Abstract
Increased expression of matrix metalloproteinases, particularly gelatinase B (MMP-9), has been described in the lungs in pulmonary fibrosis. Intratracheal bleomycin is often used experimentally to produce lesions resembling human fibrosing alveolitis. To assess the role of gelatinase B in bleomycin-induced fibrosing alveolitis, we instilled bleomycin intratracheally into gelatinase B-deficient mice and gelatinase B+/+ littermates. Twenty-one days after bleomycin the two groups of mice were indistinguishable in terms of pulmonary histology and total lung collagen and elastin. However, the lungs of gelatinase B-deficient mice showed minimal alveolar bronchiolization, whereas bronchiolization was prominent in the lungs of gelatinase B+/+ mice. Gelatinase B was identified immunohistochemically in terminal bronchiolar cells and bronchiolized cells 7 and 14 days after bleomycin in gelatinase B+/+ mice, and whole lung gelatinase B mRNA was increased at the same times. Many bronchiolized cells displayed Clara cell features by electron microscopy. Some bronchiolized cells stained with antibody to helix transcription factor 4, a factor associated with the ciliated cell phenotype. Thus, fibrosing alveolitis develops after intratracheal bleomycin irrespective of gelatinase B. However, gelatinase B is required for alveolar bronchiolization, perhaps by facilitating migration of Clara cells and other bronchiolar cells into the regions of alveolar injury.
Matrix metalloproteinases (MMPs) have been implicated in the pathogenesis of a variety of diseases through their capacity to degrade components of extracellular matrix and to modify other molecules. 1,2 Gelatinase B (MMP-9; 92-kd gelatinase), a member of the MMP family that is secreted from macrophages, polymorphonuclear leukocytes, other inflammatory cells, and epithelial cells, has proteolytic activity against a variety of substrates, including basement membrane components type IV collagen and entactin. 3 Recently, gelatinase B was identified in bronchiolar epithelial cells and alveolar epithelial cells in human idiopathic pulmonary fibrosis (IPF), but not, in normal lung tissue. 4,5 Also, alveolar macrophages in IPF were found to have marked expression of gelatinase B, 6 and tissue inhibitor of metalloproteinase-1 (TIMP-1), an endogenous inhibitor of gelatinase B, was observed to be co-expressed with gelatinase B in epithelial cells lining alveolar buds in IPF. 4 These data suggest involvement of gelatinase B in IPF, but the nature of the involvement has not been determined.
Intratracheal instillation of bleomycin is commonly used as an experimental model of lung injury having features of fibrosing alveolitis. Although many aspects of bleomycin-induced lung injury have been investigated, 7 information about gelatinase B in this model is limited. 8-10 Recently, we generated mice with a targeted mutation of gelatinase B that results in complete deficiency of gelatinase B. 11 These gelatinase B-deficient mice were used in this study to investigate the role of gelatinase B in the development of bleomycin-induced fibrosing alveolitis.
Materials and Methods
Mice
Gelatinase B null 129 SvEv mice (gelatinase B−/−) were generated by targeted mutagenesis 11 and housed with wild-type littermates (gelatinase B+/+) in a pathogen-free animal facility. Matrilysin (MMP-7) null C57BL/6 mice were kindly provided by Carole L. Wilson, Ph.D. (Washington University School of Medicine, St. Louis, MO). 12 Mice were used at 3 to 4 months of age. All procedures were approved by the Washington University Animal Studies Committee.
Experimental Protocol
Mice were anesthetized with intraperitoneal ketamine and xylazine. The trachea was surgically exposed and 50 μl of sterile isotonic saline or 0.05 U of bleomycin sulfate (Blenoxane, Nippon Kayaku Co. Ltd., Tokyo, Japan) in 50 μl sterile isotonic saline was instilled into the trachea through a 28-gauge needle inserted between the cartilaginous rings. The surgical site was then sutured. The mice were killed 3, 7, 14, or 21 days later. The lungs from different animals were processed for morphological and biochemical studies or bronchoalveolar lavage (BAL) because both lungs were required for each type of analysis considering the heterogeneous distribution of the lesions in the lungs.
Bronchoalveolar Lavage
Twenty-one days after the intratracheal instillation of bleomycin or saline, animals were killed by CO2 narcosis and the lungs were lavaged in situ with a total of 1.8 ml of isotonic saline in three aliquots of 0.6 ml, as previously described. 13 The leukocyte count and differential of the BAL fluid were determined using a hemocytometer and cytospins stained with LeukoStat (Fisher Scientific, Pittsburgh, PA).
Hydroxyproline and Desmosine
To assess the collagen and elastin content of lungs, whole lung hydrolysates were analyzed for hydroxyproline and desmosine as described. 14
Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) for Gelatinase B and TIMP-1
Immediately after animals were killed, the thorax was opened and the lungs were removed and pulverized on dry-ice using a mortar and pestle. Lung RNA was extracted by ToTally (Ambion, Austin, TX). One μg of RNA was added to each reverse transcription (RT) reaction mixture including 20 pmoles of each primer. RT was done using random hexamers by incubating at 25°C for 10 minutes, 42°C for 30 minutes, and 94°C for 5 minutes. The oligonucleotide primers for gelatinase B were derived from exon 1 and exon 3 of the murine gelatinase B gene (Table 1) ▶ . Primers for glycerylaldehyde-3-phosphate dehydrogenase (gAPDH) were amplified along with gelatinase B, but were added later in the reaction. All primers were obtained from Life Technologies, Inc., Grand Island, NY. Amplification was done for 27 cycles consisting of 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds (PTC-100; MJ Research, Inc., Waltham, MA). GAPDH primers were added after 11 cycles. The reaction products were resolved in 1.5% agarose gels, transferred onto positively charged nylon membranes (BrightStar-Plus; Ambion, Austin, TX), and hybridized with 32P-labeled oligonucleotides specific for gelatinase B or GAPDH in standard saline citrate, 1% sodium dodecyl sulfate, 1× Denhardts, and 50 μg/ml of denatured salmon sperm DNA at 43°C for overnight. Filters were washed in 6× standard saline citrate, 0.1% sodium dodecyl sulfate for 15 minutes at room temperature, followed by 15 minutes at 52°C. The intensity of the signal for gelatinase B mRNA was normalized to the signal for GAPDH mRNA using a phosphorimager (Molecular Imager System GS-525; Bio-Rad, Hercules, CA). For RT-PCR analysis of TIMP-1, the PCR annealing temperature was 68°C and the GAPDH primers were added after six cycles. The primers and probe for TIMP-1 are shown in Table 1 ▶ .
Table 1.
Primer and Probe Sequences Used for Quantitative RT-PCR
| Gelatinase B (505 bp)* | |
| Forward-primer: | 5′-CCATGAGTCCCTGGCAG-3′ |
| Reverse-primer: | 5′-ATGACAATGTCCGCTTCG-3′ |
| Probe: | 5′-TGTGATGTTATGATGGTCCC-3′ |
| TIMP-1 (490 bp) | |
| Forward-primer: | 5′-CTTTGCATCTCTGGCATCTG-3′ |
| Reverse-primer: | 5′-GAGTGTCACTCTCCAGTTTG-3′ |
| Probe: | 5′-ATGCCGCAGATATCCGGTAC-3′ |
| GAPDH (689 bp) | |
| Forward-primer: | 5′-CCCCTTCATTGACCTCAACTACATGG-3′ |
| Reverse-primer: | 5′-GACATCAAGAAGGTGGTGAAGCAGGC-3′ |
| Probe: | 5′-CCAAGGTCATCCATGACAAC-3′ |
For gelatinase β and TIMP-1 cDNA sequences, see references 55 and 56.
