Abstract
The hr (hairless) gene encodes a putative transcription factor with restricted expression in the skin and brain. Mutations in the hr locus cause papular atrichia in humans and complete hair loss in mice and other mammals. To further elucidate the role of hr in skin biology, and to identify potential target cells for hr regulation, we studied hr mRNA localization during hair follicle (HF) morphogenesis and cycling in normal C57BL/6J mice. In situ hybridization revealed that hr expression was present in the suprabasal cell layers of the epidermis, whereas the basal and highly differentiated keratinocytes of the granular layer were hr-negative. During the early stages of HF morphogenesis, hr mRNA was detected in the developing hair peg. Later, it became concentrated in the HF infundibulum, in the HF matrix, and in the inner root sheath (IRS), whereas the dermal papilla (DP) and outer root sheath were consistently hr mRNA-negative. During catagen, hr gene expression gradually declined in the regressing IRS, shortly but dramatically increased in the zone of developing club hair, and became up-regulated in the epithelial cells adjacent to the DP. The co-localization of hr mRNA with the site of the morphological defects in mutant skin implicates hr as a key factor in regulating basic cellular processes during catagen, including club hair formation, maintenance of DP-epithelial integrity, IRS disintegration, and keratinocyte apoptosis in the HF matrix.
Despite extensive functional and morphological studies, the molecular mechanisms governing hair follicle (HF) morphogenesis and cycling are not yet well understood. 1,2 Studies of genetically engineered and mutant mice with hair growth abnormalities have provided some insights into the molecular control of HF cycling. 3-7 At the same time, molecular genetic data alone are not sufficient for a complete understanding of the mechanistic role of regulatory molecules in HF neogenesis and remodeling, because the immediate cellular targets of these molecules remain unknown.
During the last decade, it has been shown that the expression patterns of many cytokines, transcription factors, and adhesion molecules are subject to significant changes during normal HF morphogenesis and cycling 8,9 thus suggesting a role in the regulation of HF transformation. Although informative, these studies are somewhat limited in that they do not illuminate the molecular pathways of gene activity and their functional role in HF biology. In contrast, expression studies may serve as a valuable extension to the molecular and genetic studies and provide substantial insights into the mechanisms of HF functioning, because they can identify the localization and immediate cellular targets of particular regulatory molecules. Therefore, the combination of genetic and functional analysis of mutant mouse models with expression studies in normal mouse skin is among the most powerful experimental approaches in hair research.
The hairless (hr) gene, which encodes for a putative zinc finger transcription factor 10 is one of the candidate genes for the regulation of basic HF functions. 6 This gene is the target of several allelic mutations in laboratory rodents, 11-13 humans, 14-16 and monkeys. 17 The attenuation of hairless gene activity in hr/hr mutants results in the progressive shedding of the infantile hairs in animals, which represents an analog of the autosomal recessive disorder papular atrichia (MIM 209500) in humans. 14,18 In addition to HF abnormalities, homozygous hr/hr mouse mutants display immunological skin dysfunction, elevated sensitivity to UV and chemically induced skin carcinogenesis, 19,20 a unique susceptibility to dioxin skin toxicity, 21 and structural abnormalities in the inner ear, retina, and colon, 13 thus suggesting possible pleiotropic effects of hr gene mutations in humans as well. 18
Our recent studies on the successive pathomorphology of hairlessness have revealed that the attenuation of hr gene activity results in premature and excessive apoptosis and discoordination of cell death, proliferation, and adhesion in selected HF cell populations during the first transition from the anagen to the catagen phase of the HF cycle. 6,22 These functional and morphological studies were supported by recent progress in understanding molecular aspects of hr gene biology. 15,23 At the same time, however, some basic questions surrounding hr gene biology remain unanswered. For example, does the expression of the hr gene co-localize to the sites of the main pathomorphological events that underlie the process of hair shedding in mutant mice? Are the cellular structures involved in the pathogenesis of the hairless phenotype direct targets of hr gene activity? What are the temporal patterns of hr gene expression in skin and which stages of HF transformation are associated with minimal and maximal gene expression? Does the hr gene serve as a regulator of HF progression during a certain stage of the cycle or is hr a key factor in modulating the transition from one stage of hair cycle to the next—a stage-switch factor?
In an initial effort to address these questions, we have used nonradioactive in situ hybridization to localize and characterize hr gene expression throughout HF morphogenesis and cycling in normal mouse skin. The combination of expression data presented here, together with our previous functional studies, allows us to posit that the hr protein is directly involved in the coordination of cell proliferation and cell death, in particular, in epithelial cell populations during HF catagen progression.
Materials and Methods
Animals and Skin Samples
C57BL/6J mice with normal HF cycling behavior were purchased from Jackson Laboratory, Bar Harbor, ME.
