Abstract
Synaptic vesicle protein 2 (SV2) is a glycoprotein identified in the nervous system of several species, including man, but its occurrence in the human neuroendocrine (NE) cell system has not been investigated. By using a monoclonal antibody to SV2, immunoreactivities were demonstrated in NE cell types in human gastrointestinal tract, pancreas, anterior pituitary gland, thyroid, parathyroid, and adrenal medulla, and also in chief cells of gastric oxyntic mucosa. Immunoelectron microscopy of pancreatic islets revealed SV2 immunoreactivity in secretory granules. Comparison of SV2, synaptophysin, and chromogranin A immunoreactivity showed more SV2- and synaptophysin- than chromogranin A-immunoreactive cells in the antrum and pancreas. In the other gastrointestinal regions and in the other endocrine organs more SV2- than synaptophysin-immunoreactive cells were seen. More chromogranin A- than SV2-immunoreactive cells were observed in duodenum, colon, and parathyroid. Various NE tumors were examined and all contained SV2-immunoreactive cells. The staining patterns with the three markers agreed well, except in hindgut carcinoids, which showed strong SV2 immunoreactivity, weak synaptophysin but no chromogranin A immunostaining. In pituitary adenomas more cells were immunoreactive to SV2 than to the other two antibodies. In conclusion, SV2 is recognized as a further broad marker for NE cells and widens the arsenal of diagnostic tools for NE tumors. It is of special importance for identifying hindgut carcinoids.
Neuroendocrine (NE) cells contain two types of vesicular structures, small clear synaptic vesicles and large electron dense secretory granules. Several proteins have been observed in these vesicular structures, and among them chromogranin A and synaptophysin have attracted great interest. Chromogranin A occurs in most NE cell types, 1,2 and has been used during the last two decades as an important broad-spectrum marker for immunocytochemical identification of normal and neoplastic NE cells. Synaptophysin, which initially was found in small-vesicle membranes of neurons, has also been demonstrated in NE cells, although in a smaller amount than chromogranin A. 3-7
Synaptic vesicle protein 2 (SV2), like synaptophysin, is an integral membrane glycoprotein. It was initially identified in the central and peripheral nervous systems of different animal species from fish to mammals, as well as in the rat pancreas, anterior pituitary lobe, and adrenal medulla, and in some murine NE cell lines. 8-10 This glycoprotein occurs in three well-characterized isoforms, SV2A, SV2B, and SV2C. SV2A is widely distributed in the nervous system, in virtually all neurons. SV2B is also widely expressed in SV2A-containing neurons, although not as widely as SV2A, whereas SV2C is only observed in a small number of neurons in a few brain areas. 11-14
Ultrastructurally, both chromogranin A and synaptophysin have been demonstrated in the secretory granules of endocrine cells. 15,16 SV2 has been observed ultrastructurally in vesicular structures in the mammalian nervous system 8 and in the membranes of the secretory granules in an NE cell line from rat pheochromocytoma. 9,17,18
In man, SV2 has been found in the nervous system, but there are no reports about its occurrence in the NE cell system. The present study was therefore undertaken to ascertain the existence of SV2 in the human NE cell system, and to evaluate the extent to which it can be used as a broad-spectrum marker in normal and neoplastic NE cells.
Materials and Methods
Light Microscopy
Tissue specimens from adult human gastric corpus and antrum, proximal duodenum, distal ileum, sigmoid colon, and pancreas were obtained from surgical samples removed at operations for adenocarcinoma. The specimens examined were taken from macroscopically normal tissue at least 2 to 5 cm from the neoplasm. Further tissue specimens from the pituitary, thyroid, parathyroid, and adrenal glands were also included in the study. The tissue specimens from the various organs were collected from three to six different cases. The pituitary glands were taken from autopsy cases without endocrine disturbances. The thyroid and adrenal tissues were from patients suffering from nonfunctional follicular and cortical adenoma, respectively. The parathyroid tissues were biopsy samples from histologically normal glands associated with a parathyroid adenoma. Hematoxylin and eosin (H&E)-stained sections from each organ showed normal histology.
Forty-four human tumor specimens were also analyzed regarding their content of SV2-immunoreactive cells. The following tumor types were studied: various carcinoid tumors from the gastrointestinal and respiratory tracts, islet cell tumors, medullary thyroid carcinomas, anterior pituitary tumors, and pheochromocytomas. The ECLomas included were associated with enterochromaffin-like cell and gastrin cell hyperplasia, and the carcinoids of the ileum, proximal colon, and appendix were of the midgut (classical) type. The hindgut carcinoids showed a predominantly ribbon pattern. The bronchial carcinoids were centrally located. All carcinoid tumors displayed synaptophysin immunoreactivity, and all of them, except hindgut carcinoids, also showed chromogranin A immunoreactivity (see Results).
Further, three cases of nesidioblastosis, in children with persistent neonatal hyperinsulinemic hypoglycemia, were included in the study; two were of the focal type and one was diffuse.
All specimens were routinely fixed in 10% buffered neutral formalin, and some pancreatic specimens also in Stefanini’s fixative (neutral picric acid-formaldehyde). 19 Some tissue specimens from the antrum, duodenum, and ileum were also fixed in Bouin’s fluid. In addition, two pancreatic specimens were also fixed in buffered 2% glutaraldehyde, or in a mixture of 0.5% glutaraldehyde/4% formaldehyde. The fixation time was 18 to 20 hours at room temperature, followed by dehydration and embedding in paraffin. Sections 5-μm thick were cut and attached to poly-l-lysine-coated or to positively charged (Superfrost+; Menzel Gläser, Braunschweig, Germany) glass slides.
The tissue sections were stained with H&E or immunostained by different methods to demonstrate various NE secretory granule products. The streptavidin-biotin-peroxidase complex technique, 20 with diaminobenzidine as chromogen, was applied as a single immunostain mainly to reveal the distribution pattern of positive endocrine cell types in the respective regions, as well as to perform the control stainings specified below. For SV2 and synaptophysin immunostaining, the formalin- and Stefanini-fixed sections were pretreated in a microwave oven (Philips Whirlpool Nordic AB, Stockholm, Sweden) for 2 × 5 minutes at 750 W, using a Tris buffer, pH 8.0, as retrieval solution; this processing step was necessary to get satisfactory immunostaining of SV2 and synaptophysin. In the Bouin’s-fixed sections the SV2 immunoreactivity seemed strong without microwave treatment.
In co-localization studies, double-immunofluorescence methods were used without microwave pretreatment. The immunofluorescence staining of SV2 was enhanced by the catalyzed reporter deposition (CARD) method with biotinyl tyramide 21,22 as described below. For double-immunofluorescence staining, the sections were incubated with a cocktail of two antibodies: SV2 (anti-mouse) plus polyclonal antibody overnight at room temperature → biotinylated goat anti-mouse IgG, 30 minutes at room temperature → streptavidin-horseradish peroxidase, 30 minutes at room temperature → biotinyl tyramide, 10 minutes at room temperature → a mixture of streptavidin-Texas Red plus fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG. Before applying the respective primary antibodies, the sections were incubated with nonimmune sera from the animal species producing the secondary antibodies at a dilution of 1:10. The secondary antibody in question was pre-incubated overnight at 4°C with 10 μl/ml normal serum both from the animal species recognized by the other secondary antibody and from the species producing the other secondary antibody.
When two primary monoclonal antibodies raised in the same species (mouse) had to be used, a double-CARD method was used as follows: primary anti-mouse SV2 antibodies were applied overnight at room temperature, followed by biotinylated goat anti-mouse IgG and the CARD method with biotinyl tyramide and streptavidin-Texas Red as described above. Thereafter, the sections were incubated first with unlabeled avidin (100 μg/ml) overnight at room temperature, and then with the second primary anti-mouse antibody, followed by biotinylated horse anti-mouse IgG and the CARD enhancement method with biotinyl tyramide and streptavidin-FITC as chromogen. The avidin at a concentration of 100 μg/ml applied overnight was found to saturate the first-step biotin. Between each of the staining steps the sections were carefully washed with phosphate-buffered saline.
The control stainings included: 1) omission of one or both of the primary antisera, 2) replacement of the first layer of antibody by nonimmune serum diluted 1:10 and by the diluent alone, and 3) preincubation (24 hours) of primary antiserum with the relevant antigen (10 nmol per ml diluted antibody solution) before application to the sections. The secondary antibodies were tested in relation to the specificity of the species in which the primary antibodies had been raised, the secondary antibody in question being replaced by secondary antibodies from different animal species. These control tests were performed with both immunofluorescence and streptavidin-biotin-peroxidase complex techniques. A neutralization test with SV2 antibodies was not performed, because we did not have access to SV2 antigen, but these antibodies have been characterized by Buckley and Kelly. 8
Electron Microscopy
Pancreatic tissue specimens, ∼1 mm 3 in size, from two adult patients with no metabolic disease, were collected and fixed in 4% paraformaldehyde/0.5% glutaraldehyde in 0.1 mol/L cacodylate buffer, pH 7.2, supplemented with 0.1 mol/L sucrose, for 4 hours at 4°C. During subsequent dehydration in 50 to 95% ethanol, the temperature was lowered to −20°C. The specimens were infiltrated at −20°C with monomeres of the low-temperature hydrophilic-embedding medium Lowicryl K4 mol/L (Agar Scientific Ltd., Stansted, Essex, UK). Polymerization was performed in ultraviolet light (360 nm) at −20°C for 24 hours and at +20°C for another 48 hours. 23,24 Islets in the adult pancreases were localized in semithin toluidine blue-stained sections. Ultrathin sections were cut with a diamond knife and placed on formvar-coated nickel grids.