*( ), predicted size of PCR products.
Tissue Processing for Light Microscopy
Lungs were perfused with saline through the right ventricle to remove blood and inflated with 10% buffered formalin or 4% paraformaldehyde at a constant pressure of 20 cm H2O via the trachea for 20 minutes. The lungs were then excised, immersion-fixed with 10% buffered formalin or 4% paraformaldehyde overnight, dehydrated and embedded in paraffin, and cut into 3-μm sections.
Antibody to Gelatinase B
A polyclonal antibody to murine gelatinase B was raised in rabbits against a recombinant protein corresponding to the catalytic domain of murine gelatinase B, Phe108-Gly444. The catalytic domain of murine gelatinase B was amplified by PCR with the primers 5′TGTCCCTTCATATGTTCCAAACCTTCAAAGGCC3′ and 5′TACCAACTCATATGTCAACCATACAGATACTGGATGC3′. The PCR product was subcloned into the NdeI site of pET-14b (Novagen, Madison, WI), and expressed in BL21 (DE3) bacterial cells as a fusion protein containing an N-terminal His-Tag having a molecular mass of 40 kd. Induction of the fusion protein was achieved with 0.4 mmol/L isopropylthio-β-D-galactosidase (IPTG) for 5 hours. Cells were harvested by centrifugation at 5,000 × g for 5 minutes, resuspended in 40 ml binding buffer (5 mmol/L imidazole, 500 mmol/L NaCl, 20 mmol/L Tris, pH 7.9), and lysed by three cycles of freezing/thawing (−80°C and 37°C), followed by sonication 8 × 20 seconds. Insoluble inclusion bodies were collected by centrifugation at 20,000 × g for 15 minutes, washed once in binding buffer, and finally resuspended in 5 ml of binding buffer containing 6 mol/L urea and incubated on ice for 1 hour. Insoluble material was removed by centrifugation at 39,000 × g for 20 minutes, and the supernatant was filtered through a 0.45-μm membrane before affinity chromatography. Rabbits were immunized with 20 μg of this protein with Freunds adjuvant for the first injection, whereas 10 μg were used for booster at 3 and 6 weeks. Specificity of the antibody for gelatinase B was confirmed using cytospins of peripheral blood leukocytes isolated from gelatinase B+/+ and gelatinase B-deficient mice. Neutrophils from gelatinase B+/+ mice showed diffuse cytoplasmic staining whereas neutrophils from gelatinase B-deficient mice showed no staining.
Gelatinase B Immunohistochemistry
Immunohistochemical staining for gelatinase B was performed on paraffin-embedded tissues that were deparaffinized and rehydrated. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in methanol for 30 minutes and microwaved for 10 minutes with 6% urea in distilled water to unmask antigenic sites. After treatment with normal goat serum for 10 minutes, lung sections were incubated with rabbit anti-murine gelatinase B antibody (1:1,500) overnight at 4°C, then incubated with biotinylated goat anti-rabbit IgG antibody (1:200), and exposed to a solution of streptavidin-biotin-peroxidase complex (DAKO, Carpinteria, CA). Peroxidase activity was localized using 3,3′-diaminobenzidine tetrahydrochloride as a chromogenic substrate. Tissue sections were counterstained with Mayer’s hematoxylin and mounted.
Assessment of Bronchiolization
Alveolar bronchiolization was identified as cells resembling bronchiolar epithelium lining normal or thickened alveolar walls, often in an acinar formation, as described by Nettesheim and Szakal. 15 The tissues were stained with elastica-Masson Goldner, which facilitated detection of bronchiolized cells. Because of the heterogeneity in amount and distribution of bronchiolization within the lungs after intratracheal bleomycin, at least five saggital sections of both lungs from each animal were analyzed at 200× for bronchiolization. Each bronchiolization lesion in each section was graded from I to IV using a modification of the grading system devised by Jensen-Taubman et al (Table 2) ▶ . 16
Table 2.
Histologic Classification of Bronchiolization after Intratracheal Bleomycin
| Grade I | Single alveolus lined by cuboidal epithelial cells or a single isolated acinar structure consisting of cuboidal epithelial cells adjacent to a terminal bronchiole |
| Grade II | 2 to 4 clustered tubular structures consisting of single-layered cuboidal epithelial cells adjacent to a terminal bronchiole |
| Grade III | More than 4 clustered tubular structures single-layered or stratified cuboidal epithelial cells |
| Grade IV | Multiple layers of cuboidal epithelial cells extending from a terminal bronchiole to distal alveolar spaces, sometimes with papillary formation |
Transmission Electron Microscopy
Transmission electron microscopy was done after standard processing. 17 Briefly, the lungs were inflated and fixed with 2.5% v/v glutaraldehyde in 0.1 mol/L phosphate buffer, pH 7.2, cut into pieces, rinsed in phosphate buffer, postfixed with 1% osmium tetroxide in phosphate buffer for 2 hours, dehydrated, and embedded in Epok 812. One-μm thick sections of the embedded tissues were stained with alkaline toluidine blue and observed with a light microscope. Appropriate areas were trimmed for ultrathin sections, which were stained with uranyl acetate and lead citrate and examined with a Hitachi H7100 transmission electron microscope (Hitachi Ltd., Tokyo, Japan).
Immunohistochemical Analysis for Proliferating Cells, Clara Cell-Specific Protein, Helix Transcription Factor 4 (HFH-4), and Surfactant Protein C (SP-C)
To assess cell proliferation after bleomycin, lung tissue was stained with a monoclonal antibody to the S phase-associated proliferating cell nuclear antigen (PCNA) (DAKO). 18 To assist in determining the phenotype of bronchiolized cells in lung tissue 21 days after bleomycin, the tissue was stained with antibodies to Clara cell-specific protein to identify Clara cells, 19 to forkhead/winged HFH-4 that is expressed by ciliated cells and cells of a ciliated lineage 20-22 and to SP-C to identify alveolar type II cells. 23 These antibodies were kindly provided by Francesco DeMayo (Baylor University, Waco, TX), Steven Brody (Washington University, St. Louis, MO), and Jeffrey Whitsett (University of Cincinnati, Cincinnati, OH), respectively.
Statistical Analysis
All data are expressed as means ± SEM. Statistical differences among groups were determined using one-factor analysis of variance and Student’s unpaired t-test between two groups when appropriate. Significance was defined at the P < 0.05 level unless otherwise stated.
Results
Inflammatory Cells in BAL Fluid
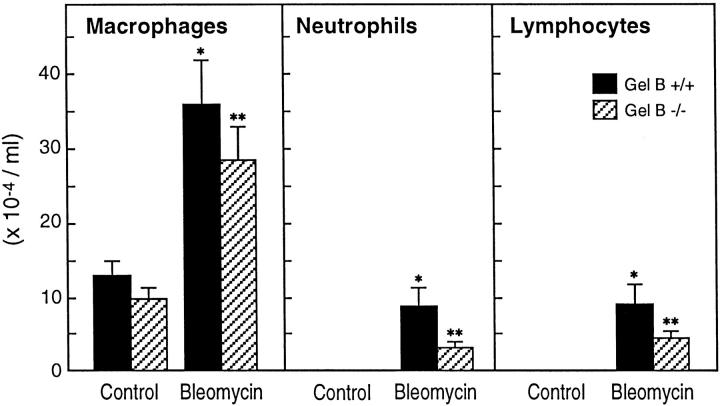
Bleomycin-treated animals showed a significant increase in the numbers of macrophages, neutrophils, and lymphocytes in BAL fluid 21 days after bleomycin instillation compared with nontreated or saline-treated controls (Figure 1) ▶ . There was no difference in the number or differential of inflammatory cells between gelatinase B+/+ and gelatinase B−/− mice. Similar to earlier findings that neutrophil emigration into tissues is preserved in gelatinase B deficiency, 13 these data suggest that gelatinase B is not critical for lymphocyte or monocyte emigration into lung.
