Abstract
To generate animal models of retinoblastoma that closely resemble metastatic and nonmetastatic human disease for the purposes of examining tumor biology and developing alternate treatments, human retinoblastoma cell lines were injected into the vitreal cavities of immunodeficient mice. Two reproducible animal models with contrasting biological behaviors analogous to human retinoblastoma have been developed. The Y79 retinoblastoma model demonstrated specific tumor evolution similar to that seen in human invasive and metastatic disease. Y79 retinoblastoma cells formed intraocular tumors that were initially confined to the vitreal cavity. Tumors progressively invaded the retina, subretinal space, choroid, optic nerve head, and anterior chamber of the eye. Tumors progressed into the subarachnoid space and focally invaded the brain. Metastases were detected in the contralateral optic nerve. Large tumors developed extraocular extensions. The histology of the tumors showed a poorly differentiated pattern with high mitotic rate, foci of necrosis, and calcification. The WERI-Rb model more closely resembled nonmetastatic human retinoblastoma. WERI- Rb tumors were localized in the eye with only anterior choroidal invasion at late stages. To examine potential biological differences in vitro, the retinoblastoma cell lines were cocultured with adherent choroid cells or adherent glioma cells which represent the targets of invasive retinoblastoma in vivo. Consistent with the in vivo observations, Y79 cells but not WERI-Rb cells adhere specifically to both the choroidal and the glioma cell lines.
Retinoblastoma is the most common primary intraocular tumor in children. In the United States, this disease presents most frequently as unilateral sporadic tumors and less frequently as bilateral hereditary tumors. When left untreated, retinoblastoma is almost always fatal. Prognosis is affected by many risk factors, the most important of which is the extent of invasion of the retinoblastoma into ocular coats and the optic nerve. 1 Although many tumors are not detected until they are large enough to be visible to the parents, very small tumors can sometimes effectively be treated using laser therapy or cryotherapy. More commonly detected larger tumors often require removal by enucleation of the affected eye. When retinoblastoma is treated in the early stages by enucleation, the cure rates approach 95%. 2-4 Besides the loss of vision, this therapeutic approach may leave the child with a facial deformity that worsens throughout life. 5 More advanced disease may require radiotherapy or chemotherapy in addition to enucleation. Both of these additional therapeutic regimes increase the probability that the surviving child will develop additional malignancies later in life. 2 There is currently no successful therapy for the treatment of patients who develop metastatic disease.
Human retinoblastomas exhibit four patterns of invasion and metastasis. 1 The first, direct invasive spread along the optic nerve to the brain, can also seed the orbital tissue and adjacent bone, the nasopharynx via the sinuses, or the cranium via the foramina. Tumor cells that have invaded the optic nerve and leptomeninges and then disperse into the circulating subarachnoid fluid are characteristic of the second pattern of metastasis. This may occur even when there is no tumor detected at the cut end of the optic nerve. Via the circulating subarachnoid fluid, tumor cells can also reach the spinal cord, distant sites of brain, and the contralateral optic nerve. Tumor formation in these sites represents true metastasis rather than local invasion. The third pattern of metastasis is hematogenous dissemination that results in widespread metastasis to the lungs, bones, brain, and other viscera. Metastasis after orbital invasion, and to a lesser degree choroidal invasion, is often via this route. The fourth pattern of metastasis, characterized by lymphatic spread, occurs when tumor is located anteriorly or massive extraocular invasion has occurred. Only tumors with these characteristics can spread via the lymphatic system, because there are no lymphatic vessels in the eye or orbit. Only the conjunctiva and eyelids have lymphatic drainage. When tumor reaches regional lymph nodes, hematogenous spread can also occur.
Retinoblastoma is a uniquely human disease. Attempts to develop animal models have provided a number of model systems each with distinct advantages but also with limitations. The majority of the animal models are xenograft models that have been created by injecting human retinoblastoma tumor cells into either the anterior chamber or the subretinal space of the eyes of immunodeficient mice or rats. Injection of tumor cells into the anterior chamber is favored because of that site’s accessibility for both the injection and the follow-up observation. 6 Anterior chamber injection of Y79 cells has previously been reported to result in the invasion of the optic nerve and brain. 7 These studies did not attempt to clarify whether the tumor spread by nonspecific extension or by specific migration of the tumor through the optic nerve to the central nervous system as is characteristic of human retinoblastoma. Tumor involvement of the anterior chamber is a late occurrence in human disease and the physiological environment of the anterior chamber differs significantly from that of the vitreal cavity in which naturally occurring tumors form. 8,9 The anterior chamber xenograft model therefore has limited utility for the study of metastatic behavior. Another animal model in which retinoblastoma cells are injected into the subretinal space produces tumors that more closely resemble human retinoblastoma in location. 10,11 However, injections into the subretinal space disrupt the choroid and the retina; therefore, use of this model to study invasiveness and progression of retinoblastoma is limited.