The immunogold labeling method used has been described in detail previously. 25 The sections were blocked for unspecific binding by applying nonimmune serum at a dilution of 1:10, followed by incubation overnight with the primary antibody diluted 1:50 in 0.05 mol/L Tris-buffered saline (TBS), pH 7.2, supplemented with 0.1 mol/L bovine serum albumin (BSA; Sigma, St. Louis, MO). After thorough rinsing in 0.05 mol/L TBS, pH 7.2, with 0.2% BSA, and in TBS, pH 8.2, with 1% BSA, the sections were incubated with 10- or 15-nm gold-conjugated goat anti-mouse IgG diluted 1:20 in TBS, pH 8.2, with 1% BSA, for 2 hours at 20°C. Again, the sections were thoroughly rinsed in TBS, pH 7.2, and finally were contrasted with 4% aqueous uranyl acetate and Reynolds lead citrate before examination in a Philips 201 electron microscope (Philips Industrial Electronics AB, Eindoven, The Netherlands). For controls, the primary antibody was omitted or was replaced with nonimmune serum. To improve the immunoreactivity, different dilutions of antibodies, durations of the incubation, and microwave pretreatments of the sections (at 150 to 750 W, for 30 seconds to 5 minutes) were tested.
Chemicals Used
The primary antibodies are characterized in Table 1 ▶ . The monoclonal antibodies to SV2 were a generous gift from Dr. R. B. Kelly (Department of Biochemistry and Biophysics, University of California, San Francisco, CA) and have been characterized by Buckley and Kelly. 8 The other primary antibodies used have been characterized previously. 7
Table 1.
Antisera Used
| Antibody raised to | Code no. | Directed to amino acid sequence | Working dilution | Source |
|---|---|---|---|---|
| Synthetic adrenocorticotropin† | M3501 clone 02A3 | 1–39 | 1:10 | DAKO, Santa Barbara, CA |
| Synthetic human calcitonin‡ | A0576 | 1–32 | 1:80 | DAKO |
| Synthetic cholecystokinin‡ | B 38-1 | 10–20 | 1:40 | Eurodiagnostica, Malmö, Sweden |
| Human chromogranin A† | LK2H10 | - | 1:20 | Boehringer Mannheim, Mannheim, Germany |
| Purified human follicle-stimulating hormone† | M3504 clone C10 | β-subunit | 1:5 | DAKO |
| Synthetic porcine gastric inhibitory polypeptide‡ | B35-1 | - | 1:80 | Eurodiagnostica |
| Synthetic human gastrin I‡ | B36-1 | C terminal | 1:80 | Eurodiagnostica |
| Synthetic human gastrin I‡ | 61050 | N terminal (2–17) | 1:800 | Peninsula Laboratories Europe, Merseyside, UK |
| Synthetic human gastrin I§ | B-GP360-1 | C terminal | 1:240 | Eurodiagnostica |
| Synthetic porcine glucagon‡ | A565 lot 081 | - | 1:60 | DAKO |
| Synthetic human glucagon/glicentin† | Glu-001 7360061 | Midportion (5–15) | 1:20 | Novo Nordisk S/A, Bagsvaerd, Denmark |
| Purified human growth hormone§ | 4749-9509 | - | 1:200 | Biogenesis Ltd., Poole, UK |
| Synthetic human insulin§ | Ma 47 | A chain | 1:80 | P. Westermark, Dept. of Pathology, Uppsala, Sweden |
| Purified human luteinizing hormone† | 5720-1004 | - | ’pre-diluted’ 1:2 | Biogenesis |
| Synthetic neurotensin‡ | 6-8208 | C terminal (8–13) | 1:50 | E. Theodorsson, Dept. of Clin. Chemistry, Linköping, Sweden |
| Synthetic human pancreatic polypeptide‡ | A619 lot 105 | - | 1:50 | DAKO |
| Synthetic parathyroid hormone‡ | 41P | 1–34 | 1:5 | BioGenex, San Ramon, CA |
| Synthetic porcine PYY§ | B-GP520-1 | - | 1:800 | Eurodiagnostica |
| Purified human prolactin† | MCA713 clone INN–hPRL-3 | - | 1:15 | Serotec, Oxford, UK |
| Purified bovine S-100‡ | Z-311, lot 113 | - | 1:80 | DAKO |
| Synthetic porcine secretin‡ | B-33-1 | - | 1:10 | Eurodiagnostica |
| Synthetic serotonin* | YC5/45 | - | 1:20 | Medicorp, Montreal, Canada |
| Synthetic human somatostatin‡ | A 566, lot 72 | 1–14 | 1:100 | DAKO |
| Synaptic vesicle protein 2 (SV2)† | - | - | 1:10 | R.B. Kelly, Dept. of Biochemistry and Biophysics, UCSF, CA |
| Bovine synaptophysin† | SY38 | - | 1:20 | Boehringer Mannheim |
| Purified human thyroid stimulating hormone† | 8920-0584 | - | ’pre-diluted’ | Biogenesis |
| Purified rat tyrosine hydroxylase† | 1017 381 | - | 1:5 | Boehringer Mannheim |
Dilutions used in immunofluorescence staining; for the ABC staining the dilutions were 10 to 20 times higher.
*Antisera raised in rat (monoclonal).
†antisera raised in mouse (monoclonal),
‡antisera raised in rabbit;
§antisera raised in guinea-pig.
The labeled secondary antisera were as follows: biotinylated swine anti-rabbit IgG, biotinylated goat anti-mouse IgG, streptavidin biotin complex kit (DAKO, Glostrup, Denmark), unlabeled avidin, biotinylated horse anti-mouse and Texas Red- and FITC-labeled streptavidin (Vector Laboratories, Burlingame, CA), FITC-conjugated goat anti-rabbit IgG (Sigma Chemical Co.), biotinyl tyramide (Dupont-New England Nuclear Research Products, Boston, MA), and 10- or 15-nm gold-conjugated goat anti-mouse IgG (GAM-G10 and GAM-G15; Amersham International, Amersham, Bucks, England).
Results
Light Microscopy
Normal Tissue
SV2-immunoreactive cells were demonstrated with both streptavidin-biotin-peroxidase complex and immunofluorescence methods with the fixatives used, except glutaraldehyde. The strongest immunoreactivity was seen in the Bouin’s-fixed tissues. The staining intensity became gradually weaker with Stefanini’s fixative and formalin, and was only faint with the glutaraldehyde/paraformaldehyde mixture. When either microwave pretreatment of the sections or the CARD technique was used, the staining intensity in the Stefanini- and formalin-fixed tissues increased to a level similar to that seen in Bouin’s-fixed tissue. The enhancement of the staining intensity did not, however, influence the frequency of immunoreactive cells. Microwave pretreatment of pancreatic sections fixed in 2% glutaraldehyde or in a glutaraldehyde/paraformaldehyde mixture did not improve the immunostaining.
Control Stainings: In double-immunofluorescence staining, omission of one of the primary antibodies gave a staining pattern corresponding to that obtained with the remaining primary antibody. The other staining controls were negative.
When using double-CARD immunostaining, biotin blocking is necessary, to avoid unspecific binding of the secondary antibody of the second staining sequence, as demonstrated in Figures 1 and 2 ▶ ▶ .
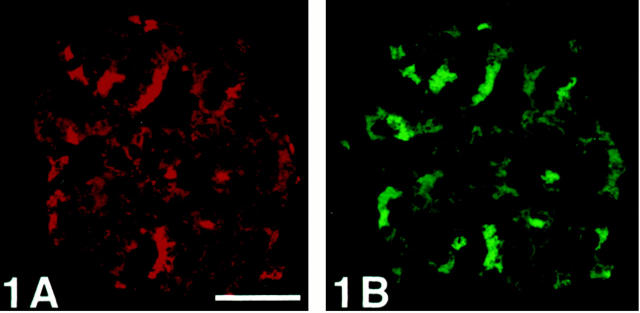
Figure 1.
Human pancreatic islet immunostained with double-CARD for SV2 and chromogranin A without avidin blocking of biotin after the first staining sequence. The staining pattern in A and B are similar, which reflects unspecific binding of the secondary antibodies of the second staining sequence. Compare with Figure 2 ▶ . Scale bar, 50 μm.
