Abstract
The effector hormone of the renin-angiotensin system, angiotensin II, plays a major role in cardiovascular regulation. In rats, both angiotensin receptor subtypes, AT1 and AT2, are up-regulated after myocardial infarction but previous studies failed to identify the cell types which express the AT2 receptor in the heart. To address this question we established a single-cell reverse transcriptase-polymerase chain reaction for AT1 and AT2 receptors to determine whether these receptor subtypes are expressed in adult rat cardiomyocytes before and 1 day after myocardial infarction. By laser-assisted cell picking, section profiles of single cells without genomic DNA contamination were isolated. After dividing samples into two identical aliquots, polymerase chain reaction amplification for AT1 and AT2 receptors was carried out and polymerase chain reaction products were subjected to gel electrophoresis. Compared to control (n = 4) and sham-operated animals (n = 4), the number of cardiomyocytes expressing the AT1 receptor mRNA 1 day after myocardial infarction (n = 4) was not changed (42% and 33% versus 45%, respectively). On the other hand, AT2 receptor mRNA was expressed in 8% and 13%, respectively, of cardiomyocytes gained from control (n = 4) and sham-operated animals (n = 4) and in 14% isolated after myocardial infarction (n = 4). These results demonstrate for the first time that the AT2 receptor is expressed in adult cardiomyocytes in vivo. They further suggest that the previously observed up-regulation of cardiac AT1 and AT2 receptors after myocardial infarction involves cell types other than cardiomyocytes.
The octapeptide angiotensin II (Ang II) exerts a variety of actions via its AT1 and AT2 receptors. Most of the so far known effects of Ang II are mediated by AT1 receptors including vasoconstriction, renal salt retention, aldosterone and vasopressin release, and cell proliferation. 1,2
Although the exact physiological role of the AT2 receptor still remains to be defined it has been widely accepted that these binding sites are involved in processes precipitated by tissue injury. For example, AT2 receptor expression is significantly increased after myocardial infarction 3,4 and peripheral nerve injury. 5 Further, AT2 receptor stimulation promotes optic nerve regeneration. 6 However, AT2 receptors have not only been associated with cell protection but there is also strong evidence that Ang II acting through the AT2 receptor induces apoptosis. 7-11 At present, it is not understood in which particular physiological or pathophysiological situation activation of the AT2 receptor leads to cell death or cell survival.
In the heart, angiotensin receptors are involved in a variety of processes. Stimulation of normal and postinfarcted ventricular myocytes with Ang II induces hypertrophy via AT1 receptors. 12 Although AT1 receptor-dependent mechanisms mediate fibroblast and vascular smooth muscle cell proliferation 13 as well as collagen synthesis, 14 AT2 receptor activation inhibits cell proliferation. 15-17 The expression of AT1 and AT2 receptors and the Ang II-induced growth or anti-growth effects in cardiomyocytes in culture 18 depend on the developmental stage at which the cells are prepared and on the ratio between those receptor subtypes. Depending on experimental conditions, AT1 receptors have been demonstrated to mediate apoptosis in cultured cardiomyocytes 19,20 or to promote hypertrophy 12 via paracrine release of transforming growth factor-β1 and endothelin-1. 21
To investigate the angiotensin receptor expression in the heart, a variety of techniques such as receptor binding or reverse transcriptase-polymerase chain reaction (RT-PCR) on tissue homogenates have been used in the past. Altogether, these studies could not answer the question as to which specific cardiac cell type(s) express(es) the AT1 and AT2 receptor. In the present study, we applied a laser-assisted cell-picking technique and combined it with a single-cell RT-PCR for both angiotensin receptor subtypes. These experiments served to determine whether adult rat cardiomyocytes express AT1 and AT2 receptors under physiological conditions and 1 day after myocardial infarction.
Materials and Methods
Induction of Myocardial Infarction and Sham Surgery
All experiments were performed in male Wistar rats (250 to 300 g). Animals were randomly divided into three experimental groups. Myocardial infarction was induced by permanent ligation of the left descending coronary artery by a modified technique described by Johns and Olson. 22 Briefly, after induction of anesthesia with an intraperitoneal injection of chloral hydrate, rats were intubated, artificially ventilated, and connected to an electrocardiogram (ECG) recorder for continuous monitoring during surgery. A left thoracotomy was carried out by cutting the third and fourth rib and a rib-spreading chest retractor was inserted. Then, the left descending coronary artery was ligated intrathoracically using sterile 6-0 suture material under a microstereoscope. Successful ligation of the coronary artery was verified by the occurrence of arrhythmia in the ECG and, visually, by the color change of the ischemic area. In rats with sham surgery, the ligation was placed beside the coronary artery. The thoracic cavity was closed during respiration hold, and analgesia was induced by a subcutaneous injection of buprenorphin-HCl (0.2 mg/kg). Finally, animals that were neither subjected to anesthesia nor to thoracotomy served as additional controls.
