Abstract
Pbx proteins are a family of TALE-class transcription factors that are well characterized as Hox co-factors acting to impart segmental identity to the hindbrain rhombomeres. However, no role for Pbx in establishing more anterior neural compartments has been demonstrated. Studies done in Drosophila show that Engrailed requires Exd (Pbx orthologue) for its biological activity. Here, we present evidence that zebrafish Pbx proteins cooperate with Engrailed to compartmentalize the midbrain by regulating the maintenance of the midbrain-hindbrain boundary (MHB) and the diencephalic-mesencephalic boundary (DMB). Embryos lacking Pbx function correctly initiate midbrain patterning, but fail to maintain eng2a, pax2a, fgf8, gbx2, and wnt1 expression at the MHB. Formation of the DMB is also defective as shown by a caudal expansion of diencephalic epha4a and pax6a expression into midbrain territory. These phenotypes are similar to the phenotype of an Engrailed loss-of-function embryo, supporting the hypothesis that Pbx and Engrailed act together on a common genetic pathway. Consistent with this model, we demonstrate that zebrafish Engrailed and Pbx interact in vitro, and that this interaction is required for both the eng2a overexpression phenotype and Engrailed’s role in patterning the MHB. Our data support a novel model of midbrain development in which Pbx and Engrailed proteins cooperatively pattern the mesencephalic region of the neural tube.
Keywords: zebrafish, Engrailed, Pbx, midbrain, midbrain-hindbrain boundary, diencephalic-mesencephalic boundary, hexapeptide, lineage restriction, neural patterning
Introduction
Over the course of vertebrate development, the neural plate is progressively subdivided into functionally specialized, lineage restricted compartments (Kiecker and Lumsden, 2005). Tissue compartmentalization is important to specify cell position, identity and function during vertebrate patterning. The seven rhombomeres of the hindbrain were the first observed lineage-restricted compartments in the vertebrate nervous system (Fraser et al., 1990; von Baer, 1828). Hindbrain segmentation has since been shown to occur downstream of Hox proteins and their DNA binding co-factors Pbx and Meis. Lineage-restriction has also been observed at the diencephalic-mesencephalic boundary (DMB) and the midbrain-hindbrain boundary (MHB), which enclose the midbrain at its rostral and caudal ends respectively. In this regard, the vertebrate neural tube is an excellent system in which to study the formation and maintenance of lineage-restricted boundaries.
The Pbx (pre-B-cell leukemia transcription factor) family of TALE class homeodomain transcription factors are best characterized as heterodimeric partners for Hox proteins (Mann and Chan, 1996; Moens and Selleri, 2006). Pbx proteins are hypothesized to reveal intrinsic DNA-binding specificity within the Hox proteins, as well as to coordinately bind an adjacent Pbx recognition site in the promoter of target genes (Chan et al., 1996; Knoepfler et al., 1996; Mann and Chan, 1996). As such, Pbx-Hox complexes often have a much higher DNA binding specificity and affinity than either Pbx or Hox alone. A zebrafish mutant in the pbx4 gene (lazarus or lzr) was identified in a genetic screen for embryos that fail to properly express the rhombomere 3 (r3) and r5-specific transcription factor egr2b (krox20) (Popperl et al., 2000). Two partially redundant zebrafish pbx genes, pbx2 and pbx4, are expressed during early embryogenesis at a time when the hindbrain is being patterned. These two Pbx proteins cooperate with Hox proteins to drive expression of early hindbrain patterning genes such as fgf3, fgf8, hoxb1a, and vhnf1 (Hernandez et al., 2004; Maves et al., 2002; Popperl et al., 1995; Walshe et al., 2002; Waskiewicz et al., 2002). In the absence of Pbx2 and Pbx4 proteins, the region of hindbrain normally fated to give rise to r2-r6 is deprogrammed to adopt the default groundstate identity of r1, a segment that lacks expression of any hox gene (Waskiewicz et al., 2002). As such, the hindbrain region of Pbx-less embryos mimics the loss of all hindbrain hox gene function, demonstrating the importance of Pbx proteins in tissue compartmentalization during vertebrate hindbrain development. However, although Pbx genes are expressed ubiquitously throughout the developing zebrafish nervous system, no role for Pbx proteins in the formation or patterning of either forebrain or midbrain has been described.
Within the Hox proteins themselves, a motif called the hexapeptide is required for cooperative DNA binding with Pbx (Chang et al., 1995; Neuteboom et al., 1995). This evolutionarily conserved consensus motif, located just N-terminal of the Hox homeodomain, consists of the residues YQWPM. The hexapeptide motif, particularly the tryptophan residue, binds within a hydrophobic pocket formed by the extended loop between helix 1 and 2 in the Pbx homeodomain (LaRonde-LeBlanc and Wolberger, 2003; Piper et al., 1999). The mechanism of the homeodomain-hexapeptide interaction is conserved in fly Exd and Hox proteins as well (Passner et al., 1999), illustrating the importance of Pbx-Hox interactions during development.
Other hexapeptide-containing transcription factors have been found to bind Pbx proteins (In der Rieden et al., 2004). Amongst these Pbx-interacting proteins is the homeodomain transcription factor Engrailed (abbreviated Eng or En). In Engrailed proteins, a hexapeptide motif (WPAWVY) is located just upstream of the EH2 (Eng Homology-2) domain. The hexapeptide, along with the EH2 and EH3 domains, is required for the Pbx - Eng interaction (Peltenburg and Murre, 1996). Within the Engrailed hexapeptide itself, the two tryptophan residues are of particular importance in mediating cooperative binding between Pbx and Eng. Additionally, the three amino acid extension of the Pbx homeodomain is also required for the Pbx–Eng interaction (Peltenburg and Murre, 1997). All domains necessary for the Pbx-Eng interaction are conserved in flies and vertebrates, pointing to the importance of this interaction for metazoan development.
Engrailed was originally identified in Drosophila as a factor required for the maintenance of cellular compartments during fly development (Hidalgo, 1996). In Drosophila, a genetic interaction between engrailed and the pbx orthologue extradenticle (exd) has been established based on the similarity in phenotypes between maternal, zygotic exd mutants and those of en mutant flies (Alexandre and Vincent, 2003; Kobayashi et al., 2003; Peifer and Wieschaus, 1990). Biochemical evidence suggests that the Pbx / Exd family of TALE-class homeodomain proteins can directly bind Engrailed in vitro and in vivo (Kobayashi et al., 2003; Peltenburg and Murre, 1996; Serrano and Maschat, 1998; van Dijk and Murre, 1994; van Dijk et al., 1995). Experimentally, Engrailed’s role as a transcriptional regulator has been shown to require the presence of functional Exd and Homothorax (Hth; vertebrate Meis) proteins (Alexandre and Vincent, 2003; Kobayashi et al., 2003; Rieckhof et al., 1997). A trimeric complex of En, Exd, and Hth can cooperatively bind DNA and either activate or repress transcription of target genes (Alexandre and Vincent, 2003; Kobayashi et al., 2003). En expression is autoregulatory and is not maintained in maternal, zygotic exd mutants, suggesting that en requires exd to positively regulate its own expression (Peifer and Wieschaus, 1990). These studies have established a genetic and biochemical pathway involving Engrailed and TALE-class transcription factors. However, vertebrate developmental pathways involving a Pbx-Eng interaction have not been investigated.
In vertebrates, the best-described role for Engrailed is in patterning the mesencephalic region of the developing neural tube, especially the midbrain-hindbrain boundary (MHB). Formed at the interface between anterior (otx2-expressing) and posterior (gbx2-expressing) neural tissue, the isthmic organizer (IsO) at the MHB has been identified as an important source of signals required for specification of the mesencephalon and the rostral metencephalon, as well as formation and maintenance of the DMB and MHB (Alvarado-Mallart et al., 1990; Raible and Brand, 2004; Wurst and Bally-Cuif, 2001). Fgf8 is likely the main IsO signaling molecule as ectopic Fgf8 protein can mimic the organizer activity of the MHB (Crossley et al., 1996; Martinez et al., 1999) Although the interface of otx2 and gbx2 expression correlates with the position of the MHB, it is unclear how gene expression at the MHB organizer is initiated. In mice, expression of MHB markers can be initiated in the absence of otx2 and gbx2 function (Li and Joyner, 2001). This suggests that other factors are involved in MHB establishment, such as Wnt8 signals originating from the lateral mesendodermal cells (Rhinn et al., 2005), and transcriptional regulation by pou5f1 (spg) and sp5 (bts1) (Burgess et al., 2002; Tallafuss et al., 2001). Although MHB initiation is not well understood, it is clear that following establishment there is considerable transcriptional interdependence amongst the MHB patterning factors. Maintenance appears to involve a complicated cross-regulatory loop involving the secreted factors Wnt1 and Fgfs 8, 17, and 18, as well as transcriptional regulators including the Pax2/5/8 family, Irx1b, Irx7, Lmx1b.1, Lmx1b.2, and Engrailed proteins (Brand et al., 1996; Itoh et al., 2002; McMahon and Bradley, 1990; McMahon et al., 1992; O’Hara et al., 2005; Reifers et al., 1998). Functional perturbations in any of these genes can lead to a depletion of all other MHB markers and a loss of tectal and cerebellar structures.
Besides being a primary player in the cross-regulatory loop that maintains the isthmic organizer, Engrailed also performs more specialized functions in midbrain development. Specifically, Engrailed is required to position the caudal extent of the forebrain by maintaining the DMB and to polarize gene expression in the optic tectum (Araki and Nakamura, 1999; Liu and Joyner, 2001; Logan et al., 1996; Scholpp and Brand, 2001; Scholpp et al., 2003). Additionally, Engrailed can act as a cell-cell signaling molecule to guide retinal ganglion cell axons via a novel secretory mechanism (Brunet et al., 2005; Maizel et al., 1999). In mouse and zebrafish embryos lacking Engrailed function, expression of the forebrain markers pax6a and epha4a are expanded caudally, implying Eng proteins are required to maintain the integrity of the DMB. Conversely, ectopic overexpression of Engrailed can repress pax6 expression in the forebrain and cause a rostral expansion of midbrain identity (Araki and Nakamura, 1999; Scholpp and Brand, 2001; Scholpp et al., 2003). Furthermore, Araki and Nakamura present evidence in chick that the repression of pax6 by ectopic En-2 occurs prior to the induction of pax2, pax5 and fgf8, suggesting that the foremost function of Engrailed is to maintain the DMB. Taken together, these studies highlight the importance of Engrailed protein function in the formation, patterning and maintenance of the vertebrate midbrain.
Here we present evidence that zebrafish Pbx proteins are important regulators of MHB and DMB formation by acting as biochemical partners with Engrailed proteins. Zebrafish embryos that lack Pbx2 and Pbx4 function initiate MHB development normally, but progressively lose eng2a, pax2a, fgf8, gbx2, and wnt1 expression as well as the corresponding midbrain-derived structures. Likewise, we show that in the absence of Pbx function, the forebrain domain of pax6a expression is caudally expanded, suggesting that Pbx proteins are required to maintain the integrity of the DMB. We also show in vitro that zebrafish Pbx4 interacts biochemically with the zebrafish Eng2a protein and that this physical interaction is required for the biological activity of eng2a overexpression in vivo. Based on these results, we favor a model where Eng requires Pbx as a co-factor in the midbrain to properly pattern the MHB and DMB.
