Abstract
Extensive limbal injury is a leading cause of irreversible blindness. The destruction of corneal limbal stem cells often results in corneal neovascularization and an optically inferior epithelium. Previous work has shown that the neovascularization after limbal injury is vascular endothelial growth factor (VEGF)-dependent, with much of the VEGF emanating from the inflammatory cells that invade the cornea. Using a relevant mouse model of limbal injury, we examined the role of CD18 and intercellular adhesion molecule-1 (ICAM-1) in limbal injury-induced neovascularization. The results show that CD18- and ICAM-1-deficient mice developed 35% (n = 5, P = 0.003) and 36% (n = 5, P = 0.002) less neovascularization than strain-specific normal controls, respectively. The corneal neutrophil counts were similarly reduced by 51% (n = 5, P < 0.003) and 46% (n = 5, P < 0.006), respectively. When VEGF mRNA levels were analyzed, they were reduced by 66% (n = 3, P = 0.004) and 48% (n = 3, P = 0.024), respectively. Taken together, these data identify CD-18 and ICAM-1 as mediators of the inflammatory and VEGF-dependent corneal neovascularization that follows limbal injury. The targeting of CD18 and ICAM-1 may prove useful in the treatment of inflammation-associated neovascularization in the cornea and elsewhere.
When the ocular surface suffers extensive damage, as occurs with alkali injury, corneal limbal stem cells are destroyed. When the corneal limbal stem cells are depleted below a certain threshold, the cornea becomes covered by an abnormal conjunctiva-like epithelium. The process has been termed “conjunctivalization.” 1 A conjunctivalized corneal epithelium lacks the smoothness and cohesion of the normal corneal epithelium, making it optically inferior and prone to erosions. It is also heavily vascularized.
Corneal neovascularization may be required for conjunctivalization. When a laser was used to selectively photothrombose corneal vessels in an animal model of limbal injury, the conjunctivalized corneal epithelium reverted to a more normal corneal epithelial phenotype. 2 Although the epithelium covering the cornea remained conjunctival in origin, it adopted many of the phenotypic characteristics of corneal epithelium.
The neovascularization that follows limbal injury requires, in part, vascular endothelial growth factor (VEGF). 3 Immunolocalization studies in rats have demonstrated that transmigrating and invading corneal leukocytes provide much of the requisite VEGF that drives corneal neovascularization. 3 Because leukocytes use adhesion molecules in the course of the inflammatory response, the role of these molecules, specifically CD18 and intercellular adhesion molecule-1 (ICAM-1), was explored in a mouse model of limbal injury and corneal neovascularization.
Materials and Methods
Corneal Neovascularization Model
All animal experiments were approved by the Children’s Hospital Animal Care and Use Committee and conformed to the Association for Research in Vision and Ophthalmology guidelines. Male CD 18-deficient mice (C57BL/6J-Itgb2tm1bay) 4 and ICAM-1-deficient mice (C57BL/6J-Icam1tm1Bay) 5 were purchased from Jackson Labs. (Bar Harbor, ME), as were normal male C57BL/6J mice, which served as controls. The mice were anesthetized with 70 to 80 mg/kg intraperitoneal Nembutal sodium solution (Abbott Laboratories, North Chicago, IL). NaOH (1.2 μl of 0.15 mol/L) was applied topically and the corneal and limbal epithelia were removed using a Tooke corneal knife (Arista Surgical Supply, Inc., New York, NY). A rotary motion parallel to limbus was used to remove the limbal epithelium. Erythromycin ophthalmic ointment (Pfizer Inc., New York, NY) was instilled immediately after epithelial debridement.