Figure 1.
Inflammatory cells in BAL fluid 21 days after bleomycin. Bleomycin-treated animals showed significant increases in macrophages, neutrophils, and lymphocytes in BAL fluid at 21 days after bleomycin instillation as compared with nontreated or saline-treated controls. No significant difference was observed in the number of inflammatory cells in BAL fluid between gelatinase B+/+ and −/− mice. Each bar represents the mean of four or five animals ± SEM. *, Indicates P < 0.05 versus gelatinase B+/+ controls; **, P < 0.05 versus gelatinase B−/− controls.
Hydroxyproline and Desmosine
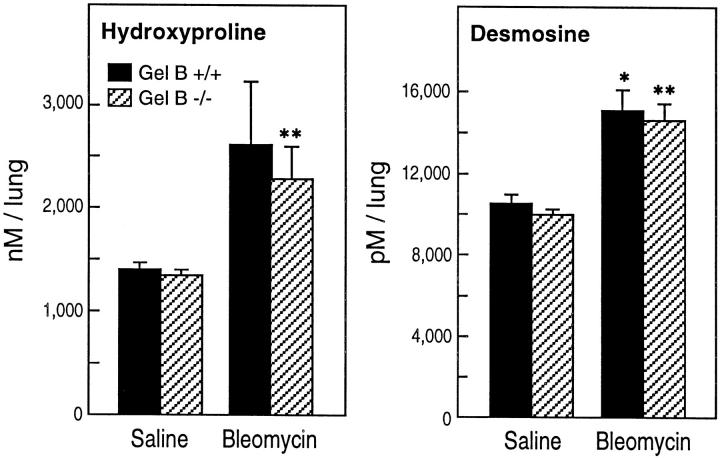
Whole lung collagen and elastin, as determined by hydroxyproline and desmosine content, respectively, were increased comparably in gelatinase B+/+ and gelatinase B−/− mice 21 days after bleomycin (Figure 2) ▶ , ∼50%, a change that is consistent with the findings of others. 24 Thus, gelatinase B does not seem to influence the net accumulation of collagen or elastin after intratracheal bleomycin.
Figure 2.
Hydroxyproline and desmosine content of lung 21 days after bleomycin. Lung collagen and elastin, as measured by the amount of hydroxyproline and desmosine, respectively, were increased comparably in gelatinase B+/+ and gelatinase B−/− mice 21 days after bleomycin treatment compared to lungs in saline-treated mice. Each bar represents the mean of four or five animals ± SEM.
Gelatinase B mRNA and TIMP-1 mRNA
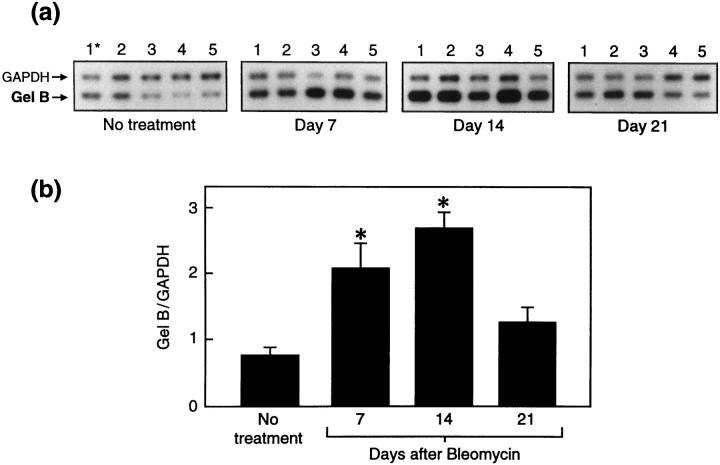
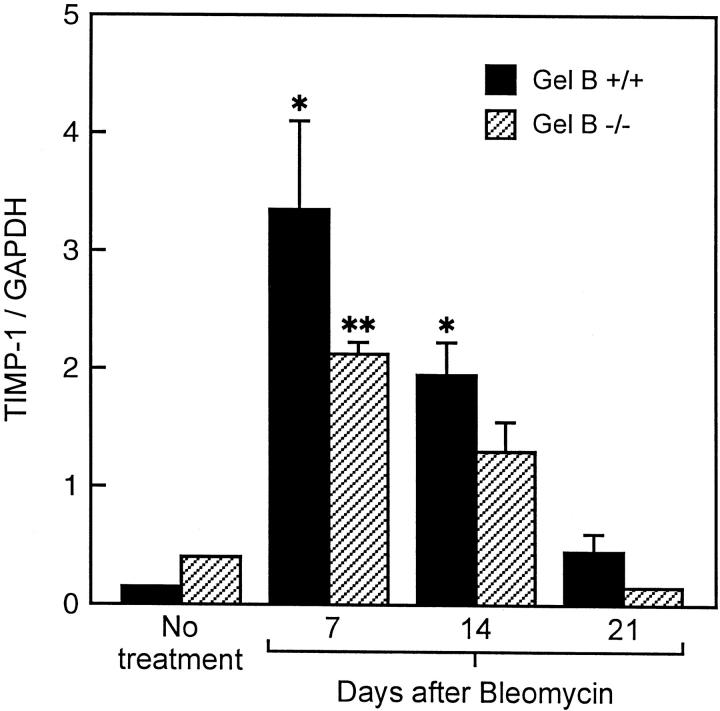
In gelatinase B+/+ mice, the RT-PCR-derived signal for lung gelatinase B mRNA, normalized for GAPDH mRNA, was present at low levels in controls at day 0, was increased threefold 14 days postbleomycin and returned to normal levels by 21 days (Figure 3, a and b) ▶ . As anticipated, lung RNA obtained from gelatinase B−/− mice did not show gelatinase B signal at any time point after bleomycin (data not shown). To determine whether TIMP-1 mRNA was altered during the development of bleomycin-induced fibrosis and affected by the absence of gelatinase B expression, we compared TIMP-1 mRNA levels between gelatinase B+/+ and gelatinase B−/− mice by quantitative RT-PCR. TIMP-1 mRNA was increased comparably at 7 days and 14 days after bleomycin instillation in both gelatinase B+/+ and gelatinase B−/− mice, and returned to near-baseline levels by 21 days after treatment in both types of mice (Figure 4) ▶ . Accordingly, the absence of gelatinase B did not influence the expression of TIMP-1 elicited by bleomycin.
Figure 3.
RT-PCR analysis of lung gelatinase B mRNA after bleomycin treatment in gelatinase B+/+ mice. a: Autoradiograph of RT-PCR of whole lung RNA isolated from nontreated or bleomycin-treated mice at 7, 14, and 21 days after bleomycin. Each lane represents RT-PCR products from a single mouse. b: The mean values (± SEM) of five mice expressed as the ratio of the signal intensity of gelatinase B mRNA to that of GAPDH at each time point. Gelatinase B mRNA in the lungs was significantly up-regulated by bleomycin treatment at 7 and 14 days, and returned to normal levels at 21 days. *, Indicates P < 0.05 versus nontreated mice.
Figure 4.
RT-PCR analysis of TIMP-1 mRNA after bleomycin treatment in gelatinase B+/+ and gelatinase B−/− mice expressed as the ratio of TIMP-1 mRNA to GAPDH mRNA. Gelatinase B+/+ (black bar), gelatinase B−/− (hatched bar). Each group consisted of four or five animals ± SEM. *, Indicates P < 0.05 versus gelatinase B+/+ nontreated mice; **, P < 0.05 versus gelatinase B−/− nontreated mice.
