of the chemokines examined here in normal Lewis rat brain (data not shown). These results suggest that KC, IP-10, MIP-1α, and MCP-1 participate in inflammatory cell recruitment into experimental brain abscesses. In turn, newly recruited neutrophils and macrophages can amplify the immune response through the synthesis of proinflammatory cytokines and chemokines.
Proinflammatory Cytokines Are Expressed during the Acute Phase of Experimental Brain Abscesses in Vivo
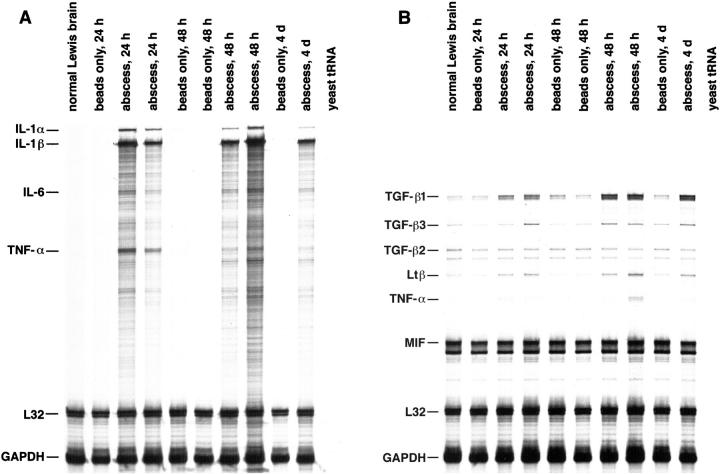
To define the array of cytokines produced during the early phase of brain abscess development, RNase protection assays (RPA) were performed on total RNA isolated from S. aureus- and sterile bead-implanted tissues. Figure 2A ▶ shows the proinflammatory cytokines that are expressed within 24 hours after S. aureus exposure. These include IL-1α, IL-1β, IL-6, and TNF-α. We also found elevated levels of TGF-β1 and lymphotoxin β (Ltβ) in abscess tissues at all time points examined (Figure 2B) ▶ . During the acute phase of abscess induction, we did not observe the expression of any T cell-derived cytokines, such as IL-2 and IFN-γ. This finding is consistent with the absence of a significant lymphocytic infiltrate early in abscess pathogenesis. S. aureus- and sterile bead-implanted tissues and normal Lewis rat brain were found to express equivalent amounts of mRNA for TGF-β2, TGF-β3, and MIF (Figure 2B) ▶ . Collectively, these findings reveal activation of a potent proinflammatory response to S. aureus in the brain.
Figure 2.
Proinflammatory cytokines are expressed during the acute phase of experimental brain abscess. Adult Lewis rats were implanted with S. aureus-containing or sterile beads. Tissues were collected from the lesion site of individual animals at the indicated time points for RNA extraction. A total of 10 μg of RNA from S. aureus- or sterile bead-implanted tissues was used in RPA with 33P-labeled ribroprobe templates. Controls included yeast tRNA and normal Lewis rat brain RNA. The housekeeping genes L32 and GAPDH serve as internal controls for the assay. A: RPA of S. aureus- or sterile bead-implanted tissues with the rat CK1 probe set. This probe set detects mRNAs for IL-1α and -1β, TNF-τ and -β, IL-2, -3, -4, -5, -6, and -10, and IFN-γ. B: RPA of S. aureus or sterile bead implanted tissues with the rat CK3 probe set. This probe set detects mRNAs for TNF-α and β, GM-CSF, IFN-β and -γ, TGF-β1, -β2, and -β3, Ltβ, and MIF. The identity of RNase-protected bands in the experimental samples are determined by plotting a standard curve of the migration distance versus log nucleotide length of the undigested probes (not shown). The size of experimental mRNAs are extrapolated from this standard curve and are denoted at the left.