For studies of HF morphogenesis, newborn pups (0, 3, 5, 12, and 18 days postpartum) were used. For synchronization of the hair cycle, 8-week-old female mice with all back skin HFs in telogen were depilated with a wax and rosin mixture. 24 Animals were sacrificed by CO2 asphyxiation at defined stages of the HF cycle (days 1, 3, 5, 12, and 18 after depilation corresponding to telogen; anagen II, IV, VI; and catagen, respectively). Three mice were studied at every stage of HF morphogenesis and cycling. The perfusion of mice was performed with ice-cold phosphate-buffered saline (PBS) (Gibco BRL, Grand Island, NY) and 4% paraformaldehyde (EM Science, Gibbstown, NJ) in accordance with standard protocols. 25 The dorsal skin samples were fixed in cold 4% paraformaldehyde overnight, washed in PBS, and embedded in paraffin according to standard procedures. Five-μm sections were mounted on silane-coated glass slides (six slides per mouse with three sections each) and prepared for in situ hybridization as previously described. 26
The stages of HF morphogenesis were assessed according to Hardy’s classification 27 with modifications suggested by Philpott and Paus. 2 The stages of HF cycle were assessed according to Paus et al. 28 On every section, five to fifteen well-sectioned HFs were analyzed.
Probes
The hr cDNA fragment was obtained by polymerase chain reaction using primers spanning nucleotides 3212 to 3233 and 3657 to 3678 (GenBank accession number Z32657) and mouse skin cDNA as a template. The fragment was ligated into a pCRII-TOPO vector (Invitrogen, Carlsbad, CA), and propagated using One Shot TOP10 competent cells (Invitrogen, Carlsbad, CA). The clones encoding the specific cDNA were identified by direct sequencing on both strands using ABI Prism 310 Genetic Analyzer (Applied Biosystems, Foster City, CA).
Digoxigenin-labeled sense and antisense riboprobes were produced in vitro using Sp6 and T7 RNA polymerases (DIG RNA Labeling Kit; Boehringer-Mannheim, Mannheim, Germany) according to the manufacturer’s protocol. The mouse keratin 1 (MK1) riboprobe was obtained as described. 21
In Situ Hybridization
In situ hybridization was performed as previously described. 26,29 Briefly, deparaffinized skin sections were acetylated in acetic anhydride solution (EM Science) and then dehydrated. Hybridization with 50 ng/section of freshly denatured cRNA probes was performed at 50°C for 17 hours in the humidified chambers. The mouse hr sense probes were used as a negative control, and the mouse keratin 1 antisense probe as a positive control. Incubation with sheep alkaline phosphatase-labeled anti-digoxigenin antibodies (DIG Nucleic Acid Detection Kit, Boehringer-Mannheim) was performed for 3 hours in humidified chambers at room temperature. Some control slides were incubated in the absence of antibodies. Then the slides were stained by incubation in nitro blue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate solution (Boehringer-Mannheim) for 16 to 20 hours in complete darkness at room temperature.
Results
hr Gene Expression during HF Morphogenesis in Neonatal Mouse Skin
In the epidermis of neonatal mouse skin (day 1 postpartum), hr mRNA reactivity was present in the suprabasal cell layers with a gradual decrease of intensity in the spinous compartment, concomitant with keratinocyte differentiation (Figure 1A) ▶ . During the initiation of HF morphogenesis, no hr mRNA immunoreactivity was found in the epidermal placode nor in the dermal fibroblast condensation (not shown).
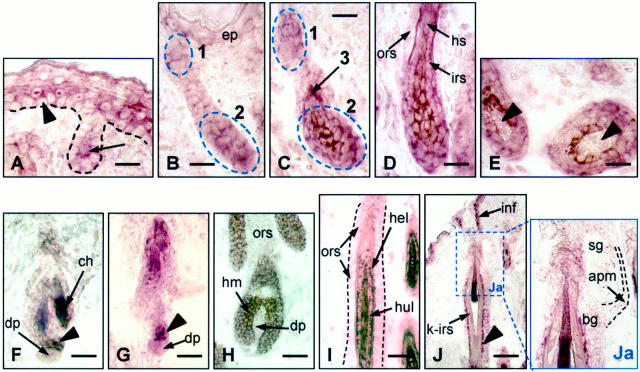
Figure 1.