Several transgenic models of retinoblastoma have been developed. These models have recently been reviewed. 12 Each of the successful models developed used viral oncogene products, most notably the SV40-T antigen, to bind to and interrupt the function of the endogenous tumor suppressor proteins pRb or p53. Several of the viral oncogene constructs used to create the transgenic mice used eye-specific promoters (IRBP, opsin, crystallin), 13-17 although the model most often used for therapeutic studies was created using the promoter for the human luteinizing protein β subunit. 18,19 The tumors formed in the eyes of these transgenic mice resemble human retinoblastoma in many histopathological characteristics, however, they often lack the photoreceptor phenotype seen in the naturally occurring human disease and there is a higher frequency of tumors developing in the brain and other extraocular locations. A transgenic model generated by inactivating both alleles of the retinoblastoma gene has not been established; the homozygous Rb negative genotype is incompatible with embryonic development. The heterozygous Rb genotype seen in children with inherited retinoblastoma does not result in intraocular tumor formation in the mouse. 20-22
Animal models of retinoblastoma that contain the naturally occurring mutated Rb gene and that accurately reproduce the tumors’ biological behaviors are critical for the examination of new therapeutic approaches. This paper reports two murine models of intraocular retinoblastoma, one that effectively resembles the histopathological and biological behavior of an aggressively invasive human tumor with metastatic potential and the other that mimics nonmetastatic disease. Therefore, these models may also lead to a better understanding of the mechanisms of retinoblastoma metastasis and allow the development of innovative therapies for metastatic retinoblastoma.
Materials and Methods
Cell Culture
The human retinoblastoma Y79 and WERI- Rb (ATCC HTB 18 and ATCC HTB 169, respectively), the human embryonic kidney HEK293 (ATCC CRL 1573), the rhesus monkey choroidal RF/6A (ATCC CRL 1780), and the rat C6 glioma (ATCC CCL 107) cell lines (all from American Type Culture Collection, Manassas, VA) were cultured in GVL modified Eagle’s minimum essential medium supplemented with fetal calf serum (5%; Hyclone), streptomycin (100 μg/ml; Irvine Scientific, Santa Ana, CA) and penicillin (100 units/ml; Irvine Scientific). Y79 and WERI-Rb cells were grown in suspension at a concentration of 105−10 6 cells/ml. Passages 2 through 8 were used for the in vivo studies. Adherent choroidal and glioma cells were propagated by diluting trypsin-treated cells 1:10 in medium when the cultures reached 80% confluence.
Development of Intraocular Tumors
Adult transgenic Rag-2 knockout immunodeficient mice 23 were used for the study. Animals were handled at all times in accordance with the Association for Research in Vision and Ophthalmology Statement of the Use of Animals in Ophthalmology and Vision Research. The right eyes of mice were injected with either Y79 or WERI-Rb human retinoblastoma cells. Each animal was first anesthetized with an intraperitoneal injection of 20–30 μl of sodium pentobarbital (Nembutal) solution, 50 mg/ml. The pupil was then dilated with 2 to 3 drops of 2.5% phenylephrine hydrochloride solution, and a drop of topical anesthetic proparacaine hydrochloride (0.5%) was applied. Cellulose eye drops (2.5%) and a glass contact lens were applied to the cornea to aid visualization of the surgical procedures. Injections were performed using a surgical microscope. The conjunctiva of the temporal area of the eye was dissected and an incision was made at the scleral sulcus with a #11 disposable scalpel. Two microliters of sterile phosphate buffered saline containing 2 × 10 4 cells were injected into the vitreal cavity through the scleral sulcus using a Hamilton syringe with a 33 gauge cannula. Special care was taken to prevent lens damage or posterior retinal punctures. The animals were examined at 1 and 24 hours and then weekly after surgery. Clinical findings regarding the presence of tumor were recorded. After 2 weeks, animals from the Y79 and WERI-Rb groups were sacrificed weekly. Necropsies were performed on every animal. The brain, eyes, mediastinum, lungs, heart, and liver were examined microscopically. The brain was serially cross-sectioned to obtain coronal sections from frontal to occipital lobes and cross-sections of the cerebellum, medulla, and spinal cord.
Histopathology
After dissection, the organs were immediately fixed in 10% formalin. The tissues were processed and embedded in paraffin using conventional automated systems. The blocks were sectioned to obtain levels and serial sections 4 to 5 microns thick and stained with conventional hematoxylin-eosin (H&E). The slides were examined and scored by an unbiased pathologist.
Binding of Retinoblastoma Cell Lines to Choroidal and Glioma Cell Lines
Monkey choroid, rat C6 glioma, or human embryonic kidney cells (1 × 105) were plated in a six-well microtiter plate and allowed to adhere overnight. Y79 or WERI-Rb retinoblastoma cells (1 × 106) were washed in 2 ml PBS and pelleted by centrifugation. The cells were resuspended in 0.2 ml trypsin-EDTA solution (0.25% trypsin and 1 mmol/L EDTA, Gibco BRL, Rockville, MD) for times varying from 0 to 20 minutes. The reaction was terminated by the addition of 2 ml culture media. Cells (0.2 ml or 1 × 10 5 cells) were then layered over the adherent choroid, glioma, or embryonic kidney cell cultures and incubated at 37°C in humidified air containing 5% CO2 for 3 hours. The culture fluid was decanted and the culture washed twice by vigorous shaking with culture medium (0.2 ml) warmed to 37°C. The three aliquots were pooled and the cells counted using a hemacytometer.
