Abstract
The urokinase-type plasminogen activator (uPA) system has been implicated in tumor spread. We have used immunohistochemistry to examine three components of this system, ie, uPA, uPA receptor (uPAR), and plasminogen activator inhibitor-1 (PAI-1), in a pilot study on 142 cases of breast carcinoma. We wished to determine whether there were any relationships between expression of the proteins in either tumor cells or fibroblasts and clinical and pathological features. Strong uPA expression in each cell type was significantly related to high tumor grade (P = 0.013 and 0.008, respectively), and was more common in invasive than in in situ carcinomas (P < 0.0001). Fibroblastic expression of uPAR was only related to the presence of invasion (P < 0.0001). Strong PAI-1 expression in both cell types was seen in high-grade tumors (tumor cells, P = 0.012; fibroblasts, P < 0.001), but only fibroblastic expression was related to the presence of invasion (P = 0.042). Fibroblastic expression of both uPA and uPAR were positively correlated with tumor size. Although patients with strong fibroblastic expression of uPA showed a tendency toward a shorter time to relapse, none of the plasminogen activator proteins were significantly associated with relapse-free survival. These results suggest that strong expression of uPA, uPAR, and PAI-1 in fibroblasts rather than in tumor cells have the most impact on the clinical behavior of breast cancer. Larger prospective studies are needed to confirm these findings.
In common with other tumor types, it is usually the metastases in breast cancer that eventually kills the patient. Consequently the search for new predictive and prognostic tumor markers has widened to include an increasingly intense examination of interactions between tumors and their surrounding, ostensibly normal, stromal tissue elements. Understanding the mechanisms involved in the metastatic process may help us to find ways of predicting which tumors are likely to spread and to identify possible sites for therapeutic intervention. The urokinase-type plasminogen activator (uPA) system, which activates the serine protease plasmin from its precursor plasminogen, is one of several enzyme systems that play an important role in the degradation of extracellular matrix central to tumor invasion. 1-4
The activity of uPA is normally under strict control, being one of several enzymes required for normal physiological processes such as tissue remodeling. 5 uPA itself initially exists as a proenzyme form (pro-uPA), which after proteolysis, by, for example, matrix metalloproteinases becomes the active form. 6 This is termed high-molecular weight uPA and it undergoes a further proteolysis step to produce the 33-kd low-molecular weight uPA and an amino terminal fragment. 4,7 Low-molecular weight uPA itself is capable of eliciting matrix degradation responses in a variety of different physiological processes as well as in tumor invasion and metastases. 1 The amino-terminal fragment molecule is activated on binding to the uPA receptor (uPAR) via its growth factor domain before promoting cell migration and adhesion. 1,2 The uPA receptor is a highly glycosylated protein that binds to the cell membrane via a carboxy terminal anchor, and contains the uPA-binding domain in the amino terminal. 8 This cascade can be amplified by plasmin, the end-product of uPA activity on plasminogen. Inhibitory regulatory steps are required to prevent the reaction from getting out of control, most notably by inactivation of uPA by plasminogen activator inhibitors (PAIs)-1 and -2 in the cytoplasm, after endocytosis of uPA/uPAR complexes. 9 During this process, PAI-1 is degraded, uPA is inactivated, and the receptor is partially recycled. 1,2
Several studies assessing both mRNA and protein levels have found that increases in the plasminogen activator (PA) components uPA, its receptor uPAR, and PAI-1 are associated with either aggressive tumor characteristics or a poor prognosis. 10 However, it is unclear whether it is their (relative) levels in the stroma or in the tumor cells themselves that is the most relevant to patient outcome. Immunohistochemistry-based studies are especially useful in determining the cellular locations of these proteins and so may help to clarify this point. In this immunohistochemical study, we have stained a series of invasive and in situ breast tumors of varying types and grade with monoclonal antibodies to uPA, uPAR, and PAI-1. Assessment was made of their cellular localization and immunostaining was related to clinical and pathological data including relapse-free survival
Materials and Methods
Patients
There were 142 breast tumors including 89 invasive ductal carcinomas, 28 invasive lobular carcinomas, and 25 cases of ductal carcinoma in situ (DCIS), from excision biopsy and mastectomy specimens taken from women diagnosed at the Imperial Cancer Research Fund Clinical Oncology Unit, Guy’s Hospital. As far as was possible, and where material was available, the majority of these were consecutive cases taken from 1990 to 1991. However, to obtain approximately equal numbers of each tumor grade and type, some of the cases were diagnosed in the period 1992 to 1994. Median follow-up time was 6.5 years (range, 1.2 to 10 years). The median patient age was 56 years (range, 28 to 86 years). Histological grade of infiltrating ductal carcinomas (IDC) was determined according to a modification of the Bloom and Richardson system, 11 and DCIS was typed according to Holland et al. 12 Tumor size was known for 138 cases. Nodal status was available on those patients in which axillary clearances had been performed (all of the invasive tumors and three of the DCIS tumors, n = 120). In addition, sections from 18 samples of normal breast, inflammatory disease, and benign lesions (papilloma, fibroadenoma, sclerosing adenosis) were also stained.