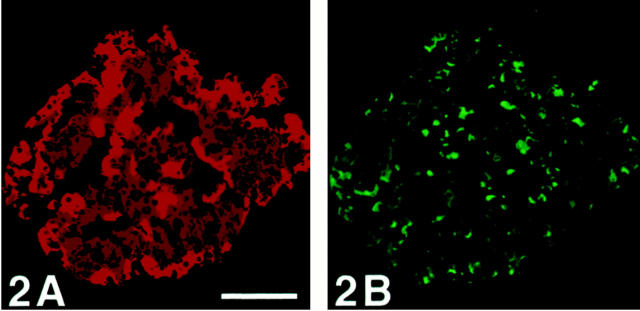
Figure 2.
Human pancreatic islet immunostained with double-CARD for SV2 (A) and chromogranin A (B) with avidin blocking of biotin after the first staining sequence. The staining pattern in A differs from that in B. After the avidin blocking of biotin, there is a distinct difference in the staining pattern between A and B. Scale bar, 170 μm.
Distribution of SV2-Immunoreactive Endocrine Cells of Various Organs: SV2-immunoreactive cells were observed in all organs examined, ie, in the gastrointestinal tract, pancreas, pituitary, thyroid, parathyroid glands, and adrenal medulla, but not in the adrenal cortex. SV2 immunoreactivity was diffusely distributed in the entire cytoplasm, involving processes of cells when present. In addition, SV2 immunostaining visualized nerve structures in all organs examined.
Gastrointestinal Tract
The whole gastrointestinal tract contained scattered SV2-immunoreactive cells, localized at all levels of the mucosa, but these cells were most numerous in the middle third portion. The highest frequency was found in the antrum, followed by the duodenum and colon. Few SV2-immunoreactive cells were observed in Brunner’s glands and in the gastric corpus and ileum.
The results concerning the co-localization of SV2 with hormones in the gastrointestinal endocrine cells are summarized in Table 2 ▶ . In the corpus, the serotonin (enterochromaffin) cells and occasionally somatostatin cells displayed SV2 immunoreactivity. In the antrum, most enterochromaffin cells, as well as virtually all somatostatin and gastrin cells (Figure 3) ▶ , were also immunoreactive. In the duodenum, only some of the enterochromaffin cells but virtually all gastrin, cholecystokinin (CCK), secretin, and enteroglucagon cells were SV2-immunoreactive; occasionally gastric inhibitory polypeptide cells were immunoreactive, whereas somatostatin cells were negative (Figure 4) ▶ . In Brunner’s glands the gastrin and CCK cells were SV2-immunoreactive, as well as most enterochromaffin cells. The sparse SV2-immunoreactive cells seen in the ileum represented enterochromaffin or enteroglucagon cells, and a few of them neurotensin cells, but the somatostatin cells were nonimmunoreactive. In the colon, enteroglucagon and peptide tyrosine tyrosine cells and most enterochromaffin cells were SV2-positive, but not the somatostatin cells.
Table 2.
Co-Localization of SV2 with Hormones in Various Endocrine Cell Types in Different Parts of the Human Gastrointestinal Tract and in the Pancreas
| Gastrointestinal level | Cell type | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serotonin (EC) | Gastrin | Somato- statin | CCK | GIP | Secretin | EG | EG//PYY | Neuro- tensin | Insulin +Glucagon +PP | |
| Corpus | ++ | (+) | ||||||||
| Antrum | ++/+++ | ++/+++ | ++/+++ | |||||||
| Duodenum: | ||||||||||
| villi+ crypts | + | +++ | — | +++ | (+) | ++ | ++/+++ | |||
| Brunner’s glands | ++ | +++ | — | +++ | ||||||
| Ileum: | ||||||||||
| villi+ crypts | ++ | — | ++ | + | ||||||
| Colon | +/++ | +++ | ||||||||
| Pancreas | ++/+++ | +++ | ||||||||
EC, enterochromaffin cells; CCK, cholecystokinin; GIP, gastric inhibitory polypeptide; EG, enteroglucagon (glucagon/glicentin); PYY, peptide tyrosine tyrosine; PP, pancreatic polypeptide.
The number of SV2-immunoreactive cells is graded in relation to the total number of corresponding endocrine cells at each gastrointestinal level: +++, more than 50% of the endocrine cells were SV2-immunoreactive (positive); ++, 30–50% of the cells were positive; (+), occasional cells were positive; −, no SV2-immunoreactive endocrine cells.
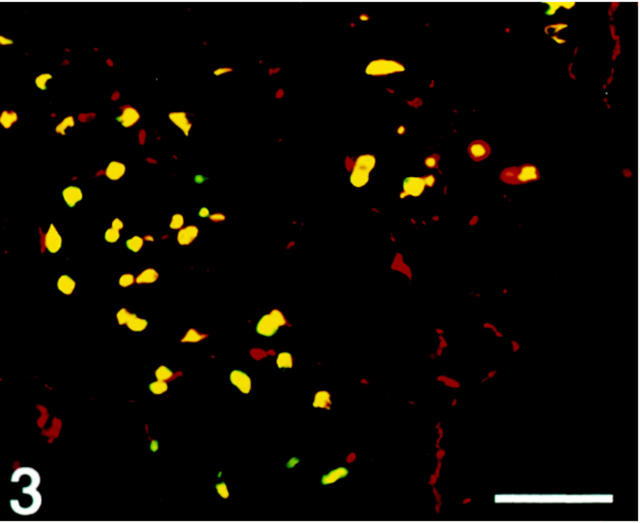
Figure 3.
Human antral mucosa double-immunostained for SV2 (Texas Red) and gastrin (FITC). Co-localization, illustrated by the yellow color (double-band filter set), shows SV2 immunoreactivity in the gastrin cells. SV2-immunoreactive nerve structures are also demonstrated. Scale bar, 160 μm.
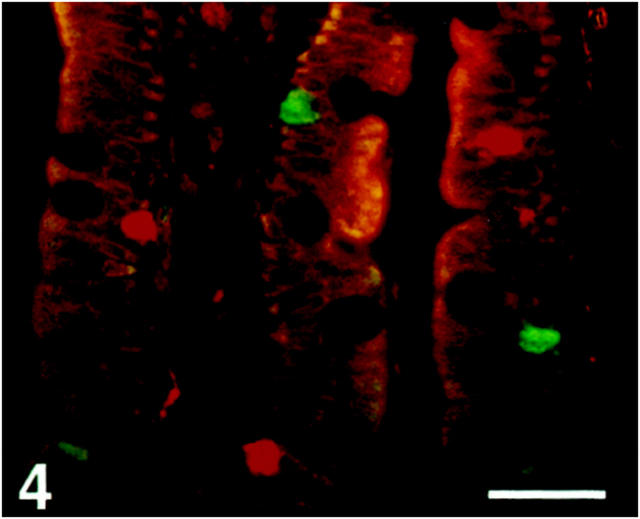
Figure 4.
Human duodenal villi double-immunostained for SV2 (Texas Red) and somatostatin (FITC), showing that the somatostatin cells are SV2-nonreactive. Scale bar, 170 μm.
Pancreas
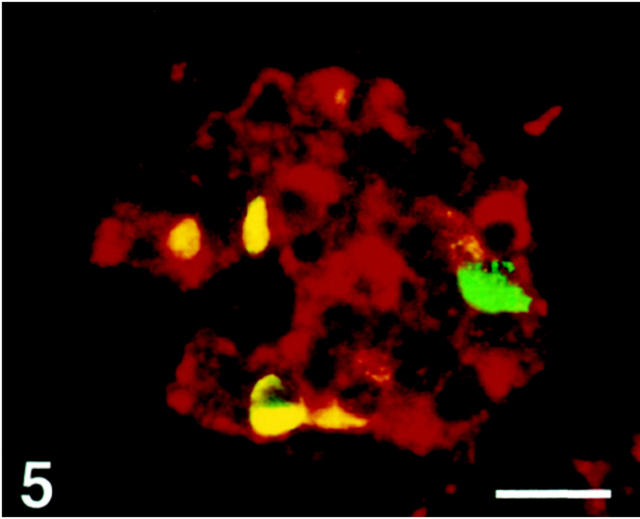
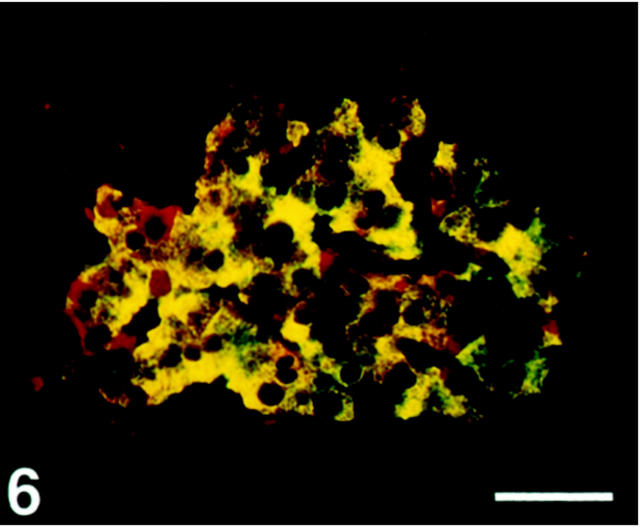
SV2 immunoreactivity was observed in all four major endocrine cell types, except in a fraction of somatostatin cells, which were nonreactive (Figures 5 and 6) ▶ ▶ . Glucagon cells displayed a stronger staining intensity than the other cell types.