Results
Y79 Retinoblastoma Tumors
The Y79 retinoblastoma cell line was derived from the tumor of a 21/2-year-old Caucasian female with a strong maternal history of retinoblastoma. The original tumor was mostly undifferentiated and, although intraocularly invasive, no extraocular extensions were evident. 24 A total of 24 mice received Y79 retinoblastoma cells. Systematic in vivo examination of the mouse eyes using an operating microscope first revealed tumors in the vitreal cavity 2 weeks after injection of the cells. Small cortical cataracts were found in the lens of all mice in the study; however, the cataracts did not interfere with adequate evaluation of the vitreal tumors. By 5 weeks, the tumors had obliterated the anterior chamber with the appearance of a whitish, focally neovascularized cornea. After 7 weeks, some eyes also showed proptosis. Contralateral eyes were unremarkable.
Histopathological Findings
Eyes
Serial sections and levels of the eyes were obtained to evaluate the extent of tumor involvement. Early retinoblastomas (2 weeks after injection) showed vitreal tumors that were located in the posterior and mid-equatorial regions of the cavity (Figure 1A ▶ 1). The tumors were composed of small to medium size cells with scanty cytoplasm, hyperchromatic nuclei, frequent mitoses, and rare necrotic cells (Figure 1A ▶ 2). No rosettes or fleurettes were present. The observed features are similar to those of a poorly differentiated human retinoblastoma. The site of injection was identified as a fibroblastic scar in the sclera with attached peripheral retina and an occasional small focal vitreal hemorrhage (data not shown). As the time between the tumor cell injection and the sacrifice of the mice lengthened, tumors were larger and invaded various ocular structures (Table 1) ▶ . By 3 weeks, the tumor showed subretinal and focal retinal invasion. By 3 to 4 weeks, focal subretinal and ciliary body invasion was observed (Figure 1A ▶ 3). By 5 to 6 weeks, retinoblastoma had invaded the choroid, retina, subretinal space, and optic nerve of most of the eyes studied (Figures 1A ▶ 4, 1A5). At this time the vitreal tumor showed foci of necrosis. The tumor was focally present in the anterior chamber by 7 weeks (Figure 1A ▶ 6). Between weeks 5 and 7, the tumors showed involvement of the ocular coats with focal corneal ulceration and invasion of the sclera. The sclera showed invasion by the tumor primarily at the injection site and extraocular tumor was also seen at this area. At 9 weeks, one mouse had evidence of tumor invading the lens. Two of the contralateral eyes of animals with tumors involving the optic nerve showed subarachnoid, perineural metastasis by retinoblastoma (Figure 1A ▶ 7), however, no intraocular tumor was observed in any of the contralateral eyes.
Figure 1.
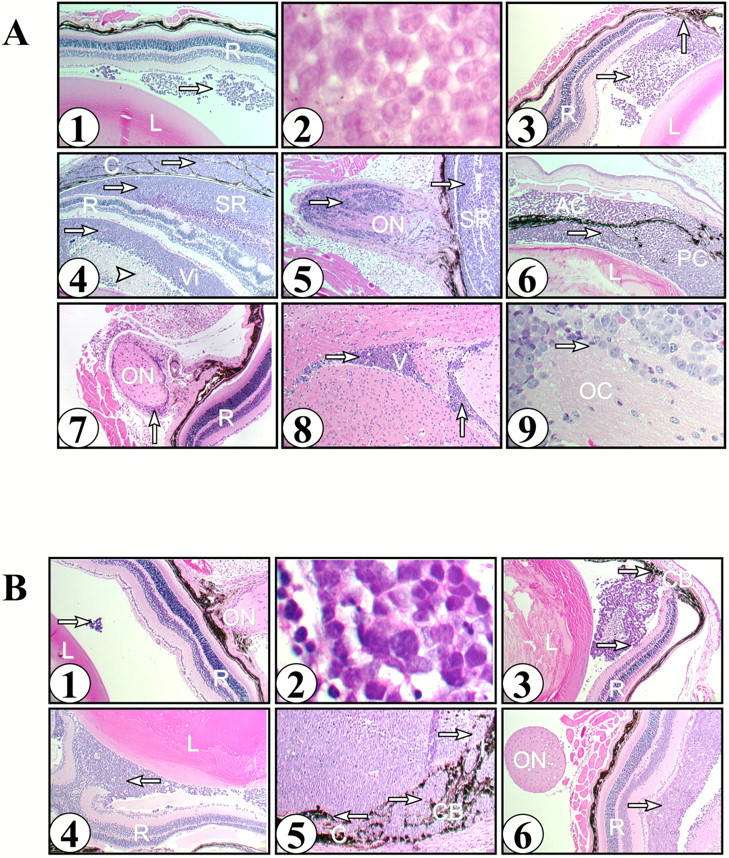
A: Histology of Y79 retinoblastoma mouse model. Y79 retinoblastoma cells (2 × 104) were injected into the vitreal space of transgenic Rag-2 knockout immunodeficient mice. Tissues were histologically examined at the indicated times postinjection as described in Methods. Tumor is indicated by the arrows in each panel (→). 1, Eye at 2 weeks with a tumor in the vitreal cavity between a normal lens (L) and a normal retina (R). Original magnification, ×40. 2, Tumor in the vitreal space showing small to medium size cells with scanty cytoplasm, hyperchromatic nuclei, and frequent mitoses. Original magnification, ×400. 3, Eye at 3 weeks showing normal lens (L), retina (R), tumor in the vitreous and tumor invasion of the ciliary body (↑). Original magnification, ×40. 4, Eye at 6 weeks showing tumor in the vitreal space (Vi) with an area of necrosis ([trioro]) and tumor invading the retina (R), subretinal space (SR), and choroid (C). Original magnification, ×40. 5, Eye at 6 weeks showing tumor in the subretinal space (SR) and tumor invading the optic nerve (ON). Original magnification, ×40. 6, Eye at 7 weeks showing lens (L) with tumor in the anterior (AC) and posterior (PC) chambers. Original magnification, ×40. 