Kinetics of Proinflammatory Cytokine Induction after S. aureus Exposure
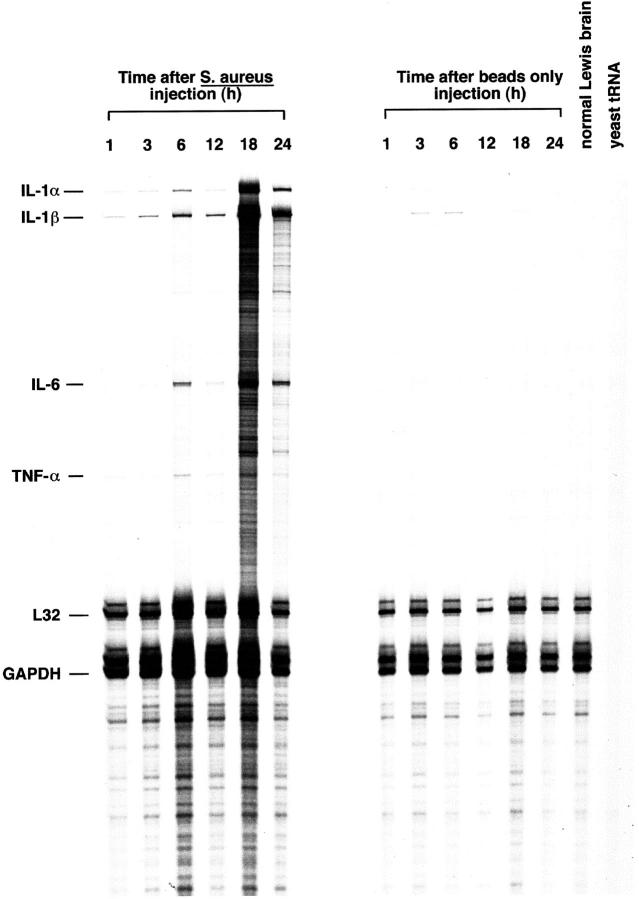
To define the early kinetics of proinflammatory cytokine induction in response to S. aureus, RNA was isolated from S. aureus- or sterile bead-implanted tissues from 1 to 24 hours after treatment and evaluated by RPA. As shown in Figure 3 ▶ , the induction of IL-1α and β was immediate, with detectable levels observed as early as 1 hour after S. aureus exposure. The amount of IL-1α and β detected in S. aureus-implanted tissues progressively increased with time and still remained strong at 4 days (Figures 3 and 2A) ▶ ▶ . Sterile beads induced a transient increase in IL-1α and -1β that was first evident 3 hours after treatment. However, the amount of IL-1α and -1β detected in sterile bead-implanted tissues was significantly less than that observed after S. aureus exposure, and the response was short-lived in the former, with expression disappearing within 18 hours (Figure 3) ▶ . Both TNF-α and IL-6 were induced within 3 to 6 hours after S. aureus exposure with levels remaining elevated until 4 days (Figures 3 and 2A) ▶ ▶ . As expected, normal Lewis rat brain did not express any of these proinflammatory cytokines (Figure 3) ▶ . The immediate induction of IL-1α and β, TNF-α, and IL-6 suggest that these mediators play an important role in the early response to S. aureus in the brain.
Figure 3.
S. aureus leads to the rapid induction of proinflammatory cytokine gene expression. Adult Lewis rats were implanted with S. aureus-containing or sterile beads. Tissues were collected from the lesion site of individual animals at the indicated time points for RNA extraction. A total of 5 μg of RNA from S. aureus or sterile bead implanted tissues was used in RPA with 33P-labeled ribroprobe templates. Controls included yeast tRNA and normal Lewis rat brain RNA. The housekeeping genes L32 and GAPDH serve as internal controls for the assay. The identity of RNase-protected bands in the experimental samples are determined by plotting a standard curve of the migration distance versus log nucleotide length of the undigested probes (not shown). The size of experimental mRNAs are extrapolated from this standard curve and are denoted at the left.
Identification of Cell Types Producing IL-1β in Early Brain Abscess Lesions
We have established by RPA that S. aureus leads to the induction of proinflammatory cytokine and chemokine expression in the brain. In an attempt to identify the cellular source(s) of IL-1β, ISH and immunohistochemical staining of S. aureus-implanted tissues were conducted. IL-1β was selected because of its rapid induction and high levels of mRNA expression based on RPA analysis. As shown in Figure 4 ▶ , IL-1β-positive cells were found to colocalize with CD11b (OX-42) staining, suggesting that they are of the monocyte/macrophage lineage (ie, resident microglia and infiltrating monocytes) or infiltrating polymorphonuclear leukocytes (PMNs). Specificity was confirmed by the inability of an IL-1β sense riboprobe to react with abscess tissue (Figure 4B) ▶ . MCP-1 was not detected in abscess lesions by ISH, possibly a result of the decreased sensitivity of this method compared to RT-PCR (data not shown). To confirm the presence of IL-1β protein within the developing abscess, tissues were stained with an IL-1β-specific antibody. As shown in Figure 5 ▶ , numerous IL-1β-positive cells were associated with the area of active inflammation in S. aureus-implanted tissues at both 24 and 48 hours. In contrast, no IL-1β-positive cells were observed in tissues receiving sterile beads (Figure 5) ▶ . These results indicate that IL-1β mRNA and protein are abundantly expressed within early abscess lesions by CD11b-positive cells.
Figure 4.