Materials and Methods
Whole mount in situ hybridization and mRNA injection
Examination of gene expression by whole-mount in situ hybridization was carried out essentially as described (Prince et al., 1998). For two-color in situs, we also labeled probes with fluorescein-UTP and detected alkaline phosphatase with either Fast-Red (Sigma) or Iodonitrotetrazolium-violet (Sigma). Embryos were manually deyolked, and photographed using a Zeiss Axioskop, Axioplan, or Axio Imager Z1 compound microscope and digital camera (SPOT RT, Retiga Exi, or Axiocam HR). Images were assembled in Photoshop (Adobe).
To make eng2a mRNA, a full-length eng2a ORF was cloned into pCS3+MT. eng2a mRNA was synthesized as a N-terminal 6X myc-tagged mRNA using the mMessage mMachine kit (Ambion). Following synthesis, mRNA was purified using four consecutive YM-30 microcon columns. mRNA concentration was estimated by spectrophotometry, and RNA was diluted to appropriate concentration in DEPC-treated H2O. For eng2a mRNA injections, 25 pg was the minimal quantity required to cause profound defects in eye formation and repress pax6a expression. The eng2a,2bMO and pbx2,4MO rescue experiments used 100 pg of mRNA.
Zebrafish strains, genotyping, and morpholinos
The pbx4 allele b557 (also known as lazarus or lzr) mutation was identified by aberrations in the expression pattern of egr2b (krox20) as described previously (Popperl et al., 2000). We used the following Pbx translation-blocking morpholinos in this paper:
Pbx2MO1: CCGTTGCCTGTGATGGGCTGCTGCG
Pbx2MO2: GCTGCAACATCCTGAGCACTACATT
Pbx4MO1: AATACTTTTGAGCCGAATCTCTCCG
Pbx4MO2: CGCCGCAAACCAATGAAAGCGTGTT
Pbx-depleted embryos were created using three methods. In the first, zebrafish embryos lacking maternal and zygotic pbx4 gene function was accomplished by germ line transplantation as described previously (Waskiewicz et al., 2002). We generated Pbx-depleted embryos by injecting pbx2MO1 into mzpbx4 one-cell stage embryos. In the second, we injected pbx2MO1 and pbx4MO1 into one-cell stage lzr (pbx4−/−) embryos. In the third method, all four pbx morpholinos were injected into one-cell stage AB embryos. The phenotypes produced from all three methods were indistinguishable from one another. We used the morphological features of a small ear and malformed midbrain to identify live Pbx-depleted embryos at 24 hpf. By in situ analysis, we assayed for the absence of egr2b (krox20) expression in the hindbrain to identify Pbx-depleted embryos.
The no isthmus (noi / pax2a−/−) b539 strain of fish was acquired from the Zebrafish International Resource Center. To create Engrailed-null embryos, we used eng2a (eng2) (CGCTCTGCTCATTCTCATCCATGCT) and eng2b (eng3) (CTATGATCATTTTCTTCCATAGTGA) morpholinos, as described previously (Scholpp and Brand, 2001).
Mobility shift assays and western immunoblotting
Electrophoretic mobility shift assays were performed using the EMSA core kit (Promega) and precast EMSA gels (Invitrogen) according to manufacturers recommendations. eng2a and pbx4 open reading frames were subcloned into pCS3MT and pCS2MT, respectively and their sequences confirmed. Protein was synthesized using a coupled in vitro transcription and translation system (SP6 Wheat germ lysate TnT, Promega). Point mutations in Eng2a were created using the Quickchange site directed mutagenesis procedure according to manufacture’s recommendations (Stratagene). For the Eng-Pbx cooperative binding experiments, the following oligonucleotide was synthesized and labeled using T4 polynucleotide kinase and 32[P]-ATP: 5’-GTCAATTAAATGATCAATCAATTTCG-3’.
To assay for the in vivo translation efficiency of the various eng2a mRNAs, we injected one-cell embryos with 100pg of each mRNA construct, and extracted protein from 70% epiboly embryos essentially as previously described (Link et al., 2006). Western immunoblots were performed using precast 4–12% gradient gels (Invitrogen), followed by blotting to PVDF membranes (Millipore). Western detection for the myc-Eng fusion proteins were done using a 1:5000 dilution of anti-Myc 1° antibody (9E10, Covance), a 1:7500 dilution of sheep anti-mouse IgG HRP (Amersham), followed by chemiluminescent detection with Pierce Supersignal West Pico Chemiluminescent Substrate.
Results
Pbx proteins are required for the proper formation of the midbrain and for maintenance of gene expression at the MHB
Given the established genetic and biochemical interactions between Engrailed and Exd proteins in flies, we wanted to see if Pbx proteins cooperated with Engrailed to pattern the vertebrate midbrain. As a first step, we examined midbrain morphology in live wild type, lazarus (lzr / pbx4−/−), and Pbx-depleted embryos at 24 hours post-fertilization (hpf). In wild type embryos, the characteristic isthmic constriction has formed at the MHB with the tectum and the cerebellum located rostrally and caudally to the MHB respectively. (Fig.1A, A′). In lzr embryos, the isthmus is poorly formed and the size of tectum is diminished (Fig.1 B, B′; n=15). To further reduce Pbx function, we injected lzr embryos with both pbx2 and pbx4 morpholinos (lzr;pbx2,4MO). In all Pbx-depleted embryos examined, the isthmic constriction is almost completely absent and the tectum is further reduced (Fig. 1C, C′; n=10). The phenotype of the Pbx-depleted embryos is similar to that of eng2a morphants, although not as severe as eng2a/2b double morphants or noi (no isthmus / pax2a−/−) embryos (Fig.1 D, D′) (Brand et al., 1996; Scholpp and Brand, 2001). These results suggest that vertebrate midbrain development requires Pbx proteins in a dose dependent fashion, and that pbx genes may act on the same genetic or biochemical MHB patterning pathway as the engrailed family of genes.
Fig. 1.
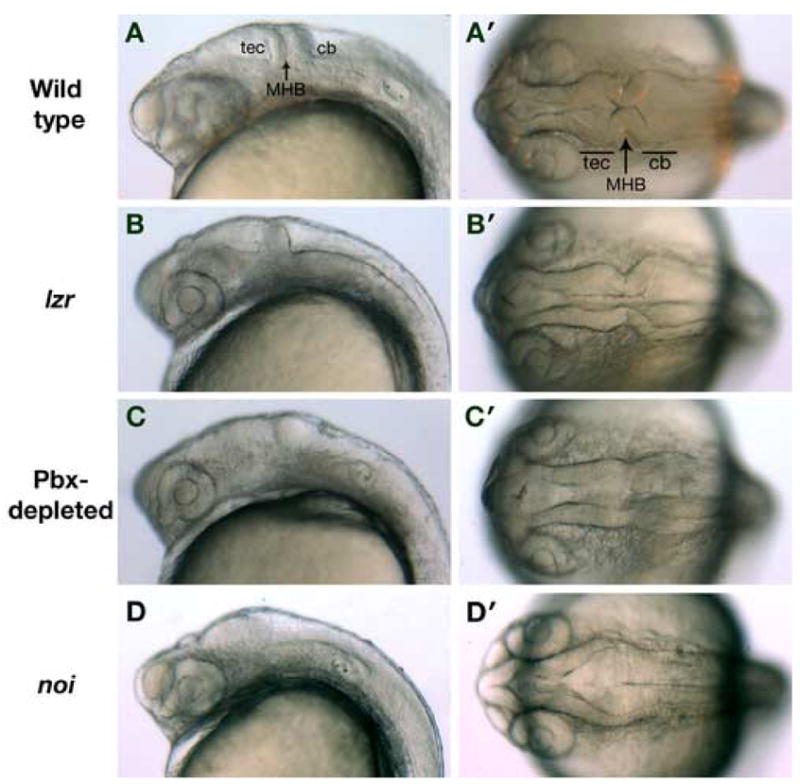
The morphology of the MHB, tectum and cerebellum is defective in Pbx-depleted embryos at 24 hpf. (A, A′) Wild type embryos at 24 hpf possess a well formed tectum (tec) and cerebellum (cb) separated by the isthmic constriction at the MHB. (B, B′) lzr (pbx4−/−) embryos have a normal cerebellum, but the size of the tectum is diminished and the isthmus is not as well formed. (C, C′) In Pbx-depleted embryos, the isthmic constriction at the MHB is indistinct, and neither the tectum nor cerebellum has formed properly. (D, D′) By way of comparison, noi (pax2a−/−) embryos lack all midbrain-derived structures. The isthmus is complete absent and the tectum and cerebellum are unrecognizable. Anterior is to the left; panels A–D are lateral views and panels A′– D′ are dorsal views.
The MHB promotes separation between midbrain and hindbrain identities by restricting cell movements between the mesencephalon and metencephalon (Langenberg and Brand, 2005). MHB development consists of two early phases: initiation and maintenance. The genes involved in MHB maintenance are initiated largely independently of one another, and later become transcriptionally interdependent (Raible and Brand, 2004). To determine if Pbx proteins are required to initiate MHB gene expression, we compared the expression of eng2a, pax2a, fgf8, gbx2, and wnt1 in wild type and Pbx-depleted embryos at 11 hpf (3 somite stage; Fig. 2A–J). Pbx-depleted embryos were identified by the absence of egr2b (krox20) expression in rhombomeres 3 and 5 of the presumptive hindbrain. eng2a and pax2a are expressed broadly across the MHB region, fgf8 and gbx2 are expressed in the posterior half of the MHB, while wnt1 is expressed in the anterior region of the mesencephalon. We can detect no difference in the level or pattern of MHB gene expression between wild type and Pbx-depleted embryos at this stage (n=30). These data suggest that Pbx-proteins are not involved in the specification or positioning of the MHB and the immediately adjacent regions. Therefore, the loss of tectal and isthmic structures observed at 24 hpf may be due to a subsequent failure to maintain MHB gene expression.
Fig. 2.