Measurement of Corneal Neovascularization
On days 2, 4, and 7 after epithelial debridement, cohorts of mice received 8 μg/g tail vein injections of an endothelial-specific fluorescein-conjugated lectin (Lycopersicon esculentum) (Vector Laboratories, Burlingame, CA). Thirty minutes later, the eyes were harvested and fixed with 10% neutral buffered formalin for 24 hours. The corneas were isolated and flat-mounted on glass slides. The fluorescence in the perfused vessels was captured using a CD-330 charge-coupled device camera (Dage-MTI, Inc., Michigan, IN) attached to a Leica MZ FLIII fluorescence microscope (Leica Microsystems Inc., Deerfield, IL) controlled by Openlab software (Improvision Inc., Lexington, MA). The images were fed into a Macintosh 6500 computer (Apple, Cupertino, CA) and were resolved at 624 × 480 pixels and converted to tagged information file format (.tiff) files. NIH Image 1.62 (National Institutes of Health, Bethesda, MD) was used for the image analysis and a modified quantitation protocol was used. 6 Briefly, the neovascularization was quantified by setting a threshold level of fluorescence, above which only vessels were captured. The entire flat-mounted cornea was analyzed to minimize sampling bias. The neovascularization quantitation was performed in a masked manner. The total corneal area was outlined using the innermost vessel of the limbal arcade as the border. The total neovascularization area was then normalized to the total corneal area. The percent of the cornea covered by vessels was then calculated.
Corneal Polymorphonucleocyte (PMN) Counts
PMN were quantified in the corneas on days 2, 4, and 7 after epithelial debridement. The corneas were fixed in 10% neutral buffered formalin, embedded in paraffin, cut into 5-μm-thick sections and transferred to glass slides. The cornea sections were stained in modified Geimsa (Sigma Diagnostics, St. Louis, MO) diluted 1:20 with distilled water for 30 minutes followed by rinsing in distilled water. A cell was deemed a PMN when a multilobed nucleus was clearly identified in cross-section. One cross-sectional slide from the anatomical center of each cornea was analyzed. Five standardized high-powered fields per tissue section were counted using a ×100 objective (two peripheral, two mid-peripheral, and one central). The two peripheral high-powered fields abutted the limbus. The central high-powered fields encompassed the anatomical center of the cornea. The two mid-peripheral high-powered fields were midpoint between the central and peripheral high-powered fields The PMN counts from all five high-powered fields were combined and expressed as PMN counts/five high-powered fields The counts were performed in a masked manner.
Measurement of Corneal VEGF mRNA Levels
On days 2, 4, and 7 after epithelial debridement, total RNA was isolated using the RNAqueous (Ambion Inc., Austin, TX) or RNAzol (Tel-Test, Inc., Friendswood, TX) kits, according to the manufacturer’s instructions. One microgram of total RNA was reverse-transcribed using the RETROscript kit (Ambion Inc.) according to manufacturer’s instructions. Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed on corneal VEGF mRNA using the Gene-Specific Relative RT-PCR system (Ambion Inc.) according to the manufacturer’s instructions. Briefly, the VEGF and 18S primer pairs produced products of 322 bp or 495 bp, respectively. To compensate for the variations in RT and PCR reactions, multiplex RT-PCR was performed using the VEGF and 18S primer sets in a single PCR reaction. The 18S cDNA served as an endogenous control. The level of VEGF product was normalized against the amplified 18S control. A preliminary PCR amplification experiment was performed to determine the range of cycles over which the PCR reaction was quantifiable. The amplification efficiency of the 18S cDNA was titrated to that of VEGF cDNA using the Competimer supplied in the kit (Ambion Inc.). The exponential phases of the amplifications were made to overlap. All PCR quantitation data were obtained from the exponential phase of the amplification. The PCR reaction took place in a 50-μl total volume containing 1-μl cDNA solution, 10× PCR buffer, 2.5 mmol/L each of dNTPs, the supplied VEGF primer pair, the supplied 18S primer:Competimer mixture, 5 U of Taq polymerase, and distilled water. The amplification was performed in a GeneAmp PCR System 2400 (Perkin Elmer Inc., Norwalk, CT) using one cycle at 94°C for 5 minutes, 29 cycles at 94°C for 30 seconds, annealing at 59°C for 30 seconds, extension at 72°C for 30 seconds, and one cycle of final extension at 72°C for 7 minutes. Twenty μl of PCR product was electrophoresed in a 5% acrylamide gel and was stained with CYBR Green (FMC BioProducts, Rockland, ME). The optical density of the bands was quantitated using NIH image 1.62 and expressed in arbitrary units as a ratio of VEGF:18S electrophoretic band optical density.
Statistical Analysis
Statistical analysis was performed using the two-way factorial analysis of variance test followed by Fisher’s protected least significant difference for multiple comparisons. All tests were completed using StatView 5.0 (SAS Institute Inc., Cary, NC). A P value of <0.05 was deemed significant.