Isolation and Treatment of Tissues
Twenty-four hours after permanent coronary ligation, rats were sacrificed and the hearts were immediately excised. After removal of the atria and large vessels, the ventricles were cut in a standardized fashion into transversal slices from the apex to the basis. This was achieved using a special Plexiglas box, adapted to the hearts of rats, which contains slits for a microtome knife at 3-mm intervals. The five slices obtained were immediately frozen in isopentane on dry ice (−30°C) and stored at −80°C until further processing. The use of isopentane protected the tissue against freezing-induced bursts and damage of the histological ultrastructure, making it possible to separate the different cell types by laser-assisted cell picking.
Micromorphometrical Evaluation of Infarct Sizes
The morphometrical determination of infarct sizes was performed according to Klein et al. 23
Laser-Assisted Cell Picking for Isolation of Nucleus-Free Single Cardiomyocytes
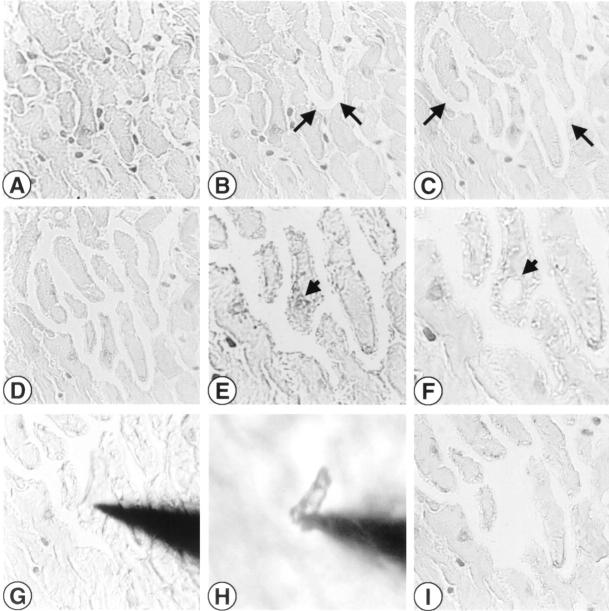
To obtain isolated, nucleus-free cardiomyocyte section profiles from infarcted and noninfarcted areas of the adult rat heart, a laser-assisted cell-picking technique (P.A.L.M., Bernried, Germany) was applied. 24 The dissection and cell harvesting (cell picking) procedures are illustrated and explained in detail in the legend to Figure 1 ▶ . Briefly, two to three samples containing 10 cardiomyocyte profiles were harvested from the noninfarcted myocardium of the posterior left ventricular wall of the respective hearts after photolysing the entire interstitial tissue between these cardiomyocytes. Microscopically visible nuclei of cardiomyocytes were photolysed by UV laser to destroy genomic DNA. Section profiles of these cells were collected with a syringe needle in first strand buffer (see Reverse Transcription) and subjected to RT-PCR for analysis of AT1 and AT2-receptor mRNA expression.
Figure 1.
Laser-assisted picking of cardiomyocytes. Example of laser-assisted cell picking of isolated, nucleus-free cardiomyocyte profiles from a noninfarcted area of the rat heart. A: Cardiomyocytes can be discriminated from interstitial nuclei. B–D: Ongoing separation of cardiomyocytes by UV laser photolysis of the interstitium surrounding cardiomyocytes (arrows indicate some areas of photolysis). E–F: A cardiomyocyte nucleus (E, short arrow) is selectively removed (F, short arrow) to minimize DNA contamination during harvesting for RT-PCR. G: The cardiomyocyte is attached to the micromanipulator-driven steel needle and is lifted (H) from the object slide under microscopic control. I: Same area after cell picking. Frozen sections, hemalaun stained. Original magnifications: ×40 (A–D), ×100 (E–F), ×64 (G–I).
Single-Cell RT-PCR
Reverse Transcription
Ten myocyte section profiles from each sample were transferred into 10 μl of First Strand Buffer (modified from Brady and Iscove 25 ; 1% Igepal CA-630, 4% RNase inhibitor in 52 mmol/L Tris-HCl, pH 8.3, 78 mmol/L KCl, 3.1 mmol/L MgCl2). The samples were cooled on ice for 5 minutes and then immediately snap-frozen.
Preceding reverse transcription, the samples were heated (70°C for 10 minutes) and then cooled on ice (5 minutes). To confirm the absence of any genomic DNA contamination, each sample was divided into two aliquots of 5 μl each and then subjected to reverse transcription in the presence or absence of the enzyme. The cDNA synthesis was carried out using 1 μl dNTP (10 mmol/L each), 1 μl random hexamers (50 μmol/L), 0.5 μl (10 U) RNase inhibitor, and 1 μl (50 U) murine leukemia virus reverse transcription in a total volume of 17.5 μl. The reaction was performed at 20°C for 10 minutes, 43°C for 60 minutes, 99°C for 5 minutes, and finally for 5 minutes on ice.