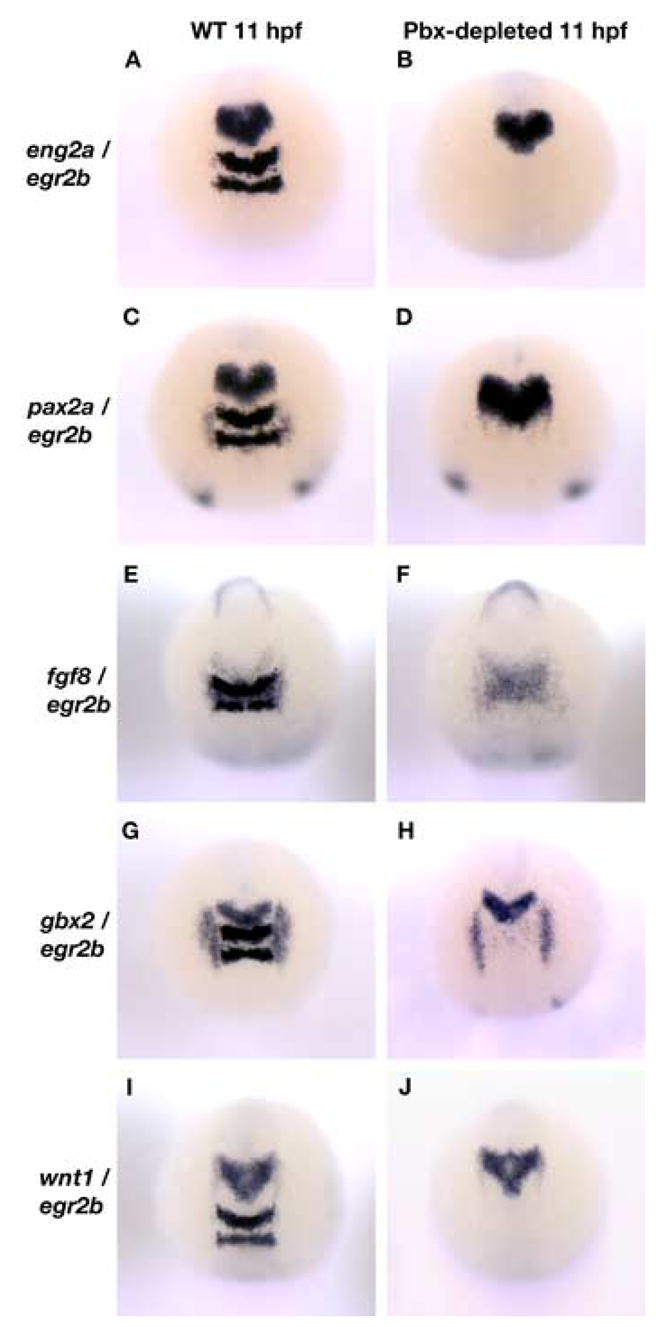
The establishment of the midbrain region of the neural tube is normal in Pbx-depleted embryos. (A – F): eng2a (A, B), pax2a (C, D), fgf8 (E, F), gbx2 (G, H) and wnt1 (I, J) expression at the MHB is normal in both wild type (WT) and Pbx-depleted embryos at 11 hpf. The absence of egr2b (krox20) expression in the rhombomeres 3 and 5 of the presumptive hindbrain was used as an indicator of Pbx loss-of-function. All embryos are shown in dorsal view.
In 18 hpf wild type embryos, a cross-regulatory loop between eng2a, eng2b, pax2a, fgf8, and wnt1 maintains gene expression at the MHB and shapes the morphology of the isthmic constriction. We tested whether Pbx function is required to maintain the MHB by examining the expression of MHB marker genes in wild type, lzr and Pbx-depleted embryos (Fig. 3A–O). We used eng2a to mark the mesencephalon, MHB and metencephalon. In wild type embryos, eng2a is expressed in a wedge shape centered about the MHB (Fig. 3A). This domain is diminished slightly in lzr embryos (Fig. 3B). In Pbx-depleted embryos, this wedge-shaped domain of expression is greatly reduced and expression anterior to the MHB (the presumptive tectum) is absent (Fig. 3C). A similar loss of ventral expression is observed for pax2a expression at the MHB (Fig. 3D–F). We also performed in situs for fgf8 and gbx2 to examine the effects of Pbx depletion on the rostral metencephalon. fgf8 expression is not changed in lzr mutants as compared to wild type (Fig. 3G, H). However, in Pbx-depleted embryos, the ventral domain is expanded caudally while medial expression is absent (Fig. 3I). Similar results were recorded for gbx2 expression, although it appears to be more sensitive to Pbx-depletion (Fig. 3J–L). In wild type embryos, wnt1 is expressed in the caudal mesencephalon and dorsal midbrain (Fig. 3M). In lzr and Pbx-depleted embryos, wnt1 ventral expression is progressively lost, while the dorsal domain is unchanged. To summarize, in all cases where egr2b expression was completely or nearly absent in hindbrain rhombomeres 3 and 5, we observed a general decrease in the level of MHB marker expression and loss of medio-ventral gene expression at the MHB (n>300). Although less severe, the perturbation of MHB gene expression in Pbx-depleted embryos is similar to a pax2a, fgf8 or eng2a/2b loss-of-function (Lun and Brand, 1998; Reifers et al., 1998; Scholpp and Brand, 2001). This comprehensive decrease in MHB gene expression supports the hypothesis that Pbx proteins act within the same regulatory pathway as eng to maintain the MHB.
Fig. 3.
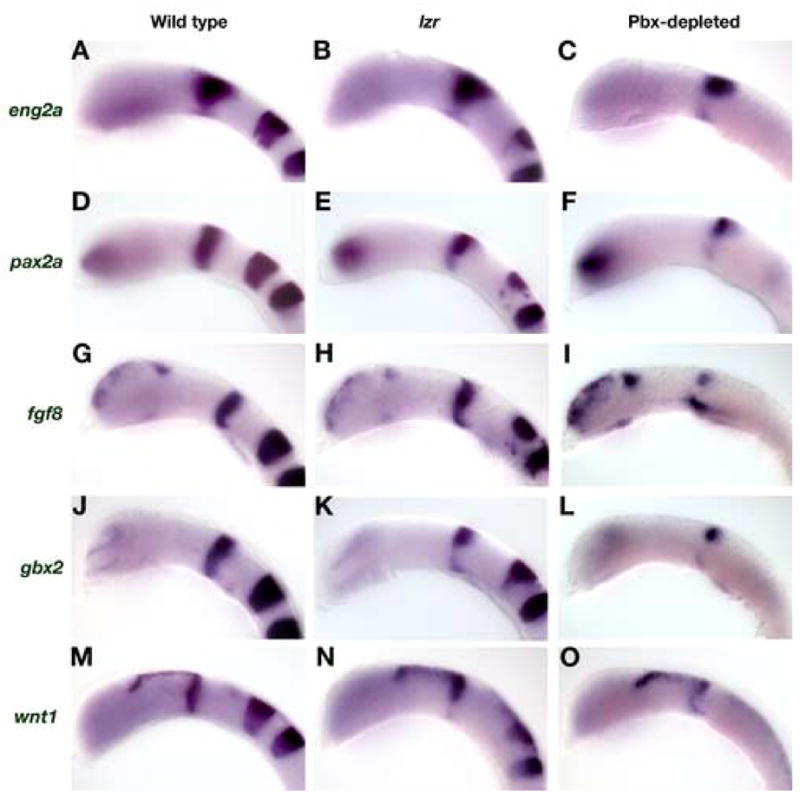
Pbx-depleted embryos do not maintain gene expression at the MHB. (A–C) eng2a: In 18 hpf wild type embryos, eng2a is expressed broadly across the MHB in a wedge-shaped domain (A). eng2a expression is decreased slightly in lzr embryos (B). In Pbx-depleted embryos, the ventral expression of eng2a is greatly decreased and the rostral domain is diminished. (D–F) pax2a: At 18 hpf, pax2a expression at the MHB is normally restricted to a narrow stripe with approximately equal expression over the dorsal-ventral axis (D). In lzr embryos, pax2a expression is decreased in the ventral domain (E). In Pbx-depleted embryos, the ventral expression of pax2a is also completely absent (F). (G–L) fgf8 and gbx2: Both fgf8 and gbx2 are expressed in the caudal half of the MHB at 18 hpf (G, J). fgf8 expression is normal in lzr embryos (H), whereas the ventral domain of gbx2 expression is decreased (K). In Pbx-depleted embryos, the medial domain of fgf8 expression is lost, while the dorsal domain is decreased and the ventral domain is expanded caudally (I). The effect of Pbx-depletion on gbx2 expression is more severe with only a residual dorsal patch remaining (L). (M–O) wnt1: In 18 hpf wild type embryos, wnt1 is expressed in the rostral half of the MHB and the dorsal diencephalons (M). The ventral domain of wnt1 expression at the MHB is decreased in lzr embryos, whereas the dorsal domains remain unchanged (N). In Pbx-depleted embryos, wnt1 expression is decreased at the MHB and expanded caudally, but is expressed in the dorsal diencephalon at near normal levels. All embryos are shown in lateral view with anterior to the left.
The diencephalic-mesencephalic boundary is compromised in Pbx-depleted embryos
The diencephalic-mesencephalic boundary (DMB) is a lineage-restricted boundary that maintains separation between forebrain and midbrain identities. A loss of eng, pax2a or fgf8 expression at the MHB has been shown to cause a caudal expansion of the forebrain at the expense of midbrain territory (Araki and Nakamura, 1999; Liu and Joyner, 2001; Scholpp and Brand, 2001; Scholpp and Brand, 2003; Scholpp et al., 2003). To determine the effect of Pbx-depletion on the DMB, we analyzed epha4a, pax6a, and fgf8 expression by in situ hybridization on wild type and Pbx-depleted embryos (Fig. 4). In 16.5 hpf and 18 hpf Pbx-depleted embryos, forebrain-specific expression of epha4a (Fig. 4A, B) and pax6a (Fig. 4C, D) extends beyond its normal posterior limit while the distance between the DMB and MHB is reduced (all Pbx-null embryos affected, n>100). To quantify this, we compared the rostro-caudal extent of the pax6a domain and found it to be expanded by an average of 15% in Pbx-depleted embryos (P<0.025; n=5). Similarly, the distance between the MHB (fgf8 expression) and the caudal limit of pax6a expression is reduced by 44% in Pbx-depleted embryos (P<0.01; n=5). Zebrafish embryos depleted of both Eng2a and Eng2b also exhibit a caudal expansion of forebrain markers pax6a and epha4a and a loss of midbrain territory. This demonstrates a strong similarity between the DMB defects in Pbx-depleted embryos and those lacking Engrailed function, implying that pbx and eng genes may function on a common genetic pathway in vertebrates.
Fig. 4.