In situ hybridization with hr mRNA probe in C57BL mouse skin during HF morphogenesis (days 1 to 8 postpartum; A–E) and telogen-anagen stages of depilation-induced HF cycling (days 1 to 12 after depilation; F–J). A: Stage 2 of morphogenesis: the hr mRNA is absent in the basal keratinocytes (arrowheads) but present in the suprabasal cell layer and in the innermost keratinocytes (arrow) of the HF peg thus forming the upper zone of hr expression in the HF (zone 1). B: Stage 4: the downward growing portion of the HF peg below zone 1 remains hr-negative, whereas another zone (zone 2) of hr expression occurs in the bulbar HF portion. C: Stage 5: the cone-shaped IRS (zone 3) is highly positive. D: Stage 7: strong hr-mRNA positivity is observed in IRS keratinocytes, whereas the ORS remains hr-negative. E: Stages 7 to 8: the DP (arrowheads) remains hr-negative. F: Telogen: high hr mRNA-positivity is seen in the secondary HF germ (arrowhead). G: Anagen II: the keratinocytes of the downward growing HF are hr mRNA-negative, except the small cell cluster above the DP (arrowhead). H: Mid-anagen: this small zone of hr expression spreads over the HF matrix (hm) and IRS. I: in the middle portion of the HF, hr expression in Huxley’s layer (hul) of the IRS gradually decreases, along with its gradual cornification. The ORS and Henley’s layer remain hr mRNA-negative. J–Ja: In the upper portion of the anagen VI HF, hr mRNA reactivity is localized to the HF infundibulum (inf) and to the innermost cell layer of the restricted portion of the ORS (arrowhead). The sebaceous gland (sg) and the bulge (bg) area of the ORS remain negative. (apm, arrector pili muscle; ch, club hair; ep, interfollicular epidermis; hel, Henley’s layer; hul, Huxley’s layer; hm, HF matrix; hs, hair shaft; inf, HF infundibulum; k-irs, keratinized IRS). Scale bar, 23 μm.
With the formation of the convex HF peg, hr mRNA immunoreactivity was detected in the innermost, suprabasal keratinocytes (Figure 1A) ▶ , similar to hr expression patterns in the interfollicular epidermis. These hr mRNA-positive follicular keratinocytes remained in the upper, epidermis-associated portion of the follicle that corresponds to the infundibulum of the mature HF (zone 1; Figure 1, B and C ▶ ). This pattern of hr expression in the infundibulum persists throughout HF morphogenesis.
The lower, downward-growing portion of the HF peg remained hr-negative at the earliest stages of HF morphogenesis. Later, at stage 4 of HF morphogenesis, along with formation of the hair matrix, the hr mRNA-positive staining appeared (Figure 1B) ▶ and progressively increased (Figure 1C) ▶ in the lower HF portion (zone 2 of expression). During the formation of the precortex and cone-shaped inner root sheath (IRS), and consequent initiation and progression of hair shaft growth, strong hr mRNA positivity was observed in IRS keratinocytes (zone 3; Figure 1, C and D ▶ ).
Thus, in addition to the consistent expression in the interfollicular epidermis, three zones of cell type-specific expression of hr gene were established during HF morphogenesis: in the HF infundibulum (zone 1), in the hair matrix (zone 2), and in the IRS (zone 3). No expression was observed in the lower and middle outer root sheath (ORS) or the dermal papilla (DP) fibroblasts throughout the entire process of HF morphogenesis (Figure 1, D and E) ▶ .
hr Gene Expression during Depilation-Induced HF Cycling
Adolescent mouse skin displayed consistent hr expression in the suprabasal cell layers of the interfollicular epidermis. The suprabasal keratinocytes of the HF infundibulum were also consistently hr mRNA-positive during the entire HF cycle (Figure 1J) ▶ .
In contrast to the epidermis and HF infundibulum, the expression patterns of hr mRNA in the lower portion of the HF epithelia were strictly hair cycle-dependent, and are summarized below.
Telogen
In the telogen or resting phase of HF cycling, prominent hr mRNA immunoreactivity was found in the cells localized to the lowermost portion of the follicle bulb in contact with the DP (the zone of the secondary follicle germ). Some other keratinocytes of the HF, primarily localized to the lower portion of the bulb, were slightly or moderately hr mRNA-positive as well (Figure 1F) ▶ .
Anagen
During the telogen-anagen transition, the hr mRNA-positive keratinocytes of the lower portion of the HF moved downward (Figure 1G) ▶ , and in anagen III, gave rise to prominent hr gene expression in keratinocytes of the HF matrix (Figure 1H) ▶ and Huxley’s layer of the IRS, excluding the completely cornified IRS portion (Figure 1I) ▶ . During the advanced stages of anagen HF development, hr mRNA immunoreactivity was also localized to the thin innermost cell layer of the upper ORS (Figure 1J ▶ , arrowhead). The sebaceous gland cells and keratinocytes of the bulge region were consistently hr mRNA-negative (Figure 1J ▶ a).
Catagen
The most prominent changes in hr mRNA expression patterns were observed during the catagen phase of HF transformation. The spontaneous switch from depilation-induced anagen to catagen was associated with a rapid decline of hr mRNA immunoreactivity in the HF matrix (Figure 2,A ▶ and B). hr mRNA-positivity in IRS keratinocytes during the initial and mid-catagen phases (catagen I to V) remained high (Figure 2, B and C) ▶ , but declined along with progressive IRS cornification and disintegration in catagen VI to VII (Figure 2, D and E) ▶ .
Figure 2.