Polymerase Chain Reaction
For the subsequent AT1 receptor PCR, the following protocol was used: 4 μl buffer, 2 μl MgCl2, 1 μl dNTP (10 mmol/L each), 1 μl of each AT1 receptor-specific primer (20 μmol/L; for details see Table 1 ▶ ), 8 μl cDNA, 0.5 μl (2.5 U) AmpliTaq Gold polymerase and 32.5 μl H2O. The PCR was performed at 94°C for 2.45 minutes, followed by 63 cycles at 94°C for 0.45 minute, 57°C for 0.45 minute, and 72°C for 0.45 minute. After a final extension of 72°C for 7 minutes the PCR products were electrophoresed on a 1.8% agarose gel and ethidium bromide stained. As internal positive control, total RNA isolated from control hearts was used.
Table 1.
Primer Sequences Used for AT1 and AT2 Receptor PCR
| AT1 receptor | Sense d(cagcttggtggtgattgtc) |
| Antisense d(gccatcggtattccatagc) | |
| AT2 receptor | Sense d(tagtctctctcttgccttgg) |
| Antisense d(ctgaccttcttggatgctct) | |
| GAPDH | Sense d(ttcaccaccatggagaaggc) |
| Antisense d(ggcatggactgtggtcatga) |
For the AT2 receptor PCR, conditions were chosen as follows: 2 μl buffer, 1 μl MgCl2, 1.5 μl of each AT2 receptor-specific primer (10 μmol/L; for details see Table 1 ▶ ), 5 μl cDNA, 0.25 μl (1.25 U) AmpliTaq Gold polymerase and 13.15 μl H2O. The PCR was carried out at 94°C for 5 minutes, followed by 70 cycles at 94°C for 0.45 minute, 63°C for 1.45 minutes, and 72°C for 2.10 minutes. After a final extension of 72°C for 10 minutes the PCR products were electrophoresed on a 1.8% agarose gel and ethidium bromide stained. Total RNA isolated from PC12W cells (passage <18) which are known to abundantly express AT2 receptors served as internal control for RT-PCR amplification.
For amplification of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), PCR reaction mixtures were identical to those for the AT1 receptor. Amplification reactions were performed at 95°C for 6 minutes followed by a total of 45 three-temperature cycles (20 seconds at 95°C, 30 seconds at 61°C, and 30 seconds at 73°C) and a final extension at 73°C for 5 minutes. The sequences of GAPDH PCR primers used 26,27 are given in Table 1 ▶ .
Materials
Gene-Amp RT-PCR kits and all reagents for RT-PCR were purchased from Perkin Elmer Applied Biosystems (Überlingen, Germany). One percent Igepal CA-630, Tris-HCl, KCl, and MgCl2 were obtained from Sigma (Deisenhofen, Germany). Specific primers for the rat AT1 and AT2 receptor gene were synthesized by Pharmacia (Erlangen, Germany). The UV laser microbeam (337-nm wavelength) was manufactured by P.A.L.M. (Bernried, Germany), and the inverted microscope Axiovert 135 was made by Zeiss (Jena, Germany). All RT-PCR reactions were performed on a GeneAmp PCR System 9600 manufactured by Perkin Elmer Applied Biosystems (Überlingen, Germany).
Results
To determine the AT1- and AT2-receptor gene expression in cardiomyocytes before and after myocardial infarction, cardiomyocytes were isolated by laser-assisted cell picking (Figure 1) ▶ . For RT-PCR, 10 genomic DNA-free section profiles, which equal approximately two entire cardiomyocytes, were pooled in First Strand Buffer to form one sample. After dividing samples into two identical aliquots, they were subjected to reverse transcription with or without reverse transcriptase for subsequent PCR amplification in a blinded fashion. Furthermore, as an additional negative control RT-PCR, amplifications were always carried out in the absence of cell material to exclude any possibility of sample contamination. These reactions invariably proved to be negative for both AT1 and AT2 receptors.
AT1 Receptor Single-Cell PCR
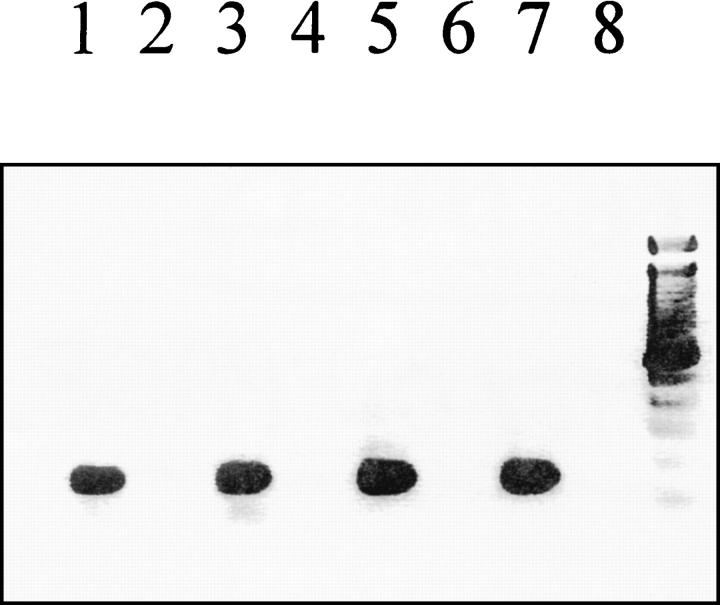
PCR amplification of AT1 receptor mRNA resulted in a single band of the predicted size (146 bp). As internal positive control, total RNA isolated from whole control hearts was used for AT1 receptor PCR (Figure 2) ▶ .