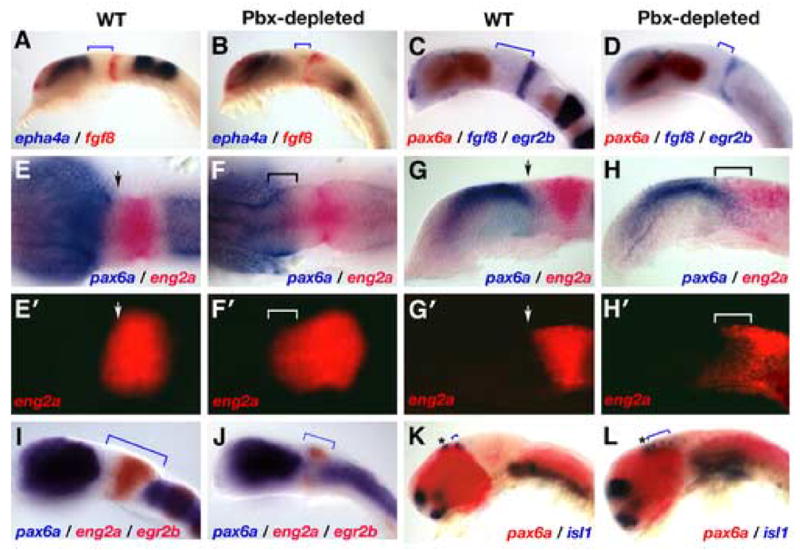
The boundary between diencephalon and mesencephalon (DMB) is not formed properly in zebrafish embryos lacking Pbx proteins. (A–D): We examined the size of forebrain and midbrain domains in 16.5 hpf (A, B) and 18 hpf (C, D) Pbx-depleted embryos. The expression domains of epha4a (A, B) and pax6a (C, D) are expanded caudally at the expense of midbrain territory, as indicated by the blue brackets. (E–H′): Forebrain and midbrain cells no longer exist as separate populations in Pbx-depleted embryos. In wild type (WT) 16.5 hpf embryos, pax6a (blue) and eng2a (red) expressing cells are separated by a sharp boundary at the DMB (arrow heads E, E′, G, G′). 16.5 hpf Pbx-depleted embryos exhibit a loss of DMB integrity, a caudal expansion of pax6a and a rostral expansion of eng2a expression (F, F′, H, H′). There is a region of overlap between these two cell populations as indicated by the brackets in F, F′, H, and H′. Note that identical embryos are shown in E–H and E′–H′. (I – L): Midbrain territory is lost in older Pbx-depleted embryos. Wild type 20 hpf embryos have well defined forebrain and hindbrain pax6a domains separated by eng2a positive cells of the midbrain. In 20 hpf Pbx-depleted embryos, the forebrain and hindbrain domains of pax6a expression have moved into the mesencephalic region (compare brackets in I and J) while eng2a expression is limited to residual dorsal and ventral patches. To determine the state of the DMB in 28 hpf Pbx-depleted embryos, we analyzed the expression of pax6a (red) and visualized the position of the epiphysis (marked with an asterisk) and posterior commissure interneurons by analyzing expression of isl1 (blue). In wild type embryos, the isl1-positive neurons of the posterior commissure are tightly grouped (blue brackets in K). In 28 hpf Pbx-depleted embryos, the position of the posterior commissure neurons is caudally expanded (marked by blue brackets in L), while the isl1-positive neurons of the epiphysis are unaffected. Embryos were imaged either using DIC microscopy (A–H, I–L)) or using fluorescent emission of Fast-Red stain (E′–H′). All embryos are deyolked and shown as either lateral or dorsal views with anterior to the left, except the embryos shown in G, G′, H, and H′, which are saggital sections.
To more closely examine the integrity of the DMB in Pbx-depleted embryos, we analyzed the expression of pax6a and eng2a in 16.5 hpf embryos (Fig. 4E-H′). In wild type embryos, cells expressing forebrain (pax6a) and midbrain (eng2a) markers exist as separate populations (arrows in Fig. 4E, G). In 16.5 hpf Pbx-depleted embryos, the integrity of the DMB has been compromised, as indicated by the region of overlap between forebrain and midbrain cells due to a caudal expansion of pax6a and a rostral expansion of eng2a (brackets in Fig. 4F, F′, H, H′) Analysis of eng2a expression using fluorescent visualization of Fast Red-labeled eng2a probe details this anterior-ward expansion of the midbrain domain (Fig. 4E′-H′). These results demonstrate that Pbx proteins are required to maintain separate populations of forebrain and midbrain cells, a critical element of DMB formation and positioning.
Given the overlap between forebrain and midbrain genetic markers at 16.5 hpf, we examined 20 hpf and 28 hpf embryos to determine if cells were able to subsequently reorganize into proper domains. In 20 hpf Pbx-depleted embryos, the overlap between pax6a and eng2a expressing cells observed earlier at 16.5 hpf has diminished (Fig. 4J). pax6a-expressing cells from the diencephalon and hindbrain have encroached into the former mesencephalon and eng2a expression has retreated to small dorsal and ventral domains (compare brackets in Fig. 4I, J). At 28 hpf, we used pax6a as a marker for forebrain identity and included isl1 to label interneurons of the posterior commissure, located just anterior to the DMB. In wild type embryos, the forebrain and hindbrain expression domains of pax6a are separated by the midbrain (Fig. 4K). However, like the 20 hpf Pbx-depleted embryos, these two domains of pax6a expression are nearly fused in 28 hpf Pbx-depleted embryos, and the caudal limit of forebrain pax6a has an obvious bulge midway along the dorsal-ventral axis (Fig. 4J, L). Additionally, the number and caudal position of the posterior commissure cell bodies are markedly expanded (brackets in Fig. 4I–L), whereas the neuronal cell population at the epiphysis is mostly unaffected (marked with an asterisk). This suggests, that the dorsal pretectal area (marked by the posterior commissure) expands posteriorly in Pbx-depleted embryos, whereas the more anteriorly positioned epithalamus (epiphysis) is less affected.
These data show that Pbx function is required to maintain the distinction between the forebrain and midbrain. In Pbx-depleted embryos, MHB development is initiated correctly, but expression of MHB patterning genes is not maintained. There is a transient period during which pax6a and eng2a expressing cells can share the same region of the midbrain, but eventually the mesencephalic region adopts a forebrain fate. It has been demonstrated in noi and eng2a/2bMO embryos that the midbrain adopts a forebrain fate (Scholpp and Brand, 2003; Scholpp et al., 2003). Other studies have demonstrated that a loss of MHB gene expression leads to a decrease in cell proliferation and / or an increase in cell death in the mesencephalon (Brand et al., 1996; Chi et al., 2003; Jaszai et al., 2003), and this may account for the eventual replacement of the mesencephalon with forebrain cells in Pbx-depleted embryos. In wild type embryos, Engrailed proteins are believed to be the principle factor that prevents the rostral midbrain from adopting a forebrain identity. From an early stage, eng2a is expressed immediately adjacent to the forebrain pax6a domain, thus placing it in an excellent position to repress diencephalic gene expression (Scholpp et al., 2003). Furthermore, in chick, ectopic Engrailed can repress pax6a expression in the forebrain, and does so before leading to the activation of other MHB genes (Araki and Nakamura, 1999). Therefore, together with the established genetic and biochemical interactions between fly Engrailed and Exd / Pbx proteins, the failure to maintain the MHB and DMB in Pbx-depleted embryos strongly suggests that zebrafish Pbx and Engrailed proteins cooperate to pattern the midbrain region of the neural tube.
Eng protein activity is dependent on the presence of Pbx proteins
Our finding that Pbx-depleted embryos resemble a loss of Engrailed function suggests a biochemical dependence of Eng function on Pbx proteins. To test this hypothesis directly, we determined whether Eng function is dependent on the presence of Pbx. We used an Eng overexpression assay in which we injected eng2a mRNA into single-cell zebrafish embryos and examined the resulting change in forebrain pax6a expression (Fig. 5) (Araki and Nakamura, 1999; Scholpp et al., 2003). Injection of low doses of eng2a mRNA caused strong reduction of pax6a expression, with only a vestigial stripe of pax6a typically remaining at the anterior-most region of the injected embryo (67.7%, n=341; Fig. 5A, B). The eng2a-dependent repression of pax6a in the forebrain was accompanied by a marked shortening of the forebrain region and a loss of eye formation. To determine whether the biological activity of ectopic Eng2a is dependent on the presence of Pbx4 protein, we also injected eng2a into maternal, zygotic lzr (mzlzr) mutant embryos. We chose to use mzlzr embryos to avoid the difficulty in scoring expression domains caused by the expansion of pax6a that is seen in Pbx-less embryos. The loss of maternal and zygotic Pbx4 potently attenuated the biological activity of injected eng2a mRNA. mzlzr, eng2a-injected embryos possessed both eyes, and near-normal levels of pax6a expression (strongly reduced in only 1.3% of injected embryos, n=75; Fig. 5C, D). The presence of Pbx2 protein in these embryos may account for some of the residual pax6a repressing activity of ectopic Eng2a. These results show that the ability of overexpressed Eng2a to repress pax6a expression is largely dependent upon the presence of Pbx4 protein. These data suggest that Eng and Pbx proteins act together to repress diencephalic fate in the vertebrate midbrain.
Fig. 5.
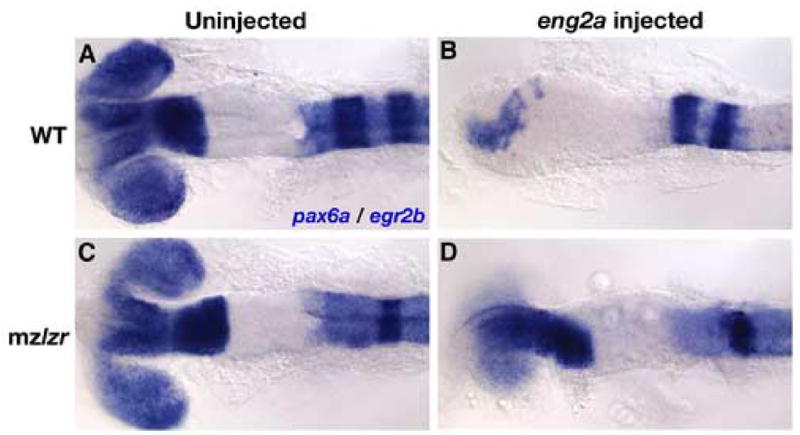
Activity of ectopically expressed eng2a mRNA is dependent on presence of Pbx4 protein. We analyzed the expression of pax6a by in situ hybridization to visualize the effect of ectopic eng2a overexpression in both 18 hpf wild type (WT) (A,B) or mzlzr embryos (C,D). We also analyzed the expression of the rhombomere 3 and 5 marker egr2b to distinguish which embryos had the mzlzr genotype (lack of r3 egr2b expression). Injection of 50 pg eng2a mRNA causes profound defects in the formation of the forebrain, including a reduction in pax6a expression and the loss of eye formation (compare A and B). These effects are strongly attenuated in embryos lacking Pbx4 (compare C and D). All embryos are deyolked and mounted dorsally with anterior to the left.
A biochemical interaction between Pbx and Eng is required for Eng function
A biochemical interaction between both vertebrate and Drosophila Eng and Pbx/Exd proteins has been documented previously (Peltenburg and Murre, 1996; van Dijk et al., 1995). To confirm that the zebrafish proteins possess similar biochemical properties, we performed EMSA using in vitro translated zebrafish Eng2a (fused to a 6X myc epitope) and Pbx4 (Fig. 6). We assayed for cooperative binding by mixing proteins together with a 32P-labeled oligonucleotide that binds both Eng2a and Pbx4 (van Dijk et al., 1995). Whereas Eng2a has the ability to bind the oligo in the absence of Pbx4, we find that zebrafish Pbx4 will bind the oligo only in the presence of Eng2a proteins (compare lanes 1, 2 and 7 in Fig. 6). To examine which residues are required for an interaction between zebrafish Pbx and Eng, we mutated the orthologous residues to those which are required for mouse Pbx-Eng interactions, tryptophan residues 145 and 148 within the hexapeptide motif of Eng2a. We found that mutation of either tryptophan (W145K, W145S, or W148K) completely eliminated cooperative DNA binding with Pbx4 protein, implying that these mutated Eng proteins cannot bind effectively to Pbx (Fig. 6 lanes 8–11). The ability of Eng2a to bind the oligo was not affected by mutation of either tryptophan residue. However, we observed a general decrease in the ability of Eng2a with nonfunctional hexapeptide motifs to bind the oligo in the presence of Pbx4 (Fig. 6 lanes 8–11). We expect that this is a result of a residual in vitro interaction between Pbx4 and Eng2a that leaves both proteins in a conformation that is unfavorable for binding DNA. This incomplete interaction may involve the EH2 and EH3 domains of Eng that have previously been shown to be required for the Eng-Pbx interaction (Peltenburg and Murre, 1996). Our EMSA results show that there is an evolutionarily conserved biochemical interaction between zebrafish Eng2a and Pbx4 mediated by the tryptophan residues of the hexapeptide domain, agreeing with previous work performed on the orthologous murine and Drosophila proteins.
