Abstract
Aldose reductase (AR; AKR1B1) a member of aldoketo reductase super family, that we had shown earlier mediates cytotoxic signals induced by high glucose, cytokines and growth factors, also mediates the inflammatory signals induced by Gram-negative bacterial endotoxin, lipopolysaccharide (LPS). Inhibition of AR by three distinct AR inhibitors sorbinil, tolrestat or zopolrestat suppressed the LPS-induced production of inflammatory cytokines such as TNF-α, IL-6, IL-1β, IFN-γ, and chemokine MCP-1 in murine peritoneal macrophages. Inhibition of AR also prevented the production of nitric oxide, and prostaglandin E2 and expression of iNOS and Cox-2 proteins. The LPS-induced DNA binding activity of NF-κB and AP1 were significantly inhibited by AR inhibitors, and this effect was mediated through the inhibition of phosphorylation of IκB-α, IKK α/β and PKC. These results suggest the therapeutic use of AR inhibitors as anti-inflammatory drugs.
Keywords: Aldose reductase, sepsis, inflammation, lipopolysaccharide and NF-κB
1. Introduction
Septic shock is the major cause of morbidity and mortality in patients with Gram-negative bacterial infections [1, 2]. Lipopolysaccharide (LPS), a major component of outer membrane of Gram-negative bacteria, is the key molecule for triggering innate immune and inflammatory responses during sepsis [3]. LPS triggers the production of proinflammatory cytokines and chemokines such as TNF-α, IL-1, IL-6, Il-12, IFN-γ and MCP-1 and proinflammatory nitric oxide (NO) and prostaglandin E2 (PGE2) [4, 5]. Excessive production of cytokines and chemokines by macrophages that is further increased by autocrine and paracrine manners tremendously increases severity of immune response that causes inflammation [6, 7]. It is well known that redox-sensitive transcription factors, NF-κB and AP1 play an important role in the expression of pro-inflammatory cytokines and chemokines and other inflammatory markers [8]. LPS via increase in the production of reactive oxygen species activates various protein kinases that stimulate the phosphorylation and ubiquitination of IκB-α and lead to the activation of NF-κB [9]. Several lines of evidence indicate that antioxidants, flavinoids, over expression of SOD and catalase, and inhibition of NADPH oxidase could prevent LPS-induced activation of NF-κB and thereby prevent LPS-induced cytotoxicity [10–13]. These studies suggest that increased production of ROS is the major culprit in the LPS-induced cytotoxic effects. The increased generation of ROS due to oxidative stress causes peroxidation of membrane lipids leading to the production of toxic lipid aldehydes. 4-hydroxy-trans-2-nonenal (HNE) is one of the most abundant and toxic lipid aldehyde generated during lipid peroxidation, which has been shown to be cytotoxic, mutagenic and genotoxic in a variety of cell types [14, 15].
We have recently demonstrated that aldose reductase (AR) is an excellent catalyst for the reduction of HNE and its glutathione conjugate with Km in micro molar range [16, 17]. Inhibition of AR prevents HNE-, growth factors- such as FGF, PDGF, cytokines- such as TNF-α, and high glucose-induced proliferation of vascular smooth muscle cells (VSMC) and apoptosis of vascular endothelial (VEC) and lens epithelial cells (HLEC) [18–22]. Inhibition AR also prevents the oxidative stress-induced activation of redox-sensitive transcription factors NF-κB and AP1 in cultured cells [18–22]. The role of AR in the mediation of oxidative stress-induced signaling was further confirmed in an animal model of restenosis. Restenosis of balloon –injured rat carotid arteries was significantly blocked by AR inhibitors [18, 23]. Recently, we have shown that GS-DHN formed by the reduction of GS-HNE by AR could mediate cell signaling leading to activation of NF-κB and proliferation of cultured VSMC [24]. We now, for the first time demonstrate that AR could mediate LPS-induced production of inflammatory markers and activation of NF-κB in isolated murine peritoneal macrophages and suggests the development of AR inhibition as a therapeutic strategy in preventing Gram-negative bacterial infection-induced inflammation, such as sepsis.
2. MATERIALS AND METHODS
2.1. Materials
Dulbecco's modified Eagle's medium (DMEM), phosphate-buffered saline (PBS), penicillin/streptomycin solution, trypsin, and fetal bovine serum (FBS) were purchased from Invitrogen. Sorbinil and Zopolrestat were gifts from Pfizer and Tolrestat was obtained from American Home Products. Normal or phosphospecific antibodies against IKKα/β and IκB-α were obtained from Cell Signaling Inc. Mouse anti-rabbit glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies were obtained from Research Diagnostics Inc. Cyclooxygenase (Cox) activity assay and prostaglandin E2 (PGE2) assay kits were obtained from Cayman chemical company. Consensus oligonucleotides for NF-κB (5'-AGTTGAGGGGACTTTCCCAGGC-3') and AP1 (5’-TTCCGGCTGACTCATCAAGCG-3’) transcription factors were obtained from Promega Corp. Lipopolysaccharide (E.coli) and the reagents used in the electrophoretic mobility shift assay (EMSA) and Western blot analysis were obtained from Sigma. All other reagents used were of analytical grade.