7, Contralateral eye at 8 weeks showing normal retina (R) and tumor surrounding the optic nerve (ON). Original magnification, ×40. 8, Brain at 6 weeks showing tumor in the ventricular space (V). Original magnification, ×40. 9, Optic chiasm (OC) at 6 weeks showing tumor invasion of the neural tissue. Original magnification, ×100. B: Histology of WERI-Rb retinoblastoma mouse model. WERI-Rb cells (2 × 104) were injected into the vitreal space of transgenic Rag-2 knockout immunodeficient mice. Tissues were histologically examined at the indicated times postinjection as described in Methods. Tumor is indicated by the arrows in each panel (→). 1, Eye at 2 weeks with normal retina (R), lens (L), and optic nerve (ON) with a small tumor in the vitreal space. Original magnification, ×40. 2, Tumor showing small to medium size cells with scanty cytoplasm and hyperchromatic nuclei. Original magnification, ×400. 3, Eye at 4 weeks with lens (L) and tumor in the vitreal space exhibiting minimal invasion of the retina (R) and ciliary body (CB). Original magnification, ×40. 4, Eye at 6 weeks with large tumor in the vitreal space (↑). Original magnification, ×40. 5, Eye at 6 weeks with large tumor invading the choroid (C), ciliary body (CB), and iris (I). Original magnification, ×100. 6, Eye at 6 weeks showing large tumor in the vitreal space adjacent to a normal retina (R) and optic nerve (ON) free of tumor. Original magnification, ×40.
Table 1.
Tissue Involvement by Y79 Retinoblastoma in Mice
| Site | Weeks | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Vitreous | +2 /3 | +3 /3 | +3 /3 | +3 /3 | +6 /6 | +3 /3 | +2 /2 | +1 /1 |
| Retina | − | +2 /3 | +2 /3 | +3 /3 | +6 /6 | +3 /3 | +2 /2 | +1 /1 |
| Subretinal space | − | +2 /3 | +2 /3 | +3 /3 | +6 /6 | +3 /3 | +2 /2 | +1 /1 |
| Iris | − | − | − | − | − | − | +1 /2 | +1 /1 |
| Lens | − | − | − | − | − | − | − | +1 /1 |
| Anterior chamber | − | − | − | − | − | +1 /3 | +2 /2 | +1 /1 |
| Choroid | − | − | +1 /3 | +3 /3 | +4 /6 | +3 /3 | +2 /2 | +1 /1 |
| Optic nerve | − | − | − | +1 /3 | +3 /6 | +2 /3 | +2 /2 | +1 /1 |
| Extraocular | − | − | − | − | − | − | +1 /2 | +1 /1 |
| Brain | − | − | − | +2 /3 | +4 /6 | +1 /3 | +2 /2 | +1 /1 |
| Contralateral eye | − | − | − | − | − | − | +1 /2 | +1 /1 |
Mice were necropsied and the tissues examined histologically as described in Methods. The data are expressed as the number of animals that were positive for tumor at each site/total number of animals examined.
−, tissues without evidence of tumor in any of the examined animals.
Brain
Mice with early intraocular tumors without invasion of the subretinal space or optic nerve had no metastases to the brain. After 5 weeks, eight mice had either tumor cells in the subarachnoid space (Figure 1A ▶ 8) or focal brain parenchyma retinoblastoma invasion (Figure 1A ▶ 9). The corresponding ocular tumors had involvement of at least the choroid and, in most cases, the optic nerve and extraocular structures (Table 1) ▶ .
Heart, Lung, Mediastinum, and Liver
There was no tumor involvement at these sites by microscopic examination. Some animals had mild chronic inflammatory infiltrates in the liver at the level of the portal triads with associated microvesicular steatosis. These changes were unrelated to the extent of tumor progression of ocular retinoblastoma.
WERI-Rb Retinoblastoma Tumors
The WERI-Rb cell line was derived from the tumor of a 1-year-old Caucasian female with no family history of retinoblastoma. This tumor was also essentially undifferentiated and, although it had invaded the optic nerve head, there was no evidence of tumor at any distal site. 25 Twenty mice received intraocular injections of WERI-Rb cells and were followed in this arm of the study. Examination of mice showed small vitreal tumors, first noted at 2 weeks after injection and visible only under the operating microscope. The lens frequently had cataracts. There was tumor extension in the anterior chamber by 5 weeks. After this time point most of the eyes appeared to have extensive tumor involvement in the anterior segment.