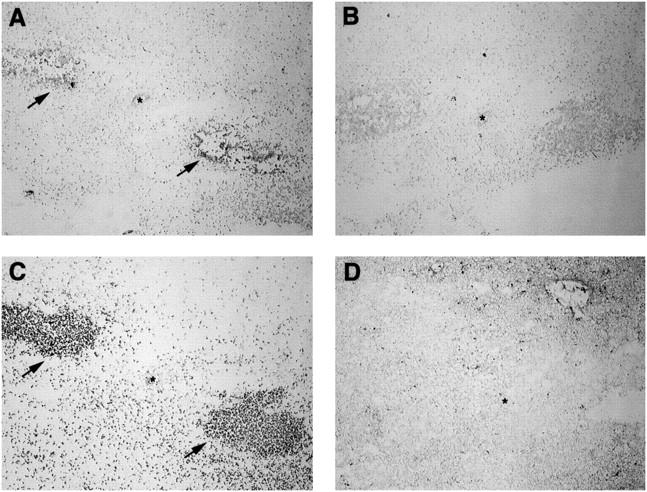
IL-1β-producing cells colocalize with CD11b (OX-42)-positive cells in brain abscess lesions. Adult Lewis rats were implanted with S. aureus-containing beads and abscess lesions collected at 24 hours for in situ hybridization (ISH) and immunohistochemical (IHC) analysis. Serial sections (5 μm) of a 24-hour abscess lesion were subjected to ISH analysis with IL-1β anti-sense (A) or IL-1β sense (B) DIG-labeled riboprobes, or IHC staining with antibodies specific for rat CD11b (OX-42; C) or GFAP (D). Asterisks denote the lumen of a small vessel in each serial section. Arrowheads in A and C denote IL-1β+ and CD11b+ cells, respectively. Original magnification, ×22.5.
Figure 5.
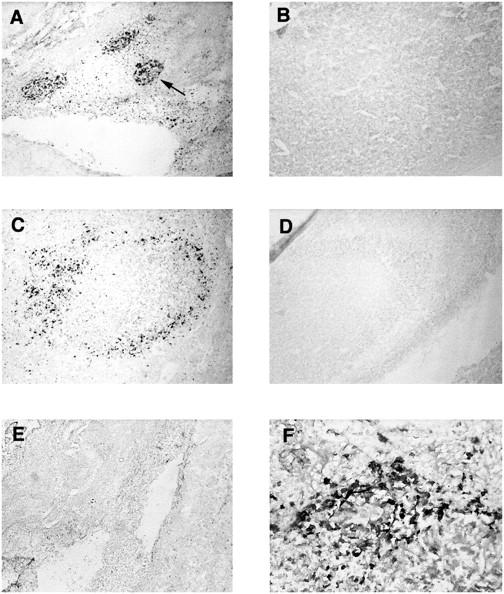
IL-1β protein is specifically expressed within S. aureus injected tissues. Adult Lewis rats were implanted with S. aureus-containing or sterile beads. Brain sections from animals perfusion-fixed with 4% paraformaldehyde/0.1 mol/L phosphate buffer were stained with antibody specific for IL-1β (A−D, F) or an isotype-matched control antibody (E) for immunohistochemical analysis. A and C: S. aureus-implanted tissues at 24 and 48 hours, respectively, stained with anti-IL-1β. B and D: Sterile bead-implanted tissues at 24 and 48 hours, respectively, stained with anti-IL-1β. E: S. aureus implanted tissue at 24 h stained with an isotype-matched control antibody F: higher magnification (×100) of IL-1β+ cells within S. aureus implanted tissue at 24 hours. The arrow in A depicts one of the three IL-1β+ foci within the area of active inflammation. Original magnification (A−E), ×22.5.
Characterization of Adhesion Molecule Expression on Brain Microvascular Endothelial Cells after S. aureus Exposure
Because proinflammatory cytokines such as TNF-α and IL-1 are known to induce adhesion molecule expression on endothelial cells 15-17 and because these cytokines are induced soon after S. aureus exposure, we were interested in examining adhesion molecule expression in S. aureus- and sterile bead-implanted tissues. The activation-induced adhesion molecules ICAM-1, P-selectin, and VCAM were evaluated, as were PECAM and the leukocyte activation marker L-selectin. As shown in Figure 6 ▶ , S. aureus induced PECAM expression on brain vascular endothelial cells at 24 and 48 hours, whereas vessels associated with sterile bead-implanted tissues were negative. Small vessels in both S. aureus- and sterile bead-implanted tissues were found to express detectable levels of ICAM-1 (Figure 6) ▶ , however, the distribution of ICAM-1-positive vessels was distinct. ICAM-1-positive vessels in S. aureus-implanted tissues were distributed diffusely throughout the injected hemisphere, compared to the restriction of ICAM-1-positive vessels associated with sterile beads to the vicinity of the stab wound. Both PECAM and ICAM-1 are important adhesion molecules for the extravasation of neutrophils and monocytes into tissues, 17,18 the predominant cell types found infiltrating early brain abscess lesions. Vessels in both S. aureus- and sterile bead-implanted tissues were negative for VCAM and P-selectin during the period examined, and L-selectin expression was not detected on infiltrating leukocytes in either tissue (data not shown). These results reveal the induction/up-regulation of PECAM and ICAM-1 expression on brain vascular endothelial cells in response to S. aureus, and suggest that they are critical to the evolving pathological process.
Figure 6.