Gelatinase B in Bleomycin-Treated Lungs
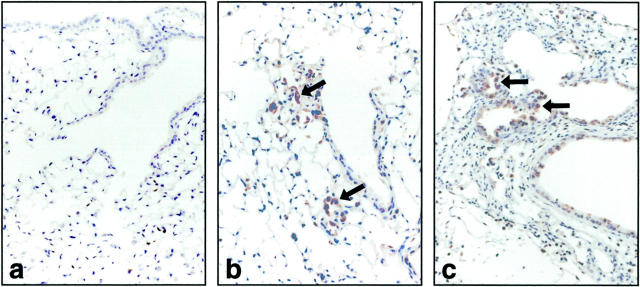
Immunoreactivity for gelatinase B was present only in occasional bronchiolar and alveolar epithelial cells of normal lungs (Figure 5a) ▶ . In contrast, gelatinase B staining was prominent in nonciliated airway epithelium and alveolar bronchiolized epithelial cells 7 and 14 days after intratracheal bleomycin (Figure 5, b and c) ▶ in gelatinase B+/+ mice. As expected, neutrophils and macrophages were positive in both untreated and bleomycin-treated lungs in gelatinase B+/+ mice, and tissues were uniformly negative in gelatinase B−/− mice (data not shown).
Figure 5.
Immunohistochemistry for gelatinase B in gelatinase B+/+ mice after bleomycin. Lung tissue was harvested from control mice (a) and at 7 (b) and 14 days (c) after bleomycin treatment. Gelatinase B was found in an occasional cell in normal lungs. Gelatinase B was present in many nonciliated airway epithelial cells at 14 days after bleomycin. Most of the bronchiolized cells were positive for gelatinase B at 7 and 14 days after bleomycin instillation (arrows) (original magnification, × 400).
Bronchiolization in Wild-Type, Gelatinase B-Deficient, and Matrilysin-Deficient Mice
The lungs of saline-treated animals appeared normal, regardless of their gelatinase B genotype. At 21 days after intratracheal bleomycin, abnormal matrix deposition was associated with numerous interstitial cells. The severity of fibrosis seemed similar in gelatinase B+/+ and gelatinase B−/− mice. In gelatinase B+/+ mice there was prominence of acinar structures lined by cuboidal epithelial cells, or conspicuously thickened interalveolar septa lined by hypertrophied and hyperplastic cuboidal epithelial cells either alongside the bronchioles or at their termination (Figure 6a) ▶ . The overall extent of this so-called bronchiolization varied from animal to animal, and a high degree of heterogeneity in the magnitude of bronchiolar epithelial cell extension into alveolar ducts was noted within the lungs of an individual animal. Bronchiolization changes have been observed in human diseases and in animal models of pulmonary fibrosis, including postbleomycin. 17 In sharp contrast, lesions of bronchiolization were hardly observed in the bleomycin-induced pulmonary fibrosis in gelatinase B−/− mice (Figure 6b) ▶ . To assess whether other MMP deficiencies would affect postbleomycin bronchiolization, bleomycin was administered to matrilysin-deficient mice and littermate controls. Matrilysin was chosen because this MMP has been implicated in airway epithelial repair. 25 Unlike in gelatinase B deficiency, bronchiolization was present in matrilysin-deficient mice 21 days after intratracheal bleomycin to the same extent as in littermate gelatinase B+/+ controls (Figure 6c) ▶ .
Figure 6.
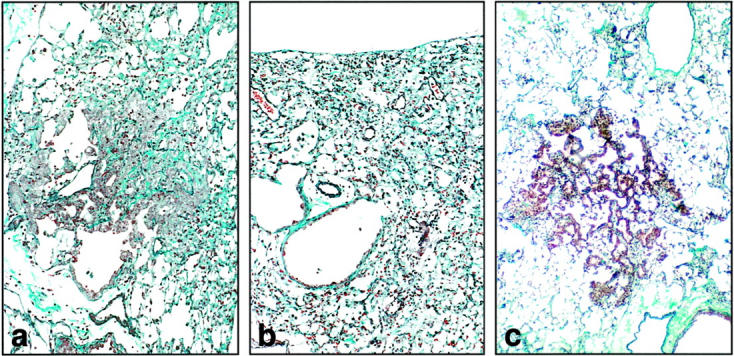
Bronchiolization 21 days after bleomycin treatment. a: In fibrotic regions of gelatinase B+/+ lung, clusters of cuboidal bronchiolar-appearing epithelium were prominent adjacent to bronchioles or at their distal termination (“bronchiolization”). b: Alveolar bronchiolization was rarely observed in the gelatinase B−/− lung. c: Bronchiolization was present in the matrilysin−/− lung. (elastica-Masson Goldner stain; original magnification, ×400)
Quantification of Bronchiolization
To confirm the impression of decreased alveolar bronchiolization in gelatinase B−/− mice, we compared the relative frequency of low- and high-grade bronchiolization in bleomycin-treated gelatinase B+/+ and −/− mice by thoroughly examining five or more Elastica-Masson Goldner-stained whole lung sections per animal in six gelatinase B+/+ and six gelatinase B−/− animals as well as one pair of matrilysin+/+ and −/− mice at 21 days after bleomycin. Bronchiolization was uniformly grade II or lower in gelatinase B−/− animals whereas grades greater than II were present in all of the gelatinase B+/+ animals (Table 3) ▶ . The composite bronchiolization score for each animal, calculated as the incidence of bronchiolization multipled by each grade and summed up in each animal, was strikingly higher in the gelatinase B+/+ animals. The gradation of bronchiolization in matrilysin−/− mice was comparable to that observed in the matrilysin+/+ littermate control as well as that seen in the gelatinase B+/+ controls. Accordingly, bronchiolization seemed unaffected in matrilysin-deficient mice whereas both the incidence and extent of bronchiolization was markedly reduced in gelatinase B-deficient mice.
Table 3.
Quantification of Bronchiolization
| Genotype | Mouse no. | Grade I | Grade II | Grade III | Grade IV | Total score |
|---|---|---|---|---|---|---|
| Gelatinase B+/+ | 1 | 20 | 11 | 8 | 7 | 94 |
| 2 | 19 | 12 | 9 | 3 | 82 | |
| 3 | 17 | 2 | 2 | 0 | 27 | |
| 4 | 24 | 15 | 6 | 1 | 76 | |
| 5 | 18 | 13 | 3 | 1 | 57 | |
| 6 | 15 | 12 | 5 | 2 | 62 | |
| Gelatinase B−/− | 1 | 7 | 1 | 0 | 0 | 9 |
| 2 | 3 | 0 | 0 | 0 | 3 | |
| 3 | 7 | 1 | 0 | 0 | 9 | |
| 4 | 5 | 2 | 1 | 0 | 12 | |
| 5 | 9 | 3 | 0 | 0 | 15 | |
| 6 | 10 | 1 | 0 | 0 | 12 | |
| Matrilysin +/+ | 1 | 20 | 17 | 5 | 3 | 81 |
| Matrilysin −/− | 1 | 17 | 18 | 8 | 1 | 81 |
Electron Microscopic Observations
Because alveolar bronchiolization might be the result of migration and proliferation of terminal airway epithelial cells, we examined the terminal airways of gelatinase B-deficient and gelatinase B+/+ mice after bleomycin. Under normal conditions, in both gelatinase B+/+ and gelatinase B−/− animals the epithelial cells lining the terminal bronchioles in areas near the alveolar duct junction were mainly Clara cells. Three days after intratracheal bleomycin, most of these single-layered cells were swollen. The histology pattern and the Clara cell ultrastructure did not vary between the two groups. (Figure 7, a and b) ▶ . Apparently, gelatinase B deficiency did not confer obvious protection against the acute effects of bleomycin on terminal airway epithelium. At 21 days, however, there was prominent bronchiolization in gelatinase B +/+ mice comprised of Clara-like cells (Figure 7c) ▶ , but bronchiolization was not seen in gelatinase B−/− animals (Figure 7d) ▶ .