Histopathological Findings
Eyes
Histological examination of early tumors (2–3 weeks) showed scanty amounts of tumor cells present mostly in the posterior vitreous (Table 2 ▶ , Figure 1B ▶ 1). The tumor cells were small to medium in size with scanty cytoplasm and hyperchromatic nuclei. The cells grew in a trabecular pattern. No fleurettes or true rosettes were found (Figure 1B ▶ 2). There were no areas of necrosis or calcification seen. By day 28, the tumor focally invaded the retina but rarely the subretinal space (Figure 1B ▶ 3). In most of the cases with subretinal involvement, tumors were contiguous with the site of injection or were evident in advanced disease. In striking contrast to the Y79 retinoblastoma cells, WERI-Rb retinoblastoma cells were predisposed to invade the anterior uveal tissues (anterior choroid, ciliary body, and iris), and lens (Figure 1 ▶ , B4 and B5). Tumor invasion of optic nerve (Figure 1B ▶ 6) or brain (data not shown) was lacking in the WERI-Rb-injected eyes. Extraocular extension was seen in advanced tumors and almost always through the injection site (data not shown).
Table 2.
Tissue Involvement by WERI-Rb Retinoblastoma in Mice
| Site | Weeks | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Vitreous | +2 /2 | +2 /2 | +2 /2 | +2 /2 | +4 /4 | +5 /5 | +2 /2 | +1 /1 |
| Retina | − | − | +1 /2 | +2 /2 | +4 /4 | +5 /5 | +2 /2 | +1 /1 |
| Subretinal space | − | − | − | − | +3 /4 | +4 /5 | +2 /2 | +1 /1 |
| Iris | − | − | − | +2 /2 | +2 /4 | +4 /5 | +2 /2 | +1 /1 |
| Lens | − | − | − | +1 /2 | +2 /4 | +2 /5 | +1 /2 | +1 /1 |
| Anterior chamber | − | − | − | +1 /2 | +2 /4 | +4 /5 | +2 /2 | +1 /1 |
| Choroid | − | − | − | − | +3 /4 | +3 /5 | +2 /2 | +1 /1 |
| Optic nerve | − | − | − | − | − | − | − | − |
| Extraocular | − | − | − | − | − | − | +1 /2 | +1 /1 |
| Brain | − | − | − | − | − | − | − | − |
| Contralateral eye | − | − | − | − | − | − | − | − |
Mice were necropsied and the tissues examined histologically as described in Methods. The data are expressed as the number of animals that were positive for tumor at each site/total number of animals examined.
−, tissues without evidence of tumor in any of the examined animals.
Brain
There were no tumor cells found in the meninges or brain parenchyma in the WERI-Rb-injected group.
Heart, Lung, Mediastinum, and Liver
No involvement of the heart, lung, mediastinum, or liver was observed. Inflammatory changes in the liver were similar to those seen in the Y79-injected group.
In Vitro Binding of Retinoblastoma Cell Lines to Choroidal and Glioma Cell Lines
One contributing characteristic to the development of metastases is the specific trafficking of tumor cells to the target organ. To test the hypothesis that Y79 cells might invade by specifically adhering to choroidal or glial cells, suspensions of either Y79 or WERI-Rb cells were incubated with the adherent RF/6A monkey choroidal cell line. The RF/6A cell line was established from a spontaneously transformed endothelial cell line derived from the choroid-retina of a rhesus macaque fetus and exhibits endothelial cell characteristics. 26 After incubation for 1 hour, non-adherent cells were removed by vigorous washing with media and the plates were observed under the microscope (Figure 2A) ▶ . Plates containing Y79 cells showed a significant percentage of the retinoblastoma cells bound to the monkey choroidal cell line, whereas plates containing WERI-Rb cells showed little binding. Similar results were found when the two retinoblastoma cell lines were incubated with the rat C6 glioma cell line 27 (Figure 2A) ▶ . Neither retinoblastoma cell line exhibited significant binding to the HEK293 human embryonic kidney cell line (Figure 2A) ▶ . Trypsin treatment of Y79 cells reduced the binding of these cells to both the choroidal and glioma cell lines (Figure 2B) ▶ , whereas similar treatment with neuraminidase had no effect on binding (data not shown). The trypsin treatment of Y79Rb cells reduced binding to either the choroid cells or the glioma cells in a time-dependent manner (data not shown).
Figure 2.