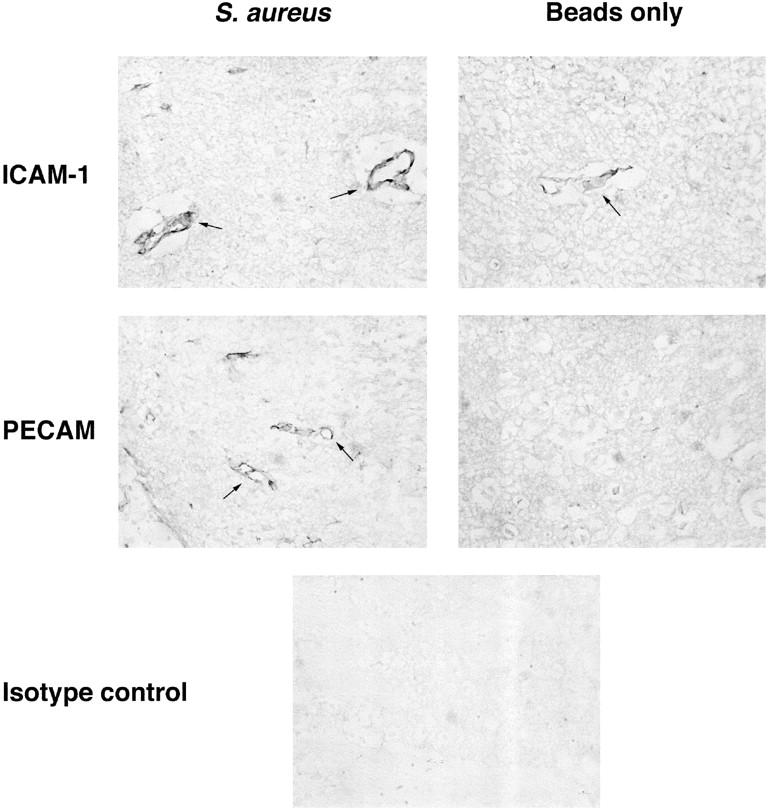
Expression of both PECAM and ICAM-1 is up-regulated on vascular endothelium in early brain abscess lesions. Adult Lewis rats were implanted with S. aureus-containing or sterile beads. Brain sections from animals perfusion-fixed with 4% paraformaldehyde/0.1 mol/L phosphate buffer were stained with antibodies specific for PECAM or ICAM-1 or an isotype-matched control antibody for immunohistochemical analysis. Images shown represent tissues collected at 48 hours after implantation of S. aureus or sterile beads. Arrows delineate vascular endothelium expressing PECAM or ICAM-1. Original magnification, ×100.
Discussion
Identification of the cytokines and chemokines expressed during the acute phase of brain abscess pathogenesis has not previously been investigated. In this paper, we have characterized a number of cytokines and chemokines that are induced immediately after the introduction of S. aureus into the brain.
Within 24 hours after S. aureus implantation, KC, IP-10, MIP-1α, and MCP-1 expression were elevated compared to animals implanted with sterile agarose beads. We found that the introduction of sterile beads into the brain induced transient chemokine expression, which began to decline around 24 hours. This effect may be a consequence of direct damage to the blood-brain barrier during bead administration. In contrast, chemokine levels in S. aureus-implanted brain remained elevated up to 4 days, suggesting the rapid and persistent establishment of a strong chemotactic gradient that parallels the continued presence of bacteria and their by-products. It appears as if the sustained production of chemokines is required for leukocyte entry into tissues because, although sterile beads induce transient chemokine expression, there is no accumulation of inflammatory cells in these tissues 4 (Kielian, unpublished observations). The chemokines we have detected in our brain abscess model most likely serve as signals for recruitment of neutrophils and monocytes, cells which are important for effective bacterial neutralization and eventual abscess resolution. Chemokines could also serve to facilitate the phagocytic function of glial cells, aiding in the destruction of S. aureus and eliminating debris from the abscess site. In addition to serving as chemoattractants, chemokines can activate target cell populations by inducing a rapid and transient calcium flux. It is possible that chemokines induced in response to S. aureus serve to regulate mediator production in responding cell populations in the brain, although this remains to be determined.