Figure 5.
Human pancreatic islet double-immunostained for SV2 (Texas Red) and somatostatin (FITC). SV2 immunoreactivity is seen in all somatostatin cells (yellow), except one (green). Scale bar, 40 μm.
Figure 6.
Human pancreatic islet double-immunostained for SV2 (Texas Red) and insulin (FITC). With the double-band filter set SV2 is shown to be co-localized with insulin (yellow). Noninsulin cells are also immunostained (red). Scale bar, 50 μm.
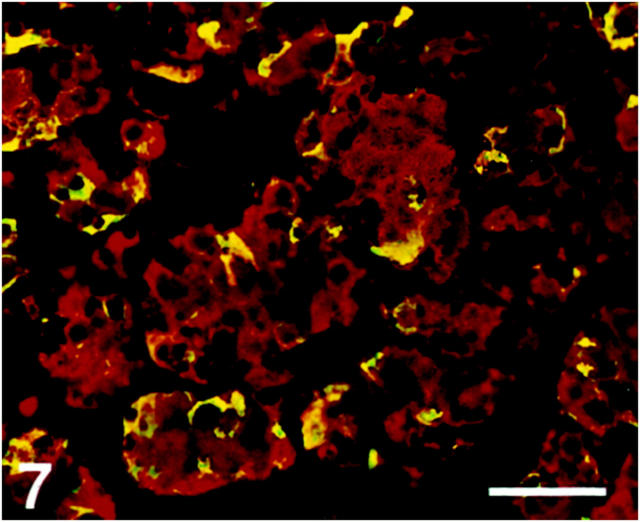
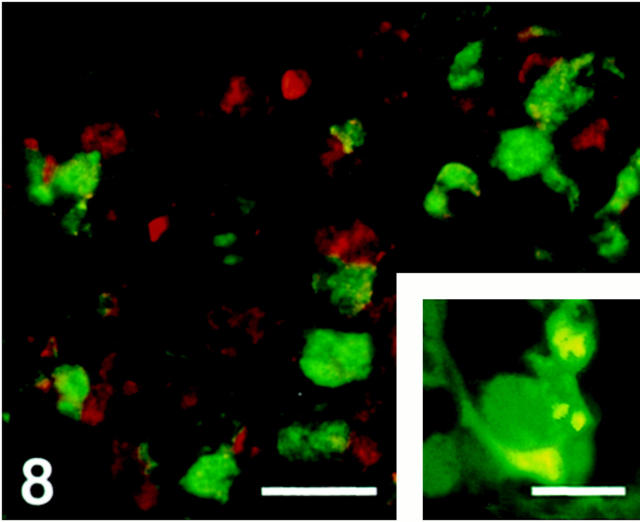
Pituitary Gland
The majority of the parenchymal cells in the anterior pituitary lobe were SV2-immunoreactive. The immunoreactivity was observed mainly in growth hormone-immunoreactive cells but also in some adrenocorticotropic hormone cells (Figure 7) ▶ . No immunoreactivity was seen in thyroid-stimulating hormone, luteinizing hormone, or follicle-stimulating hormone cells (Figure 8) ▶ . Folliculo-stellate cells, identified by S-100 antibodies, showed SV2 immunoreactivity (Figure 8 ▶ , inset).
Figure 7.
Human anterior pituitary gland. Section double-immunostained for SV2 (Texas Red) and adrenocorticotropic hormone (ACTH) (FITC), showing that adrenocorticotropic hormone (ACTH) cells display SV2 immunoreactivity (yellow). Scale bar, 60 μm.
Figure 8.
Human anterior pituitary gland. Section double-immunostained for SV2 (Texas Red) and thyroid-stimulating hormone (FITC), illustrating that SV2 and thyroid-stimulating hormone appear in different cells. Scale bar, 35 μm. Inset: Folliculo-stellate cells stained for S-100 protein (Texas Red) and SV2 (FITC). These cells contain SV2 (yellow). Scale bar, 35 μm.
Thyroid Gland
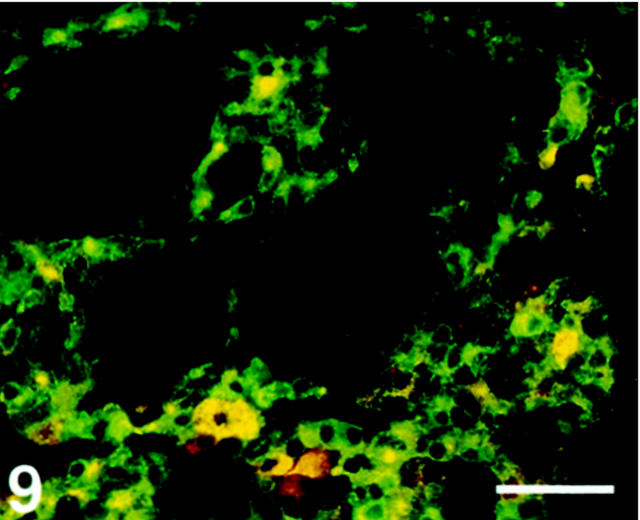
C cells, but not follicular cells, displayed SV2 immunoreactivity (Figure 9) ▶ .
Figure 9.
Human thyroid gland, double-immunostained for SV2 (Texas Red) and calcitonin (FITC). Only C-cells exhibit co-localization (yellow). Scale bar, 80 μm.
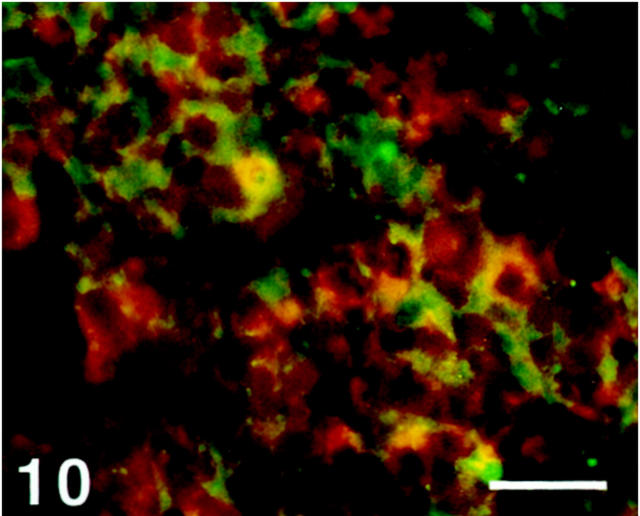
Parathyroid Gland
A minority of chief cells showed immunoreactivity to SV2, whereas the oxyphil cells were nonreactive (Figure 10) ▶ .
Figure 10.
Human parathyroid gland double-immunostained for SV2 (Texas Red) and parathyroid hormone (FITC). Some chief cells display SV2 immunoreactivity to different extents (yellow to orange color), whereas others are nonreactive (green). Scale bar, 40 μm.
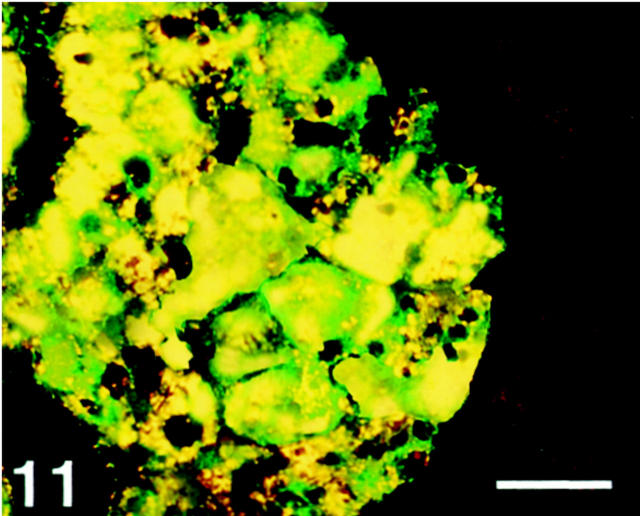
Adrenal Gland
All medullary cells, which were identified by chromogranin A and tyrosine hydroxylase antibodies, were stained with SV2 antibody, and the cortical cells were negative (Figure 11) ▶ .
Figure 11.