2.2. Isolation and culture of peritoneal macrophages
The balb/c mice (25–30 g) were obtained from Taconic laboratories and housed in pathogen-free conditions with free access to food and water at the institutional animal care facility. Mice were injected intraperitoneally with 2 ml of 3 % thioglycollate. After 4 days, peritoneal cells collected by lavage were seeded onto 6-well plates in RPMI 1640 medium with 10% calf serum and gentamicin (50 μg/ml) for 6 h to allow the macrophages to adhere to the plates. Nonadherent cells were subsequently removed by washing with HBSS solution, confirmed with F4/80 stain, and the adherent macrophages were grown in RPMI 1640 medium containing 10% fetal bovine serum and gentamicin. Macrophages were used for experiments immediately following isolation.
2.3. Determination of cytokines levels
The peritoneal macrophages were pre-incubated with 10 μM sorbinil, tolrestat or zopolrestat for 24 h followed by incubation with 0.5 μg/ml LPS. After 12 h of incubation the cytokines (TNF-α, IL-6, IL-1β, and IFN-γ) and chemokine (MCP-1) levels were measured in the culture medium of peritoneal macrophages by using BD biosciences Mouse Inflammation Cytometric bead array kits according to manufacturer’s instructions.
2.4. PGE2 and Cox Assays
Macrophages (2x105 cells/well in 6 well plates) were growth arrested in the serum-free medium without or with AR inhibitors for 24 h followed by incubation with 0.5 μg/ml LPS for another 24 h. The medium was collected from each well and analyzed for PGE2 and Cox by using an Enzyme Immuno Assay kit according to the manufacturer's instructions (Cayman Chemical Co.). Briefly, for PGE2; 50 μl of diluted standard/sample was pipetted into a pre-coated goat polyclonal anti-mouse IgG 96-well plate. Aliquots (50 μl) of a PGE2 monoclonal antibody and PGE2 acetylcholine esterase (AChE) conjugate were added to each well and allowed to incubate at 4°C for 24 h. After incubation the wells were washed and 200 μl of Ellman’s reagent containing acetylthiocholine and 5, 5’-dithio-bis-(2-nitrobenzoic acid) were added. Samples were read after 60 min at 412 nm with an ELISA reader (Packard). For Cox; 10 μl of standard/sample was incubated in the presence of arachidonic acid and colorimetric substrate, N, N, N, N-tetra methyl-p-phenylenediamine (TMPD) in a total reaction volume of 210 μl. The Cox peroxidase activity was measured colorimetrically by monitoring appearance of oxidized TMPD at 590 nm by using ELISA reader (Packard).
2.5. Determination of nitrite/nitrate levels
The nitrate/nitrite levels in macrophages were determined using commercially available microplate assay kit from Cayman Chemical according to supplier’s instructions. Briefly, to measure nitrite (NO2), 100 μl of macrophage culture supernatant were collected, mixed with an equal volume of the Griess reagent (1% sulfanilamide/0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride/2.5% H3PO4) and incubated for 10 min at room temperature. Nitrite concentration was determined by measuring the absorbance at 540 nm in an ELISA 96 well plate spectrophotometer. NaNO2 was used for external calibration.
2.6. Electrophoretic mobility gel shift assays
The cytosolic as well as nuclear extracts were prepared as described before [18]. Consensus oligonucleotide for NF-κB and AP1 transcription factors were 5'-end labeled using T4 polynucleotide kinase. The EMSA were performed as described earlier [18].
2.7. Western blot analysis
To examine expression of iNOS and Cox-2 and phosphorylation of IKKα/β and IκB-α, Western blot analyses were carried out. Briefly, equivalent amounts of total proteins were loaded onto 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis. The proteins in the gels were transferred to nitrocellulose membrane using an electroblotter. (Bio-Rad, Richmond, CA, USA) and reacted with the appropriate primary antibodies phospho-IKKα/β, phospho-IκB-α, non-phospho-IκB-α and GAPDH. Bound antigen-antibody complexes were detected by enhanced chemiluminescence (Amersham Pharmacia Biotech, NJ). GAPDH was used to monitor equal protein sample loading. The densitometry scanning and analysis were carried out using Kodak Image Station 2000R.
2.8. Determination of PKC activity
The membrane -bound PKC activity was determined as described earlier using Promega SignaTect™ total PKC assay system [25]. Briefly, aliquots of the reaction mixture (25 mM Tris-HCl pH 7.5, 1.6 mg/ml phosphatidylserine, 0.16 mg/ml diacylglycerol, and 50 mM MgCl2) were mixed with [γ-32P] ATP (3,000 Ci/mmol, 10 μCi/μl) and incubated at 30°C for 10 min. The extent of phosphorylation was detected by measuring radioactivity retained on the paper.
2.9. Determination of ROS levels and α, β-unsaturated lipid aldehydes
The serum- starved peritoneal macrophages (1.5 × 104 cells/well in a 24-well plate) without or with 10 μM of sorbinil or tolrestat were treated with the ROS-sensitive fluorophore 2', 7’-dichlorofluorescein diacetate for 30 min. Subsequently, the macrophages were exposed to LPS (0.5 μg/ml) for 60 min and fluorescence was measured with a CytoFluorII fluorescence plate reader (PerSeptive Biosystems, Inc., Framingham, MA) at excitation of 485 nm and emission of 528 nm. The levels of α, β-unsaturated aldehydes such as HNE were quantified colorimetrically by using a lipid peroxidation kit (Bioxytech LPO-586™) obtained from Oxford Biomedical Research, Oxford, MI, as per the supplier’s instructions. Briefly, the determination is based on the reaction of the chromogenic reagent, methanesulfonic acid with α, β-unsaturated aldehydes such as HNE at 45 °C. One molecule of aldehyde reacts with two molecules of reagent to yield a stable chromophore with maximal absorbance at 586 nm. The protein-HNE adducts formation was determined by performing Westen blot analysis by using polyclonal antibodies against HNE-KLH. The densitometric scanning and analysis were carried out using Kodak Image Station 2000R. Data are presented as mean ± SEM (n=4) and the P values were determined using the unpaired student’s t-test. P<0.05 was considered as statistically significant.