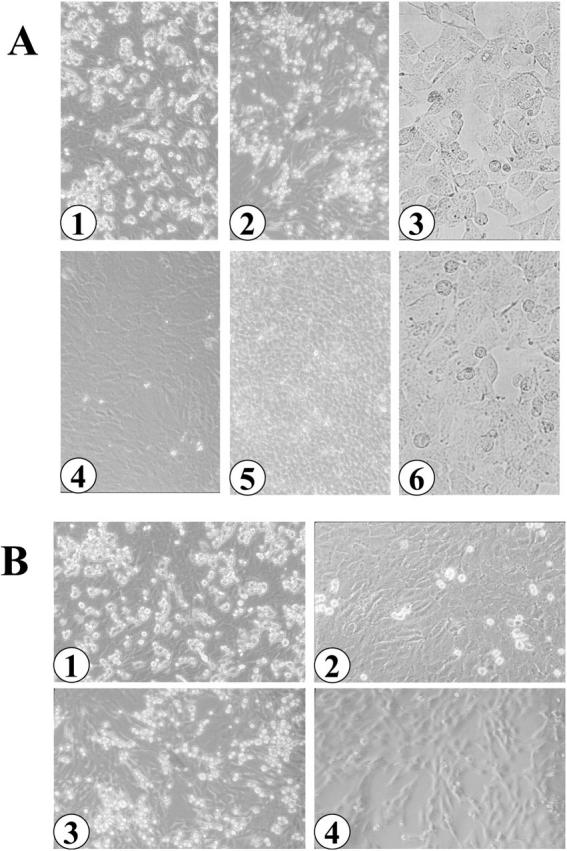
Binding of suspended retinoblastoma cells to adherent choroidal and glioma cells. A: Y79Rb (1, 2, 3) or WERI Rb (4, 5, 6) cells were suspended over adherent monkey choroid (1, 4), rat C6 glioma (2, 5), or human embryonic kidney (3, 6) cells as described in Methods. B: Y79Rb cells were pretreated without (1, 3) or with (2, 4) trypsin for 5 minutes. The cells were suspended over adherent choroid (1, 2) or glioma (3, 4) cells for 3 hours as described in Methods. Original magnifications, ×200.
Discussion
The retinoblastoma tumors that develop after intravitreal injections of Y79 human retinoblastoma cells into immunodeficient mice closely resemble human tumors with aggressive behavior and metastatic potential. An injection of Y79 retinoblastoma cells causes the initial formation of a small intravitreal tumor. Although vitreal seedings are considered a feature of a large human retinoblastoma, the tumor formed in this murine model closely approaches the naturally occurring tumor in anatomical site without disrupting the choroid/sclera or involving the anterior chamber. Only minimal trauma to the lens, peripheral uvea, and retina occurs, thus allowing a histological examination of the invasive and metastatic behavior of the tumors without remarkable interference due to the injection technique. Tumors invade the subretinal and choroidal space and eventually invade the optic nerve. As the tumor progresses, the brain shows evidence of tumor, and, finally, the tumor metastasizes to the contralateral optic nerve. Successful development of tumor with reproducible size and aggressive invasive behavior occurs over a predictable time span after the intravitreal injection of the Y79 retinoblastoma cells. The Y79 tumors exhibit two of the four patterns of invasion and metastasis described for human retinoblastomas (see Introduction): i) direct invasive spread and ii) dispersion of tumor cells resulting in metastasis to the brain and contralateral optic nerve. The remaining two routes of spread, hematogenous and lymphatic, are not evident in the Y79 tumor model at 9 weeks after injection (the longest time point reported here) or even in animals used in survival studies during preclinical evaluation of adenoviral-mediated gene therapy for retinoblastoma. 28 A parallel murine retinoblastoma model was developed using intravitreal injections of another human retinoblastoma cell line (WERI-Rb). The WERI-Rb model shows a retinoblastoma tumor that behaves as a localized tumor that invades only the anterior structures of the eye without extraocular spread or metastasis.
This manuscript aims to describe not only two models of retinoblastoma but also, and maybe more importantly, some of the striking differences between the two models. Although the procedures used to induce tumor formation are identical and tumors that closely resembled human retinoblastoma result from injection of either retinoblastoma cell line, the characteristics of the tumors formed are remarkably and reproducibly different. The WERI-Rb tumors exhibit characteristics of localized, nonmetastatic human retinoblastoma, whereas the Y79 tumors exhibit the histopathological characteristics of aggressive human retinoblastoma with invasion of the optic nerve and brain that would likely result in distant metastatic disease and metastasis to the contralateral optic nerve. Differences in the growth rates of these two cell lines cannot completely explain the histopathological differences exhibited by the tumors because, by the end of the experiments, animals in both groups had tumors that had completely replaced the normal ocular tissues in the affected eye. Only animals in the Y79 tumor group had tumors that appeared to mimic the aggressive, rapidly growing, metastatic retinoblastomas only occasionally observed in North America and Europe but more commonly seen in Mexico, Central and South America, Saudi Arabia, and India. 29 The WERI-Rb animal model behaved like patients with nonmetastatic retinoblastoma more commonly seen in Europe and North America.
In vitro binding studies suggested that a specific difference in the membrane protein structure of the cells may, at least in part, play a role with respect to the observed difference in metastatic behavior of the two animal models. Adherent cell lines derived from monkey choroid or rat C6 glioma (derived from tissues that are similar in origin to the target tissues of invasive retinoblastoma), were found to rapidly bind Y79 cells. Y79 cells also bound to human Hs 683 glioma cells (data not shown). Specificity was suggested since Y79 cells did not bind to human embryonic kidney cells. Trypsin abolished this binding suggesting protein on the surface of Y79 cells is apparently involved in the cell-cell interaction. Treatment with neuraminidase had no effect. WERI-Rb cells did not appear to interact with choroidal, glioma, or embryonic kidney cell lines under the same experimental conditions. Understanding the mechanism of this binding may aid in understanding the biochemical events related to metastasis of retinoblastoma. Furthermore, this simple in vitro binding assay may help predict which patients with retinoblastoma might be prone to develop metastatic disease.