Fig. 6.
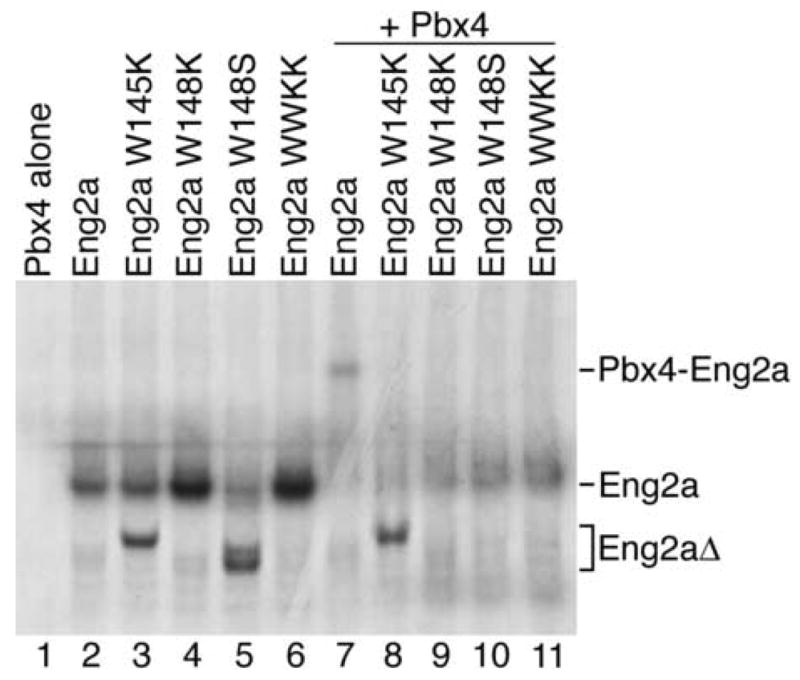
Eng2a requires a functional Pbx-binding hexapeptide to bind Pbx4 in vitro. Zebrafish Pbx4 and myc-Eng2a were synthesized using a wheat germ in vitro transcription and translation system. Crude in vitro translated proteins were mixed with a 32P-labeled oligo containing both Pbx and Eng consensus binding sites: 5’-GTCAATTAAATGATCAATCAATTTCG-3’ (van Dijk et al., 1995). myc-Eng2a proteins with mutations in the hexapeptide motif were also tested for cooperative binding with Pbx4. By site-directed mutagenesis, zebrafish Eng2a tryptophan residues W145 and W148 were changed to either lysine (W145K, W148K, WWKK), or a serine (W148S) residues. In lanes containing Pbx4 (Lanes1, 7–11), only the sample containing both Pbx4 and myc-Eng2a was capable of binding the 32P-labeled oligo (Lane 7). Mutations in the Eng2a hexapeptide abrogated cooperative Eng2a-Pbx4 binding to the oligo (Lanes 8–11).
According to our experiments with Pbx-depleted embryos, reduction of pax6a expression by overexpressed Eng2a requires the presence of Pbx proteins (Fig. 5). To directly test whether ectopically expressed Eng2a must have the ability to bind Pbx proteins in order to reduce pax6a expression, we injected one-cell wild type embryos with the same mRNAs used in our gel shift assays. We then assayed for pax6a expression to compare the biological activity of wild type Eng2a with that of the hexapeptide mutants which cannot bind directly to Pbx proteins. We find that all of the Eng2a hexapeptide mutants have dramatically lowered biological activity. Whereas 76.2% (n=126) of eng2a WT injected embryos show reduced expression of pax6a (Fig. 7B, G), only 6.6% of embryos injected with eng2a-W148K and 17.2% of embryos injected with eng2a-W148S show any observable reduction of pax6a (Fig. 7C, F, G). Mutation of both tryptophan residues together (WWKK) was similar to mutation of the W148 alone (7.9% showing reduced expression; Fig. 7E, G). Mutation of the other conserved Eng2a tryptophan residue alone (W145) leads to a subtly smaller attenuation of biological activity (24% showing reduced pax6a; Fig. 7D, G). All eng2a mRNA constructs were expressed as full-length proteins and translated at similar efficiencies (Fig. 7H), showing that the point mutations introduced into the eng2a coding region did not affect the translation or stability of the protein product. Both wild type and WWKK forms of Eng were also correctly localized to the nucleus (data not shown). Taken together, these results agree with our in vitro gel-shift assays and show that the repression of pax6a expression by Eng2a in vivo requires the ability to directly bind Pbx4.
Fig. 7.
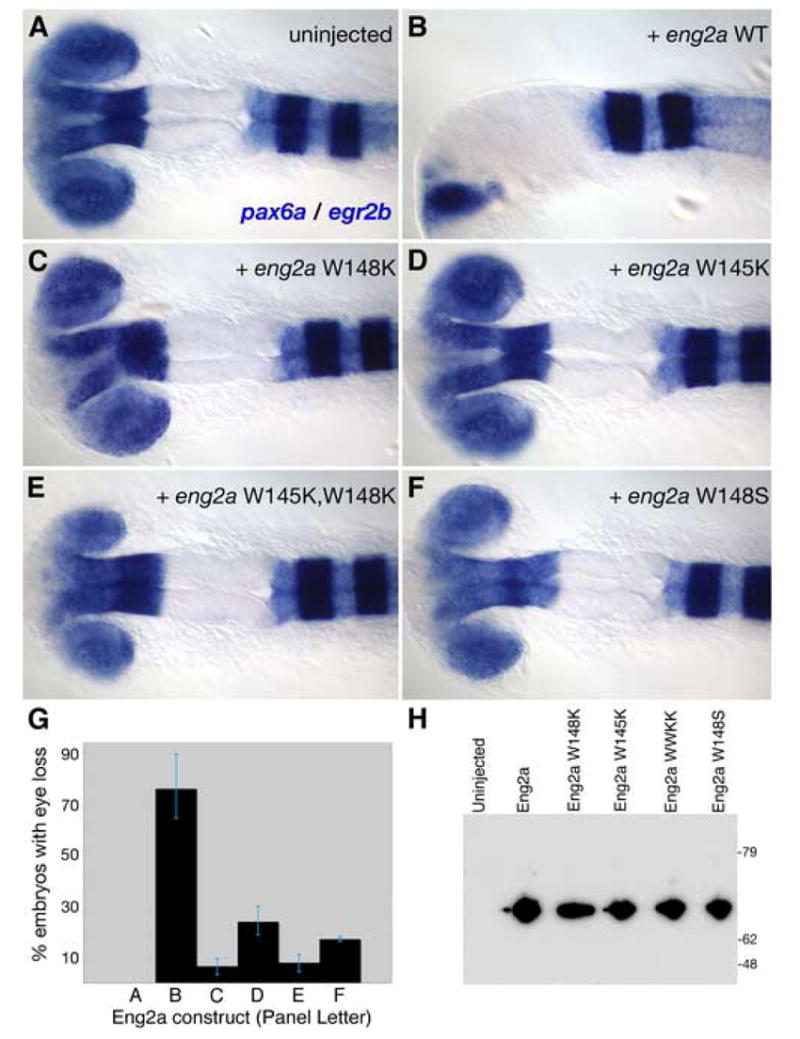
Eng2a constructs with point mutations in the hexapeptide exhibit attenuated pax6a-reducing activity. To determine whether mutations in the Eng2a hexapeptide affected the in vivo activity of overexpressed eng2a, we injected single-cell zebrafish embryos with mRNAs coding for the same myc-Eng2a proteins that we used in our EMSA assay and assayed for pax6a and egr2b expression by in situ analysis. (A, B) Uninjected wild type embryos never exhibited eye loss, while the majority of embryos injected with eng2a mRNA displayed a loss of eye formation and greatly reduced pax6a expression. (C–F) Mutations in the Eng2a hexapeptide, W148K (C), W145K (D), W145KW148K (WWKK) (E), W148S (F), resulted in strongly reduced biological activity compared to the wild type Eng2a (B). (G) Biological effects of each construct were quantified and are shown with the error bars denoting the range of values from two separate experiments. Compared to the activity of wild type Eng2a, mutations in the hexapeptide caused a 3-11X reduction in activity. (H) To rule out the possibility that the reduced biological activity of the Eng2a point mutants was due to inefficient translation or preferential degradation of the protein products, we performed a Western analysis using a monoclonal antibody (9E10) against the myc-epitope to which the various eng2a coding regions were fused. All Eng2a constructs were full length and present at similar levels, showing that the point mutations introduced into the eng2a coding region did not affect the translation or stability of the protein product.
To establish whether Eng and Pbx proteins cooperate to pattern the MHB, we examined the ability of wild type and tryptophan-mutant forms of eng2a mRNA to rescue the MHB defects of eng2a,2b morphants and pbx2,4 morphants (Fig. 8). First, we examined the effects of overexpressing the WT and WWKK forms of eng2a mRNA on the MHB by assaying for pax2a expression. As shown previously, the ectopic expression of wild type eng2a causes a loss of eye formation. In 65% embryos that exhibit this phenotype, we also observe a slight expansion of pax2a expression at the MHB (n=37) (Fig. 8B). On the other hand, overexpression of eng2a WWKK does not lead to an expansion of the MHB. Furthermore, in 47% of these embryos (n=73) we observe a decrease in pax2a expression, suggesting the tryptophan-mutant forms of Eng2a can act as a dominant negative (Fig. 8C). We speculate that Eng2a WWKK can bind to promoter sites normally occupied by Eng-Pbx heterodimers and prevent the normal regulation of target genes, thereby causing the dominant negative effect. Similar results were observed for both WT and WWKK forms of Eng2a when we assayed for fgf8 and wnt1 expression (data not shown). These results suggest that Eng2a requires an intact hexapeptide motif in order to properly regulate MHB development. To further test this, we attempted to rescue eng2a and eng2b double morphants (eng2a,2bMO) with wild type and WWKK forms of eng2a mRNA. At the dose of morpholino we used (8ng of each MO), knockdown of both Eng2a and Eng2b lead to a dramatic decrease in pax2a expression at the MHB (Fig. 8D). Injection of wild type eng2a RNA resulted in near normal levels of pax2a expression in 67% of eng2a,2bMO embryos (n=15) (Fig. 8E). eng2a WWKK was unable to rescue the MHB phenotype of eng2a,2bMO embryos (100%, n=20) (Fig. 8F). To see if eng2a RNA is able to rescue the MHB phenotype of Pbx-depleted embryos, we injected pbx2 and pbx4 double morphant embryos with either WT or WWKK forms of eng2a mRNA. The loss of pax2a expression at the MHB in Pbx-depleted embryos (Fig. 8G) cannot be fully rescued by injection of WT eng2a mRNA (n=14), though we do observe a partial rescue in 36% of embryos (Fig. 8H). We attribute this partial rescue to incomplete knockdown of Pbx proteins by morpholino treatment in some embryos, since the degree of rescue correlates with the amount of egr2b remaining in the hindbrain of Pbx-depleted embryos (data not shown). Injection of Pbx-depleted embryos with eng2a WWKK cannot rescue pax2a expression at the MHB (100%, n=26) (Fig. 8I). Taken together, the dominant negative effect of eng2a WWKK mRNA at the MHB, its inability to rescue eng2a,2b morphants, and the inability of WT eng2a mRNA to rescue Pbx-depleted embryos all suggest that Engrailed function at the MHB requires a biochemical interaction with Pbx proteins.