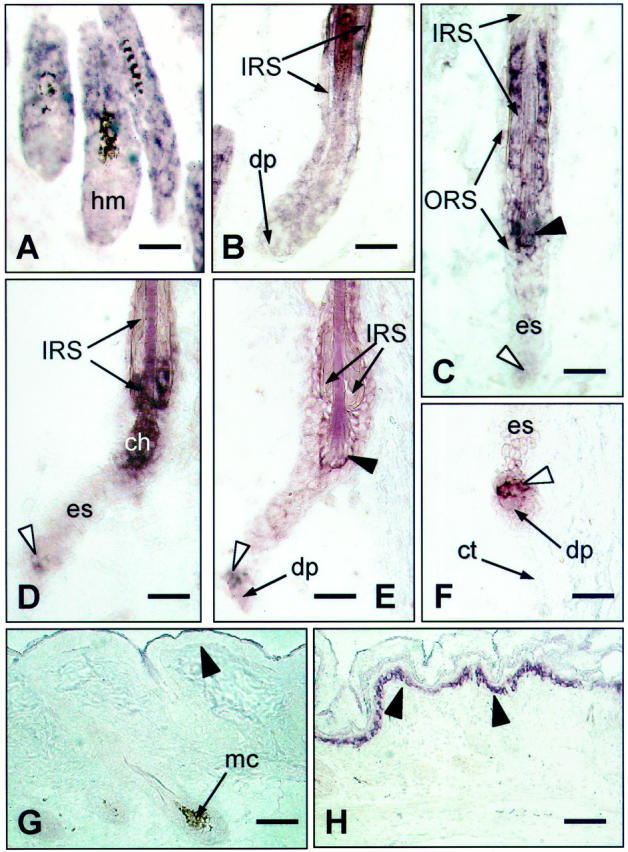
In situ hybridization with hr mRNA probe in C57BL mouse skin during catagen progression of depilation-induced HF cycling (days 17 to 21 after depilation). A: At the transition from anagen to catagen, the decline of hr expression in the HF matrix (hm) is noted (compared to Figure 3C ▶ ). B: Catagen III: the middle portion of the IRS remains hr mRNA-positive. C: Catagen V, hr expression in the upper IRS continues to decline, while it begins in the zone of club hair formation (black arrowhead). The epithelial strand (es) between the DP and the zone of club hair remains hr-negative. D: Catagen VI: hr expression in the zone of club hair formation (ch) is increased. In the IRS, it persists in the restricted lowermost still unkeratinized portion. Some faint hr mRNA positivity occurs in the epithelial strand keratinocytes adjacent to the DP (white arrowhead). E: Catagen VII: with the completion of club hair formation, hr expression in this zone decreased (black arrowhead), but slowly increased in DP-associated keratinocytes (white arrowhead). F: Catagen VII to VIII: hr expression is still present in cell cluster above the DP (white arrowhead). In situ hybridization with sense hr mRNA probe (negative control) (G ) and antisense MK1 mRNA probe (positive control, H) in C57BL mouse skin during HF morphogenesis. Basal cell layer is marked with black arrowheads. (apm, arrector pili muscle; ch, club hair; es, epithelial strand; hm, HF matrix; mc, melanocytes). Scale bars: 23 μm (A–F); 46 μm (G–H).
In catagen V, a new zone of moderate hr mRNA immunoreactivity occurred in the thin layer of ORS keratinocytes around the lower end of the hair shaft (Figure 2C ▶ , black arrowhead). At that point, the club hair begins to form by means of trichilemmal keratinization of ORS keratinocytes and their interdigitation with the cells of the hair shaft. 30 At the next stage of catagen (VI), a significant increase of positive staining in this zone was observed (Figure 2D) ▶ . The completion of club hair formation in catagen VII was associated with a rapid decline of hr mRNA expression and its complete disappearance during HF progression into telogen (Figure 2E) ▶ .
In late catagen, hr mRNA was localized in a few cells of the regressing epithelial strand just above the DP (Figure 2D ▶ , white arrowhead). The epithelial strand contraction and upward movement of the DP during catagen VII to VIII was associated with an increase in hr mRNA expression in this particular keratinocyte population. This population also moved upward and apparently provided a source of hr-positive keratinocytes in the telogen HF (Figure 2, D–F) ▶ . These DP-associated epithelial cells remained highly hr mRNA-positive throughout the entire telogen stage (Figure 1F) ▶ .
MK1 Expression in C57BL/6 Mouse Skin
In situ hybridization with a digoxigenin-labeled riboprobe specific for MK1 was used as positive control. The positive MK1 mRNA staining was clearly seen in the suprabasal cell layers of the interfollicular epidermis as previously reported by Schweizer et al 31 (Figure 2H) ▶ . This MK1 expression pattern along with the results of application of the sense hr riboprobe (Figure 2G ▶ ; negative control) confirms the specificity and sensitivity of our in situ hybridization technique. The slides processed in the absence of anti-digoxigenin alkaline phosphatase-labeled antibodies displayed no staining after the final stage of detection (not shown).