Immunohistochemistry
Immunostaining was performed on 3-μm sections taken from formalin-fixed paraffin-embedded breast tumor blocks. Nils Brünner (Finsen Institute, Copenhagen, Denmark) kindly provided the three primary mouse monoclonal antibodies (respectively, anti-uPAklon16, anti-uPAR2, and anti-PAI-1klon1). The specificity of the antibodies had previously been tested and confirmed with Western blotting (Nils Brünner, personal communication). They were used at the following concentrations in 0.015 mol/L Tris-buffered saline (pH 7.6): uPAklon16 (10 μg/ml), uPAR2 (4.7 μg/ml), and PAI-1klon1 (0.8 μg/ml). Sections were blocked in 20% normal rabbit serum (DAKO Ltd., Cambridge, UK). Primary antibody was applied and incubations were performed overnight at room temperature. Biotinylated rabbit anti-mouse immunoglobulins (DAKO Ltd.) diluted 1/400 in 3% normal human serum was applied, followed by peroxidase-conjugated preformed streptavidin biotin complex (DAKO Ltd.) each for 30 minutes at room temperature. Reaction sites were visualized using diaminobenzidine as the chromogen, and nuclei were counterstained with hematoxylin. Sections of breast tumor previously found to be positive with each of the primary antibodies were included with each staining run. For negative controls, primary antibody was omitted on duplicate test sections.
Statistics
The relationships between expression of uPA, uPAR, and PAI-1 in tumor cells and fibroblasts were evaluated by the Spearman’s rank correlation test. Pearson’s chi-square test was used to examine associations of the PA components with tumor grade, tumor type, the presence or absence of invasion, tumor size, and nodal status. Kaplan-Meier survival curves were constructed and log-rank analyses performed to assess whether any level of uPA, uPAR, or PAI-1 expression had any effect on relapse-free survival of those patients with invasive carcinoma.
Results
Assessment
Expression of uPA, uPAR, and PAI-1 was based on the intensity of staining, and was assessed in the malignant epithelial cells and tumor-associated fibroblasts within each section. The degree of expression was graded as negative (no immunostaining in either tumor cells or fibroblasts), weak (definite but weak staining), or strong (intense staining easily seen under low power on a microscope).
Immunostaining Patterns
Positive immunostaining with all three antibodies was observed in the cytoplasm of tumor cells. This was often heterogeneous throughout the tumor area, with some negative areas within positive tumors. Strong cases were positive in as many as 100% of cells, whereas most of the weakly staining cases were positive in <10%. Normal and benign breast epithelia (and occasionally myoepithelium) adjacent to tumor were sometimes positive with each of the antibodies, but this staining was never as intense as that seen in tumors. Occasional staining of blood vessel walls was also seen. For all three antibodies, expression in tumor cells was usually stronger or equivalent to that seen in accompanying stromal elements and in nonmalignant epithelium (Figure 1, A–D) ▶ .
Figure 1.
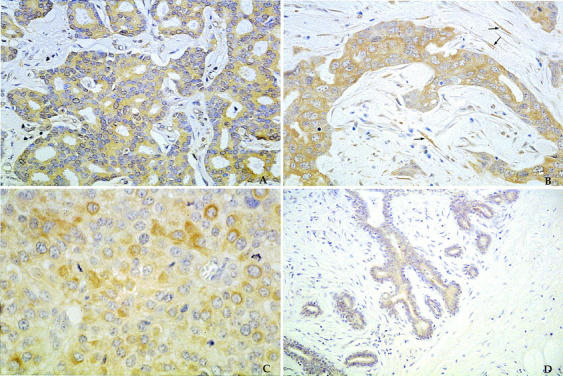
Immunoperoxidase staining with diaminobenzidine as the brown chromogen showing strong uPA expression in a grade I invasive ductal carcinoma (A); strong uPAR expression in the tumor cells and fibroblasts (arrows) of a grade III ductal carcinoma (B); strong PAI-1 expression in a grade III ductal carcinoma (C); and weak uPA expression in the epithelial cells of a fibroadenoma (D). Original magnification, ×40.
Correlations between uPA, uPAR, and PAI-1 Immunostaining
The statistical data with correlation coefficients and P values are outlined in Table 1 ▶ . There was a statistically significant association between tumor cell and fibroblast expression for all three proteins. Ninety percent (128 of 142) of cases had expression of uPA in their tumor cells (weak = 29, strong = 99), and in 69% of cases (98 of 142) it was present in the fibroblasts (weak = 44, strong = 54). There was a significant positive correlation between tumor epithelial and fibroblast expression (r = 0.73, P < 0.005). Tumor epithelial expression of uPA was significantly correlated to the other markers with the exception of fibroblastic expression of PAI-1. Fibroblastic uPA expression was significantly associated with uPAR and PAI-1 in fibroblasts, but not in tumor cells. Expression of the receptor protein uPAR was seen in the tumor cells of 97% (138 of 142) of cases (weak = 55, strong = 83), with 86% (122 of 142) of cases expressing it in the fibroblasts (weak = 49, strong = 73). As with uPA, there was a positive correlation between tumor cell and fibroblastic uPAR expression (r = 0.38, P = 0.02) (Table 1) ▶ . uPAR expression in tumor cells also correlated with PAI-1 in tumor cells but not fibroblasts. However, its expression in fibroblasts was significantly associated with PAI-1 fibroblastic expression. Ninety-three percent (132 of 142) of cases were PAI-1-positive in tumor cells (weak = 72, strong = 60), with 47% (67 of 142) expressing it in their fibroblasts (weak = 49, strong = 18). There was a positive correlation between tumor cell and fibroblastic expression (r = 0.57, P < 0.005).
Table 1.