Fig. 8.
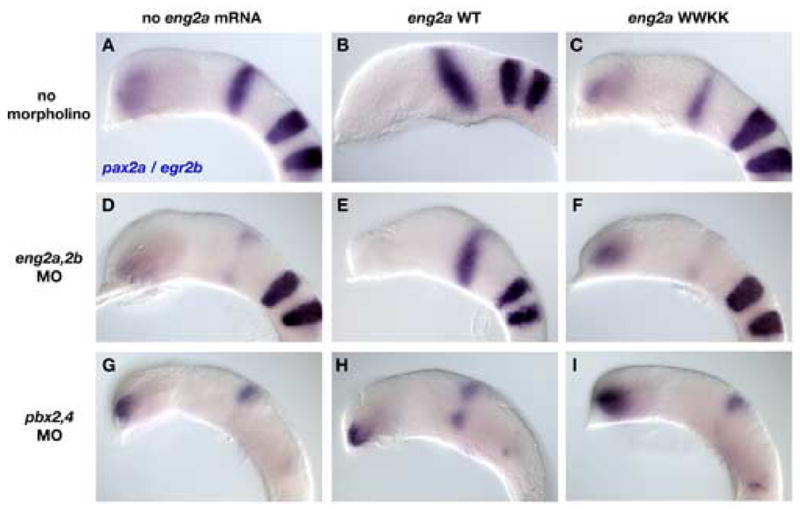
Engrailed and Pbx cooperatively regulate midbrain-hindbrain boundary development. (AC) We used pax2a as a marker for the MHB in wild type, eng2a WT, and eng2a WWKK injected embryos. Overexpression of wild type Eng2a causes a slight expansion of the MHB, together with a loss of eye formation (compare A and B). Overexpression of a hexapeptide mutated form of Eng2a (WWKK) has a dominant negative effect on MHB development, as shown by the decrease in pax2a expression (compare A and C). (D–F) eng2a,2b morphant embryos (D) can be rescued by injection with wild type eng2a mRNA (E), but not by eng2a WWKK (F). (G–I) The MHB defect in Pbx-depleted embryos (G) cannot be rescued by injection of either eng2a WT (H) or eng2a WWKK (I). All embryos are deyolked and mounted laterally with anterior to the left.
Discussion
In this paper, we present evidence that zebrafish Pbx2 and Pbx4 proteins act as biochemical partners with Eng proteins to pattern the mesencephalic territory of the developing vertebrate neural tube. This new role as a midbrain patterning factor expands upon the previously reported role of zebrafish Pbx2 and Pbx4 as Hox co-factors in patterning the hindbrain. We show that the expression of MHB markers is initiated, but not maintained in Pbx-depleted embryos, suggesting that Pbx participates in the cross-regulatory loop that maintains MHB gene expression. Furthermore, diencephalic markers pax6a and epha4a are expanded caudally and that there is an anomalous overlap between pax6a and eng2a expressing cells at the DMB in Pbx-depleted embryos. We used an Eng2a overexpression assay to demonstrate that the pax6a repressing activity of Eng2a depends largely upon the presence of Pbx4 protein. Lastly, we show that zebrafish Pbx4 and Eng2a interact biochemically in vitro via Engrailed’s hexapeptide motif, and that this biochemical interaction is required for Engrailed’s role in regulating MHB development. Taken together, these data suggest a model whereby Pbx and Eng proteins cooperate biochemically to pattern the developing vertebrate midbrain.
The interaction between Eng and Pbx/Exd is conserved in vertebrates
Drosophila Engrailed is an important factor in establishing and maintaining cellular compartments during development (Hidalgo, 1996). Engrailed fulfills this role in part through its biochemical interaction with Exd, the fly orthologue of Pbx (Alexandre and Vincent, 2003; Kobayashi et al., 2003; Peifer and Wieschaus, 1990). Engrailed’s role in forming lineage restricted compartments is highly conserved in vertebrates, as evidenced by the Engrailed loss-of-function phenotype in the mesencephalon (Scholpp and Brand, 2001; Wurst et al., 1994). In this paper, we show that the ability of Engrailed to pattern the MHB and DMB is dependent on its interaction with Pbx proteins. Thus, the partnership between Engrailed and Pbx/Exd, their mechanism of biochemical interaction, and their role in the transcriptional regulation of boundary formation are all conserved between flies and vertebrates.
Pbx proteins act outside of the hindbrain to pattern the zebrafish embryo
Pbx proteins are well characterized as Hox co-factors that function to compartmentalize the vertebrate hindbrain. However, previous research has demonstrated that Pbx proteins do function outside of the hindbrain, sometimes in a Hox-independent fashion. Pbx-deficient mice have defects in Hox-dependent processes such as organogenesis (Manley et al., 2004; Schnabel et al., 2003), hematopoiesis (DiMartino et al., 2001), limb formation (Capellini et al., 2006), and skeletal and cartilage formation (Selleri et al., 2001). Other studies in zebrafish and mouse have demonstrated Hox-independent functions for Pbx proteins as cofactors for MyoD in muscle cells (Berkes et al., 2004), Pdx1/Ipf1 in pancreatic development (Dutta et al., 2001; Kim et al., 2002; Peers et al., 1995), and for the metaHox protein Rnx/Hox11L2/Tlx-3 to control development of the medullary respiratory control mechanisms (Rhee et al., 2004). Our finding that Pbx cooperates with Eng proteins to pattern the midbrain adds to this growing body of evidence that vertebrate Pbx proteins are involved in a myriad of developmental processes in multiple tissue types, and that some functions of Pbx proteins are Hox-independent.
Our results also suggest that Pbx proteins are key regulators of compartmental boundaries. In the absence of Pbx function, the primary division of the neuroectoderm into presumptive fore-, mid-, and hindbrain regions still occurs normally. Subsequent to this, these boundaries are reinforced and maintained while the tissues are secondarily subdivided. Our results show that, in the absence of Pbx function, the midbrain region is initiated correctly, but that secondary maintenance of the MHB and DMB is compromised. This result is consistent with the role already described for Pbx in the hindbrain. A Pbx (Waskiewicz et al., 2002), Meis (Choe and Sagerstrom, 2004) or Pknox (Deflorian et al., 2004) loss-of-function prevents the rhombomere boundaries from ever forming, but does not prevent the initial specification of the hindbrain region. Thus, it appears that a general role for Pbx proteins during vertebrate development is to act as transcriptional co-factors throughout the midbrain and hindbrain in the formation and maintenance of lineage-restricted boundaries. Whether or not Pbx participates in lineage restriction between compartments within the forebrain has not been investigated.
The requirement for zebrafish Pbx proteins in regulating midbrain gene expression
A loss of pax2a or engrailed function in the vertebrate midbrain is characterized by a reduction of the tectum and cerebellum, and a failure to maintain the morphological and genetic characteristics of the isthmic organizer at the MHB. In fish, it is possible to study a partial loss of engrailed function by using morpholinos against one of the two eng paralogues, eng2a (eng2) or eng2b (eng3) (Scholpp and Brand, 2001). Eng2b knockdown has very limited phenotypic effects, but the loss of eng2a function leads to a morphological phenotype that is intermediate between wild type and eng2a/2bMO or noi embryos. The ventral region of the MHB is especially sensitive to Eng2a knockdown, as the ventral domains of pax2a and eng2b expression are lost. Similar DV patterning defects can be observed in weak alleles of pax2a (Lun and Brand, 1998). We observe similar defects in Pbx-depleted embryos. The medio-ventral domain of MHB gene expression preferentially lost (Fig. 3), and the isthmic constriction is diminished, but not completely eliminated (Fig. 1). This result shows that a Pbx loss-of-function is not equivalent to a complete Engrailed loss-of-function, suggesting that some activities of Engrailed are Pbx-independent. The idea that Engrailed can act independently of Pbx is also supported by the observation that Engrailed proteins with mutated hexapeptide domains still possess some ability to repress pax6a expression in zebrafish embryos (Fig. 7). These data suggest either of two possibilities: that Engrailed can act independently of Pbx, or that the Pbx-Engrailed interaction in vivo is not solely dependent on a functional hexapeptide domain in Engrailed. Although either theory is possible, it has been demonstrated in flies that En requires Exd for activation of some targets, but not the repression of others (Alexandre and Vincent, 2003; Kobayashi et al., 2003; Serrano and Maschat, 1998). Therefore, it is likely that some functions of Eng are Pbx-independent.
Downstream effects of Pbx depletion on midbrain structures and function
Besides patterning the DMB and MHB regions of the neural tube, Engrailed function is also required to establish spatial polarity in the optic tectum. A rostrocaudal gradient of Engrailed expression in the tectum is necessary for correct topographic targeting of the retinal ganglion cell axons (Friedman and O’Leary, 1996; Itasaki et al., 1991; Itasaki and Nakamura, 1996; Logan et al., 1996; Nakamura and Sugiyama, 2004). Engrailed likely exerts that effect by regulating the gradient expression of Ephs and Ephrins in the tectum (Logan et al., 1996). The Eph family of RTKs and their Ephrin ligands are essential components in establishing tectal polarity, mediating axon guidance and forming the retinotectal topographic map (Drescher et al., 1997). Our analysis of Pbx-depleted embryos shows that the rostrocaudal gradient of eng2a is abolished by 21 hpf, suggesting that Pbx-depletion may cause tectal patterning defects later in development. We found that the normal patterns of epha4a (Fig. 1A–D) and efna2 (French et al., in preparation) gene expression in the presumptive tectum are disrupted in Pbx-depleted embryos. Both epha4a and efna2 have been implicated in retinal ganglion axon guidance (Marin et al., 2001; Pfeiffenberger et al., 2005; Walkenhorst et al., 2000). This result implies that, together with defects in the DMB, Pbx-depleted embryos may also exhibit abnormalities in tectal patterning and retinal ganglion cell axon projection defects.