Discussion
Using in situ hybridization, we have shown that the hr gene displays specific patterns of expression in distinct HF cell populations. These patterns are subject to significant changes during HF morphogenesis and cycling, with the most striking alterations during the catagen phase.
hr Expression in the Interfollicular Epidermis Is Stable
The expression patterns of the hr gene in the suprabasal keratinocytes of the interfollicular epidermis and in the suprabasal keratinocytes of the HF infundibulum, which is normally characterized by epidermal patterns of keratinization, 32 were found to be identical. These findings suggest that the characteristic dilation of utriculi in the skin of mutant hairless mice reflects hr-related abnormalities in the interfollicular epidermis rather than in the HF itself. The interfollicular epidermis and utricular epithelium in hairless HRS/J hr/hr mutant mice are characterized by excessive cornification. 22,33 This feature is associated with simultaneous up-regulation of both keratinocyte proliferation 22 and apoptosis, 34 suggesting an elevated rate of cell turnover in the epidermis and utricular epithelium of hairless mouse skin. Because hr is actively expressed in both of these cell populations (in normal interfollicular epidermis and in the HF infundibulum, which corresponds to the utricular epithelium), it might be implicated in the regulation of the balance of cell proliferation and terminal differentiation in selected epithelial cells.
hr Gene Is not Involved in the Initiation of HF Morphogenesis
During the earliest stages of HF morphogenesis (stages 0 to 2), hr mRNA immunoreactivity was observed in neither the epidermal placode nor in the dermal fibroblast condensation and the DP was consistently hr mRNA-negative, thus essentially excluding hr from among the regulatory factors involved in the initiation of HF morphogenesis. This observation is consistent with the entirely normal development of the first pelage hairs in homozygous hr/hr hairless mutants until the onset of first catagen. 6
hr Expression in the Anagen-Catagen Transition
The initial stages of catagen in normal C57BL mouse skin are associated with a decline of hr mRNA immunoreactivity in the keratinocytes of the hair matrix (Figure 3) ▶ . As we have shown previously, in hairless mouse skin the switch from anagen to catagen is associated with a dramatic and premature up-regulation of apoptosis in the HF matrix. 6 These observations, together with data on the discoordination of keratinocyte apoptosis and differentiation in epidermis and utricular epithelium of hairless mouse skin, 22,34 suggest that normal expression of the hr gene seems to regulate the balance between apoptosis and differentiation in selected HF keratinocyte populations.
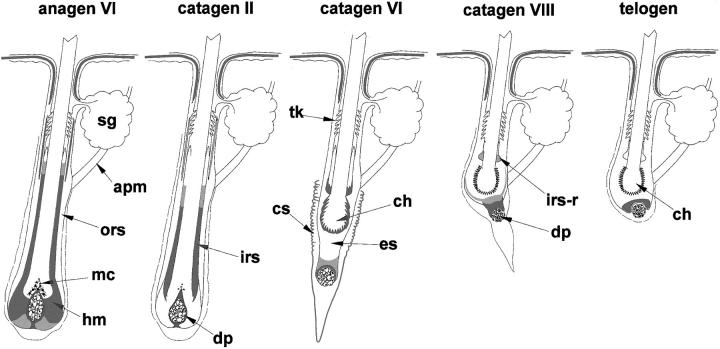
Figure 3.
Schematic representation of hr gene expression patterns (two different intensities, low and high, are shown in gray and black, respectively) during late anagen-catagen-telogen progression of depilation-induced HF cycling. (apm, arrector pili muscle; ch, club hair; cs, perifollicular connective tissue sheath; es, epithelial strand; hm, HF matrix; irs-r, remnants of inner root sheath; mc, melanocytes; sg, sebaceous gland; tk, zone of trichilemmal keratinization).
This hypothesis is consistent with the sequence of cellular events that determine the HF dysfunction in hr skin (Figure 4) ▶ . 6
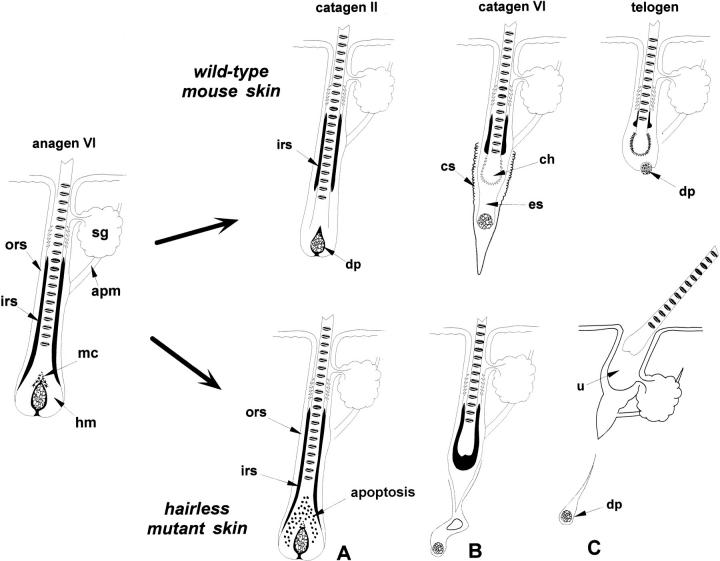
Figure 4.