Statistical Relationships between uPA, uPAR, PAI-1 Expression in Tumor Cells and Fibroblasts
| uPA(F) | uPAR | uPAR(F) | PAI-1 | PAI-1(F) | |
|---|---|---|---|---|---|
| uPA | r = 0.73 | r = 0.48 | r = 0.48 | r = 0.43 | n.s. |
| P < 0.005 | P < 0.005 | P = 0.005 | P = 0.01 | ||
| uPA(F) | n.s. | r = 0.56 | n.s. | r = 0.35 | |
| — | P < 0.005 | P = 0.01 | |||
| uPAR | r = 0.38 | r = 0.59 | n.s. | ||
| — | — | P = 0.02 | P < 0.005 | ||
| uPAR(F) | r = 0.38 | r = 0.55 | |||
| — | — | — | P = 0.01 | P < 0.005 | |
| PAI-1 | r = 0.57 | ||||
| — | — | — | — | P < 0.005 |
(F) refers to expression of the specified protein in fibroblasts. n.s., not significant.
Associations between Immunostaining Patterns and Histological Type and Grade
uPA
Strong uPA expression in IDC was seen in the tumor cells of 71 of 89 cases (79%) and the fibroblasts of 42 of 89 (47%) cases (Table 2) ▶ . In both instances, strong expression was significantly related to high tumor grade (tumor cells, chi-square = 12.7, P = 0.013; fibroblasts, chi-square = 13.79, P = 0.008) (Tables 3 and 4) ▶ ▶ . There was no statistically significant difference in uPA expression between the two invasive tumor types (ductal and lobular) in either the tumor cells or fibroblasts (Table 4) ▶ . However, there were differences between invasive and in situ tumors. Significantly fewer DCIS cases expressed uPA strongly in tumor cells compared to invasive tumors (28% versus 78%, chi-square = 25.03, P < 0.0001). Similarly in fibroblasts, only two cases (8%) were strongly positive in contrast to 44% of the invasive tumors (chi-square = 39.89, P < 0.0001) (Table 4) ▶ . Because of the relatively small numbers of each DCIS subtype it was not possible to produce meaningful statistics to ascertain any differences in expression between the subtypes. (This was also true for uPAR and PAI-1.)
Table 2.
Proportion of Cases Showing Strong Expression of uPA, uPAR, and PAI-1 According to Tumor Type, Tumor Size, and Nodal Status
| Proportion of cases with strong expression, n (%) | ||||||
|---|---|---|---|---|---|---|
| uPA | uPAR | PAI-1 | ||||
| Tumor | Fibroblasts | Tumor | Fibroblasts | Tumor | Fibroblasts | |
| Histological tumor type* | ||||||
| IDC (n = 89) | 71 (79) | 42 (47) | 53 (59) | 57 (64) | 41 (46) | 17 (19) |
| Grade I (28) | 19 (68) | 8 (28) | 18 (64) | 18 (64) | 17 (61) | 8 (29) |
| Grade II (31) | 25 (81) | 15 (49) | 20 (65) | 15 (48) | 7 (22) | 1 (3) |
| Grade III (30) | 27 (90) | 19 (64) | 15 (50) | 24 (80) | 17 (57) | 8 (27) |
| ILC (n = 28) | 21 (75) | 10 (36) | 16 (57) | 12 (43) | 10 (28) | 1 (4) |
| DCIS (n = 25) | 7 (28) | 2 (8) | 14 (56) | 4 (16) | 9 (36) | 0 |
| WD (7) | 4 (57) | 1 (14) | 5 (71) | 2 (29) | 4 (57) | 0 |
| ID (9) | 2 (22) | 1 (11) | 5 (55) | 2 (22) | 3 (33) | 0 |
| PD (9) | 1 (11) | 0 | 4 (44) | 0 | 2 (22) | 0 |
| Tumor size | ||||||
| ≤2 cm (80) | 53 (66) | 28 (35) | 48 (60) | 35 (44) | 30 (38) | 12 (15) |
| >2 cm (58) | 44 (76) | 26 (44) | 32 (55) | 36 (62) | 28 (48) | 6 (10) |
| Nodal status | ||||||
| Negative (44) | 37 (84) | 18 (41) | 27 (61) | 49 (66) | 19 (43) | 5 (11) |
| Positive (76) | 56 (74) | 35 (46) | 43 (57) | 41 (54) | 33 (43) | 13 (17) |
Table 3.
Relationship between Tumor Cell/Fibroblastic Expression of uPA, uPAR, and PAI-1 and Tumor Grade in Invasive Ductal Carcinoma (IDC)
| IDC I n = 28 (%) | IDC II n = 31 (%) | IDC III n = 30 (%) | Chi-square value | P value | |
|---|---|---|---|---|---|
| uPA | |||||
| Negative | 5 (18) | 0 | 0 | ||
| Weak | 4 (14) | 6 (19) | 3 (10) | ||
| Strong | 19 (68) | 25 (81) | 27 (90) | 12.7 | 0.013 |
| uPA(F) | |||||
| Negative | 11 (40) | 5 (16) | 1 (3) | ||
| Weak | 9 (32) | 11 (35) | 10 (33) | ||
| Strong | 8 (28) | 15 (49) | 19 (64) | 13.79 | 0.008 |
| uPAR | |||||
| Negative | 2 (7) | 0 | 1 (3) | ||
| Weak | 8 (29) | 11 (35) | 14 (47) | ||
| Strong | 18 (64) | 20 (65) | 15 (50) | 4.23 | 0.376 |
| uPAR(F) | |||||
| Negative | 1 (4) | 3 (10) | 2 (7) | ||
| Weak | 9 (32) | 13 (42) | 4 (13) | ||
| Strong | 18 (64) | 15 (48) | 24 (80) | 7.58 | 0.108 |
| PAI-1 | |||||
| Negative | 0 | 3 (10) | 3 (10) | ||
| Weak | 11 (39) | 21 (68) | 10 (33) | ||
| Strong | 17 (61) | 7 (22) | 17 (57) | 12.87 | 0.012 |
| PAI-1(F) | |||||
| Negative | 6 (21) | 22 (71) | 9 (30) | ||
| Weak | 14 (50) | 8 (26) | 13 (43) | ||
| Strong | 8 (29) | 1 (3) | 8 (27) | 18.82 | <0.001 |
(F) refers to expression of the specified protein in fibroblasts.