Figure 7.
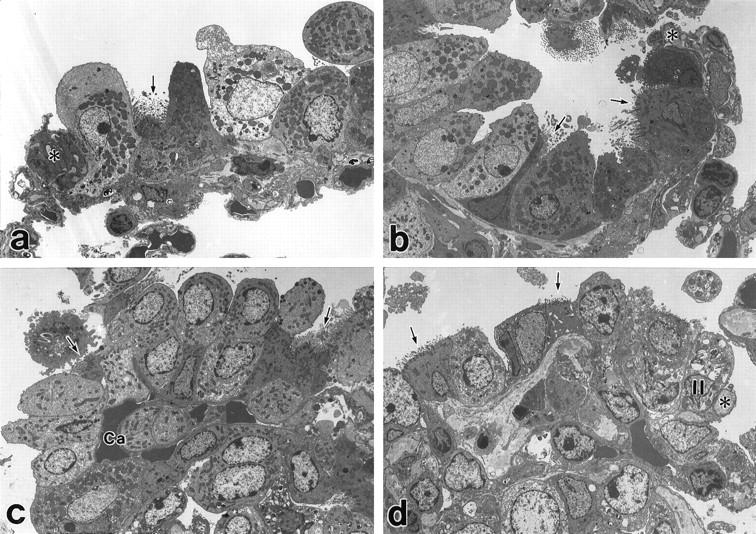
Electron micrographs of terminal bronchioles after intratracheal bleomycin. Uranyl acetate and lead citrate. 1500×. a: Three days after bleomycin in gelatinase B+/+ lung there is swelling of Clara cells and a ciliated cell (arrow). *, Indicates tip of septum of alveolar duct at the entrance to the alveolus. b: Three days after bleomycin in gelatinase B−/− lung, there are swollen Clara cells and ciliated cells (arrows). *, Indicates entrance to the alveolus. c: Twenty-one days after bleomycin in gelatinase B+/+ there is bronchiolization consisting of many Clara cells and some ciliated cells (arrows). The capillary (Ca) of the original alveolar wall is covered with bronchiolized epithelial cells. d: Twenty-one days after bleomycin in gelatinase B−/− Clara cells and some ciliated cells (arrows) are located in the original terminal bronchiole. An alveolar type I cell and an alveolar type II cell (II) are observed in the region of alveoli. * indicates entrance to the alveolus.
Cell Proliferation and Immunohistochemical Characteristics of Bronchiolized Cells
Because reduced bronchiolization in gelatinase B−/− mice after bleomycin might be the result of reduced proliferation of airway and/or alveolar epithelium, cell proliferation was evaluated by PCNA staining at 3 and 21 days. Three days after bleomycin some of the terminal bronchiolar epithelial cells were PCNA-positive in both gelatinase B+/+ and −/− mice (data not shown). Subsequently, in gelatinase B+/+ mice, PCNA immunoreactivity was found in cells located in the distal parts of high-grade bronchiolization lesions, and was not seen in cells in bronchiolization immediately adjacent to terminal bronchioles or in epithelium lining the bronchioles themselves (Figure 8a) ▶ . At this time point, proliferation seemed to be occurring mainly in bronchiolized cells within alveolar tissue. In gelatinase B−/− mice, PCNA-positive cells were seen in low-grade bronchiolization and in some terminal airway epithelial cells (Figure 8b) ▶ . The presence of active cell proliferation in gelatinase B−/− mice suggests that the minimal bronchiolization in these animals is not because of a gross decrease in epithelial cell proliferative activity. Further analysis would be necessary to exclude differences in proliferation at earlier time points. Most of the bronchiolized cells were positive for Clara cell-specific protein. They were uniformly negative for SP-C, although alveolar epithelial cells in normal appearing alveoli on the same sections did stain (not shown). Thus, by this marker bronchiolized cells did not seem to be alveolar type II cells.
Figure 8.
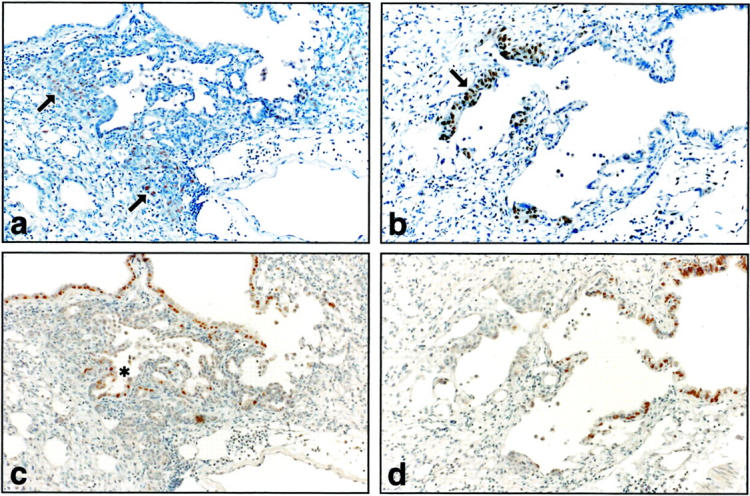
Expression of PCNA and HFH-4 21 days after bleomycin treatment. a: PCNA-positive cells are widespread in distal lesions (arrows) of grade IV bronchiolization, but not in the proximal lesions in gelatinase B+/+ mice. b: PCNA-stained cells are limited in low-grade bronchiolization (arrow) immediately adjacent to conducting airway in gelatinase B−/− mice. c: HFH-4 expression is present in ciliated airway epithelial and proximal sites (*) of high-grade alveolar bronchiolization in gelatinase B+/+. d: HFH-4 expression in gelatinase B−/− lung is limited to ciliated airway epithelium. Original magnification, ×400.
To help distinguish between Clara cells and immature ciliated epithelial cells, immunohistochemistry was performed with antibody to HFH-4. HFH-4 is a member of the winged-helix/forkhead family of transcription factors that is expressed postnatally by ciliated bronchial and bronchiolar epithelium, coincident with β-tubulin. At 21 days after bleomycin in gelatinase B+/+ tissue HFH-4 expression was evident in ciliated airway epithelium and in proximal sites of high-grade alveolar bronchiolization, whereas most of the cells involved in bronchiolization did not stain for HFH-4 (Figure 8c) ▶ . It seems therefore that bronchiolized cells in alveolar areas are mainly the result of Clara cell migration and proliferation. In gelatinase B−/− tissue HFH-4 staining at 21 days was restricted to airway epithelium which was negative for PCNA (Figure 8, b and d) ▶ .
Discussion
For more than 2 decades bleomycin-induced lung injury in experimental animals has been used as a model to investigate the cell biology and histopathology of pulmonary lesions that resemble human IPF. 7 Studies of this model have helped uncover the complexity of mechanisms involved in the human disease. Large differences exist between strains of mice in their susceptibility to bleomycin-induced lung injury 26,27 and susceptibility correlates with production of cytokines and other endogenous factors. 28 Moreover, the injury can be attenuated or even prevented in susceptible strains in various ways, for example, by gene targeting of transforming growth factor-α 29 or plasminogen activator inhibitor-1, 30 by intravenous administration of antibodies against transforming growth factor-β, 31 and by intrapulmonary instillation of keratinocyte growth factor. 24 Although bleomycin may damage structural cells of the lungs directly 32,33 its principal mode of action in leading to IPF-like pathology seems to be via endogenous mediators of inflammation, fibrinolysis, and proliferation.