Figure 2.
AT1 receptor mRNA expression in adult rat cardiomyocytes. The AT1 receptor gene was expressed in 42% (five out of 12) of these cells obtained from control animals (n = 4) and in 33% (eight out of 24) isolated from sham-operated animals (n = 4), whereas AT1 receptor mRNA was detected in 45% (nine out of 20) of cardiomyocytes after myocardial infarction (n = 4). Displayed is a representative ethidium bromide-stained 1.8% agarose gel showing the AT1 receptor RT-PCR results in the presence (+RT) or absence (−RT) of reverse transcriptase. Lane 1, control animals (+RT); lane 2, control animals (−RT); lane 3, sham-operated animals (+RT); lane 4, sham-operated animals (−RT); lane 5, myocardial infarction (+RT); lane 6, myocardial infarction (−RT); lane 7, whole heart (+RT); lane 8, negative control.
In control animals (n = 4), 14 samples were investigated. Two of them had to be excluded from the study because they showed an AT1 receptor signal in the absence of reverse transcriptase. Five out of the remaining 12 samples were AT1 receptor-positive (42%) (Figure 2) ▶ .
Three samples gained from sham-operated animals (n = 4) were discarded because of positive AT1 receptor PCR in the absence of reverse transcriptase. Eight out of 24 remaining samples were AT1 mRNA-positive (33%) (Figure 2) ▶ .
Finally, 1 day after myocardial infarction (n = 4), the number of cardiomyocytes expressing AT1 receptor mRNA was not significantly changed. Because of a positive signal in the absence of reverse transcriptase two samples had to be excluded. Nine out of the remaining 20 samples (45%) were AT1 receptor mRNA-positive (Figure 2) ▶ .
AT2 Receptor Single-Cell PCR
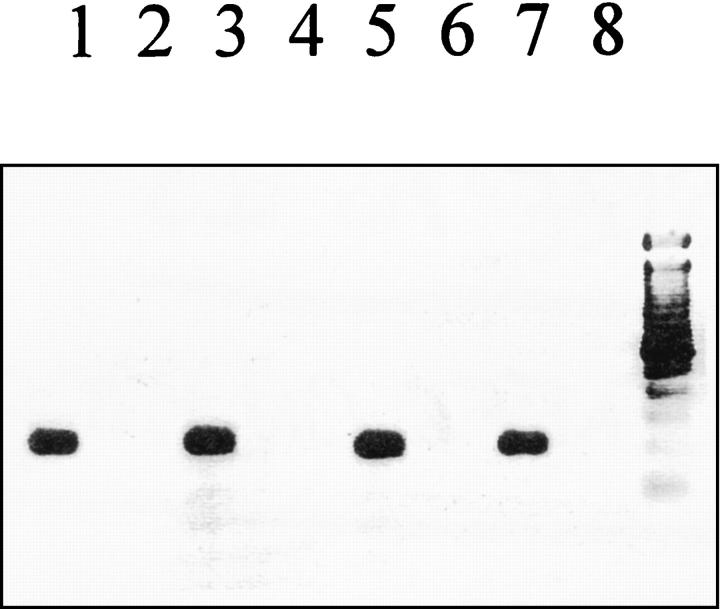
One single band of the expected size (217 bp) was gained from the AT2 receptor PCR. Total RNA isolated from PC12W cells (passage <18) was used as an internal positive control for the AT2 receptor PCR in each experiment (Figure 3) ▶ .
Figure 3.
AT2 receptor mRNA expression in adult rat cardiomyocytes. Using single-cell RT-PCR it was observed that the AT2 receptor gene was expressed in 8% (one out of 13) of cardiomyocytes obtained from control animals (n = 4), in 13% (three out of 23) of these cells from sham-operated animals, and in 14% (three out of 21) of those cells isolated after myocardial infarction (n = 4). Displayed is a representative ethidium bromide-stained 1.8% agarose gel showing the AT2 receptor RT-PCR results in the presence (+RT) or absence (−RT) of reverse transcriptase. Lane 1, control animals (+RT); lane 2, control animals (−RT); lane 3, sham-operated animals (+RT); lane 4, sham-operated animals (−RT); lane 5, myocardial infarction (+RT); lane 6, myocardial infarction (−RT); lane 7, PC12W cells (+RT); lane 8, negative control.