3. RESULTS
3.1. Effect of AR inhibition on LPS-induced production of inflammatory cytokines and chemokines in peritoneal macrophages
To analyze the mechanism of anti-inflammation by AR inhibitors, we examined the effect of the AR inhibition on LPS-induced production of cytokines and chemokines in isolated mouse peritoneal macrophages. The levels of TNF-α, IFN-γ, IL-1β, IL-6 and MCP-1 proteins in untreated peritoneal macrophages were low but detectable (Fig. 1A–E). Addition of LPS to the incubation media for 12 h caused significant 18-, 34-, 6-, 10- and 16-fold increases of TNF-α, IL-6, IL-1β, IFN-γ and MCP-1 levels in the culture medium, respectively (Fig. 1A–E). However, preincubation of peritoneal macrophages with three structurally distinct pharmacological inhibitors of AR, sorbinil, tolrestat, or zopolrestat followed by incubation with LPS significantly (80–90 %) prevented the LPS-enhanced cytokines and chemokines production. AR inhibitors alone had no effect on the basal levels of these inflammatory cytokines. These results suggest that AR inhibition could prevent LPS -induced production of inflammatory cytokines and chemokines in peritoneal macrophages.
Fig. 1.
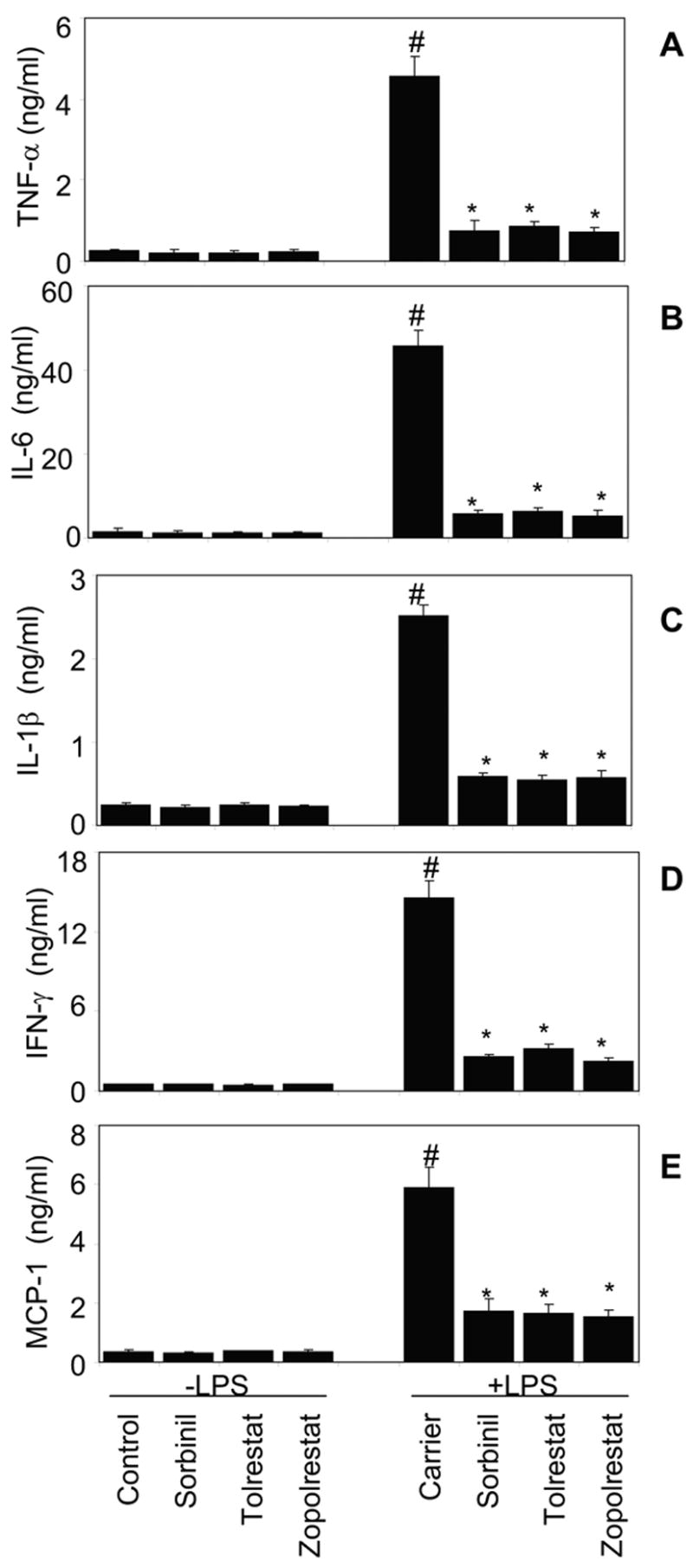
Effect of AR inhibition on LPS-induced production of inflammatory cytokines in isolated peritoneal macrophages. A–E) Growth -arrested macrophages in 0.1% serum medium were incubated with indicated AR inhibitors (10 μM) for 24 h followed by stimulation with LPS (0.5 μg/ml). The cytokine and chemokine levels were measured at 12 h in the culture media of macrophages by using BD Biosciences Mouse Inflammation Cytometric Bead array kit as described in the Experimental Procedures. Data are expressed as Mean ± SEM (N = 4). *P < 0.001 as compared to LPS-treated cells#P < 0.001 control cells.