Pyogenic bacteria, such as S. aureus, elicit a potent innate immune response typified by the production of proinflammatory cytokines such as IL-1α, IL-1β, TNF-α, and IL-6. 19,20 Indeed, we have detected these proinflammatory cytokines in the rat brain immediately after S. aureus exposure. These cytokines can also exert pleiotropic effects on cells within the CNS. For example, both IL-1β and TNF-α act as potent stimulators of cytokine and chemokine expression for both astrocytes and microglia. 21-24 TNF-α and IL-1 can also stimulate IL-6 production by astrocytes which, in turn, acts in an autocrine matter to potentiate IL-6 release. 25 Due to this complex regulation of proinflammatory mediators, it is not unreasonable to predict that if not tightly controlled, amplification of the immune response would result in damage to normal host tissue. Indeed, in addition to bacterial neutralization, the immune response induced by S. aureus leads to the destruction of a significant portion of normal brain tissue in proximity to the bacteria. Evidence in support of this is demonstrated by several studies revealing the detrimental effects of IL-1β, TNF-α, and IL-6 on the CNS. For example, in concert with IFN-γ, TNF-γ activates murine microglia to produce toxic reactive nitrogen intermediates. 26,27 Exposure of the CNS vasculature to IL-1β and TNF-α leads to increased adhesion and migration of inflammatory cells into the CNS and alterations in vascular permeability which may persist for several weeks. 28,29 TNF-α has been shown to indirectly mediate neuronal cell injury 30 and is toxic to oligodendrocytes. 31,32 Finally, overexpression of IL-6 in the CNS can lead to several detrimental conditions including reactive gliosis, neurodegeneration, breakdown of the blood-brain barrier, angiogenesis, and chronic expression of C3. 33,34 Studies are ongoing to directly assess the importance of individual proinflammatory cytokines in the initiation and progression of brain abscess using in vivo Ab neutralization experiments. Nevertheless, it is likely that a complex interaction exists between IL-1α, β, TNF-α, and IL-6, bacterial neutralization, and destruction of normal brain parenchyma.
Previous studies have described chemokine receptor expression on those cell types associated with brain abscesses in vivo. 35 For example, monocytes express CCR5 whose natural ligands include MIP-1α, MIP-1β, and RANTES. 6,7 Microglia 36-38 and astrocytes 39,40 have also been shown to express CCR5, which may contribute, in part, to the activation of these cells in areas immediately surrounding the developing abscess. One characteristic of chemokines is their promiscuity in terms of their ability to bind multiple receptors. In addition to CCR5, monocytes and microglia express CCR1 which also binds MIP-1α with high affinity. Both microglia and monocytes express CCR2, the only known receptor for MCP-1. We do not currently know whether CCR1, CCR2, and/or CCR5 are important for monocyte recruitment into evolving brain abscesses. Receptors for the proinflammatory cytokines TNF-α and IL-1 have been shown to be constitutively expressed on some neurons and glial cells in the rat brain. 41 Based on this pattern of receptor expression, it is intriguing to consider what effect IL-1 and TNF-α might exert on neurons in our brain abscess model. In this case, receptor expression may be detrimental, leading to neuronal cell death via signaling through TNFR. In addition to glial and neuronal cells, TNFR is expressed by monocytes/macrophages which are found infiltrating abscess lesions. 42,43 Activation of monocytes/macrophages via TNF-α-dependent signaling leads to the synthesis of nitric oxide 44 and cytokine and chemokine mediators 45-47 which can amplify the immune response. In the CNS, some neurons and astrocytes express IL-6R. However, the amount of IL-6R on astrocytes is not sufficient by itself to deliver an activation signal, and requires the presence of a soluble form of the IL-6R generated by shedding of the membrane-bound receptor or by mRNA alternative splicing. 25,48 Interestingly, astrocytes and sympathetic neurons can produce IL-6 and may respond to it in an autocrine/paracrine manner if sIL-6R is present, 25,48,49 a mechanism for amplifying IL-6 expression in the CNS. We are actively investigating proinflammatory cytokine receptor expression to identify which cell populations are competent to respond to the mediators detected in early brain abscess lesions.
The cell types responsible for proinflammatory cytokine and chemokine production in experimental brain abscesses remain to be defined. Their rapid induction suggests that the response is driven by resident CNS glia or cells associated with the ensuing inflammatory infiltrate (ie, neutrophils and/or monocytes/macrophages). Results from ISH analysis revealed that CD11b+ cells are the source of IL-1β within early brain abscesses. This population includes resident microglia and infiltrating monocytes and neutrophils. In agreement with our findings, it has been shown that human microglia can produce IL-1β, 50,51 and S. aureus has been shown to directly stimulate IL-1β production by neonatal rat microglia in vitro. 24 We have preliminary data (Kielian, unpublished observations) which demonstrates that S. aureus can directly induce neonatal mouse microglia and astrocytes to produce numerous proinflammatory cytokines and chemokines including IL-1β, TNF-α, IL-6, MCP-1, MIP-1α, MIP-1β, MIP-2, RANTES, and MIP-1α. Studies are ongoing to further identify the cell types within developing brain abscesses responsible for mediator production.