In control animals (n = 4), one sample out of the 14 investigated samples elicited an AT2 receptor signal in the absence of reverse transcriptase and had, thus, to be excluded. Out of the remaining 13 samples, one was positive for the AT2 receptor (8%) (Figure 3) ▶ .
In the sham-operated group (n = 4), positive AT2 receptor mRNA signals were observed in three out of 23 samples (13%) and no PCR signals were detected in the absence of reverse transcriptase (Figure 3) ▶ .
One day after myocardial infarction (n = 4), one sample out of 22 had to be excluded from the study because of a positive signal in the absence of reverse transcriptase. Three out of the remaining 21 samples (14%) showed a strong AT2 receptor mRNA signal (Figure 3) ▶ revealing that cardiomyocytes from adult rats do express AT2 receptors. The RT-PCR data for AT1 and AT2 receptors are summarized in Table 2 ▶ .
Table 2.
Expression of AT1 and AT2 Receptor mRNA in Adult Cardiomyocytes Before and After Myocardial Infarction
| AT1 receptor mRNA | AT2 receptor mRNA | |
|---|---|---|
| Control animals | 5 /12 (42%) | 1 /13 (8%) |
| Sham-operated animals | 8 /24 (33%) | 3 /23 (13%) |
| Myocardial infarction (1 day) | 9 /20 (45%) | 3 /21 (14%) |
GAPDH Single-Cell RT-PCR
To demonstrate the general sensitivity of the described single-cell RT-PCR assay, the mRNA expression of the housekeeping gene GAPDH was investigated in cardiomyocyte section profiles gained from control animals. In none of the 54 samples studied, were PCR signals observed in the absence of reverse transcriptase. Out of these 54 samples, 52 were GAPDH-positive (96%) underlining the sensitivity and reproducibility of this assay (data not shown).
Determination of Infarct Sizes
To verify that myocardial infarctions were of similar degrees, the infarction sizes were micromorphometrically evaluated. These experiments revealed similar infarction sizes among the four animals of the myocardial infarction group (45.7%, 47.3%, 48.6%, and 49.1%, respectively) demonstrating the consistency of the procedure used (data not shown).
Discussion
Previous studies have revealed that AT1 and AT2 receptors are up-regulated after myocardial infarction. However, because of the limitations of the techniques formerly applied (binding assays, autoradiography, RT-PCR using tissue homogenates) the cellular cardiac distribution of AT2 receptors has not yet been determined. Therefore, we established a single-cell RT-PCR for AT1 and AT2 receptors and investigated the expression of both receptor subtypes in the heart of adult rats under physiological and pathophysiological conditions. As determined by the mRNA expression of the housekeeping gene GAPDH the sensitivity and reproducibility of the assay used equaled 96%.
The present study demonstrates for the first time that adult rat cardiomyocytes not only express the AT1 but also the AT2 receptor gene. Approximately 10 percent of cardiomyocytes isolated from adult rats expressed the AT2 receptor gene under physiological conditions as well as 1 day after myocardial infarction. Thus, in contrast to a previous study, 28 we have demonstrated for the first time that adult rat cardiomyocytes, at least to a certain percentage, contain the AT2 receptor mRNA. Also, we could demonstrate that the number of cardiomyocytes that express the AT1 receptor mRNA is not increased after myocardial infarction.
In our study, a laser-assisted cell-picking technique 24,29 was used to obtain cardiomyocyte samples without genomic DNA contamination. Because genomic DNA can serve as a template in RT-PCR reactions its absence must be verified to ensure that an observed gene expression indeed reflects mRNA levels of the respective genes. To avoid DNase treatments on tiniest amounts of total RNA obtained from single cells but to overcome this particular problem, microscopically guided UV laser photolysis of cell nuclei was applied. The cell-picking technique that was used here has previously been shown to be successful for the mRNA analysis of single cells by RT-PCR and has been proven to be highly effective in eliminating genomic DNA. 30 However, to confirm the absence of genomic DNA in the samples used, different controls were performed for each sample. First, samples containing cell material were divided into two tubes and were subjected to RT-PCR, once in the presence and once in the absence of reverse transcriptase. Samples were only used for this study if they did not show any signal for both the AT1 and AT2 receptor in the absence of reverse transcriptase. Second, all negative controls, eg, samples without cell material were included in a blinded manner and did not elicit any signals for AT1 and AT2 receptors after RT-PCR.