Table 4.
Summary of Statistical Relationships between uPA, uPAR, and PAI-1 Expression and Pathological and Clinical Data
| uPA | uPAR | PAI-1 | ||||
|---|---|---|---|---|---|---|
| Tumor | Fibroblasts | Tumor | Fibroblasts | Tumor | Fibroblasts | |
| Grade | χ2 = 12.7, P = 0.013 | χ2 = 13.79, P = 0.008 | n.s. | n.s. | χ2 = 12.87, P = 0.012 | χ2 = 18.82, P < 0.001 |
| Ductal versus lobular | n.s. | n.s. | n.s. | n.s. | n.s. | χ2 = 8.45, P = 0.015 |
| In situ versus infiltrating | χ2 = 25.03, P < 0.0001 | χ2 = 39.89, P < 0.0001 | n.s. | χ2 = 22.66, P < 0.0001 | n.s. | χ2 = 6.3, P = 0.042 |
| Tumor size | n.s. | χ2 = 6.25, P = 0.044 | n.s. | χ2 = 6.03, P = 0.049 | n.s. | n.s. |
| Nodal status | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
n.s., not significant.
Note: Due to the small numbers of DCIS types, statistical correlations between these and PA component expression were not carried out.
uPAR
Strong uPAR expression in IDC was seen in the tumor cells of 59% of cases and in the fibroblasts of 64% (Table 2) ▶ . uPAR expression in both tumor cells and fibroblasts was unrelated to either tumor grade or tumor type (Tables 3 and 4) ▶ ▶ . Interestingly, there was no significant difference in uPAR expression between the tumor cells of invasive and in situ carcinomas. Contrary to this, fibroblasts in DCIS were predominantly negative or weak, with only a few (4 of 25, 16%) being strongly positive, significantly lower than the proportion (59%) seen in invasive tumors (chi-square = 22.66, P < 0.0001) (Table 4) ▶ .
PAI-1
In IDC, strong PAI-1 expression was seen in the tumor cells of 46% (41 of 89) of cases and the fibroblasts of 17 of 89 (19%) cases (Table 2) ▶ . Strong expression was significantly related to high tumor grade in both tumor cells (chi-square = 12.87, P = 0.012) and fibroblasts (chi-square = 18.82, P < 0.001) (Tables 3 and 4) ▶ ▶ . There was a significant difference in PAI-1 expression in the fibroblasts of invasive lobular and ductal carcinomas, but not in the tumor cells; in lobular carcinomas the fibroblasts were predominantly negative with only one strong case (chi-square = 8.45, P = 0.015). Similarly in DCIS, PAI-1 expression in the fibroblasts, rather than the tumor cells, was significantly lower (none were strong) than in invasive tumors where strong expression was observed in the fibroblasts in 15% of cases (chi-square = 6.3, P = 0.042) (Table 4) ▶ .
Expression of uPA, uPAR, and PAI-1 in Normal and Benign Breast Tissue
Expression of all three proteins in the normal and benign breast lesions was similar. The epithelial cells in the normal cases were either negative or occasionally weakly positive, whereas the fibroblasts rarely stained. This observation was also seen in the inflammatory cases. Weak expression was common in the fibroadenomas and papillomas, with small, focally stronger areas in sclerosing adenomas. Once again, fibroblasts rarely stained.
Correlation between uPA, uPAR, and PAI-1 Expression and Tumor Size and Nodal Status
Strong fibroblastic expression of both uPA and uPAR were significantly associated with tumor size grouped as ≤ and >2 cm (uPA: chi-square = 6.25, P = 0.044; uPAR: chi-square = 6.03, P = 0.049) (Table 4) ▶ . These associations were confirmed using Spearman’s rank correlation test with tumor size treated as a continuous variable (uPA: r = 0.27, P = 0.01; uPAR: r = 0.34. P = 0.005). Tumor cell expression of uPA and uPAR was not similarly related. There was no correlation between PAI-1 expression and tumor size, and none of the three proteins were related to nodal status.
(A summary of the statistical relationships between uPA, uPAR, and PAI-1 expression and pathological and clinical data are given in Table 4 ▶ ).
With the aim of assessing the total effect of protein expression in a manner similar to the cytosol assays, we also combined tumor cell and fibroblastic values for uPA, uPAR, and PAI-1 for each tumor and related this single score to the same parameters and clinical data. However, the values were similar and doing this did not change the magnitude of the results (data not shown).
Survival Data
Log-rank analysis for relapse-free survival among patients with invasive tumors showed a nonsignificant trend for patients with strong uPA expression in fibroblasts, but not in tumor cells, to have a shorter time to relapse (Figure 2A) ▶ . There was no relationship between relapse-free survival and expression of either uPAR or PAI-1 in either tumor cells or fibroblasts.