Proposed scenario of reorganization of specific HF structures during the formation of the club hair in normal (C57BL or wild-type) and hairless (HRS/J hr/hr) mouse skin. During catagen in wild-type mouse skin, the degradation and shortening of IRS starts before the cessation of activity in the precortex. As a result of early IRS shortening, the lower end of the hair shaft comes into direct contact with ORS cells inducing trichilemmal keratinization of the club hair. In hairless mutant skin, dysregulation of apoptosis in HF matrix (A) results in early termination of hair shaft production and in delay of IRS disintegration. As a result, the IRS coalesces around the end of hair shaft (B) and the normal serrated club hair never forms. Note the loss of contact between the DP and HF in hairless skin (C). Legends as in Figures 1 to 3 ▶ ▶ ▶ . IRS is marked with black.
hr Gene Is Expressed in the Zone of Club Hair Formation
The onset of club hair formation was associated with a rapid increase in hr gene expression in the ORS cells surrounding the zone of interdigitation between the ORS keratinocytes and the hair shaft cells. As soon as the club hair is formed, hr gene expression in this region rapidly declined (Figure 3) ▶ . In hairless mouse skin, the normal club hair does not form, and instead it turns into an amorphous bulbous structure, in contrast to the serrated club hair in normal skin (Figure 4) ▶ . 6 It is widely accepted that trichilemmal keratinization is the main mechanism of club hair formation. 30 The possible involvement of hr in the regulation of trichilemmal keratinization is also supported by its elevated expression in a spatially restricted portion of the isthmus ORS between the upper end of the IRS and the sebaceous gland duct, which is also characterized by formation of a specific keratinous comb because of trichilemmal keratinization of ORS keratinocytes. 35
hr Gene May Be Involved in Upward Movement of the DP
In late catagen, a small cluster of epithelial strand cells adjacent to the DP exhibits a substantial level of hr mRNA positivity. Other cells of the epithelial strand are weakly positive or negative (Figure 3) ▶ . Recently, we proposed that the keratinocytes in this particular zone of the epithelial strand may be implicated in the continuity of DP-epithelial integrity in the catagen HF. 6 The hr gene expression in these cells also supports this notion. In hairless mutants, the loss of hr gene activity in this particular cell cluster may be the reason for the loss of integrity between DP and the rest of the ascending epithelial strand. These findings, along with the absence of hr expression in the DP throughout all stages of HF cycling, suggest that the mechanism of DP separation in hr skin and the subsequent failure of HF cycling is not intrinsic to the DP itself, but rather to its epithelial mooring.
Major Defects in Hairless Skin Coincide with hr Expression
Our previous studies of hairless mouse skin during the initiation and progression of hair loss (days 14 to 21 postpartum) revealed several characteristic abnormalities. 6 First, the dysregulation of cell proliferation and death in the HF matrix resulted in mispositioning of the ORS, IRS, and hair shaft (Figure 4) ▶ . Second, the club hair is excessively large and lacks its normal serrated appearance. Third, the secondary HF germ fails to form. Fourth, the epithelial strand in hr/hr skin is not able to undergo its normal contraction, and instead disintegrates into separate cell clusters. Finally, the DP fibroblasts remain stranded in the dermis, surrounded by a few epithelial cells. As shown in this study, the temporal and spatial patterns of hr gene expression in the normally haired skin of C57BL mice coincide with most morphological defects in hairless mutant skin, thus implicating the hr gene as one of the key factors in coordinating basic cellular processes during HF catagen, including club hair formation, maintenance of DP-epithelial integrity, IRS disintegration, and keratinocyte apoptosis in the HF matrix.
At the same time, the diminution of hr gene activity in the matrix during the HF morphogenesis does not result in any apparent abnormalities. In normally haired skin of C57BL mice, hr is actively expressed in the matrix keratinocytes during first postnatal anagen hair growth. In hairless and rhino mice with reduced or absent activity of this gene, HF morphogenesis is apparently normal. Perhaps in this particular zone of HF epithelia, hr may share functional redundancy with other genes, and hr gene functions are not critical for the support of matrix cell homeostasis during HF anagen development.
Conclusions
The changes in hr mRNA expression during the resting (telogen) and growing (anagen) stages of the HF cycle mirror the quantitative changes in HF structure and cell proliferation-differentiation rates. In contrast, during HF transition into catagen, and consequent catagen progression, hr mRNA expression undergoes significant qualitative and quantitative changes that are strictly associated with major catagen-driven cellular processes. Most likely, hr plays a role in cell-type-specific coordination of the expression of genes required for the maintenance of the balance between proliferation, differentiation, and/or apoptosis in selected cell populations of the epidermis and HF. Specifically, in the HF, hr may be a member of the cascade that is triggered by a putative clock factor, which governs the switch of the HF from anagen growth into catagen transformation. Thus, hr may not be a repressor of catagen, but instead, a key regulator of the earliest catagen-associated events, whose absence results in a dramatic, uncontrolled up-regulation of apoptosis in defined populations of hair matrix cells.