3.2. Effect of AR inhibition on LPS-induced production of PGE2 and expression of Cox-2 in peritoneal macrophages
We next examined the effect of AR inhibitors on PGE2 production and COX-2 protein expression following LPS stimulation in peritoneal macrophages. LPS caused an ~5-fold increase in the biosynthesis of PGE2 as compared to untreated cells (Fig 2A). However when the macrophages were incubated with LPS in the presence of AR inhibitors only ~2- fold increase in the biosynthesis of PGE2 was observed. AR inhibitors alone did not affect the basal biosynthesis of PGE2 in macrophages (Fig. 2A). Since Cox-1 and Cox-2 enzymes catalyze the biosynthesis of PGE2, we next measured the effect of AR inhibition on LPS-induced total Cox activity. LPS increased Cox activity by 4-fold and inhibition of AR significantly (>80%) prevented the LPS-induced increase of Cox activity (Fig. 2B). Because the activity of Cox is contributed by Cox-1 (constitutive) and Cox-2 (inducible), we next measured the effect of AR inhibition on LPS-induced expression of these proteins in peritoneal macrophages. As shown in Fig 2C, the levels of Cox-1 protein were not affected by LPS but that of Cox-2 increased by ~3 fold (Fig. 2D). The LPS-induced increase in Cox-2 protein was attenuated by AR inhibition.
Fig. 2.

Effect of AR inhibition on LPS-induced biosynthesis of PGE2 and expression of Cox-2 in isolated peritoneal macrophages. A–E) Growth -arrested macrophages in 0.1% serum medium were incubated with indicated AR inhibitors (10 μM) for 24 h followed by stimulation with LPS (0.5 μg/ml) for 12 h. The A) PGE2 levels and B) total Cox activity in the cultured media were measured by using specific ELISA kits as described in the Methods. Equal amounts of cell extracts were subjected to Western Blot analysis using antibodies against C) Cox-1, D) Cox-2 and E) GAPDH. The antibody binding was detected by enhanced pico chemiluminescence (Pierce). Data are expressed as Mean ± SEM (N = 4). *P < 0.001 as compared to LPS-treated cells# P < 0.001 control cells. The -fold change determined by densitometric scanning, is indicated at the bottom of the Western blot.
3.3. Effect of AR inhibition on LPS-induced production of Nitric oxide and expression of iNOS in peritoneal macrophages
Since, NO is the major inflammatory marker produced during inflammation and is well known for its mediation in cytotoxic events [4,5], we next examined the effect of AR inhibition on NO production. As shown Fig. 3A, NO (nitrite/nitrate) levels were detectable in untreated macrophages, and AR inhibitors alone did not affect the basal NO production. Administration of LPS caused a 4-fold increase in NO levels in the medium with macrophages (Fig. 3A) and AR inhibitors significantly (80 %) reduced LPS-augmented NO levels in macrophages. We next examined the expression of iNOS protein in peritoneal macrophages. As shown in Fig. 3B, AR inhibitors did not influence basal iNOS protein, but LPS increased the protein levels of iNOS in macrophages. Further treatment of macrophages with AR inhibitors before LPS challenge significantly inhibited LPS-induced iNOS protein by 65%.
Fig. 3.

Effect of AR inhibition on LPS-induced levels of NO and expression of iNOS in isolated peritoneal macrophages. A–C) Growth -arrested macrophages in 0.1% serum medium were incubated with indicated AR inhibitors (10 μM) for 24 h followed by stimulation with LPS (0.5 μg/ml) for 12 h. The A) Nitrite/nitrate levels in the cultured media were measured by using specific ELISA kit as described in the Methods. Equal amounts of cell extracts were subjected to Western Blot analysis using antibodies against B) iNOS and C) GAPDH. The antibody binding was detected by enhanced pico chemiluminescence (Pierce). Data are expressed as Mean ± SEM (N = 4). *P < 0.001 as compared to LPS-treated cells# P < 0.001 control cells. The -fold change determined by densitometric scanning, is indicated at the bottom of the Western blot.
3.4. Effect of AR inhibition on LPS-induced activation of NF-kB and AP1 signaling in peritoneal macrophages
Redox- sensitive transcription factors NF-κB and AP-1 transcribe the genes necessary for induction of inflammatory Cox-2, iNOS, cytokines, and chemokines [9]. We therefore examined the effect of inhibition of AR on LPS –induced NF-κB and AP1 activation in peritoneal macrophages. Within 1 h of LPS addition to peritoneal macrophages activation of NF-κB was observed and the increase was significantly attenuated by AR inhibitors (Fig. 4A&B). However, AR inhibitors alone had no effect on basal NF-κB or AP1 activities in macrophages. Further, LPS caused phosphorylation of IκB-α within 5 min of LPS challenge that maximized in 10 min (Fig. 4C) and AR inhibition by sorbinil prevented it (Fig. 4D). We next examined the effect of AR inhibition on IκB-α upstream kinases such as IKK and PKC. As shown in Fig. 4E & G, LPS caused ~5- and 7 fold activation of IKKα/β and PKC, respectively and inhibition of AR prevented it. These results suggest that inhibition of AR prevents activation of LPS-induced PKC, which could be responsible for the activation of NF-κB and AP1 that leads to the transcriptional activation of various inflammatory markers observed during Gram-negative bacterial infections.
Fig. 4.