Human adrenal gland, double-immunostained for SV2 (Texas Red) and tyrosine hydroxylase (FITC). All tyrosine hydroxylase-immunoreactive cells (medullary cells) show co-localization with SV2 (yellow). Scale bar, 40 μm.
Comparison of SV2, Synaptophysin and Chromogranin A Immunoreactivities: All endocrine organs examined contained cells that displayed SV2, synaptophysin, and chromogranin A immunoreactivity (Table 3) ▶ . The number of SV2-immunoreactive cells exceeded that of synaptophysin-immunoreactive cells except in the gastric antrum and in the pancreas, where a reverse staining pattern was observed. This latter finding was because of the fact that a larger number of somatostatin cells showed synaptophysin than SV2 immunoreactivity. The immunoreactivity to SV2 was usually slightly stronger than that to synaptophysin.
Table 3.
SV2 Immunoreactivity Compared with the Immunoreactivity to Synaptophysin (Sy) and Chromogranin A (CgA) in the Adrenal, Anterior Pituitary, Thyroid, and Parathyroid Glands
| SV2 | Sy | Cg A | |
|---|---|---|---|
| Gastrointestinal tract: | |||
| Stomach | |||
| Corpus | ++ | + | ++ |
| Antrum | +++ | +++ | ++ |
| Duodenum | ++/+++ | +/++ | +++ |
| Ileum | +/++ | + | +++ |
| Colon | ++/+++ | + | +++ |
| Pancreas | +++ | +++ | ++ |
| Anterior pituitary | ++ | + | ++ |
| Thyroid (C cells) | +++ | + | +++ |
| Parathyroid (chief cells) | + | − | ++ |
| Adrenal medulla | +++ | + | ++ |
The number of SV2-immunoreactive cells is graded in relation to the total number of endocrine cells in each endocrine gland: +++, more than 50% of the endocrine cells were SV2-immunoreactive (positive); ++, 30–50% of the cells were positive; (+), occasional cells were positive;—, no SV2-immunoreactive endocrine cells.
The chromogranin A-immunoreactive cells were more numerous than the cells immunoreactive to SV2, except in the antrum and pancreas. In the antrum, a fraction of the somatostatin cells displayed SV2 but not chromogranin A immunoreactivity. Furthermore, in the antrum the immunoreactivity to SV2 was usually stronger than that to chromogranin A.
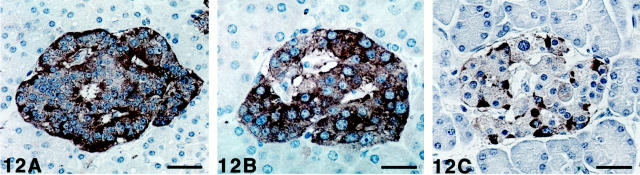
In the pancreas, a minority cell population showed strong immunoreactivity to SV2 and chromogranin A, with a distribution pattern corresponding to that of glucagon cells, whereas synaptophysin immunoreactivity seemed more uniform (Figure 12 ▶ , A–C). The insulin cells displayed weaker immunoreactivity, whereas this cell population was still more weakly immunoreactive or was nonreactive to chromogranin A. In the gastrointestinal tract, SV2- and synaptophysin-immunoreactive nerve structures were present in the myenteric and submucosal plexus, whereas in the lamina propria, SV2 but not synaptophysin nerve fibers were found. The synaptophysin-containing nerves (ganglion cells and bundles of nerve fibers) were fewer than those containing SV2 (ganglion cells, bundles of fibers, and thin nerve fibers), which displayed stronger immunoreactivity. Chromogranin A-immunoreactive nerve structures were also seen, but showed weaker immunostaining than SV2.
Figure 12.
Human pancreatic islets immunostained for SV2 (A), synaptophysin (B), and chromogranin A (C), using the streptavidin-biotin complex method with diaminobenzidine as chromogen, and counterstained with Mayer’s hematoxylin. A: All islet cells are SV2-immunoreactive, but those located at the periphery and close to vessels (glucagon and PP cells) display stronger immunoreactivity. Scale bar, 34 μm. B: More even immunostaining is observed with synaptophysin, ie, insulin cells show stronger immunoreactivity to synaptophysin than to SV2. Scale bar, 21 μm. C: Strong immunoreactivity to chromogranin A is seen in only few cells, with a localization corresponding to glucagon and PP cells. Some of the other cells display weak chromogranin A immunoreactivity. Scale bar, 32 μm.
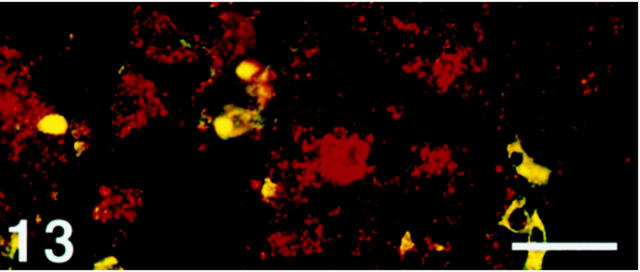
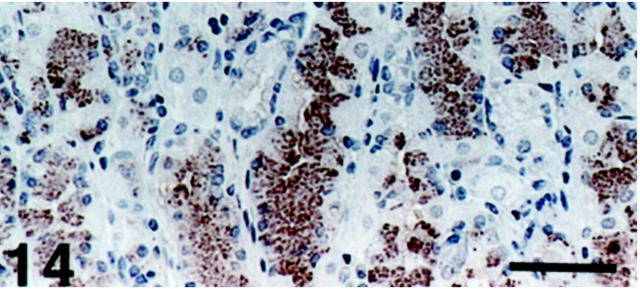
Distribution of SV2-Immunoreactive Exocrine Cells: Among all organs examined, the only SV2 immunoreactivity observed in exocrine cells was in the oxyntic mucosa of the stomach. These numerous nonchromogranin A-immunoreactive cells with basally located nuclei showed a distribution pattern virtually identical to that of chief cells (Figures 13 and 14) ▶ ▶ .
Figure 13.
Human oxyntic mucosa. Section double-immunostained with SV2 (Texas Red) and chromogranin A (FITC), showing co-localization of these two glycoproteins in NE cells (yellow). There are numerous SV2 nonchromogranin A cells (red), indicating the occurrence of SV2 immunoreactivity in exocrine cells. Scale bar, 30 μm.
Figure 14.
Human oxyntic mucosa. The numerous SV2-immunoreactive cells have a distribution pattern corresponding to that of chief cells. Scale bar, 64 μm.
NE Tumors
All tumors examined contained SV2-immunoreactive cells (Table 4 ▶ and Figures 15, 16, 17, 18, 19, 20, and 21 ▶ ▶ ▶ ). This reaction was observed in a majority of the neoplastic cells except in the vipoma. The staining varied in intensity, but was usually moderate to strong, and occurred in the whole cytoplasm as seen in normal cells. Synaptophysin and chromogranin A immunoreactivities were also demonstrated in all of the tumors except in hindgut carcinoids.
Table 4.
Semiquantitative Grading of the Number of Tumor Cells Displaying Immunoreactivity to SV2, Synaptophysin, and Chromogranin A in Various Neuroendocrine Tumors (n = 46)
| Tumor type | Immunoreactivity to | ||
|---|---|---|---|
| SV2 | Synaptophysin | Chromogranin A | |
| Carcinoid of | |||
| Stomach | |||
| ECLoma (2) | +++ | +++ | +++ |
| Gastrinoma (1) | +++ | +++ | +++ |
| Duodenum | |||
| Somatostatinoma (1) | +++ | +++ | +/+++ |
| Ileum/proximal colon (6) | +++ | +++ | +++ |
| Appendix (4) | +++ | +++ | +++ |
| Rectum (8) | +++ | ++ | − |
| Bronchus (3) | +++ | +++ | +++ |
| Islet cell tumors | |||
| Insulinoma (4) | ++/+++ | ++/+++ | ++/+++ |
| Vipoma (1) | ++/+++ | +++ | +++ |
| Nesidioblastosis (3) | ++/+++ | +++ | ++/+++ |
| Medullary thyroid carcinoma (4) | +++ | +/+++ | +++ |
| Anterior pituitary tumors | |||
| ACTH (Cushing) (1) | +++ | ++ | ++ |
| GH (acromegaly) (1) | +++ | ++ | ++ |
| GH/PL (acromegaly) (1) | +++ | ++ | ++ |
| Nonfunctional (2) | +++ | ++/+++ | ++/+++ |
| Pheochromocytoma (4) | +++ | +++ | +++ |
Number of tumor cells displaying immunoreactivity: +++, >90% of the endocrine cells were immunoreactive (positive); ++, approximately 50–90% of the cells positive; +, <50% of the cells positive, (+), a few scattered cells positive; −, no immunoreactive cells.
ECL, enterochromaffin-like; ACTH, adrenocorticotropin; GH, growth hormone; PL, prolactin.
The figures within parentheses refer to the number of cases examined.
Figure 15.