Results
Corneal Neovascularization Is Reduced in CD18- and ICAM-1-Deficient Mice
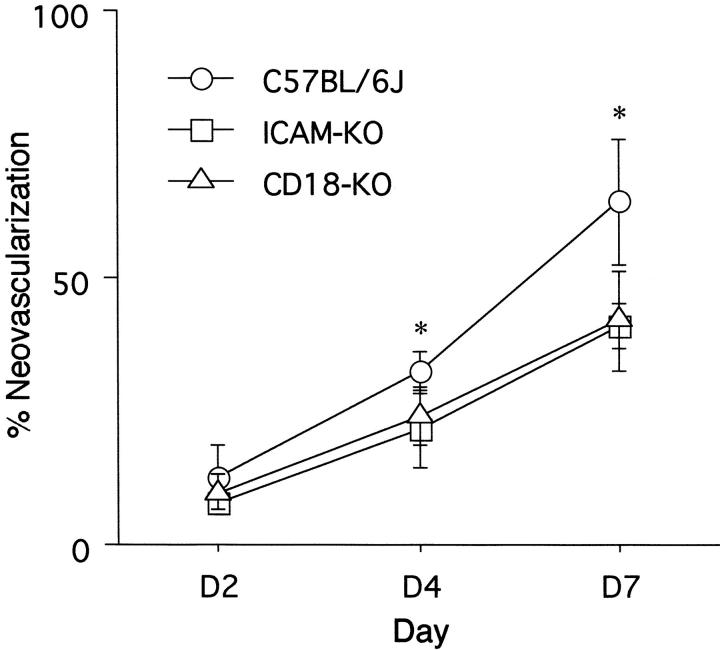
To determine whether CD18 and ICAM-1 are important in the development of the corneal neovascularization after limbal injury, neovascularization was quantitated 2, 4, and 7 days after epithelial debridement. Compared to strain-specific controls, the CD18-deficient mice had 35% less neovascularization (day 7, n = 5, P = 0.003) (Figures 1 and 2) ▶ ▶ . Similarly, the ICAM-1 deficient mice had 36% less neovascularization than the control mice (day 7, n = 5, P = 0.002) (Figures 1 and 2) ▶ ▶ . Uninjured corneas did not exhibit any neovascularization.
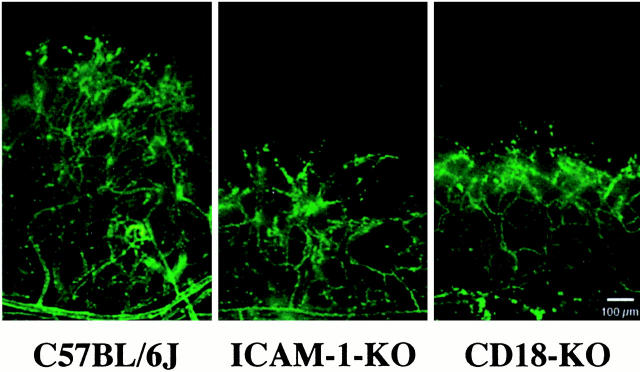
Figure 1.
Examples of corneal neovascularization 7 days after epithelial debridement. The corneas come from a normal C57BL6J mouse (left), an ICAM-1-deficient mouse (middle), and a CD18-deficient mouse (right). Vessels were highlighted via intravenous injections of fluorescein-linked Lycopersicon esculentum lectin. KO, knockout. Original magnification, ×5.12; scale bar, 100 μm. Image size of 200 × 300 pixels is shown from the acquired image of 624 × 480 pixels.
Figure 2.
Percent area of corneal neovascularization 2, 4, and 7 days after epithelial debridement. The area of neovascularization is shown as the percent of total corneal area. KO, knockout. *, Denotes P < 0.05 versus other conditions at same time point.