Collectively, our findings provide new insights into the pathobiology of the hr mutation, and suggest that the normal hr gene product is involved in the spatial and temporal coordinating of the expression of genes required for regulation of cell proliferation, differentiation, and death which together maintain the normal tissue architecture of the HF during the catagen progression.
Acknowledgments
We thank Andrey Panteleyev, Jr., and Dmitry Panteleyev for their help with artwork.
Footnotes
Address reprint requests to Angela M. Christiano, Ph.D., Department of Dermatology, Columbia University, College of Physicians & Surgeons, 630 W 168th Street, Vanderbilt Clinic VC-1526, New York, NY 10032. E-mail: amc65@columbia.edu.
Supported in part by grants from the Dermatology Foundation (to A. A. P.), the National Alopecia Areata Foundation (A. M. C.), and from Wella AG and Deutsche Forschungsgemeinschaft (Pa 345/8–1; to R. P.).
References
- 1.Oro AE, Scott MP: Splitting hairs: dissecting roles of signaling systems in epidermal development. Cell 1998, 95:575-578 [DOI] [PubMed] [Google Scholar]
- 2.Philpott M, Paus R: Principles of hair follicle morphogenesis. Choung C-M eds. Molecular Basis of Epithelial Appendage Morphogenesis. 1998, :pp 75-110 R. G. Landes Company, Austin [Google Scholar]
- 3.Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T: New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature 1994, 372:103-107 [DOI] [PubMed] [Google Scholar]
- 4.Gat U, DasGupta R, Degenstein L, Fuchs E: De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 1998, 95:605-614 [DOI] [PubMed] [Google Scholar]
- 5.St. Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, McMahon JA, Lewis PM, Paus R, McMahon AP: Sonic hedgehog signaling is essential for hair development. Curr Biol 1998, 8:1058-1068 [DOI] [PubMed] [Google Scholar]
- 6.Panteleyev AA, Botchkareva NV, Sundberg JP, Christiano AM, Paus R: The role of the hairless (hr) gene in the regulation of hair follicle catagen transformation. Am J Pathol 1999, 155:159-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botchkarev VA, Botchkareva NV, Welker P, Metz M, Lewin GR, Subramaniam A, Bulfone-Paus S, Hagen E, Braun A, Lommatzsch M, Renz H, Paus AR: A new role for neurotrophins: involvement of brain-derived neurotrophic factor and neurotrophin-4 in hair cycle control. FASEB J 1999, 13:395-410 [DOI] [PubMed] [Google Scholar]
- 8.Stenn KS, Combates NJ, Eilertsen KJ, Gordon JS, Pardinas JR, Parimoo S, Prouty SM: Hair follicle growth controls. Dermatol Clin 1996, 14:543-558 [DOI] [PubMed] [Google Scholar]
- 9.Paus R: Principles of hair cycle control. J Dermatol 1998, 25:793-802 [DOI] [PubMed] [Google Scholar]
- 10.Cachon-Gonzalez MB, Fenner S, Coffin JM, Moran C, Best S, Stoye JP: Structure and expression of the hairless gene of mice. Proc Natl Acad Sci USA 1994, 91:7717-7721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad W, Panteleyev AA, Sundberg JP, Christiano AM: Molecular basis for the rhino (hrrh-8J) phenotype: a nonsense mutation in the mouse hairless gene. Genomics 1998, 53:383-386 [DOI] [PubMed] [Google Scholar]
- 12.Panteleyev AA, Ahmad W, Malashenko AM, Ignatieva EL, Paus R, Sundberg JP, Christiano AM: Molecular basis for the rhino Yurlovo (hrrhY) phenotype: severe skin abnormalities and female reproductive defects associated with an insertion in the hairless gene. Exp Dermatol 1998, 7:281-288 [DOI] [PubMed] [Google Scholar]
- 13.Cachon-Gonzalez MB, San-Jose I, Cano A, Vega JA, Garcia N, Freeman T, Schimmang T, Stoye JP: The hairless gene of the mouse: relationship of phenotypic effects with expression profile and genotype. Dev Dyn 1999, 216:113-126 [DOI] [PubMed] [Google Scholar]
- 14.Ahmad W, Faiyaz ul Haque M, Brancolini V, Tsou HC, ul Haque S, Lam HM, Aita VM, Owen J, deBlaquiere M, Frank J, Cserhalmi-Friedman PB, Leask A, McGrath JA, Peacocke M, Ahmad M, Ott J, Christiano AM: Alopecia universalis associated with a mutation in the human hairless gene. Science 1998, 279:720-724 [DOI] [PubMed] [Google Scholar]
- 15.Ahmad W, Panteleyev A, Christiano AM: Molecular basis of congenital atrichia in humans and mice. Cutis 1999, 64:269-276 [PubMed] [Google Scholar]