Figure 2.
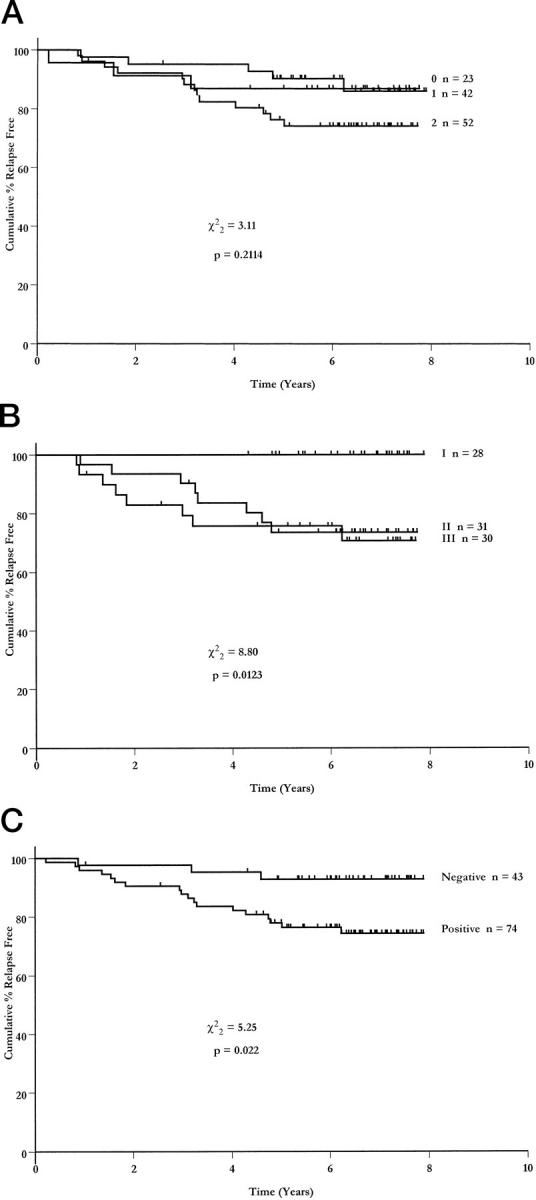
Relapse-free survival curves stratified according to intensity of uPA expression (0 = negative, 1 = weak, 2 = strong) (A), tumor grade (B), and nodal status (C).
However, despite this being a selected group of patients, with a higher than usual proportion of tumors with good prognostic characteristics, two established prognostic parameters, namely tumor grade and nodal status, were both significantly associated with relapse-free survival (chi-square = 8.8, P = 0.012, Figure 2B ▶ and chi-square = 5.25, P = 0.022, Figure 2C ▶ , respectively).
Discussion
Biochemical assays using cytosols prepared from mammary carcinomas show that high levels of expression of uPA and other components of the urokinase system are associated with a poor prognosis. 3,10 We undertook the present immunohistochemical study, one of the largest to date to assess simultaneously uPA, uPAR, and PAI-1, to determine whether it was tumor cell, fibroblastic expression, or both that was most closely correlated to breast tumor pathology. We found that all three proteins were expressed more widely in breast tumors than in normal and benign breast tissues. Significantly stronger expression was noted in invasive compared to in situ tumors, and strong expression was a feature of high-grade tumors. There were significant associations between strong expression of uPA and uPAR and tumor size, but none of the proteins were associated with nodal status. Although there was a trend for patients with strong uPA expression to have a shorter time to relapse, this was not significant. Interestingly, all of these associations were stronger when expression in fibroblasts rather than tumor cells was examined, thus reflecting the importance and hinting at the collaboration of the stroma and its components in the invasive process.
Immunostaining for all three antibodies was cytoplasmic. The lack of membrane expression with uPAR was a little unexpected as it is a trans-membrane receptor molecule. The antibody uPAR2 could be recognizing cytosolic uPAR or a cytoplasmic domain. It is also possible that either antibody binding was inhibited by uPAR binding to its ligand, uPA, or that the epitope recognized by the antibody at the membrane was hidden by conformational changes in the uPAR molecule. 13 Antigen-retrieval techniques have been used by others to produce membrane immunostaining in addition to the cytoplasmic staining that we observed. 14 However, on our material such treatment rendered tissue sections unreadable with a very high background. Another group also using the antibody uPAR2 used pronase digestion but do not describe membrane staining. 15
It is not surprising that these proteins are present in nonmalignant breast tissue as they all have roles in normal physiology. 5 However, the increased expression seen in the tumoral fibroblasts as well as the malignant epithelial cells in tumors implies that there is a specific response to tumor growth. The lower levels found in fibroblasts within the actively fibrosing benign conditions also indicates that there is more to the tumor/stroma response than is suggested by the concept that tumors are wounds that do not heal. 16 The differences between invasive tumors and DCIS suggest that loss of the basement membrane and a myoepithelial layer are necessary for full expression of these molecules, which is possibly mediated through local cytokines and/or changes in the spectrum of adhesion molecules expressed and interacting with the stroma.