The identification of cardiomyocytes was carried out by histological means and not via cardiomyocyte-specific gene expression because this approach is often not conclusive. This is particularly the case after a traumatic tissue injury like myocardial infarction (MI) that causes massive alterations in the gene expression patterns of different cell types. Even if a certain gene was exclusively expressed in cardiomyocytes before MI this would not necessarily be the case after MI resulting in possible false-positive identification. On the other hand, cardiomyocytes exhibit a characteristic and distinct morphology and are microscopically clearly distinguishable from cells such as neurons or fibroblasts which makes it possible to conclusively identify and isolate this particular cell type. Therefore, microdissection experiments combined with RT-PCR are the method of choice to isolate a certain cell type and to identify a cell type-specific gene expression.
In previous studies it has been demonstrated that myocardial infarction evokes a dramatic up-regulation of both angiotensin receptor subtypes. Whereas Zhu et al 4 observed a pronounced increase in the AT1 and AT2 receptor gene expression as soon as 24 hours after myocardial infarction, Nio et al 3 reported a maximal expression after 7 days. Both studies used tissue homogenates and, therefore, did not discriminate between different cell types. By applying a single-cell RT-PCR technique, we show here that cardiomyocytes in vivo express AT1 and AT2 receptor mRNA under physiological conditions but that the number of cells expressing these receptors is not increased after myocardial infarction. However, with respect to the study performed by Nio et al 3 the possibility cannot be excluded that a greater number of cardiomyocytes may express the AT2 receptor gene at a later time point.
With regard to the RT-PCR data presented the previously reported increase in AT1- and AT2-receptor mRNA levels after myocardial infarction might be explained in two different ways but it should be noted that the assay used in this study was not carried out under quantitative conditions: first, the AT1 and AT2 receptor mRNA expression is increased in those cardiomyocytes which already express these receptors under physiological conditions. However, the assay used in our study cannot be applied to address this particular question. Under the experimental conditions used here for the detection of both angiotensin receptor genes in single cells, saturation phases of PCR are reached making it extremely difficult to quantitate the gene expression. A second explanation is that other cell types within the heart such as fibroblasts, neurons, or endothelial cells are responsible for the observed AT1- and AT2-receptor mRNA up-regulation. Unfortunately, compared to cardiomyocytes these cell types are much smaller. Using the laser-assisted cell-picking technique, it is not yet possible to photolyse the nuclei of these cells without photolysing the entire cell. Therefore, this method is, at least at present, restricted to the larger cardiomyocytes. To determine the angiotensin receptor gene expression in other cell types, other improved experimental approaches have to be used in the future. Also, these studies will have to address the question whether adult rat cardiomyocytes do express the AT2 receptor protein or just the AT2 receptor mRNA.
The question which specific cell type is responsible for the AT1 and AT2 receptor expression in the heart is of major importance because Ang II plays an important role in cardiovascular function. In neonatal rats, AT2 receptor protein is detectable in cardiac myocytes but not in fibroblasts. 31 Mechanical stretch of myocytes evokes increases in both AT1- and AT2-receptor mRNA levels 32 indicating that nonsecretory pathways activated by myocyte stretching are involved in angiotensin receptor regulation. In the human heart, the level of expression of AT1 receptor genes seems to decrease in the failing ventricle whereas the level of AT2 receptor expression is unaffected. 33 Finally, after heart transplantation, both angiotensin receptors are down-regulated. 34
Several studies elucidated the physiological role of AT2 receptors in the heart but the available data are still controversial. Angiotensin receptors have both been demonstrated to induce apoptosis in different cell types. With respect to the heart, it has been reported that AT1 but not AT2 receptor stimulation induces programmed cell death in cardiomyocytes 19,20 by activating p53. 35 On the other hand, Ang II is capable of promoting both apoptosis and neuronal regeneration 6,10 via its AT2 receptor. In PC12W cells, AT2 receptor-mediated apoptosis is paralleled by inactivation of Bcl-2 8 as well as generation of ceramides in PC12W cells. 10,11 Thus, the observation that adult cardiomyocytes not only express AT1 but also AT2 receptors may have important consequences for the heart, under physiological and pathophysiological conditions.
The data presented here clearly indicate that cardiomyocytes in the adult organism are capable of expressing AT2 receptors. Therefore, when evaluating Ang II effects on the heart, AT2 receptor-mediated effects have to be taken into account. This is, in particular, the case when discussing potential side effects of AT1 receptor antagonists. One major concern about the use of these compounds for the treatment of hypertension is that they engender an indirect overstimulation of the unopposed AT2 receptor by increasing Ang II plasma levels. Because AT2 receptors have been identified as a receptor being able to induce apoptosis it was argued that one potential serious consequence of AT1 receptor blockade might be the AT2 receptor-mediated cell death of cardiomyocytes. Because our studies clearly demonstrate that cardiomyocytes express AT2 receptor mRNA under physiological and pathophysiological conditions the possibility of an AT1 receptor antagonist-induced and AT2 receptor-mediated apoptosis does exist.