- 16.Zlotogorski A, Ahmad W, Christiano AM: Congenital atrichia in five Arab Palestinian families resulting from a deletion mutation in the human hairless gene. Hum Genet 1998, 103:400-404 [DOI] [PubMed] [Google Scholar]
- 17.Panteleyen AA, Ahmad W, Ratterree MS, Aita VM, Sundberg JP, Christiano AM. Evolutionary Aspects of the hairless phenotype in a Rhesus macaque (macaca mulatta). J Invest \E Dermatol 114:869
- 18.Panteleyev AA, Paus R, Ahmad W, Sundberg JP, Christiano AM: Molecular and functional aspects of the hairless (hr) gene in laboratory rodents and humans. Exp Dermatol 1998, 7:249-267 [DOI] [PubMed] [Google Scholar]
- 19.Morrissey PJ, Parkinson DR, Schwartz RS, Waksal SD: Immunologic abnormalities in HRS/J mice. I. Specific deficit in T lymphocyte helper function in a mutant mouse. J Immunol 1980, 125:1558-1562 [PubMed] [Google Scholar]
- 20.Meier H, Myers DD, Huebner RJ: Genetic control by the hr-locus of susceptibility and resistance to leukemia. Proc Natl Acad Sci USA 1969, 63:759-766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panteleyev AA, Thiel R, Wanner R, Zhang J, Roumak VS, Paus R, Neubert D, Henz BM, Rosenbach T: 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCCD) affects keratin 1 and keratin 17 gene expression and differentially induces keratinization in hairless mouse skin. J Invest Dermatol 1997, 108:330-335 [DOI] [PubMed] [Google Scholar]
- 22.Panteleyev AA, van der Veen C, Rosenbach T, Muller-Rover S, Sokolov VE, Paus R: Towards defining the pathogenesis of the hairless phenotype. J Invest Dermatol 1998, 110:902-907 [DOI] [PubMed] [Google Scholar]
- 23.Thompson CC: Thyroid hormone-responsive genes in developing cerebellum include a novel synaptotagmin and a hairless homolog. J Neurosci 1996, 16:7832-7840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paus R, Hofmann U, Eichmuller S, Czarnetzki BM: Distribution and changing density of gamma-delta T cells in murine skin during the induced hair cycle. Br J Dermatol 1994, 130:281-289 [DOI] [PubMed] [Google Scholar]
- 25.Zeller R: Fixation, embedding, and sectioning of tissues, embryos, and single cells. Current Protocols in Molecular Biology. 1990, R Brent, RE Kingston, DD Moore, JG Seidman, JA Smith, K Struhl. New York, Greene Publishing Associates & Wiley-Interscience, Edited by FM Ausubel
- 26.Panteleyev AA, Paus R, Wanner R, Nurnberg W, Eichmuller S, Thiel R, Zhang J, Henz BM, Rosenbach T: Keratin 17 gene expression during the murine hair cycle. J Invest Dermatol 1997, 108:324-329 [DOI] [PubMed] [Google Scholar]
- 27.Hardy MH: The secret life of the hair follicle. Trends Genet 1992, 8:55-61 [DOI] [PubMed] [Google Scholar]
- 28.Paus R, Muller-Rover S, Van Der Veen C, Maurer M, Eichmuller S, Ling G, Hofmann U, Foitzik K, Mecklenburg L, Handjiski B: Review article: a comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol 1999, 113:523-532 [DOI] [PubMed] [Google Scholar]
- 29.Breitschopf H, Suchanek G, Gould RM, Colman DR, Lassmann H: In situ hybridization with digoxigenin-labeled probes: sensitive and reliable detection method applied to myelinating rat brain. Acta Neuropathol 1992, 84:581-587 [DOI] [PubMed] [Google Scholar]
- 30.Pinkus H, Iwasaki T, Mishima Y: Outer root sheath keratinization in anagen and catagen of the mammalian hair follicle. A seventh distinct type of keratinization in the hair follicle: trichilemmal keratinization. J Anat 1981, 133:19–35 [PMC free article] [PubMed]
- 31.Schweizer J: Murine epidermal keratins. Darmon M Blumenberg M eds. Molecular Biology of the Skin: The Keratinocyte. 1993, :pp 33-78 Academic Press, New York [Google Scholar]
- 32.Leigh I, Lane E, Watt F: The Keratinocyte Handbook. 1994. Cambridge University Press, Cambridge
- 33.Mann SJ: Hair loss and cyst formation in hairless and rhino mutant mice. Anat Rec 1971, 170:485-499 [DOI] [PubMed] [Google Scholar]
- 34.Seiberg M, Siock P, Wisniewski S, Cauwenbergh G, Shapiro SS: The effects of trypsin on apoptosis, utriculi size, and skin elasticity in the Rhino mouse. J Invest Dermatol 1997, 109:370-376 [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto K, Ito M: Keratinization of outer root sheath of human anagen hair. Marks R Plewig G eds. Acne and Related Disorders. 1989, :pp 3-17 Martin Dunitz, London [Google Scholar]