Recent observations have demonstrated gelatinase B in human IPF lesions, 5 increased gelatinase B expression by alveolar macrophages from lungs affected by IPF, 7 and gelatinolytic activity in BAL fluid in experimental bleomycin-induced lung injury. 9 Consistent with these findings, we have detected gelatinase B by immunohistochemistry in terminal airway epithelial cells and bronchiolized epithelial cells at 7 and 14 days after bleomycin administration, but not in lung tissue of saline-treated animals. Moreover, we detected twofold to threefold increases in gelatinase B mRNA by RT-PCR in the lungs at 7 and 14 days with a return to baseline by 21 days. These data are important because neutrophils do not contain gelatinase B mRNA although they do contain gelatinase B protein. 34,35 Thus, the increased gelatinase B associated with bleomycin-induced lung injury is not simply release of gelatinase B from neutrophils in the lungs.
In contrast to our findings, others have not detected induction of gelatinase B mRNA in whole lung as determined by Northern analysis after receiving serial injections of bleomycin intraperitoneally. 10 Because we found that gelatinase B mRNA increased only transiently and the increase was modest, peaking around day 14 with a threefold increase over the baseline, we would suggest that the increase might not be obvious by Northern analysis. In addition to increased gelatinase B mRNA levels, we also observed gelatinase B in terminal airway epithelial cells and bronchiolized epithelial cells by immunohistochemistry whereas in control lung tissues there was no staining of epithelial cells. Therefore, we are confident that gelatinase B was induced in epithelial cells in bleomycin-treated gelatinase B+/+ mice. We did not examine lung tissue for expression of other MMPs. In the same study in which gelatinase B induction was not observed, 10 inductions of interstitial collagenase (MMP-13) and stromelysin-1 (MMP-3) expression were also not found, but macrophage metalloelastase (MMP-12) mRNA increased 10-fold at 21 days, and macrophages demonstrated macrophage metalloelastase prominently by immunohistochemistry. The significance of increased macrophage metalloelastase in this context remains to be determined.
Despite their inability to express gelatinase B mRNA, gelatinase B-deficient mice showed increased TIMP-1 mRNA levels after intratracheal bleomycin equivalent to the levels seen in gelatinase B+/+ mice, and similar to results of others. 10 Clearly, the induction of TIMP-1 after bleomycin is not triggered by gelatinase B expression. It is well recognized that the regulation of expression of MMPs and TIMPs is not necessarily parallel. 36
By two criteria, gelatinase B deficiency did not seem to affect the fibro-inflammatory response to intratracheal bleomycin. First, the lungs of deficient animals showed alveolar inflammation comparable to that seen in gelatinase B+/+ animals. Second, the lungs of deficient animals had increased collagen and elastin content at 21 days that matched the increases observed in the gelatinase B+/+ controls. Thus, gelatinase B does not play an essential role in the initiation or evolution of the inflammatory and fibrotic changes that develop after intratracheal bleomycin, and gelatinase B deficiency does not ameliorate these characteristic lesions of bleomycin-induced lung injury.
An intriguing histopathological finding in bleomycin-treated mice, 9,37,38 rats, 19 and baboons 39 is clusters of cuboidal epithelial cells, commonly in acinar or papillary formations, in regions of alveolar injury. This pathological pattern, called alveolar bronchiolization, 15 is not restricted to bleomycin-induced lung injury, as it is also seen in human IPF 5 and lung cancer 16,40 and in various types of experimental lung injury, including viral infection, 41 and exposure to ozone, 42 chromate, 15 or paraquat. 43 Alveolar bronchiolization in IPF is thought to be permanent as it is observed in honeycomb lesions, but it is considered a temporary phenomenon after alveolar damage in experimental fibrosing alveolitis. 17,43
The histogenesis of the cells comprising alveolar bronchiolization is uncertain despite many years of study. One speculation is that these cells arise from colonization and proliferation of differentiated epithelial cells that have migrated from the distal conducting airways 15,44 or by migration and then differentiation of progenitor cells located in terminal airways. An alternative concept is that the cells arise from alveolar epithelial cells or their progenitors. Findings in rats of prominent terminal bronchiolar epithelial cell proliferation by bromodeoxyuridine labeling at 3 days after intratracheal bleomycin when alveolar type II cell labeling is minimal would favor the hypothesis that the bronchiolized cells arise from the terminal airways. 17
Recent studies of bleomycin-induced lung injury in C57BL/6 mice suggest that bronchiolized cells and the adjacent distal airway epithelial cells have a complex phenotype, showing co-expression of markers of both alveolar type II cells and of Clara cells 37,38 and in some instances even having cilia. These data would support the concept that there is a population of progenitor cells that are important in alveolar epithelial repair which are capable of differentiating into either Clara cells or alveolar type II cells, as well as into cells with features of both types of cells. 45 Our data confirm the presence of Clara cell-specific protein-positive cells within areas of alveolar bronchiolization, agreeing with earlier work pointing to a bronchiolar origin of bronchiolized alveolar cells. We have not observed SP-C-positive cells in sites of alveolar bronchiolization, but recognize that a stem cell for both Clara cells and alveolar type II cells might originate in distal airways and be the source of alveolar bronchiolized cells. 46,47
Alveolar bronchiolization was markedly reduced in gelatinase B-deficient mice compared to gelatinase B+/+ controls, pointing strongly to a role for gelatinase B in alveolar bronchiolization (Figure 9). Although alveolar bronchiolization has been considered beneficial by helping to cover denuded alveolar walls in alveolar injury, there was no evidence that lack of bronchiolization in gelatinase B−/− mice resulted in worse fibrosing alveolitis. It is intriguing to speculate that gelatinase B facilitates distal airway epithelial cells, including epithelial stem cells, to migrate into sites of alveolar injury. Because gelatinase B has proteolytic activity against a number of extracellular matrix components, 3 release of gelatinase B from epithelial cells could disrupt cell-cell and cell-matrix attachment and enable bronchiolar epithelial cells to migrate into zones of alveolar injury.
Recent evidence shows that the extracellular matrix is not only a structural support, but also a storage site for many secreted and bound growth factors. 48,49 Gelatinase activity may provide a stimulus to tissue repair by releasing cytokines and/or growth factors from extracellular matrix, and the expression of gelatinase B has been reported to correlate with epithelial cell proliferation in bleomycin-induced lung injury. 9 Considering the degradative capacity of gelatinase B for some components of basement membrane, the presence of gelatinase B in terminal airway and bronchiolized epithelial cells suggests that basement membrane damage is involved in the process of alveolar bronchiolization.
Alveolar and airway epithelial cells can synthesize gelatinase B in vitro. 50,51 Wound repair of human respiratory epithelium in vitro requires gelatinase B which seems to operate by facilitating epithelial cell migration. 52,53 The possibility that gelatinase B might function to promote cell migration has support from studies with other MMPs. For example, collagenase-1 (MMP-1) acting on type I collagen is required for keratinocyte migration during cutaneous wound repair. 54 Matrilysin (MMP-7) is primarily expressed in ciliated airway epithelial cells and facilitates re-epithelialization of tracheal wounds. 25 Interestingly, we found that matrilysin deficiency does not impair alveolar bronchiolization in bleomycin-induced lung injury suggesting that different MMPs may function in repair at different levels of the airways.