Effect of AR inhibition on LPS-induced activation of NF-κB and AP-1 signals in isolated peritoneal macrophages. A–G) Growth -arrested macrophages in 0.1% serum medium were incubated with indicated AR inhibitors (10 μM) for 24 h followed by stimulation with LPS (0.5 μg/ml) for 1 h. The A and B) Equal amounts of nuclear extracts were subjected to EMSA to measure activation of NF-κB and AP-1 as described in the methods. C–F) Equal amounts of cytoplasmic extracts were subjected to Western Blot analysis using antibodies against C and D) phospho-IκB-α, E) phospho-IKKα/β and F) GAPDH. The antibody binding was detected by enhanced pico chemiluminescence (Pierce). G) Total membrane bound PKC activation was measured by using Promega SignaTect™ PKC Assay System. Data are expressed as Mean ± SEM (N = 4). *P < 0.001 as compared to LPS-treated cells# P < 0.001 control cells. The -fold change determined by densitometric scanning, is indicated at the bottom of the Western blot.
3.5. Effect of AR inhibition on LPS-induced Oxidative stress in peritoneal macrophages
Since it is well known that AR protein levels increase under oxidative stress conditions, we next measured the effect of LPS on AR activity as well as protein expression in mouse peritoneal macrophages. As Shown in Fig. 5A and B, the AR activity and protein levels increased ~2.5- and ~3.2- fold respectively, under LPS stimulus in 12 h. Pretreatment with AR inhibitors significantly (>85%) prevented LPS-induced AR activity as well as protein levels. Similarly in the cells treated with AR inhibitors alone, the AR activity but not protein levels was significantly decreased as compared to untreated control. Since LPS is known to increase ROS, we next examined the effect of AR inhibition on LPS-induced generation of ROS in peritoneal macrophages. As shown in Fig. 5D, treatment of macrophages with LPS caused a significant increase in ROS levels in 60 min and AR inhibition prevented it. Since ROS cause peroxidation of membrane lipids resulting in the formation of α, β-unsaturated lipid aldehydes, HNE being the most abundant, which could readily conjugate with glutathione (GSH) and both HNE and GS-HNE can be reduced by AR, we investigated the effect of AR inhibition on LPS-induced lipid aldehydes in macrophages. As expected, LPS increased the levels of lipid aldehydes and protein-HNE adducts by ~2.2 and ~3.5 –folds, respectively within 12 h (Fig. 5E and F).
Fig. 5.
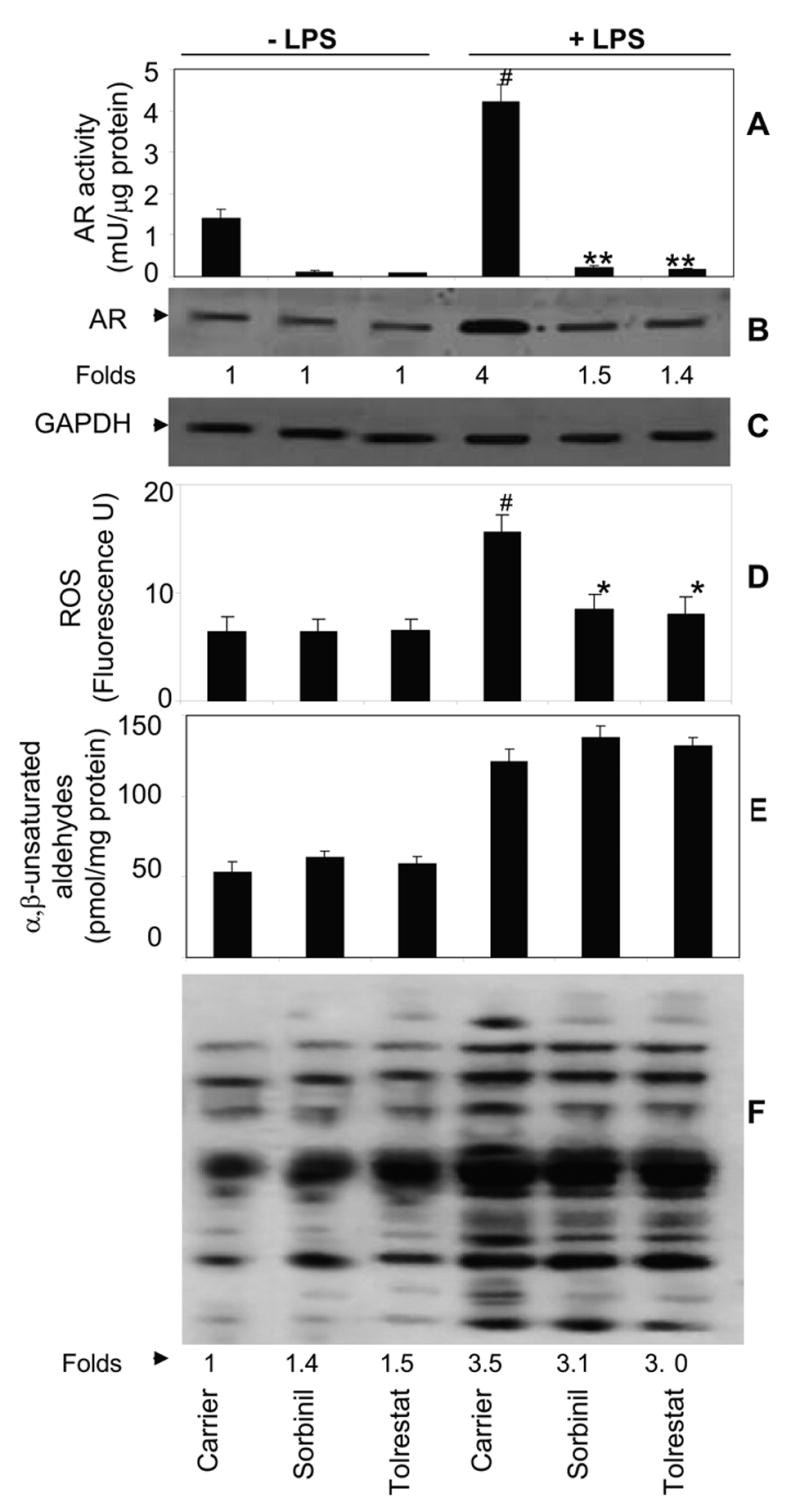
Effect of AR inhibition on LPS-induced oxidative stress in macrophages. Growth -arrested macrophages in 0.1% serum medium were incubated with indicated AR inhibitors (10 μM) for 24 h followed by stimulation with LPS (0.5 μg/ml) for 12 h. A) AR activity was measured in the cell lysates spectrophotometrically by using glyceraldehyde as a substrate at 340 nm. B, C) Western blots were developed using antibodies against AR and GAPDH. D) The levels of ROS were measured fluorometrically. The levels of E) α, β-unsaturated aldehydes and F) protein-HNE adducts were measured as described in the Methods. Data are expressed as Mean ± SEM (N = 4). **P < 0.001, *P<0.01 as compared to LPS-treated cells#P < 0.001 control cells.