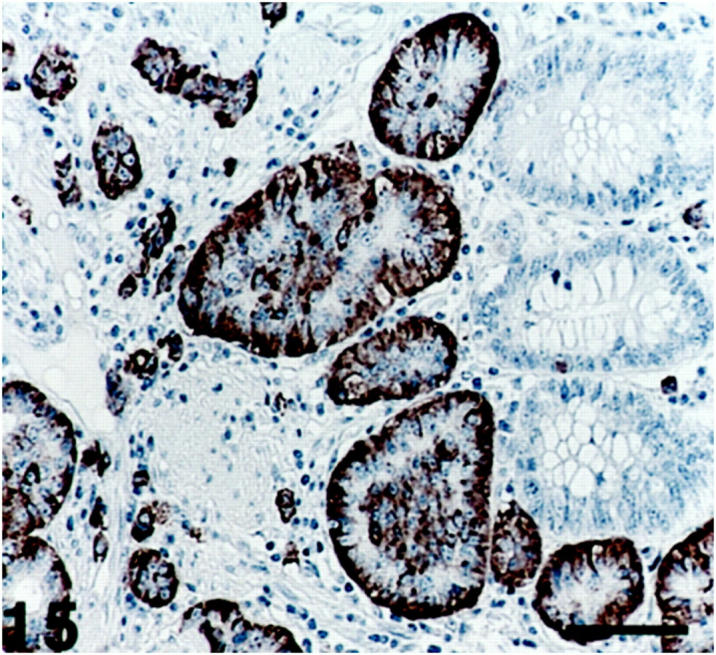
Ileum (midgut) carcinoid, where the tumor cells, arranged in an insular pattern, show SV2 immunoreactivity. Right: A remnant of normal glandular structures. Scale bar, 80 μm.
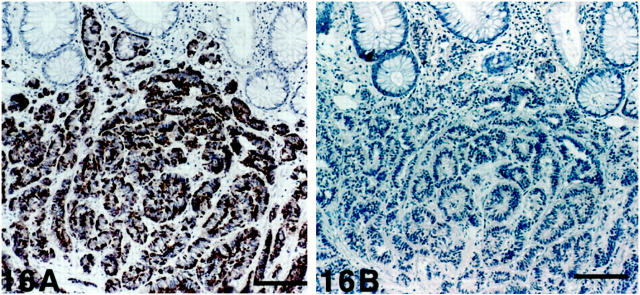
Figure 16.
Rectal (hindgut) carcinoid. A: The tumor cells display SV2 immunoreactivity. Top: Exocrine glands are seen. B: The tumor cells are nonreactive with chromogranin A antibodies. Scale bars, 160 μm.
Figure 17.
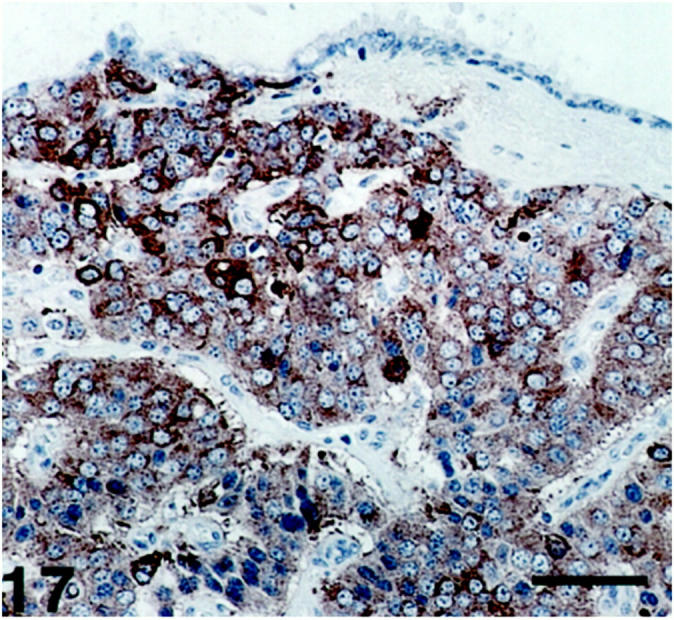
Lung (foregut) carcinoid showing SV2 immunoreactivity of varying intensity. The tumor surface is partly covered by bronchial epithelium. Scale bar, 64 μm.
The relative frequencies of SV2, synaptophysin, and chromogranin A-immunoreactive cells were similar in the different tumors, with some exceptions. The difference in staining pattern was most obvious in hindgut carcinoids, where the majority of tumor cells displayed strong SV2 immunoreactivity, whereas the immunoreactivity to synaptophysin was weak and that to chromogranin A was negative.
The intensity of the immunoreactivity varied in the SV2- and chromogranin A-immunostained tumor cells, but often two cell populations could be distinguished, one displaying stronger immunoreactivity, the other moderate. In contrast, synaptophysin showed a more homogeneous staining pattern in the individual cells in the same tumor, but the intensity varied between the tumors.
In bronchial and midgut carcinoids, pituitary NE tumors, and medullary thyroid carcinomas, the immunoreactivity to SV2 was more intense than that to synaptophysin. The vipoma was the only tumor in which the immunostaining with SV2 seemed weaker than that with synaptophysin and chromogranin A, but this tumor contained sustentacular light cells which stained strongly for SV2. The islets in the nesidioblastosis cases showed a staining pattern similar to that of islets in normal pancreas.
Electron Microscopy
Ultrastructurally, insulin and glucagon cells were identified, as well as solitary somatostatin cells, but no pancreatic polypeptide cells, although numerous sections were examined.
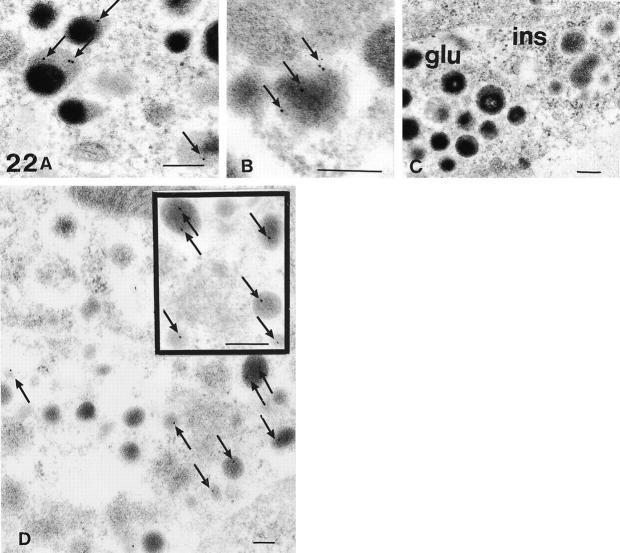
Numerous, but not all, glucagon and insulin secretory granules showed labeling with one to four gold markers (Figure 22, A, B, and D) ▶ . In the glucagon secretory granules, these markers were localized in the lucent peripheral halo, whereas in the insulin secretory granules they were present in the central electron-dense core. Sparse labeling was also seen in somatostatin cell granules (not shown). No gold particles were observed in other intracellular compartments.
Figure 22.
Electron microscopic micrographs from Lowicryl-embedded pancreatic islets, demonstrating the presence of SV2 immunoreactivity (arrows) in the halo of the glucagon cell secretory granules (GAM-G15) (A) and in the core of insulin cell secretory granules (GAM-G10) (B). C: The negative immunoreactivity noted after omission of the SV2 antibody is evident in both the glucagon cell (left) and the insulin cell (right) (GAM-G15). D: This micrograph demonstrates the granular specificity and low background labeling of the SV2 antibodies in an insulin cell (GAM-G15), and the inset shows the preferably core localization of SV2 (GAM-G15). Original magnifications, ×54,000 (A and inset); ×84,000 (B); ×36,000 (C); ×30,000 (D). Scale bars, 200 nm.
Different variations in the microwave pretreatment of the sections before the immunoreaction did not improve the labeling. No gold markers were seen in the negative control sections (Figure 22C) ▶ .
Discussion
To our knowledge, this is the first report on SV2 immunoreactivity in human NE cells and in NE tumors. SV2-immunoreactive cells were found in all organs examined and in most NE cell types, but not in exocrine tissue, except in chief cells of gastric oxyntic mucosa. The frequency of SV2 immunoreactivity in each NE cell type varied from a majority to a minority cell population. The frequency of SV2-immunoreactive somatostatin cells varied with their localization; they were numerous in the pancreas and antrum, but sparse in the remaining gastrointestinal tract.