In conclusion, bleomycin induced-lung injury is associated with transient up-regulation of gelatinase B in terminal airway epithelium and cells comprising alveolar bronchiolization. Although typical pulmonary inflammation and fibrosis occur in gelatinase B deficiency, alveolar bronchiolization is essentially absent whereas it is a prominent feature of bleomycin-induced lung injury in gelatinase B+/+ mice. Because bronchiolized cells in alveoli have morphological features of Clara cells and ciliated cells and display markers for these cell types but not for alveolar type II cells, it seems that they arise from bronchiolar cells or progenitors of bronchiolar cells. The molecular mechanisms linking gelatinase B expression to alveolar bronchiolization in bleomycin-induced lung injury are not yet established, but facilitating cell migration and proliferation by disrupting cell attachments seems likely.
Acknowledgments
We thank Steven L. Brody, M.D., for assistance with immunohistochemistry for HFH-4, Barry C. Starcher, Ph.D., for desmosine and hydroxyproline assays, Mrs. Clarina Tisdale for rabbit immunizations, the Morphology Core at Barnes-Jewish Hospital for tissue embedding and sectioning, and Ms. Arimi Ishikawa and Ms. Naomi Tamura for technical assistance with histological studies.
Footnotes
Address reprint requests to Robert M. Senior, M.D., Department of Medicine, Barnes-Jewish Hospital (North Campus), 216 South Kingshighway, St. Louis, MO 63110. E-mail:seniorr@msnotes.wustl.edu.
Supported by grants HL47328 and HL29594 from the National Heart, Lung, and Blood Institute of the National Institutes of Health, the Alan A. and Edith L. Wolff Charitable Trust (to R. M. S.), and a Research Fellowship from the Japan Society for the Promotion of Science for Young Scientists (to T. B.).
References
- 1.Woessner JF Jr: The matrix metalloproteinase family. Edited by WC Parks, RP Mecham. San Diego, Academic Press, 1998, pp 1–14
- 2.Shapiro SD, Senior RM: Matrix metalloproteinases. Matrix degradation and more. Am J Respir Cell Mol Biol 1999, 20:1100-1102 [DOI] [PubMed] [Google Scholar]
- 3.Vu TH, Werb Z: Gelatinase B: structure, regulation, and function. Parks WC Mecham RP eds. Matrix Metalloproteinases. 1998, :pp 115-148 Academic Press, San Diego [Google Scholar]
- 4.Hayashi T, Stetler-Stevenson WG, Fleming MV, Fishback N, Koss MN, Liotta LA, Ferrance VJ, Travis WD: Immunohistochemical study of metalloproteinases and their tissue inhibitors in the lungs of patients with diffuse alveolar damage and idiopathic pulmonary fibrosis. Am J Pathol 1996, 149:1241-1256 [PMC free article] [PubMed] [Google Scholar]
- 5.Fukuda Y, Ishizuka M, Kudoh S, Kitaichi M, Yamanaka N: Localization of matrix metalloproteinase-1, -2, and -9 and tissue inhibitor of metalloproteinase-2 in interstitial lung diseases. Lab Invest 1998, 78:687-698 [PubMed] [Google Scholar]
- 6.Lemjabbar H, Gosset P, Lechapt-Zalcman E, Franco-Montoya ML, Wallaert B, Harf A, Lafuma C: Overexpression of alveolar macrophage gelatinase B (MMP-9) in patients with idiopathic pulmonary fibrosis: effects of steroid and immunosuppressive treatment. Am J Respir Cell Mol Biol 1999, 20:903-913 [DOI] [PubMed] [Google Scholar]
- 7.Thrall RS, Scalise PJ: Bleomycin. Phan SH eds. Pulmonary Fibrosis. 1995, :pp 231-292 Marcel Dekker, New York [Google Scholar]
- 8.Yaguchi T, Fukuda Y, Ishizaki M, Yamanaka N: Immunohistochemical and gelatin zymography studies for matrix metalloproteinases in bleomycin-induced pulmonary fibrosis. Pathol Int 1998, 48:954-963 [DOI] [PubMed] [Google Scholar]
- 9.Bakowska J, Adamson IY: Collagenase and gelatinase activities in bronchoalveolar lavage fluids during bleomycin-induced lung injury. J Pathol 1998, 185:319-323 [DOI] [PubMed] [Google Scholar]
- 10.Swiderski RE, Dencoff JE, Floerchinger CS, Shapiro SD, Hunninghake GW: Differential expression of extracellular matrix remodeling genes in a murine model of bleomycin-induced pulmonary fibrosis. Am J Pathol 1998, 152:821-828 [PMC free article] [PubMed] [Google Scholar]
- 11.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z: MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998, 93:411-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson CL, Heppner KJ, Labosky PA, Hogan BLM, Matrisian LM: Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci USA 1997, 94:1402-1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Betsuyaku T, Shipley JM, Liu Z, Senior RM: Neutrophil emigration in the lungs, peritoneum, and skin does not require gelatinase B. Am J Respir Cell Mol Biol 1999, 20:1303-1309 [DOI] [PubMed] [Google Scholar]
- 14.Koh DW, Roby JD, Starcher B, Senior RM, Pierce RA: Postpneumonectomy lung growth: a model of reinitiation of tropoelastin and type I collagen production in a normal pattern in adult rat lung. Am J Respir Cell Mol Biol 1996, 15:611-623 [DOI] [PubMed] [Google Scholar]
- 15.Nettesheim P, Szakal AK: Morphogenesis of alveolar bronchiolization. Lab Invest 1972, 26:210-219 [PubMed] [Google Scholar]
- 16.Jensen-Taubman SM, Steinberg SM, Linnoila RI: Bronchiolization of the alveoli in lung cancer: pathology, patterns of differentiation and oncogene expression. Int J Cancer 1998, 75:489-496 [DOI] [PubMed] [Google Scholar]
- 17.Kawamoto M, Fukuda Y: Cell proliferation during the process of bleomycin-induced pulmonary fibrosis in rats. Acta Pathol Jpn 1990, 40:227-438 [DOI] [PubMed] [Google Scholar]
- 18.Muglia LJ, Bae DS, Brown TT, Vogt SK, Alvarez JG, Sunday ME, Majzoub JA: Proliferation and differentiation defects during lung development in corticotropin-releasing hormone-deficient mice. Am J Respir Cell Mol Biol 1999, 20:181-188 [DOI] [PubMed] [Google Scholar]
- 19.Ray MK, Wang G, Barrish J, Finegold MJ, DeMayo FJ: Immunohistochemical localization of mouse Clara cell 10-KD protein using antibodies raised against the recombinant protein. J Histochem Cytochem 1996, 44:919-927 [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Knowles H, Hebert JL, Hackett BP: Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left-right asymmetry. J Clin Invest 1998, 102:1077-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tichleaar JW, Wert SE, Costa RH, Kimura S, Whitsett J: HNF-3/forkhead homologue-4 (HFH-4) is expressed in ciliated epithelial cells in the developing mouse lung. J Histochem Cytochem 1999, 47:823-831 [DOI] [PubMed] [Google Scholar]
- 22.Blatt EN, Yan XH, Wuerffel MK, Hamilos DL, Brody SL: Forkhead transcription factor HFH-4 expression is temporally related to ciliogenesis. Am J Respir Cell Mol Biol 1999, 21:168-176 [DOI] [PubMed] [Google Scholar]
- 23.Zhou L, Lim L, Costa RH, Whitsett JA: Thyroid transcription factor-1, hepatocyte nuclear factor-3beta, surfactant protein B, C, and Clara cell secretory protein in developing mouse lung. J Histochem Cytochem 1996, 44:1183-1193 [DOI] [PubMed] [Google Scholar]