Acknowledgments
We wish to thank members of the Waskiewicz and Moens laboratories for technical assistance and comments. Excellent fish care was provided by Erin Pemberton, Robyn Shortt, Lisa Prichard, Jennifer Stout, and Sean Rhodes. This work was funded by Canadian Institutes of Health Research (A.J.W.), Alberta Ingenuity Fund (A.J.W., T.E.), Natural Sciences and Engineering Research Council (T.E.), National Institutes of Health Grant 1R01HD37909 (C.B.M.), and Howard Hughes Medical Institute (C.B.M.). A.J.W. is a recipient of a Canada Research Chair and an Alberta Ingenuity New Faculty Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexandre C, Vincent JP. Requirements for transcriptional repression and activation by Engrailed in Drosophila embryos. Development. 2003;130:729–39. doi: 10.1242/dev.00286. [DOI] [PubMed] [Google Scholar]
- Alvarado-Mallart RM, Martinez S, Lance-Jones CC. Pluripotentiality of the 2-day-old avian germinative neuroepithelium. Dev Biol. 1990;139:75–88. doi: 10.1016/0012-1606(90)90280-v. [DOI] [PubMed] [Google Scholar]
- Araki I, Nakamura H. Engrailed defines the position of dorsal di-mesencephalic boundary by repressing diencephalic fate. Development. 1999;126:5127–35. doi: 10.1242/dev.126.22.5127. [DOI] [PubMed] [Google Scholar]
- Berkes CA, Bergstrom DA, Penn BH, Seaver KJ, Knoepfler PS, Tapscott SJ. Pbx marks genes for activation by MyoD indicating a role for a homeodomain protein in establishing myogenic potential. Mol Cell. 2004;14:465–77. doi: 10.1016/s1097-2765(04)00260-6. [DOI] [PubMed] [Google Scholar]
- Brand M, Heisenberg CP, Jiang YJ, Beuchle D, Lun K, Furutani-Seiki M, Granato M, Haffter P, Hammerschmidt M, Kane DA, Kelsh RN, Mullins MC, Odenthal J, van Eeden FJ, Nusslein-Volhard C. Mutations in zebrafish genes affecting the formation of the boundary between midbrain and hindbrain. Development. 1996;123:179–90. doi: 10.1242/dev.123.1.179. [DOI] [PubMed] [Google Scholar]
- Brunet I, Weinl C, Piper M, Trembleau A, Volovitch M, Harris W, Prochiantz A, Holt C. The transcription factor Engrailed-2 guides retinal axons. Nature. 2005;438:94–8. doi: 10.1038/nature04110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Reim G, Chen W, Hopkins N, Brand M. The zebrafish spiel-ohne-grenzen (spg) gene encodes the POU domain protein Pou2 related to mammalian Oct4 and is essential for formation of the midbrain and hindbrain, and for pre-gastrula morphogenesis. Development. 2002;129:905–16. doi: 10.1242/dev.129.4.905. [DOI] [PubMed] [Google Scholar]
- Capellini TD, Di Giacomo G, Salsi V, Brendolan A, Ferretti E, Srivastava D, Zappavigna V, Selleri L. Pbx1/Pbx2 requirement for distal limb patterning is mediated by the hierarchical control of Hox gene spatial distribution and Shh expression. Development. 2006;133:2263–73. doi: 10.1242/dev.02395. [DOI] [PubMed] [Google Scholar]
- Chan SK, Popperl H, Krumlauf R, Mann RS. An extradenticle-induced conformational change in a HOX protein overcomes an inhibitory function of the conserved hexapeptide motif. Embo J. 1996;15:2476–87. [PMC free article] [PubMed] [Google Scholar]
- Chang CP, Shen WF, Rozenfeld S, Lawrence HJ, Largman C, Cleary ML. Pbx proteins display hexapeptide-dependent cooperative DNA binding with a subset of Hox proteins. Genes Dev. 1995;9:663–74. doi: 10.1101/gad.9.6.663. [DOI] [PubMed] [Google Scholar]
- Chi CL, Martinez S, Wurst W, Martin GR. The isthmic organizer signal FGF8 is required for cell survival in the prospective midbrain and cerebellum. Development. 2003;130:2633–44. doi: 10.1242/dev.00487. [DOI] [PubMed] [Google Scholar]
- Choe SK, Sagerstrom CG. Paralog group 1 hox genes regulate rhombomere 5/6 expression of vhnf1, a repressor of rostral hindbrain fates, in a meis-dependent manner. Dev Biol. 2004;271:350–61. doi: 10.1016/j.ydbio.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martinez S, Martin GR. Midbrain development induced by FGF8 in the chick embryo. Nature. 1996;380:66–8. doi: 10.1038/380066a0. [DOI] [PubMed] [Google Scholar]
- Deflorian G, Tiso N, Ferretti E, Meyer D, Blasi F, Bortolussi M, Argenton F. Prep1.1 has essential genetic functions in hindbrain development and cranial neural crest cell differentiation. Development. 2004;131:613–27. doi: 10.1242/dev.00948. [DOI] [PubMed] [Google Scholar]
- DiMartino JF, Selleri L, Traver D, Firpo MT, Rhee J, Warnke R, O’Gorman S, Weissman IL, Cleary ML. The Hox cofactor and proto-oncogene Pbx1 is required for maintenance of definitive hematopoiesis in the fetal liver. Blood. 2001;98:618–26. doi: 10.1182/blood.v98.3.618. [DOI] [PubMed] [Google Scholar]
- Drescher U, Bonhoeffer F, Muller BK. The Eph family in retinal axon guidance. Curr Opin Neurobiol. 1997;7:75–80. doi: 10.1016/s0959-4388(97)80123-7. [DOI] [PubMed] [Google Scholar]
- Dutta S, Gannon M, Peers B, Wright C, Bonner-Weir S, Montminy M. PDX:PBX complexes are required for normal proliferation of pancreatic cells during development. Proc Natl Acad Sci U S A. 2001;98:1065–70. doi: 10.1073/pnas.031561298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser S, Keynes R, Lumsden A. Segmentation in the chick embryo hindbrain is defined by cell lineage restrictions. Nature. 1990;344:431–5. doi: 10.1038/344431a0. [DOI] [PubMed] [Google Scholar]
- Friedman GC, O’Leary DD. Retroviral misexpression of engrailed genes in the chick optic tectum perturbs the topographic targeting of retinal axons. J Neurosci. 1996;16:5498–509. doi: 10.1523/JNEUROSCI.16-17-05498.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez RE, Rikhof HA, Bachmann R, Moens CB. vhnf1 integrates global RA patterning and local FGF signals to direct posterior hindbrain development in zebrafish. Development. 2004;131:4511–20. doi: 10.1242/dev.01297. [DOI] [PubMed] [Google Scholar]
- Hidalgo A. The roles of engrailed. Trends Genet. 1996;12:1–4. doi: 10.1016/0168-9525(96)81373-4. [DOI] [PubMed] [Google Scholar]
- In der Rieden PM, Mainguy G, Woltering JM, Durston AJ. Homeodomain to hexapeptide or PBC-interaction-domain distance: size apparently matters. Trends Genet. 2004;20:76–9. doi: 10.1016/j.tig.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Itasaki N, Ichijo H, Hama C, Matsuno T, Nakamura H. Establishment of rostrocaudal polarity in tectal primordium: engrailed expression and subsequent tectal polarity. Development. 1991;113:1133–44. doi: 10.1242/dev.113.4.1133. [DOI] [PubMed] [Google Scholar]
- Itasaki N, Nakamura H. A role for gradient en expression in positional specification on the optic tectum. Neuron. 1996;16:55–62. doi: 10.1016/s0896-6273(00)80023-9. [DOI] [PubMed] [Google Scholar]
- Itoh M, Kudoh T, Dedekian M, Kim CH, Chitnis AB. A role for iro1 and iro7 in the establishment of an anteroposterior compartment of the ectoderm adjacent to the midbrain-hindbrain boundary. Development. 2002;129:2317–27. doi: 10.1242/dev.129.10.2317. [DOI] [PubMed] [Google Scholar]
- Jaszai J, Reifers F, Picker A, Langenberg T, Brand M. Isthmus-to-midbrain transformation in the absence of midbrain-hindbrain organizer activity. Development. 2003;130:6611–23. doi: 10.1242/dev.00899. [DOI] [PubMed] [Google Scholar]
- Kiecker C, Lumsden A. Compartments and their boundaries in vertebrate brain development. Nat Rev Neurosci. 2005;6:553–64. doi: 10.1038/nrn1702. [DOI] [PubMed] [Google Scholar]
- Kim SK, Selleri L, Lee JS, Zhang AY, Gu X, Jacobs Y, Cleary ML. Pbx1 inactivation disrupts pancreas development and in Ipf1-deficient mice promotes diabetes mellitus. Nat Genet. 2002;30:430–5. doi: 10.1038/ng860. [DOI] [PubMed] [Google Scholar]
- Knoepfler PS, Lu Q, Kamps MP. Pbx-1 Hox heterodimers bind DNA on inseparable half-sites that permit intrinsic DNA binding specificity of the Hox partner at nucleotides 3′ to a TAAT motif. Nucleic Acids Res. 1996;24:2288–94. doi: 10.1093/nar/24.12.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Fujioka M, Tolkunova EN, Deka D, Abu-Shaar M, Mann RS, Jaynes JB. Engrailed cooperates with extradenticle and homothorax to repress target genes in Drosophila. Development. 2003;130:741–51. doi: 10.1242/dev.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg T, Brand M. Lineage restriction maintains a stable organizer cell population at the zebrafish midbrain-hindbrain boundary. Development. 2005;132:3209–16. doi: 10.1242/dev.01862. [DOI] [PubMed] [Google Scholar]
- LaRonde-LeBlanc NA, Wolberger C. Structure of HoxA9 and Pbx1 bound to DNA: Hox hexapeptide and DNA recognition anterior to posterior. Genes Dev. 2003;17:2060–72. doi: 10.1101/gad.1103303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Joyner AL. Otx2 and Gbx2 are required for refinement and not induction of mid-hindbrain gene expression. Development. 2001;128:4979–91. doi: 10.1242/dev.128.24.4979. [DOI] [PubMed] [Google Scholar]
- Link V, Shevchenko A, Heisenberg CP. Proteomics of early zebrafish embryos. BMC Dev Biol. 2006;6:1. doi: 10.1186/1471-213X-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Joyner AL. EN and GBX2 play essential roles downstream of FGF8 in patterning the mouse mid/hindbrain region. Development. 2001;128:181–91. doi: 10.1242/dev.128.2.181. [DOI] [PubMed] [Google Scholar]
- Logan C, Wizenmann A, Drescher U, Monschau B, Bonhoeffer F, Lumsden A. Rostral optic tectum acquires caudal characteristics following ectopic engrailed expression. Curr Biol. 1996;6:1006–14. doi: 10.1016/s0960-9822(02)00645-0. [DOI] [PubMed] [Google Scholar]
- Lun K, Brand M. A series of no isthmus (noi) alleles of the zebrafish pax2.1 gene reveals multiple signaling events in development of the midbrain-hindbrain boundary. Development. 1998;125:3049–62. doi: 10.1242/dev.125.16.3049. [DOI] [PubMed] [Google Scholar]