The question remains, what is the impetus driving abscess formation and can the progression of the abscess and immunopathological destruction of surrounding normal brain tissue be minimized by altering the production of proinflammatory cytokines? The chronic overexpression of proinflammatory cytokines initially induced in response to S. aureus may itself have a direct detrimental effect on the surrounding normal brain parenchyma, ultimately leading to tissue destruction. The uncontrolled amplification of the immune response to S. aureus can be explained by the autocrine/paracrine action of proinflammatory cytokines on resident glial cell populations in the brain. For example, IL-1 could stimulate astrocyte activation and production of TNF-α and IL-6, 21,52-54 whereas activated microglia produce IL-1 and TNF-α. 50,51 It is possible that a tissue toxic microenvironment might evolve from the robust production of proinflammatory cytokines immediately following exposure to S. aureus, as shown in this study. This overproduction of proinflammatory cytokines could result in a detrimental or cytocidal bystander effect, leading to the damage of surrounding normal brain parenchyma. Thus, the process of neural tissue necrosis may be underway before the arrival of large numbers of neutrophils. By controlling the amount of proinflammatory cytokine production in response to S. aureus, this could lead to bacterial neutralization while minimizing tissue destruction due to the chronic overactive immune response.
In summary, the results presented in this report establish the expression of several proinflammatory cytokines and chemokines within early brain abscess lesions which may participate in cell activation and amplification of the immune response initiated by S. aureus. Characterization of inflammatory mediators produced in response to S. aureus in the brain may lead to novel therapies for the clinical management of brain abscesses.
Acknowledgments
We thank Dr. Joyce DeLeo for providing the IL-1β antibody and critical reading of the manuscript, John Hutchins for image analysis, and Dr. William F. Wade for helpful discussions.
Footnotes
Address reprint requests to Tammy Kielian, Ph.D., Dartmouth Medical School, Department of Pathology, HB 7600, One Medical Center Drive, Lebanon, NH 03756. E-mail: tammy.kielian@dartmouth.edu.
Supported in part by a grant from the National Institutes of Health/National Institute of NDS (NS-27321) and The Hitchcock Foundation.
References
- 1.Wispelwey B, Dacey RG, Scheld WM: Brain abscess. Scheld WM Whitely RJ Durack DT eds. Infections of the Central Nervous System. 1991, :457-486 Raven Press, New York [Google Scholar]
- 2.Mathisen GE, Johnson JP: Brain abscess. Clinical Infect Dis 1997, 25:763-781 [DOI] [PubMed] [Google Scholar]
- 3.Townsend GC, Scheld WM: Infections of the central nervous system. Adv Internal Med 1998, 43:403-447 [PubMed] [Google Scholar]
- 4.Flaris N, Hickey WF: Development and characterization of an experimental model of brain abscess in the rat. Am J Pathol 1992, 141:1299-1307 [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Baruch A, Michiel DF, Oppenheim JJ: Signals and receptors involved in recruitment of inflammatory cells. J Biol Chem 1995, 270:11703-11706 [DOI] [PubMed] [Google Scholar]
- 6.Luster AD: Chemokines: chemotactic cytokines that mediate inflammation. New Engl J Med 1998, 338:436-445 [DOI] [PubMed] [Google Scholar]
- 7.Rollins BJ: Chemokines. Blood 1997, 90:909-928 [PubMed] [Google Scholar]
- 8.Glabinski AR, Balasingam V, Tani M, Kunkel SL, Strieter RM, Yong VW, Ransohoff RM: Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J Immunol 1996, 156:4363-4368 [PubMed] [Google Scholar]
- 9.Ghimikar RS, Lee YL, He TR, Eng LF: Chemokine expression in rat stab wound brain injury. J Neurosci Res 1996, 46:727-733 [DOI] [PubMed] [Google Scholar]
- 10.Karpus WJ, Lukacs NW, McRae BL, Strieter RM, Kunkel SL, Miller SD: An important role for the chemokine macrophage inflammatory protein-1α in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J Immunol 1995, 155:5003-5010 [PubMed] [Google Scholar]
- 11.Glabinski AR, Tani M, Strieter RM, Tuohy VK, Ransohoff RM: Synchronous synthesis of α- and β-chemokines by cells of diverse lineage in the central nervous system of mice with relapses of chronic experimental autoimmune encephalomyelitis. Am J Pathol 1997, 150:617-630 [PMC free article] [PubMed] [Google Scholar]