- 24.Deterding RR, Havill AM, Yano T, Middleton SC, Jacoby CR, Shannon JM, Simonet WS, Mason RJ: Prevention of bleomycin-induced lung injury in rats by keratinocyte growth factor. Proc Assoc Am Phys 1997, 109:254-268 [PubMed] [Google Scholar]
- 25.Dunsmore SE, Saarialho-Kere UK, Roby JD, Wilson CL, Matrisian LM, Welgus HG, Parks WC: Matrilysin expression and function in airway epithelium. J Clin Invest 1998, 102:1321-1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrier DJ, Kunkel RG, Phan SH: The role of strain variation in murine bleomycin-induced pulmonary fibrosis. Am Rev Respir Dis 1983, 127:63-66 [DOI] [PubMed] [Google Scholar]
- 27.Haston CK, Amos CI, King TM, Travis EL: Inheritance of susceptibility to bleomycin-induced pulmonary fibrosis in the mouse. Cancer Res 1996, 56:2596-2601 [PubMed] [Google Scholar]
- 28.Marshall RP, McAnulty RJ, Laurent GJ: The pathogenesis of pulmonary fibrosis: is there a fibrosis gene? Int J Biochem Cell Biol 1997, 29:107-120 [DOI] [PubMed] [Google Scholar]
- 29.Madtes DK, Elston AL, Hackman RC, Dunn AR, Clark JG: Transforming growth factor-alpha deficiency reduces pulmonary fibrosis in transgenic mice. Am J Respir Cell Mol Biol 1999, 20:924-934 [DOI] [PubMed] [Google Scholar]
- 30.Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH: Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest 1996, 97:232-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giri SN, Hyde DM, Hollinger MA: Effect of antibody to transforming growth factor beta on bleomycin induced accumulation of lung collagen in mice. Thorax 1993, 48:959-966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato E, Koyama S, Masubuchi T, Takamizawa A, Kubo K, Nagai S, Izumi T: Bleomycin stimulates lung epithelial cells to release neutrophil and monocyte chemotactic activities. Am J Physiol 1999, 276:L941-L950 [DOI] [PubMed] [Google Scholar]
- 33.Simon RH, Paine R: Participation of pulmonary alveolar epithelial cells in lung inflammation. J Lab Clin Med 1995, 126:108-118 [PubMed] [Google Scholar]
- 34.Borregaard N, Cowland JB: Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997, 89:3503-3521 [PubMed] [Google Scholar]
- 35.Betsuyaku T, Liu F, Senior RM, Haug JS, Brown EJ, Jones SL, Matsushima K, Link DC: A functional granulocyte colony-stimulating factor receptor is required for normal chemoattractant-induced neutrophil activation. J Clin Invest 1999, 103:825-832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lacraz S, Nicod LP, Chicheportiche R, Welgus HG, Dayer JM: IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J Clin Invest 1995, 96:2304-2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daly HE, Baecher-Allan CM, Barth RK, D’Angio CT, Finkelstein JN: Bleomycin induces strain-dependent alterations in the pattern of epithelial cell-specific marker expression in mouse lung. Toxicol Appl Pharmacol 1997, 142:303-310 [DOI] [PubMed] [Google Scholar]
- 38.Daly HE, Baecher-Allan CM, Paxhia AT, Ryan RM, Barth RK, Finkelstein JN: Cell-specific gene expression reveals changes in epithelial cell populations after bleomycin treatment. Lab Invest 1998, 78:393-400 [PubMed] [Google Scholar]
- 39.Collins JF, Orozco CR, McCullough B, Coalson JJ, Johanson WG, Jr: Pulmonary fibrosis with small-airway disease: a model in nonhuman primates. Exp Lung Res 1982, 3:91-108 [DOI] [PubMed] [Google Scholar]
- 40.Jensen SM, Jones JE, Pass H, Steinberg SM, Linnoila RI: Clara cell 10 kDa protein mRNA in normal and atypical regions of human respiratory epithelium. Int J Cancer 1994, 58:629-637 [DOI] [PubMed] [Google Scholar]
- 41.Baskerville A, Thomas G, Wood M, Harris WJ: Histology and ultrastructure of metaplasia of alveolar epithelium following infection of mice and hamsters with influenza virus. Br J Exp Pathol 1974, 55:130-137 [PMC free article] [PubMed] [Google Scholar]
- 42.Pinkerton KE, Dodge DE, Cederdahl-Demmler J, Wong VJ, Peake J, Haselton CJ, Mellick PW, Singh G, Plopper CG: Differentiated bronchiolar epithelium in alveolar ducts of rats exposed to ozone for 20 months. Am J Pathol 1993, 142:947-956 [PMC free article] [PubMed] [Google Scholar]
- 43.Fukuda Y, Takemura T, Ferrans VJ: Evolution of metaplastic squamous cells of alveolar walls in pulmonary fibrosis produced by paraquat. Virchows Arch B Cell Pathol 1989, 58:27-43 [DOI] [PubMed] [Google Scholar]
- 44.Kawanami O, Ferrans VJ, Crystal RG: Structure of alveolar epithelial cells in patients with fibrotic lung disorders. Lab Invest 1982, 46:39-53 [PubMed] [Google Scholar]
- 45.Mason RJ, Williams MC, Moses HL, Mohla S, Berberich MA: Stem cells in lung development, disease, and therapy. Am J Respir Cell Mol Biol 1997, 16:355-363 [DOI] [PubMed] [Google Scholar]
- 46.Evans MJ, Johnson LV, Stephens RJ, Freeman G: Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Lab Invest 1976, 35:246-257 [PubMed] [Google Scholar]
- 47.Evans MJ, Cabral-Anderson LJ, Freeman G: Role of the Clara cells in renewal of the bronchiolar epithelium. Lab Invest 1978, 38:648-655 [PubMed] [Google Scholar]
- 48.Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH: Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res 1992, 202:1-8 [DOI] [PubMed] [Google Scholar]
- 49.Taipale J, Keski-Oja J: Growth factors in the extracellular matrix. FASEB J 1997, 11:51-59 [DOI] [PubMed] [Google Scholar]
- 50.Pardo A, Ridge K, Uhal B, Sznajder JI, Selman M: Lung alveolar epithelial cells synthesize interstitial collagenase and gelatinase A and B in vitro. Int J Biochem Cell Biol 1997, 29:901-910 [DOI] [PubMed] [Google Scholar]
- 51.Yao PM, Delclaux C, d’Ortho MP, Matre B, Harf A, Lafuma C: Cell-matrix interactions modulate 92-kD gelatinase expression by human bronchial epithelial cells. Am J Respir Cell Mol Biol 1998, 18:813-822 [DOI] [PubMed] [Google Scholar]
- 52.Buisson A-C, Zahm J-M, Polette M, Pierrot D, Bellon G, Puchelle E, Birembaut P, Tournier J-M: Gelatinase B is involved in the in vitro wound repair of human respiratory epithelium. J Cell Physiol 1996, 166:413-426 [DOI] [PubMed] [Google Scholar]
- 53.Legrand C, Gilles C, Zahm JM, Polette M, Buisson AC, Kaplan H, Birembaut P, Tournier JM: Airway epithelial cell migration dynamics. MMP-9 role in cell-extracellular matrix remodeling. J Cell Biol 1999, 146:517-529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG: The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol 1997, 137:1445-1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graubert T, Johnston J, Berliner N: Cloning and expression of the cDNA encoding mouse neutropil gelatinase: demonstration of coordinate secondary granule protein gene expression during terminal neutrophil maturation. Blood 1993, 82:3192-3197 [PubMed] [Google Scholar]
- 56.Gewert DR, Coulombe B, Castelino M, Skup D, Williams BR: Characterization and expression of a murine gene homologous to human EPA/TIMP: a virus-induced gene in the mouse. EMBO J 1987, 6:651-657 [DOI] [PMC free article] [PubMed] [Google Scholar]