In the IDCs strong expression of uPA, uPAR, and PAI-1 was associated with high tumor grade. Although few other immunohistochemical studies looked at tumor grade specifically, our results are broadly in accordance with other studies using immunohistochemistry. 17-21 Christensen et al 22 found a subgroup of poorly differentiated tumors in their study in which the fibroblasts stained intensely. Umeda et al 18 found significantly more uPA staining in IDC (NOS) containing abundant stroma compared to those with scanty stroma, whereas Jahkola et al, 23 found no association. These findings may contribute to the aggression of grade III tumors or could conceivably be an epi-phenomenon related to other features associated with higher grade such as enhanced proliferative activity or relative lack of tubule formation. Equally these observations may go hand in hand with observations suggesting that grade defines groups of different breast cancers with different cytogenetics and biologies. 24 The increased expression of PAI-1 at first seems paradoxical, as one might predict that an inhibitor of the plasminogen activator system would be reduced during invasion. However, it has been proposed that PAI-1 is increased at some point during the invasion process, to prevent proteolysis of the tumor itself. 25 There is also evidence for increased expression of PAI-1 in lymph node metastases. 22,23
One might also expect to see associations between high levels of the proteins and clinicopathological markers of tumor spread such as tumor size or nodal status. We observed significant increases in the fibroblastic, but not tumor cell, expression of uPA and uPAR within larger tumors. Protein levels showed an exponential increase with tumor size as well as a significant difference in levels of expression between tumors >2 cm and those measuring less than this. This suggests that not only do uPA and uPAR fibroblastic expression have a direct association with increasing tumor size but that they also may have a potential prognostic role, because larger tumors generally have a worse prognosis. These findings are supported by a study using mouse models injected with rat breast-cancer cell lines transfected with uPAR-overexpressing plasmids, which were found to grow larger tumors compared to those injected with vector cells alone. 26 Few studies have used immunohistochemistry to assess this relationship in clinical material. One study failed to find an association between uPAR expression and either tumor size or nodal status. 21 Another found no correlation between uPA or PAI-2 and nodal status. 18 In the present study, we found no association between uPA, uPAR, or PAI-1 and nodal status.
The main purpose of this study was to determine which particular cell types expressed uPA, uPAR, and PAI-1, and to ascertain which of them was most im-portant in aiding tumor growth, as judged by their relationship to traditional clinicopathological parameters. This aim has become more important in recent years as evidence grows for components of the PA system to have potential prognostic or therapeutic uses in breast cancer. Until now, the larger studies have used techniques such as enzyme immunoassay and enzyme-linked immunosorbent assay on cytosol material. 19,27-32 The results of these studies regarding interassociations between the various PA components and also with clinicopathological and prognostic data have subsequently been reproduced to varying degrees in immunohistochemical studies. Importantly, where studies have directly compared results between cytosolic and immunohistochemical assays, there is broad agreement between protein levels and degree of immunostaining. 19,22,33 Although one study 33 did point to an overlap in individual values, suggesting that the two methods could not easily be interchanged. However, although cytosol assays do find differences between samples from normal, benign, and malignant breast tissues, they are unable to determine the cellular location of the PA components.
Unlike many of these cytosol-based studies, and a small number of the immunohistochemical studies, we found no significant association between PA component expression and patient outcome. It is likely that this is a reflection of our study population that has relatively few events (relapses and deaths) occurring during the follow-up period (median, 6.5 years), probably because of the relatively high proportion of tumors with a good prognosis within the group. However, because there was a trend for patients with strong uPA-expressing tumors to have a shorter time to relapse, longer follow-up or increased patient numbers may result in associations becoming more evident.
We observed that stromal fibroblasts expressed these proteins as well as tumor cells. In fact, our results suggest that it is this stromal expression, rather than tumor expression, which is more directly related to tumor behavior (a distinction that could not be observed using cytosol-based assays). Whenever a correlation was found between uPA, uPAR, and PAI-1 and any of the parameters studied, the relationship was strongest with the fibroblastic expression and, in the case of uPA, this expression was strongest in those patients who had a shorter relapse-free survival. There is evidence that in breast cancer the PA components are synthesized by both epithelial and stromal cells. 34,35 This is consistent with findings that underline the importance in tumor development and metastases of the production and activity of various matrix-degrading enzymes such as stromelysin-3 (one of the matrix metalloproteinases) and regulators by stromal cells. 6,36-38
This study builds on earlier work that has found that expression of uPA, uPAR, and PAI-1 differ significantly between benign and malignant breast disease. We have also found a difference between in situ and invasive tumors with stronger expression in high-grade tumors. Interestingly, we noted that expression in fibroblasts rather than tumor cells might be more important in this regard. These findings could have implications for treatment decisions in patients and also for novel therapy regimens in the future that exploit components of the plasminogen activator system.
Acknowledgments
We thank Dr. Nils Brünner for the donation of his antibodies and also for his encouragement and support during this work; and Dr. Cheryl Gillett and Professor Ian Hart for critical reading of the manuscript.
Footnotes
Address reprint requests to Mr. Edwin A. Dublin, Hedley Atkins/Imperial Cancer Research Fund Breast Pathology Laboratory, 3rd Floor Thomas Guy House, Guy’s Hospital, London SE1 9RT, UK. E-mail: dublin@lcrf.icnet.uk.
Supported by the Imperial Cancer Research Fund.