- 12.Ransohoff RM, Glabinski A, Tani M: Chemokines in immune-mediated inflammation of the central nervous system. Cytokine Growth Factor Rev 1996, 7:35-46 [DOI] [PubMed] [Google Scholar]
- 13.Hickey WF, Gonatas NK, Kimura H, Wilson DB: Identification and quantitation of T-lymphocyte subsets found in the spinal cord of Lewis rats with acute EAE. J Immunol 1983, 131:2805-2809 [PubMed] [Google Scholar]
- 14.Kielian T, Nagai E, Ikubo A, Rasmussen CA, Suzuki T: Granulocyte/macrophage-colony-stimulating factor released by adenovirally transduced CT26 cells leads to the local expression of macrophage inflammatory protein 1α and accumulation of dendritic cells at vaccination sites in vivo. Cancer Immunol Immunother 1999, 48:123-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vassalli P: The pathophysiology of tumor necrosis factors. Ann Rev Immunol 1992, 10:411-452 [DOI] [PubMed] [Google Scholar]
- 16.Wong D, Dorovini-Zis K: Upregulation of intercellular adhesion molecule-1 (ICAM-1) expression in primary cultures of human brain microvessel endothelial cells by cytokines and lipopolysaccharide. J Neuroimmunol 1992, 39:11-22 [DOI] [PubMed] [Google Scholar]
- 17.Bevilacqua MP: Endothelial-leukocyte adhesion molecules. Ann Rev Immunol 1993, 11:767-804 [DOI] [PubMed] [Google Scholar]
- 18.Butcher EC: Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell 1991, 67:1033-1036 [DOI] [PubMed] [Google Scholar]
- 19.Giese MJ, Sumner HL, Berliner JA, Mondino BJ: Cytokine expression in a rat model of Staphylococcus aureus endophthalmitis. Invest Ophthalmol Visual Sci 1998, 39:2785-2790 [PubMed] [Google Scholar]
- 20.Yao L, Berman JW, Factor SM, Lowy FD: Correlation of histopathologic and bacteriologic changes with cytokine expression in an experimental murine model of bacteremic Staphylococcus aureus infection. Infect Immun 1997, 65:3889-3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bethea JR, Chung IY, Sparacio SM, Gillespie GY, Benveniste EN: Interleukin-1 β induction of tumor necrosis factor-α gene expression in human astroglioma cells. J Neuroimmunol 1992, 36:179-191 [DOI] [PubMed] [Google Scholar]
- 22.Fontana A, Kristensen F, Dubs R, Gemsa D, Weber E: Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J Immunol 1982, 129:2413-2419 [PubMed] [Google Scholar]
- 23.Giulian D, Baker TJ: Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci 1986, 6:2163-2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giulian D, Corpuz M: Microglial secretion products and their impact on the nervous system. Adv Neurol 1993, 59:315-320 [PubMed] [Google Scholar]
- 25.Van Wagoner NJ, Oh J-W, Repovic P, Benveniste EN: Interleukin-6 (IL-6) production by astrocytes: autocrine regulation by IL-6 and the soluble IL-6 receptor. J Neurosci 1999, 19:5236-5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK: Activated microglia-mediated neuronal cell injury via a nitric oxide mechanism. J Immunol 1992, 149:2736-2741 [PubMed] [Google Scholar]
- 27.Zielasek J, Tausch M, Toyka KV, Hartung H-P: Production of nitrite by neonatal rat microglial cells/brain macrophages. Cell Immunol 1992, 141:111-120 [DOI] [PubMed] [Google Scholar]
- 28.Hughes CCW, Male DK, Lantos PL: Adhesion of lymphocytes to cerebral microvascular cells: effects of interferon-τ, tumor necrosis factor and interleukin-1. Immunology 1988, 64:677-681 [PMC free article] [PubMed] [Google Scholar]
- 29.Martiney JA, Litwak MS, Berman JW, Arezzo JC, Brosnan CF: Pathophysiologic effect of IL-1β in the rabbit retina. Am J Pathol 1990, 137:1411-1423 [PMC free article] [PubMed] [Google Scholar]
- 30.Chao CC, Hu S: TNF-α potentiates glutamate neurotoxicity in human fetal brain cell cultures. Dev Neurosci 1994, 16:172-179 [DOI] [PubMed] [Google Scholar]
- 31.Selmaj KW, Raine CS: Tumor necrosis factor mediated myelin and oligodendrocyte damage in vitro. Ann Neurol 1988, 23:339-346 [DOI] [PubMed] [Google Scholar]
- 32.Robbins DS, Shirazi Y, Drysdale BE, Lieberman A, Shin HS, Shin ML: Production of cytotoxic factor for oligodendrocytes by stimulated astrocytes. J Immunol 1987, 139:2593-2597 [PubMed] [Google Scholar]
- 33.Barnum SR, Jones JL, Muller-Ladner U, Samimi A, Campbell IL: Chronic complement C3 gene expression in the CNS of transgenic mice with astrocyte-targeted interleukin-6 expression. Glia 1996, 18:107–117 [DOI] [PubMed]
- 34.Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MBA, Mucke L: Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci USA 1993, 90:10061-10065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hesselgesser J, Horuk R: Chemokine and chemokine receptor expression in the central nervous system. J Neurovirol 1999, 5:13-26 [DOI] [PubMed] [Google Scholar]