Corneal PMN Density Is Reduced in CD18- and ICAM-1-Deficient Mice
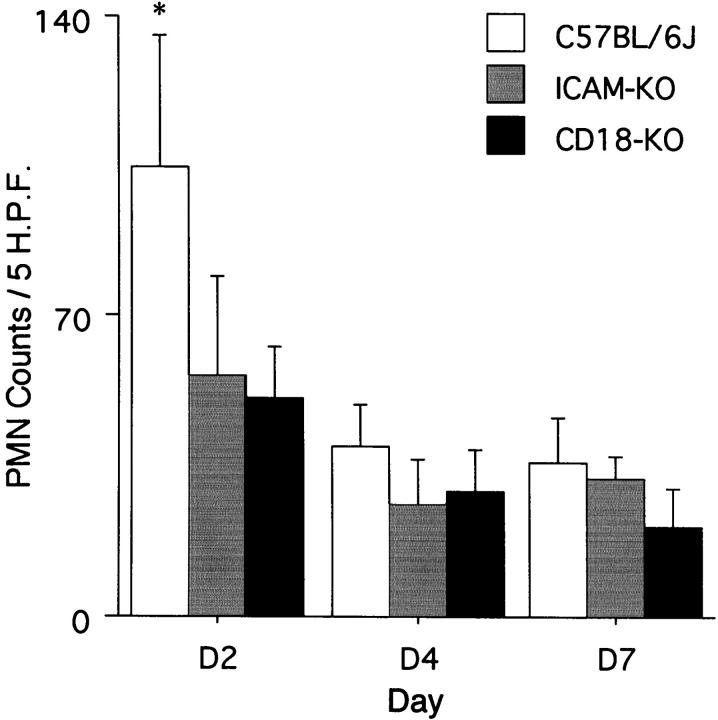
To determine whether the inhibition of corneal neovascularization was associated with decreased inflammation, corneal PMN counts were performed 2, 4, and 7 days after epithelial debridement. The 2-day time point manifested maximum corneal opacity and corneal leukocyte infiltration via slit lamp. Compared to the strain-specific controls, the CD18- and ICAM-1-deficient mice had 51% (day 2, n = 5, P < 0.003) and 46% (day 2, n = 5, P < 0.006) fewer PMN, respectively (Figure 3) ▶ . Uninjured corneas did not possess any PMN.
Figure 3.
The total number of PMN in five standardized high-power fields from corneas 2, 4, and 7 days after epithelial debridement. KO, knockout. *, Denotes P < 0.05 versus other conditions at same time point.
VEGF mRNA Levels Are Reduced in CD18- and ICAM-1-Deficient Mice
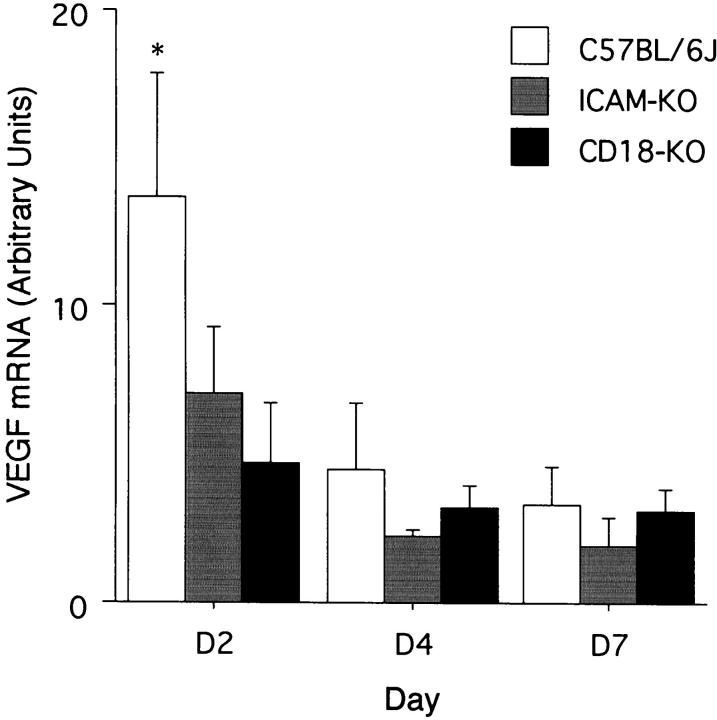
Semiquantitative PCR was performed to assess relative VEGF mRNA levels in the corneas of mice 2, 4, and 7 days after epithelial debridement. The 2-day time point previously manifested maximal VEGF expression. 3 VEGF mRNA levels were decreased by 65% (day 2, 4.72 ± 1.97, P = 0.004) and 48% (day 2, 7.07 ± 2.22, P = 0.024) in the CD18- and ICAM-1-deficient mice, respectively, as compared to the strain-specific controls (day 2, 1.318 ± 0.401) (mean ± SD) (Figure 4) ▶ . VEGF was not detected in the uninjured corneas using the RT-PCR protocol described above.