References
- 1.Rabbani SA, Xing RHM: Role of urokinase (upa) and its receptor (upar) in invasion and metastasis of hormone-dependent malignancies (review). Int J Oncol 1998, 12:911-920 [DOI] [PubMed] [Google Scholar]
- 2.Reuning U, Magdolen V, Wilhelm O, Fischer K, Lutz V, Graeff H, Schmitt M: Multifunctional potential of the plasminogen activation system in tumor invasion and metastasis (review). Int J Oncol 1998, 13:893-906 [DOI] [PubMed] [Google Scholar]
- 3.Stephens RW, Brunner N, Janicke F, Schmitt M: The urokinase plasminogen activator system as a target for prognostic studies in breast cancer. Breast Cancer Res Treat 1998, 52:99-111 [DOI] [PubMed] [Google Scholar]
- 4.Brunner N, Pyke C, Hansen CH, Romer J, Grondahl-Hansen J, Dano K: Urokinase plasminogen activator (uPA) and its type 1 inhibitor (PAI-1): regulators of proteolysis during cancer invasion and prognostic parameters in breast cancer. Dickson R Lippman M eds. Mammary Tumorigenesis and Malignant Progression. 1994, :pp 299-309 The Netherlands, Kluwer Academic Publishers, Dordrecht [DOI] [PubMed] [Google Scholar]
- 5.Mignatti P, Robbins E, Rifkin DB: Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell 1986, 47:487-498 [DOI] [PubMed] [Google Scholar]
- 6.Duffy MJ: The role of proteolytic enzymes in cancer invasion and metastasis. Clin Exp Metastasis 1992, 10:145-155 [DOI] [PubMed] [Google Scholar]
- 7.Dano K, Andreasen PA, Grondahl-Hansen K, Kristensen P, Nielsen LS, Skriver L: Plasminogen activators, tissue degradation and cancer. Adv Cancer Res 1985, 44:139-266 [DOI] [PubMed] [Google Scholar]
- 8.Ploug M, Ronne E, Behrendt N, Brunner N, Jensen AL, Blasi F, Dano K: Cellular receptor for urokinase plasminogen activator. Carboxyl terminal processing and membrane anchoring by glycosyl-phosphatidyl inositol. J Biol Chem 1991, 266:1926-1933 [PubMed] [Google Scholar]
- 9.Andreasen PA, Nielsen LS, Kristensen P, Grondahl-Hansen J, Skriver L, Dano K: Plasminogen activator inhibitor from human fibrosarcoma cells binds urokinase-type plasminogen activator, but not its proenzyme. J Biol Chem 1986, 261:7644-7651 [PubMed] [Google Scholar]
- 10.Duffy MJ, Maguire TM, McDermott EW, O’Higgins N: Urokinase plasminogen activator: a prognostic marker in multiple types of cancer. J Surg Oncol 1999, 71:130-135 [DOI] [PubMed] [Google Scholar]
- 11.Elston CW, Ellis IO: Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991, 19:403-410 [DOI] [PubMed] [Google Scholar]
- 12.Holland R, Peterse JL, Millis RR, Eusebi V, Faverley D, Vijver MJVD, Zafrani B: Ductal carcinoma in situ: a proposal for a new classification. Semin Diagn Pathol 1994, 11:167-180 [PubMed] [Google Scholar]
- 13.Hildenbrand R, Glienke W, Magdolen V, Graeff H, Stutte HJ, Schmitt M: Urokinase receptor localization in breast-cancer and benign lesions assessed by in-situ hybridization and immunohistochemistry. Histochem Cell Biol 1998, 110:27-32 [DOI] [PubMed] [Google Scholar]
- 14.Ferrier CM, Geloof WLV, Witte HHD, Kramer MD, Ruiter DJ, Muijen GNPV: Epitopes of components of the plasminogen activation system are re-exposed in formalin-fixed paraffin sections by different retrieval techniques. J Histochem Cytochem 1998, 46:469-476 [DOI] [PubMed] [Google Scholar]
- 15.Kennedy S, Duffy MJ, Duggan C, Barnes C, Rafferty R, Kramer MD: Semi-quantitation of urokinase plasminogen activator and its receptor in breast carcinomas by immunohistochemistry. Br J Cancer 1998, 77:1638-1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dvorak HF: Tumours: wounds that do not heal. Similarities between tumour stroma generation and wound healing. N Engl J Med 1986, 315:1650-1659 [DOI] [PubMed] [Google Scholar]
- 17.Costantini V, Sidoni A, Deveglia R, Cazzato OA, Ferri I, Bucciarelli E, Nenci GG: Combined overexpression of urokinase, urokinase receptor, and plasminogen activator inhibitor-1 is associated with breast cancer progression. An immunohistochemical comparison of normal, benign, and malignant breast tissues. Cancer 1996, 77:1079-1088 [DOI] [PubMed] [Google Scholar]
- 18.Umeda T, Eguchi Y, Okino K, Kodama M, Hattori T: Cellular localization of urokinase-type plasminogen activator, its inhibitors, and their mRNAs in breast cancer tissues. J Pathol 1997, 183:388-397 [DOI] [PubMed] [Google Scholar]
- 19.Janicke F, Schmitt M, Hafter R, Hollrieder A, Babic R, Ulm K, Gossmer W, Graeff H: Urokinase-type plasminogen activator (u-PA) antigen is a predictor of early relapse in breast cancer. Fibrinolysis 1990, 4:69-78 [Google Scholar]
- 20.Jankun J, Merrick HW, Goldblatt PJ: Expression and localization of elements of the plasminogen activation system in benign breast disease and breast cancers. J Cell Biochem 1993, 53:135-144 [DOI] [PubMed] [Google Scholar]
- 21.Kim SJ, Shiba E, Taguchi T, Watanabe T, Tanji Y, Kimoto Y, Izukura M, Takai S-I: Urokinase type plasminogen activator receptor is a novel prognostic factor in breast cancer. Anticancer Res 1997, 17:1373-1378 [PubMed] [Google Scholar]