In summary, we have shown that the AT2 receptor gene is expressed in ∼10% of adult rat cardiomyocytes before and 1 day after myocardial infarction. Further, AT1 receptor mRNA is present in approximately every second cardiomyocyte with the number of cells expressing this receptor being not increased by tissue injury. Future studies will have to determine whether the observed angiotensin receptor mRNA expression in cardiomyocytes directly translates into protein expression and whether other cardiac cell types also express AT2 receptors. Also, they will have to assess whether the AT1 receptor antagonist-evoked increase in Ang II plasma levels indeed induces AT2 receptor-mediated side effects in the heart.
Footnotes
Address reprint requests to Silke Busche, Ph.D., Department of Physiology, College of Medicine, University of Florida, 1600 S.W. Archer Rd.,P.O. Box 100274, Gainesville, FL 32610. E-mail: sbusche@phys.med.ufl.edu.
S. B. and S. G. contributed equally to these studies.
References
- 1.Timmermans PBMWM, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JAM, Smith RD: Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev 1993, 45:205-251 [PubMed] [Google Scholar]
- 2.Unger T, Chung O, Csikos T, Culman J, Gallinat S, Gohlke P, Höhle S, Meffert S, Stoll M, Stroth U, Zhu YZ: Angiotensin receptors. J Hypertens 1996, 14:95-103 [PubMed] [Google Scholar]
- 3.Nio Y, Matsubara H, Murasawa S, Kanasaki M, Inada M: Regulation of gene transcription of angiotensin II receptor subtypes in myocardial infarction. J Clin Invest 1995, 95:46-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu YZ, Li J, Zhu YC, Chung O, Spitznagel H, Sandmann S, Tschöpe C, Unger T: Increased expression of angiotensin AT1 and AT2 receptors in the acute phase of myocardial infarction. Hypertension 1996, 28:541 [Google Scholar]
- 5.Gallinat S, Yu M, Dorst A, Unger T, Herdegen T: Sciatic nerve transection evokes lasting up-regulation of angiotensin AT2 and AT1 receptor mRNA in adult rat dorsal root ganglia and sciatic nerves. Mol Brain Res 1998, 57:111-122 [DOI] [PubMed] [Google Scholar]
- 6.Lucius R, Gallinat S, Rosenstiel P, Herdegen T, Sievers J, Unger T: The angiotensin II type 2 (AT2) receptor promotes axonal regeneration in the optic nerve of adult rats. J Exp Med 1998, 188:661-670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamada T, Horiuchi M, Dzau VJ: Angiotensin II type 2 receptor mediates programmed cell death. Proc Natl Acad Sci USA 1996, 93:156-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horiuchi M, Hayashida W, Kambe T, Yamada T, Dzau VJ: Angiotensin type 2 receptor dephosphorylates Bcl-2 by activating mitogen-activated protein kinase phosphatase-1 and induces apoptosis. J Biol Chem 1997, 272:19022-19026 [DOI] [PubMed] [Google Scholar]
- 9.Shenoy UV, Richards EM, Huang XC, Sumners C: Angiotensin II type 2 receptor-mediated apoptosis of cultured neurons from newborn rat brain. Endocrinology 1999, 140:500-509 [DOI] [PubMed] [Google Scholar]
- 10.Gallinat S, Busche S, Schütze S, Krönke M, Unger T: AT2 receptor stimulation induces generation of ceramides in PC12W cells. FEBS Lett 1999, 443:75-79 [DOI] [PubMed] [Google Scholar]
- 11.Lehtonen JY, Horiuchi M, Daviet L, Akishita M, Dzau VJ: Activation of the de novo biosynthesis of sphingolipids mediates angiotensin II type 2 receptor-induced apoptosis. J Biol Chem 1999, 274:16901-16906 [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Leri A, Li B, Wang X, Cheng W, Kajstura J, Anversa P: Angiotensin II stimulation in vitro induces hypertrophy of normal and postinfarcted ventricular myocytes. Circ Res 1998, 82:1145-1159 [DOI] [PubMed] [Google Scholar]
- 13.McEwan PE, Gray GA, Sherry L, Webb DJ, Kenyon CJ: Differential effects of angiotensin II on cardiac cell proliferation and intramyocardial perivascular fibrosis in vivo. Circulation 1998, 98:2765-2773 [DOI] [PubMed] [Google Scholar]
- 14.Brilla CG, Scheer C, Rupp H: Renin-angiotensin system and myocardial collagen matrix: modulation of cardiac fibroblast function by angiotensin II type 1 receptor antagonism. J Hypertens 1997, 6:S13-S19 [PubMed] [Google Scholar]