Distant metastases outside the central nervous system were not observed in these animal models. Metastases outside of the central nervous system in human disease is only rarely observed and is considered a very late event in the course of the disease. Distant metastases may be seeded via the vasculature, probably after choroidal or orbital invasion. Although choroidal invasion was eventually always observed in the Y79 cell animal model, the animals in these particular studies were not allowed to survive for longer than 9 weeks. If the animals were allowed to progress, it is possible that distant metastases outside of the central nervous system might have been observed.
All of the animal models described previously may have utility in particular research protocols, but none is perfect in all settings. The extent of choroidal, extraocular, or optic nerve invasion at the time of diagnosis 1,30 most often determines the prognosis of children with retinoblastoma. The tumors formed in the models described in this paper closely mimic human disease. We have recently used these models of retinoblastoma in preclinical evaluations of the potential efficacy of suicide gene therapy 28 and gene replacement therapy 31 for retinoblastoma. These models provide an opportunity for research into the similarities and differences in the biological behavior and the response to treatment of metastatic and nonmetastatic retinoblastoma. These two models will also allow a detailed study of the molecular biological and physiological differences between metastatic and nonmetastatic retinoblastoma originating from cells with the naturally occurring mutations in the Rb genes.
Acknowledgments
We thank Dr. Milton Finegold, Department of Pathology, Baylor College of Medicine for his assistance and support and Janice Gant, Department of Pediatrics, Baylor College of Medicine for her assistance in preparation of the manuscript.
Footnotes
Address reprint requests to Richard L. Hurwitz, 6621 Fannin Street, M.C. 3–3320, Houston, TX 77030. E-mail: rhurwitz@bcm.tmc.edu.
Supported by grants from the Foundation for Research and the Retina Research Foundation (to R. L. H.), the Lions Eye Bank and the Moran Foundation (to P. C.-B.), the Knights Templar Eye Foundation, Inc. (to M. Y. H.), and the General Clinical Research Center no. M01RR00188 (to C. E. A.-C.).
References
- 1.McClean I, Burnier M, Zimmerman L, Jakobiec F: Tumors of the retina. Tumors of the eye and adnexa. 1994, :pp 100-135 D.C., Armed Forces Institute of Pathology, Atlas of Tumor Pathology. Edited by Rosai J. Washington [Google Scholar]
- 2.Eng C, Li FP, Abramson DH, Ellsworth RM, Wong FL, Goldman MB, Seddon J, Tarbell N, Boice JD, Jr: Mortality from second tumors among long-term survivors of retinoblastoma. J Natl Cancer Inst 1993, 85:1121-1128 [DOI] [PubMed] [Google Scholar]
- 3.Advani SH, Rao SR, Iyer RS, Pai SK, Kurkure PA, Nair CN: Pilot study of sequential combination chemotherapy in advanced and recurrent retinoblastoma. Med Pediatr Oncol 1994, 22:125-128 [DOI] [PubMed] [Google Scholar]
- 4.Byrne J, Fears TR, Whitney C, Parry DM: Survival after retinoblastoma: long-term consequences and family history of cancer. Med Pediatr Oncol 1995, 24:160-165 [DOI] [PubMed] [Google Scholar]
- 5.Kaste SC, Chen G, Fontanesi J, Crom DB, Pratt CB: Orbital development in long-term survivors of retinoblastoma. J Clin Oncol 1997, 15:1183-1189 [DOI] [PubMed] [Google Scholar]
- 6.Madreperla SA, Whittum-Hudson JA, Prendergast RA, Chen P-L, Lee W-H: Intraocular tumor suppression of retinoblastoma gene-reconstituted retinoblastoma cells. Cancer Res 1991, 51:6381-6384 [PubMed] [Google Scholar]
- 7.Gallie BL, Albert DM, Wong JJ, Buyukmihci N, Pullafito CA: Heterotransplantation of retinoblastoma into the athymic “nude” mouse. Invest Ophthalmol Vis Sci 1977, 16:256-259 [PubMed] [Google Scholar]
- 8.Kaufman PL: Aqueous humor dynamics. Parrish RK, II eds. Duane’s Clinical Ophthalmology Glaucoma. 1992, :pp 1-24 J.B. Lippincott, Philadelphia [Google Scholar]
- 9.Sebag J: Vitreous biochemistry, morphology, and clinical examination. Benson WE eds. Duane’s Clinical Ophthalmology Diseases of the Retina. 1992, :pp 1-21 J.B. Lippincott, Philadelphia [Google Scholar]
- 10.Rowe SG, Lee WH, Madreperla S: Subretinal and vitreal growth of human retinoblastoma cells in the mouse eye. Invest Ophthalmol Vis Sci 1992, 33:875(abstr.) [Google Scholar]