- Maizel A, Bensaude O, Prochiantz A, Joliot A. A short region of its homeodomain is necessary for engrailed nuclear export and secretion. Development. 1999;126:3183–90. doi: 10.1242/dev.126.14.3183. [DOI] [PubMed] [Google Scholar]
- Manley NR, Selleri L, Brendolan A, Gordon J, Cleary ML. Abnormalities of caudal pharyngeal pouch development in Pbx1 knockout mice mimic loss of Hox3 paralogs. Dev Biol. 2004;276:301–12. doi: 10.1016/j.ydbio.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Mann RS, Chan SK. Extra specificity from extradenticle: the partnership between HOX and PBX/EXD homeodomain proteins. Trends Genet. 1996;12:258–62. doi: 10.1016/0168-9525(96)10026-3. [DOI] [PubMed] [Google Scholar]
- Marin O, Blanco MJ, Nieto MA. Differential expression of Eph receptors and ephrins correlates with the formation of topographic projections in primary and secondary visual circuits of the embryonic chick forebrain. Dev Biol. 2001;234:289–303. doi: 10.1006/dbio.2001.0268. [DOI] [PubMed] [Google Scholar]
- Martinez S, Crossley PH, Cobos I, Rubenstein JL, Martin GR. FGF8 induces formation of an ectopic isthmic organizer and isthmocerebellar development via a repressive effect on Otx2 expression. Development. 1999;126:1189–200. doi: 10.1242/dev.126.6.1189. [DOI] [PubMed] [Google Scholar]
- Maves L, Jackman W, Kimmel CB. FGF3 and FGF8 mediate a rhombomere 4 signaling activity in the zebrafish hindbrain. Development. 2002;129:3825–37. doi: 10.1242/dev.129.16.3825. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–85. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Joyner AL, Bradley A, McMahon JA. The midbrain-hindbrain phenotype of Wnt-1-/Wnt-1- mice results from stepwise deletion of engrailed-expressing cells by 9.5 days postcoitum. Cell. 1992;69:581–95. doi: 10.1016/0092-8674(92)90222-x. [DOI] [PubMed] [Google Scholar]
- Moens CB, Selleri L. Hox cofactors in vertebrate development. Dev Biol. 2006;291:193–206. doi: 10.1016/j.ydbio.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Sugiyama S. Polarity and laminar formation of the optic tectum in relation to retinal projection. J Neurobiol. 2004;59:48–56. doi: 10.1002/neu.10339. [DOI] [PubMed] [Google Scholar]
- Neuteboom ST, Peltenburg LT, van Dijk MA, Murre C. The hexapeptide LFPWMR in Hoxb-8 is required for cooperative DNA binding with Pbx1 and Pbx2 proteins. Proc Natl Acad Sci U S A. 1995;92:9166–70. doi: 10.1073/pnas.92.20.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara FP, Beck E, Barr LK, Wong LL, Kessler DS, Riddle RD. Zebrafish Lmx1b.1 and Lmx1b.2 are required for maintenance of the isthmic organizer. Development. 2005;132:3163–73. doi: 10.1242/dev.01898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passner JM, Ryoo HD, Shen L, Mann RS, Aggarwal AK. Structure of a DNA-bound Ultrabithorax-Extradenticle homeodomain complex. Nature. 1999;397:714–9. doi: 10.1038/17833. [DOI] [PubMed] [Google Scholar]
- Peers B, Sharma S, Johnson T, Kamps M, Montminy M. The pancreatic islet factor STF-1 binds cooperatively with Pbx to a regulatory element in the somatostatin promoter: importance of the FPWMK motif and of the homeodomain. Mol Cell Biol. 1995;15:7091–7. doi: 10.1128/mcb.15.12.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M, Wieschaus E. Mutations in the Drosophila gene extradenticle affect the way specific homeo domain proteins regulate segmental identity. Genes Dev. 1990;4:1209–23. doi: 10.1101/gad.4.7.1209. [DOI] [PubMed] [Google Scholar]
- Peltenburg LT, Murre C. Engrailed and Hox homeodomain proteins contain a related Pbx interaction motif that recognizes a common structure present in Pbx. Embo J. 1996;15:3385–93. [PMC free article] [PubMed] [Google Scholar]
- Peltenburg LT, Murre C. Specific residues in the Pbx homeodomain differentially modulate the DNA-binding activity of Hox and Engrailed proteins. Development. 1997;124:1089–98. doi: 10.1242/dev.124.5.1089. [DOI] [PubMed] [Google Scholar]
- Pfeiffenberger C, Cutforth T, Woods G, Yamada J, Renteria RC, Copenhagen DR, Flanagan JG, Feldheim DA. Ephrin-As and neural activity are required for eye-specific patterning during retinogeniculate mapping. Nat Neurosci. 2005;8:1022–7. doi: 10.1038/nn1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper DE, Batchelor AH, Chang CP, Cleary ML, Wolberger C. Structure of a HoxB1-Pbx1 heterodimer bound to DNA: role of the hexapeptide and a fourth homeodomain helix in complex formation. Cell. 1999;96:587–97. doi: 10.1016/s0092-8674(00)80662-5. [DOI] [PubMed] [Google Scholar]
- Popperl H, Bienz M, Studer M, Chan SK, Aparicio S, Brenner S, Mann RS, Krumlauf R. Segmental expression of Hoxb-1 is controlled by a highly conserved autoregulatory loop dependent upon exd/pbx. Cell. 1995;81:1031–42. doi: 10.1016/s0092-8674(05)80008-x. [DOI] [PubMed] [Google Scholar]
- Popperl H, Rikhof H, Chang H, Haffter P, Kimmel CB, Moens CB. lazarus is a novel pbx gene that globally mediates hox gene function in zebrafish. Mol Cell. 2000;6:255–67. doi: 10.1016/s1097-2765(00)00027-7. [DOI] [PubMed] [Google Scholar]
- Prince VE, Moens CB, Kimmel CB, Ho RK. Zebrafish hox genes: expression in the hindbrain region of wild-type and mutants of the segmentation gene, valentino. Development. 1998;125:393–406. doi: 10.1242/dev.125.3.393. [DOI] [PubMed] [Google Scholar]
- Raible F, Brand M. Divide et Impera--the midbrain-hindbrain boundary and its organizer. Trends Neurosci. 2004;27:727–34. doi: 10.1016/j.tins.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Reifers F, Bohli H, Walsh EC, Crossley PH, Stainier DY, Brand M. Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development. 1998;125:2381–95. doi: 10.1242/dev.125.13.2381. [DOI] [PubMed] [Google Scholar]
- Rhee JW, Arata A, Selleri L, Jacobs Y, Arata S, Onimaru H, Cleary ML. Pbx3 deficiency results in central hypoventilation. Am J Pathol. 2004;165:1343–50. doi: 10.1016/S0002-9440(10)63392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinn M, Lun K, Luz M, Werner M, Brand M. Positioning of the midbrain-hindbrain boundary organizer through global posteriorization of the neuroectoderm mediated by Wnt8 signaling. Development. 2005;132:1261–72. doi: 10.1242/dev.01685. [DOI] [PubMed] [Google Scholar]
- Rieckhof GE, Casares F, Ryoo HD, Abu-Shaar M, Mann RS. Nuclear translocation of extradenticle requires homothorax, which encodes an extradenticle-related homeodomain protein. Cell. 1997;91:171–83. doi: 10.1016/s0092-8674(00)80400-6. [DOI] [PubMed] [Google Scholar]
- Schnabel CA, Selleri L, Cleary ML. Pbx1 is essential for adrenal development and urogenital differentiation. Genesis. 2003;37:123–30. doi: 10.1002/gene.10235. [DOI] [PubMed] [Google Scholar]
- Scholpp S, Brand M. Morpholino-induced knockdown of zebrafish engrailed genes eng2 and eng3 reveals redundant and unique functions in midbrain--hindbrain boundary development. Genesis. 2001;30:129–33. doi: 10.1002/gene.1047. [DOI] [PubMed] [Google Scholar]
- Scholpp S, Brand M. Integrity of the midbrain region is required to maintain the diencephalic-mesencephalic boundary in zebrafish no isthmus/pax2.1 mutants. Dev Dyn. 2003;228:313–22. doi: 10.1002/dvdy.10384. [DOI] [PubMed] [Google Scholar]
- Scholpp S, Lohs C, Brand M. Engrailed and Fgf8 act synergistically to maintain the boundary between diencephalon and mesencephalon. Development. 2003;130:4881–93. doi: 10.1242/dev.00683. [DOI] [PubMed] [Google Scholar]
- Selleri L, Depew MJ, Jacobs Y, Chanda SK, Tsang KY, Cheah KS, Rubenstein JL, O’Gorman S, Cleary ML. Requirement for Pbx1 in skeletal patterning and programming chondrocyte proliferation and differentiation. Development. 2001;128:3543–57. doi: 10.1242/dev.128.18.3543. [DOI] [PubMed] [Google Scholar]
- Serrano N, Maschat F. Molecular mechanism of polyhomeotic activation by Engrailed. Embo J. 1998;17:3704–13. doi: 10.1093/emboj/17.13.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallafuss A, Wilm TP, Crozatier M, Pfeffer P, Wassef M, Bally-Cuif L. The zebrafish buttonhead-like factor Bts1 is an early regulator of pax2.1 expression during mid-hindbrain development. Development. 2001;128:4021–34. doi: 10.1242/dev.128.20.4021. [DOI] [PubMed] [Google Scholar]
- van Dijk MA, Murre C. extradenticle raises the DNA binding specificity of homeotic selector gene products. Cell. 1994;78:617–24. doi: 10.1016/0092-8674(94)90526-6. [DOI] [PubMed] [Google Scholar]
- van Dijk MA, Peltenburg LT, Murre C. Hox gene products modulate the DNA binding activity of Pbx1 and Pbx2. Mech Dev. 1995;52:99–108. doi: 10.1016/0925-4773(95)00394-g. [DOI] [PubMed] [Google Scholar]
- von Baer KE. Entwicklungsgeschichte der Thiere: Beobachtung und Reflexion. 1828. Bornträger, Königsberg. [Google Scholar]
- Walkenhorst J, Dutting D, Handwerker C, Huai J, Tanaka H, Drescher U. The EphA4 receptor tyrosine kinase is necessary for the guidance of nasal retinal ganglion cell axons in vitro. Mol Cell Neurosci. 2000;16:365–75. doi: 10.1006/mcne.2000.0878. [DOI] [PubMed] [Google Scholar]
- Walshe J, Maroon H, McGonnell IM, Dickson C, Mason I. Establishment of hindbrain segmental identity requires signaling by FGF3 and FGF8. Curr Biol. 2002;12:1117–23. doi: 10.1016/s0960-9822(02)00899-0. [DOI] [PubMed] [Google Scholar]
- Waskiewicz AJ, Rikhof HA, Moens CB. Eliminating zebrafish pbx proteins reveals a hindbrain ground state. Dev Cell. 2002;3:723–33. doi: 10.1016/s1534-5807(02)00319-2. [DOI] [PubMed] [Google Scholar]
- Wurst W, Auerbach AB, Joyner AL. Multiple developmental defects in Engrailed-1 mutant mice: an early mid-hindbrain deletion and patterning defects in forelimbs and sternum. Development. 1994;120:2065–75. doi: 10.1242/dev.120.7.2065. [DOI] [PubMed] [Google Scholar]
- Wurst W, Bally-Cuif L. Neural plate patterning: upstream and downstream of the isthmic organizer. Nat Rev Neurosci. 2001;2:99–108. doi: 10.1038/35053516. [DOI] [PubMed] [Google Scholar]