- 15.Stoll M, Steckelings UM, Paul M, Bottari SP, Metzger R, Unger T: The angiotensin AT2 receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest 1995, 95:651-657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakajima M, Hutchinson HG, Fujinaga M, Hayashida W, Morishita R, Zhang L, Horiuchi M, Pratt RE, Dzau VJ: The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer. Proc Natl Acad Sci USA 1995, 92:10663-10667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuzuki S, Eguchi S, Inagami T: Inhibition of cell proliferation and activation of protein tyrosine phosphatase mediated by angiotensin II type 2 (AT2) receptor in R3T3 cells. Biochem Biophys Res Commun 1996, 228:825-830 [DOI] [PubMed] [Google Scholar]
- 18.van Kesteren CA, van Heugten HA, Lamers JM, Saxena PR, Schalekamp MA, Danser AH: Angiotensin II-mediated growth and antigrowth effects in cultured neonatal rat cardiac myocytes and fibroblasts. J Mol Cell Cardiol 1997, 29:2147-2157 [DOI] [PubMed] [Google Scholar]
- 19.Cigola E, Kajstura J, Li B, Meggs LG, Anversa P: Angiotensin II activates programmed cell death in vitro. Exp Cell Res 1997, 231:363-371 [DOI] [PubMed] [Google Scholar]
- 20.Kajstura J, Cigola E, Malhotra A, Li P, Cheng W, Meggs LG, Anversa P: Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J Mol Cell Cardiol 1997, 29:859-870 [DOI] [PubMed] [Google Scholar]
- 21.Gray MO, Long CS, Kalinyak JE, Li HT, Karliner JS: Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res 1998, 40:352-363 [DOI] [PubMed] [Google Scholar]
- 22.Johns TNP, Olson BJ: Experimental myocardial infarction. A method of coronary occlusion in small animals. Ann Surg 1954, 140:675-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein HH, Pich S, Bohle RM, Wollenweber J, Nebendahl K: Myocardial protection by Na(+)-H+ exchange inhibition in ischemic, reperfused porcine hearts. Circulation 1995, 92:912-917 [DOI] [PubMed] [Google Scholar]
- 24.Fink L, Stahl U, Ermert L, Kummer W, Seeger W, Bohle RM: Rat porphobilinogen deaminase gene: a pseudogene-free internal standard for laser-assisted cell picking. BioTechniques 1999, 26:510-516 [DOI] [PubMed] [Google Scholar]
- 25.Brady G, Iscove NN: Construction of cDNA libraries from single cells. Methods Enzymol 1993, 225:611-623 [DOI] [PubMed] [Google Scholar]
- 26.Overbergh L, Valckx D, Waer M, Mathieu C: Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine 1999, 11:305-312 [DOI] [PubMed] [Google Scholar]
- 27.Zheng J, Ramirez VD: Rattus norvegicus glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA, complete cds. National Center for Biotechnology Information Entrez Accession No. AF 106860
- 28.Meggs LG, Coupet J, Huang H, Cheng W, Li P, Capasso JM, Homcy CJ, Anversa P: Regulation of angiotensin II receptors on ventricular myocytes after myocardial infarction in rats. Circ Res 1993, 72:1149-1162 [DOI] [PubMed] [Google Scholar]
- 29.Fink L, Seeger W, Ermert L, Hanze J, Stahl U, Grimminger F, Kummer W, Bohle RM: Real-time quantitative RT-PCR after laser-assisted cell picking. Nat Med 1998, 4:1329-1333 [DOI] [PubMed] [Google Scholar]
- 30.Kummer W, Fink L, Dvorakova M, Haberberger R, Bohle RM: Rat cardiac neurons express the non-coding R-exon (exon 1) of the cholinergic gene locus. NeuroReport 1998, 9:2209-2212 [DOI] [PubMed] [Google Scholar]
- 31.Wang ZQ, Moore AF, Ozono R, Siragy HM, Carey RM: Immunolocalization of subtype 2 angiotensin II (AT2) receptor protein in rat heart. Hypertension 1998, 32:78-83 [DOI] [PubMed] [Google Scholar]
- 32.Kijima K, Matsubara H, Murasawa S, Maruyama K, Mori Y, Ohkubo N, Komuro I, Yazaki Y, Iwasaka T, Inada M: Mechanical stretch induces enhanced expression of angiotensin II receptor subtypes in neonatal rat cardiac myocytes. Circ Res 1996, 79:887-897 [DOI] [PubMed] [Google Scholar]
- 33.Haywood GA, Gullestad L, Katsuya T, Hutchinson HG, Pratt RE, Horiuchi M, Fowler MB: AT1 and AT2 angiotensin receptor gene expression in human heart failure. Circulation 1997, 95:1201-1206 [DOI] [PubMed] [Google Scholar]
- 34.Gullestad L, Haywood G, Aass H, Ross H, Yee G, Ueland T, Geiran O, Kjekshus J, Simonsen S, Bishopric N, Fowler M: Angiotensin II receptor subtype AT1 and AT2 expression after heart transplantation. Cardiovasc Res 1999, 38:340-347 [DOI] [PubMed] [Google Scholar]
- 35.Leri A, Claudio PP, Li Q, Wang X, Reiss K, Wang S, Malhotra A, Kajstura J, Anversa P: Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell. J Clin Invest 1998, 101:1326-1342 [DOI] [PMC free article] [PubMed] [Google Scholar]