- 36.Jiang Y, Salafranca MN, Adhikari S, Xia Y, Feng L, Sonntag MK, deFiebre CM, Pennell NA, Streit WJ, Harrison JK: Chemokine receptor expression in cultured glia and rat experimental allergic encepholomyelitis. J Neuroimmunol 1998, 86:1-12 [DOI] [PubMed] [Google Scholar]
- 37.Boddeke EWGM, Meigel I, Frentzel S, Gourmala NG, Harrison JK, Buttini M, Spleiss O, Gebicke-Harter P: Cultured rat microglia express functional β-chemokine receptors. J Neuroimmunol 1999, 98:176-184 [DOI] [PubMed] [Google Scholar]
- 38.Albright AV, Shieh JTC, Itoh T, Lee B, Pleasure D, O’Conner MJ, Doms RW, Gonzalez-Scarano F: Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J Virol 1999, 73:205-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bajetto A, Bonavia R, Barbero S, Florio T, Costa A, Schettini G: Expression of chemokine receptors in the rat brain. Ann NY Acad Sci 1999, 876:201-209 [DOI] [PubMed] [Google Scholar]
- 40.Rottman JB, Ganley KP, Williams K, Wu LJ, Mackay CR, Ringler DJ: Cellular localization of the chemokine receptor CCR5- Correlation to cellular targets of HIV infection. Am J Pathol 1997, 151:1341-1351 [PMC free article] [PubMed] [Google Scholar]
- 41.Vitkovic L, Bockaert J, Jacque C: “Inflammatory” cytokines: neuromodulators in normal brain? J Neurochem 2000, 74:457-471 [DOI] [PubMed] [Google Scholar]
- 42.Tannenbaum CS, Major JA, Hamilton TA: IFN-gamma and lipopolysaccharide differentially modulate expresion of tumor necrosis factor receptor mRNA in murine peritoneal macrophages. J Immunol 1993, 151:6833-6839 [PubMed] [Google Scholar]
- 43.Welborn MB, Christman JW, Shepherd VL: Regulation of tumor necrosis factor-alpha receptors on macrophages: differences between primary macrophages and transformed macrophage cell lines. Regional Immunol 1993, 5:158-164 [PubMed] [Google Scholar]
- 44.Riches DW, Chan ED, Zahradka EA, Winston BW, Remigio LK, Lake FR: Cooperative signaling by tumor necrosis factor receptors CD120a (p55) and CD120b (p75) in the expression of nitric oxide and inducible nitric oxide synthase by mouse macrophages. J Biol Chem 1998, 273:22800-22806 [DOI] [PubMed] [Google Scholar]
- 45.Brieland JK, Flory CM, Jones ML, Miller GR, Remick DG, Warren JS, Fantone JC: Regulation of monocyte chemoattractant protein-1 gene expression and secretion in rat pulmonary alveolar macrophages by lipopolysaccharide, tumor necrosis factor-alpha, and interleukin-1 beta. Am J Resp Cell Mol Biol 1995, 12:104-109 [DOI] [PubMed] [Google Scholar]
- 46.Crippen TL, Riches DW, Hyde DM: Differential regulation of the expression of cytokine-induced neutrophil chemoattractant by mouse macrophages. Pathobiol 1998, 66:24-32 [DOI] [PubMed] [Google Scholar]
- 47.Rodenburg RJ, Brinkhuis RF, Peek R, Westphal JR, Van Den Hoogen FH, van Venrooij WJ, van de Putte LB: Expression of macrophage-derived chemokine (MDC) mRNA in macrophages is enhanced by interluekin-1 beta, tumor necrosis factor alpha, and lipopolysaccharide. J Leukoc Biol 1998, 63:606-611 [DOI] [PubMed] [Google Scholar]
- 48.Oh J-W, Van Wagoner NJ, Rose-John S, Benveniste EN: Role of IL-6 and the soluble IL-6 receptor in inhibition of VCAM-1 gene expression. J Immunol 1998, 161:4992-4999 [PubMed] [Google Scholar]
- 49.Marz P, Cheng J-G, Gadient RA, Patterson PH, Stoyan T, Otten U, Rose-John S: Sympathetic neurons can produce and respond to interleukin 6. Proc Natl Acad Sci USA 1998, 95:3251-3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW: Cytokine production by human fetal microglia and astrocytes: differential induction by lipopolysaccharide and IL-1 beta. J Immunol 1993, 150:2659-2667 [PubMed] [Google Scholar]
- 51.Chao CC, Hu S, Sheng WS, Peterson PK: Tumor necrosis factor-alpha production by human fetal microglial cells: regulation by other cytokines. Dev Neurosci 1995, 17:97-105 [DOI] [PubMed] [Google Scholar]
- 52.Sawada M, Suzumura A, Marunouchi T: TNFα induces IL-6 production by astrocytes but not by microglia. Brain Res 1992, 583:296-299 [DOI] [PubMed] [Google Scholar]
- 53.Benveniste EN, Sparacio SM, Norris JG, Grenett HE, Fuller GH: Induction and regulation of interleukin-6 gene expression in rat astrocytes. J Neuroimmunol 1990, 30:201-212 [DOI] [PubMed] [Google Scholar]
- 54.Chung IY, Benveniste EN: Tumor necrosis factor-α production by astrocytes: induction by lipopolysaccharide, IFN-γ, and IL-1β. J Immunol 1990, 144:2999-3007 [PubMed] [Google Scholar]