Figure 4.
VEGF mRNA levels normalized to 18S mRNA, expressed in arbitrary densitometry units, from corneas harvested 2, 4, and 7 days after epithelial debridement. KO, knockout. *, Denotes P < 0.05 versus other conditions at same time point.
Discussion
The results of the current study indicate that CD18 and ICAM-1 are required, in part, for the corneal neovascularization and inflammation that follow limbal injury. Notably, the extent of the requirement for CD18 and ICAM-1 may be greater than what was documented in these studies. This is because the mice used in these studies possess hypomorphic rather than null alleles for CD18 4 and ICAM-1. 7 In the case of the CD18-deficient mice, the homozygous mutant mice retain 2 to 16% of normal granulocyte CD18 expression, the exact amount varying with the state of granulocyte activation. 4 A mild systemic leukocytosis in both knockout mice has been previously reported 4,5 and was confirmed here (data not shown). The systemic leukocytosis makes the corneal granulocytopenia documented in the mutant mice all the more significant. Nevertheless, it is also likely that non-ICAM-1/CD18/VEGF mechanisms are operative. The continued neovascularization after the early peak in VEGF expression and PMN infiltration on day 2 suggests the participation of additional factors. However, taken together, the results strengthen the conclusion that CD18 and ICAM-1 are required for corneal inflammation in this model of limbal injury.
VEGF has previously been shown to be required for corneal neovascularization in this model. 3 The current study extends these data by showing that CD18 and ICAM-1 seem to be causally linked to the VEGF-dependent corneal neovascularization. Corneal leukocytes, via their own VEGF, have been shown to constitute the majority of the VEGF gene expression in the injured cornea. 3 The current data suggest that with the prevention of CD18- and ICAM-1-dependent leukocyte emigration, less leukocyte VEGF is present, thereby suppressing corneal neovascularization. VEGF is known to exist in leukocytes, including neutrophils, 8 monocytes, 9 eosinophils, 10 lymphocytes, 11 and platelets. 12 It is therefore not surprising that VEGF is present in the neutrophils and monocytes that infiltrate the cornea after limbal injury. 3 The fact that some leukocytes possess high-affinity VEGF receptors and migrate in response to VEGF 13 suggests a positive feedback loop may also be operative. Another potential contributor to the feedback loop is VEGF itself, as it can up-regulate ICAM-1, VCAM, and P-selectin, and E-selectin on endothelial cells. 14-16
The results of the current study are also consistent those of Sholley and co-workers. 17 In that study, leukocyte depletion via somatic irradiation and anti-neutrophil antibody infusion was shown to reduce corneal neovascularization. Although their method of inducing corneal neovascularization did not involve limbal injury, a link between inflammation and neovascularization was established.
Finally, because limbal injury characterizes a number of difficult-to-treat conditions, including alkali injury, Stevens-Johnson syndrome, and cicatricial pemphigoid, the current findings suggest that CD18 and ICAM-1 inhibition may be useful in these conditions. All three conditions are characterized by the destruction of limbal stem cells and corneal neovascularization. Small molecule CD18 and ICAM-1 inhibitors may prove useful when applied topically. The current findings may also prove relevant to neovascularization elsewhere in the body, where leukocytes and growing vessels are often found in close proximity. 18
Footnotes
Address reprint requests to Anthony P. Adamis, Massachusetts Eye and Ear Infirmary, 243 Charles St., Boston, MA 02114.
Supported by the National Eye Institute (R01 EY 12611 and EY 11627), the Roberta Siegel Fund, and the Juvenile Diabetes Foundation.