4. DISCUSSION
Macrophages play a critical role in the regulation of the immune response and cellular host defense during bacterial infections [26]. LPS is an outer membrane component of Gram-negative bacterial cell walls, which activates macrophages to produce pro-inflammatory cytokines and chemokines such as TNF-α, IL-6, IFN-γ and MCP-1, and secondary mediators, such as NO and prostaglandins [4, 5]. These substances are generally important regulators of both innate and adaptive immunity. However, their uncontrolled and excessive production can cause acute or chronic inflammatory syndromes such as sepsis, which is characterized by fever, hypotension, disseminated intravascular coagulation and multiple organ failure [5, 27]. Over -production of PGE2 and NO can mediate LPS-induced cytotoxicity in a variety of cells [28–30]. Slowing the production and/or release of these inflammatory factors from macrophages may retard the inflammatory responses to LPS stimulation. In the present study, we demonstrate that inhibition of AR prevents LPS –induced production of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IFN-γ), chemokine (MCP-1) and other inflammatory markers such as NO, PGE2 and Cox-2 in freshly isolated murine peritoneal macrophages.
LPS –induced generation of reactive oxygen species (ROS) is the major cause of cytotoxicity [31]. ROS can cause activation of redox-sensitive transcription factors such as NF-κB and AP-1 which transcribe various inflammatory genes [8]. Scavenging of ROS by antioxidants could attenuate NF-κB-dependent production of pro-inflammatory markers thereby prevent LPS toxicity [10–13]. Thus data showing prevention of LPS-induced NF-κB and AP-1 activation in peritoneal macrophages by AR inhibition indicates that AR mediates the ROS-induced activation of transcription factors that transcribe inflammatory markers. Further, the inhibition of NF-κB translocation was associated with a decrease in LPS–induced phosphorylation of IκB-α and a decrease in IKK-α/β phosphorylation (Fig 4), indicating that inhibition of AR abrogates IκB-α phosphorylation and thus prevents dissociation of the IκB-p55-p65 complex but does not directly interfere with NF-κB–dependent production of inflammatory markers. These results are consistent with our previous observations that inhibition of AR prevents TNF-α and high glucose–induced NF-κB and AP-1 activation in VSMC, VEC and HLEC [18–22]. In agreement with the view that LPS activates NF-κB by a PKC-dependent pathway [32], we have observed a significant activation of PKC by LPS in peritoneal macrophages. Further, LPS–induced PKC activation was prevented by AR inhibitors, suggesting that AR regulates the activation of PKC induced by LPS in macrophages. Although the relationship between AR and PKC remains unclear in macrophages, we have shown earlier that AR inhibition prevents high glucose –induced activation of PLC and the synthesis of diacylglycerol (DAG) in VSMC [25], which are essential endogenous activators of PKC. Furthermore, we have recently shown that AR- catalyzed reduction product of GS-HNE, GS-DHN could mediate mitogenic signals in VSMC via activation of NF-κB and PKC [24]. It is likely that similar to VSMC, the role of AR in LPS-induced cytotoxicity in peritoneal macrophages could be via reduction of lipid aldehydes such as HNE and their glutathione conjugates such as GS-HNE to corresponding alcohols, DHN and GS-DHN, respectively which could be the main molecules of ROS signaling. Although LPS-induced activation of PKC is well established, extensive investigations are required to understand the mediation of AR. Nonetheless, our current observation that inhibition of AR prevents PLC suggests that AR is upstream of PLC activation and that inhibition of AR during inflammation could provide salutary effects similar to or greater than those provided by PKC inhibitors.
Exposure of macrophages to LPS significantly increased the levels of NO and iNOS protein in macrophages and AR inhibitors attenuated such increases. The suppressive effects of AR inhibitors on LPS-induced NO and PGE2 biosynthesis are exerted at the transcriptional level since NF-κB regulates iNOS and Cox-2 gene expressions, respectively and we have shown that AR inhibitors prevent LPS-induced activation of NF-κB via PKC. Consistent with our observations there are several reports that show NF-κB -mediated activation of iNOS and Cox-2 in various cell lines including macrophages [33, 34].