The immunoelectron microscopic study of pancreatic islets showed that SV2 immunoreactivity was localized in the secretory granules, but its intragranular distribution differed between the glucagon and insulin secretory granules. In the former, the gold markers were located in the lucent peripheral halo, and in the latter in the dense core. The secretory granules showed low labeling, one to four markers per granule, a finding in accordance with an earlier report. 17 This low labeling contrasts, however, with the moderate to strong light microscopic immunostaining, especially in the glucagon cells. The reason for this discrepancy lies in the choice of fixative, glutaraldehyde obviously impairing SV2 immunostaining, but this chemical compound was necessary to preserve the ultrastructure satisfactorily. Tissue processing with the low temperature protocol was chosen to preserve the antigenicity. With 5% water left in the tissue and no postfixation with osmium tetroxide, the morphology is slightly different compared to that of fully dehydrated and postosmicated tissues. 25
The comparison of the immunostaining results between SV2 and the other two examined granule-related glycoproteins, synaptophysin and chromogranin A, showed both similarities and differences. The SV2 immunostaining visualized more NE cells than synaptophysin in all organs examined except in the antrum and pancreas. The reason for this reversed relationship in the latter organs is that a larger proportion of somatostatin cells was stained with the synaptophysin than with the SV2 antibodies. Chromogranin A, at present the broadest NE cell marker, visualizes most NE cell types; some small endocrine cell fractions are not demonstrated, particularly somatostatin cells and a fraction of insulin cells. 2 Chromogranin A identifies more NE cells than SV2 except in the gastric antrum and pancreas; in these organs, both SV2 and synaptophysin are superior to chromogranin A as NE markers. In the anterior pituitary gland and in C cells in the thyroid gland SV2 and chromogranin A identified a similar number of cells. In the adrenal medulla, more cells were immunostained with SV2 than with chromogranin A antibodies, a finding opposite to that in the parathyroid gland.
The physiological function of SV2 is still primarily unknown, but its wide occurrence in different organs indicates an important functional role in the NE cell system. Findings in recent experiments with SV2 knockout mice indicate that SV2 is required for normal neurotransmission and suggest that it plays a role in the regulation of calcium-stimulated exocytosis. 26,27 Some authors have proposed that SV2 may be a vesicle transporter protein in the nervous system, 28-30 but it is not known which molecules may be transported. SV2 is highly glycosylated, for which reason Janz et al 13 hypothesized that it may act as a stabilizing gel in the intravesicular space in the nerve cells. Possibly SV2 has a similar function in the secretory granules of the NE cell system, ie, as a regulator of exocytosis, as a transporter protein, and/or as a stabilizer of the secretory granule structure.
In each NE cell type there were both SV2-immunoreactive and nonimmunoreactive cells. The relative numbers of these two cell fractions varied from a majority to virtual absence, suggesting that SV2 has an important functional role in hormone metabolism. A further interesting finding was that SV2 occurred mainly in somatostatin cells in the antrum and pancreas, and not in the intestinal tract; thus it was found in those somatostatin cells which, according to Francis et al, 31 produce somatostatin-14, but not somatostatin-28.
The frequency of SV2-immunoreactive cells in NE tumors was in good accordance with that of chromogranin A- and synaptophysin-immunoreactive cells, except in hindgut carcinoids, where SV2 was superior as a marker compared to the other two; the tumor cells displayed strong immunoreactivity with the SV2 antibody and very weak immunoreactivity with synaptophysin, whereas chromogranin A immunoreactivity was negative. Immunoreactivity to SV2 was also apparently stronger than that to synaptophysin in bronchial and midgut carcinoids, pituitary NE tumors and medullary thyroid carcinoma. Thus, SV2 broadens the spectrum of diagnostic tools for NE tumors and seems to be of great value especially in the diagnosis of hindgut carcinoids.
Figure 18.
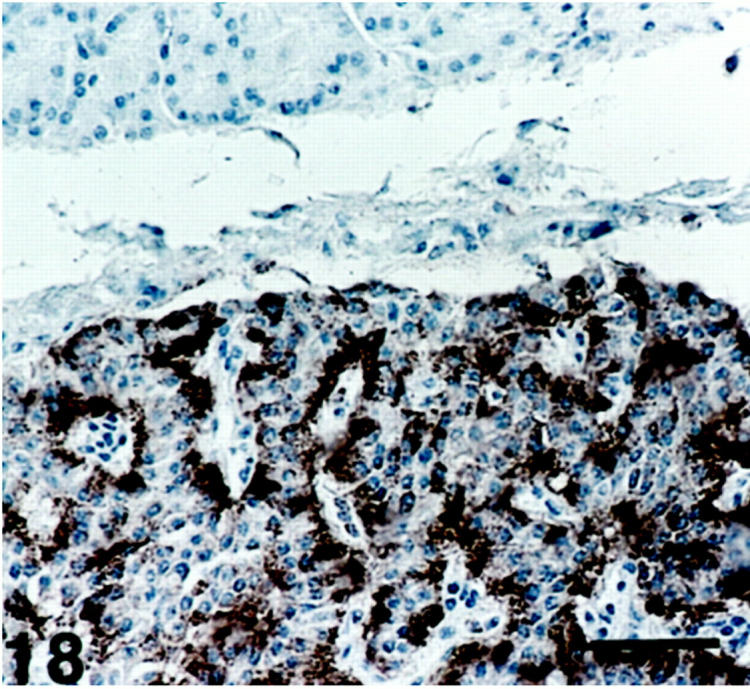
Insulinoma showing SV2 immunoreactivity. The strongest immunoreactivity is seen in cells in connection with the perivascular stroma. The exocrine acinar cells (top) are unstained. Scale bar, 80 μm.
Figure 19.
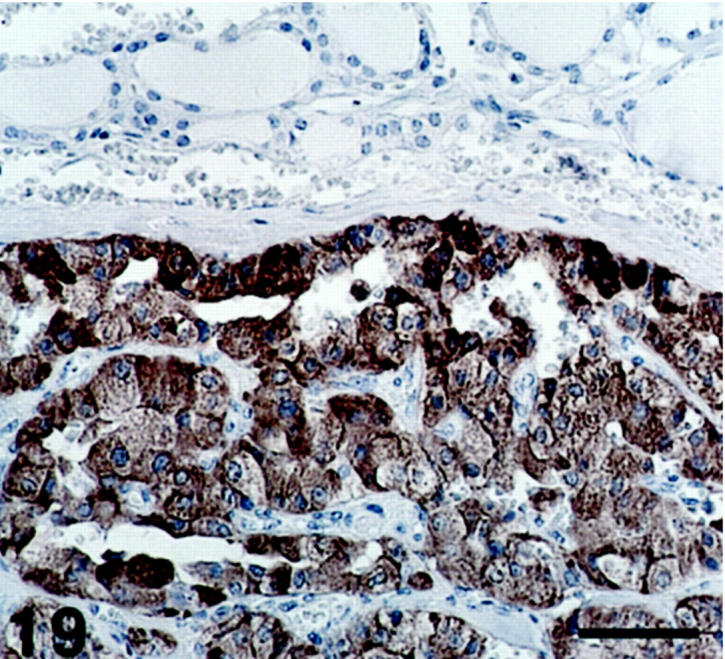
Medullary thyroid carcinoma, where all neoplastic cells are SV2-immunoreactive. At the top, some normal follicular structures are seen. Scale bar, 64 μm.
Figure 20.
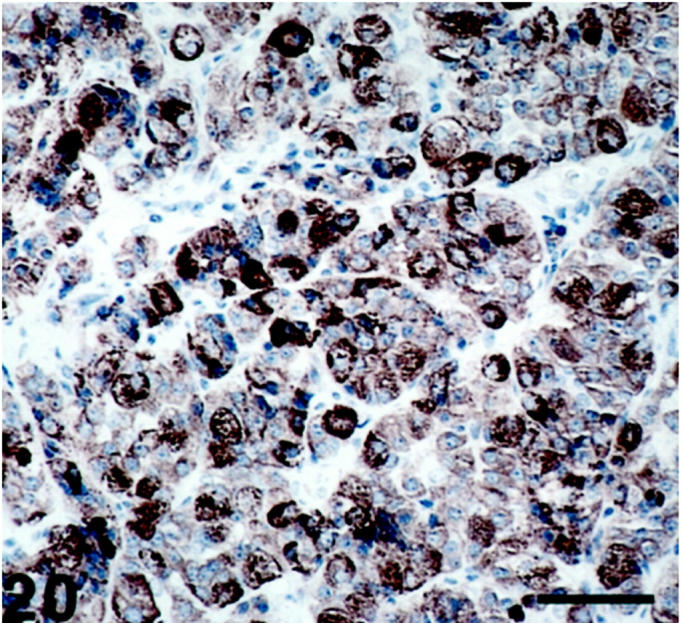
Pituitary adenoma (acromegaly) exhibiting SV2-immunoreactive neoplastic cells with varying degrees of immunoreactivity. Scale bar, 53 μm.
Figure 21.