References
- 1.Tsai RJ, Sun TT, Tseng SC: Comparison of limbal and conjunctival autograft transplantation in corneal surface reconstruction in rabbits. Ophthalmology 1990, 97:446-455 [DOI] [PubMed] [Google Scholar]
- 2.Huang AJW, Watson BD, Hernandez E, Tseng SCG: Induction of conjunctival transdifferentiation on vascularized corneas by photothrombotic occlusion of corneal neovascularization. Ophthalmology 1988, 95:228-235 [DOI] [PubMed] [Google Scholar]
- 3.Amano S, Rohan R, Kuroki M, Tolentino M, Adamis AP: Requirement for vascular endothelial growth factor in wound- and inflammation-related corneal neovascularization. Invest Ophthalmol Vis Sci 1998, 39:18-22 [PubMed] [Google Scholar]
- 4.Wilson RW, Ballantyne CM, Smith CW, Montgomery C, Bradley A, O’Brien WE, Beaudet AL: Gene targeting yields a CD18-mutant mouse for study of inflammation. J Immunol 1993, 151:1571-1578 [PubMed] [Google Scholar]
- 5.Sligh JE, Ballantyne CM, Rich SS, Hawkins HK, Smith CW, Bradley A, Beaudet AL: Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci USA 1993, 90:8529-8533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proia AD, Chandler DB, Haynes WL, Smith CF, Suvarnamani C, Erkel FH, Klintworth GK: Methods of laboratory investigation. Quantitation of corneal neovascularization using computerized image analysis. Lab Invest 1988, 58:473-479 [PubMed] [Google Scholar]
- 7.King PD, Sandberg ET, Selvakumar A, Fang P, Beaudet AL, Dupont B: Novel isoforms of murine intercellular adhesion molecule-1 generated by alternative splicing RNA splicing. J Immunol 1995, 154:6080-6093 [PubMed] [Google Scholar]
- 8.Gaudry M, Bregerie O, Andrieu V, El Benna J, Pocidalo MA, Hakim J: Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood 1997, 41:4153-4161 [PubMed] [Google Scholar]
- 9.Iijima K, Yoshikawa N, Connolly DT, Nakamura H: Human mesangial cells and peripheral blood mononuclear cells produce vascular permeability factor. Kidney Int 1993, 44:959-966 [DOI] [PubMed] [Google Scholar]
- 10.Horiuchi T, Weller PF: Expression of vascular endothelial growth factor by human eosinophils: upregulation by granulocyte macrophage colony-stimulating factor and interleukin-5. Am J Respir Cell Mol Biol 1997, 17:70-77 [DOI] [PubMed] [Google Scholar]
- 11.Freeman MR, Schneck FX, Gagnon ML, Corless C, Soker S, Niknejad K, Peoples GE, Klagsbrun M: Peripheral blood T lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: a potential role for T cells in angiogenesis. Cancer Res 1995, 55:4140-4145 [PubMed] [Google Scholar]
- 12.Mohle R, Green D, Moore MA, Nachman RL, Rafii S: Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci USA 1997, 94:663-668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen H, Clauss M, Ryan J, Schmidt AM, Tijburg P, Borden L, Connolly D, Stern D, Kao J: Characterization of vascular permeability factor/vascular endothelial growth factor receptors on mononuclear phagocytes. Blood 1993, 81:2767-2773 [PubMed] [Google Scholar]
- 14.Becker MD, Kruse FE, Azzam L, Nobiling R, Reichling J, Volcker HE: In vivo significance of ICAM-1-dependent leukocyte adhesion in early corneal angiogenesis. Invest Ophthalmol Vis Sci 1999, 40:612-618 [PubMed] [Google Scholar]
- 15.Melder RJ, Koenig GC, Witwer BP, Safabakhsh N, Munn LL, Jain RK: During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat Med 1996, 2:992-997 [DOI] [PubMed] [Google Scholar]
- 16.Detmar M, Brown LF, Schon MP, Elicker BM, Velasco P, Richard L, Fukamura D, Monsky D, Claffey KP, Jain RK: Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol 1998, 111:1-6 [DOI] [PubMed] [Google Scholar]
- 17.Sholley MM, Gimbrone MA, Cotran RS: The effects of leukocyte depletion on corneal neovascularization. Lab Invest 1978, 38:32-40 [PubMed] [Google Scholar]
- 18.Knighton DR, Hunt TK, Scheuenstuhl H, Halliday BJ, Werb Z, Banda MJ: Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science 1983, 221:1283-1285 [DOI] [PubMed] [Google Scholar]