In summary, our results reveal a novel role of AR as an inflammatory factor in cellular response to LPS and provide a new insight for understanding the role of AR in the mediation of LPS- triggered inflammatory response in macrophages. Our results also suggest a possible use of AR inhibitors as potential anti-inflammatory drugs for the pathologies associated with Gram-negative bacterial infections.
Acknowledgments
This study was supported in part by NIH grants GM 71036 (to KVR) and DK 36118 (to SKS)
The abbreviations used are
- AP1
activator protein1
- AR
aldose reductase
- Cox
cyclooxygenase
- HNE
4-hydroxytrans-2-nonenal
- GSH
glutathione
- IκB-α
inhibitor of kappa binding protein-alpha
- IKK
IκB-kinase
- iNOS
inducible nitric oxide synthase
- PGE2
prostaglandin E2
- MTT
[3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy phenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt]
- NF-κB
nuclear factor kappa binding protein
- NO
nitric oxide
- PKC
Protein kinase C
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angus DC, Pereira CA, Silva E. Epidemiology of severe sepsis around the world. Endocr Metab Immune Disord Drug Targets. 2006;6:207–212. doi: 10.2174/187153006777442332. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Heumann D, Roger T. Initial responses to endotoxins and Gram-negative bacteria. Clin Chim Acta. 2002;323:59–72. doi: 10.1016/s0009-8981(02)00180-8. [DOI] [PubMed] [Google Scholar]
- 4.Blatteis CM, Li S, Li Z, Feleder C, Perlik V. Cytokines, PGE2 and endotoxic fever: a re-assessment. Prostaglandins Other Lipid Mediat. 2005;76:1–18. doi: 10.1016/j.prostaglandins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Palsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, Trinchieri G, Caux C, Garrone P. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005;201:1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crespo A, Filla MB, Russell SW, Murphy WJ. Indirect induction of suppressor of cytokine signalling-1 in macrophages stimulated with bacterial lipopolysaccharide: partial role of autocrine/paracrine interferon-alpha/beta. Biochem J. 2000;349:99–104. doi: 10.1042/0264-6021:3490099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharyya J, Biswas S, Datta AG. Mode of action of endotoxin: role of free radicals and antioxidants. Curr Med Chem. 2004;11:359–368. doi: 10.2174/0929867043456098. [DOI] [PubMed] [Google Scholar]
- 9.Zhang G, Ghosh S. Molecular mechanisms of NF-kappaB activation induced by bacterial lipopolysaccharide through Toll-like receptors. J Endotoxin Res. 2000;6:453–457. doi: 10.1179/096805100101532414. [DOI] [PubMed] [Google Scholar]
- 10.Victor VM, Rocha M, Esplugues JV, De la Fuente M. Role of free radicals in sepsis: antioxidant therapy. Curr Pharm Res. 2005;11:3141–3158. doi: 10.2174/1381612054864894. [DOI] [PubMed] [Google Scholar]
- 11.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 12.Mirochnitchenko O, Inouye M. Effect of overexpression of human Cu,Zn superoxide dismutase in transgenic mice on macrophage functions. J Immunol. 1996;156:1578–1586. [PubMed] [Google Scholar]
- 13.Kim BH, Cho SM, Reddy AM, Kim YS, Min KR, Kim Y. Down-regulatory effect of quercitrin gallate on nuclear factor-kappa B-dependent inducible nitric oxide synthase expression in lipopolysaccharide-stimulated macrophages RAW 264.7. Biochem Pharmacol. 2005;69:1577–1583. doi: 10.1016/j.bcp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Awasthi YC, Sharma R, Cheng JZ, Yang Y, Sharma A, Singhal SS, Awasthi S. Role of 4-hydroxynonenal in stress-mediated apoptosis signaling. Mol Aspects Med. 2003;24:219–230. doi: 10.1016/s0098-2997(03)00017-7. [DOI] [PubMed] [Google Scholar]
- 15.Eckl PM. Genotoxicity of HNE. Mol Aspects Med. 2003;24:161–165. doi: 10.1016/s0098-2997(03)00010-4. [DOI] [PubMed] [Google Scholar]
- 16.Ramana KV, Dixit BL, Srivastava S, Balendiran GK, Srivastava SK, Bhatnagar A. Selective recognition of glutathiolated aldehydes by aldose reductase. Biochemistry. 2000;39:12172–12180. doi: 10.1021/bi000796e. [DOI] [PubMed] [Google Scholar]
- 17.Dixit BL, Balendiran GK, Watowich SJ, Srivastava S, Ramana KV, Petrash JM, Bhatnagar A, Srivastava SK. Kinetic and structural characterization of the glutathione-binding site of aldose reductase. J Biol Chem. 2000;275:21587–21595. doi: 10.1074/jbc.M909235199. [DOI] [PubMed] [Google Scholar]
- 18.Ramana KV, Chandra D, Srivastava S, Bhatnagar A, Aggarwal BB, Srivastava SK. Aldose reductase mediates mitogenic signaling in vascular smooth muscle cells. J Biol Chem. 2002;277:32063–32070. doi: 10.1074/jbc.M202126200. [DOI] [PubMed] [Google Scholar]