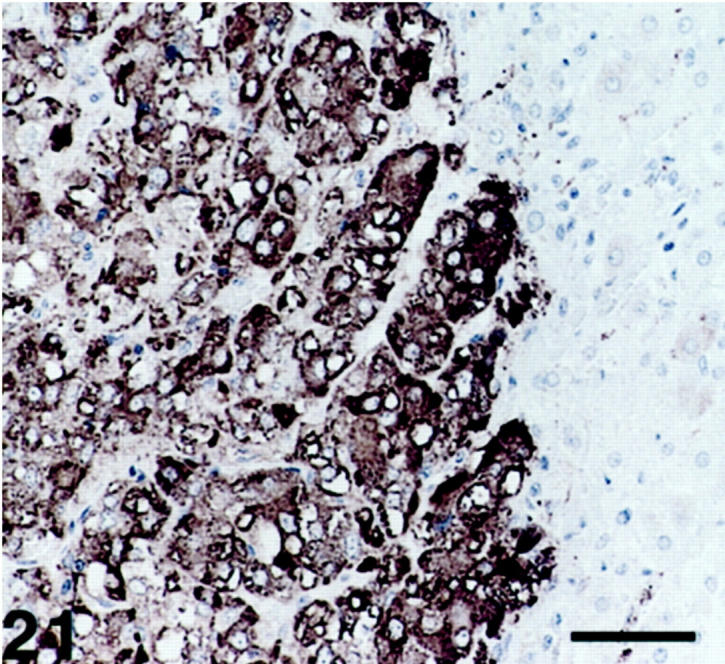
Pheochromocytoma displaying SV2-immunoreactive cells. The cortical cells (right) are nonimmunoreactive. Scale bar, 53 μm.
Acknowledgments
We thank Dr. R.B. Kelly for his generous gift of SV2 antibodies, and Professor H. Johansson for comments on the manuscript.
Footnotes
Address reprint requests to Guida Maria Portela-Gomes, Department of Genetics and Pathology, Unit of Pathology, University Hospital, S-75185 Uppsala, Sweden. E-mail: portela gomes@yahoo.com.
Supported by a grant from the Swedish Medical Research Council (project no. 102) and by the Lion’s Foundation.
References
- 1.Lloyd RV, Wilson BS: Specific endocrine tissue marker defined by a monoclonal antibody. Science 1983, 222:628-630 [DOI] [PubMed] [Google Scholar]
- 2.Portela-Gomes GM, Stridsberg M, Johansson H, Grimelius L: Complex co-localization of chromogranins and neurohormones in the human gastrointestinal tract. J Histochem Cytochem 1997, 45:815-822 [DOI] [PubMed] [Google Scholar]
- 3.Jahn R, Schiebler W, Ouimet CH, Greengard P: A 38,000-dalton membrane protein (p 38) present in synaptic vesicles. Proc Natl Acad Sci USA 1985, 82:4137-4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiedenmann B, Franke WW: Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell 1985, 41:1017-1028 [DOI] [PubMed] [Google Scholar]
- 5.Wiedenmann B, Waldherr R, Buhr H, Hille A, Rosa P, Huttner WB: Identification of gastroenteropancreatic neuroendocrine cells in normal and neoplastic human tissue with antibodies against synaptophysin, chromogranin A, secretogranin I (chromogranin B), and secretogranin II. Gastroenterology 1988, 95:1364-1372 [DOI] [PubMed] [Google Scholar]
- 6.Buffa R, Rindi G, Sessa F, Gini A, Capella C, Jahn R, Navone F, De Camilli P, Solcia E: Synaptophysin immunoreactivity and small clear vesicles in neuroendocrine cells and related tumours. Mol Cell Probes 1988, 2:367-381 [DOI] [PubMed] [Google Scholar]
- 7.Portela-Gomes GM, Stridsberg M, Johansson H, Grimelius L: Co-localization of synaptophysin with different neuroendocrine hormones in the human gastrointestinal tract. Histochem Cell Biol 1999, 111:49-54 [DOI] [PubMed] [Google Scholar]
- 8.Buckley K, Kelly RB: Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol 1985, 100:1284-1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowe W, Madeddu L, Kelly RB: Endocrine secretory granules and neuronal synaptic vesicles have three integral membrane proteins in common. J Cell Biol 1988, 106:51-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou XE, Dahlström A: Synaptic vesicle proteins in cells of the sympathoadrenal lineage. J Auton Nerv Syst 1996, 61:301-312 [DOI] [PubMed] [Google Scholar]
- 11.Bajjalieh SM, Peterson K, Linial M, Scheller RH: Brain contains two forms of synaptic vesicle protein 2. Proc Natl Acad Sci USA 1993, 90:2150-2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bajjalieh SM, Frantz GD, Weimann JM, McConnel SK, Scheller RH: Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci 1994, 14:5223-5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janz R, Hofmann K, Südhof C: SVOP, an evolutionarily conserved synaptic vesicle protein, suggests novel transport functions of synaptic vesicles. J Neurosci 1998, 18:9269-9281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janz R, Südhof C: SV2C is a synaptic vesicle protein with an unusually restricted localization: anatomy of a synaptic vesicle protein family. Neuroscience 1999, 94:1279-1290 [DOI] [PubMed] [Google Scholar]
- 15.Varndell IM, Lloyd RV, Wilson BS, Polak JM: Ultrastructural localization of chromogranin: a potential marker for the electron microscopic recognition of endocrine cell secretory granules. Histochem J 1985, 17:981-992 [DOI] [PubMed] [Google Scholar]
- 16.Kalina M, Lukinius A, Grimelius L, Höög A, Falkmer S: Ultrastructural localization of synaptophysin to the secretory granules of normal glucagon and insulin cells in human islets of Langerhans. Ultrastruct Pathol 1991, 15:215-219 [DOI] [PubMed] [Google Scholar]
- 17.Tanner VA, Plough T, Tao-Cheng JH: Subcellular localization of SV2 and other secretory vesicle components in PC12 cells by an efficient method of preembedding EM immunocytochemistry for cell cultures. J Histochem Cytochem 1996, 12:1481-1488 [DOI] [PubMed] [Google Scholar]
- 18.Marxen M, Maienschein V, Volknandt W, Zimmermann H: Immunocytochemical localization of synaptical proteins at vesicular organelles in PC12 cells. Neurochem Res 1997, 22:941-950 [DOI] [PubMed] [Google Scholar]
- 19.Stefanini M, De Martino C, Zamboni L: Fixation of ejaculated spermatozoa for electron microscopy. Nature 1967, 216:173-174 [DOI] [PubMed] [Google Scholar]
- 20.Hsu SM, Raine T, Fanger H: Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled (PAP) procedures. J Histochem Cytochem 1981, 29:577-580 [DOI] [PubMed] [Google Scholar]
- 21.Bobrow MN, Harris TD, Shaughnessy KJ, Litt GJ: Catalyzed reporter deposition, a novel method of signal amplification. Application to immunoassays. J Immunol Methods 1989, 125:279-285 [DOI] [PubMed] [Google Scholar]
- 22.Adams JC: Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J Histochem Cytochem 1992, 40:1457-1463 [DOI] [PubMed] [Google Scholar]
- 23.Kellenberger E, Carlemalm E, Villiger W, Roth J, Garavito RM: Low Denaturation Embedding for Electron Microscopy of Thin Sections. 1980. Chemishe Werke Lowi, Waldkreiburg
- 24.Lukinius A, Wilander E, Westermark G, Engström U, Westermark P: Co-localization of islet amyloid polypeptide and insulin in the B cell secretory granules of the human pancreatic islets. Diabetologia 1989, 32:240-244 [DOI] [PubMed] [Google Scholar]
- 25.Lukinius A, Ericsson JLE, Grimelius L, Korsgren O: Ultrastructural studies of the ontogeny of fetal human and porcine endocrine pancreas, with special reference to colocalization of the four major islet hormones. Dev Biol 1992, 153:376-385 [DOI] [PubMed] [Google Scholar]
- 26.Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, Scheller RH, Chavkin C, Bajjalieh S: Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A). Proc Natl Acad Sci USA 1999, 96:15268-15273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janz R, Goda Y, Geppert M, Missler M, Südhof TC: SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron 1999, 24:1003-1016 [DOI] [PubMed] [Google Scholar]
- 28.Bajjalieh SM, Peterson K, Shinghal R, Scheller RH: SV2, a brain vesicle protein homologous to bacterial transporters. Science 1992, 257:1271-1273 [DOI] [PubMed] [Google Scholar]
- 29.Feany MB, Lee S, Edwards RH, Buckley KM: The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell 1992, 70:861-867 [DOI] [PubMed] [Google Scholar]
- 30.Schivell AE, Batchelor RH, Bajjalieh SM: Isoform-specific, calcium-regulated interaction of the synaptic vesicle proteins SV2 and synaptotagmin. J Biol Chem 1996, 271:27770-27775 [DOI] [PubMed] [Google Scholar]
- 31.Francis BH, Baskin DG, Saunders DR, Ensinck JW: Distribution of somatostatin-14 and somatostatin-28 gastrointestinal-pancreatic cells of rats and humans. Gastroenterology 1990, 99:1283-1291 [DOI] [PubMed] [Google Scholar]