- 22.Christensen L, Simonsen ACW, Heegaard CW, Moestrup SK, Andersen JA, Andreasen PA: Immunohistochemical localization of urokinase-type plasminogen activator, type-1 plasminogen-activator inhibitor, urokinase receptor and α2-macroglobulin receptor in human breast carcinomas. Int J Cancer 1996, 66:441-452 [DOI] [PubMed] [Google Scholar]
- 23.Jahkola T, Toivonen T, von Smitten K, Virtanen I, Wasenius VM, Blomqvist C: Cathepsin-D, urokinase plasminogen activator and type-1 plasminogen activator inhibitor in early breast cancer: an immunohistochemical study of prognostic value and relations to tenascin-C and other factors. Br J Cancer 1999, 80:167-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roylance R, Gorman P, Harris W, Liebman R, Barnes D, Hanby A, Sheer D: Comparative genomic hybridisation of breast tumours stratified by histological grade reveals new insights into the biological progression of breast cancer. Cancer Res 1999, 59:1433-1436 [PubMed] [Google Scholar]
- 25.Bianchi E, Cohen RL, Dai A, Thor AT, Shuman MA, Smith HS: Immunohistochemical localization of the plasminogen activator inhibitor-1 in breast cancer. Int J Cancer 1995, 60:597-603 [DOI] [PubMed] [Google Scholar]
- 26.Xing RH, Rabbani SA: Overexpression of urokinase receptor in breast cancer cells results in increased tumor invasion, growth and metastasis. Int J Cancer 1996, 67:423-429 [DOI] [PubMed] [Google Scholar]
- 27.Grondahl-Hansen J, Hilsenbeck SG, Christensen IJ, Clark GM, Osborne CK, Brunner N: Prognostic significance of PAI-1 and uPA in cytosolic extracts obtained from node-positive breast cancer patients. Breast Cancer Res Treat 1997, 43:153-163 [DOI] [PubMed] [Google Scholar]
- 28.Janicke F, Schmitt M, Pache L, Ulm K, Harbeck N, Hofler H, Graeff H: Urokinase (uPA) and its inhibitor PAI-1 are strong and independent prognostic factors in node-negative breast cancer. Breast Cancer Res Treat 1993, 24:195-208 [DOI] [PubMed] [Google Scholar]
- 29.de Witte JH, Sweep CGJ, Klijn JGM, Grebenschikov N, Peters HA, Look MP, van Tienoven TH, Heuvel JJTM, van Putten WLJ, Benraad TJ, Foeken JA: Prognostic impact of urokinase-type plasminogen activator (uPA) and its inhibitor (PAI-1) in cytosols and pellet extracts derived from 892 breast cancer patients. Br J Cancer 1999, 79:1190-1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harbeck N, Dettmar P, Thomssen C, Henselmann B, Kuhn W, Ulm K, Janicke F, Hofler H, Graeff H, Schmitt M: Prognostic impact of tumor biological factors on survival in node-negative breast-cancer. Anticancer Res 1998, 18:2187-2197 [PubMed] [Google Scholar]
- 31.Thomssen C, Oppelt P, Janicke F, Ulm K, Harbeck N, Hofler H, Kuhn W, Graeff H, Schmitt M: Identification of low-risk node-negative breast-cancer patients by tumor biological factors pai-1 and cathepsin-l. Anticancer Res 1998, 18:2173-2180 [PubMed] [Google Scholar]
- 32.Schmitt M, Thomssen C, Ulm K, Seiderer A, Harbeck N, Hofler H, Janicke F, Graef H: Time-varying prognostic impact of tumour biological factors urokinase (uPA), PAI-1 and steroid hormone receptor status in primary breast cancer. Br J Cancer 1997, 76:306-311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrier CM, Witte HHD, Straatman H, Tienoven DHV, Geloof WLV, Rietveld FJR, Sweep CGJ, Ruiter DJ, Muijen GNPV: Comparison of immunohistochemistry with immunoassay (ELISA) for the detection of components of the plasminogen activation system in human tumour tissue. Br J Cancer 1999, 79:1534-1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolf C, Rouer N, Lutz Y, Adida C, Loriot M, Bellocq JP, Chambon P, Basset P: Stromelysin 3 belongs to a sub-group of proteinases in breast carcinoma fibroblastic cells and possibly implicated in tumor progression. Proc Natl Acad Sci USA 1993, 90:1843-1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen BS, Sehested M, Timshel M, Pyke C, Dano K: Messenger RNA for urokinase plasminogen activator is expressed in myofibroblasts adjacent to cancer cells in human breast cancer. Lab Invest 1996, 74:168-177 [PubMed] [Google Scholar]
- 36.Basset P, Bellocq JP, Wolf C, Stoll I, Hutin P, Limacher JM, Podhajcer OL, Chenard MP, Rio MC, Chambon P: A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature 1990, 348:699-704 [DOI] [PubMed] [Google Scholar]
- 37.Ahmad A, Hanby A, Dublin E, Poulsom R, Smith P, Barnes D, Rubens R, Anglard P, Hart I: Stromelysin-3—an independent prognostic factor for relapse-free survival in node-positive breast-cancer and demonstration of novel breast-carcinoma cell expression. Am J Pathol 1998, 152:721-728 [PMC free article] [PubMed] [Google Scholar]
- 38.van den Hooff A: The role of stromal cells in tumor metastasis: a new link. Cancer Cells 1991, 3:65-71 [PubMed] [Google Scholar]