- 11.del Cerro M, Seigel GM, Lazar E, Grover D, del Cerro C, Brooks DH, DiLoreto D, Jr, Chader G: Transplantation of Y79 cells into rat eyes: An in vivo model of human retinoblastomas. Invest Ophthalmol Vis Sci 1993, 34:3336-3346 [PubMed] [Google Scholar]
- 12.Mills M, Windle JJ, Albert DM: Retinoblastoma in transgenic mice: models of hereditary retinoblastoma. Surv Ophthalmol 1999, 43:508-518 [DOI] [PubMed] [Google Scholar]
- 13.Baehr W, Falk JD, Bugra K, Triantafyllos JT, McGinnis JF: Isolation and analysis of the mouse opsin gene. FEBS Lett 1988, 238:253-256 [DOI] [PubMed] [Google Scholar]
- 14.Borst DE, Nickerson JM: The isolation of a gene encoding interphotoreceptor retinoid-binding protein. Exp Eye Res 1988, 47:825-838 [DOI] [PubMed] [Google Scholar]
- 15.Fong SL, Fong WB, Morris TA, Kedzie KM, Bridges CD: Characterization and comparative structural features of the gene for human interstitial retinol-binding protein. J Biol Chem 1990, 265:3648-3653 [PubMed] [Google Scholar]
- 16.Nathans J, Hogness DS: Isolation and nucleotide sequence of the gene encoding human rhodopsin. Proc Natl Acad Sci USA 1984, 81:4851-4855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.al-Ubaidi MR, Font RL, Quiambao AB, Keener MJ, Liou GI, Overbeek PA, Baehr W: Bilateral retinal and brain tumors in transgenic mice expressing simian virus 40 large T antigen under control of the human interphotoreceptor retinoid-binding protein promoter. J Cell Biol 1992, 119:1681-1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howes KA, Lasudry JGH, Albert DM, Windle JJ: Photoreceptor cell tumors in transgenic mice. Invest Ophthalmol Vis Sci 1994, 35:342-351 [PubMed] [Google Scholar]
- 19.Albert DM, Griep AE, Lambert PF, Howes KA, Windle JJ, Lasudry JG: Transgenic models of retinoblastoma: what they tell us about its cause and treatment. Trans Am Acad Ophthalmol Soc 1994, 92:385-401 [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke AR, Maandag ER, van Roon M, van der Lugt NM, van der Valk M, Hooper ML, Berns A, te Riele H: Requirement for a functional Rb-1 gene in murine development. Nature 1992, 359:328-330 [DOI] [PubMed] [Google Scholar]
- 21.Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA: Effects of an Rb mutation in the mouse. Nature 1992, 359:295-300 [DOI] [PubMed] [Google Scholar]
- 22.Lee EY, Chang CY, Hu N, Wang YC, Lai CC, Herrup K, Lee WH, Bradley A: Mice deficient for RB are nonviable and show defects in neurogenesis and haematopoiesis. Nature 1992, 359:288-294 [DOI] [PubMed] [Google Scholar]
- 23.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW: RAG-2 deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 1992, 68:855-867 [DOI] [PubMed] [Google Scholar]
- 24.Reid TW, Albert DM, Rabson AS, Russell P, Craft J, Chu EW, Tralka TS, Wilcox JL: Characteristics of an established cell line of retinoblastoma. J Natl Cancer Inst 1974, 53:347-360 [DOI] [PubMed] [Google Scholar]
- 25.McFall RC, Sery TW, Makadon M: Characterization of a new continuous cell line derived from a human retinoblastoma. Cancer Res 1977, 37:1003-1010 [PubMed] [Google Scholar]
- 26.Lou D-A, Hu F: Specific antigen and organelle expression of a long-term rhesus endothelial cell line. In Vitro Cell Dev Biol 1987, 23:75-85 [DOI] [PubMed] [Google Scholar]
- 27.Benda P, Lightbody J, Sato G, Levine L, Sweet W: Differentiated rat glial cell strain in tissue culture. Science 1968, 161:370-371 [DOI] [PubMed] [Google Scholar]
- 28.Hurwitz MY, Marcus KT, Chévez-Barrios P, Louie K, Aguilar-Cordova E, Hurwitz RL: Suicide gene therapy for treatment of retinoblastoma in a murine model. Hum Gene Ther 1999, 10:441-448 [DOI] [PubMed] [Google Scholar]
- 29.Sahu S, Banavali SD, Pai SK, Nair CN, Kurkure PA, Motwani SA, Advani SH: Retinoblastoma: problems and perspectives from India. Pediatr Hematol Oncol 1998, 15:501-508 [DOI] [PubMed] [Google Scholar]
- 30.Shields JA: The expanding role of laser photocoagulation for intraocular tumors. The1993 H. Christian Zweng Memorial Lecture. Retina 1994, 14:310-322 [DOI] [PubMed] [Google Scholar]
- 31.Hurwitz RL, Rivera AL, Holcombe VN, Chevez-Barrios P, Hurwitz MY: Gene therapy for retinoblastoma: comparison of gene replacement with the retinoblastoma gene to suicide gene therapy with herpes simplex thymidine kinase and ganciclovir. Invest Ophthalmol Vis Sci 1999, 40:S761(abstr.) [Google Scholar]