- 19.Ramana KV, Friedrich B, Bhatnagar A, Srivastava SK. Aldose reductase mediates cytotoxic signals of hyperglycemia and TNF-alpha in human lens epithelial cells. FASEB J. 2003;17:315–317. doi: 10.1096/fj.02-0568fje. [DOI] [PubMed] [Google Scholar]
- 20.Ramana KV, Bhatnagar A, Srivastava SK. Aldose reductase regulates TNF-alpha-induced cell signaling and apoptosis in vascular endothelial cells. FEBS Lett. 2004;570:189–194. doi: 10.1016/j.febslet.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 21.Ramana KV, Bhatnagar A, Srivastava SK. Inhibition of aldose reductase attenuates TNF-alpha-induced expression of adhesion molecules in endothelial cells. FASEB J. 2004;18:1209–1218. doi: 10.1096/fj.04-1650com. [DOI] [PubMed] [Google Scholar]
- 22.Ramana KV, Friedrich B, Srivastava S, Bhatnagar A, Srivastava SK. Activation of nuclear factor-kappaB by hyperglycemia in vascular smooth muscle cells is regulated by aldose reductase. Diabetes. 2004;53:2910–2920. doi: 10.2337/diabetes.53.11.2910. [DOI] [PubMed] [Google Scholar]
- 23.Srivastava S, Ramana KV, Tammali R, Srivastava SK, Bhatnagar A. Contribution of aldose reductase to diabetic hyperproliferation of vascular smooth muscle cells. Diabetes. 2006;55:901–910. doi: 10.2337/diabetes.55.04.06.db05-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramana KV, Bhatnagar A, Srivastava S, Yadav UC, Awasthi S, Awasthi YC, Srivastava SK. Mitogenic responses of vascular smooth muscle cells to lipid peroxidation-derived aldehyde 4-hydroxy-trans-2-nonenal (HNE): Role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth. J Biol Chem. 2006;281:17652–17660. doi: 10.1074/jbc.M600270200. [DOI] [PubMed] [Google Scholar]
- 25.Ramana KV, Friedrich B, Tammali R, West MB, Bhatnagar A, Srivastava SK. Requirement of aldose reductase for the hyperglycemic activation of protein kinase C and formation of diacylglycerol in vascular smooth muscle cells. Diabetes. 2005;54:818–829. doi: 10.2337/diabetes.54.3.818. [DOI] [PubMed] [Google Scholar]
- 26.Twigg HL., 3rd Macrophages in innate and acquired immunity. Semin Respir Crit Care Med. 2004;25:21–31. doi: 10.1055/s-2004-822302. [DOI] [PubMed] [Google Scholar]
- 27.Guidet B, Aegerter P, Gauzit R, Meshaka P, Dreyfuss D CUB-Rea Study Group. Incidence and impact of organ dysfunctions associated with sepsis . Chest. 2005;127:942–951. doi: 10.1378/chest.127.3.942. [DOI] [PubMed] [Google Scholar]
- 28.Walton LJ, Franklin IJ, Bayston T, Brown LC, Greenhalgh RM, Taylor GW, Powell JT. Inhibition of prostaglandin E2 synthesis in abdominal aortic aneurysms: implications for smooth muscle cell viability, inflammatory processes, and the expansion of abdominal aortic aneurysms. Circulation. 1999;100:48–54. doi: 10.1161/01.cir.100.1.48. [DOI] [PubMed] [Google Scholar]
- 29.Tiwari MM, Messer KJ, Mayeux PR. Inducible nitric oxide synthase and apoptosis in murine proximal tubule epithelial cells. Toxicol Sci. 2006;91:493–500. doi: 10.1093/toxsci/kfj168. [DOI] [PubMed] [Google Scholar]
- 30.Yook YH, Kang KH, Maeng O, Kim TR, Lee JO, Kang KI, Kim YS, Paik SG, Lee H. Nitric oxide induces BNIP3 expression that causes cell death in macrophages. Biochem Biophys Res Commun. 2004;32:1298–305. doi: 10.1016/j.bbrc.2004.06.144. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez PK, Zhuang J, Doctrow SR, Malfroy B, Benson PF, Menconi MJ, Fink MP. Role of oxidant stress in the adult respiratory distress syndrome: evaluation of a novel antioxidant strategy in a porcine model of endotoxin-induced acute lung injury. Shock. 1996;6:S23–S26. [PubMed] [Google Scholar]
- 32.Chen CC, Wang JK, Lin SB. Antisense oligonucleotides targeting protein kinase C-alpha, -beta I, or -delta but not -eta inhibit lipopolysaccharide-induced nitric oxide synthase expression in RAW 264.7 macrophages: involvement of a nuclear factor kappa B-dependent mechanism. J Immunol. 1998;161:6206–6214. [PubMed] [Google Scholar]
- 33.Han YJ, Kwon YG, Chung HT, Lee SK, Simmons RL, Billiar TR, Kim YM. Antioxidant enzymes suppress nitric oxide production through the inhibition of NF-kappa B activation: role of H(2)O(2) and nitric oxide in inducible nitric oxide synthase expression in macrophages. Nitric Oxide. 2001;5:504–513. doi: 10.1006/niox.2001.0367. [DOI] [PubMed] [Google Scholar]
- 34.Yan Z, Stapleton PP, Freeman TA, Fuortes M, Daly JM. Enhanced expression of cyclooxygenase-2 and prostaglandin E2 in response to endotoxin after trauma is dependent on MAPK and NF-kappaB mechanisms. Cell Immunol. 2004;232:116–126. doi: 10.1016/j.cellimm.2005.03.001. [DOI] [PubMed] [Google Scholar]


